Ultrasound and clinicopathological characteristics of breast cancer for predicting axillary lymph node metastasis
Abstract
OBJECTIVES:
The goal of this study was to assess the clinicopathological and ultrasound (US) features of breast cancer for predicting the risk of axillary lymph node metastasis.
METHODS:
Patients with breast cancer were included in this retrospective, monocentric, observational study. Their preoperative ultrasound features, clinical data, laboratory results and postoperative pathologic results and immunophenotyping were collected. The association of these factors of breast cancer with axillary lymph node metastasis was evaluated by univariate and multivariate analysis.
RESULTS:
In this study, 471 patients diagnosed with breast cancer at the First Affiliated Hospital of Xi’an Jiaotong University between July 2016 and September 2019 were collected, with a total of 471 nodules, of which 231(49.0%) had axillary lymph node metastasis, and 240(51.0%) did not. The parameters of hyperechoic halo, posterior acoustic decrease, microcalcification, carcinogenic embryonic antigen (CEA), cancer antigen-153 (CA153), CK5/6 (+), Ki67 (≥40%), AR (+) and histological grade (grade II and grade III) were significantly and independently associated with axillary lymph node metastasis (p < 0.05 for all).
CONCLUSIONS:
The combination of ultrasound features, tumor markers, pathology, and immunohistochemistry can predict axillary lymph node metastasis in breast cancer patients.
1Introduction
Breast cancer is one of the most common female malignant tumors that seriously threaten women’s health and life worldwide, and its incidence is increasing annually. According to statistics, more than 400,000 patients die from breast cancer every year [1]. The presence of axillary lymph node metastasis (ALNM) plays a crucial role in determining the prognosis of breast cancer and significantly influences decisions related to treatment options [2]. Consequently, the development of diagnostically precise techniques for identifying ALNM has consistently been of paramount significance.
Axillary lymph node dissection following mastectomy or breast conserving surgery has been widely prescribed for disease staging, prediction of prognosis, local tumor control, and determination of adjuvant treatment [3]. Usually, patients with clinical positive (cN+) axillary lymph node directly was performed axillary lymph node dissection (ALND) [4]. Unfortunately, the anatomical disruption caused by axillary lymph node dissection can result in side effects, such as nerve injury, lymph edema and decreased range of motion in the shoulder, and paresthesia, other complications [5]. Therefore, finding an accurate, simple and effective and noninvasive predicting axillary lymph node metastasis to attenuate operational injury is very important.
Recent data [6–11] demonstrate that some ultrasonic features and Clinicopathologic characteristics of breast cancer might be associated with axillary lymph node metastases and can help to better predict ALN status, for example, the tumor size, tumor quadrant, local invasion status, pathologic type, and molecular subtypes, tumor shape, growth orientation, margin, posterior features, calcifications, and echogenicity. However, whether the image Characteristics of breast lesions are correlated with axillary lymph node metastasis has still not been fully elucidated in patients with breast cancer. In the present study, we retrospectively investigated the US features and clinicopathologic results to explore the value of US features and clinicopathologic results of breast cancer for predicting axillary lymph node metastasis for guidance in clinical practice.
2Materials and methods
2.1Ethics
The Ethics committee of the hospital approved the design process (approval number: XJTU1AF2019LSK-279) and waived the requirement for written informed consent, since this study was characterized by noninvasive anonymous retrospective analysis. Verbal informed consent was obtained by phone for using data from all of the patients who were recruited in this study.
2.2Inclusion and exclusion criteria
The inclusion and exclusion criteria were as follows.
Inclusion criteria: (1) patients pathologically diagnosed as having breast cancer; (2) axillary lymph node status clearly illustrated by pathology after sentinel lymph node biopsy or axillary lymph node dissection; (3) US examination was performed before mastectomy or breast-conserving surgery and axillary lymph node dissection.
Exclusion criteria: (1) multiple malignant lesions; (2) target neoplasms that could not be visualized on US; (3) patients who received any treatments before surgery or had distant metastases.
2.3Patients
Data from 471 female patients with breast cancer from July 2016 to September 2019 were retrospectively analyzed in this study. The clinical data, US images and pathological results were reviewed. The clinical information included age, age at menarche, the times of fertility, oral contraceptives, hormone replacement therapy, history of breast diseases, family history of breast cancer, tumor size, nodule location. The US images included echogenicity, Orientation, internal echo, margin, morphology, spiculated margins, angled edge, hyperechoic halo, posterior acoustic decrease, calcification, color doppler flow, Breast Imaging Reporting and Data System (BI-RADS). The pathological data included tumor histologic type, tumor histologic grade, molecular subtype, E- cadherin, ER, PR, Her-2, CK5/6, Ki67, p53, AR.
Finally, a total of 471 patients were included in this study. Based on axillary status evaluated on final histopathology, all patients were divided into 2 groups: axillary lymph nodes metastasis positive and negative.
2.4Ultrasound examination and analysis
Preoperatively, all patients underwent breast ultrasound. All examinations were performed by the two sonographer who had a minimum of 5 years of experience with breast ultrasound. Breast ultrasound was performed on all patients with GE LOGIC E9 ultrasound device (GE Healthcare, Milwaukee, WI, USA) with 15 MHz linear transducer (type ML6–15). Acquired US images were stored in the picture archiving and communication system (PACS).
Two-dimensional and Doppler images of lesions were observed multidimensionally for the subsequent evaluation. In this study, all image analysis was performed independently in a blind fashion by two physicians with more than 5 years of diagnostic experience in breast ultrasound.
Ten ultrasound phantoms characteristics of each breast lesion were analysed, including tumor size, tumor shape (regular or irregular), growth orientation (horizontal or vertical), Echogenicity (Hypoechoic, hyperechoic, heterogeneous), internal echo (homogeneous or inhomogeneous), margin (clear or unclear, speculated, angled edge), posterior acoustic decrease, hyperechoic halo, calcification(no, micro or macro), Color Doppler flow (No flow, minimal or Moderate, abundant). Afterwards, Lesions were classified according to the ultrasound BI-RADS lexicon of American College of Radiology (ACR) of fifth edition [12].
2.5Pathologic features
The pathological features of the patients, including tumor histologic type (Carcinoma in situ, Invasive in carcinoma or other type), tumor histologic grade (Carcinoma in situ, Grade I, Grade II, Grade III), immunohistochemical analyses (E-cadherin, ER, PR, Her-2, CK5/6, P53, ki67,AR, Molecular subtype), breast cancer molecular subtypes were categorized as Luminal A, Luminal B, Her2 enriched, Triple negative according to the result of ER, PR, Her-2 and Ki67. Ki67 was considered positive if it was equal to or greater than 40%. HER2 positivity was defined as HER2 protein3+ or HER2 gene amplification.
2.6Tumor markers
The content of tumor markers Carcinoembryonic antigen (CEA), carbohydrate antigen (CA125), and carbohydrate antigen (CA153) can be used as the basis for evaluating the prognosis of breast cancer. CEA, CA125, and CA153 were determined by the direct chemiluminescence method (Beckman). The result of Serum CEA, CA153 and CA125 concentrations were extracted from routine clinical records.
