Association of coagulation factors profile with clinical outcome in patient with COVID-19 and acute stroke: A second wave cohort study
Abstract
INTRODUCTION:
The second wave of COVID-19 in Indonesia occurred due to delta variant transmission with up to 2266 cases. This variant could cause higher rate of morbidities and mortalities. This study reported coagulation profile of COVID-19 patients with acute stroke and its association with patients’ outcome.
METHOD:
This is a cohort-retrospective study conducted during the second wave of COVID-19, June-August 2021 in Cipto Mangunkusumo General Hospital. Inclusion criteria were adult patients with confirmed COVID-19 and diagnosed with acute stroke confirmed by radiological evidences. Exclusion criteria were COVID-19 patients with prior diagnosis of acute stroke. Coagulation factors were analyzed and presented with tables and graphs.
RESULTS:
A total of 33 patients included in this study with majority experienced ischemic stroke (84.8%), followed by ischemic with haemorrhagic transformation (9.1%), and the rest with haemorrhagic stroke. The median of fibrinogen and D-dimer was 487.1(147–8,943)mg/dL and 2,110(250–35,200)ug/L respectively. Prothrombin time (PT) ratio was 0.95(0.82–1.3) and activated partial thromboplastin time (APTT) ratio was 1.01(0.64–2.72). On observation, 33.3% died during hospitalization, D-dimer value in these patients was significantly higher with 9,940ug/L compared to those who survived with 1,160ug/L(p = 0.009). The highest D-dimer value during hospitalization was also significantly higher with the median of 14,395ug/L compared to 3,740 ug/L (p = 0.014).
DISCUSSION:
D-dimer value on initial assessment and its highest value during hospitalization were significantly higher in patient with poor outcome, showing that D-dimer can be one predictor of mortality in COVID-19 patients with acute stroke
1Introduction
The World Health Organization (WHO) proclaimed the COVID-19 pandemic in 2020. It was caused by infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which has a significant fatality rate worldwide, reaching 4.9 million [1, 2]. At the end of 2020, a new variety, the B.1.617.2 variant, commonly known as the delta variant, was detected. This variation was discovered in India and has since spread worldwide, resulting in the pandemic’s second wave. As a result, the WHO has designated this variety as one of the variants of concern (VOC) [3]. Numerous studies indicate that this variety increases symptoms, complications, and death and is more easily transmitted [4, 5]. In Indonesia, the pandemic’s second wave occurred between June and August 2021, with the delta variant suspected of being the primary cause, accounting for 2266 cases [6].
Systemic inflammation impacts have been observed to cause coagulation abnormalities in individuals with COVID-19 by systemic activation of the coagulation cascade. This can result in coagulopathy, disseminated intravascular coagulation (DIC), cerebrovascular strokes, pulmonary embolism, and multisystem failure [7–9]. This condition is indicated by a rise in the patient’s coagulation factor values, such as fibrinogen and D-dimer [10, 11]. Additionally, cells infected with COVID-19 are considered depleted of the ACE2 protein, resulting in an extracellular buildup of angiotensin II (AT II). Due to its interaction with platelets and endothelial cells, this buildup will also develop clots. Proinflammatory substances such as IL-6, TNF-a, and AT II activation have also damaged microvascular endothelial cells. They activate tissue factors (TF), further strengthening the coagulation cascade and perhaps forming microthrombus in tiny blood vessels [12, 13].
As previously established, activation of the coagulation cascade causes cerebrovascular events, one of which is acute stroke. Increased fibrinogen and D-dimer levels are established predictors of stroke in individuals with COVID-19. Additionally, multiple researches has stated that elevated coagulation markers might be a predictor of patient death in patients with COVID-19. D-dimer levels of more than 1000 ng/mL were identified as a risk factor for mortality. Additionally, substantial variations in fibrinogen and prothrombin time (PT) levels have been documented between patients who died and those who did not [14, 15].
Numerous case reports and research have established that increased coagulation parameters are associated with the development of cerebrovascular events such as ischemic stroke and mortality in patients with COVID-19. However, the importance of this parameter in mortality, particularly in COVID-19 patients who also have an acute stroke, has not been widely reported, particularly in individuals in the second wave of the COVID-19 pandemic, when the delta variation accounts for the majority of variants discovered. This study aims to examine the coagulation profile of COVID-19 patients who were also diagnosed with acute stroke at Cipto Mangunkusumo General Hospital Jakarta during the second wave of the pandemic and determine how it influences patient outcomes.
