Complement-mediated Extracellular Vesicle release as a measure of endothelial dysfunction and prognostic marker for COVID-19 in peripheral blood - Letter to the Editor
Dear Editor,
The recent articles in Clin. Hemorheol. Microcirc. by Jung et al. [1–3], elegantly highlighted the thrombotic complications that arise in severe coronavirus disease 2019 (COVID-19) and discussed the role vascular injury and associated hyper inflammation play in bringing about multi-organ failure in severe disease. Coagulation and venous thromboembolism (VTE) in COVID-19 commonly presents as deep vein thrombosis (DVT) or pulmonary embolism (PE), and occurs because of inflammation, blood vessel injury and associated endothelial dysfunction. Likely contributors to this thrombotic milieu, hitherto little discussed in this COVID-19 pandemic, include Extracellular Vesicles (EVs), nanosized, cell-derived intercellular communicative vesicles, carrying proteins, bioactive lipids and miRNAs. Endothelial cell- (EC-) derived EVs (EEVs) are often released because of endothelial injury [4] and also likely to contribute to this prothrombotic environment. Whilst EVs and VTE in cancer has been much described, there is a significant knowledge gap concerning EVs and VTE in infectious disease. This letter considers how, as part of ongoing inflammation, complement may be activated in SARS-CoV-2 infection and so mediate EV biogenesis. It also assesses the role procoagulant EVs play in the context of coagulopathy and VTE in COVID-19, and their potential as a prognostic peripheral blood marker.
During a viral infection, complement activation resulting in membrane attack complex- (C5b-9-) mediated damage of ECs will bring about the release of EEVs. However, in COVID-19 there are scarce few preprints describing complement activation [5], and EVs have so far only been mentioned in the literature as prospective therapeutic tools. A local prothrombotic environment is initiated early in infection (Fig. A(1)), as Angiotensin-converting enzyme 2 is taken up during Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) entry, causing AngII-mediated activation of ECs, platelets and the release of pro-inflammatory cytokines. Triggering of complement via MBL recognition of damaged cells, such as ECs (Fig. A(2)), in COVID-19, would generate the Lectin Pathway (LP)/Classical Pathway (CP) C3 convertase on these cells, Ca2+-mediated release of EVs (Fig. A(3 and 4iii)) and release of anaphylatoxins, C3a and C5a. Besides recruiting inflammatory cells, C3a/C5a activate ECs and platelets, promoting proinflammatory cytokine release, coagulation and TF expression [6], as does MBL-associated Serine Protease (MASP-1) cleavage of Protease-Activated Receptor 4 on ECs [7].
Fig. A(1)
Fig. Extracellular Vesicles and coagulopathy in COVID-19 SARS-CoV-2 stimulate endocytosis via angiotensis-converting enzyme 2 (ACE2), initiates the release of proinflammatory cytokines and induces endothelial damage (1). Complement is activated (2). The resulting Ca2+ influx (3) through deposited C5b-9 (and the SARS-CoV-2 E-protein Ca2+ channel from ERGIC/ER, not shown) results in a Ca2+ mediated Extracellular Vesicle (EV) release. (4): (i) Weibel Palade Body exocytosis releasing vWF and CD63+/CD9–/CD81– small EVs; (ii) MultiVesicular Body exocytosis releasing CD63+/CD9+/CD81+ small EVs; (iii) release of plasma membrane-derived medium EVs carrying coagulation proteins. In severe COVID-19 (A), where there is increased circulating EV levels, with their known function in coagulopathy, EVs could play an important role in COVID-related deaths through VTE. Coagulation factors including vWF, and EVs with more procoagulant morphalogies, as markers of endothelial damage, are released during severe COVID-19 (A) as opposed to those released during recovery (B).