2.7Statistical analyses
Normally distributed continuous variables were presented as mean±SD, compared by Student’s t-test. Categorical variables were presented as frequencies and percentages. Categorical variables were compared by Chi-square test or the Fisher’s exact test. First, univariable logistic regression analysis was performed, then, variables with P values < 0.10 in the univariable analysis were entered into multivariate logistic regression analysis to predict the best risk factors of axillary lymph node metastasis of breast cancers. The ROC curve was used to analyze the predictive factors, and the area under the receiver operating characteristic (ROC) curve (AUC) was calculated. All statistical analysis was performed by the statistical software packages R (http://www.R-project.org, The R Foundation) and the EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA). P < 0.05 was considered statistically significant.
3Results
3.1Clinicopathologic characteristics and US features and axillary lymph nodes
A total of 471 patients were retrospectively enrolled in this study. Clinicopathologic characteristics are showed in Tables 1 and 2. From Table 1, we can see that among people over 50 years old, the probability of breast cancer is higher. Among people with axillary lymph node metastasis, people over 50 years old have a higher proportion of metastasis, breast cancer has a larger focus, and left breast cancer has a higher proportion of axillary lymph node metastasis. 231 (49.0%) cases had axillary lymph nodal metastases, 240 (51.0%) did not. Of all patients, there were 59 patients with the Luminal A subtype, 215 patients with the Luminal B subtype, 52 patients with the Her2 enriched subtype, 73 patients with the Triple negative subtype. There were 7 with Carcinoma in situ, 441 cases were invasive in carcinoma, 7 cases were others type.
Table 1
Clinicopathologic characteristics of patients with breast cancer who presented with and without axillary lymph node metastasis
| Characteristic | Lymph node negative, n (%) n = 240 | Lymph node positive, n (%) n = 231 | P |
| Age, y | 0.759 | ||
| <50 | 101 (42.1%) | 94 (40.7%) | |
| ≥50 | 139 (57.9%) | 137 (59.3%) | |
| Carcinoembryonic antigen | 2.5±2.1 | 7.3±38.9 | 0.002 |
| CA125 | 25.7±57.0 | 30.1±99.0 | 0.012 |
| CA153 | 16.3±12.4 | 33.6±162.7 | <0.001 |
| Age at menarche, y | 0.173 | ||
| ≤13 | 102 (42.5%) | 84 (36.4%) | |
| >13 | 138 (57.5%) | 147 (63.6%) | |
| The times of fertility | 0.845 | ||
| No previous childbirth | 6 (2.5%) | 5 (2.2%) | |
| Once | 82 (34.2%) | 86 (37.2%) | |
| Twice | 116 (48.3%) | 106 (45.9%) | |
| Three times | 29 (12.1%) | 26 (11.3%) | |
| Four times | 6 (2.5%) | 7 (3.0%) | |
| Five times | 0 (0.0%) | 1 (0.4%) | |
| Six times | 1 (0.4%) | 0 (0.0%) | |
| Oral contraceptives | 0.978 | ||
| No | 239 (99.6%) | 230 (99.6%) | |
| Yes | 1 (0.4%) | 1 (0.4%) | |
| Hormone replacement therapy | 0.234 | ||
| No | 234 (97.5%) | 228 (98.7%) | |
| Yes (levothyroxine tablets) | 6 (2.5%) | 2 (0.9%) | |
| Yes (estrogen) | 0 (0.0%) | 1 (0.4%) | |
| History of breast diseases | 0.761 | ||
| No | 228 (95.0%) | 218 (94.4%) | |
| Yes | 12 (5.0%) | 13 (5.6%) | |
| Family history of breast cancer | 0.833 | ||
| No | 233 (97.1%) | 225 (97.4%) | |
| Yes | 7 (2.9%) | 6 (2.6%) | |
| Tumor size, mm | 22.8±13.6 | 23.6±12.9 | 0.268 |
| Nodule location | 0.839 | ||
| Left | 114 (47.5%) | 116 (50.2%) | |
| Right | 125 (52.1%) | 114 (49.4%) | |
| Not recorded | 1 (0.4%) | 1 (0.4%) | |
| E- cadherin | 0.008 | ||
| Negative | 2 (0.8%) | 1 (0.4%) | |
| Positive | 13 (5.4%) | 30 (13.0%) | |
| Not recorded | 225 (93.8%) | 200 (86.6%) | |
| ER | 0.345 | ||
| Negative | 71 (29.6%) | 67 (29.0%) | |
| Positive | 134 (55.8%) | 140 (60.6%) | |
| Not recorded | 35 (14.6%) | 24 (10.4%) | |
| PR | 0.049 | ||
| Negative | 92 (44.9%) | 114 (54.5%) | |
| Positive | 113 (55.1%) | 95 (45.5%) | |
| Her-2 | 0.416 | ||
| Negative | 149 (62.1%) | 152 (65.8%) | |
| Positive | 47 (19.6%) | 47 (20.3%) | |
| Not recorded | 44 (18.3%) | 32 (13.9%) | |
| Molecular subtype | 0.493 | ||
| Luminal A | 34 (14.2%) | 25 (10.8%) | |
| Luminal B | 103 (42.9%) | 112 (48.5%) | |
| Her2 enriched | 24 (10.0%) | 28 (12.1%) | |
| Triple negative | 38 (15.8%) | 35 (15.2%) | |
| Not recorded | 41 (17.1%) | 31 (13.4%) | |
| CK5/6 | 0.002 | ||
| Negative | 124 (51.7%) | 142 (61.5%) | |
| Positive | 54 (22.5%) | 24 (10.4%) | |
| Not recorded | 62 (25.8%) | 65 (28.1%) | |
| Ki67 | 0.009 | ||
| <40% | 119 (49.6%) | 93 (40.3%) | |
| ≥40% | 80 (33.3%) | 109 (47.2%) | |
| Not recorded | 41 (17.1%) | 29 (12.6%) | |
| P53 | 0.430 | ||
| Negative | 33 (13.8%) | 23 (10.0%) | |
| Positive | 134 (55.8%) | 132 (57.1%) | |
| Not recorded | 73 (30.4%) | 76 (32.9%) | |
| AR | 0.032 | ||
| Negative | 44 (18.3%) | 54 (23.4%) | |
| Positive | 36 (15.0%) | 18 (7.8%) | |
| Not recorded | 160 (66.7%) | 159 (68.8%) | |
| Tumor histologic grade | <0.001 | ||
| Carcinoma in situ | 27 (11.2%) | 2 (0.9%) | |
