Predictive value of inflammatory and coagulation biomarkers for venous thromboembolism in COVID-19 patients
Abstract
BACKGROUND:
The predictive value of coagulation markers for venous thromboembolism (VTE) in COVID-19 patients has been investigated with conflicting results.
OBJECTIVE:
Our aim was to investigate the correlation between biomarkers and VTE and the predictive value of D-dimer for VTE in hospitalized COVID-19 patients.
METHODS:
Complete blood count, inflammatory and coagulation biomarkers at admission were collected. VTE was defined as diagnosed pulmonary embolism or deep vein thrombosis. Events were defined as in-hospital death or ICU admission. Predictors of VTE were identified with Pearson prediction models. A ROC curve was constructed to assess the predictive value of D-dimer.
RESULTS:
1651 participants were included, 111 VTE were identified. Events incidence was higher in the VTE group (49.5% vs 28.2%, p < 0.001). Neutrophil-lymphocyte ratio (NLR, 0.001; 95% CI 0.000–0.002; p 0.019) and D-dimer (0.00005; 95% CI 0.00002–0.00008; p < 0.001), Geneva score (0.026; 95% CI 0.012–0.040; p < 0.001) and Wells score (0.047; 95% CI 0.033–0.061; p < 0.001) were associated with VTE. D-dimer had a goor predictive value for VTE (ROC area 0.85, 95% CI 0.816–0.893), with an optimal cut-off value of 2677μg/L (Youden index of 0,602).
CONCLUSIONS:
Among coagulation biomarkers D-dimer had the best predictive value for VTE, but higher cut-off values should be used in COVID-19.
1Introduction
Coronavirus disease 2019 (COVID-19) pandemic has caused dramatic consequences on health-care systems and society with more than 611 million infections and over 6.5 million deaths as on September 19, 2022 [1]. In the majority of cases COVID-19 has a favourable outcome, however around 20% of patients develop severe disease, characterised by a massive inflammatory response and a hypercoagulable state [2–5]. The inflammatory response is appraised by changes in complete blood count (decreased haemoglobin, lymphocytes and platelet count, increase in neutrophils), inflammatory markers (increase in C-reactive protein (CRP), lactate dehydrogenase (LDH), procalcitonin and interleukin-6 (IL-6)), and combined biomarkers [2]. Among those, neutrophil to lymphocyte ratio (NLR) emerged as a cost-effective predictor of complications and high values suggest poor prognosis in patients with severe COVID-19 [2,6]. The hypercoagulable state is characterised by an increased D-dimer, fibrinogen and prothrombin time, which along with blood hyperviscosity leads to venous and arterial thrombotic complications [7–14]. Among COVID-19 patients a higher incidence of venous thromboembolisms (VTE) has been observed across studies, however the reported prevalence rate varies greatly, ranging from 8 to 76% for pulmonary embolism (PE) and from 7 to 85% for deep vein thrombosis (DVT), with a higher prevalence among ICU patients [15–20]. VTE in COVID-19 patients have been shown to carry a worse prognosis with an increase in mortality as high as two-fold [18,21] and should be therefore managed in a timely manner. Diagnosing VTE in COVID-19 patients, however, can be challenging. On one side, clinical features and symptoms of COVID-19 pneumonia and VTE greatly overlap, preventing us from assessing effectively the pre-test probability of VTE in COVID-19 patients [22,23]. On the other, D-dimer, which has a great negative predictive value for VTE in general population [24], is usually elevated in COVID-19 patients even in absence of VTE [8,25–27]. In COVID-19 patients D-dimer has been shown to correlate with disease’s severity and has an important prognostic value, with a 3 to 6-fold increase leading to poor prognosis and a hazard ratio of 1.7 for in-hospital mortality [11,28–31]. Nonetheless, higher values of D-dimer have been shown to correlate with an increased probability of VTE, therefore there is a general recommendation to use higher cut-off values of D-dimer to trigger VTE diagnostic work-up. However, the optimal D-dimer cut-off value is still unknown and proposed values range from 1000 to over 5000μg/L [32–34].
Our aim was to investigate the correlation between inflammation and coagulation biomarkers and VTE, the incidence of adverse events and the predictive value of D-dimer for VTE in hospitalized COVID-19 patients.
2Methods
2.1Study design and population
Consecutive patients hospitalized in general ward for COVID-19 between December 1st, 2020 and February 28th, 2021 were screened for inclusion. Inclusion criteria were 18 years of age or older, clinical and risk factors information available.
Patients with missing data were excluded.
