ADAMTS13 factor deficiency in severe COVID-19 may not be immune mediated – report from a pilot study
Abstract
BACKGROUND:
The assessment of ADAMTS13 factor activity and inhibitor levels was conducted in severe COVID-19 patients as an observational study.
RESULTS:
A total of 14 patients were included and the average ADAMTS13 activity level at the time of admission was 28.54±30.74% (range 1.83–86.67%) which was reduced compared to controls (88.09±14.77). Nine patients had reduced ADAMTS13 factor activity (<40%) and 77.7% among them had severe deficiency (<10% activity). ADAMTS13 inhibitor was positive (>15 IU/mL) only in two patients and an overall mean value was 8.15±5.8. Elevated D-Dimer and length of hospital stay had significant correlation with ADAMTS13 activity (–0.247 and 0.306 respectively). No features of thrombotic microangiopathy were observed and hence no plasma exchange was performed.
CONCLUSION:
Reduced ADAMTS13 factor activity without inhibitor development may give a clue to the disease progress in COVID-19.
1Introduction
ADAMTS13 (A disintegrin and metalloproteinase with thrombospondin type 1 motif, member 13) is a protease that cleaves the large multimers of von Willebrand factor (vWF) which mediate platelet adhesion and activation. ADAMTS13 activity regulates the size of vWF multimers by cleaving the A2 domain of vWF to prevent platelet driven thrombus formation in physiological conditions [1]. This is possible because of the closed conformation of ADAMTS13 in circulation. Severe deficiency of ADAMTS13 is classically associated with thrombotic incidents as seen in the thrombotic thrombocytopenic purpura (TTP) spectrum of thrombotic microangiopathy (TMA). A severe deficiency is defined as <10% ADAMTS13 factor activity in plasma, which leads to large vWF multimers and thus platelet driven thrombi formation [2, 3]. When the deficiency of factor associated with presence of inhibitors to ADAMTS13, it is addressed as acquired or immune TTP [3]. Apart from TTP, there are secondary TMAs where ADAMTS13 activity levels are reduced, but generally in the range of 10–40% [3]. The reduced ADAMTS13 activity in secondary TMAs is usually associated with a concurrent presence of inhibitor to ADAMTS13 [3].
COVID-19 is associated with micro as well as macro vascular thrombi formation in the lungs, heart, kidney, and brain leading to multiorgan dysfunction[4–6]. The coagulopathy in COVID-19 is typically a hypercoagulable stage causing either arterial or venous thromboembolisms [4–6]. The damage on the endothelial cells plays a primary role in mediating such coagulation changes. Studies are ongoing to understand the physiology of microvascular thrombus formation in COVID-19 and one of the possible mechanisms is imbalance in the vWF/ADAMTS13 levels [1, 7–10]. A few reports also suggest the presence of TTP in COVID-19 patients [11–14]. We conducted a pilot study to establish the ADAMTS13 factor activity and inhibitor levels in patients with severe SARS CoV-2 infection and to generate evidence for further studies.
2Methodology
We carried out a prospective observational study after obtaining Institutional Ethics Committee (703/2021) approval. The study duration was two weeks. All participants were severe COVID-19 admitted in critical care units recruited after obtaining informed consent. Below 18 years of age, known case of coagulation disorders, patients who were on anti-platelets or anticoagulants and patients not admitted to intensive care units (ICUs) were excluded. Samples for ADAMTS13 activity and inhibitor assay were collected at the time of admission in 3.2% sodium citrate vacutainer (BD Vacutainer system, USA) prior to administration of anticoagulant.
Plasma was separated within four hours of collection after centrifugation at 3000 rpm for 10 minutes, stored in–80° C and batch testing was performed at a later stage. ADAMTS13 activity and inhibitor assay was measured by enzyme linked immunosorbent assay (ELISA) technique using Technozym kits (Technoclone, Austria). The kit reference range for factor activity was 40–150% and less than 40% was considered to be deficient ADAMTS13 activity and less than 10% was severe deficiency. The kit identifies IgG inhibitors, and the level was positive if the obtained value was above 15 IU/mL, borderline when observed value was between 12–15 IU/mL and negative if the concentrations were less than 12 IU/mL. The ELISA was validated and performed in the department of Transfusion Medicine and reading was done at 450 nm as per manufacturer’s instruction. Laboratory data collected were complete blood count (CBC), prothrombin time (PT), activated partial thromboplastin time (aPTT) and D-Dimer. Patient’s clinical outcome, survival rate, and thromboembolic incidents during the current admission were also noted.