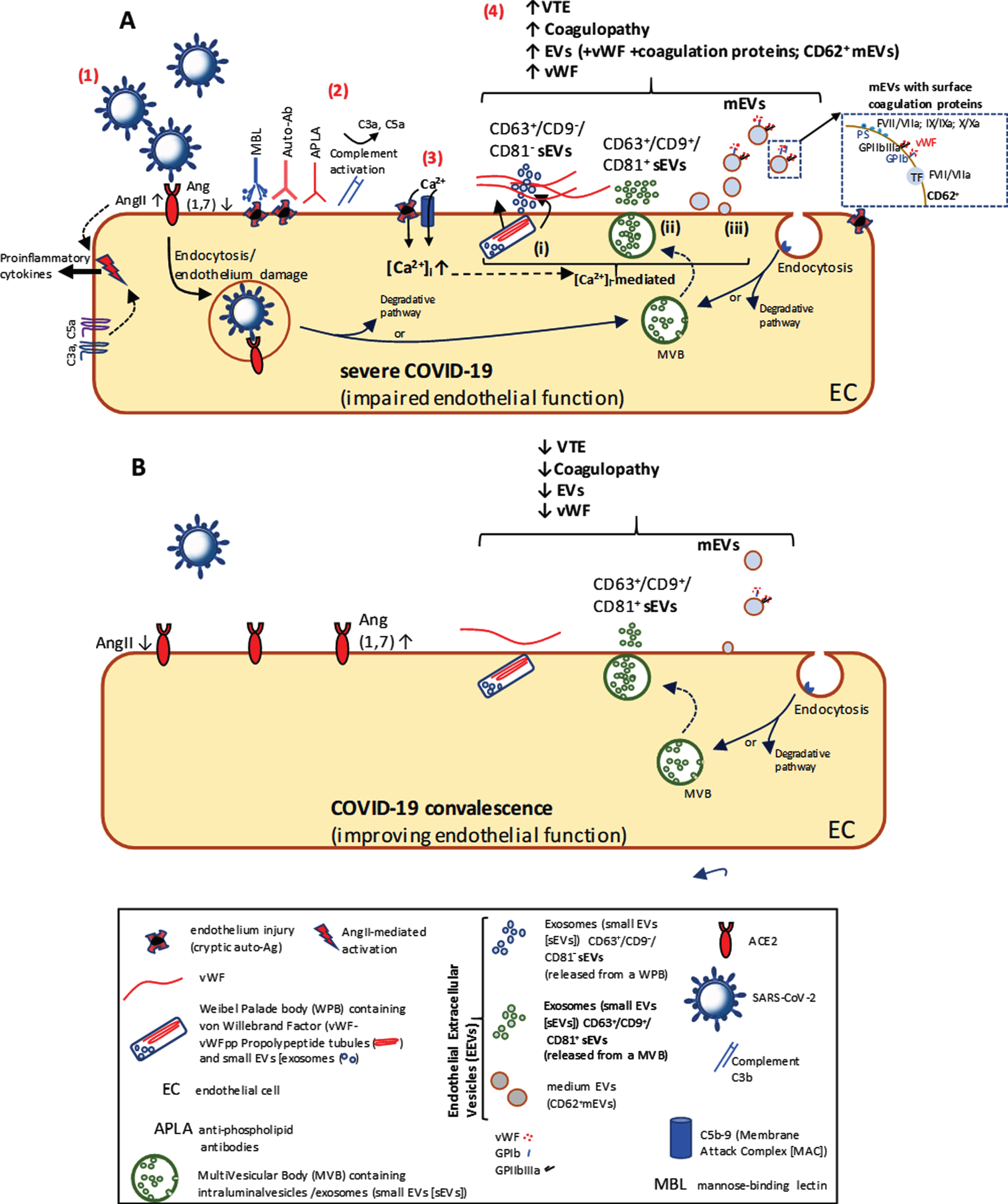
As for SARS-CoV infection, where autoantibodies are formed against cryptic autoantigens, revealed upon endothelial damage [8], in SARS-CoV-2 infection, where endothelialitis occurs [9], similar autoantibodies must stimulate CP-mediated complement activation and release of EEVs (Fig. A(2–4)). With certain viral infections, a transitory increase in pathogenic anti-phospholipid (aPL) antibodies (APLA) may occur, and if present in COVID-19, result in thrombotic complications. Recently, three COVID-19 patients with advanced coagulopathy and several cerebral infarctions were found to have aPL antibodies (anticardiolipin IgA and anti-β2-glycoprotein I IgA and IgG) [10]. Although the anti-β2GPI IgG suggests autoantibodies present before COVID-19, it is also likely that the anti-β2GPI component of APLA stimulates EV release from ECs [11], monocytes and platelets. The implication of antiphospholipid syndrome could explain the coagulopathy, damage to ECs and cerebral ischaemia, as seen in severe COVID-19.