The study was conducted in accordance with the Declaration of Helsinki (1964) and approved by the National Medical Ethics Committee (approval number 0120-414/2021/6).
Baseline data, comorbidities and laboratory tests at admission were collected.
Comorbidities were defined as known diagnosis of arterial hypertension, hear failure, cardiovascular disease, chronic obstructive pulmonary disease, asthma, chronic kidney disease, diabetes mellitus, chronic liver disease and chronic hematologic disease.
Geneva prediction score for PE and Wells prediction score for DVT have been calculated based on patient’s history and physical exam.
We collected data about lactate dehydrogenases (LDH, μkat/L), ferritin (μg/L), C-reactive protein (mg/L), procalcitonin (μg/L), leukocytes (109/L), haemoglobin (1012/L), platelets (109/L), neutrophils (109/L), lymphocytes (109/L), neutrophil-lymphocyte ratio (NLR), prothrombin time, international normalized ratio (INR), activated thromboplastin time (s), fibrinogen (g/L), D-dimer (μg/L) and interleukin-6 (ng/L). Blood samples were analysed using Advia® 2120i (Siemens Healthineers, Elden, Germany) for blood cell count, Advia® 2400/1800 (Siemens Healthineers, Elden, Germany) for biochemical analysis, Cobas e 411 (Roche Diagnostics, Basel, Switzerland), for IL6, BCS® XP (Siemens Healthineers, Elden, Germany) for coagulation tests.
Venous thromboembolism (VTE) was defined as PE, DVT or both. PE was verified with a CT pulmonary angiography scan performed due to out of proportion worsening of respiratory insufficiency or because of a high clinical suspicion for PE. DVT was verified with a Doppler ultrasound compression exam due to unilateral limb swelling or unilateral pitting oedema.
Event was defined as a combination of death from any cause and ICU admission.
2.2Statistical analysis
Baseline characteristics are expressed as mean (±standard deviation) for normally distributed continuous variables, as median (interquartile range) for non-normally distributed continuous variables and as frequency (%) for categorical variables. Between-group differences were assessed by t-test for normally distributed variables, by Mann-Whitney U test for non-normally distributed variables and proportions were compared using the χ2 test. Linear regression models accounting for gender, age and comorbidities as independent variables were constructed and Pearson correlation coefficients were calculated to identify independent predictors of VTE and event. A ROC curve was constructed to assess the predictive value of D-dimer and the optimal cut off value was determined using the Youden index. A gender specific and age specific sub analysis was performed. A 2-tailed p < 0.05 was considered significant. Statistical analysis was carried out using SPSS Statistics version 23 (SPSS Inc, Chicago, USA).
3Results
A total of 1713 patients were screened for inclusion; 62 (3.6%) were excluded (14 did not meet inclusion criteria, 48 had missing data), 1651 participants were included. Baseline demographics and characteristics are presented in Table 1. VTE was identified in 111 (6.7%) participants: 76 (68.5%) had PE, 19 (17.1%) had DVT and 15 (13.5%) had both. There were no differences in age and gender representation between VTE and non-VTE group. The VTE group had a higher Wells score for DVT (DVT likely in 11 (9.9%) vs 63 (4.1%) participants, p < 0.001) and a higher Geneva score for PE (PE likely in 43 (38.7%) vs 294 (19.1%) participants, p < 0.001).
Table 1
Baseline characteristics and treatment
| All (n = 1651) | VTE (n = 111) | Non-VTE (n = 1540) | p-value | |
| Age, years, mean (SD) | 74.0±14 | 73.8±13 | 74.1±14 | 0.875 |
| Female, n (%) | 747 (45.2) | 44 (39.6) | 705 (45.8) | 0.114 |
| Comorbidities, n (%) | <0.001 | |||
| 0 | 50 (3.0) | 11 (9.9) | 39 (2.5) | |
| 1 | 246 (14.9) | 20 (18.0) | 226 (14.7) | |
| 2 | 212 (12.8) | 17 (15.3) | 195 (12.7) | |
| 3 or more | 1143 (69.2) | 63 (56.8) | 1080 (70.1) |
VTE –venous thromboembolism, SD –standard deviation.
