Cerebrovascular comorbidity, high blood levels of C-reactive protein and D-dimer are associated with disease outcomes in COVID-19 patients
Abstract
The emerging coronavirus disease (COVID-19) swept the world, affecting more than 200 countries and territories. As of August 22, 2020, the pandemic infected more than 23,329,752 including 807,054 patients who have died. Although the main clinical features of the pandemic disease are respiratory, cerebrovascular comorbidities emerged as one of the leading causes of death associated with COVID-19. Different case reports have indicated that C-reactive protein (CRP) and D-dimer (pro-inflammatory biomarkers) were elevated in COVID-19 patients, which can significantly increase the risk of ischemic stroke. Available data on cerebrovascular complications in COVID-19 patients were collected and a meta-analysis was designed and carried out to evaluate the risk of severity and mortality associated with high levels of CRP and D-dimer levels in COVID-19 patients. In addition, we aimed to describe the overall event rate of pre-existing cerebrovascular disease in COVID-19 patients. In our analysis, 5,614 cases have been studied, out of these patients 164 cases have developed cerebrovascular comorbities. Cerebrovascular comorbidity increased the risk of disease severity (odd ratio = 4.4; 95% CI: 1.48 to 12.84) and mortality (odd ratio = 7.0; 95% CI: 2.56 to 18.99). Statistical analyses showed that CRP and D-dimer serum levels were elevated by six-folds in the severe cases of COVID-19 patients. This significant increase in these two proteins levels can serve as a vital indicator for COVID-19 patients who are at increased risk of severe COVID-19 cerebrovascular complications, such as stroke.
1Introduction
The novel coronavirus disease (COVID-19) was first identified in patients with severe respiratory illness in Wuhan, China, in December of 2019. The causative agent was a novel coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1–4]. The worrisome features of COVID-19 are its ability to spread readily and its propensity to cause severe disease in older adults and patients with pre-existed health conditions. These cases are often associated with a high risk of cerebrovascular diseases, cardiovascular disease, hypertension, diabetes, and even death [5, 6].
Cerebrovascular and cardiovascular comorbidities are among the most common cause of death associated with COVID-19 [6]. Stroke incidence and cerebrovascular disease have been reported in 5% and 5.7% of COVID-19 pateints, repectively [7]. However, the thrombotic changes are generalized and occur in all regions of the body - not only in the brain), as it was demonstrated by previous studies like thromboses in the lung [8], kidney infractions [9], as well as overt intravascular disseminated coagulation [10]. The pro-inflammatory biomarkers were elevated signicantly due to the COVID-19 infection. Increased levels of pro-inflammatory markers are known to increase the risk of ischemic stroke, particularly in the elderly and chronically ill patients [11]. Although there are no supporting evidences to the role of inflammation in stroke pathogenesis, it was suggested that inflammation might cause a stroke by several mechanisms such as genetic susceptibility, presence of traditional risk factors (hypertension, diabetes mellitus, smoking, and cardiac diseases), and infectious diseases [12].
C-reactive protein (CRP) is a protein synthesized in the liver as an acute-phase reactant in response to an inflammation; and it plays a significant role in the pathophysiological process of stroke [13]. D-dimer is a small protein fragment produced by fibrinolysis’s degradation of the blood clot that might be used to diagnose thrombosis by measuring its concentration [14]. The elevation of circulated D-dimer plasma concentration indicates activation in blood coagulation thrombin formation, particularly in the intra-arterial that is associated with ischemia [15]. Several studies also have reported associations between ischemic cerebrovascular disease and inflammatory indexes such as CRP and fibrinogen [16, 17]. In addition, other studies have reported an increase in stroke severity with elevated D-dimer concentration [18, 19]. The associations between serum levels of CRP and D-dimer and stroke suggest a relation between the inflammatory process and the risk of stroke. Accordingly, this meta-analysis study was performed (i) to investigate overall event rate of pre-exicisting cerbrovavcualr disease in pateints with COVID-19, (ii) to find the association between pre-existing cerebrovascular diseases and disease severity and mortality in COVID-19 patients; and (iii) to evaluate the risk of severity and mortality associated with high levels of CRP and D-dimer levels in COVID patients.
2Methods
2.1Data search
Three databases including PubMed, Science Direct, and Scopus were searched between 1 January 2020 and 30 May 2020 to find studies that reported pre-excisiting cerebrovascular diaseases, CRP levels, or D-dimer levels in COVID-19 pateints. The following combined keywords were used for searching the databases: CRP, Stroke, COVID-19, D-dimer, and SARS-CoV-2. Besides, the lists of references of all relevant studies were also manually checked to identify further studies.
2.2Study selection
The language was limited to English. Studies were selected if they had case series designs and provided adequate details on clinical symptoms and laboratory results, particularly CRP and D-dimer protein levels.
2.3Data abstraction
For studies that met the inclusion criteria, the following data were extracted from each included study using a standardized form: the surname of the first author; the design of the study; number, age and sex of patients; ratios of clinical and laboratory characteristics of interest; and data relevant to cerebrovascular comorbidities factor. All concentration units of CRP or D-dimer protein levels in all studies were justified and converted to unify unit each, mg/L, and ng/ml, respectively. Values 10 mg/L and more identified high levels of CRP, and the high level of D-dimer were identified by values≥0.5 mg/L. The severity of the disease validation, as reported in the included studies, was determined if patients needed to be admitted to the intensive care unit, needed vital life support, required mechanical ventilation, or death. Data extraction was carried out by FA and double-checked by MA.
2.4Quality assessment
The methodological quality was assessed with the National Institutes of Health Joanna Briggs Institute (JBI) quality assessment tool for case series studies [20]. The quality of case series studies was investigated by one reviewer (SA). Quality checklist of the JBI tool is based on ten criteria including: inclusion criteria; the method of measurement of the condition, the method of measurement of the outcomes; whether inclusion of case was consecutive or not; the completeness of a case series; reporting of relevant participant’s demographics; reporting of clinical information of the participants; description of the clinical condition post-intervention; reporting of the presenting site(s)/clinic(s) demographic information; and consideration of statistical analysis.