| Grade I | 1 (0.4%) | 0 (0.0%) | |
| Grade II | 52 (21.7%) | 58 (25.1%) | |
| Grade III | 90 (37.5%) | 109 (47.2%) | |
| Not recorded | 70 (29.2%) | 62 (26.8%) | |
| Tumor histologic type | 0.044 | ||
| Carcinoma in situ | 7 (2.9%) | 0 (0.0%) | |
| Invasive in carcinoma | 220 (91.7%) | 221 (95.7%) | |
| Others type | 5 (2.1%) | 2 (0.9%) | |
| Not recorded | 8 (3.3%) | 8 (3.5%) |
Table 2
Correlation between US characteristics and axillary lymph node metastasis in patients with breast cancer
| Characteristic | Lymph node negative, n (%) | Lymph node positive, n (%) | P |
| Echogenicity | 0.016 | ||
| Hypoechoic | 206 (85.8%) | 171 (74.0%) | |
| Hyperechoic | 1 (0.4%) | 2 (0.9%) | |
| Heterogeneous | 4 (1.7%) | 7 (3.0%) | |
| Not recorded | 29 (12.1%) | 51 (22.1%) | |
| Orientation | 0.07 | ||
| Horizontal | 235 (97.9%) | 219 (94.8%) | |
| Vertical | 5 (2.1%) | 12 (5.2%) | |
| Internal echo | 0.127 | ||
| Homogeneous | 12 (5.0%) | 10 (4.3%) | |
| Inhomogeneous | 144 (60.0%) | 119 (51.5%) | |
| Not recorded | 84 (35.0%) | 102 (44.2%) | |
| Margin | 0.022 | ||
| Clear | 49 (20.4%) | 34 (14.7%) | |
| Unclear | 150 (62.5%) | 135 (58.4%) | |
| Not recorded | 41 (17.1%) | 62 (26.8%) | |
| Morphology | 0.004 | ||
| Regular | 13 (5.4%) | 5 (2.2%) | |
| Irregular | 186 (77.5%) | 160 (69.3%) | |
| Not recorded | 41 (17.1%) | 66 (28.6%) | |
| Spiculated margins | 0.014 | ||
| No | 149 (62.1%) | 120 (51.9%) | |
| Yes | 50 (20.8%) | 46 (19.9%) | |
| Not recorded | 41 (17.1%) | 65 (28.1%) | |
| Angled edge | 0.015 | ||
| No | 151 (62.9%) | 123 (53.2%) | |
| Yes | 48 (20.0%) | 43 (18.6%) | |
| Not recorded | 41 (17.1%) | 65 (28.1%) | |
| Hyperechoic halo | 0.001 | ||
| No | 194 (80.8%) | 153 (66.2%) | |
| Yes | 5 (2.1%) | 13 (5.6%) | |
| Not recorded | 41 (17.1%) | 65 (28.1%) | |
| Posterior acoustic decrease | <0.001 | ||
| No | 189 (78.8%) | 165 (71.4%) | |
| Yes | 10 (4.2%) | 1 (0.4%) | |
| Not recorded | 41 (17.1%) | 65 (28.1%) | |
| Calcification | 0.012 | ||
| No | 153 (63.7%) | 122 (52.8%) | |
| Micro | 87 (36.2%) | 106 (45.9%) | |
| Macro | 0 (0.0%) | 3 (1.3%) | |
| Color Doppler flow | 0.774 | ||
| No flow, minimal | 113 (47.3%) | 116 (50.2%) | |
| Moderate, abundant | 9 (3.8%) | 7 (3.0%) | |
| Not recorded | 117 (49.0%) | 108 (46.8%) | |
| BI-RADS | <0.001 | ||
| 0 | 1 (0.4%) | 0 (0.0%) | |
| 3 | 6 (2.5%) | 4 (1.7%) | |
| 4a | 35 (14.6%) | 11 (4.8%) | |
| 4b | 34 (14.2%) | 24 (10.4%) | |
| 4c | 119 (49.6%) | 94 (40.7%) | |
| 5 | 41 (17.1%) | 88 (38.1%) | |
| 6 | 4 (1.7%) | 10 (4.3%) |
3.2Univariate analyses of clinicopathologic characteristics and US characteristics in predicting axillary lymph nodes metastases
The result of the correlation analysis between Clinicopathologic characteristics and US characteristics is shown in Table 3. There were significant differences between the lymph node-positive and lymph node-negative groups for hyperechoic halo (p = 0.026), posterior acoustic decrease (p = 0.040), microcalcification (p = 0.025), Carcinoembryonic antigen (p = 0.005), CA153 (p = 0.003), ck5/6 (p < 0.001), ki67≥40% (p = 0.006), AR positive (p = 0.011), tumor histologic grade II and III (p < 0.001).
Table 3
Univariate logistic regression analysis of the association of clinicopathologic and US characteristics with axillary lymph node metastases in patients with breast cancer
| Feature | Statistics | OR (95% CI) | P |
| Age, y | |||
| <50 | 195 (41.4%) | 1.0 | |
| ≥50 | 276 (58.6%) | 1.1 (0.7, 1.5) | 0.759 |
| Age at menarche, y | |||
| ≤13 | 186 (39.5%) | 1.0 | |
| >13 | 285 (60.5%) | 1.3 (0.9, 1.9) | 0.174 |
| The times of fertility | |||
| No previous childbirth | 11 (2.3%) | 1.0 | |
| Once | 168 (35.7%) | 1.3 (0.4, 4.3) | 0.713 |
| Twice | 222 (47.1%) | 1.1 (0.3, 3.7) | 0.882 |
| Three times | 55 (11.7%) | 1.1 (0.3, 3.9) | 0.912 |
| Four times | 13 (2.8%) | 1.4 (0.3, 7.0) | 0.682 |
| Five times | 1 (0.2%) | 2541816.0 (0.0, Inf) | 0.987 |
| Six times | 1 (0.2%) | 0.0 (0.0, Inf) | 0.987 |
| Oral contraceptives | |||
| No | 469 (99.6%) | 1.0 | |
| Yes | 2 (0.4%) | 1.0 (0.1, 16.7) | 0.978 |
| Hormone replacement therapy | |||
| No | 462 (98.1%) | 1.0 | |
| Yes (levothyroxine tablets) | 8 (1.7%) | 0.3 (0.1, 1.7) | 0.192 |
| Yes (estrogen) | 1 (0.2%) | 799740.0 (0.0, Inf) | 0.980 |
| History of breast diseases | |||
| No | 446 (94.7%) | 1.0 | |
| Yes | 25 (5.3%) | 1.1 (0.5, 2.5) | 0.761 |
| Family history of breast cancer | |||
| No | 458 (97.2%) | 1.0 | |
| Yes | 13 (2.8%) | 0.9 (0.3, 2.7) | 0.833 |
| Nodule location | |||
| Left | 230 (48.8%) | 1.0 | |
| Right | 239 (50.7%) | 0.9 (0.6, 1.3) | 0.554 |
| Not recorded | 2 (0.4%) | 1.0 (0.1, 15.9) | 0.990 |
| Orientation | |||
| Horizontal | 454 (96.4%) | 1.0 | |
| Vertical | 17 (3.6%) | 2.6 (0.9, 7.4) | 0.080 |
| Tumor size, mm | 23.2±13.3 | 1.0 (1.0, 1.0) | 0.520 |
| Echogenicity | |||
| Hypoechoic | 377 (80.0%) | 1.0 | |
| Hyperechoic | 3 (0.6%) | 2.4 (0.2, 26.8) | 0.474 |
| Heterogeneous | 11 (2.3%) | 2.1 (0.6, 7.3) | 0.240 |