Table 2
Laboratory tests. Values are displayed as median (interquartile range)
| All (n = 1651) | VTE (n = 111) | Non-VTE (n = 1540) | P-value | |
| Lactate dehydrogenase, μkat/L | 4.8 (2.94) | 5.7 (3.3) | 4.8 (2.9) | 0.001 |
| Ferritin, μg/L | 636 (885) | 646 (1206) | 636 (974) | 0.541 |
| C-reactive protein, mg/L | 77 (108) | 80.5 (108) | 76 (35.3) | 0.196 |
| Procalcitonin, μg/L | 0.09 (0.23) | 0.09 (0.27) | 0.09 (0.23) | 0.736 |
| Leukocytes, 109/L | 6.7 (4.6) | 10.3 (5.8) | 6.5 (4.3) | <0.001 |
| Haemoglobin, 1012/L | 128 (30) | 130.5 (25) | 128 (31) | 0.293 |
| Platelets, 109/L | 206 (124) | 223 (150) | 205 (122) | 0.040 |
| Neutrophils, 109/L | 5.0 (4.3) | 8.4 (5.1) | 4.9 (4.1) | <0.001 |
| Lymphocytes, 109/L | 0.81 (0.64) | 0.7 (0.5) | 0.82 (0.65) | 0.274 |
| Neutrophil-lymphocyte ratio | 6.1 (7.4) | 10.2 (11.9) | 5.9 (6.9) | <0.001 |
| Prothrombin time | 1.0 (0.3) | 1.01 (0.3) | 1.01 (0.3) | 0.254 |
| international normalized ratio | 1.0 (0.17) | 1.01 (0.1) | 1.01 (0.2) | 0.321 |
| Activated thromboplastin time, s | 33 (9.0) | 32 (8.1) | 33 (9) | 0.507 |
| Fibrinogen, g/L | 5.3 (1.9) | 4.8 (2.0) | 5.4 (1.7) | 0.012 |
| D-dimer, μg/L | 1307 (2619) | 11341 (27487) | 1218.5 (1974) | <0.001 |
| Interleukin 6, ng/L | 61 (113.5) | 82.9 (128.5) | 58 (112.1) | 0.421 |
VTE –venous thromboembolism.
3.1Events
A total of 490 (29.7%) events occurred: 320 (19.4%) participants died and 170 (10.3%) participants were admitted to ICU. There was a higher incidence of events in the VTE group (55 (49.5%) vs 435 (28,2%), p < 0.001), the difference was mainly driven by ICU admission (35 (31.5%) vs 135 (8.8%), p < 0.001), while the death rate was similar ((20 (18%) vs 300 (19.5%), p = 0.900).
3.2Predictors of VTE
Univariate correlation analysis showed a significant correlation between VTE and LDH (τb = 0.073, p = 0.001), leucocytes (τb = 0.166, p < 0.001), platelets (τb = 0.042, p = 0.040), neutrophils (τb = 0.169, p < 0.001), NLR (τb = 0.127, p < 0.001), fibrinogen (τb = –0.108, p = 0.012), D-dimer (τb = 0.256, p < 0.001), Geneva prediction score (τb = 0.098, p < 0.001) and Wells prediction score (τb = 0.214, p < 0.001) retained a strong predictive value for VTE.
In the multivariate model, adjusted for age, gender and comorbidities, NLR (0.001; 95% CI 0.000 –0.002; p = 0.019), D-dimer (0.00005; 95% CI 0.000 –0.000; p < 0.001), Geneva prediction score (0.026; 95% CI 0.012 –0.040; p < 0.001) and Wells prediction score (0.047; 95% CI 0.033 –0.061; p < 0.001) retained a strong predictive value for VTE.
3.3Predictors of event
Univariate correlation analysis showed a significant correlation between events and age (τb = 0.129, p < 0.001), male gender (τb = 0.049, p = 0.045), VTE (τb = 0.117, p < 0.001), comorbidities (τb = 0.049, p = 0.039), LDH (τb = 0.261, p < 0.001), ferritin (τb = 0.161, p < 0.001), C-reactive protein (τb = 0.224, p < 0.001), procalcitonin (τb = 0.300, p < 0.001), leucocytes (τb = 0.147, p < 0.001), haemoglobin (τb = –0.077, p < 0.001), platelets (τb = –0.052, p = 0.011), neutrophils (τb = 0.188, p < 0.001), lymphocytes (τb = –0.205, p < 0.001), NLR (τb = 0.274, p < 0.001), prothrombin time (τb = –0.107, p < 0.001), fibrinogen (τb = 0.132, p = 0.002), D-dimer (τb = 0.205, p < 0.001), IL6 (τb = 0.162, p = 0.004).
In the multivariate model, adjusted for age, gender and comorbidities, NLR (0.009; 95% CI 0.007 –0.010; p < 0.001), D-dimer (0.00003; 95% CI 0.000 –0.000; p = 0.007), VTE (0.122; 95% CI 0.030 –0.214; p = 0.010) emerged as strong predictors of event.