3Results
A total of 14 patients were included in the study and out of them, ten patients were males (71.42%) with a mean age of 57.07±15.8 years. For the 14 patients, the average ADAMTS13 activity level at the time of admission was 28.54±30.74% (range 1.83–86.17%). Other laboratory parameters at the time of admission are provided in Table 1. Compared to age matched normal controls (88.09±14.77), ADAMTS13 in COVID-19 was found to be significantly reduced (P < 0.0001). The average length of hospital stay was 11.14±5.46 days. Mortality rate was 50% and the mean ADAMTS13 levels among non-survivors were 27.27±31.31%.
Table 1
Laboratory parameters of 14 patients at the time of admission and at endpoint
| Parameters (n = 14) | Normal range | Observed value at admission (mean &sd) | Observed value at end point* (mean &sd) |
| Hb | 13–17 g/dL | 11.43±2.66 g/dL | 11.27±2.03 g/dL |
| Hct | 40–50% | 34.21±8.1% | 34.6±6.07% |
| WBC | 4–10×103 cells/μL | 8.65±3.97×103 cells/μL | 20.99±17.22×103 cells/μL |
| PLT | 150–400×103 cells/μL | 206.07±85.9×103 cells/μL | 238.81±126.39×103 cells/μL |
| PT | 9.6–12.5 seconds | 11.39±1.17 seconds | NA |
| aPTT | 26.8–33.2 seconds | 31.12±7.04 seconds | NA |
| D-Dimer | <0.5μg FEU/mL | 1.96±2.65μg FEU/mL | 1.98±2.02μg FEU/mL |
*Endpoint–Discharge or death whichever happened earlier.
The mean antibody level was 8.15±5.8 IU/mL with only two patients had a positive antibody result (>15 IU/mL). One patient had borderline antibody level (12.87 IU/mL) and the rest of them were negative values. The factor activity and the corresponding antibody levels are depicted in Fig. 1. The other laboratory profile of these 14 patients at the time of admission and end point are provided in Table 1. We have not observed microangiopathic anemia, thrombocytopenia, prolonged coagulation screen times and therapeutic plasma exchange was not performed for any of these patients.
Fig. 1
ADAMTS13 factor activity and Inhibitor levels in severe COVID19. ADAMTS13 factor activity is plotted on left y axis and Inhibitor levels is plotted on right y axis.
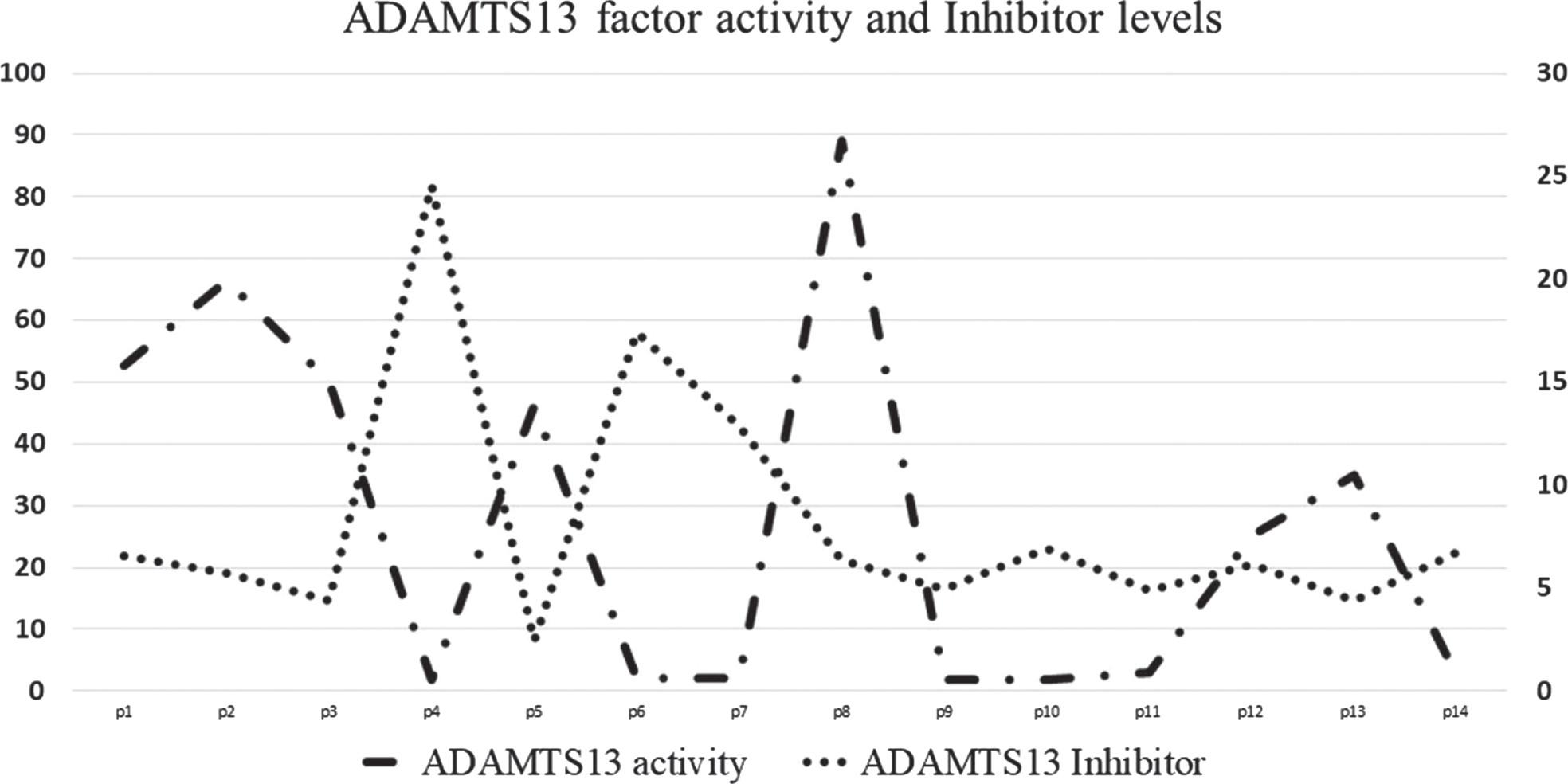