2.5Quantitative data synthesis and analysis
Random-effects meta-analysis was used to calculate pooled crude prevalence rate, with 95% confidence intervals of high levels of CRP and D-dimer measures, besides cerebrovascular comorbidities of COVID-19 patients. A pooled analysis was carried out to assess the odds ratio (OR) and 95% confidence interval (CI) of high levels of CRP and D-dimer, as well as pre-existing cerebrovascular disease in COVID-19 patients with or without severe disease and in non-survivors versus survivors. All statistical analyses were carried out by Comprehensive Meta-Analysis V2 (Biostat, USA). A p-value of <0.05 was considered statistically significant. Heterogeneity in study results was tested by using the I2 statistic (p-value of < 0.1). I2 provides an estimate of the percentage of variation in study results that is explained by between-study heterogeneity rather than sampling error. An I2 value >50% indicates considerable heterogeneity [21]. The presence of publication bias was examined for using a funnel plot.
3Results
A total of 851 articles were identified from the three databases examined and other sources. The initial searched included 683 articles; after excluding duplicated or overlapping articles and removing reviews and editorials, a total number of 168 publications could be initially identified. A hundred Fifty-three studies were excluded because they did not have a data about pre-existing cerebrovascular disease in COVID patients or no data about the levels of CRP and D-dimer protein among patients who positively diagnosed with COVID-19. For the quantitative assessment of our study, sixteen studies were included in the meta-analysis that reported either the event rate of pre-existing cerebrovascular disease, high CRP levels, and/or high D-dimer levels (Fig. 1).
Fig. 1
Flow diagram of the study selection process.
Table 1 shows the details and the characteristics of the studies used in the pooled-analysis. Studies included in the meta-analysis were conducted mostly in China and published between 1 January 2020 and 30 May 2020. The sixteen included studies examined 5,611 cerebrovascular patients’ records. These 16 studies were based on data collected worldwide with clinical symptoms observed in patients with COVID-19, with a clear explanation of eligibility criteria and diagnosis criteria.
Table 1
Pre-existing cerebrovascular and high levels of CRP and D-dimer distribution in the included studies of patients with COVID-19
| Country | Condition | Sample size | Events (n) | Non-events (n) | Severe cases ratio | Non-severcases ratio | Non-survivors | Survivors | |