| Not recorded | 80 (17.0%) | 2.1 (1.3, 3.5) | 0.003 |
| Internal echo | |||
| Homogeneous | 22 (4.7%) | 1.0 | |
| Inhomogeneous | 263 (55.8%) | 1.0 (0.4, 2.4) | 0.985 |
| Not recorded | 186 (39.5%) | 1.5 (0.6, 3.5) | 0.406 |
| Margin | |||
| Clear | 83 (17.6%) | 1.0 | |
| Unclear | 285 (60.5%) | 1.3 (0.8, 2.1) | 0.303 |
| Not recorded | 103 (21.9%) | 2.2 (1.2, 3.9) | 0.010 |
| Morphology | |||
| Regular | 18 (3.8%) | 1.0 | |
| Irregular | 346 (73.5%) | 2.2 (0.8, 6.4) | 0.134 |
| Not recorded | 107 (22.7%) | 4.2 (1.4, 12.6) | 0.011 |
| Spiculated margins | |||
| No | 269 (57.1%) | 1.0 | |
| Yes | 96 (20.4%) | 1.1 (0.7, 1.8) | 0.577 |
| Not recorded | 106 (22.5%) | 2.0 (1.2, 3.1) | 0.004 |
| Angled edge | |||
| No | 274 (58.2%) | 1.0 | |
| Yes | 91 (19.3%) | 1.1 (0.7, 1.8) | 0.695 |
| Not recorded | 106 (22.5%) | 1.9 (1.2, 3.1) | 0.004 |
| Hyperechoic halo | |||
| No | 347 (73.7%) | 1.0 | |
| Yes | 18 (3.8%) | 3.3 (1.2, 9.4) | 0.026 |
| Not recorded | 106 (22.5%) | 2.0 (1.3, 3.1) | 0.002 |
| Posterior acoustic decrease | |||
| No | 354 (75.2%) | 1.0 | |
| Yes | 11 (2.3%) | 0.1 (0.0, 0.9) | 0.040 |
| Not recorded | 106 (22.5%) | 1.8 (1.2, 2.8) | 0.008 |
| Calcification | |||
| No | 275 (58.4%) | 1.0 | |
| Micro | 193 (41.0%) | 1.5 (1.1, 2.2) | 0.025 |
| Macro | 3 (0.6%) | 2656406.0 (0.0, Inf) | 0.977 |
| Color Doppler flow | |||
| No flow, minimal | 229 (48.7%) | 1.0 | |
| Moderate, abundant | 16 (3.4%) | 0.8 (0.3, 2.1) | 0.594 |
| Not recorded | 225 (47.9%) | 0.9 (0.6, 1.3) | 0.572 |
| BI-RADS | |||
| 0 | 1 (0.2%) | 1.0 | |
| 3 | 10 (2.1%) | 519489.2 (0.0, Inf) | 0.980 |
| 4a | 46 (9.8%) | 244902.1 (0.0, Inf) | 0.982 |
| 4b | 58 (12.3%) | 550047.4 (0.0, Inf) | 0.980 |
| 4c | 213 (45.2%) | 615529.3 (0.0, Inf) | 0.980 |
| 5 | 129 (27.4%) | 1672501.9 (0.0, Inf) | 0.979 |
| 6 | 14 (3.0%) | 1948084.7 (0.0, Inf) | 0.978 |
| Carcinoembryonic antigen | 4.9±27.4 | 1.1 (1.0, 1.2) | 0.005 |
| CA125 | 27.9±80.4 | 1.0 (1.0, 1.0) | 0.568 |
| CA153 | 24.8±114.7 | 1.0 (1.0, 1.0) | 0.003 |
| E-cadherin | |||
| Negative | 3 (0.6%) | 1.0 | |
| Positive | 43 (9.1%) | 4.6 (0.4, 55.5) | 0.228 |
| Not recorded | 425 (90.2%) | 1.8 (0.2, 19.8) | 0.640 |
| ER | |||
| Negative | 138 (29.3%) | 1.0 | |
| Positive | 274 (58.2%) | 1.1 (0.7, 1.7) | 0.626 |
| Not recorded | 59 (12.5%) | 0.7 (0.4, 1.3) | 0.311 |
| PR | |||
| Negative | 206 (49.8%) | 1.0 | |
| Positive | 208 (50.2%) | 0.7 (0.5, 1.0) | 0.050 |
| Her-2 | |||
| Negative | 301 (63.9%) | 1.0 | |
| Positive | 94 (20.0%) | 1.0 (0.6, 1.6) | 0.933 |
| Not recorded | 76 (16.1%) | 0.7 (0.4, 1.2) | 0.192 |
| Molecular subtype | |||
| Luminal A | 59 (12.5%) | 1.0 | |
| Luminal B | 215 (45.6%) | 1.5 (0.8, 2.6) | 0.187 |
| Her2 enriched | 52 (11.0%) | 1.6 (0.7, 3.4) | 0.228 |
| Triple negative | 73 (15.5%) | 1.3 (0.6, 2.5) | 0.523 |
| Not recorded | 72 (15.3%) | 1.0 (0.5, 2.1) | 0.937 |
| CK5/6 | |||
| Negative | 266 (56.5%) | 1.0 | |
| Positive | 78 (16.6%) | 0.4 (0.2, 0.7) | <0.001 |
| Not recorded | 127 (27.0%) | 0.9 (0.6, 1.4) | 0.683 |
| Ki67 | |||
| <40% | 212 (45.0%) | 1.0 | |
| ≥40% | 189 (40.1%) | 1.7 (1.2, 2.6) | 0.006 |
| Not recorded | 70 (14.9%) | 0.9 (0.5, 1.6) | 0.721 |
| P53 | |||
| Negative | 56 (11.9%) | 1.0 | |
| Positive | 266 (56.5%) | 1.4 (0.8, 2.5) | 0.246 |
| Not recorded | 149 (31.6%) | 1.5 (0.8, 2.8) | 0.206 |
| AR | |||
| Negative | 98 (20.8%) | 1.0 | |
| Positive | 54 (11.5%) | 0.4 (0.2, 0.8) | 0.011 |
| Not recorded | 319 (67.7%) | 0.8 (0.5, 1.3) | 0.363 |
| Tumor histologic grade | |||
| Carcinoma in situ | 29 (6.2%) | 1.0 | |
| Grade I | 1 (0.2%) | 0.0 (0.0, Inf) | 0.984 |
| Grade II | 110 (23.4%) | 15.1 (3.4, 66.4) | <0.001 |
| Grade III | 199 (42.3%) | 16.4 (3.8, 70.6) | <0.001 |
| Not recorded | 132 (28.0%) | 12.0 (2.7, 52.3) | <0.001 |
| Tumor histologic type | |||
| Carcinoma in situ | 7 (1.5%) | 1.0 | |
| Invasive in carcinoma | 441 (93.6%) | 5783984.7 (0.0, Inf) | 0.977 |
| Others type | 7 (1.5%) | 2303125.1 (0.0, Inf) | 0.979 |
| Not recorded | 16 (3.4%) | 5757812.8 (0.0, Inf) | 0.977 |
3.3Multiple logistic regression analysis of the association of clinicopathologic and US characteristics with axillary lymph node metastases in patients with breast cancer
Table 4 shows the results of the multivariate logistic regression that can predict the axillary lymph node metastases. hyperechoic halo, posterior acoustic decrease, microcalcification, Carcinoembryonic antigen, CA153, ck5/6, ki67≥40%, AR positive, tumor histologic grade II and III were significantly and independently associated with axillary lymph node metastases. A receiver operating characteristic curve was drawn, and area under the curve was 0.774 (Fig. 1). Figure 2 represents the typical “microcalcification” on ultrasound in breast cancer patient. Figure 3 represents the typical “posterior acoustic decrease” in ultrasound in breast cancer patient. Figure 4 represents the typical “hyperechoic halo” sign on ultrasound in breast cancer patient.
