Modified contrast-enhanced ultrasonography with the new high-resolution examination technique of high frame rate contrast-enhanced ultrasound (HiFR-CEUS) for characterization of liver lesions: First results
Abstract
AIM:
To examine to what extent the high frame rate contrast-enhanced ultrasound (HiFR) diagnostic enables the conclusive diagnosis of liver changes with suspected malignancy.
MATERIAL/METHODS:
Ultrasound examinations were performed by an experienced examiner using a multifrequency probe (SC6-1) on a high-end ultrasound system (Resona 7, Mindray) to clarify liver changes that were unclear on the B-scan. A bolus of 1–2.4 ml of the Sulphur hexafluoride ultrasound microbubbles contrast agent SonoVue™ (Bracco SpA, Italy) was administered with DICOM storage of CEUS examinations from the early arterial phase (5–15 s) to the late phase (5–6 min). Based on the image files stored in the PACS, an independent reading was performed regarding image quality and finding-related diagnostic significance (0 not informative/non-diagnostic to 5 excellent image quality/confident diagnosis possible). References were clinical follow-up, if possible, comparison to promptly performed computed tomography or magnetic resonance imaging, in some cases also to histopathology.
RESULTS:
We examined 100 patients (42 women, 58 men, from 18 years to 90 years, mean 63±13 years) with different entities of focal and diffuse liver parenchymal changes, which could be detected in all cases with sufficient image quality with CEUS and with high image quality with HiFR-CEUS. Proportionally septate cysts were found in n = 19 cases, scars after hemihepatectomy with local reduced fat in n = 5 cases, scars after microwave ablation in n = 19 cases, hemangiomas in n = 9 cases, focal nodular hyperplasia in n = 8 cases, colorectal metastases in n = 15 cases, hepatocellular carcinoma (HCC) in n = 11 cases, Osler disease in n = 8 cases. The size of lesions ranged from 5 mm to 200 mm with a mean value of 33.1±27.8 mm. Conclusive diagnoses could be made by the experienced investigator in 97/100 cases with CEUS, confirmed by reference imaging, in parts by histopathology or follow-up. The image quality for HiFR CEUS was rated with a score of 3 to 5; 62 cases were assessed with an average of good (4 points), 27 cases with very good (5 points), and in 11 cases (3 points) still satisfactory despite aggravated acoustic conditions. The specificity of HIFR-CEUS was 97%, the sensitivity 97%, the positive predictive value 94%, the negative predictive value 99% and the accuracy 97%.
CONCLUSION:
HIFR-CEUS has demonstrated has demonstrated an improved image quality resulting in a high diagnostic accuracy. In the hands of an experienced investigator, HiFR-CEUS allows the assessment of focal and diffuse unclear liver parenchymal changes on B-scan and dynamic assessment of microcirculation in solid and vascular changes.
1Introduction
Contrast-enhanced sonography (CEUS) of the liver has a high diagnostic value in the hands of experienced examiners [1–7]. Already in the DEGUM multicenter study, under appropriate examination conditions, CEUS was able to achieve a diagnostic reliability comparable to contrast-enhanced computed tomography (ceCT) with regard to the detection and characterization of solid liver lesions [8–13]. Compared with contrast-enhanced magnetic resonance imaging (ceMRI), there may be diagnostic advantages for MRI over CEUS with respect to the detection and characterization of solid liver lesions due to the use of liver-specific MRI contrast agents.
Ultrasound contrast agents of the 2nd generation are based on the principle of echo signal amplification by oscillation of microbubbles using Contrast Harmonic Imaging (CHI) with a low mechanical index (MI) < 0.2 [5]. The EFSUMB Guidelines describe a large number of applications relating to liver diagnostics and non-liver indications [1]. Regarding liver tumor diagnostics, there is a high classification of diagnostic relevance also by the FDA with recommendations and own experience also for applications in pediatric questions [1, 17].
The possible side effects of CEUS are considered to be low with increasingly worldwide clinical use. There is no restriction of renal perfusion [14, 15] or renal function and no influence of ultrasound contrast agents on thyroid metabolism. This still allows CEUS diagnosis of the liver even when impaired renal function makes this no longer feasible for the use of CT or MRI contrast agents.
The most common ultrasound contrast agent currently used in Europe is based on the Sulphur hexafluoride microbubbles contrast agent SonoVue™ (BRACCO, Italy), which are usually administered intravenously as a bolus of 1 to 2.4 ml followed by 5 to 10 ml of NaCL. Liver-specific ultrasound contrast agents are being researched but are currently not approved for routine use for liver ultrasound diagnosis in Germany and many European countries [1].
In order to optimize CEUS in the application of Sulphur-hexafluoride microbubbles contrast agent with respect to image quality, a large number of technical developments have been carried out recently. Increasingly, high-resolution multi-frequency transducers as sector or linear transducers with significantly increased crystal number, modified programs of the CHI with improved amplitude modulation or pulse inversion techniques (PIHI) and improved digital image spreading especially of CINE sequences are used [5]. Furthermore, efforts are being made to increase the frame rate to achieve even greater detail. A possible novel contrast medium ultrasound technique, which tries to take advantage of all the latest technical developments, is the new technique of high frame rate contrast-enhanced ultrasound (HiFR).
Whether there may be diagnostic advantages for CEUS with the novel mode of investigation such as HiFR must be based on prior studies regarding diagnostic confidence. The extent to which the detection and characterization of solid liver tumors is possible must be clarified, complicated cystic lesions can be differentiated, and the dynamics of lesions unclear on CT or MRI can be dynamically recorded and digitally stored from the early arterial phase after 5–15 s to a late phase of 5 to 6 min for independent assessment.
The following clinical investigation examined the extent to which the new technique of HiFR-CEUS detects and characterizes complicate cystic, solid, and microvascular liver lesions?
2Material and methods
Written informed consent was obtained from patients for all examinations using contrast-enhanced sonography (CEUS). Evaluation was performed independently using the image data stored in PACS by experienced readers in consensus. For the scientific evaluation of the data of contrast medium sonography for the diagnosis of liver lesions, an ethics vote of the ethics committee of the University Hospital Regensburg is available (approval number: 21-2761-104). The procedure for CEUS liver tumor diagnostics complies with the guidelines of the DEGUM studies [1].
N = 100 Patients with unclear liver lesions were subsequently included in the study. The examinations were performed on referral of the liver consultations for the clarification of unclear liver lesions on the basis of previous findings of B-mode sonography, computed tomography (CT) or magnetic resonance imaging (MRI). The aim of CEUS was to allow a final diagnosis, to describe the type, location and number of possible liver lesions or perfusion changes and, if necessary, to determine the further procedure. In the case of clearly benign findings, a follow-up after 3–6 months was performed as a reference, and in the case of suspected lesions corresponding to hepatocellular carcinoma (HCC) a grading according to LI-RADS IV-V was performed according to the probability of malignancy, and reference imaging with contrast-enhanced multislice CT and/or MRI with liver-specific contrast agent (Primovist™, Bayer, Germany) was performed promptly. If metastases were indicated, a primary tumor search was performed with CT and, if necessary, positron emission tomography (PET-CT). If necessary, a biopsy was performed after tumor board decision. If these were available, comparison to the surgical result of a liver tumor resection was performed.
All ultrasound examinations were performed by an experienced examiner (> 3000 examinations/year > 20 years) with a multifrequency convex probe (C1-6 MHz) on a high-end ultrasound machine (Resona 7, Mindray, China). The detectable lesions were digitally documented in B-mode in two planes under optimization of the examinations depth-dependent over the frequency, complementary with Tissue Harmonic Imaging (THI), ultrasound CT technique and averting Photopic technique (B-Colour). Assessment of macrovascularization of the liver was performed with color-coded duplex sonography (CCDS) via optimizations with the HR flow technique and glazing flow [34] for the hepatic veins (LV), hepatic artery (HA), portal vein (PV), and, if possible, tumor lesions with parameters mostly adapted to low flow (low scale/pulse repetition frequency (PRF), highest possible color gain, and low wall filter). Diagnostic indications were a wheel spoke pattern in focal nodular hyperplasia (FNH), marginal vascularization in adenomas (hepatic adenoma) or irregular central vascularization in HCC. It was also necessary to detect vascular tumor infiltration, especially in relation to the hepatic veins and portal vein intrahepatic. In the case of vascular changes, it was necessary to detect shunt with CCDS or a vascular breakdown in emboli and infarcts.