| Wang D, et al. [29] | China | Cerebrovascular | 138 | 7 | 131 | 3/36 | 1/102 | ||
| Zhang G, et al. [37] | China | Cerebrovascular | 221 | 15 | 206 | 11/55 | 4/166 | ||
| Hu L, et al. [38] | USA | Cerebrovascular | 323 | 7 | 316 | 3/172 | 4/151 | ||
| Zhang J, et al. [40] | China | Cerebrovascular | 140 | 3 | 125 | 2/58 | 1/82 | ||
| Du Y, et al. [51] | China | Cerebrovascular | 85 | 7 | 78 | ||||
| Lei S, et al. [52] | China | Cerebrovascular | 34 | 2 | 32 | 2/15 | 0/19 | ||
| Guan W, et al. [5] | China | Cerebrovascular | 1099 | 15 | 1084 | 4/173 | 11/926 | ||
| Qin C, et al. [28] | China | Cerebrovascular | 452 | 11 | 441 | 8/286 | 3/166 | ||
| Shi S, et al. [30] | China | Cerebrovascular | 416 | 22 | 394 | 13/82 | 9/334 | ||
| Lagi F, et al. [31] | Italy | Cerebrovascular | 84 | 2 | 82 | 0/16 | 2/68 | ||
| Wang D, et al. [53] | China | Cerebrovascular | 107 | 6 | 101 | 3/19 | 3/88 | ||
| Chen TL, et al. [32] | China | Cerebrovascular | 406 | 18 | 388 | 3/19 | 5/36 | ||
| Yang X, et al. [34] | China | Cerebrovascular | 52 | 7 | 45 | 7/32 | 0/20 | ||
| Yan Y, et al. [36] | China | Cerebrovascular | 193 | 8 | 185 | 8/108 | 0/85 | ||
| Guan WJ, et al. [27] | China | Cerebrovascular | 1590 | 30 | 1560 | 7/30 | 23/1560 | 6/30 | 24/1560 |
| Chen T [33] | China | Cerebrovascular | 274 | 4 | 270 | 4.0/113 | 0/161 | ||
| Guan W et al. [5] | China | CRP | 793 | 481 | 312 | 110/135 | 371/658 | ||
| Huang Y, et al. [39] | China | CRP | 208 | 72 | 136 | 58/89 | 14/119 | ||
| Zhang J, et al. [40] | China | CRP | 81 | 35 | 46 | 23/38 | 12/43 | ||
| Guan W et al. [5] | China | D-dimer | 560 | 260 | 300 | 65/109 | 195/451 | ||
| Huang Y, et al. [389] | China | D-dimer | 195 | 110 | 85 | 69/79 | 41/116 | ||
| Zhou F, et al. [41] | China | D-dimer | 172 | 117 | 55 | 50/54 | 67/118 |
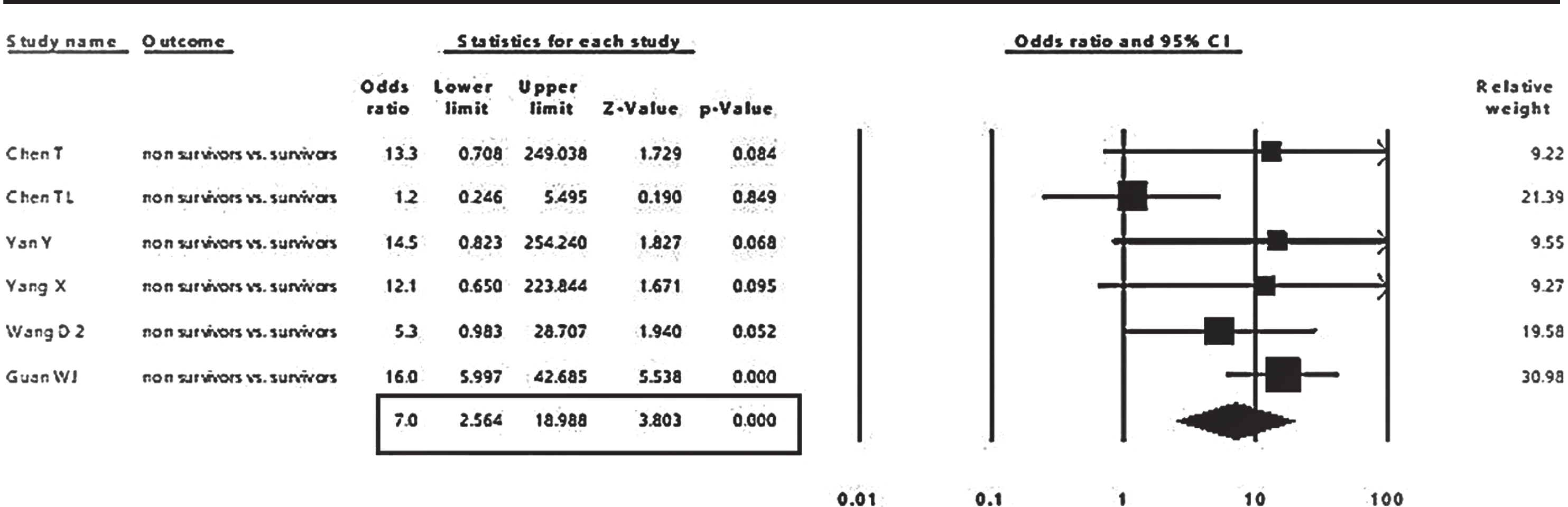
The pooled event rate from 16 studies of cerebrovascular comorbidity in COVID-19 patients revealed a prevalence of 4 % (95% CI: 2.8 to 5.9%), as shown in Fig. 2. The associations of cerebrovascular comorbidity with disease outcomes are shown in Figs. 3, 4. Cerebrovascular comorbidity increased the risk of disease severity more than four folds (OR = 4.4; 95% CI: 1.48 to 12.84) (Fig. 3). The cerebrovascular disease comorbidity was associated with about seven times the risk of non-surviving COVID-19 (odd ratio = 7.0; 95% CI: 2.56 to 18.99) (Fig. 4).
Fig. 2
Pooled analysis of the event rate of cerebrovascular comorbidity in COVID-19 patients.
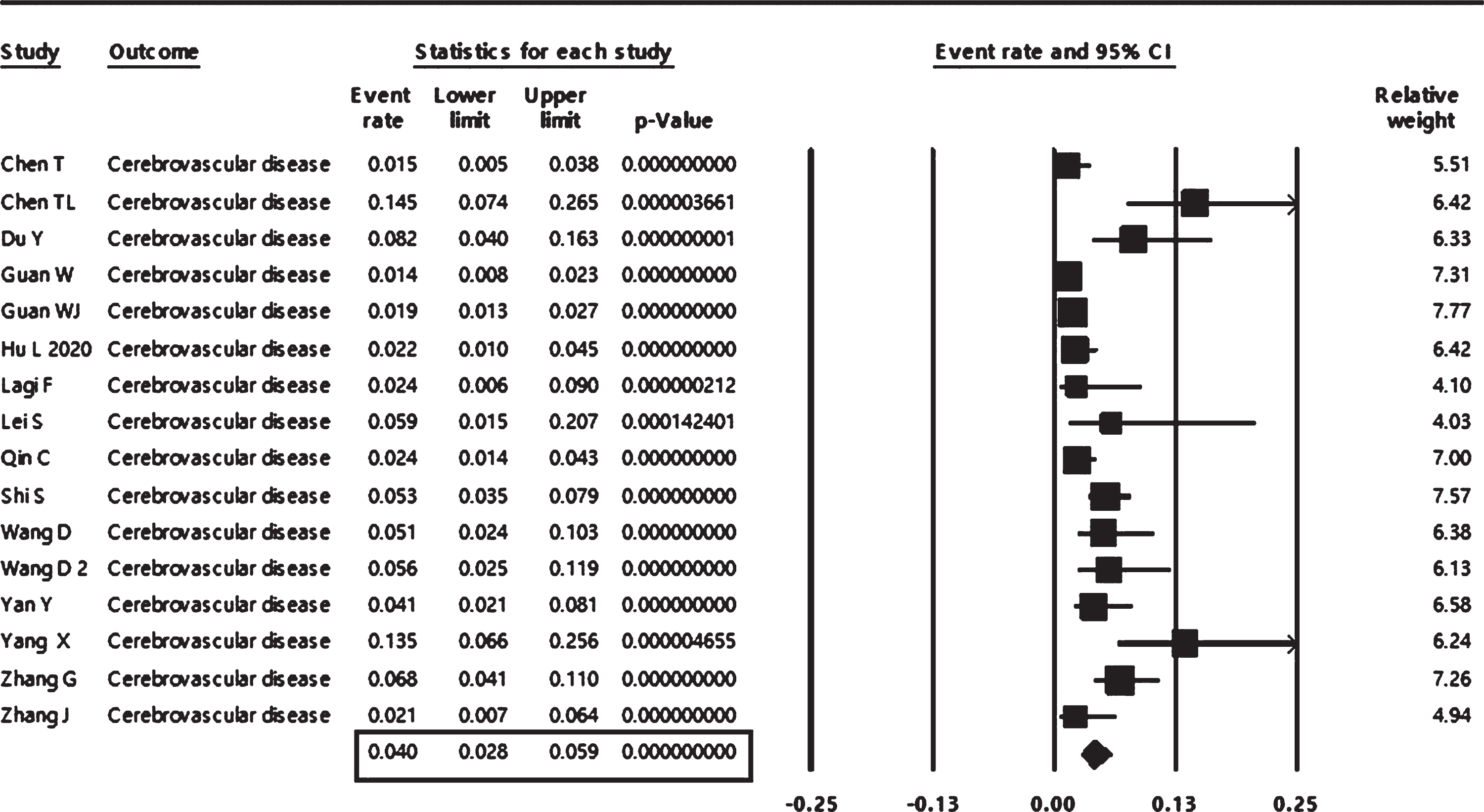
Fig. 3
The pooled analysis of the association of cerebrovascular comorbidity with COVID-19 severity in adult patients.