2Material and methods
This retrospective cohort study was conducted in the Cipto Mangunkusumo Hospital, Faculty of Medicine, the University of Indonesia, from June 2021 to August 2021, during the second wave of the COVID-19 pandemic. The Ethics Committee approved the experimental protocols of the Faculty of Medicine, the University of Indonesia, with protocol number 917/ UN2.F1/ETIK/PPM.00.02/2021 in May 2021. The study was undertaken with the understanding and written consent of each subject. The study conforms to The Code of Ethics of the World Medical Association (Declaration of Helsinki) [16].
Total sampling was used to determine the sample size, considering the inclusion and exclusion criteria. Adult patients > 18 years of age who were diagnosed with COVID-19 with positive PCR swab findings, (3) those diagnosed with acute stroke with confirmation of radiological examination results, and (4) Willing to participate in this investigation were included in this study. Incomplete data and patients diagnosed with ischemic stroke prior to the diagnosis of COVID-19 were excluded from this investigation.
The study collected data on patients’ demographic, clinical, laboratory, and radiological characteristics. A comprehensive coagulation factor examination is conducted when a patient is hospitalized for the first time or is diagnosed with acute stroke for the first time. Several repeated assessments of coagulation factors, particularly D-dimer, were also included in the recording of this investigation throughout patient therapy. The outcomes examined in patients were death in care, deemed a negative outcome, and survival throughout treatment and outpatient therapy, deemed positive outcomes.
Data was collected using Microsoft Excel and placed on the main table (Microsoft Corp, Redmond, WA, USA). The tabulated data were processed and shown using SPSS version 20.0 (IBM Corp, Armonk, NY, USA). The statistical analysis was done using the unpaired t-test and Mann-Whitney test.
3Results
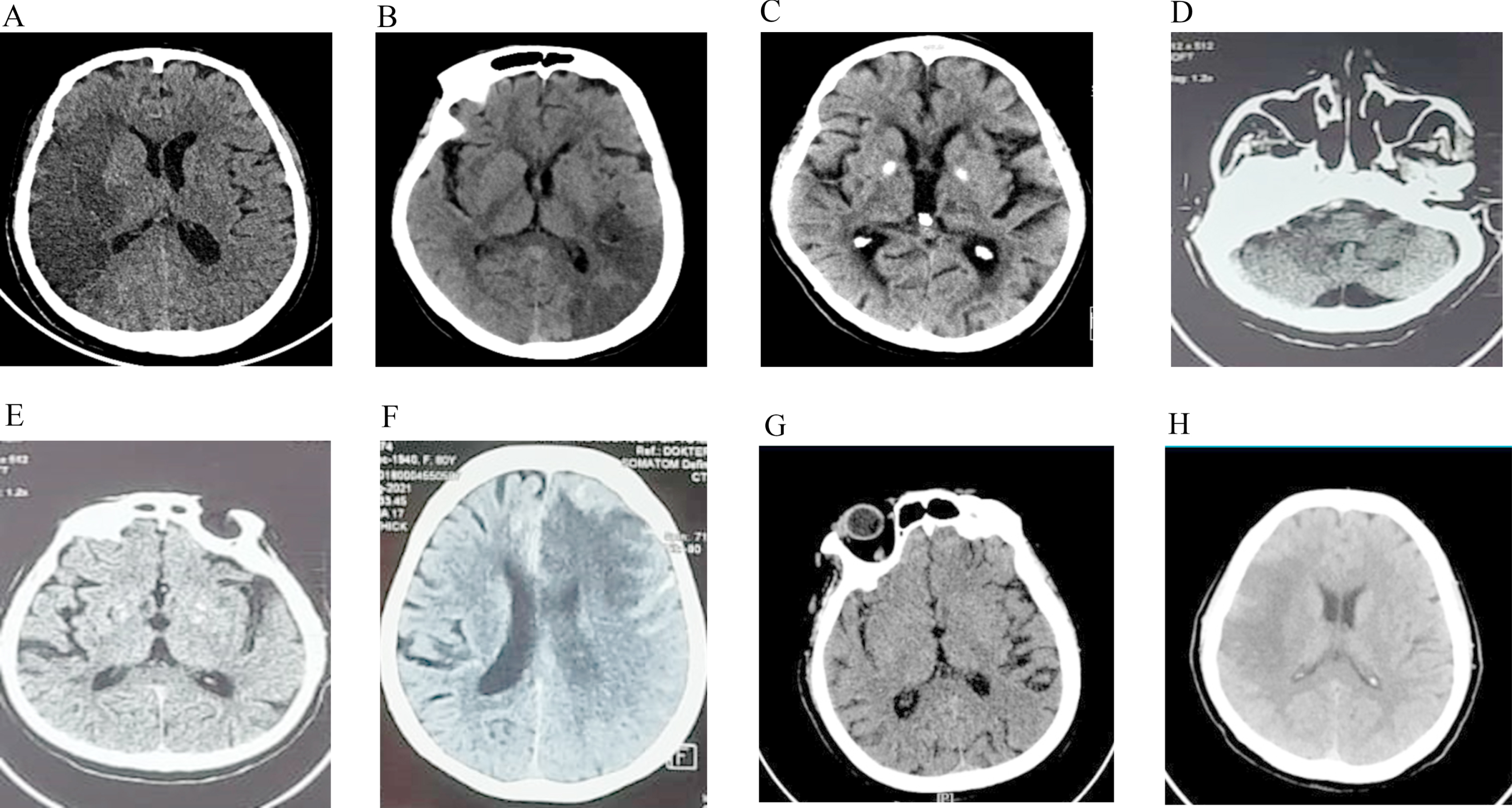