2.1ZONE Sonography Technology Plus (ZST+) technology
The ZST + technology adopts the weak focused transmission, generating wider physical beams. The area scanned in each transmission is significantly larger than that in traditional focused transmission. The plane wave technology applies the non-focused whole-zone transmission mode. The entire imaging area is scanned in one transmission. Therefore, one frame of image can be generated with one transmission. In addition, due to the weak focused transmission and non-focused transmission modes, the both two zone transmission technologies require post processing at the receive end, to achieve a higher frame rate without compromising the image quality of the contrast imaging. For focused imaging, the lateral resolution and signal-to-noise ratio (SNR) of the sound field at the focus are the best, but the performance is gradually degraded away from the focus. The lateral resolution of the entire field can be improved by increasing the number of transmission foci or adopting continuous focused transmission at the cost of an inevitably decreased frame rate. This is infeasible for the focused imaging with a low upper limit of the frame rate. Although the overall lateral resolution is poorer than that at the focus of the focused transmission, the sound field is more uniform. With the reconstruction of the receiving sound field, the continuous transmission focused synthetic sound field could be achieved. In addition, with the coherent transmission synthesis technology, the number of transmissions is significantly reduced. Furthermore, in order to tackle the problem of line artifact and SNR loss caused by decreased transmissions, each receiving line is generated through optimized compounding of echoes from adjacent multiple transmissions. To sum up, the ZST + technology is the key to HiFR-CEUS by increasing the frame rate of CEUS without compromising image quality.
HIFR-CEUS was performed in bolus technique as intravenous administration of 1–2.4 ml Sulphur hexafluoride microbubbles contrast agent SonoVue™ (Bracco, Italy) with 10 ml NaCL (saline solution) via cubital vein. Possible history of contrast intolerance was considered a contraindication. Impaired renal function or renal insufficiency or changes in thyroid function were not considered contraindications according to the EFSUMB guidelines. The examinations were performed dynamically with the new HiFR technique depth-adapted with the modalities general (GEN), resolution (RES) or penetration (PEN) and digitally documented. The CEUS examinations of the entire liver performed in sweep technique included continuous acquisition of the arterial phase 10 to 15 s after bolus administration to the portal venous phase over 1 minute, in case of vascular changes with optimal acquisition of the hepatic hilus to be able to detect possible arteriovenous or portal venous macro- or microshunts. Then, lesion- and findings-related documentation of a possible wash-out was performed with short cine loops over 5 to 10 s up to a late phase of 5 to 6 minutes, supplemented by single images that could be stored during the cine sequences specifically related to findings. The images were automatically sent to PACS for independent analysis.
According to the EFSUMB guidelines and the results of the DEGUM studies, continuous contrast enhancement in CEUS, marginal nodular enhancement, regular marginal contrast enhancement were considered typical for benign lesions such as FNH, typically with central scar, hemangiomas and adenomas. Typical for malignant lesions such as metastases, CCC or HCC lesions were irregular arterial vascularization with increasing wash out of tumor lesions to late phase, in metastases already in PV phase, in HCC not rarely starting after 3 minutes, in CCC in between. In complicated cysts, attention was paid to possible contrast enhancement of the septa, whether regular linear to a maximum of 2 mm in width benign, so post-inflammatory, or irregular nodular > 3 mm with wash out as a sign of cystic necrotic disintegrating tumors. For vascular changes, contrast of unaffected liver tissue and contrast of hepatic artery portal vein and hepatic veins formed the reference.
Early contrast was considered to be < 10 s after bolus administration of the contrast agent, regular contrast was considered to be 10 to 20 s depending on the cycle time, and delayed contrast was considered to be > 20 s after bolus administration. A contrast of the portal vein after 20 s and of the hepatic veins after 30 s was considered typical for shunts. After completion of the documentation of the late phase after 5 to 6 min, this could be checked with the technique of replenishment (FLASH kinetics) specifically on certain liver segments with the detection of a reflux of the microbubbles, how extensive the shunts were. If necessary, hemodynamics was then again specifically assessed with CCDS. Follow-up with CEUS was performed after 3 to 6 months for benign lesions with comparable documentation. Malignancy was verified with reference imaging of CT, MRI, or by biopsy/surgery by independent investigation.
For CT, multislice CT examinations in arterial and portal-venous contrast phase would be the reference with 80 to 120 ml iodine-containing contrast medium (Accupaque® 350, GE Healthcare Buchler GmbH & Co.KG, Germany) in 5 mm reconstructions axial and coronary, considering possible contraindications. MRI was performed with 1.5 and 3 Tesla scanners of the latest generation with native T1 and T2 sequences in 5 mm slices, diffusion sequences with b values up to 1800 and ADC evaluation, and with liver-specific contrast agent (Gd-EOB-DTPA, Primovist®, Bayer, Germany) enhanced MRI as 3 D vibe sequences in 3 mm reconstructions. Impaired renal function, GFR < 30, and known intolerances to contrast agents were considered contraindications.
Biopsies of the liver were performed in an inpatient setting with all safety measures observed, and written informed consent was obtained 24 hours before the intervention. A representative cylinder 2 cm from the tumor area was taken after surgical disinfection under sterile conditions with a 16 G semi-automatic biopsy needle under US guidance with puncture line and a second sample from the marginal tissue.
Mindray’s latest high frame rate contrast enhanced ultrasound (HiFR-CEUS) technology can significantly increase the frame rate of CEUS. The frame rate of the convex array probe can be increased to 4 –8 times while comparing to conventional CEUS technology. In the linear array probes, the highest frame rate can exceed 100 FPS. As a result, the improvement of time resolution could be helpful for better tracking microbubbles movement, which is conducive to the observation of microvascular perfusion details, especially in small focal liver lesions or lesions with rich blood supply. In particular, it could better display vascular morphology by accurately delineating blood perfusion paths of the lesion, thereby improving the diagnostic efficiency of liver CEUS. On the basis of the image files stored in the PACS, an independent reading was performed with regard to image quality and finding-related diagnostic significance (0 not meaningful, 1 only conditionally assessable, 2 limited, 3 satisfactory but impeded, 4 good to 5 excellent image quality, diagnosis possible with certainty).
The final independent evaluation of the constellation of findings CEUS, CT, MRI was performed by 2 independent readers in consensus. The gold standard was the histology of the target lesion, if available, above the reference findings of MRI with liver-specific contrast agent, in case of HCC by 2 independent imaging (LI-RADS classification), in case of benign lesions alternatively the follow-up with CEUS for at least 6 months with unchanged findings (Osler‘s disease).
2.2Statistics
Numerical data were expressed as arithmetic means with standard deviation, and the categorical variables as numbers and percentages. Specificity, sensitivity, positive and negative prognostic value as well as diagnostic accuracy were calculated on the basis of the following table.
Table 4
| disease existent | disease not existent | ||
| Test positive | a | b | a + b |
| Test negative | c | d | c + d |
| a + c | b + d |
With:
a: number of patients with existing disease and positive result of the test (true positive)
b: number of patients with no disease, but positive result of the test (false positive)
c: number of patients with existing disease and negative result of the test (false negative)
d: number of patients with no disease and negative result of the test (true negative)
Sensitivity and specificity were calculated as follows:
1 Sensitivity = a / (a + c)
2 Specificity = d / (b + d)
3 Positive predictive value = a / (a + b)
4 Negative predictive value = d / (d + c)
5 Accuracy = (a + d) / (a + b + c + d)
3Results
N = 100 patients (42 women, 58 men, from 18 years to 90 years, mean 63±13 years) with various entities of focal and diffuse liver parenchymal changes were studied, all of which could be detected with high image quality with HiFR-CEUS. There were n = 19 cases with proportionally septated cysts, in n = 5 cases scars after hemihepatectomy with local reduced fat, in n = 19 cases scars after microwave ablation, in n = 9 cases hemangiomas, focal nodular hyperplasia (FNH) in n = 8 cases, colorectal metastases in n = 15 cases, hepatocellular carcinoma (HCC) in n = 11 cases n = 8 cases, Osler disease in n = 8 cases. Table 1 shows the classification by the diagnostic procedures used in the 32 patients with malignant tumors of the 100 patients with suspected liver tumor.
Table 1
Diagnoses according to the four methods used
| Not unequivocal | Benign | Malignant | Patients examined | |
| HiFR-CEUS | 0 | 67 | 33 | 100 |
| CT | 0 | 46 | 28 | 74 |
| MRI | 0 | 32 | 13 | 45 |
| Biopsy | 0 | 3 | 17 | 20 |
The size of the lesions ranged from 5 mm to 200 mm, with a mean of 33.1±27.8 mm. The tumor sizes are classified in Table 2 for the three imaging methods.
Table 2
Tumor sizes according to the different measuring methods
| n | < 10 mm | 10–15 mm | 15–20 mm | 20–30 mm | > 30 mm | |
| HiFR-CEUS | 78 | 9 | 12 | 14 | 10 | 33 |
| CT | 39 | 9 | 4 | 10 | 4 | 12 |
| MRI | 30 | 5 | 6 | 5 | 2 | 12 |
The tumor sizes correlated quite well: CEUS –CT: 0.743 and CEUS –MRI: 0.745.