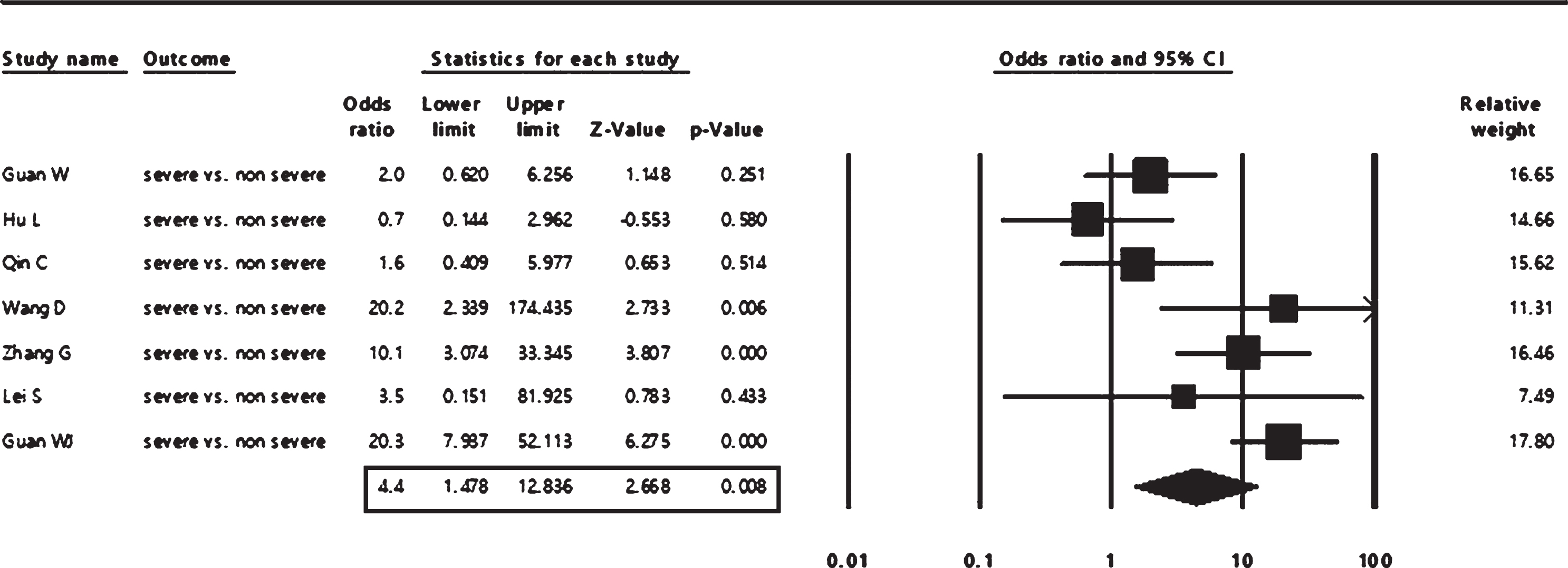
Fig. 4
The pooled analysis of the association of cerebrovascular comorbidity with COVID-19 mortality in adult patients.
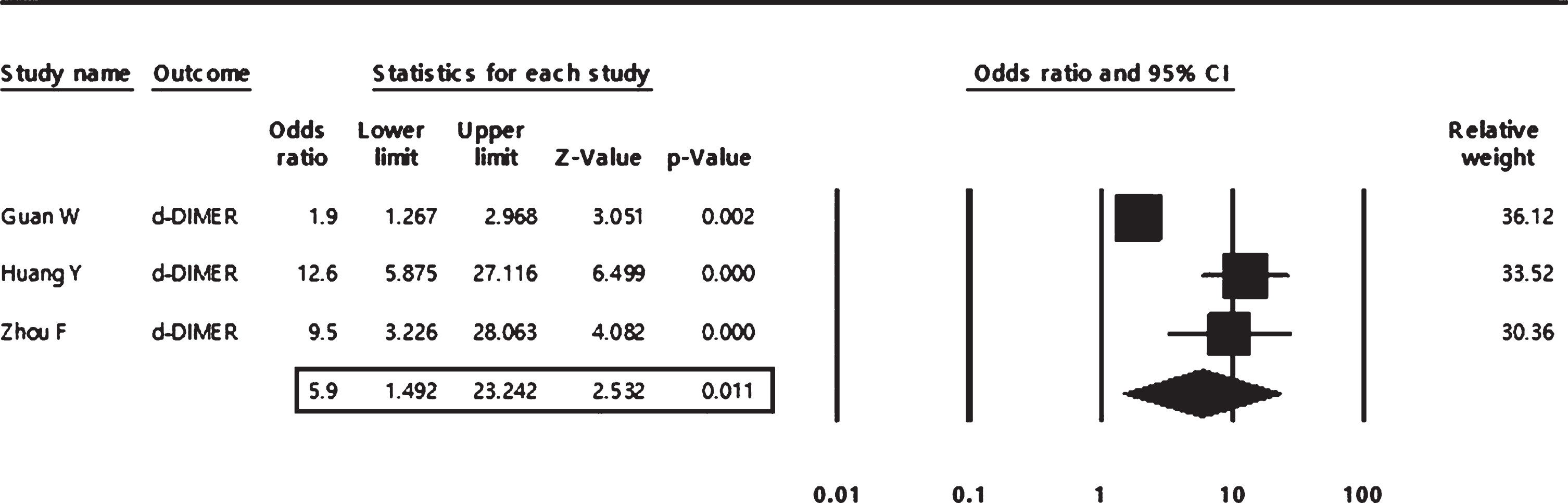
In the pooled analysis, high levels of D-dimer and CRP were found to be significantly associated with about six-folds increased risk of COVID severity (odds ratio = 5.90; 95% CI: 1.49 to 23.24 and 5.70;95% CI: 1.93 to 16.79 respectively), as shown in both Figs. 5, 6.
Fig. 5
The pooled analysis of the association of high levels of C-reactive protein with COVID-19 severity in adult patients.
