Impaired fibrinolysis in severe Covid-19 infection is detectable in early stages of the disease
Abstract
BACKGROUND:
A significant degree of mortality and morbidity in Covid-19 is due to thromboembolic disease. Coagulopathy has been well described in critically unwell patients on ICU. There is less clear evidence regarding these changes at the time of presentation to the Emergency Department and the progression of disease over time.
OBJECTIVE:
We sought to investigate whether coagulation markers can predict severity and how they change over the disease course.
METHODS:
Patients presenting to a single University Teaching Hospital were recruited and followed up if PCR was positive. Alongside routine blood testing, Rotational Thromboelastometry (ROTEM) was performed. Outcome data was recorded for all patients, and ROTEM values were compared across outcome groups.
RESULTS:
Extem and Intem Maximum Lysis were significantly reduced in those who died or required an ICU admission, indicating a reduced ability to break down clot mass in the most critically unwell patients.
CONCLUSION:
Comparisons between groups demonstrated that one distinguishing feature between those who require ICU admission or die of Covid-19 compared with those who survive a hospital stay to discharge was the extent to which fibrinolysis could occur. Mortality and morbidity in Covid-19 infection appears in part driven by an inability to break down clot mass.
1Introduction
A major cause of mortality and morbidity in Covid-19 infection has been micro-thromboembolic disease leading to multi-organ failure [1–4]. Covid-19 associated coagulopathy appears to generate thrombotic complications at a rate out of proportion with comparable critically unwell patient cohorts cared for on Intensive Care Units (ICU) [5, 6]. Observational studies utilising viscoelastic testing such as Rotational Thromboelastometry (ROTEM) have demonstrated that there is a hypercoagulable state seen in the most critically unwell Covid-19 patients that is not seen in those patients with a less severe Covid-19 infection who have been managed on general medical wards [7]. Comparably little has been published to date regarding ROTEM in the early stages of disease before patients progress to a critically unwell state. Some studies in ICU have recruited patients at the time of admission to ICU, however only one small study describes changes to coagulation at the time of presentation to the Emergency Department (ED) [8]. As such, the body of evidence described in systematic reviews on thromboelastography in Covid-19 is weighted towards ICU cohorts with a lack of understanding of the early changes seen in Covid-19 [9, 10]. In addition to this focus on ICU cohorts, the data shows a degree of heterogeneity in terms of the specific changes in ROTEM parameters seen in the hypercoagulable state. With the speed and scale of the spread of the virus in early 2020 there was an urgent need for immediate results to guide clinical management. As such, much of the early data in Covid-19 has been lower quality evidence from small scale studies or non-peer reviewed publications, and there is therefore a need to consolidate these findings with more comprehensive studies.
One suggested mechanism for the hypercoagulable state is fibrinolysis shutdown –initially posed in trauma [11] –and was subsequently implicated as a mechanism for disease in other inflammatory states [12, 13]. Impaired fibrinolysis has now also been associated with mortality and morbidity in severe Covid-19 infection. Previous studies have demonstrated that a high proportion of Covid-19 patients with an objective diagnosis of a major VTE event also meet the criteria for diagnosis of fibrinolytic shutdown [14, 15]. And while there is a growing body of literature demonstrating hypercoagulable changes as assessed by ROTEM, studies attempting to generate guidelines for clinical practice have in some cases found that the evidence does not support the implementation of ROTEM based guidelines for practice [16, 17].
A number of studies have utilised other coagulation and inflammatory markers to prognosticate and evaluate risk of complication in Covid-19. Clot breakdown products such as D-Dimer and fibrin degradation product are seen to be elevated in more severe cases of Covid-19 [18, 19], and this has been linked to subsequent outcome [20]. It is therefore clear that a crucial part of the pathophysiology of Covid-19 is derangement of the coagulation system.
The aims of this study were to establish what parameters in clotting profile were deranged early in the progression of the disease, to provide information to clinicians as to the potential severity of infection at an early stage and to track these changes over progression of the disease to assess the utility of ROTEM in ongoing monitoring.