Final diagnoses could be made by the experienced examiner in 97/100 cases with CEUS, confirmed by reference imaging, in parts by histopathology or follow-up. Image quality for HiFR CEUS was rated 3 to 5, with an average of good 4 in a total of 62 cases, very good in 27 cases, and still satisfactory in 11 cases despite severe sonic conditions.
Fig. 1
Case of a 76 years old patient with colorectal cancer. Detection of a small liver metastasis of the right liver lobe, less than 1 cm in diameter using the HiFR CEUS technology after bolus injection of 1.5 ml ultrasound contrast agent. Wash out of the non-cystic lesion, image after 3 minutes in the late venous phase (left side, arrow). The lesion is not visible on fundamental B-mode (right side).
In all cases, contrast-enhanced sonography (CEUS) was technically feasible with good image quality in 62% (scored 4), very good image quality in 27% (scored 5). Due to difficult sonographic conditions, such as meteorism, colon interposition, inhomogeneous clear steatosis hepatis in obesity per magna and due to motion unrest, the digital cine sequences were limited in image quality but still sufficient image quality in 11 % (evaluation with 3 points). In some cases with extremely difficult sonic conditions for segment VIII lesions, image quality was significantly limited by distances to lesions > 20 cm (lesions were very difficult to detect). In MRI, limited image quality occurred in 20 % due to respiratory artifacts especially in the contrast sequences with a rating < 3 points. In CT, inhomogeneous contrast with limited assessment due to mixed contrast of the liver occurred in 15% even when using fast multislice technique.
High frame rate contrast-enhanced ultrasonography (HiFR-CEUS) was able to detect the typical contrast uptake from the rim to the center, nodular in hemangiomas, and from the center to the rim in the sense of a wheel spoke pattern in FNH in all cases of benign liver lesions, such as hemangiomas and focal nodular hyperplasia (FHN), leading to diagnosis. Contrast uptake increased toward the late phase to 5 min and was complete in typical hemangiomas, whereas a central recess remained in partially thrombosed atypical hemangiomas. In typical cases of FNH, a central scar was comparably found.
Typical for scarring changes after micro wave ablation or postoperatively in CEUS was the lack of contrast enhancement from the arterial to the late phase. In marginal recurrences, irregular nodular contrast enhancement arterially in the marginal area and a wash out of these nodular tumor parts beginning in the portal-venous phase would typically occur. With HiFR-CEUS, the defects were correctly assessed in all cases. This was also true for postoperative defects with local reduced fat. In these cases, the echo-deficient areas on the B-scan appeared garland-shaped with wavy margins. MRI examinations with dynamic contrast sequences (Vibe-3D) and diffusion sequences at 1.5 or 3 Tesla with specific contrast agent (Primovist™) or a 2-phase multislice CT with arterial and later portal venous phase served as reference.
Typical for malignant lesions was an arterial irregular contrast enhancement in HiFR-CEUS and a wash out starting in the portal venous phase and increasing to the late phase. Thereby, in all cases of colorectal metastases the more reliable detection was achieved in correlation to CT or MRI. Evidence of irregular marginal vascularization was detectable for all lesions. With high image quality, metastases as small as 5 mm in diameter could be delineated based on the wash out in the portal venous phase after 60 s up to the late phase of 5 to 6 min. The image quality was in some cases superior to CT and reached that of MRI with liver-specific contrast agent in almost all cases. In cases of small septated cysts up to 10 mm in diameter, HiFR-CEUS proved superior to CT in differentiation from metastases.
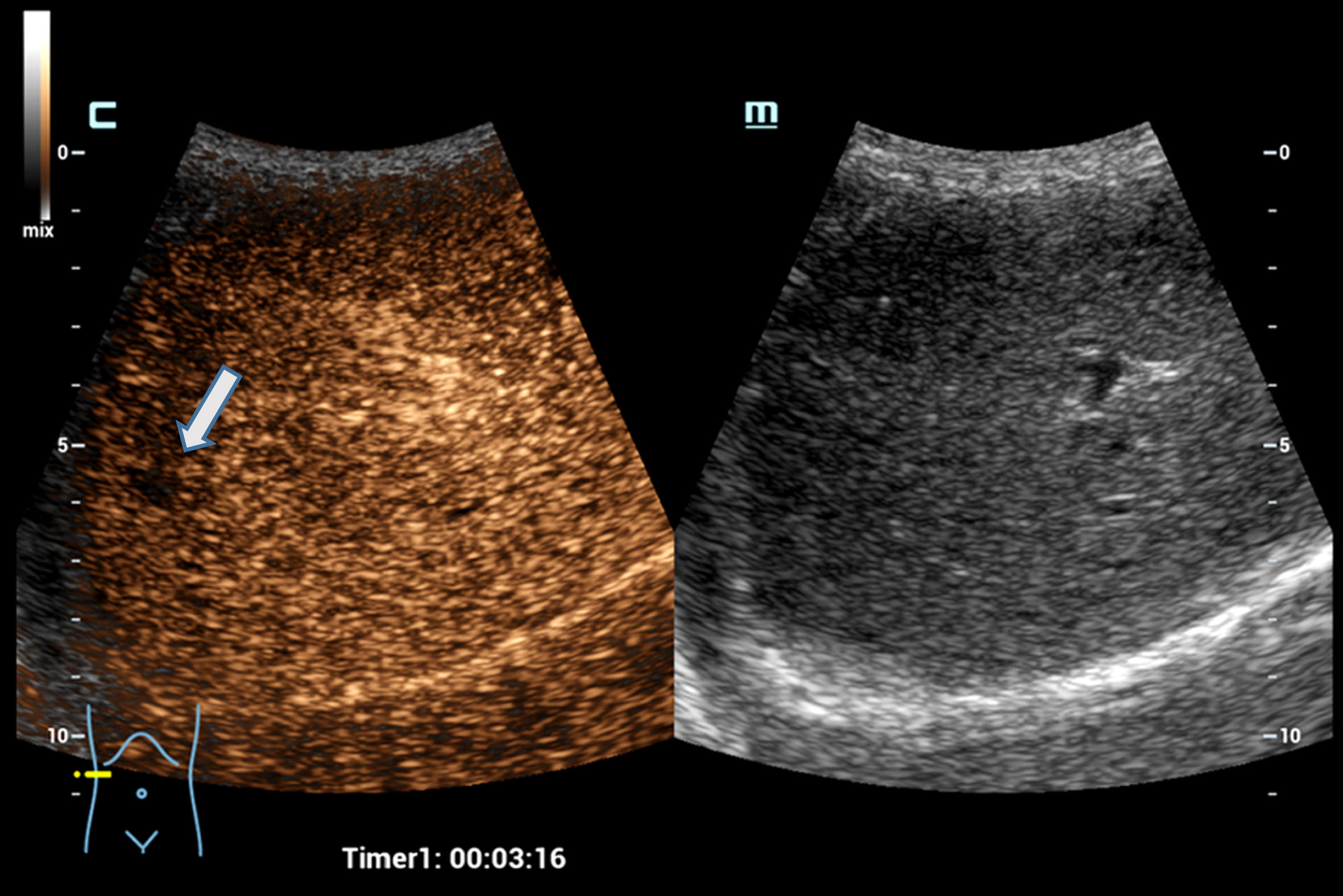
Fig. 2
Case of a 34 years old female patient with focal nodular hyperplasia (FNH). Detection in the early arterial phase using the HiFR CEUS technology after bolus injection of 1.0 ml ultrasound contrast agent hyperenhancement from the center to the margin (arrow). Excellent visualization by HiFR CEUS. The lesion is difficult to detect on fundamental B-mode (right side).