3.4ROC analysis
Based on the multivariate analysis we constructed ROC curves for NLR and D-dimer. Of the two, D-dimer emerged as the best biomarker to predict VTE (ROC area for NLR 0.67, 95% CI 0.623 –0.722; ROC area for D-dimer 0.85, 95% CI 0.816 –0.893), Fig. 1. A d-dimer value of 2677μg/L was found to yield the best balance between sensitivity and specificity (85.0% and 75.1% respectively, Youden index of 0,602).
Fig. 1
ROC curve for d-dimer.
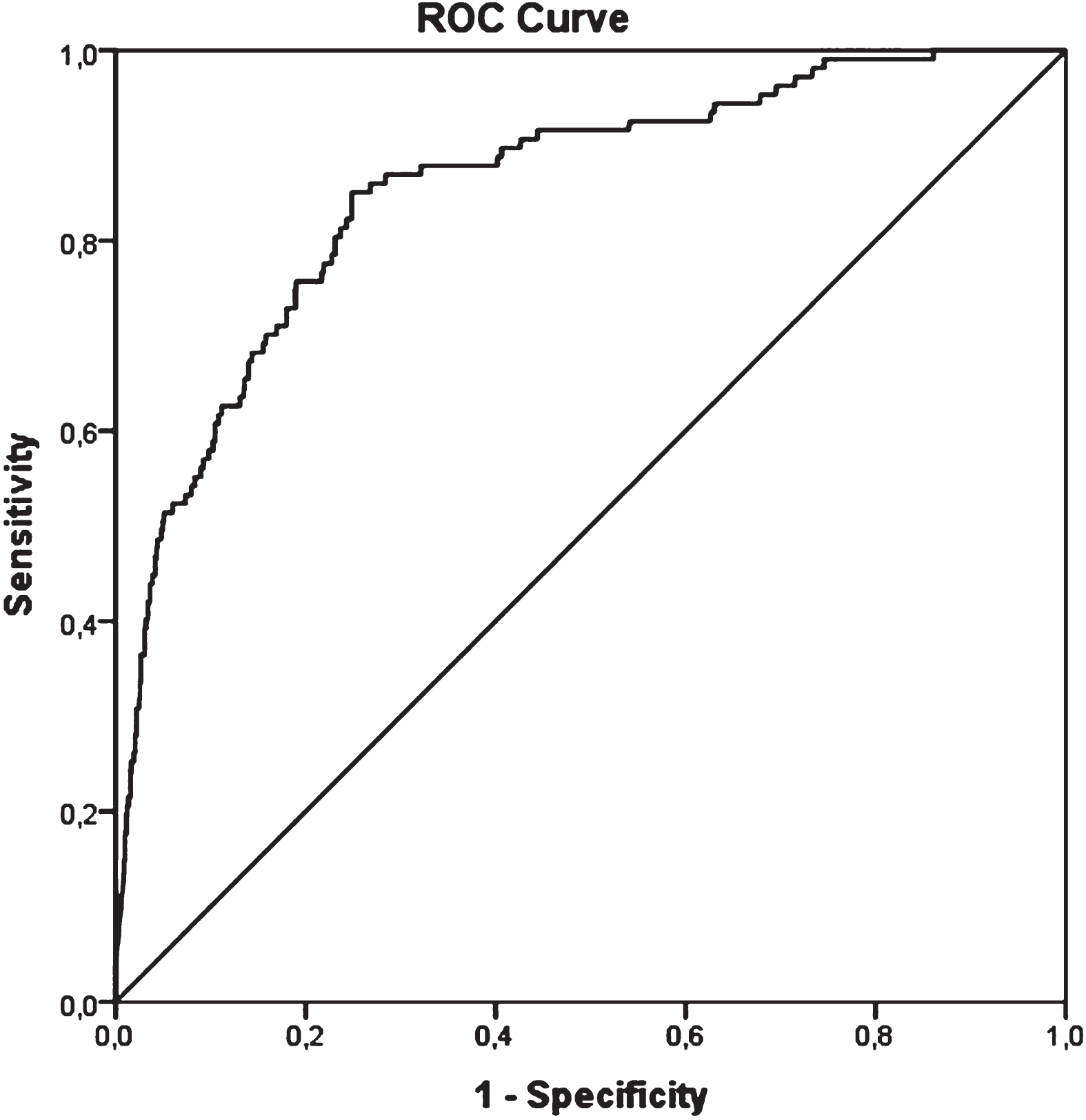
ROC curves for women (ROC area 0.87, 95% CI 0.813 –0.924) and men (ROC area 0.85, 95% CI 0.801 –0.901) did not show significant differences (pairwise comparison difference 0.018; 95% CI –0.058 –0.093, p = 0.649). We identified a D-dimer cut-off value of 2782μg/L for women (sensitivity 92.5%, specificity 73.0%, Youden index of 0.655) and of 2320μg/L for men (sensitivity 83.6%, specificity 74.8%, Youden index of 0.584).