This research included a total of 33 people. Table 1 summarizes the characteristics of all participants. Most individuals were male, with a mean age of 63.45 years. 84.8% of participants had an ischemic stroke, 9.1% had hemorrhagic transformation ischemic stroke, and the remainder had a hemorrhagic stroke. Figure 1 summarizes the radiological images most representative of the patient. The coagulation levels evaluated were fibrinogen, d-dimer, haemoglobin, platelets, as well as PT and APTT, which were performed at the time of the patient’s first diagnosis or early admission.
Table 1
Clinical characteristics (n = 33)
| Characteristics | n | % |
| Gender | ||
| Male | 19 | 57.60 % |
| Female | 14 | 42.40 % |
| Age (Mean±SD) | 63.45 + 10.66 | |
| Type of Stroke | ||
| Ischemic stroke | 28 | 84.80 % |
| Hemorrhagic Stroke | 2 | 6.10 % |
| Ischemic stroke with hemorrhagic transformation | 3 | 9.10 % |
| CT -scan infarct extension in regions | ||
| (some cases were overlapped) | ||
| Frontal, parietal, temporal, occipital | 2 | 5.13% |
| Frontal, parietal, temporal | 3 | 7.69% |
| Frontal, parietal | 4 | 10.26% |
| Occipital, temporal | 2 | 5.13% |
| Parietal | 6 | 15.38% |
| Thalamus | 6 | 15.38% |
| Basal Ganglia | 14 | 35.89% |
| Pons, Cerebellum | 2 | 5.13% |
| Patient Outcome | ||
| Discharged | 22 | 66.66% |
| Dead | 11 | 33.33% |
| Initial Fibrinogen (Median, min-max) | 487.10 (147–8,943) | |
| Initial D-dimer (Median, min-max) | 2 110 (250–35,200) | |
| Hb (Mean±SD) | 13.49 (±2.51) | |
| Thrombocyte count (Mean±SD) | 255,392 (±105,431) | |
| PT ratio (Median, min-max) | 0.95 (0.82–1.30) | |
| APTT ratio (Median, min-max) | 1.01 (0.64–2.72) |
SD: standard deviation; PT: prothrombin time; APTT: activated partial thromboplastine time; Hb: Hemoglobin.
Fig. 1
Representative image of the CT-scan infarct extension region. A: frontal, parietal, temporal, occipital; B: parietal; C: thalamus; D: pons and cerebellum; E: basal ganglia; F: frontal, temporal, parietal: G: oksipital, temporal; H: frontal, parietal.