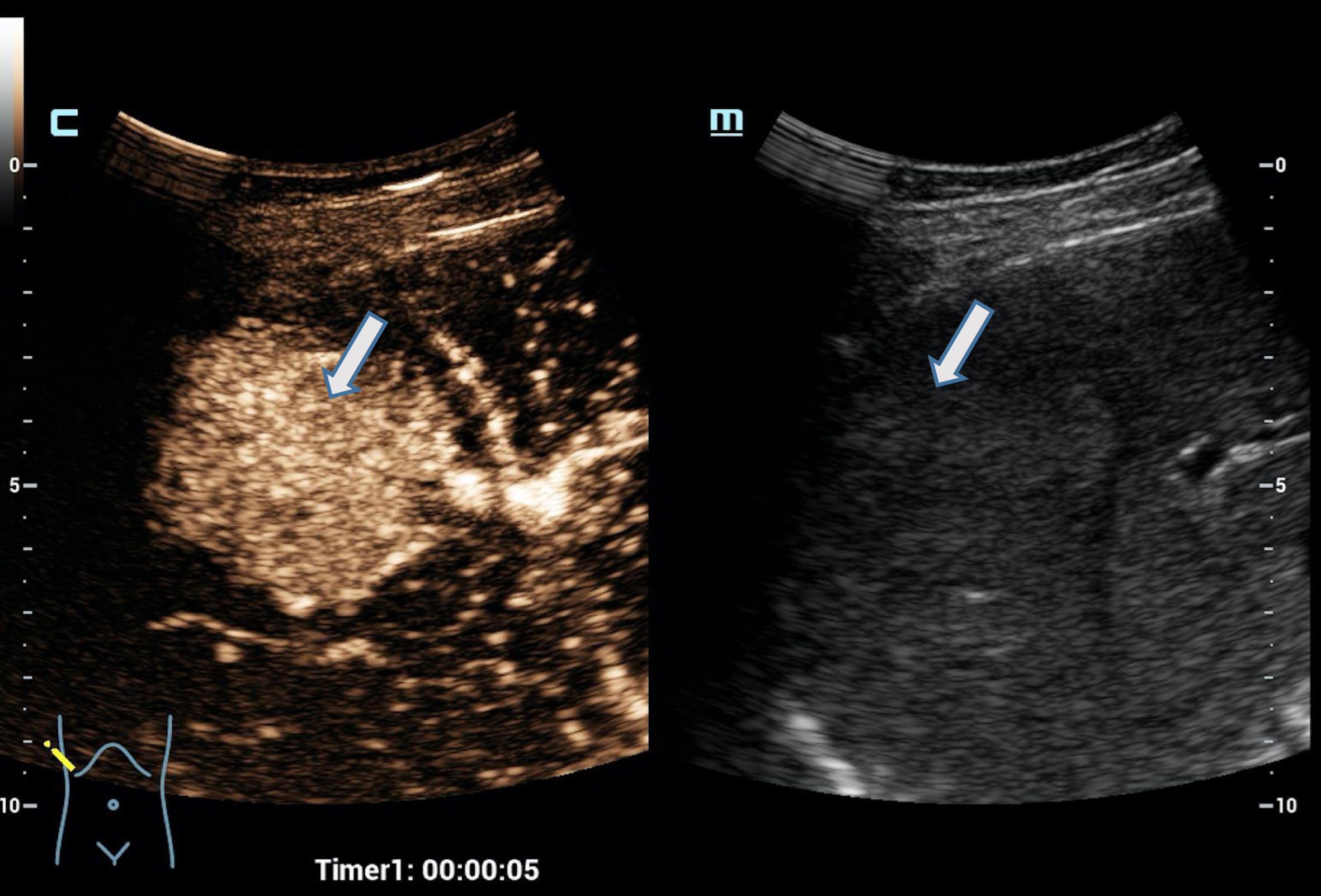
In the detection of hepatocellular carcinoma (HCC) HiFR-CEUS was able to detect irregular, almost chaotic early hypervascularization in the tumor foci and wash out from the portal-venous phase, in HCC often clearly only in the late phase from 4 min, when the tumor foci were > 15 mm in diameter with good or very good image quality. In the case of multiple suspicious foci, the detection of even small tumor foci > 10 mm in the late phase is crucial for the detection of all malignant lesions of a CCC or HCC in HiFR-CEUS. This was possible with HiFR-CEUS in almost all cases with good image quality, in some cases even very good. However, the image quality was often significantly limited by the echo inhomogeneous cirrhosis.
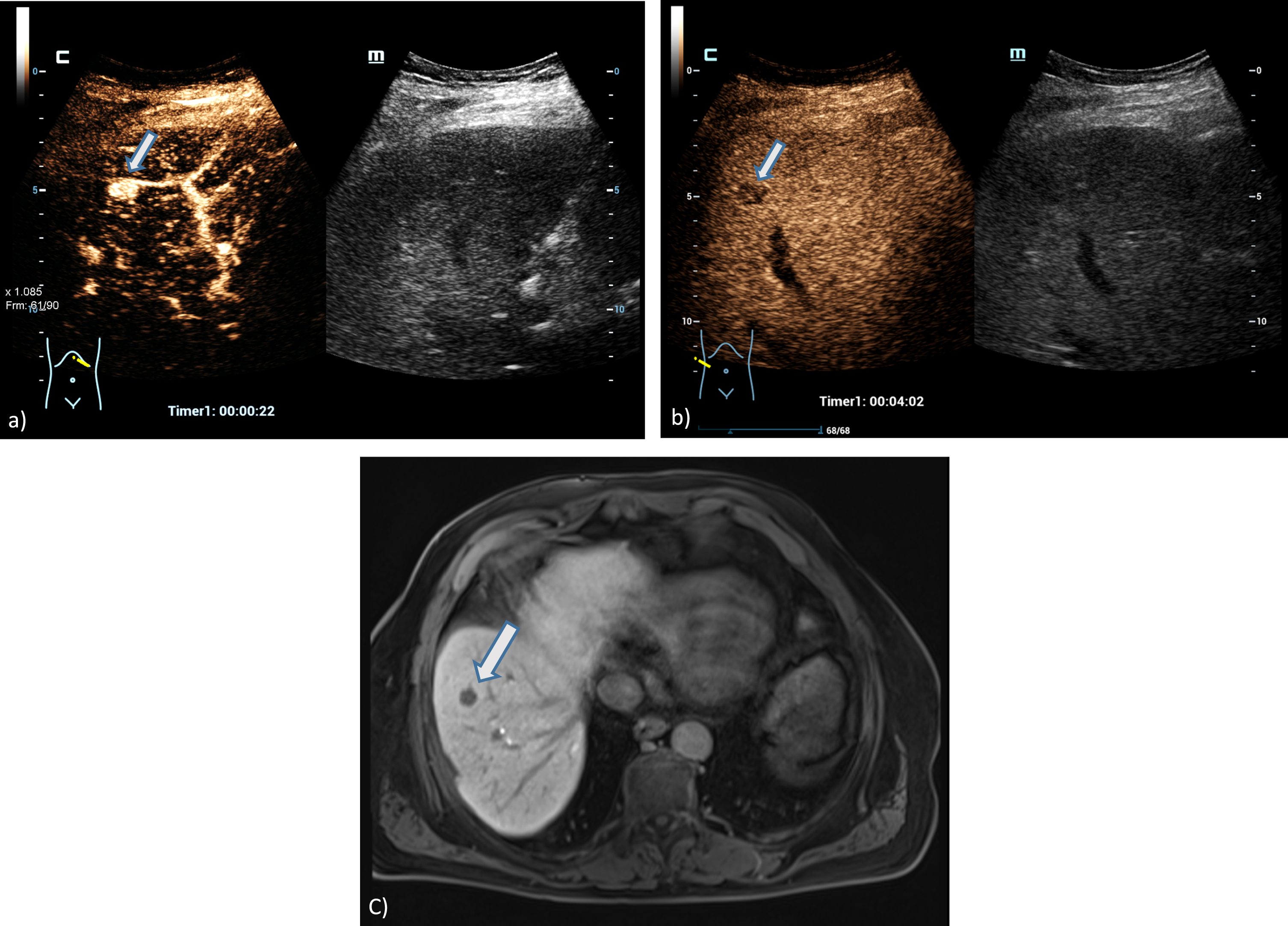
Fig. 3
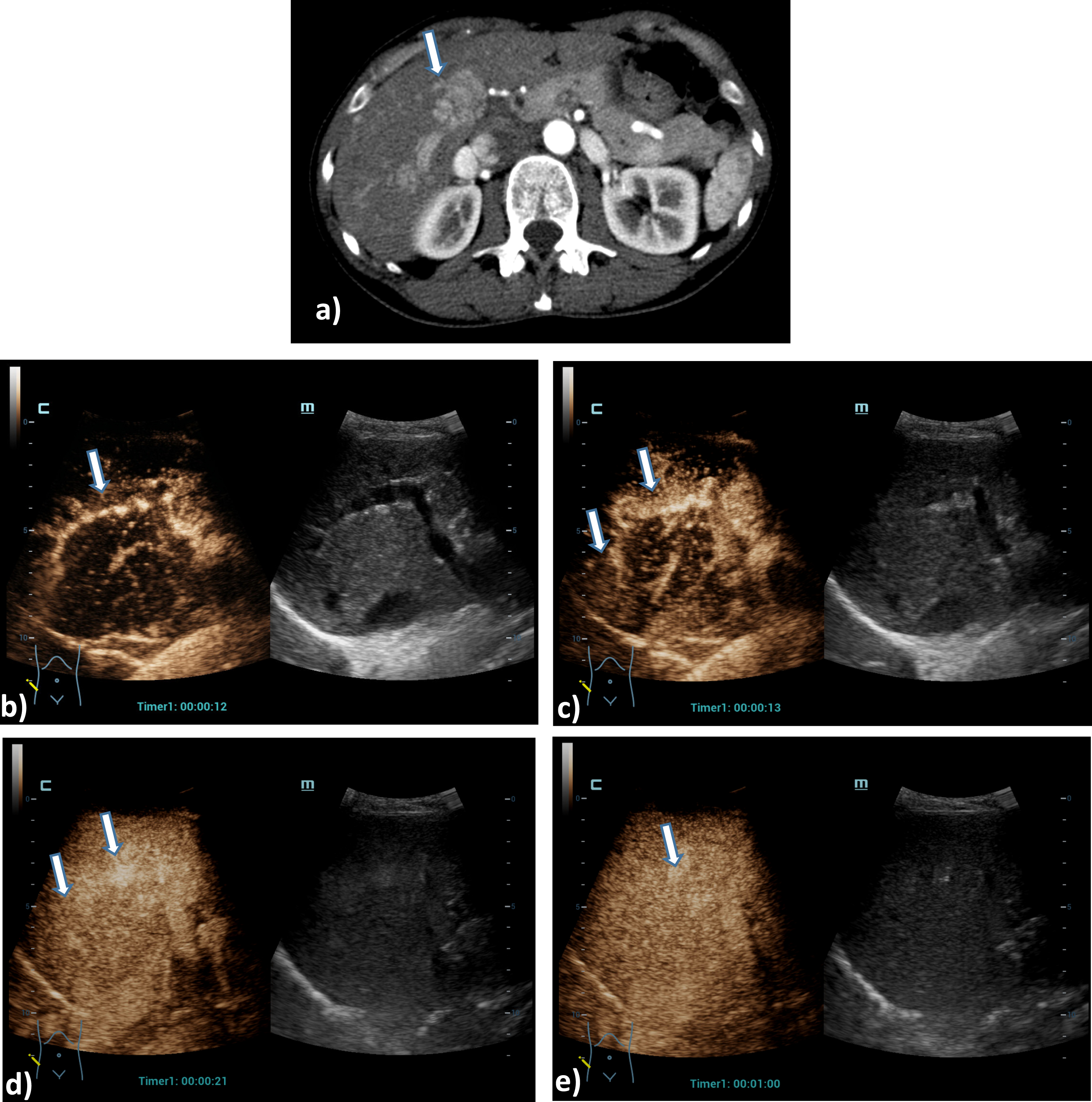
A 73 years old patient with a small lesion of a hepatocellular carcinoma. Detection of a small tumor lesion of the right liver lobe, at maximum 1 cm in diameter using the HiFR CEUS technology after bolus injection of 1.5 ml ultrasound contrast agent (left side, arrow). Clear detectable by HiFR CEUS with irregular arterial hypervascularization (a) and wash out after 4 minutes (b) and by MRI using liver specific contras agent (c). The lesion is not visible on fundamental B-mode (right side).