ROC curves for younger than 74 years (ROC area 0.87, 95% CI 0.817 –0.914) and older than 75 years (ROC area 0.85, 95% CI 0.793 –0.911) did not show significant differences (pairwise comparison difference 0.014; 95% CI –0.064 –0.091, p = 0.649). We identified a D-dimer cut-off value of 2511μg/L for younger than 74 years (sensitivity 85.7%, specificity 79.3%, Youden index of 0.650) and of 4236μg/L for older than 75 years (sensitivity 80.4%, specificity 82.7%, Youden index of 0.650).
4Discussion
Our study shows a moderate prevalence of VTE in hospitalized COVID-19 patients. VTE correlated with worse prognosis and higher ICU admission rate. A higher NLR, D-dimer, Geneva score and Wells score emerged as predictors of VTE in the multivariate model, while other inflammation and coagulation biomarkers did not show a correlation with VTE. Based on Roc curve analysis, D-dimer had the best predictive value and we found a cut-off of 2677μg/L to yield the best balance between sensitivity and specificity. We explored possible gender and age specific cut-off values and found a slightly higher cut-off for women and a markedly higher cut-off for older people.
The reported prevalence of VTE in COVID-19 patients varies greatly from 7% to 85% [15–18], in our study we found a prevalence of 7%, in line with the lower reported prevalence. Such differences can be explained by different study designs and participants’ cohorts: studies where VTE diagnostic work-up was triggered by signs and symptoms, like ours, report lower prevalence than studies, where VTE was actively investigated with serial Doppler ultrasound and CT pulmonary angiography [17–19]. All studies show however a higher prevalence in patients with more severe disease and in ICU patients. In our cohort the prevalence of VTE was in line with the reported data from similar designed studies [18]. Compared to data from the literature, where VTE have been shown to worsen the prognosis by even doubling the mortality rate [18], in our cohort VTE carried a 12% increase in death and ICU admission. The magnitude of effect was smaller, possibly due to the combination of observational design of our study, a high number of events in the study group, a smaller pool of patients compared to meta-analysis data sets and differences in unmeasured variables, however still present, reinforcing the prognostic importance of VTE in COVID-19 patients.
Severe COVID-19 is caused by a massive inflammatory response and is therefore characterised by changes in complete blood count and inflammatory markers [2]. We showed that higher leucocytes, neutrophils, LDH, CRP, procalcitonin, IL-6 and lower haemoglobin, platelets and lymphocytes correlated with worse prognosis. Recently combined laboratory parameters have been introduced to allow for a more precise risk assessment and stratification [2]. Among others NLR has been shown to indicate marked inflammation [6] and as such in our study correlated with a worse prognosis. Interestingly, NLR emerged also as an independent predictor of VTE, reinforcing the theory of inflammation and coagulation interplay that ultimately leads to thrombotic events in COVID-19. Besides NLR, D-dimer was also markedly elevated in participants with VTE and it has been shown to still be the best prediction marker for VTE despite being elevated in all COVID-19 patients [11]. Authors concur that higher values should be used to trigger diagnostic investigations for VTE, however the proposed cut-off values differ markedly from 1000 to over 5000μg/L [32,34]. According to our results the best value for our cohort was 2677μg/L with an 85.0% sensitivity and 75.1% specificity. To further increase the diagnostic accuracy, we explored gender and age specific D-dimer values and cut-offs. Our results show that D-dimer retains its good predictive value regardless of gender and age. No significant difference in cut-off values was found between genders, however in participants older than 75 years the cut-off value emerged to be significantly higher –4236μg/L reflecting the known increase with age in general population [35].