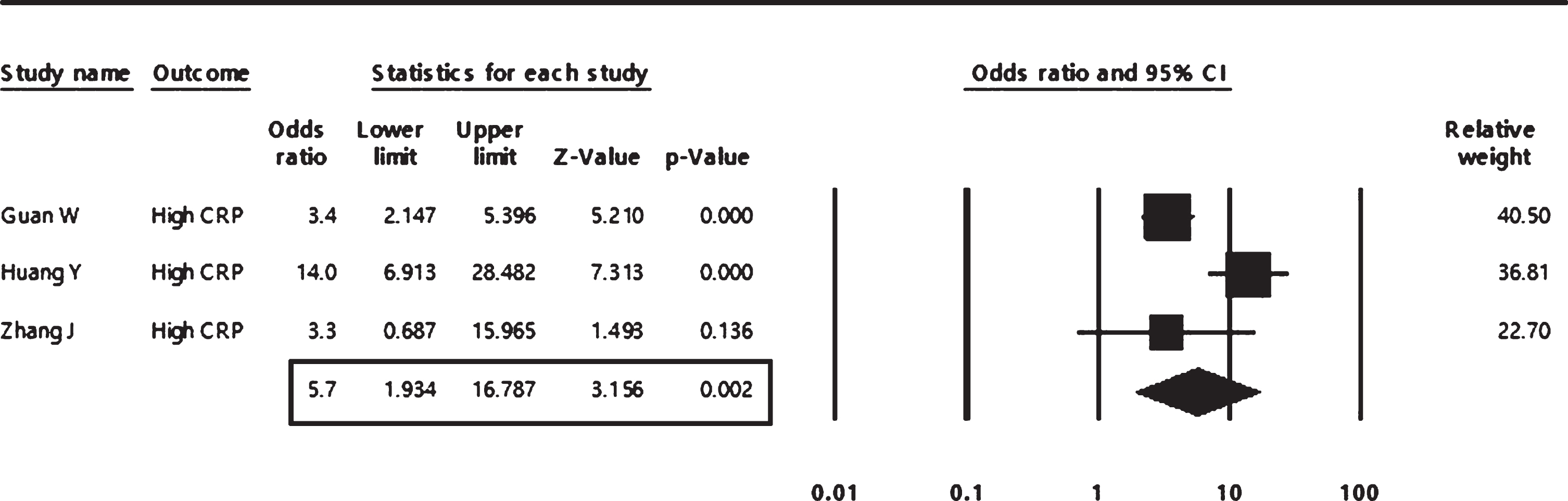
A random-effect model was used for all pooled analyses as a significant heterogeneity was observed across all included studies.
Fig. 6
The pooled analysis of the association of high levels of D-dimer with COVID-19 severity in adult patients.
As shown Fig. 7 that evaluates publication bias using a funnel plot based on the event rate of cerebrovascular comorbidity, a visual symmetry indicates the absence of publication bias. Also, the Egger’s test revealed no significant publication bias (Egger’s test: p = 0.16593)
Fig. 7
Funnel plot for publication bias based on cerebrovascular comorbidity.
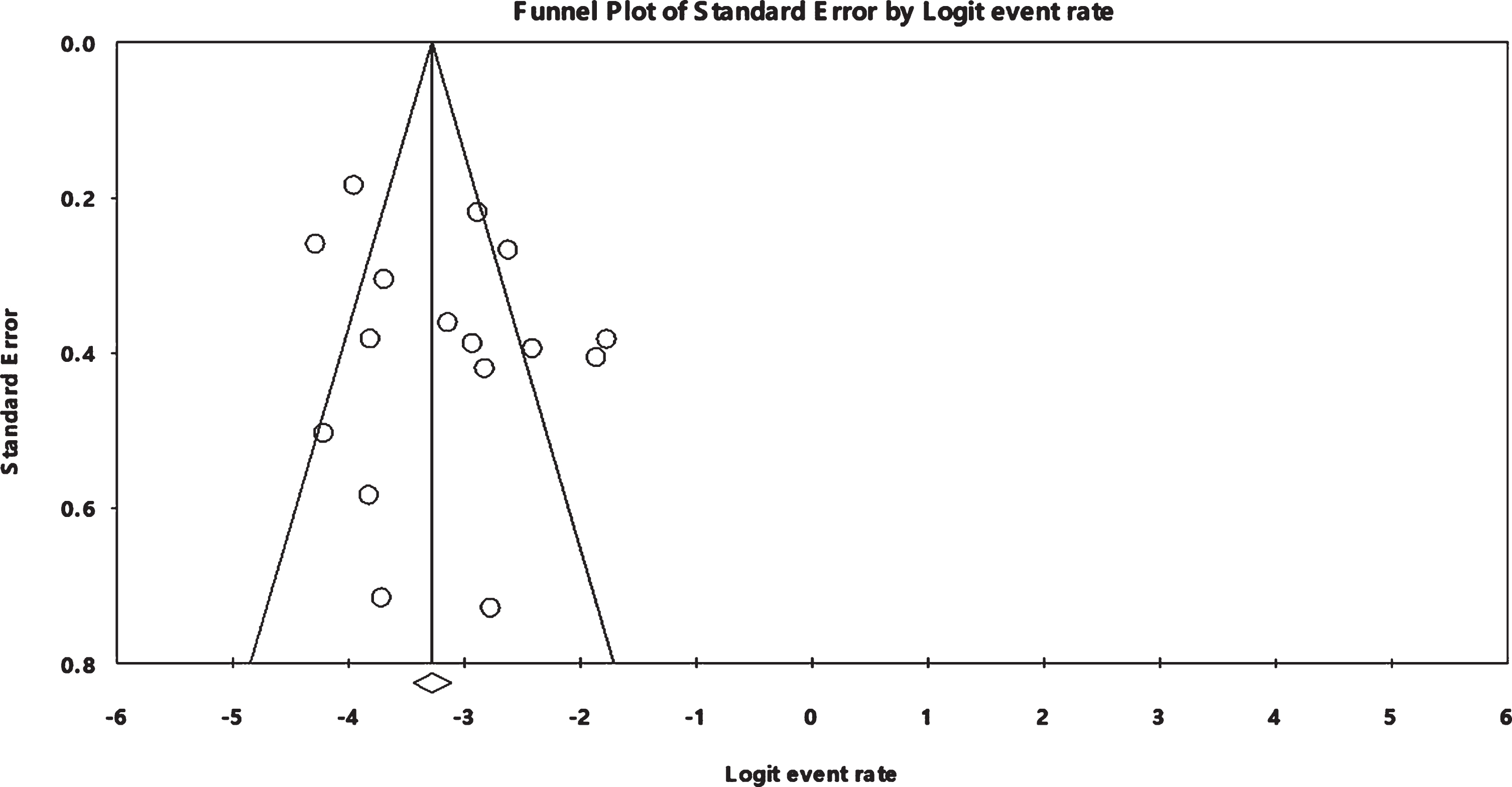
Table 2 shows the methodological quality of all studies included in the meta-analyses. Defined inclusion criteria were not there in 13 studies. The measurement method of the condition was reported in all studies, whereas the method of measurement of the outcomes was published in the majority of studies. Whether cases were recruited consecutively is not clear in five studies and two studies. Similarly, the complete inclusion of participants is not explicit in the majority of the included studies. On the other hand, all studies reported relevant participant demographics, clinical information of the participants; description of the clinical condition post-intervention; the presenting site(s)/clinic(s) demographic information; and the statistical analysis.