More difficult was the evaluation of tumor foci with a maximum diameter of only 10 mm. Under difficult sonic conditions, it may be difficult to assess both hypervascularization and partial washout as reliably malignant at depths < 15 cm. Thus, one case was evaluated as regenerative dysplastic with HiFR-CEUS. In this case, the evaluation as HCC lesion was finally performed by MRI with liver-specific contrast agent. The diagnostic test performance of the methods used is shown in Table 3.
Table 3
Results of the evaluation of liver lesion by HIFR CEUS, CT, MRI or histopathology (Standard of diagnosis according to LI-RADS)
| Specificity | Sensitivity | Positive Predictive Value | Negative Predictive Value | Diagnostic Accuracy | |
| HiFR-CEUS | 97% | 97% | 94% | 99% | 97% |
| CT | 90% | 100% | 82% | 100% | 93% |
| MRI | 91% | 91% | 77% | 97% | 91% |
| Biopsy | 50% | 100% | 82% | 100% | 85% |
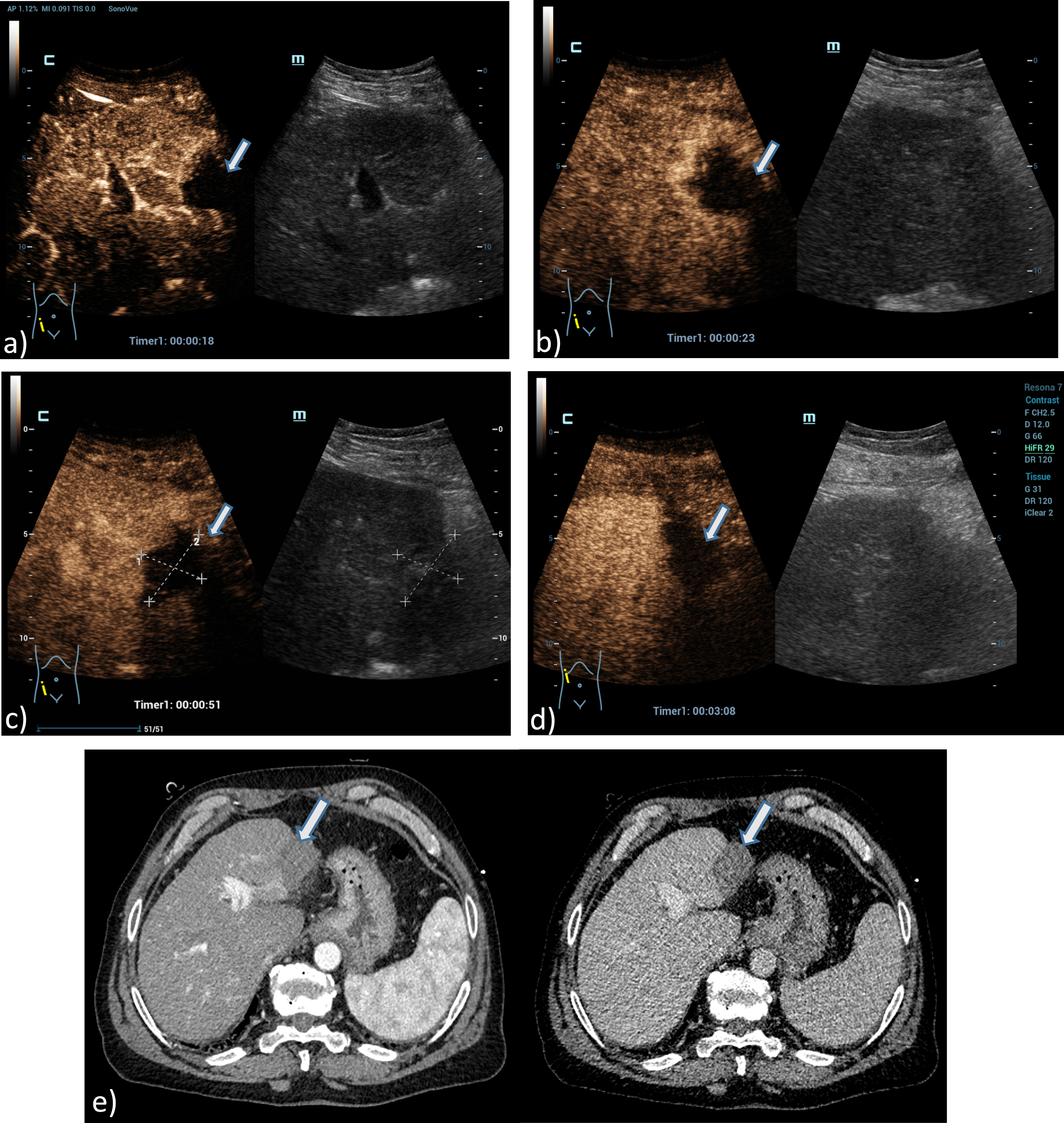
Fig. 4
A 61 years old patient with post ablation defect after MWA. Detection of an avascular defect on the left liver lobe, at maximum 4 cm in diameter using the HiFR CEUS technology after bolus injection of 1.5 ml ultrasound contrast agent (left side, arrow). Clear detectable MWA defect by HiFR CEUS with central devascularization during early arterial phase (a), arterial phase (b), portal venous phase (c) and up to late phase after 3 minutes (d) in comparison to contrast enhanced CT scans (e) during arterial phase (left) and portal venous phase (right) as a vascular defect on the left liver lobe (arrows). The lesion is not clear visible on fundamental B-mode.