Limitations of our study pertain primarily to its single centre and retrospective design. Regarding the former, albeit being ours a single centre, it was the biggest Covid hospital and the referral centre for COVID-19 in the country, hence the participants group was fairly representative of the whole population. The retrospective design is known to carry potential information bias, to minimize it we searched for data both in the Emergency department, hospital stay and demission documentation. By doing that, we were able to exclude only less than 3% of screened patients. To avoid detection bias, the collection of baseline and laboratory data was performed separately from the main diagnosis collection. Serial measurements seem to increase the predictive value of D-dimer in other setting and potentially also in COVID-19 patients [36,37], however due to the study design we were unable to explore this promising work-up strategy. Moreover, we were able to retrieve only laboratory test and parameters that were analysed during routine hospital management. We were unable to include some potentially interesting novel biomarkers, that are currently used only for research purposes. This to be said several studies have identified endothelial dysfunction and markers of extrinsic coagulation pathway to have an important role in the pathophysiology of hypercoagulation as well as a potential prognostic impact in COVID-19 [38–40]. However, since our goal was to investigate the correlation of common biomarkers with VTE in clinical practice, we are convinced that it was a drawback of minor entity.
Nonetheless, since our participants group is fairly representative, we believe that our results are applicable to hospitalized COVID-19 patients in general. Our results support the use of higher cut-off values for D-dimer and the use of multiple biomarkers to further guide our decision-making process about diagnostic work-up of VTE, with the aim of effectively initiating anticoagulation treatment in a timely manner in patients with VTE to improve their prognosis. This is of paramount importance since, despite all the efforts of the world health community, COVID-19 is still an ongoing pandemic with new variants of concern on the way and the use of efficient, economically sustainable and time effective diagnostic procedures will help us mitigate the costs both in money and lives of the pandemic. Further studies are needed, however, to define optimal cut-off values and the potential benefit of serial measurements of D-dimer and identify other useful biomarkers to better stratify according to risk patients with COVID-19.
In conclusion, VTE among COVID-19 patients is common and carries a worse prognosis. Effort should be made to identify patients at risk, using higher than usual cut-off values of D-dimer, which is still the biomarker with the best diagnostic accuracy independently from gender and age, and combining it with other biomarkers, such as NLR, to better guide our decision-making process.
Acknowledgments
We thank all the physicians, nurses, physiotherapists and supporting staff at the DTS COVID-19 clinic, that give every day their best and devotionally took and will take care of patients during this pandemic. We thank the Department of Infectious Diseases, University Medical Centre Ljubljana for supporting the study.
References
[1] | Home [homepage on the Internet]. Johns Hopkins Coronavirus Resource Center. [cited 2022 Jul 22]. Available from: https://coronavirus.jhu.edu/ |
[2] | Ghahramani S , Tabrizi R , Lankarani KB , Kashani SMA , Rezaei S , Zeidi N , et al. Laboratory features of severe vs. non-severe COVID-19 patients in Asian populations: a systematic review and meta-analysis. Eur J Med Res. (2020) ;25: :30. doi: 10.1186/s40001-020-00432-3 |
[3] | Coronavirus disease (COVID-19) [homepage on the Internet]. [cited 2022 Jul 27]. Available from: https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-covid-19 |
[4] | Sun P , Qie S , Liu Z , Ren J , Li K , Xi J . Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: A single arm meta-analysis. J Med Virol. (2020) ;92: (6):612–7. doi: 10.1002/jmv.25735 |
[5] | Becker RC . COVID-19 update: Covid-19-associated coagulopathy. J Thromb Thrombolysis. (2020) ;50: (1):54–67. doi: 10.1007/s11239-020-02134-3 |