Nine patients had activity less than 40% and out of this, seven patients had severe deficiency of ADAMTS13 activity (<10%). Out of these nine patients, 44.4% had succumbed to death. Ten (71.42%) patients received thromboprophylaxis at admission, and one (7.14%) patient among them developed deep vein thrombosis during ICU stay. Eight (57.14%) patients required mechanical ventilation. The clinical outcome categorized based on the ADAMTS13 activity has been provided in Table 2. There was a weak negative correlation between ADAMTS13 activity and the mortality of the patients (r = –0.09) and a positive correlation between ADAMTS13 and LOHS (Coefficient = 0.306, p < 0.001). D-Dimer in our study group was elevated at the time of admission (0.39–9.0μg FEU/ml) and there was a negative correlation with the ADAMTS13 activity (Coefficient = –0.247, P < 0.05).
Table 2
Comparison of patients’ clinical profile categorized based on the activity level of ADAMTS13 at the time of admission
| Parameters | ADAMTS13 < 40% (n = 9) | ADAMTS13 > 40% (n = 5) |
| Hb (g/dL) | 11.93±2.23 | 10.54±3.39 |
| HCT (%) | 35.88±7.17 | 31.2±9.62 |
| Platelets (cells/μL) | 192.78±72.03 | 230±111.92 |
| WBC (cells/μL) | 7.81±3.63 | 10.18±4.51 |
| PT (sec) | 11.33±1.14 | 11.5±1.35 |
| aPTT (sec) | 32.35±8.54 | 28.9±2.35 |
| D-Dimer (μg FEU/mL) | 2.04±2.79 | 1.7±2.30 |
| LOHS (days) | 10.44±3.2 | 12.4±6.8 |
| TE (number, %) | NIL | N = 1 |
| Mortality (number, %) | 5/9 (55.55%) | 2/5 (40%) |
4Discussion
The mean ADAMTS 13 activity level was 28.54% which was much less than healthy controls indicating that CAC influences the ADAMTS13 activity and interaction with vWF. We also observed most of the patients (77.7%, 7/9) had severe deficiency (<10%). ADAMTS13 activity was greatly reduced among non-survivors in a cohort of 181 COVID-19 patients with varying severity [8]. The impaired ADAMTS 13–vWF pathway might shed further insight into the fact that the incidence of TEs remains on the higher side even after systemic anticoagulation in COVID-19. The endotheliopathy might be a primary cause in elevated vWF/ADAMTS 13 ratio in such patients and indicate that this imbalance might play a role in the survival of the patients [15]. Reduced ADAMTS 13 activity may be due to increased ADAMTS 13 consumption along with elevated vWF, a hallmark of COVID-19 [1].