4Discussion
Numerous publications exist on the diagnosis of focal liver lesions (FLL) using contrast-enhanced ultrasound (CEUS) in comparison to CT, MRI and histopathology. A critical factor for the characterization of FLL is the assessment of the enhancement pattern of the wash-in and wash-out of the contrast agent. The novelty of the provided research is the use of a new ultrasound technique based on the ‘Zone technology’, which uses broader zones insonation instead of line-by-line insonation. This allows significantly higher frame rates (giving a better representation of the contrast inflow dynamics) and more homogeneous acoustic power distribution across the image field (reducing microbubble destruction). Such new technical developments have made contrast-enhanced sonography (CEUS) an alternative with high diagnostic accuracy and fewer risks in liver diagnostics compared with contrast-enhanced computed tomography (ceCT) or magnetic resonance imaging (ceMRI) [18–22]. However, the success of CEUS always depends on the examination conditions, patient compliance, organ structure, depth localization, and ultimately the technical capabilities in the hands of experienced investigators [23]. Under favorable conditions, CEUS can achieve diagnostic confidence significantly > 90% in the localization and characterization of focal liver lesions, comparable to reference imaging.
Fig. 5
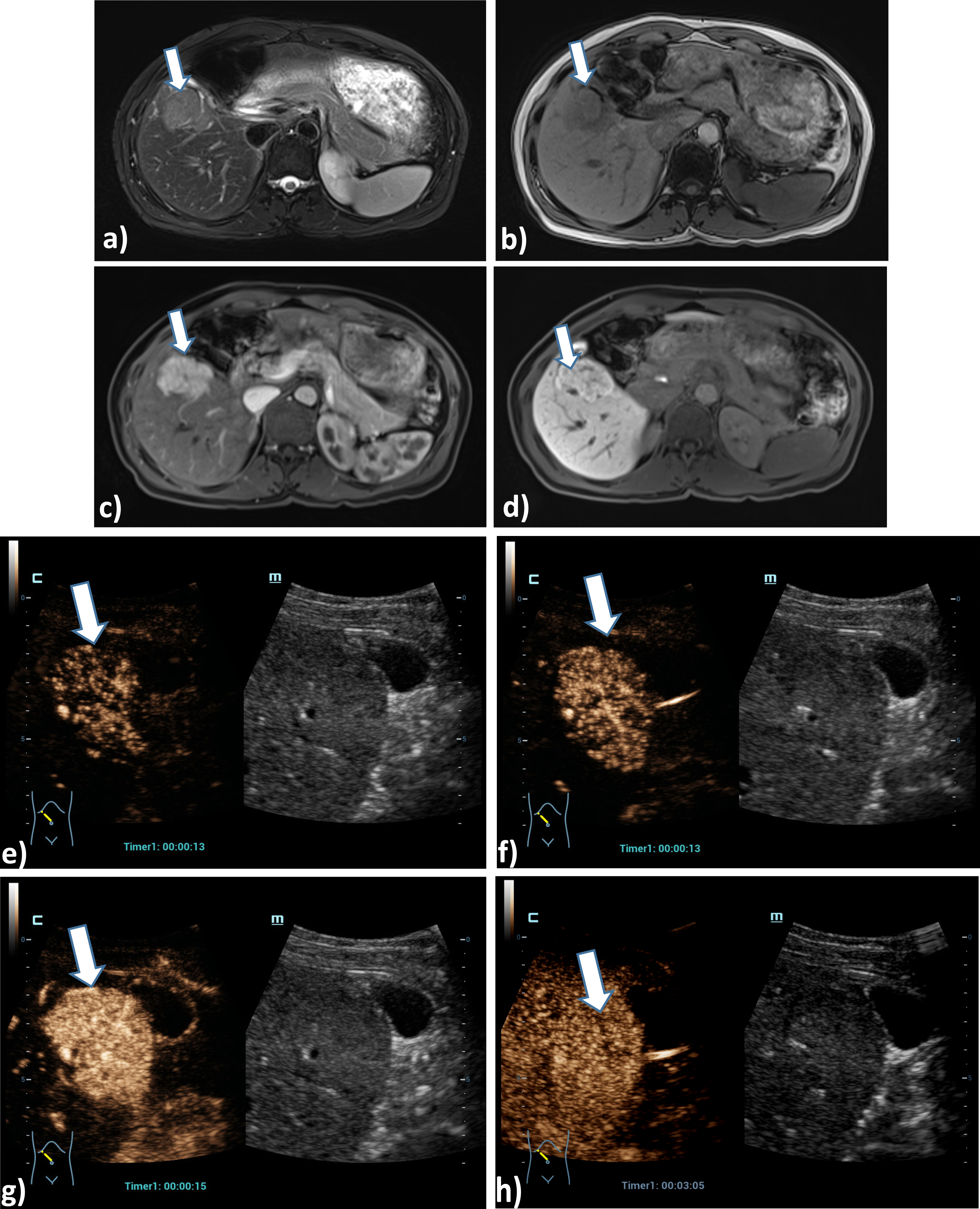
Case of a 54 years old female patient with Osler’s disease. CT scan of the lung shows irregular arterial hypervascularization of the central liver (a), after bolus injection of 1.5 ml ultrasound contrast agent HiFR-CEUS shows during early arterial phase (b), arterial phase (c), late arterial phase irregular dilatation of the hepatica artery (arrow) and hyperenhancement of hemangiomas (d). Late phase of HiFR-CEUS after 1 min shows homogenous contrast of the liver parenchyma without wash out (e).
Using second generation ultrasound contrast agents SonoVue™ as a bolus injection intravenously of only 1 to 2.4 ml, continuous dynamic acquisition of microvascularization from the early arterial phase after 5 to 15 s to the late phase of 5 to 6 min can be imaged with CEUS and stored in DICOM cine sequences. The acquisition of smaller tumor foci is directed by the use of optimized multifrequency probes, by high frame rate and optimization of the harmonic imaging of Contrast Harmonic Imaging (CHI) with pulse inversion techniques and amplitude modulation. One approach to this is offered by the new technique of HiFR.
CEUS is dynamic microvascular imaging down to the level of tumor capillaries. Detection of tumor nodular foci, complicated cysts with septal enhancement and evaluation into benign or malignant was based on wash in kinetics and regular or irregular arterial vascular patterns and wash out kinetics to late phase [1–9, 10–22, 30, 31]. Contrast CT is vascular and parenchymal and shows different dynamics. For MRI, liver-specific contrast agents are available with vascular, parenchymal, and RES-specific contrast, which may be beneficial in detecting smaller HCC foci. CEUS, however, does not affect renal function, which may be a critical advantage when creatinine clearance is altered [1, 14, 15].
Fig. 6
Case of a 45 years old female patient with a liver lesion on the right liver lobe, segment V, 5 cm in diameter. Correlation of the tumor lesion by MRI T2, T1, arterial phase and late contrast phase (a, b, c, d) using liver specific contrast agent as a benign lesion. Using HiFR-CEUS with bolus injection of 1.5 ml ultrasound contrast agent shows typical signs of a focal nodular hyperplasia (FNH) with contrast enhancement from the center to the margin during early arterial phase up to arterial phase (e, f, g) and no wash out after 3 min in the late phase with a small central scar (h) (arrow).
Characterization of malignant and benign foci of the liver is based on detailed assessment of arterial vascularization with CEUS as regular in benign lesions or irregular in malignant lesions. Regular patterns include nodular enhancement from the margin in hemangiomas, wheel spoke pattern from center to margin in an FNH, or from margin to center in adenomas. In complicated cysts that are reactively altered, narrow septal contrast enhancement is found in septa < 2 mm. These contrast agent patterns can sometimes be detected much better with HIFR-CEUS than with ceCT or ceMRI. Thus, CEUS is also important here as a reference imaging modality when the findings on CT are inconclusive and MRI with liver-specific contrast agent cannot be performed [24–28]. CEUS should always be performed before a possible biopsy, since the size, localization, and number of possible lesions, including benign ones, can be detected much better than with B-mode. In addition, CEUS enables also interventional procedures [29].
In malignant lesions, irregular hypervascularization is found in the arterial phase, such as in hepatocellular carcinoma (HCC), or irregular marginal vascularization, such as in metastases, most commonly in colorectal metastases. To detect this irregular microvascularization, the use of HiFR-CEUS may also be beneficial. This is also true for the characterization of tumor cysts with irregular septa > 3 mm or tumor nodules. Especially in smaller lesions of 10 mm or less in diameter, the detection of irregular microvascularization can be crucial in the differentiation of regenerated nodules, dysplastic foci, or HCC findings according to LI-RADS classification [5]. Thus, a high-resolution contrast mode in CEUS such as HiFR-CEUS is co-decisive in determining tumor entity.
To characterize a malignant or benign lesion, it is crucial to determine the dynamic washout kinetics. In metastases, this often begins in the portal venous phase (after 60 to 90 s), in CCC in the late portal venous phase, and in HCC not infrequently after 3 min, but may also only be detectable in the late phase after 5 min [1, 5, 27]. To detect this late washout behavior, particularly high-resolution CEUS techniques are required. HIFR-CEUS can also be helpful for this purpose. This also applies to the detection of small malignant tumor foci of less than 10 mm in diameter. Under favorable acoustic conditions, CEUS can achieve a sensitivity higher than CT, even comparable to ceMRI.