[6] | Lagunas-Rangel FA . Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): A meta-analysis. J Med Virol. (2020) ;92: (10):1733–4. doi: 10.1002/jmv.25819 |
[7] | Di Minno MND , Calcaterra I , Lupoli R , Storino A , Spedicato GA , Maniscalco M , et al. Hemostatic Changes in Patients with COVID- A Meta-Analysis with Meta-Regressions. J Clin Med. (2020) ;9: (7):E2244. doi: 10.3390/jcm9072244 |
[8] | Iba T , Levy JH , Levi M , Connors JM , Thachil J . Coagulopathy of Coronavirus Disease 2019. Crit Care Med. (2020) ;48: (9):1358–64. doi: 10.1097/CCM.0000000000004458 |
[9] | Teimury A , Khameneh MT , Khaledi EM . Major coagulation disorders and parameters in COVID-19 patients. Eur J Med Res. (2022) ;27: (1):25. doi: 10.1186/s40001-022-00655-6 |
[10] | Abou-Ismail MY , Diamond A , Kapoor S , Arafah Y , Nayak L . The hypercoagulable state in COVID- Incidence, pathophysiology, and management. Thromb Res. (2020) ;194: :101–15. doi: 10.1016/j.thromres.2020.06.029 |
[11] | Rostami M , Mansouritorghabeh H . D-dimer level in COVID-19 infection: a systematic review. Expert Rev Hematol. (2020) ;13: (11):1265–75. doi: 10.1080/17474086.2020.1831383 |
[12] | Rasyid A , Riyanto DL , Harris S , Kurniawan M , Mesiano T , Hidayat R , et al. Association of coagulation factors profile with clinical outcome in patient with COVID-19 and acute stroke: A second wave cohort study. Clin Hemorheol Microcirc. 2022. doi: 10.3233/CH-221546 |
[13] | Shaik A , Chen Q , Mar P , Kim H , Mejia P , Pacheco H , et al. Blood hyperviscosity in acute and recent COVID-19 infection. Clin Hemorheol Microcirc. (2022) ;82: (2):149–55. doi: 10.3233/CH-221429 |
[14] | Nugroho J , Wardhana A , Mulia EP , Maghfirah I , Rachmi DA , A’yun MQ , et al. Elevated fibrinogen and fibrin degradation product are associated with poor outcome in COVID-19 patients: A meta-analysis. Clin Hemorheol Microcirc. (2021) ;77: (2):221–31. doi: 10.3233/CH-200978 |
[15] | Tufano A , Rendina D , Abate V , Casoria A , Marra A , Buonanno P , et al. Venous Thromboembolism in COVID-19 Compared to Non-COVID-19 Cohorts: A Systematic Review with Meta-Analysis. J Clin Med. (2021) ;10: (21):4925. doi: 10.3390/jcm10214925 |
[16] | Klok FA , Kruip MJHA , van der Meer NJM , Arbous MS , Gommers a D MPJ , Kant KM , et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. (2020) ;191: :145–7. doi: 10.1016/j.thromres.2020.04.013 |
[17] | Mohamed MFH , Al-Shokri SD , Shunnar KM , Mohamed SF , Najim MS , Ibrahim SI , et al. Prevalence of Venous Thromboembolism in Critically Ill COVID-19 Patients: Systematic Review and Meta-Analysis. Front Cardiovasc Med. (2020) ;7: :598846. doi: 10.3389/fcvm.2020.598846 |
[18] | Kollias A , Kyriakoulis KG , Lagou S , Kontopantelis E , Stergiou GS , Syrigos K . Venous thromboembolism in COVID- A systematic review and meta-analysis. Vasc Med. (2021) ;26: (4):415–25. doi: 10.1177/1358863X21995566 |
[19] | Fauvel C , Weizman O , Trimaille A , Mika D , Pommier T , Pace N , et al. Pulmonary embolism in COVID-19 patients: a French multicentre cohort study. Eur Heart J. (2020) ;41: (32):3058–68. doi: 10.1093/eurheartj/ehaa500 |
[20] | Mazzaccaro D , Giacomazzi F , Giannetta M , Varriale A , Scaramuzzo R , Modafferi A , et al. Non-Overt Coagulopathy in Non-ICU Patients with Mild to Moderate COVID-19 Pneumonia. J Clin Med. (2020) ;9: (6):E1781. doi: 10.3390/jcm9061781 |
[21] | Panigada M , Bottino N , Tagliabue P , Grasselli G , Novembrino C , Chantarangkul V , et al. Hypercoagulability of COVID-19 patients in intensive care unit: A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. (2020) ;18: (7):1738–42. doi: 10.1111/jth.14850 |
[22] | Yüce M , Filiztekin E , Özkaya KG . COVID-19 diagnosis—A review of current methods. Biosens Bioelectron. (2021) ;172: :112752. doi: 10.1016/j.bios.2020.112752 |
[23] | Pollack CV , Schreiber D , Goldhaber SZ , Slattery D , Fanikos J , O’Neil BJ , et al. Clinical characteristics, management, and outcomes of patients diagnosed with acute pulmonary embolism in the emergency department: initial report of EMPEROR (Multicenter Emergency Medicine Pulmonary Embolism in the Real World Registry). J Am Coll Cardiol. (2011) ;57: (6):700–6. doi: 10.1016/j.jacc.2010.05.071 |