Table 2
The methodological quality of the included case series studies in the meta-analyses using the Joanna Briggs Institute critical appraisal tools
| Study | Guan W et al. [5] | Qin C et al. [28] | Wang D et al. [29] | Shi S et al. [30] | Lagi F et al. [31] | Chen T L et al. [32] | Chen T et al. [33] | Yang X et al. [34] | Xu X et al. [35] | Yan Y et al. [36] | Zhang G et al. [37] | Hu L et al. [38] | Huang Y et al. [39] | Zhang J et al. [40] | Zhou F et al. [41] |
| Were there clear | No | No | No | No | No | No | No | No | No | Yes | No | Yes | No | No | No |
| criteria for inclusion | |||||||||||||||
| in the case series? | |||||||||||||||
| Was the condition | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| measured in a standard, | |||||||||||||||
| reliable way for all | |||||||||||||||
| participants included | |||||||||||||||
| in the case series? | |||||||||||||||
| Were valid methods | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Not clear | Yes | Yes | Yes |
| used for identification | |||||||||||||||
| of the condition | |||||||||||||||
| for all participants | |||||||||||||||
| included in the case series? | |||||||||||||||
| Did the case | Not clear | Not clear | Yes | Yes | Yes | Not clear | Not clear | Yes | No | No | Yes | Yes | Not clear | Yes | Yes |
| series have consecutive | |||||||||||||||
| inclusion of participants? | |||||||||||||||
| Did the case series | Not clear | Not clear | Not clear | No | Yes | Not clear | Not clear | No | No | No | Not clear | Not clear | Not clear | Not clear | Not clear |
| have complete inclusion | |||||||||||||||
| of participants? | |||||||||||||||
| Was there clear | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| reporting of the | |||||||||||||||
| demographics of the | |||||||||||||||
| participants in the study? | |||||||||||||||
| Was there clear | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| reporting of clinical | |||||||||||||||
| information of | |||||||||||||||
| the participants? | |||||||||||||||
| Were the outcomes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| or follow up | |||||||||||||||
| results of cases | |||||||||||||||
| clearly reported? | |||||||||||||||
| Was there clear | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes |
| reporting of the | |||||||||||||||
| presenting site(s)/clinic(s) | |||||||||||||||
| demographic information? | |||||||||||||||
| Was statistical | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| analysis appropriate? |
4Discussion
In the present meta-analysis, we have explored 16 independent studies reported cerebrovascular disease as a comorbid factor on about 5,611 confirmed COVID-19 patients distributed across many countries. Results showed that the pre-existing cerebrovascular disease, as well as high levels of D-dimer or CRP, are associated with severe outcomes in COVID-19 patients.
Generally, previous studies have been shown the association between CRP, D-dimer and stroke [49,50]. A dose-response meta-analysis study about the association between CRP level and the risk of ischemic stroke showed that high level of CRP is related to increase the risk of ischemic strokes. Moreover, the result also showed that risk was significantly reduced by controlling the CRP at a low level [42]. Our results were also supported by an Italian study that was investigating the relationship between the serum concentration level of CRP and prognosis after ischemic stroke [43], the study’s findings suggested that post-stroke patients with CRP levels higher than 15 mg/L have a worse prognosis and a higher risk of subsequent cardiovascular complications which might lead to death [43]. An additional meta-analysis study demonstrated that increased CRP level was independently linked with an elevated 2.07-fold risk of mortality in patients with acute ischemic stroke [44]. Among all studies included in this meta-analysis, the highest CRP cut-off value was 31.2 mg/l [45]. Moreover, In a study that included 3653 patients with first-ever ischemic stroke, the elevated plasma CRP (>4.70 mg/l) is independently related to adverse clinical outcomes after acute ischemic stroke [46]. In a case-control study, it was reported that the concentration of CRP in mg/l among 600 patients with ischemic stroke was 3.48 (1.52–9.42), the level of CRP in mg/l among 73 patients with the large-vessel disease (LDV) was 4.66 (1.79–13.9), the concentration of CRP in mg/l among 124 patients with the small-vessel disease (SDV) was 3.08 (1.52–5.79), the level of CRP in mg/l among 98 patients with the cardio-embolic stroke (CE) was 7.07 (2.39–17.8). The study reported significant differences in CRP levels between different ischemic stroke etiological subtypes. However, the maximum level of CRP measured was 17.8 mg/l [47]. In comparison with our study, all these studies showed mildly increased CRP levels in patients with ischemic stroke. Our pooled analysis indicated that CRP levels were associated with about six-folds increased risk of COVID severity, however, due to insufficient reporting of data, we could not analysis direst association of pro-inflammatory markers in pateints with COVID-19 who developed stroke compared to those who did not. Future studies should look at the suggestion that COVID-19 disease massively increase the level of CRP that could be an excellent candidate to be a biomarker for stroke in COVID-19 patients.