Additionally, the greatest d-dimer level is observed while the patient is hospitalized. Hb, platelets, and PT data have normal distributions and are shown as means with standard deviations. However, the values of other components are not normally distributed and are displayed in median form with maximum and lowest values.
A total of 33.3% of patients died (poor outcome) in treatment, and the rest were alive and outpatient (favourable outcome). The difference in coagulation variables between patients with poor and favourable outcomes is shown in Table 2 and is examined based on their distribution. As can be shown, clinical and statistical differences exist between d-dimer levels at initial diagnosis in individuals with poor vs favourable outcomes (p = 0.09). Additionally, a significant difference in the maximum levels of d-dimer during hospitalization was observed (p = 0.14).
Table 2
Comparison of coagulation factor profiles and patient outcomes (n = 33)
| Coagulation Factors | Outcome | P-value | |
| Profile | Discharged | Dead | |
| Hb | 12.82 (2.43) | 14.44 (2.32) | 0.092* |
| Thrombocyte count | 261,850 (122,661) | 222,700 (55,377) | 0.348* |
| Patient PT | 11.01 (0.83) | 11.64 (1.48) | 0.145* |
| PT ratio | 0.95 (0.07) | 1.01 (0.13) | 0.124* |
| Patient APTT | 31.9 (23.9–87.7) | 31.9 (23.3–36.0) | 0.218# |
| APTT ratio | 1.03 (0.75–2.72) | 0.96 (0.64–1.08) | 0.124# |
| Initial Fibrinogen | 487.1 (238.1–2,809) | 555.0 (147.0–8943.0) | 0.507# |
| Initial D-dimer | 1,190 (250–26,680) | 9,940 (1 090–35,00) | 0.009# |
| Maximum in-hospital D-dimer | 3,740 (250–35,200) | 14,395 (2,120–35,200) | 0.014# |
PT: prothrombin time; APTT: activated partial thromboplastin time; Hb: Hemoglobin. Statistical analysis was performed using the *Unpaired t-test or #Mann-Whitney. p-value less than 0.05 is considered statistically significant.
4Discussion
This study enrolled 33 patients, the majority of whom were male, with a mean age of 63.45 years. The age range of patients entering the geriatric age category (>60 years) is comparable to that reported by Qureshi et al. in a large-scale multicenter retrospective cohort analysis from December 2019 to April 2020 [17]. There was a substantial difference in mean age between COVID-19 patients who did not have a stroke and those who did, with the patient who had a stroke having an average age of 68.8 years [17]. According to these findings, people with COVID-19 who have had a stroke are above 60.
The most common stroke among COVID-19 patients was an ischemic stroke, followed by ischemic stroke with bleeding transformation and hemorrhagic stroke. This is consistent with a comprehensive review published in 2020 by Bhatia et al., which found that 84.8% of COVID-19 patients who had a stroke had an ischemic stroke. Meanwhile, cerebral haemorrhage, including subarachnoid haemorrhage (SAH) and intracranial haemorrhage (ICH), occurs in around 7% of individuals [18]. The pathophysiology of stroke in COVID-19 is mostly due to activation of the coagulation cascade, which results in blood vessel thrombosis. On the other hand, the pathophysiology of hemorrhagic stroke in COVID-19 patients has not been well investigated in investigations. One element is the SARS-CoV 2 virus’s affinity for the ACE 2 receptor. ACE 2 receptors are also located on endothelial cells and arterial smooth muscle cells in the brain. Its activation by this virus can cause vessel wall damage and rupture, resulting in hemorrhagic stroke [14, 19]. Additionally, excessive cytokine activation during COVID-19 infection might result in capillary and blood-brain barrier disruption, resulting in blood vessel rupture [14].
Additionally, individuals with ischemic stroke bleeding transformation were identified in this investigation. Although this variety is less often reported, examples have been discovered. Patients with ischemic stroke who also have endothelial impairment may be prone to this hemorrhagic transition, it is postulated. Additionally, consumptive coagulopathy is believed to contribute to the development of this hemorrhagic metamorphosis [20].
Clinically and statistically significant differences were discovered between D-dimer levels at the stroke diagnosis and the highest D-dimer levels during therapy and patient outcome in this investigation. Patients who died had significantly higher D-dimer levels at first diagnosis, 9940ug/L, than patients who lived, 1160ug/L (p = 0.009). Additionally, the maximum D-dimer level throughout therapy was 14395ug/L in patients who died, compared to 3740ug/L in survivors (p = 0.014).