In the evaluation of vascular changes with CEUS, it is crucial to detect reduced perfusion, such as in infarcts or behind tumors, increased perfusion, such as in hyperemia, or micro- and macro-shunts or tumor thrombosis with transfer of microbubbles into the thrombus. In Osler's disease, signs of hepatic changes include elongation and dilatation of the hepatic artery (A hepatica) with early contrast often < 10 s, rapid contrast of the portal vein (V portae through shunts) < 40s, and hepatic veins < 50 s. Infarcts and scars appear as avascular wedge-shaped defects [32, 33]. Comparably, postoperative or post-ablative defects may impose after radiofrequency ablation (RFA), microwave ablation (MWA), or electroporation (IRE). In tumor recurrences, irregular nodular marginal changes arterially hyper-vascularized with wash out in the late phase are then not uncommon [24, 26]. HiFR-CEUS can be helpful to visualize micro-shunts, smaller tumor focus findings and to visualize the penetration of microbubbles into possible tumor thromboses in cases of aggressive malignant tumors.
Atypical partially thrombosed hemangiomas may be difficult to classify species-diagnostically, because the central thrombus may suggest a partial wash out. This is also true of adenomas with central fat or necrosis. The central scar of FNH cannot always be imaged. After chemotherapy, partial necrosis may occur in metastases, with an avascular appearance. Similarly, partial avascular necrosis occurs after chemoembolization (TACE) for HCC [1, 24, 26]. Under favorable acoustic conditions, HiFR-CEUS can image these partial necroses with high detail.
In cases where a defect was detected after ablation, dynamic CEUS was able to assess the dynamic microvascularization of only one defect continuously from the early arterial phase to the portal venous phase over one minute. Late washout is typical for malignant tumors. In small HCC lesions, only irregular arterial enhancement could be detected by CEUS or CT with contrast. In these cases, MRI with liver-specific contrast agents might be superior, or biopsies after 3 to 6 months. New liver-specific ultrasound contrast agents are prepared for in vivo or clinical evaluation but could not be used in clinical practice in Europe so far.
Limitations of CEUS examinations even with HiFR are the clear dependence on the experience of the examiner [23]. HIFR-CEUS was interpreted in independent blinded fashion by the MRI, CT or biopsy. The depth localization of possible foci at a depth of more than 15 cm, liver parenchymal changes due to fatty liver tissue (steatosis hepatis) or liver fibrosis up to cirrhosis, especially in ascites, worsen the acoustic conditions. In some cases, HiFR can still help to map the relevant findings. Unfavorable ultrasound conditions due to air superposition, meteorism, adiposity per magna and poor compliance cannot be changed.
5Conclusion
Based on the results the study indicates that the new HiFR-CEUS technology (high frame rate contrast-enhanced ultrasound) might further improve the diagnostic accuracy of CEUS leading to comparable diagnostic accuracy as with other imaging techniques.
References
[1] | Dietrich CF , Nolsøe CP , Barr RG , Berzigotti A , Burns PN , Cantisani V , Chammas MC , Chaubal N , Choi BI , Clevert DA , Cui X , Dong Y , D’Onofrio M , Fowlkes JB , Gilja OH , Huang P , Ignee A , Jenssen C , Kono Y , Kudo M , Lassau N , Lee WJ , Lee JY , Liang P , Lim A , Lyshchik A , Meloni MF , Correas JM , Minami Y , Moriyasu F , Nicolau C , Piscaglia F , Saftoiu A , Sidhu PS , Sporea I , Torzilli G , Xie X , Zheng R . Guidelines and Good Clinical Practice Recommendations for Contrast-Enhanced Ultrasound (CEUS) in the Liver-Update WFUMB in Cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound Med Biol. (2020) ;46: (10):2579–2604. doi: 10.1016/j.ultrasmedbio.2020.04.030. |
[2] | Huang JY , Li JW , Lu Q , Luo Y , Lin L , Shi YJ , Li T , Liu JB , Lyshchik A . Diagnostic Accuracy of CEUS LI-RADS for the Characterization of Liver Nodules 20 mm or Smaller in Patients at Risk for Hepatocellular Carcinoma. Radiology.. (2020) ;294: (2):329–339. doi: 10.1148/radiol.2019191086. |
[3] | Geyer T , Clevert DA , Schwarz S , Reidler P , Gassenmaier S , Knösel T , Rübenthaler J , Schwarze V , Armbruster M . Diagnostic Value of CEUS Prompting Liver Biopsy: Histopathological Correlation of Hepatic Lesions with Ambiguous Imaging Characteristics. Diagnostics (Basel).. (2020) ;11: (1):35. doi: 10.3390/diagnostics11010035. |
[4] | Şirli R , Sporea I , Popescu A , Dănilă M , Săndulescu DL , Săftoiu A , Moga T , Spârchez Z , Cijevschi C , Mihai C , Ioaniţescu S , Nedelcu D , Iacob N , Miclăuş G , Brisc C , Badea R . Contrast-enhanced ultrasound for the assessment of focal nodularhyperplasia - results of a multicentre study. Med Ultrason. (2021) ;23: (2):140–146. doi: 10.11152/mu-2912. |
[5] | Schellhaas B , Bernatik T , Bohle W , Borowitzka F , Chang J , Dietrich CF , Dirks K , Donoval R , Drube K , Friedrich-Rust M , Gall C , Gittinger F , Gutermann M , Haenle MM , von Herbay A , Ho CH , Hochdoerffer R , Hoffmann T , Hüttig M , Janson C , Jung EM , Jung N , Karlas T , Klinger C , Kornmehl A , Kratzer W , Krug S , Kunze G , Leitlein J , Link A , Lottspeich C , Marano A , Mauch M , Moleda L , Neesse A , Petzold G , Potthoff A , Praktiknjo M , Rösner KD , Schanz S , Schultheiß M , Sivanathan V , Stock J , Thomsen T , Vogelpohl J , Vogt C , Wagner S , Wiegard C , Wiesinger I , Will U , Ziesch M , Zimmermann P , Strobel D . Contrast-Enhanced Ultrasound Algorithms (CEUS-LIRADS/ESCULAP) for the Noninvasive Diagnosis of Hepatocellular Carcinoma –A Prospective Multicenter DEGUM Study. Ultraschall Med. (2021) ;42: (2):178–186. doi: 10.1055/a-1198-4874. |
[6] | Strobel D , Jung EM , Ziesch M , Praktiknjo M , Link A , Dietrich CF , Klinger C , Schultheiß M , Jesper D , Schellhaas B . Real-life assessment of standardized contrast-enhanced ultrasound (CEUS) and CEUS algorithms (CEUS LI-RADS/ESCULAP) in hepatic nodules in cirrhotic patients-a prospective multicenter study. Eur Radiol. (2021) ;31: (10):7614–7625. doi: 10.1007/s00330-021-07872-3. |
[7] | Sporea I , Badea R , Popescu A , Spârchez Z , Sirli RL , Dănil M , Săndulescu L , Bota S , Calescu DP , Nedelcu D , Brisc C , Ciobâca L , Gheorghe L , Socaciu M , Martie A , Ioaniţescu S , Tamas A , Streba CT , Iordache M , Simionov I , Jinga M , Anghel A , Cijevschi Prelipcean C , Mihai C , Stanciu SM , Stoicescu D , Dumitru E , Pietrareanu C , Bartos D , Manzat Saplacan R , Pârvulescu I , Vădan R , Smira G , Tuţ L , Contrast-enhanced ultrasound (CEUS) for the evaluationof focal liver lesions - a prospective multicenter study of itsusefulness in clinical practice. Ultraschall Med. (2014) ;35: (3):259–66. doi: 10.1055/s-0033-1355728. |
[8] | Wildner D , Bernatik T , Greis C , Seitz K , Neurath MF , Strobel D . CEUS in hepatocellular carcinoma and intrahepatic cholangiocellular carcinoma in 320 patients - early or late washout matters: a subanalysis of the DEGUM multicenter trial. Ultraschall Med. (2015) ;36: (2):132–9. doi: 10.1055/s-0034-1399147. |
[9] | Bernatik T , Schuler A , Kunze G , Mauch M , Dietrich CF , Dirks K , Pachmann C , Börner N , Fellermann K , Menzel J , Strobel D . Benefit of Contrast-Enhanced Ultrasound (CEUS) in the Follow-Up Care of Patients with Colon Cancer: A Prospective Multicenter Study. Ultraschall Med. (2015) ;36: (6):590–3. doi: 10.1055/s-0041-107833. |