2Methodology
120 patients were recruited from a single large University Teaching Hospital ED in South Wales, UK. Inclusion criteria were first presentation to the ED with febrile or respiratory symptoms suspicious of Covid-19. Patients were excluded if they were on anticoagulant medication, had a previous diagnosis of a genetic disorder, chronic liver or kidney disease, or previous vascular disease, or if they declined to participate. Recruitment for this study occurred before the roll out of the Covid vaccination in the UK, as such all patients were unvaccinated.
PCR testing was performed on all participants and those with a negative result had no further follow up on receipt of result. 24 patients from the initial cohort tested negative on PCR and 1 further patient was retrospectively excluded due to being on warfarin, leaving a remaining cohort of 95 positive cases for analysis. Those who were positive were followed up through their hospital stay and their outcome was recorded. Alongside baseline characteristics such as age, weight and past medical history, routine haematological, biochemical and coagulation assays were performed on all participants. Rotational Thromboelastometry (ROTEM) was performed to evaluate the coagulation profile of all participants.
Repeat sampling was taken 24 hours after admission, at 3–5 days, and at 7 days. Further samples were not taken if the patient had already been discharged from hospital by the clinicians responsible for their care.
Once outcomes were known for a patient, their coagulation profiles were retrospectively analysed based on the severity of their disease. Those who were admitted to ICU or died were classed as ‘Critical’, those with a hospital stay of 5 days or more were classed as ‘Severe’, those with a hospital stay between 24 hours and 5 days were classed as ‘Moderate’ and those discharged directly from ED or within 24 hours of admission were classed as ‘Mild’. The Mild and Moderate groups were pooled for statistical analysis.
The investigating team played no part in the clinical care for the patient. Corticosteroids were given if the participant had an oxygen requirement as per standard clinical pathways, dexamethasone was used as standard unless the responsible clinician deemed other corticosteroids were more appropriate. Low Molecular Weight Heparin (LMWH) was also given to patients based on clinician decision. Where possible blood sampling was performed before the administration of these medications, but their administration was not delayed to wait for research study related investigations. Timing of administration of medications was recorded in order to account for this in analysis. In the case of LMWH, Anti-Xa sampling allowed us to measure the activity of LMWH at the time of sampling.
Blood samples were taken from the antecubital vein where possible using a 21 G butterfly needle. Two 2.7 mL samples were immediately transferred into PET 0.109M 3.2% citrated vacutainers (Becton Dickinson, Plymouth, UK Ref: 363095). Routine coagulation studies including PT, APTT and Clauss fibrinogen and anti-Xa assay were performed on the first citrated vacutainer measured using a Sysmex CA1500 analyser within two hours of collection. D-dimer analysis was carried out using Latex immunoturbidimetric assay (Instrumentation Laboratory, Warrington, UK). 4 ml was collected into a plastic dipotassium EDTA vacuette (Becton Dickinson, Plymouth, UK Ref 367839) for FBC analysis using a Sysmex XE 2100 (Sysmex UK, Milton Keynes, UK). 5 mL was collected into a SST II Advance (Becton Dickinson, Plymouth, UK Ref 367954), testing of U&Es, LFTs, Bone Profile and CRP were performed on a Roche-Cobas 8000 Modular Analyser (Roche Diagnostics Ltd, UK).
The second citrated sample was used for viscoelastic testing via Rotational Thromboelastometry. This was performed using a ROTEM Delta Whole Blood Haemostasis System (TEM Innovations GmbH, 2011) with testing carried out according to manufacturer recommendations and analysed on ROTEM Delta Software v1.6.3. The markers selected for analysis were Extem and Intem Clotting Time (CT), Clot Formation Time (CFT), Maximum Clot Firmness (MCF), Alpha Angle (Alpha) and Maximum Lysis (ML). This was designed to evaluate clot formation kinetics, mechanical structure and clot breakdown, mirroring the values selected by other studies.
Blood sampling at later time points was only performed if the patient remained in hospital. As such, the mild and moderate cases of Covid-19 did not have sampling performed at all time points. ROTEM values were compared between each group for each time point, and also within each group values were compared over time to assess for any progressive changes in time.