D-dimer levels have been extensively studied to measure coagulation activation in COVID-19 patients. D-dimer results from fibrin breakdown and may be detected in the circulation following the fibrinolysis of blood clots. D-dimer is a marker or indicator of coagulation activity and thrombus development. The greatly enhanced activation of proinflammatory cytokines (IL-2, IL-6, IL-8, IL-17, and TNF-) plays a critical role in activating the coagulation cascade in COVID-19. Sufficient anti-inflammatory proteins do not accompany this activation in the circulation to initiate the coagulation cascade and result in widespread thrombus formation. Subsequent thrombus breakdown results in a noticeable rise in the amount of D-dimer [21, 22].
Before the COVID-19 pandemic, D-dimer levels were often employed as markers of pulmonary embolism, venous thromboembolism, and disseminated intravascular coagulation (DIC) but were never utilized to measure viral or bacterial infection severity. However, because COVID-19 is caused by activation of the coagulation cascade, D-dimer has been one of the most investigated indicators and has been shown to have a substantial correlation with the severity of the illness. Additionally, some studies have shown that D-dimer can be used to predict death during therapy with a D-dimer cut-off of > 2000 mg/mL [23, 24]. Patients with D-dimer readings greater than this cut-off point had an odds ratio of 10.17 (95% CI 1.10–94.38) for death. Another research conducted in India had a different cut-off of 1440 mg/mL [25]. Although a comprehensive analysis published in 2020 discovered a strong, consistent association between D-dimer levels and poor prognosis in patients, the cut-off values used in the research varied.
Studies examining this association in people infected during the second wave remain underreported. The second wave, which began in 2021, was dominated by delta variant transmission. According to research conducted in India, the severity of COVID-19 infection and the fatality rate rose during the pandemic’s second wave [5]. However, this study also found that patients’ mean D-dimer levels were lower during the second wave [5].
Acknowledgments
None.
Conflict of interest
The authors declare that there is no conflict of interest.
Funding
No funding to be declared.
Data Statement
All data generated or analyzed during the study are included in this published article.
References
[1] | Zhu N , Zhang D , Wang W , Li X , Yang B , Song J , et al., A Novel Coronavirus from Patients with Pneumonia in China, N Engl J Med (2020) ;382: (8):727–33. |
[2] | Dong E , Du H , Gardner L , An interactive web-based dashboard to track COVID-19 in real time, Lancet Infect Dis (2020) ;20: (5):533–4. |
[3] | Rocha ICN , Goyal S , Rackimuthu S , Jain S . SARS-CoV-2 variants of concern: implications on the second wave of COVID-19 in India, Infez Med (2021) ;29: (3):492–4. |
[4] | Tareq AM , Emran TB , Dhama K , Dhawan M , Tallei TE , Impact of SARS-CoV-2 delta variant (B, 1.617.2) in surging second wave of COVID-19 and efficacy of vaccines in tackling the ongoing pandemic. Hum Vaccin Immunother (2021) ;17: (11):4126–7. |
[5] | Khedar RS , Mittal K , Ambaliya HC , Mathur A , Gupta JB , Sharma KK , et al., Greater Covid-19 Severity and Mortality in Hospitalized Patients in Second (Delta Variant) Wave Compared to the First: Single Centre Prospectiv e Study in India. medRxiv. 2021:2021.09.03.21263091. |
[6] | Song H , Fan G , Liu Y , Wang X , He D , The Second Wave of COVID-19 in South and Southeast Asia and the Effects of Vaccination, Front Med (Lausanne) (2021) ;8: , 773110. |