Table 4
Multiple logistic regression analysis of the association of clinicopathologic and US characteristics with axillary lymph node metastases in patients with breast cancer
| Feature | OR (95% CI) | P |
| Orientation | ||
| Horizontal | 1.0 | |
| Vertical | 2.6 (0.9, 7.4) | 0.080 |
| Hyperechoic halo | ||
| No | 1.0 | |
| Yes | 3.3 (1.2, 9.4) | 0.026 |
| Posterior acoustic decrease | ||
| No | 1.0 | |
| Yes | 0.1 (0.0, 0.9) | 0.040 |
| Calcification | ||
| No | 1.0 | |
| Micro | 1.5 (1.1, 2.2) | 0.025 |
| Macro | 2656406.0 (0.0, Inf) | 0.977 |
| Carcinoembryonic antigen | 1.1 (1.0, 1.2) | 0.005 |
| CA153 | 1.0 (1.0, 1.0) | 0.003 |
| PR | ||
| Negative | 1.0 | |
| Positive | 0.7 (0.5, 1.0) | 0.050 |
| CK5/6 | ||
| Negative | 1.0 | |
| Positive | 0.4 (0.2, 0.7) | <0.001 |
| Ki67 | ||
| Negative | 1.0 | |
| Positive | 1.7 (1.2, 2.6) | 0.006 |
| AR | ||
| Negative | 1.0 | |
| Positive | 0.4 (0.2, 0.8) | 0.011 |
| Tumor histologic grade | ||
| Carcinoma in situ | 1.0 | |
| Grade I | 0.0 (0.0, Inf) 0.984 | |
| Grade II | 15.1 (3.4, 66.4) | <0.001 |
| Grade III | 16.4 (3.8, 70.6) | <0.001 |
Fig. 1
Receiver operating characteristic curve for the predictive capacity of hyperechoic halo, posterior acoustic decrease, microcalcification, Carcinoembryonic antigen, CA153, Ck5/6, ki67≥40%, AR positive and tumor histologic grade II and III on axillary lymph node metastasis. AUC indicates area under the curve.
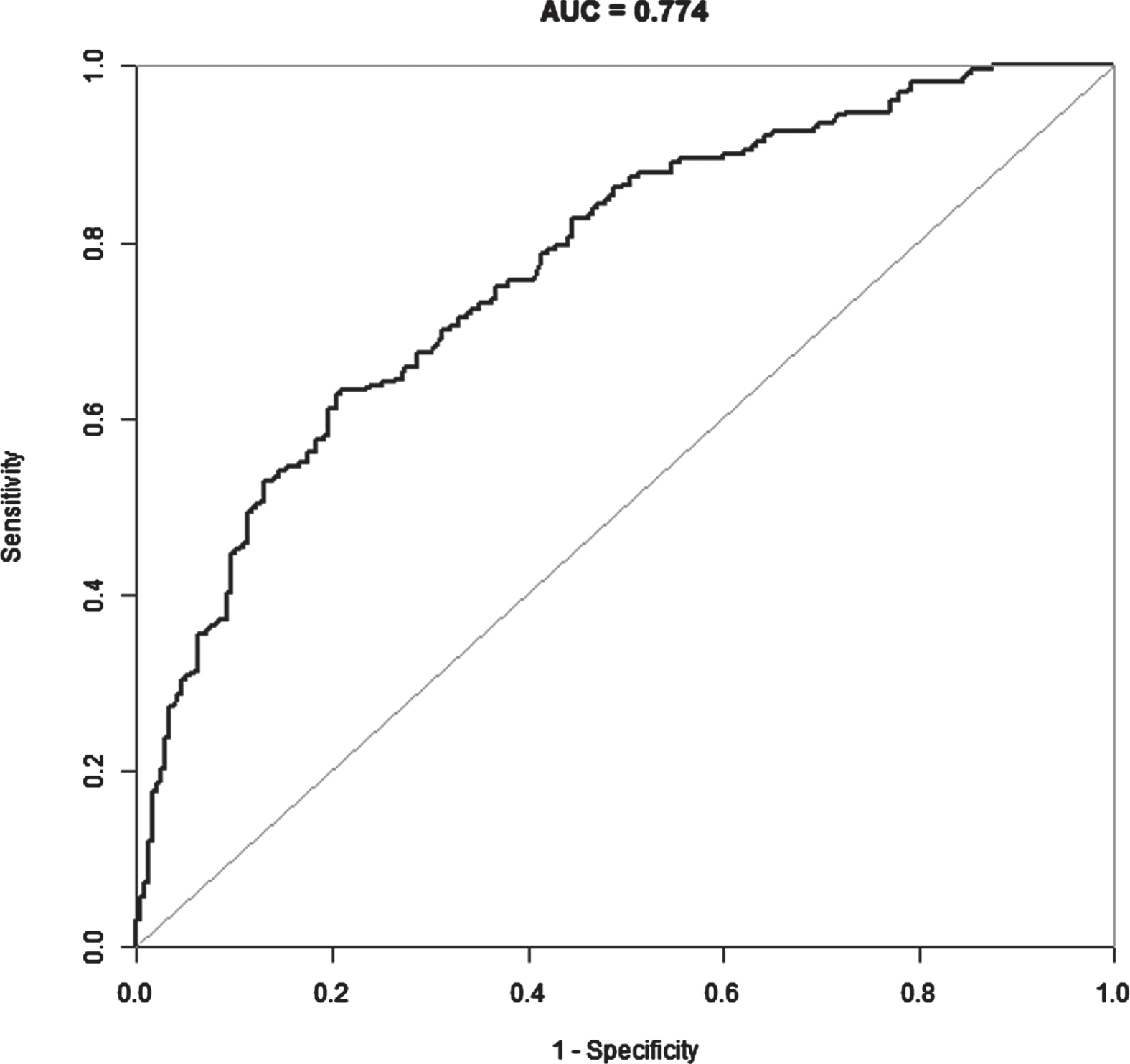
Fig. 2
(a) A 45-year-old female patient who had been diagnosed with an invasive carcinoma in the left breast. Ultrasound of left breast showing a hypoechoic nodule with a maximum diameter of 20 mm with ill defined margins, Internal microcalcifications are seen within the nodule. The patient had left axillary lymph node metastasis. Immunohistochemistry showed that ER(+80%), PR(+80%), AR(+80%), HER2(2+), CK5/6(-), P53(+30%), Ki67(+30%). FISH showed that amplification of the HER-2 gene was negative. (b) A 59-year-old female patient who had been diagnosed with a non-special invasive carcinoma grade II in the left breast. Ultrasound of left breast showing a hypoechoic nodule with a maximum diameter of 12 mm with ill defined margins, Internal microcalcifications are seen within the nodule. The patient had left axillary lymph node metastasis. Immunohistochemistry showed that ER(+90%), PR(+10%), HER2(1+), CK5/6(-), P53(+5%), AR(+20%), Ki67(+30%).