Statistical analysis was performed on IBM SPSS Statistics v28.0.0.0(190), Shapiro-Wilk testing was performed to assess for normality, and t-tests or ANOVA were used to assess for differences in groups when appropriate. These data are presented as a mean±standard deviation. Where non-parametric testing was appropriate, data is presented as a median±interquartile range and Mann-Witney or Kruskal-Wallis testing performed in place of a t-test or ANOVA respectively. Statistical significance throughout the paper is defined as p < 0.05. In tables and figures *, ** and *** signify p < 0.05, p≤0.01 and p≤0.001 respectively. Where comparisons between 3 groups indicate 1 group is significantly different to both other groups, but those two groups do not have a significant difference, the significant differences are denoted by a and b next to the values which differ with the p value indicated below.
Graphs and tables were produced in GraphPad Prism 9 v9.3.1(471).
Ethical approval was given by the South West Wales Research Ethics Committee (Wales REC 6), IRAS 216266, REC reference 17/WA/0123 [21].
3Results
The cohort characteristics are described in Table 1a. There was a significant difference in the age of mild-moderate group compared to the other groups (p < 0.001). There was no difference in the age between severe and critical groups (p = 0.537). Age is a well-known risk factor for severe disease, as are chronic health conditions such as hypertension and diabetes, which were seen in the greatest frequencies in the most unwell cohorts. The relatively low rates of conditions such as CVA and PE likely reflect the fact these conditions are treated with anticoagulant drugs which would then be an exclusion criterion for this study. Table 1b details the results of the routine bloods taken at presentation. The only statistically significant result was a difference in CRP between the mild-moderate group and both other groups (p = 0.009 vs severe, p = 0.002 vs critical), the severe and critical groups did not have a statistically significant difference. As discussed in the methodology, some patients received treatment with LMWH before recruitment, however there was not a significant difference in Anti-Xa levels across groups (p = 0.651) and there was a similar percentage of patients in each group who had received treatment.
Table 1a
Patient cohort characteristics for each group
| Mild-Moderate | Severe | Critical | |
| n | 29 | 46 | 20 |
| Age (yrs) | 50.0±14.6ab | 64.3±12.5a | 69.2±13.6b |
| Male % | 38 | 39 | 55 |
| Diabetes % | 17 | 22 | 25 |
| COPD % | 3 | 4 | 30 |
| Hypertension % | 14 | 30 | 50 |
| IHD / Angina % | 7 | 4 | 20 |
| Heart failure % | 3 | 0 | 0 |
| Hypercholesterolaemia | 7 | 7 | 5 |
| CVA % | 0 | 2 | 5 |
| PE / DVT % | 3 | 2 | 5 |
| Cancer % | 7 | 11 | 5 |
| Received LMWH before recruitment % | 31 | 39 | 30 |
a and b, p < 0.001.
Table 1b
Standard blood testing results taken on arrival to the ED
| Mild-Moderate | Severe | Critical | |
| Full Blood Count | |||
| Haemoglobin (g/dL) | 139±16 | 134±18 | 137±18 |
| Platelets (x109/L) | 238±74 | 282±97 | 228±80 |
| White Cell Count | 6.7 (5.15 – 8.55) | 7.25 (5.325 – 9.850) | 7.9 (6.525 – 11.075) |
| Neutrophils | 4.9 (3.55 – 7.05) | 6.15 (4.15 – 8.65) | 6.4 (4.85 – 10.4) |
| Coagulation Screen | |||
| PT (s) | 10.6 (10.2 – 11.15) | 10.6 (10.4 – 11.0) | 10.9 (10.225 – 11.2) |
| APTT (s) | 23.8 (21.7 – 25.45) | 24.6 (20.35 – 25.6) | 24.05 (22.3 – 26.9) |
| Fibrinogen (g/L) | 5.3 (4.1 – 6.15) | 5.65 (4.8 – 6.625) | 5.9 (4.025 – 6.35) |
| D-dimer (μg/L) | 731 (544 – 1456.5) | 1149 (597.5 – 2182.75) | 981.5 (632 – 1520.75) |
| Anti-Xa (units/mL) | 0.03 (0.01 – 0.0775) | 0.04 (0.01 – 0.275) | 0.04 (0.01 – 0.1675) |
| Inflammatory Markers | |||
| CRP (mg/L) | 44 (5 – 101)ab | 88 (38.25 – 229)a | 149.5 (63.5 – 200.25)b |
a and b, p < 0.01.