EVs budding from the EC plasma membrane (medium EVs [mEVs] or microvesicles), expressing E-selectin (CD62E+), tissue factor (TF+) and other coagulation factors, are known to be raised in patients with cancer/autoimmune disease and associated VTE [12]. They may also be raised in infectious diseases as in meningococcal sepsis, where prothrombotic events are common, and where raised TF+-EVs are derived from monocytes stimulated by endotoxin [13]. In severe COVID-19, which may lead to acute respiratory distress syndrome, sepsis, septic shock and eventual death, raised TF+-EVs are also likely. However, besides EVs stimulating thrombosis through TF-dependent mechanisms, in COVID-19, their procoagulant nature is also due to phosphatidylserine (PtdSer) exposed on the outer leaflet of the EV lipid bilayer. With exposed negatively charged PtdSer, EEVs and platelet-derived EVs (PEVs) are able to electrostatically bind various cationic coagulation cascade proteins.
Markers of EC injury and inflammation such as circulating EVs, but also including vWF and Factor VIII [14], all important contributors to coagulopathy, VTE and increased mortality, are largely raised in COVID-19 [14]. Besides furthering understanding of pathology and mortality risk in COVID-19, this capacity to measure EC dysfunction in the systemic vasculature (Fig. A(4)), namely EEVs with associated surface-bound coagulation factors (prothrombin and factors VII, IX and X) and vWF, could be used for stratification of COVID-19 severity or clinical prognosis in treatment (comparing dysfunctional endothelium (Fig. A) with convalescent endothelium (Fig. B)). Other EEV phenotypes which are useful in stratification of thrombotic disorders and could similarly be used in monitoring clinical progression of COVID-19 include CD31 (PECAM-1)/CD105 (endoglin) markers of myocardial infarction/acute coronary syndromes and CD54 (ICAM-1)/CD63E (E-selectin)/CD105 (endoglin), markers of acute ischaemic stroke.
Recently however, some exciting new markers of endothelial injury have emerged. This includes CD63+ small EVs (sEVs or exosomes), discovered in Weibel-Palade bodies (WPBs) as intraluminal vesicles [15]. Released from ECs following endothelial injury, during WPB exocytosis, along with vWF and P-selectin (Fig. A(4i), WPB-sEVs have a unique molecular signature (CD63+/CD9–/CD81–). Importantly, these WPB-derived sEVs are distinguishable from sEVs (CD63+/CD9+/CD81+) constitutively released through exocytosis of multivesicular bodies (Fig. A(4ii) and B). As WPB-derived sEVs are only released with vWF upon WPB exocytosis, they could represent an excellent novel marker of endothelial dysfunction in COVID-19. PEVs already make ideal diagnostic markers of PE; as specific EVs can be immunoaffinity purified from plasma [16] and generally make optimal diagnostic tools for non-invasive liquid biopsy [17], EEV phenotypes typical of thrombotic disorders, should now be used as an important new predictive biomarker monitoring disease progression and therapy response in COVID-19 patients.
Author contributions
The author confirms to be the sole contributor to this manuscript.
Conflicts of interest
The author confirms there are no conflicts of interest.