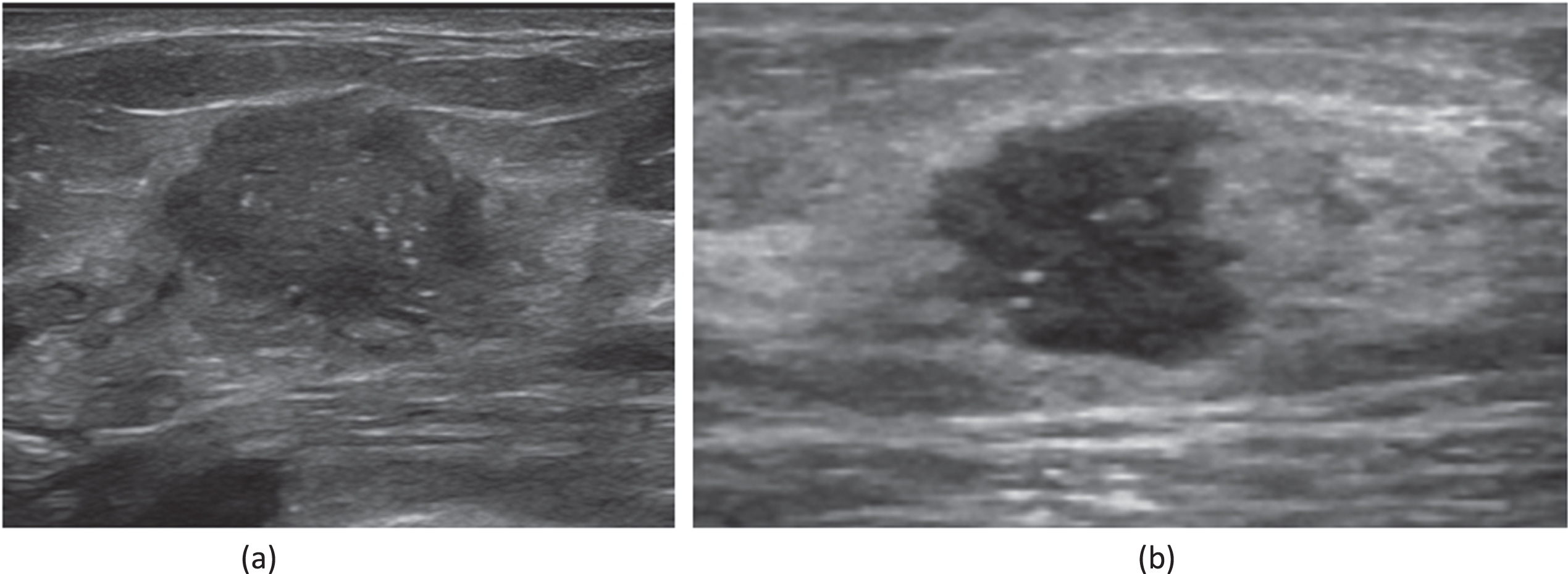
Fig. 3
(c) A 69-year-old female patient who had been diagnosed with an invasive carcinoma in the left breast. Ultrasound of left breast showing a hypoechoic nodule with a maximum diameter of 17 mm with ill defined margins, ultrasound attenuation in the posterior fields are seen within the nodule. The patient had left axillary lymph node metastasis. (d) A 66-year-old female patient who had been diagnosed with a non-special invasive carcinoma grade II in the left breast. Ultrasound of left breast showing a hypoechoic nodule with a maximum diameter of 28 mm with ill defined margins, ultrasound attenuation in the posterior fields are seen within the nodule. The patient had left axillary lymph node metastasis. Immunohistochemistry showed that ER(+80%), PR(+20%), HER2(0), KI67(+20%).
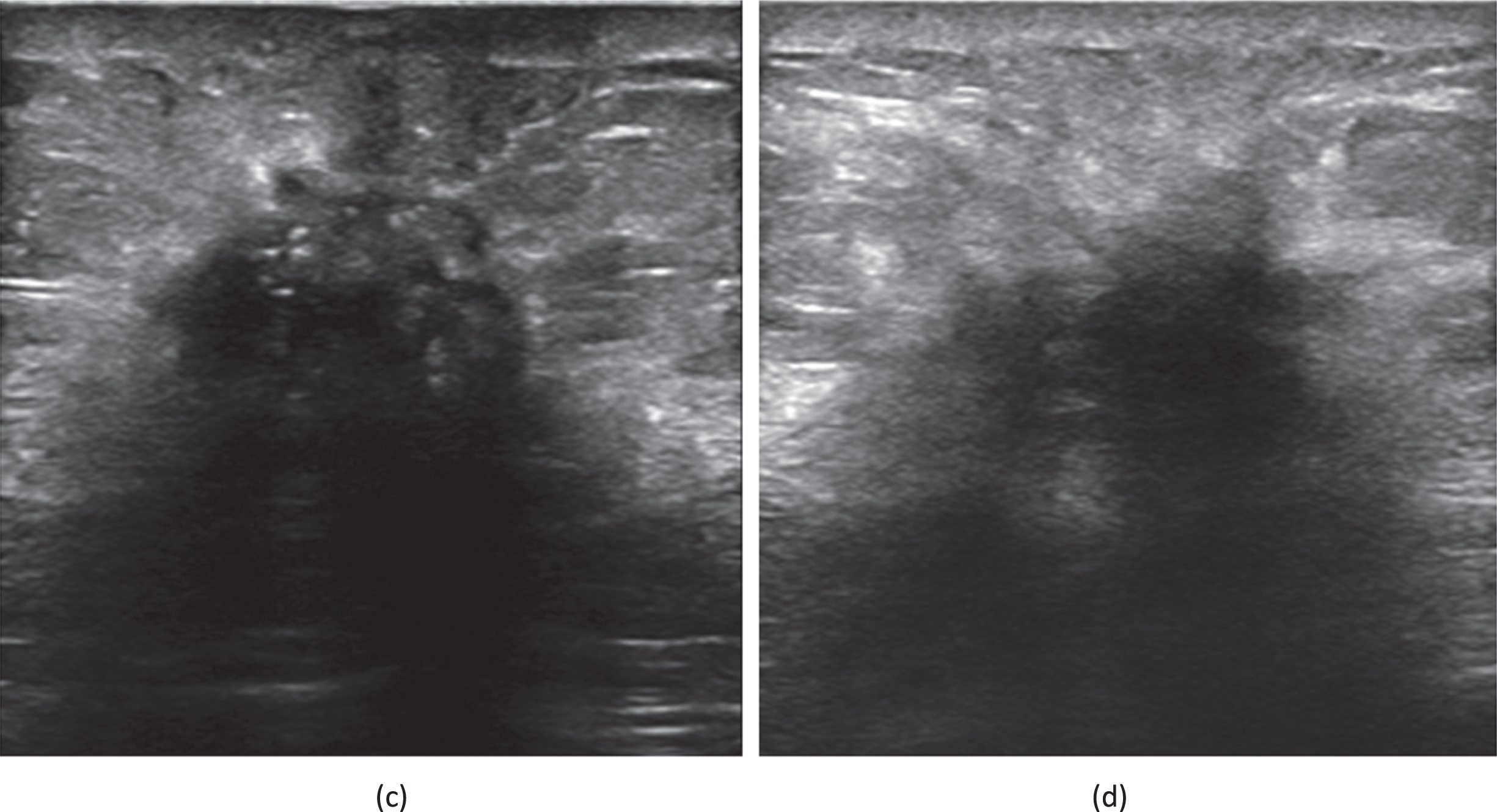
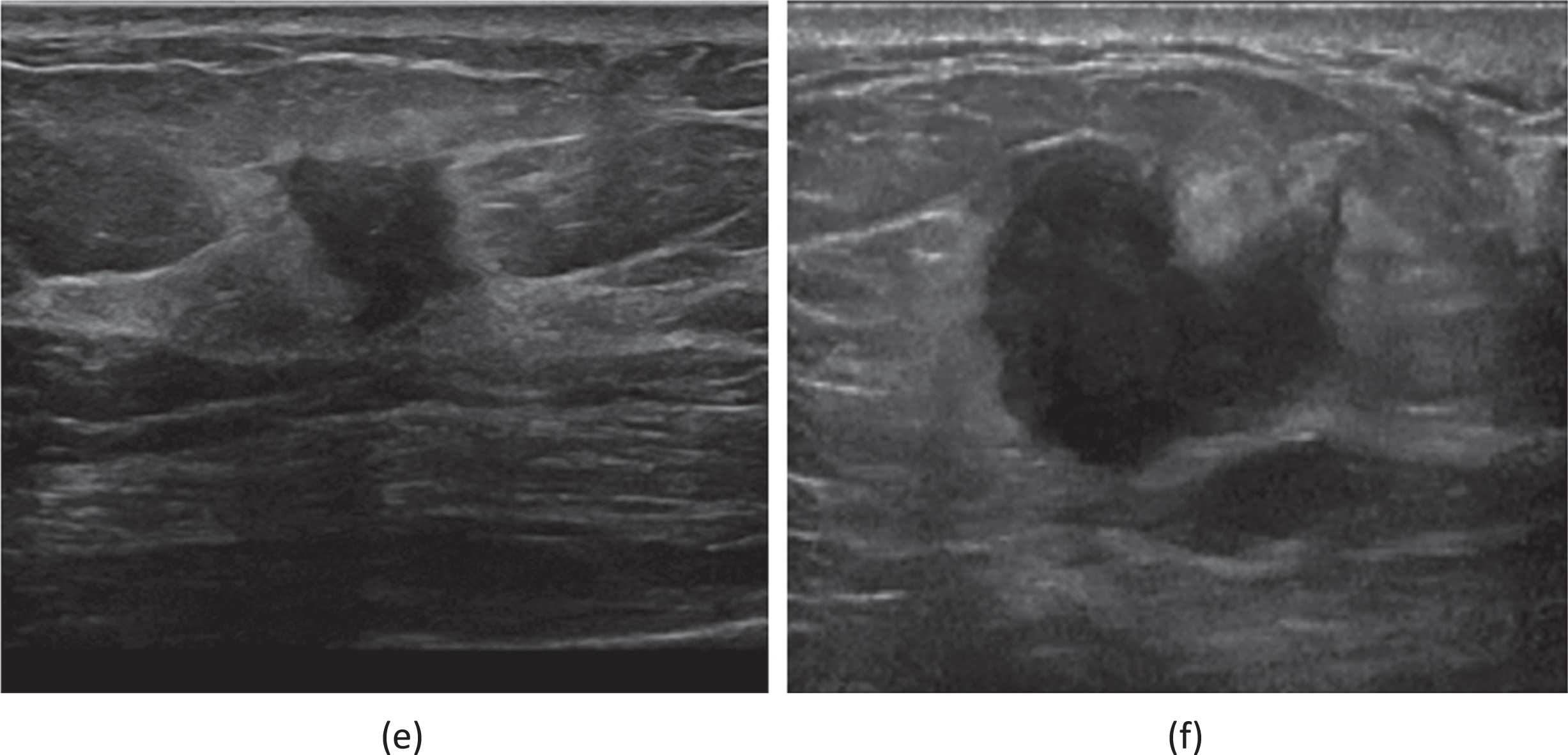
Fig. 4
(e) A 59-year-old female patient who had been diagnosed with an invasive carcinoma in the right breast. Ultrasound of right breast showing a hypoechoic nodule with a maximum diameter of 20 mm with ill defined margins, hyperechoic halo are seen within the nodule. The patient had right axillary lymph node metastasis. Immunohistochemistry showed that ER(+80%), PR(+5%), HER2(1+), CK5/6(-), P53(+80%), Ki67(+40%), AR(+10%). (f) A 62-year-old female patient who had been diagnosed a non-special invasive carcinoma grade II in the right breast. Ultrasound of right breast showing a hypoechoic nodule with a maximum diameter of 16 mm with ill defined margins, hyperechoic halo are seen within the nodule. The patient had right axillary lymph node metastasis. Immunohistochemistry showed that ER(+80%), PR(-),Her-2(0), Ki67(+50%).
3.4Limitations
Our study has some limitations that warrant mention. Firstly, it was a retrospective study and not a prospective study. Secondly, the number of axillary lymph node metastasis cases was not further split into different groups to discriminate the degree of metastasis. Finally, the sample size in our study was small, and a further analysis with more patients should be carried out.
4Discussion
In recent years, the early detection of breast cancer imaging is one of the important reasons for the significant decline in its mortality. Breast cancer has different TNM stages, and its treatment plan and prognosis are also very different. Axillary lymph node metastasis is an important factor affecting the prognosis of breast cancer patients. Therefore, it is particularly important to determine whether there is axillary lymph node metastasis in breast cancer patients early and correctly.