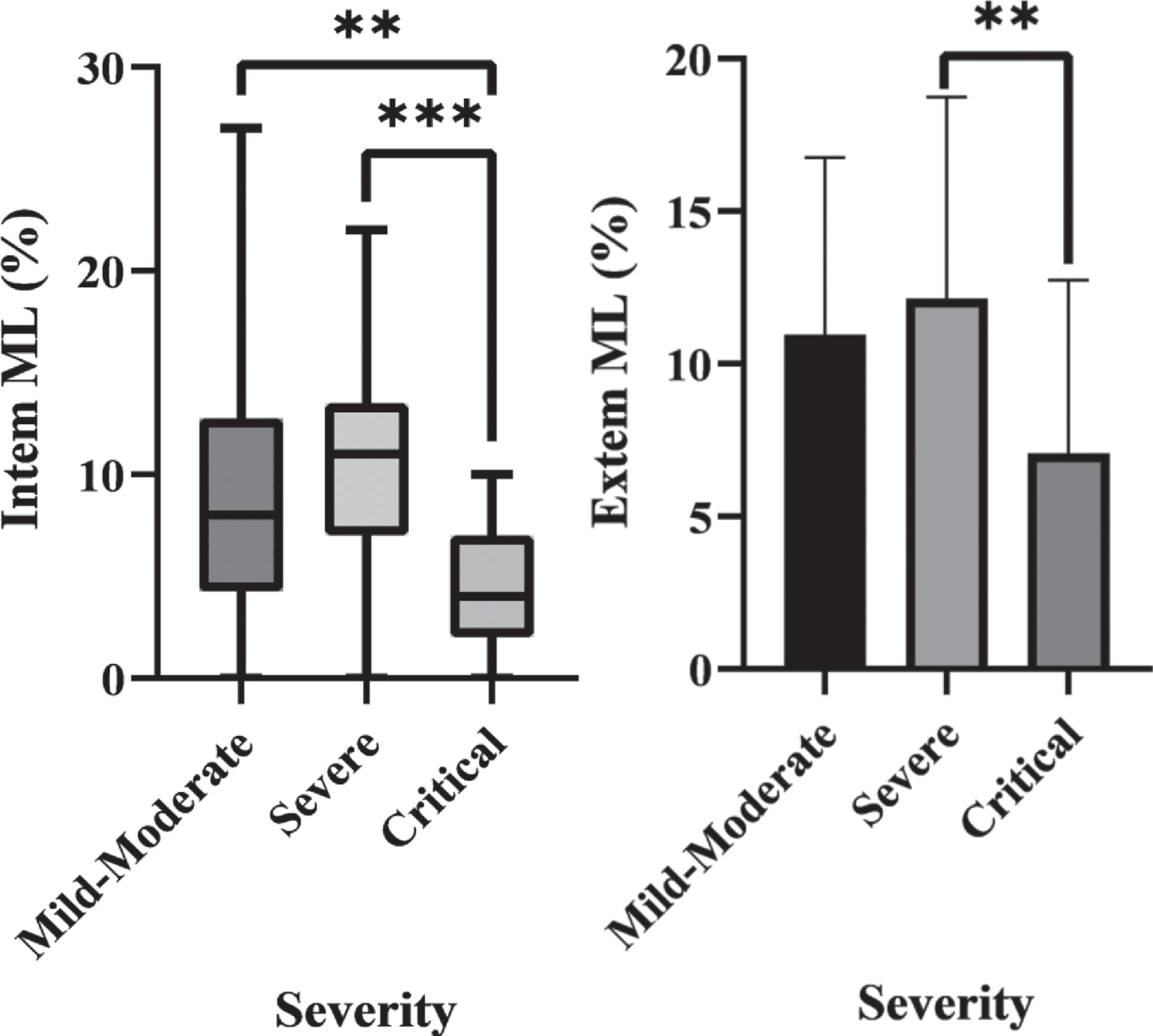
The ROTEM values on arrival for each group are shown in Table 2. Extem ML was significantly different between the severe and critical groups (p = 0.026). Intem ML was significantly different between the critical group and both other groups (p = 0.005 vs mild-moderate, p < 0.001 vs severe), but non-significant in mild-moderate vs severe groups (p = 0.450). These significant differences are shown in Fig. 1.