The mean antibody levels were in the normal range among 14 patients, only two patients had positive ADAMTS13 inhibitor levels. This may suggest that the reduction in ADAMTS13 factor activity may not be due to inhibitor development in COVID-19. One among the common causes of reduced ADAMTS 13 activity in critically ill patients is TTP or secondary TMAs. Congenital TTP is associated with a severe deficiency of ADAMTS13 activity without inhibitors and manifests at a younger age. Acquired / Immune TTP is characteristically seen when patients develop inhibitors to ADAMTS13 [2, 3]. There are reports of immune TTP induced by COVID-19 which may suggest patients are prone to develop TTP in COIVD-19 [14]. The study indicated that a severe ADAMTS13 factor deficiency might be due to the endothelial injury and not because of COVID-19 induced inhibitor development. Second point to differentiate COVID-19 induced deficiency from TTP is the closed conformation of ADAMTS13 in COVID-19 as opposed to the open conformation in TTP [1]. The abnormal high levels of vWF propeptide (vWF:pp) in COVID-19 also helps to differentiate from TTP [1, 8]. Other screening tools like schistocytes and thrombocytopenia also favors a diagnosis of TTP, however we have not observed any of these changes in our study group [2, 3].
Reduced ADAMTS13 activity leads to elevated large multimers of vWF which in turn causes platelet adhesion and aggregation (microvascular thrombi). Breakdown of this clot leads to elevated D-Dimer levels as proposed by Iba et al. [4]. Different studies have proven that raised vWF is associated with a bad outcome in COVID-19 [16] and it was later established that elevated vWF antigen is associated with a reduced ADAMTS13 factor activity [1, 2]. Correlating D-Dimer with ADAMTS13 activity revealed that as ADAMTS13 activity decreased, D-Dimer was increasing [1].
The study was limited by the financial considerations of performing ADAMTS13 activity and inhibitor analysis. It would have been better if we were able to find out the vWF levels and correlating that with the clinical outcome along with the ADAMTS13 activity levels.
This study has shown that ADAMTS13 activity is reduced in severe COVID-19 without a significant inhibitor development. One must keep in mind that reduced ADAMTS13 may be observed in TTP or TMAs which can co-exist in COVID-19 and appropriate clinical and laboratorial work up with a multidisciplinary team will help in establishing the coagulopathy associated with COVID-19 and differentiate from the other possibilities.
Author contributions
The study was conceptualized by GM and SS. The tests were performed by RS and validated by DC. Data collection was done by AG and RS and data analysis and interpretation was done by DC and VR. JMB and SR were involved in patient management and recruitment. Manuscript was prepared by GM and AG. Critical review of the manuscript was done by SS and JMB.
Funding
The authors report no funding.
Conflicts of interest
We do not have any conflicts of interest to report.