[7] | Watson O , Pillai S , Howard M , Cezar-Zaldua J , Whitley J , Burgess B , et al., Impaired fibrinolysis in severe Covid-19 infection is detectable in early stages of the disease. LID - 10.3233/CH-221491 [doi]. (1875-8622 (Electronic)). |
[8] | Tripolino C , Pizzini AM , Zaccaroni S , Cicognani C , Dapporto S , Cipollini ML , et al., Is SARS-CoV-2 infection an emerging risk factor for splanchnic venous thrombosis? Clin Hemorheol Microcirc (2021) ;79: (2):347–55. |
[9] | Jung EM , Stroszczynski C , Jung F , Contrast enhanced ultrasonography (CEUS) to detect abdominal microcirculatory disorders in severe cases of COVID-19 infection: First experience, Clin Hemorheol Microcirc (2020) ;74: (4):353–61. |
[10] | Xing Y , Yang W , Jin Y , Wang C , Guan X D-dimer daily continuous tendency predicts the short-term prognosis for COVID-19 independently: A retrospective study from Northeast China. (1875-8622 (Electronic)). |
[11] | Alzoughool F , Alanagreh L , Abumweis S , Atoum M , Cerebrovascular comorbidity, high blood levels of C-reactive protein and D-dimer are associated with disease outcomes in COVID-19 patients, Clin Hemorheol Microcirc (2021) ;77: (3):311–22. |
[12] | Eslamifar Z , Behzadifard M , Soleimani M , Behzadifard S , Coagulation abnormalities in SARS-CoV-2 infection: overexpression tissue factor, Thromb J (2020) ;18: (1):38. |
[13] | Martín-Rojas RM , Pérez-Rus G , Delgado-Pinos VE , Domingo-González A , Regalado-Artamendi I , Alba-Urdiales N , et al., COVID-19 coagulopathy: An in-depth analysis of the coagulationsystem, Eur J Haematol (2020) ;105: (6):741–50. |
[14] | Spence JD , de Freitas GR , Pettigrew LC , Ay H , Liebeskind DS , Kase CS ,et al., Mechanisms of Stroke in COVID-19, Cerebrovascular Diseases (2020) ;49: (4):451–8. |
[15] | Xiang G , Hao S , Fu C , Hu W , Xie L , Wu Q , et al., The effect of coagulation factors in novel coronavirus patients: A systematic review and meta-analysis, Medicine (Baltimore) (2021) ;100: (7):e24537. |
[16] | Rickham PP HUMAN EXPERIMENTATION. CODE OF ETHICS OF THE WORLD MEDICAL ASSOCIATION. DECLARATION OF HELSINKI. Br Med J. (1964) ;2: (5402):177. |
[17] | Qureshi AI , Baskett WI , Huang W , Shyu D , Myers D , Raju M , et al., Acute Ischemic Stroke and COVID- An Analysis of 27 676 Patients, Stroke (2021) ;52: (3):905–12. |
[18] | Bhatia R , Pedapati R , Komakula S , Srivastava MVP , Vishnubhatla S , Khurana D , Stroke in Coronavirus Disease A Systematic Review, J Stroke (2020) ;22: (3):324–35. |
[19] | Carod-Artal FJ , Neurological complications of coronavirus and COVID-19, Rev Neurol (2020) ;70: (9):311–22. |
[20] | Valderrama EV , Humbert K , Lord A , Frontera J , Yaghi S Severe Acute Respiratory Syndrome Coronavirus 2 Infection and Ischemic Stroke, Stroke (2020) ;51: (7):e124–e7. |
[21] | Paliogiannis P , Mangoni AA , Dettori P , Nasrallah GK , Pintus G , Zinellu A , D-Dimer Concentrations and COVID-19 Severity: A Systematic Review and Meta-Analysis. Frontiers in Public Health. 2020;8. |
[22] | Poudel A , Poudel Y , Adhikari A , Aryal BB , Dangol D , Bajracharya T , et al., D-dimer as a biomarker for assessment of COVID-19 prognosis: D-dimer levels on admission and its role in predicting disease outcome in hospitalized patients with COVID-19, PLOS ONE (2021) ;16: (8):e0256744. |
[23] | Yao Y , Cao J , Wang Q , Shi Q , Liu K , Luo Z , et al., D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: a case control study, Journal of Intensive Care (2020) ;8: (1):49. |
[24] | Zhang L , Yan X , Fan Q , Liu H , Liu X , Liu Z , et al., D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19, J Thromb Haemost (2020) ;18: (6):1324–9. |
[25] | Soni M , Gopalakrishnan R , Vaishya R , Prabu P , D-dimer level is a useful predictor for mortality in patients with COVID- Analysis of 483 cases, Diabetes Metab Syndr (2020) ;14: (6):2245–9. |