Table 2
ROTEM parameters for each of the severity groups
| Mild-Moderate | Severe | Critical | |
| Extem CT | 81 (67 – 106) | 81 (74 – 93) | 77 (68.5 – 90.25 |
| Extem CFT | 61 (44 – 72) | 56 (44.5 – 74.5) | 57.5 (49 – 85.25) |
| Extem MCF | 71 (65 – 77) | 73 (69.5 – 76) | 72 (65.75 – 78) |
| Extem Alpha | 78 (76 – 81) | 79 (76 – 81) | 78.5 (74 – 80.25) |
| Extem ML | 11.0±5.8a | 12.1±6.1b | 7.1±5.7ab |
| Intem CT | 187 (169 – 247) | 183 (155 – 205.5) | 179.5 (152.25 – 255.5) |
| Intem CFT | 56 (49 – 75) | 57 (47.5 – 78) | 70.5 (54.75 – 115.25) |
| Intem MCF | 70 (66 – 77) | 74 (69 – 76) | 68.5 (59 – 76) |
| Intem Alpha | 79 (76 – 80) | 79 (74.5 – 80) | 75.5 (68.5 – 79.5) |
| Intem ML | 8 (4.25 – 12.75) | 11 (7 – 13.5)a | 4 (2 – 7)a |
a = p≤0.01; b = p≤0.001.
Fig. 1
a) Box plot of Intem ML in each severity group which demonstrates statistically significant differences between the Critical group and both other groups. b) A bar chart of Extem ML in each severity group which shows a statistically significant difference between the Critical and Severe groups.
Where patients in these groups had a CT Pulmonary Angiogram (CTPA) performed, this was recorded and results detailed in Table 3. The ROTEM results of those with a Pulmonary Embolus (PE) identified on CTPA were compared to those with a negative scan. CTPAs were performed at the discretion of the clinical team where a PE was considered part of a differential diagnosis based on the patients clinical presentation. The results are detailed in Table 3. Across the 95 patients included, there were 32 CTPAs requested but only patients 7 had a CTPA proven PE. Those patients were compared to those with a negative CTPA but there were no significant changes in any ROTEM value between those with a PE and those without. Compared to a meta-analysis of the incidence of PE in hospitalised Covid-19 patients, the proportion of patients in this study undergoing CTPA and subsequently having a positive finding were broadly comparable[22].
Table 3
Percentage of patients in each severity group who had a CTPA performed whilst an inpatient, and the percentage of those CTPAs which were subsequently positive for a PE
| Mild-moderate | Severe | Critical | |
| CTPAs performed | 34% | 30% | 40% |
| Positive rate | 20% | 21% | 25% |
ROTEM values taken at later time points were also analysed, however there were no significant trends in terms of any progressive changes –either in the subgroups or the cohort as a whole. By the second time point all patients had received LMWH, and continued on it throughout the remainder of the study period and this did not alter the observed effect.
4Discussion
In this dataset there was an extremely wide range in outcomes from those who were discharged immediately from ED, to those with prolonged hospital stays associated with significant mortality and morbidity. Corresponding with this was an association of age and co-morbidity with worsening outcomes. This is a widely accepted feature of Covid-19 infection. The significant heterogeneity in patient groups warranted selective analysis of patients in specific outcome groups. Those who survived a prolonged hospital stay were compared to those who died or spent time on ICU as this reflects a divide in patients who recovered from serious illness vs those with greater mortality and morbidity. We were careful to limit the extent of statistical analysis to prevent the introduction of a statistical type 1 error from over-analysis based on the number of patients recruited to the study.
The significant differences seen in clot lysis markers in the most critically unwell patients would suggest that impaired ability to breakdown clot mass rather than excessive clot formation is the predominant clotting abnormality. The mortality and morbidity in the critically unwell group may therefore be a result of a failure to effectively match clot breakdown with formation. The microthomboembolic disease seen at post-mortem in Covid-19 patients would support this [1]. LMWH therapy throughout admission does not appear to have any impact on this effect. Further research is needed to establish what effect LMWH has on clot microstructure and activity. However, fibrinolytic shutdown would not explain the elevated levels of clot breakdown products such as D-dimer, so it is likely there are a number of additional factors involved in the coagulopathy.