References
[1] | Ward SE , Fogarty H , Karampini E , Lavin M , Schneppenheim S , Dittmer R , et al. ADAMTS13 regulation of VWF multimer distribution in severe COVID-19. Journal of Thrombosis and Haemostasis. (2021) Jun 20;jth.15409. |
[2] | Zheng XL , Vesely SK , Cataland SR , Coppo P , Geldziler B , Iorio A , et al. ISTH guidelines for the diagnosis of thrombotic thrombocytopenic purpura. Journal of Thrombosis and Haemostasis. (2020) ;18: (10):2486–95. |
[3] | Scully M , Hunt BJ , Benjamin S , Liesner R , Rose P , Peyvandi F , et al. Guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. British Journal of Haematology. (2012) ;158: (3):323–35. |
[4] | Iba T , Levy JH , Connors JM , Warkentin TE , Thachil J , Levi M . The unique characteristics of COVID-19 coagulopathy. Vol. 24, Crit Care. BioMed Central; (2020) . |
[5] | Levi M , Thachil J , Iba T , Levy JH . Coagulation abnormalities and thrombosis in patients with COVID-19. The Lancet Haematology. (2020) ;7: (6):e438–40. |
[6] | Jung EM , Stroszczynski C , Jung F . Contrast enhanced ultrasonography (CEUS) to detect abdominal microcirculatory disorders in severe cases of COVID-19 infection: First experience. Clin Hemorheol Microcirc. (2020) ;74: (4):353–61. |
[7] | Joly BS , Darmon M , Dekimpe C , Dupont T , Dumas G , Yvin E , et al. Imbalance of von Willebrand factor and ADAMTS13 axis is rather a biomarker of strong inflammation and endothelial damage than a cause of thrombotic process in critically ill COVID-19 patients. Journal of Thrombosis and Haemostasis. (2021) ;19: (9):2193–8. |
[8] | Sweeney J , Barouqa M , Krause G , Lugo JG , Rahman S , Reyes M . Low ADAMTS13 Activity Correlates with Increased Mortality in COVID-19 Patients. American Journal of Clinical Pathology. (2021) ;156: (Supplement_1):S6–7. |
[9] | Favaloro EJ , Henry BM , Lippi G . Increased VWF and Decreased ADAMTS-13 in COVID-19: Creating a Milieu for (Micro) Thrombosis. Vol. 47, Seminars in Thrombosis and Hemostasis. Thieme Medical Publishers, Inc.; (2021) . pp. 400–18. |
[10] | Panigada M , Bottino N , Tagliabue P , Grasselli G , Novembrino C , Chantarangkul V , et al. Hypercoagulability of COVID-19 patients in intensive care unit: A report of thromboelastography findings and other parameters of hemostasis. Journal of Thrombosis and Haemostasis. (2020) ;18: (7):1738–42. |
[11] | Hindilerden F , Hindilerdem I , Akar E , Yasar K . Covid-19 associated autoimmune thrombotic thrombocytopenic purpura: Report of a case. Thrombosis Research. (2020) ;(July):136–8. |
[12] | Altowyan E , Alnujeidi O , Alhujilan A , Alkathlan M . COVID-19 presenting as thrombotic thrombocytopenic purpura (TTP). BMJ Case Reports. (2020) ;13: (12):1–4. |
[13] | Nicolotti D , Bignami EG , Rossi S , Vezzani A . A case of thrombotic thrombocytopenic purpura associated with COVID-19. Journal of Thrombosis and Thrombolysis. (2021) ;52: (2):468–70. |
[14] | Schwaegermann MK , Hobohm L , Rausch J , Reuter M , Griemert TF , Sivanathan V , Falter T , Sprinzl MF , Lackner KJ , Galle PR , Konstantinides S , Theobald von M AC . COVID-19 as a Potential Trigger for Immune Thrombotic Thrombocytopenic Purpura and Reason for an Unusual Treatment: A Case Report. Hamostaseologie [Internet]. (2021) ;29:E pub. Available from: https://pubmed.ncbi.nlm.nih.gov/34327693/ |
[15] | Thomas VV , Kumar SE , Alexander V , Nadaraj A , Vijayalekshmi B , Prabhu S , et al. PlasmaVonWillebrand Factor Levels Predict Survival in COVID-19 Patients Across the Entire Spectrum of Disease Severity. Indian Journal of Hematology and Blood Transfusion. (2021) ;(June). |
[16] | Nugroho J , Wardhana A , Prasetya E , Maghfirah I . Elevated fibrinogen and fibrin degradation product are associated with poor outcome in COVID-19 patients: A meta-analysis. Clin Hemorheol Microcirc. (2021) ;77: :221–31. |