[10] | Seitz K , Strobel D , Bernatik T , Blank W , Friedrich-Rust M , Herbay Av , Dietrich CF , Strunk H , Kratzer W , Schuler A . Contrast-Enhanced Ultrasound (CEUS) for the characterization of focal liver lesions - prospective comparison in clinical practice: CEUS vs. CT (DEGUM multicenter trial). Ultraschall Med. (2009) ;30: (4):383–9. doi: 10.1055/s-0028-1109673. |
[11] | Seitz K , Bernatik T , Strobel D , Blank W , Friedrich-Rust M , Strunk H , Greis C , Kratzer W , Schuler A . Contrast-enhanced ultrasound (CEUS) for the characterization of focal liver lesions in clinical practice (DEGUM Multicenter Trial): CEUS vs. MRI–a prospective comparison in 269 patients. Ultraschall Med. (2010) ;31: (5):492–9. doi: 10.1055/s-0029-1245591. |
[12] | Bernatik T , Seitz K , Blank W , Schuler A , Dietrich CF , Strobel D . Unclear focal liver lesions in contrast-enhanced ultrasonography–lessons to be learned from the DEGUM multicenter study for the characterization of liver tumors. Ultraschall Med. (2010) ;31: (6):577–81. doi: 10.1055/s-0029-1245649. |
[13] | Strobel D , Bernatik T , Blank W , Schuler A , Greis C , Dietrich CF , Seitz K . Diagnostic accuracy of CEUS in the differential diagnosis of small (20 mm) and subcentimetric (10 mm) focal liver lesions in comparison with histology. Results of the DEGUM multicenter trial. Ultraschall Med. (2011) ;32: (6):593–7. doi: 10.1055/s-0031-1271114. |
[14] | Lamby P , Jung F , Graf S , Schellenberg L , Falter J , Platz-da-Silva N , Schreml S , Prantl L , Franke RP , Jung EM . Effect of iodinated contrast media on renal perfusion: A randomized comparison study in pigs using quantitative contrast-enhanced ultrasound (CEUS). Sci Rep. (2017) ;7: (1):13125. |
[15] | Lamby P , Krüger-Genge A , Franke RP , Mrowietz C , Falter J , Graf S , Schellenberg EL , Jung F , Prantl L . Effect of iodinated contrast media on the oxygen tension in the renal cortico-medullary region of pigs. Clin Hemorheol Microcirc. (2019) ;73: (1):261–270. |
[16] | Squires JH , McCarville MB . Contrast-Enhanced Ultrasound in Children: Implementation and Key Diagnostic Applications. AJR Am J Roentgenol. (2021) ;217: (5):1217–1231. doi: 10.2214/AJR.21.25713. |
[17] | Pschierer K , Grothues D , Rennert J , Platz Batista da Silva N , Schreyer AG , Melter M , Stroszczysnski C , Jung EM . Evaluation of the diagnostic accuracy of CEUS in children with benign and malignant liver lesions and portal vein anomalies. Clin Hemorheol Microcirc. (2015) ;61: (2):333–45. doi: 10.3233/CH-152003. |
[18] | Auer TA , Fischer T , Garcia SRM , Penzkofer T , Jung EM , Hamm B , Lerchbaumer MH . Value of contrast-enhanced ultrasound (CEUS) in Focal Liver Lesions (FLL) with inconclusive findings on cross-sectional imaging. Clin Hemorheol Microcirc. (2020) ;74: (3):327–339. doi: 10.3233/CH-190718. |
[19] | Dong Y , Wang WP , Mao F , Zhang Q , Yang D , Tannapfel A , Meloni MF , Neye H , Clevert DA , Dietrich CF . Imaging Features of Fibrolamellar Hepatocellular Carcinoma with Contrast-Enhanced Ultrasound. Ultraschall Med. (2021) ;42: (3):306–313. doi: 10.1055/a-1110-7124. |
[20] | Schwarze V , Marschner C , Völckers W , de Figueiredo GN , Rübenthaler J , Clevert DA . The diagnostic performance of contrast-enhanced ultrasound (CEUS) for evaluating hepatocellular carcinoma (HCC) juxtaposed to MRI findings; a retrospective single-center analysis of 292 patients. Clin Hemorheol Microcirc. (2020) ;76: (2):155–160. doi: 10.3233/CH-209213. |
[21] | Marschner CA , Zhang L , Schwarze V , Völckers W , Froelich MF , von Münchhausen N , Schnitzer ML , Geyer T , Fabritius MP , Rübenthaler J , Clevert DA . The diagnostic value of contrast-enhanced ultrasound (CEUS) for assessing hepatocellular carcinoma compared to histopathology; a retrospective single-center analysis of 119 patients1. Clin Hemorheol Microcirc. (2020) ;76: (4):453–458. doi: 10.3233/CH-209221. |
[22] | Schwarze V , Lindner F , Marschner C , Negrão de Figueiredo G , Rübenthaler J , Clevert DA . Single-center study: The diagnosticperformance of contrast-enhanced ultrasound (CEUS) for assessingfocal splenic lesions compared to CT and MRI. Clin HemorheolMicrocirc. (2019) ;73: (1):65–71. doi: 10.3233/CH-199204. |
[23] | Putz FJ , Verloh N , Erlmeier A , Schelker RC , Schreyer AG , Hautmann MG , Stroszczynski C , Banas B , Jung EM . Influence of limited examination conditions on contrast-enhanced sonography for characterising liver lesions. Clin Hemorheol Microcirc. (2019) ;71: (2):267–276. doi: 10.3233/CH-189417. |
[24] | Jung EM , Weber MA , Wiesinger I . Contrast-enhanced ultrasound perfusion imaging of organs. Radiologe. (2021) ;61: (Suppl 1):19–28. doi: 10.1007/s00117-021-00891-7. |
[25] | Rennert J , Grosse J , Einspieler I , Bäumler W , Stroszczynski C , Jung EM . Complementary imaging of ultrasound and PET/CT: A new opportunity? Clin Hemorheol Microcirc. (2021) ;79: (1):39–54. doi: 10.3233/CH-219105. |
[26] | Wiesinger I , Jung F , Jung EM . Contrast-enhanced ultrasound (CEUS) and perfusion imaging using VueBox. Clin Hemorheol Microcirc. (2021) ;78: (1):29–40. doi: 10.3233/CH-201040. |
[27] | Dong Y , Qiu Y , Yang D , Yu L , Zuo D , Zhang Q , Tian X , Wang WP , Jung EM . Potential application of dynamic contrast enhanced ultrasound in predicting microvascular invasion of hepatocellular carcinoma. Clin Hemorheol Microcirc. (2021) ;77: (4):461–469. doi: 10.3233/CH-201085. |
[28] | Werner JM , Zidek M , Kammerer S , da Silva NPB , Jung F , Schlitt HJ , Hornung M , Jung EM . Intraoperative contrast-enhanced ultrasound can have a crucial role in surgical decision-making during hepato-pancreatico-biliary surgery - Analysis of impact and input. Clin Hemorheol Microcirc. (2021) ;78: (1):103–116. doi: 10.3233/CH-201031. |
[29] | Jung EM , Jung F , Stroszczynski C , Wiesinger I . Dynamic endoluminal contrast enhanced ultrasound (CEUS) for display of drainages in inflammatory abdominal fluid collections. Clin Hemorheol Microcirc. (2022) ;80: (2):49–59. doi: 10.3233/CH-211370. |
[30] | Müller-Peltzer K , Rübenthaler J , Negrao de Figueiredo G , Clevert DA . [CEUS-diagnosis of benign liver lesions]. Radiologe. (2018) ;58: (6):521–527. doi: 10.1007/s00117-018-0390-8. |
[31] | Corvino A , Sandomenico F , Setola SV , Corvino F , Tafuri D , Catalano O . Morphological and dynamic evaluation of complex cystic focal liver lesions by contrast-enhanced ultrasound: current state of the art. J Ultrasound. (2019) ;22: (3):251–259. doi: 10.1007/s40477-019-00385-2. |
[32] | Schelker RC , Barreiros AP , Hart C , Herr W , Jung EM . Macro- and microcirculation patterns of intrahepatic blood flow changes in patients with hereditary hemorrhagic telangiectasia. World J Gastroenterol. (2017) ;23: (3):486–495. doi: 10.3748/wjg.v23.i3.486. |
[33] | Schelker RC , Andorfer K , Putz F , Herr W , Jung EM . Identification of two distinct hereditary hemorrhagic telangiectasia patient subsets with different hepatic perfusion properties by combination of contrast-enhanced ultrasound (CEUS) with perfusion imaging quantification. PLoS One. (2019) ;14: (4):e178. doi: 10.1371/journal.pone.0215178. |
[34] | Jung EM , Kammerer S , Brandenstein M , Putz FJ , Stroszczynski C , Jung F . High resolution flow (HR Flow) and Glazing Flow in cases of hepatic flow changes: Comparison to color-coded Doppler sonography (CCDS). Clin Hemorheol Microcirc. (2021) ;79: (1):3–17. doi: 10.3233/CH-219102. |