There are a range of additional mechanisms suggested to form a part of Covid-19 associated coagulopathy, Neutrophil Extracellular Traps (NETs) are proinflammatory intracellular contents released by neutrophils and incorporated into clot structure [23]. They have been associated with coagulopathy, and in particular with a resistance to anticoagulant therapy [24–26]. However, NETs have also been implicated in driving the acute respiratory distress syndrome (ARDS) seen in Covid-19 [27, 28]. Management strategies for ARDS such as mechanical ventilation and extra-corporeal membrane oxygenation (ECMO) are then associated with greater risk of thromboembolic disease, so the association of NETs with coagulopathy may be confounded to some extent.
One initial aim of the study was to provide information to clinicians that might indicate a patient at higher risk of deterioration. Patients who present to hospital with normal physiological measurements are typically discharged with advice regarding worsening symptoms. Some of these patients may be in the very early stages of the disease and subsequently re-attend following a deterioration. It was hoped this study might allow comparisons between those who were discharged and did not re-attend compared to those who presented to the ED for a second time. This might therefore allow for closer monitoring of patients deemed at high risk of the need for hospitalisation based on anticipated clinical course. Only 1 patient in the study re-presented following discharge, and were admitted to hospital for a 9 day stay, they did not require ICU and survived to discharge. On first presentation their ROTEM parameters were all within the normal ranges. As such it remains unknown if there are factors that can predict re-attendance for patients who present at the very earliest stages.
Another related finding was that over time there were not significant changes in ROTEM parameters. Patients who arrived to ED with evidence of coagulopathy on ROTEM tended to have a persistent coagulopathy throughout their treatment –regardless of therapeutic intervention –and patients without evidence of coagulopathy did not tend to develop a coagulopathy. Those who died of Covid-19 in the absence of a coagulopathy may have died due to the other pathophysiological mechanisms of the virus such as ARDS. As the Mild-Moderate group had been discharged before the later time points, it is not known whether ROTEM parameters might change over time in those with a milder Covid-19 infection. However, as they did not re-present to hospital it is likely that they do not develop a clinically significant coagulopathy.
While this study was not designed to evaluate treatment options, it does offer an indication that on arrival to hospital, coagulation analysis can predict who is most at risk of severe disease. As a follow on from this finding, it would be worthwhile investigating whether these patients would benefit from more aggressive anticoagulation therapy. However, it is also possible that these patients remain hypercoagulable in spite of anticoagulation, and these changes simply predict risk of mortality and morbidity.
4.1Limitations
All patients were recruited in the ED at presentation, however, those who presented out of standard working hours were only recruited the following morning. Therefore they were significantly more likely to have had treatment before blood sampling. In terms of the study aim to identify derangements in coagulation at the time of presentation, this may have introduced a selection bias in which patients presenting to ED overnight were more severely unwell but were subsequently commenced on appropriate management before the research team were able to assess coagulation markers. Anti-Xa levels were not different between severity groups, and similar proportions in each group had received treatment before blood sampling. Nevertheless we are unable to completely exclude this as a potential source of bias.
We also were unable to account for the duration or progression of symptoms before presentation to the ED. Some patients may have remained at home for longer before presenting later in the disease progression.
As the final samples were taken 7 days after admission, it is also unclear whether those who died much later in the disease course continued to have a progressive derangement of any clotting parameters later in the disease progression. Of those who died, the longest duration of stay was 25 days before death.
5Conclusion
This paper not only adds to the growing body of evidence which suggests that thromboembolic disease in Covid-19 is exacerbated by a failure to breakdown clots due to hypofibrinolysis. But it also demonstrates this finding can be seen early in the course of disease progression. Identifying high risk patients as they arrive in the ED allows clinicians to better triage patients who require early senior clinician input or escalation to higher level care. These patients may also benefit from early and aggressive anticoagulation therapy, and further research should be targeted at evaluating this treatment option.
This study does not provide evidence that the use of ROTEM later into the hospital stay can provide an insight into a patients improvement or deterioration. And further research would be needed to establish whether this is an inherent characteristic of Covid-19 infection, or whether current licensed therapeutics are insufficient to combat Covid-19 associated coagulopathy.