Ultrasound is the first choice to evaluate the condition of axillary lymph nodes in patients with breast cancer, which can accurately reflect the status of axillary lymph node metastasis. When axillary lymph node metastasis occurs in breast cancer, the criteria are asymmetric cortical thickening of axillary lymph nodes, homogeneous hypoechoic in lymph nodes, disappearance of lymph node hilum, and increase of peripheral blood flow. Asymmetric thickening of the axillary lymph node cortex is considered a characteristic morphological change of early metastasis.
However, in some patients with axillary lymph node metastasis of breast cancer, the ultrasonographic image lacks the above characteristic metastasis signs. Therefore, the author retrospectively analyzed the cancer focus of breast cancer patients without characteristic lymph node metastasis signs. Two dimensional ultrasound image features are expected to provide valuable information for preoperative lymph node status of breast cancer patients.
The axillary lymph node status is an important prognostic factor in patients with breast cancer [13]. In this study, we evaluated the value of using clinicopathologic and US characteristics of breast cancers in clinical practice to predict the axillary lymph node metastases. The results showed that a lesion with US features of hyperechoic halo, posterior acoustic decrease and microcalcification were significantly and independently associated with axillary lymph node metastases. The analysis of the clinicopathological characteristics also demonstrated that CA153, ck5/6, ki67≥40%, and AR expression was correlated with axillary lymph node metastasis. Histological grade II and III is a risk factors for axillary lymph node metastases. Our study also indicated that Carcinoembryonic antigen and CA153 expression is correlated with axillary lymph node metastasis.
In our study, we found that elevated expression of Ki67 (≥40%) has been associated with axillary lymph node metastasis. This result is consistent with findings of previous study. Ki67 is a Cell Proliferation Index, Ki67 correlates with the mitotic index and has been used in breast cancer as a prognostic marker and high invasiveness.
In our study, we also found that tumor histologic grade II and III were significantly and independently associated with axillary lymph node metastases. The histological grading system in breast cancer is based on differentiation of tumor cells, which is an important factor in predicting prognosis of breast cancer patients and tumor aggressiveness. Previous studies showed the higher histological grade were associated with axillary lymph node metastasis and the poor prognosis [14].
Contrast-enhanced ultrasound (CEUS) is a highly accurate, non-invasive, and effective examination method that utilizes the state of microcirculation perfusion to reflect the circulatory status of tissues and lesions. It can predict axillary lymph node metastasis based on the development mode of different lesions.
CEUS is based on two-dimensional ultrasound and improves the diagnostic value of lymph node properties to some extent by real-time dynamic imaging of the microcirculation inside lymph nodes. It can serve as a good supplement to preoperative examination and help us better evaluate the status of ALN before surgery.
Ultrasonic elastography is affected by many factors, such as biomechanics and Linear elasticity. Combined with digital imaging technology, it can more accurately evaluate the internal anatomical structure of the tested tissue, and then feedback the internal elastic modulus index of the tissue, so as to achieve quantitative analysis of the degree of softness and hardness of the tissue, which has an ideal guiding value for identifying lymph node metastasis.
In future studies, we will add contrast-enhanced ultrasound and elastography to predict axillary lymph node metastasis in breast cancer patients.
In conclusion, The combination of ultrasound features, tumor markers, pathology, and immunohistochemistry can predict axillary lymph node metastasis in breast cancer patients. The results of this study show that the US feature hyperechoic halo, posterior acoustic decrease and microcalcification were significantly correlated with axillary lymph node metastasis. Therefore, tumor US features should be taken into account for additional determination of axillary lymph node metastasis in patients with breast cancer.
Authors’contributions
BXF contributed to data collection, analysis and writing of the manuscript. RLT contributed to study design and writing of the manuscript. ZQL contributed to revise the manuscript. The author(s) read and approved the final manuscript.
Disclosures
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
[1] | Sung H , et al. Global Cancer Statistics GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. (2021) ;71: (3):209–49. |
[2] | Akissue de Camargo Teixeira P , et al. Axillary Lymph Node Sonographic Features and Breast Tumor Characteristics as Predictors of Malignancy: A Nomogram to Predict Risk. Ultrasound Med Biol. (2017) ;43: (9):1837–45. |
[3] | Huang TW , et al. Recommendation for axillary lymph node dissection in women with early breast cancer and sentinel node metastasis: A systematic review and meta-analysis of randomized controlled trials using the GRADE system. Int J Surg. (2016) ;34: :73–80. |
[4] | Lucci A , et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. (2007) ;25: (24):3657–63. |
[5] | Maeseele N , et al. Axillary lymph node dissection on the run? Facts Views Vis Obgyn. (2017) ;9: (1):45–9. |
[6] | Yun SJ , Sohn YM , Seo M . Risk Stratification For Axillary Lymph Node Metastases in Breast Cancer Patients: What Clinicopathological and Radiological Factors of Primary Breast Cancer Can Predict Preoperatively Axillary Lymph Node Metastases? Ultrasound Q. (2017) ;33: (1):15–22. |
[7] | Han L , et al. Radiomic nomogram for prediction of axillary lymph node metastasis in breast cancer. Eur Radiol. (2019) ;29: (7):3820–9. |
[8] | Abass MO , et al. Axillary Lymph Node Dissection for Breast Cancer: Efficacy and Complication in Developing Countries. J Glob Oncol. (2018) ;4: :1–8. |
[9] | Burkett BJ , Hanemann CW . A Review of Supplemental Screening Ultrasound for Breast Cancer: Certain Populations of Women with Dense Breast Tissue May Benefit. Acad Radiol. (2016) ;23: (12):1604–9. |
[10] | Duan Y , Zhu Y , Nie F , Guan L , Jia Y , Chen K , Wang W . Predictive value of combining clinicopathological, multimodal ultrasonic characteristics in axillary lymph nodal metastasis burden of patients with cT1-2N0 breast cancer. Clin Hemorheol Microcirc. (2022) ;81: (3):255–69. |
[11] | Chen J , Li CX , Shao SH , Yao MH , Su YJ , Wu R . The association between conventional ultrasound and contrast-enhanced ultrasound appearances and pathological features in small breast cancer. Clin Hemorheol Microcirc. (2022) ;80: (4):413–22. |
[12] | Shi XQ , Dong Y , Tan X , Yang P , Wang C , Feng W , Lin Y , Qian L . Accuracy of conventional ultrasound, contrast-enhanced ultrasound and dynamic contrast-enhanced magnetic resonance imaging in assessing the size of breast cancer. Clin Hemorheol Microcirc. (2022) ;82: (2):157–68. |
[13] | Guo Q , et al. Ultrasound Features of Breast Cancer for Predicting Axillary Lymph Node Metastasis. J Ultrasound Med. (2018) ;37: (6):1354–1353. |
[14] | Qiu SQ , et al. A nomogram to predict the probability of axillary lymph node metastasis in early breast cancer patients with positive axillary ultrasound. Sci Rep. (2016) ;6: :21196. |