High levels of D-dimer is also prevelant in COVID-19 pateints. Consistent with our study, a case-control study performed on 240 Chinese patients with acute ischemic stroke (AIS) showed that plasma D-dimer levels increased with increasing stroke severity, besides it significantly increased in AIS patients compared to healthy controls [18]. The results showed that the D-dimer levels in AIS patients range from 280–2110 ng/ml compared to healthy controls that range from 170–740 ng/ml [18]. Moreover, the mean D-dimer level at the admission of 59 acute ischemic stroke patients was 626 mg/ml (range, 77–4,752 mg/ml), and the mean level measured after seven days of treatment was 238.3 mg/ml (range, 50–924 mg/ml), besides that D-dimer level has reported positively correlated with infarction volume and suggested to be used to predict infarction-volume [48]. Another meta-analysis study which investigated the association between D-dimer level and the risk of stroke included seven prospective observational studies with a total of 22,207 patients and three case-control studies with 2,248 patients; the study showed a significantly higher risk of stroke associated with the increased level of D-dimer [19] which support our results. Our pooled analysis indicated that D-dimer levels were associated with almost six-fold increased risk of COVID-19 severity, which also suggests that COVID-19 disease with stroke enormous increased the level of D-dimer protein suggesting that D-dimer may be used to screen patients at risk of stroke in COVID-19 patients.
Systematic reviews and meta-analyses on case series studies with low risk of bias are essential to describe and understand new diseases, including COVID-19. A rationalay of our analysis is the absent of publication bias, meaning that all relevant studies have been identified. A common limitation of the studies included in our analysis is that it was not clear what the eligibility criteria were and if participants’ selection was consecutive and complete. Other biases in the included studies are less likely since all studies reported adequately addresses other items in the JBI checklist.
5Conclusion
CRP and D-dimer serum levels are prevelant in patients who are positively diagnosed Covid-19 and may put them at increased risk of stroke development. Patients with pre-existing cerebrovascular disease are at increased risk of severe COVID-19 outcomes as well as mortality. High levels of D-dimer and CRP are associated with a higher risk of severe outcomes of COVID-19, suggesting that they may serve as biomarkers to identify patients at risk of undesirable outcomes. Nevertheless, prospective cohort studies are needed to confirm such associations.
Conflicts of interest
The authors declare no conflict of interest.
Funding
This research received no external funding.
References
[1] | Huang C , Wang Y , Li X , Ren L , Zhao J , Hu Y , et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) . |
[2] | Zhou P , Yang X-L , Wang X-G , Hu B , Zhang L , Zhang W , et al. discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat origin. Nature. (2020) . |
[3] | Alanagreh L , Alzoughool F , Atoum M . The Human Coronavirus Disease COVID-19 Its Origin, Characteristics, and Insights into Potential Drugs and Its Mechanisms. Pathogens. (2020) . |
[4] | Alzoughool F , Alanagreh L . Coronavirus drugs: Using plasma from recovered patients as a treatment for COVID-19. Int J Risk Saf Med. (2020) . |
[5] | Guan W , Ni Z , Hu Y , Liang W , Ou C , He J , et al. Clinical characteristics of 2019 novel coronavirus infection in China. N Engl J Med. (2020) . |
[6] | Viguier A , Delamarre L , Duplantier J , Olivot JM , Bonneville F . Acute ischemic stroke complicating common carotid artery thrombosis during a severe COVID-19 infection. Journal of neuroradiology=Journal de neuroradiologie. (2020) . |
[7] | Mao L , Jin H , Wang M , Hu Y , Chen S , He Q , et al. Neurologic Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. (2020) . |
[8] | Jung EM , Stroszczynski C , Jung F . Contrast enhanced ultrasonography (CEUS) to detect abdominal microcirculatory disorders in severe cases of COVID-19 infection: First experience. Clin Hemorheol Microcirc. (2020) ;74: (4):353–61. |
[9] | Jung EM , Stroszczynski C , Jung F . Contrast enhanced ultrasound (CEUS) to assess pleural pulmonal changes in severe COVID-19 infection: First results. Clin Hemorheol Microcirc. (2020) ;75: (1):19–26. |
[10] | Martini R . The compelling arguments for the need of microvascular investigation in COVID-19 critical patients. Clin Hemorheol Microcirc. (2020) . DOI: 10.3233/CH-200895 |
[11] | Siniscalchi A , Gallelli L . Could COVID-19 represents a negative prognostic factor in patients with stroke. Infection Control and Hospital Epidemiology. (2020) . |
[12] | Lindsberg PJ , Grau AJ . Inflammation and infections as risk factors for ischemic stroke. Stroke. (2003) . |
[13] | Wang A , Xu T , Xu T , Zhang M , Li H , Tong W , et al. Hypertension and elevated C-reactive protein: Future risk of ischemic stroke in a prospective cohort study among inner Mongolians in China. Int J Cardiol. (2014) . |
[14] | Adam SS , Key NS , Greenberg CS . D-dimer antigen: Current concepts and future prospects. Blood. (2009) . |
[15] | Lippi G , Filippozzi L , Montagnana M , Salvagno GL , Guidi GC . Diagnostic value of D-dimer measurement in patients referred to the emergency department with suspected myocardial ischemia. J Thromb Thrombolysis. (2008) . |
[16] | Grau AJ , Buggle F , Becher H , Werle E , Hacke W . The association of leukocyte count, fibrinogen and C-reactive protein with vascular risk factors and ischemic vascular diseases. Thromb Res. (1996) . |
[17] | Kuo HK , Yen CJ , Chang CH , Kuo CK , Chen JH , Sorond F . Relation of C-reactive protein to stroke, cognitive disorders, and depression in the general population: Systematic review and meta-analysis. Lancet Neurology. (2005) . |
[18] | Zi WJ , Shuai J . Plasma D-dimer levels are associated with stroke subtypes and infarction volume in patients with acute ischemic stroke. PLoS One. (2014) . |