[24] | Konstantinides SV , Meyer G , Becattini C , Bueno H , Geersing G-J , Harjola V-P , et al. ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur Respir J. (2019) ;54: (3). doi: 10.1183/13993003.01647-2019 |
[25] | Hong L-Z , Shou Z-X , Zheng D-M , Jin X . The most important biomarker associated with coagulation and inflammation among COVID-19 patients. Mol Cell Biochem. (2021) ;476: (7):2877–85. doi: 10.1007/s11010-021-04122-4 |
[26] | Devreese KMJ . COVID-19-related laboratory coagulation findings. Int J Lab Hematol. (2021) ;43 Suppl 1: :36–42. doi: 10.1111/ijlh.13547 |
[27] | Vandenbriele C , Gorog DA . Screening for venous thromboembolism in patients with COVID-19. J Thromb Thrombolysis. (2021) ;52: (4):985–91. doi: 10.1007/s11239-021-02474-8 |
[28] | Shah S , Shah K , Patel SB , Patel FS , Osman M , Velagapudi P , et al. Elevated D-Dimer Levels Are Associated With Increased Risk of Mortality in Coronavirus Disease A Systematic Review and Meta-Analysis. Cardiol Rev. (2020) ;28: (6):295–302. doi: 10.1097/CRD.0000000000000330 |
[29] | García de Guadiana-Romualdo L , Morell-García D , Favaloro EJ , Vílchez JA , Bauça JM , Alcaide Martín MJ , et al. Harmonized D-dimer levels upon admission for prognosis of COVID-19 severity: Results from a Spanish multicenter registry (BIOCOVID-Spain study). J Thromb Thrombolysis. (2022) ;53: (1):103–12. doi: 10.1007/s11239-021-02527-y |
[30] | Xing Y , Yang W , Jin Y , Wang C , Guan X . D-dimer daily continuous tendency predicts the short-term prognosis for COVID-19 independently: A retrospective study from Northeast China. Clin Hemorheol Microcirc. (2021) ;79: (2):269–77. doi: 10.3233/CH-201071 |
[31] | Alzoughool F , Alanagreh L , Abumweis S , Atoum M . Cerebrovascular comorbidity, high blood levels of C-reactive protein and D-dimer are associated with disease outcomes in COVID-19 patients. Clin Hemorheol Microcirc. (2021) ;77: (3):311–22. doi: 10.3233/CH-201002 |
[32] | Kwee RM , Adams HJA , Kwee TC . Pulmonary embolism in patients with COVID-19 and value of D-dimer assessment: a meta-analysis. Eur Radiol. (2021) ;31: (11):8168–86. doi: 10.1007/s00330-021-08003-8 |
[33] | Artifoni M , Danic G , Gautier G , Gicquel P , Boutoille D , Raffi F , et al. Systematic assessment of venous thromboembolism in COVID-19 patients receiving thromboprophylaxis: incidence and role of D-dimer as predictive factors. J Thromb Thrombolysis. (2020) ;50: (1):211–6. doi: 10.1007/s11239-020-02146-z |
[34] | Mestre-Gómez B , Lorente-Ramos RM , Rogado J , Franco-Moreno A , Obispo B , Salazar-Chiriboga D , et al. Incidence of pulmonary embolism in non-critically ill COVID-19 patients. Predicting factors for a challenging diagnosis. J Thromb Thrombolysis. (2021) ;51: (1):40–6. doi: 10.1007/s11239-020-02190-9 |
[35] | Righini M , Van Es J , Den Exter PL , Roy P-M , Verschuren F , Ghuysen A , et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA. (2014) ;311: (11):1117–24. doi: 10.1001/jama.2014.2135 |
[36] | Palareti G , Legnani C , Antonucci E , Cosmi B , Poli D , Testa S , et al. D-dimer testing, with gender-specific cutoff levels, is of value to assess the individual risk of venous thromboembolic recurrence in non-elderly patients of both genders: a post hoc analysis of the DULCIS study. Intern Emerg Med. (2020) ;15: (3):453–62. doi: 10.1007/s11739-019-02216-y |
[37] | Patil S . Role of ‘Serial D-Dimer Level’ in predicting Severity and outcome in COVID-19 pneumonia: A Prospective multicentric Observational Study of 1000 cases in Tertiary Care Setting in India. EJMA. 2022. doi: 10.14744/ejma.2022.36854 |
[38] | Jung F , Krüger-Genge A , Franke RP , Hufert F , Küpper J-H . COVID-19 and the endothelium. Clin Hemorheol Microcirc. (2020) ;75: (1):7–11. doi: 10.3233/CH-209007 |
[39] | Subramaniam S , Kothari H , Bosmann M . Tissue factor in COVID-19-associated coagulopathy. Thromb Res. (2022) ;220: :35–47. doi: 10.1016/j.thromres.2022.09.025 |
[40] | Mavraganis G , Dimopoulou M-A , Delialis D , Bampatsias D , Patras R , Sianis A , et al. Clinical implications of vascular dysfunction in acute and convalescent COVID- A systematic review. Eur J Clin Invest. (2022) ;52: (11):e13859. doi: 10.1111/eci.13859 |