References
[1] | Iba T , Levy JH , Levi M , Connors JM , Thachil J . Coagulopathy of Coronavirus Disease 2019. Crit Care Med. (2020) ;48: (9):1358–64. doi: 10.1097/CCM.0000000000004458 |
[2] | de Roquetaillade C , Bredin S , Lascarrou JB , Soumagne T , Cojocaru M , Chousterman BG et al. Timing and causes of death in severe COVID-19 patients. Crit Care. (2021) ;25: (1):224. doi: 10.1186/s13054-021-03639-w. |
[3] | Chen T , Wu D , Chen H , Yan W , Yang D , Chen G , et al. Clinical characteristics of 113 deceased patients with coronavirus 2019: retrospective study. BMJ. (2020) ;368: :m1295. doi: 10.1136/bmj.m1091. |
[4] | Jung EM , Stroszczynski C , Jung F . Contrast enhanced ultrasonography (CEUS) to detect abdominal microcirculatory disorders in severe cases of COVID-19 infection: First experience. Clin Hemorheol Microcirc. (2020) ;74: (4):353–61. doi: 10.3233/CH-209003. |
[5] | Boss K , Kribben A , Tyczynski B . Pathological findings in rotation thromboelastometry associated with thromboembolic events in COVID-19 patients. Thromb J. (2021) ;19: (1):10. doi: 10.1186/s12959-021-00263-0. |
[6] | Pavoni V , Gianesello L , Pazzi M , Horton A , Suardi LR . Derangement of the coagulation process using subclinical mark253 ers and viscoelastic measurements in critically ill patients with coronavirus disease 2019 pneumonia and non-coronavirus disease 2019 pneumonia. Blood Coagul Fibrinolysis. (2021) ;32: (2):80–86. doi: 10.1097/MBC.0000000000000971. |
[7] | Almskog LM , Wikman A , Svensson J , Wanecek M , Bottai M , van der Linden J et al. Rotational thromboelastometry results are associated with care level in COVID-19. J Thromb Thrombolysis. (2021) ;51: (2):437–45. doi: 10.1007/s11239-020-02312-3. |
[8] | Gonenli MG , Komesli Z , Incir S , Yalcin O , Akay OM . Rotational Thromboelastometry Reveals Distinct Coagulation Profiles for Patients With COVID-19 Depending on Disease Severity. Clin Appl Thromb Hemost. (2021) . doi: 10.1177/10760296211027653. |
[9] | Hartmann J , Ergang A , Mason D , Dias JD . The Role of TEG Analysis in Patients with COVID-19 Associated Coagulopathy: A Systematic Review. Diagnostics (Basel). (2021) ;11: (2):172. doi: 10.3390/diagnostics11020172. |
[10] | Bareille M , Hardy M , Douxfils J , Roullet S , Lasne D , Levy JH et al. Viscoelastometric Testing to Assess Hemostasis of COVID-19: A Systematic Review. J Clin Med. (2021) ;10: (8):1740. doi: 10.3390/jcm10081740. |
[11] | Gomez-Builes JC , Acuna SA , Nascimento B , Madotto F , Rizoli SB . Harmful or Physiologic: Diagnosing Fibrinoysis Shutdown in a Trauma Cohort With Rotational Thromboelastometry. Anesth Analg. (2018) ;127: (4):840–9. doi: 10.1213/ANE.0000000000003341. |
[12] | Davies GR , Lawrence M , Pillai S , Mills GM , Aubrey R , Thomas D et al. The effect of sepsis and septic shock on the viscoelastic properties of clot quality and mass using rotational thromboelastometry: A prospective observational study. J Crit Care. (2018) ;44: :7–11. doi: 10.1016/j.jcrc.2017.09.183. |
[13] | Matsumoto H , Ishimaru K , Kikuchi S , Akita S , Yamamoto Y , Yoshida M et al. Perioperative coagulofibrinolytic responses in colorectal surgery patients without chemical thromboprophylaxis: a retrospective observational study. Surg Today. (2021) . doi: 10.1007/s00595-021-02393-4. |
[14] | Creel-Bulos C , Auld SC , Caridi-Scheible M , Barker NA , Friend S , Gaddh M . Fibrinolysis Shutdown and Thrombosis in a COVID-19 ICU. Shock. (2021) ;55: (3):316–20. doi: 10.1097/SHK.0000000000001635. |