[19] | Zhang J , Song Y , Shan B , He M , Ren Q , Zeng Y , et al. Elevated level of D-dimer increases the risk of stroke. Oncotarget. (2018) . |
[20] | Moola S , Munn Z , Tufanaru C , Aromataris E , Sears K , Sfetcu R , et al. Chapter 7 Systematic reviews of etiology and risk. Joanna Briggs Inst Rev Man. (2017) . |
[21] | Higgins JPT , Thompson SG . Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) . |
[22] | Morassi M , Bagatto D , Cobelli M , D’Agostini S , Gigli GL , Bnà C , et al. Stroke in patients with SARS-CoV-2 infection: case series. J Neurol. (2020) . |
[23] | Avula A , Nalleballe K , Narula N , Sapozhnikov S , Dandu V , Toom S , et al. COVID-19 presenting as stroke. Brain Behav Immun. (2020) . |
[24] | Gonzalez-Pinto T , Luna-Rodríguez A , Moreno-Estebanez A , Agirre-Beitia G , Rodríguez-Antigüedad A , Ruiz-Lopez M . Emergency Room Neurology in times of COVID-19 Malignant Ischemic Stroke and SARS-COV2 Infection. European Journal of Neurology. (2020) . |
[25] | Beyrouti R , Adams ME , Benjamin L , Cohen H , Farmer SF , Goh YY , et al. Characteristics of ischaemic stroke associated with COVID-19. Journal of Neurology, Neurosurgery and Psychiatry. (2020) . |
[26] | Tunc A , Ünlübas Y , Alemdar M , Akyüz E . Coexistence of COVID-19 and acute ischemic stroke report of four cases. J Clin Neurosci. (2020) . |
[27] | Guan W , Ni Z , Hu Y , Liang W , Ou C , He J , et al. Clinical characteristics of coronavirus disease in 2019 China. N Engl J Med. (2020) . |
[28] | Qin C , Zhou L , Hu Z , Zhang S , Yang S , Tao Y , et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. (2020) . |
[29] | Wang D , Hu B , Hu C , Zhu F , Liu X , Zhang J , et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA - J Am Med Assoc. (2020) . |
[30] | Shi S , Qin M , Shen B , Cai Y , Liu T , Yang F , et al. Association of Cardiac Injury with Mortality in Hospitalized Patients with COVID-19 in Wuhan, China. JAMA Cardiol. (2020) . |
[31] | Lagi F , Piccica M , Graziani L , Vellere I , Botta A , Tilli M , et al. Early experience of an infectious and tropical diseases unit during the coronavirus disease (COVID-19) pandemic, Florence, Italy, February to March 2020. Eurosurveillance. (2020) . |
[32] | Chen TL , Dai Z , Mo P , Li X , Ma Z , Song S , et al. Clinical characteristics and outcomes of older patients with coronavirus disease (COVID-19) in Wuhan, China (2019) a single-centered, retrospective study. J Gerontol A Biol Sci Med Sci. (2020) . |
[33] | Chen T , Wu D , Chen H , Yan W , Yang D , Chen G , et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019 Retrospective study. BMJ. (2020) . |
[34] | Yang X , Yu Y , Xu J , Shu H , Xia J , Liu H , et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. (2020) . |
[35] | Xu X , Han M , Li T , Sun W , Wang D , Fu B , et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. (2020) . |
[36] | Yan Y , Yang Y , Wang F , Ren H , Zhang S , Shi X , et al. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Res Care. (2020) . |
[37] | Zhang G , Hu C , Luo L , Fang F , Chen Y , Li J , et al. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J Clin Virol. (2020) . |
[38] | Hu L , Chen S , Fu Y , Gao Z , Long H , Wang J , et al. Risk Factors Associated with Clinical Outcomes in 323 COVID-19 Hospitalized Patients in Wuhan, China. Clin Infect Dis. (2020) . |
[39] | Huang Y , Lyu X , Li D , Wang Y , Wang L , Zou W , et al. A cohort study of 223 patients explores the clinical risk factors for the severity diagnosis of COVID-19. medRxiv. (2020) . |
[40] | Zhang jin J , Dong X , Cao yuan Y , Yuan dong Y , Yang bin Y , Yan qin Y , et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy Eur J Allergy Clin Immunol. (2020) . |
[41] | Zhou F , Yu T , Du R , Fan G , Liu Y , Liu Z , et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) . |
[42] | Xu T , Ke K . C-reactive protein and ischemic stroke risk in general population: A dose-response meta-analysis of prospective studies. Int J Cardiol. (2015) . |
[43] | Di Napoli M , Papa F , Bocola V . C-reactive protein in ischemic stroke an independent prognostic factor. Stroke. (2001) . |
[44] | Yu B , Yang P , Xu X , Shao L . C-reactive protein for predicting all-cause mortality in patients with acute ischemic stroke: A meta-analysis. Biosci Rep. (2019) . |
[45] | Elkind MSV , Tai W , Coates K , Paik MC , Sacco RL . High-sensitivity C-reactive protein, lipoprotein-associated phospholipase A2, and outcome after ischemic stroke. Arch Intern Med. (2006) . |
[46] | Matsuo R , Ago T , Hata J , Wakisaka Y , Kuroda J , Kuwashiro T , et al. Plasma C-reactive protein and clinical outcomes after acute ischemic stroke: A prospective observational study. PLoS One. (2016) . |
[47] | Ladenvall C , Jood K , Blomstrand C , Nilsson S , Jern C , Ladenvall P . Serum C-reactive protein concentration and genotype in relation to ischemic stroke subtype. Stroke. (2006) . |
[48] | Park YW , Koh EJ , Choi HY . Correlation between serum D-Dimer level and volume in acute ischemic stroke. J Korean Neurosurg Soc. (2011) . |
[49] | Welsh P , Barber M , Langhorne P , Rumley A , Lowe GDO , Stott DJ . Associations of inflammatory and haemostatic biomarkers with poor outcome in acute ischaemic stroke. Cerebrovasc Dis. (2009) . |
[50] | Montaner J , Perea-Gainza M , Delgado P , Ribó M , Chacón P , Rosell A , et al. Etiologic diagnosis of ischemic stroke subtypes with plasma biomarkers. Stroke. (2008) . |
[51] | Du Y , Tu L , Zhu P , Mu M , Wang R , Yang P , et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan: A retrospective observational study. Am J Respir Crit Care Med. (2020) . |
[52] | Lei S , Jiang F , Su W , Chen C , Chen J , Mei W , et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinicalMedicine. (2020) . |
[53] | Wang D , Yin Y , Hu C , Liu X , Zhang X , Zhou S , et al. Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS-CoV-2, discharged from two hospitals in Wuhan, China. Crit Care. (2020) . |