References
[1] | Jung F , Krüger-Genge A , Franke RP , Hufert F , Küpper JH . COVID-19 and the endothelium. Clinical Hemorheology and Microcirculation. (2020) ;75: (1):7–11. |
[2] | Jung EM , Stroszczynski C , Jung F . Contrast enhanced ultrasonography (CEUS) to detect abdominal microcirculatory disorders in severe cases of COVID-19 infection: First experience. Clinical Hemorheology and Microcirculation. (2020) ;74: (4):353–61. |
[3] | Jung EM , Stroszczynski C , Jung F . Contrast enhanced ultrasound (CEUS) to assess pleural pulmonal changes in severe COVID-19 infection: First results. Clinical Hemorheology and Microcirculation. (2020) ;75: (1):19–26. |
[4] | Jung KH , Chu K , Lee ST , Park HK , Bahn JJ , Kim DH , et al. Circulating endothelial microparticles as a marker of cerebrovascular disease. Annals of Neurology. (2009) ;66: (2):191–9. |
[5] | Mobini M , Ghasemian R , Larijani LV , Mataji M , Maleki I . Vasculitis-Associated Auto-antibodies and Complement Levels in patients with COVID-19 Infection. 2020. |
[6] | Oikonomopoulou K , Ricklin D , Ward PA , Lambris JD . Interactions between coagulation and complement–their role in inflammation. Seminars in Immunopathology. (2012) ;34: (1):151–65. |
[7] | Megyeri M , Makó V , Beinrohr L , Doleschall Z , Prohászka Z , Cervenak L , et al. Complement protease MASP-1 activates human endothelial cells: PAR4 activation is a link between complement and endothelial function. Journal of Immunology (Baltimore, Md: 1950). (2009) ;183: (5):3409–16. |
[8] | Yang YH , Huang YH , Chuang YH , Peng CM , Wang LC , Lin YT , et al. Autoantibodies against human epithelial cells and endothelial cells after severe acute respiratory syndrome (SARS)-associated coronavirus infection. Journal of Medical Virology. (2005) ;77: (1):1–7. |
[9] | Varga Z , Flammer AJ , Steiger P , Haberecker M , Andermatt R , Zinkernagel AS , et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet (London, England). (2020) ;395: (10234):1417–8. |
[10] | Zhang Y , Xiao M , Zhang S , Xia P , Cao W , Jiang W , et al. Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. The New England Journal of Medicine. (2020) ;382: (17):e38. |
[11] | Holnthoner W , Bonstingl C , Hromada C , Muehleder S , Zipperle J , Stojkovic S , et al. Endothelial Cell-derived Extracellular Vesicles Size-dependently Exert Procoagulant Activity Detected by Thromboelastometry. Scientific Reports. (2017) ;7: (1):3707. |
[12] | Zarà M , Guidetti GF , Camera M , Canobbio I , Amadio P , Torti M , et al. Biology and Role of Extracellular Vesicles (EVs) in the Pathogenesis of Thrombosis. International Journal of Molecular Sciences. (2019) ;20: (11). |
[13] | Iba T , Ogura H . Role of extracellular vesicles in the development of sepsis-induced coagulopathy. Journal of Intensive Care. (2018) ;6: :68. |
[14] | Escher R , Breakey N , Lämmle B . Severe COVID-19 infection associated with endothelial activation. Thrombosis Research. (2020) ;190: :62. |
[15] | Streetley J , Fonseca AV , Turner J , Kiskin NI , Knipe L , Rosenthal PB , et al. Stimulated release of intraluminal vesicles from Weibel-Palade bodies. Blood. (2019) ;133: (25):2707–17. |
[16] | Whiteside TL . Proteomic Analysis of Plasma-Derived Exosomes in Defining Their Role as Biomarkers of Disease Progression, Response to Therapy and Outcome. Proteomes. (2019) ;7: (3). |
[17] | Dickhout A , Koenen RR . Extracellular Vesicles as Biomarkers in Cardiovascular Disease; Chances and Risks. Frontiers in Cardiovascular Medicine. (2018) ;5: :113. |