[15] | Wright FL , Vogler TO , Moore EE , Moore HB , Wohlauer MV , Urban S et al. Fibrinolysis 276 Shutdown Correlation with Thromboembolic Events in Severe COVID-19 Infection. J Am Coll Surg. (2020) ;231: (2):193–203. doi: 10.1016/j.jamcollsurg.2020.05.007. |
[16] | van Veenendaal N , Scheeren TWL , Meijer K , van der Voort PHJ . Rotational thromboelastometry to assess hypercoagulability in COVID-19 patients. Thromb Res. (2020) ;196: :379–81. doi: 10.1016/j.thromres.2020.08.046. |
[17] | Pavoni V , Gianesello L , Pazzi M , Stera C , Meconi T , Covani Frigieri F . J Thromb Thrombolysis. (2020) ;50: (2):281–6. doi: 10.1007/s11239-020-02130-7. |
[18] | Xing Y , Yang W , Jin Y , Wang C , Guan X . D-dimer daily continuous tendency predicts the short-term prognosis for COVID-19 independently: A retrospective study from Northeast China. Clin Hemorheol Microcirc. (2021) ;79: (2):269–77. doi: 10.3233/CH-201071 |
[19] | Nugroho J , Wardhana A , Mulia EP , et al. Elevated fibrinogen and fibrin degradation product are associated with poor outcome in COVID-19 patients: A meta-analysis. Clin Hemorheol Microcirc. (2021) ;77: (2):221–31. doi:10.3233/CH-200978 |
[20] | Alzoughool F , Alanagreh L , Abumweis S , Atoum M . Cerebrovascular comorbidity, high blood levels of C-reactive protein and D-dimer are associated with disease outcomes in COVID-19 patients. Clin Hemorheol Microcirc. (2021) ;77: (3):311–22. doi: 10.3233/CH-201002 |
[21] | Health Research Authority. UK [https://www.hra.nhs.uk]. Retrieved 30/05/2022. Available from: https://www.hra.nhs.uk/planning-and-improving-research/application-summaries/research-summaries/thrombogenicity-in-acuteexacerbation-of-copd/ |
[22] | Roncon L , Zuin M , Barco S , Valerio L , Zuliani G , Zonzin P et al. Incidence of acute pulmonary embolism in COVID-19 patients: Systematic review and meta-analysis. Eur J Intern Med. (2020) ;82: :29–37. doi: 10.1016/j.ejim.2020.09.006. |
[23] | Zhu Y , Chen X , Liu X . NETosis and Neutrophil Extracellular Traps in COVID-19: Immunothrombosis and Beyond. Front. Immunol. (2022) ;13: :838011. Doi: 10.3389/fimmu.2022.838011. |
[24] | Zuo Y , Zuo M , Yalavarthi S , Gockman K , Madison JA , Shi H , et al. Neutrophil extracellular traps and thrombosis in COVID-19. J Thromb Thrombolysis. (2021) ;51: (2):446–53. 10.1007/s11239-020-02324-z. |
[25] | Ligi D , Maniscalo R , Plebani M , Lippi G , Mannello . Do Circulating Histones Represent the Missing Link among COVID-19 Infection and Multiorgan Injuries, Microvascular Coagulopathy and Systemic Hyperinflammation? J Clin Med. (2022) ;11: (7):1800. doi: 10.3390/jcm11071800. |
[26] | Bautista-Becerril B , Campi-Caballero R , Sevilla-Fuentes S , Hernandex-Regino LM , Hanono A , Flores-Bustamente A et al. Immunothrombosis in COVID-19: Implications of Neutrophil Extracellular Traps. Biomolecules. (2021) ;11: (5):694. doi: 10.3390/biom11050694. |
[27] | Narasaraju T , Tang BM , Herrmann M , Muller S , Chow VTK , Radic M . Neutrophilia and NETopathy as Key Pathologic Drivers of Progressive Lung Impairment in Patients With COVID-19. Front Pharmacol. (2020) ;11: :870. doi: 10.3389/fphar.2020.00870. |
[28] | Ouwendijk WJD , Raadsen MP , van Kampen JJA , Verdijk RM , von der Thusen JH , Guo L , et al. High Levels of Neutrophil Extracellular Traps Persist in the Lower Respiratory Tract of Critically Ill Patients With Coronavirus Disease 2019. J Infect Dis. (2021) ;223: (9):1512–21. doi: 10.1093/infdis/jiab050. |




