Is SARS-CoV-2 infection an emerging risk factor for splanchnic venous thrombosis?
Abstract
OBJECTIVE:
Thrombosis represents one of the most feared complications of the COronaVIrus Disease-2019 (COVID-19). Although pulmonary embolism and deep venous thrombosis are the most described complications, some investigations reported thrombotic localization also in the splanchnic venous district.
METHODS:
We describe the case of a patient with SARS-CoV-2 infection presenting with abdominal pain and diagnosed with portal vein thrombosis. In addition, we shortly review available literature supporting the possible role of COVID-19 as leading cause of splanchnic venous thrombosis.
RESULTS:
After in-depth diagnostic workup, we excluded the commonest causes of portal thrombosis and concluded that SARS-CoV-2 infection represented the main explanation of this finding.
CONCLUSIONS:
Our study warns the clinicians to maintain a high index of suspicion for thrombosis in patients diagnosed with SARS-CoV-2 infection manifesting gastrointestinal symptoms. An appropriate diagnostic work-up could allow to obtain an early diagnosis and consequently improve the clinical outcome of patients.
1Introduction
Systemic hypercoagulable state and coagulation disorders represent the hallmark of the COronaVIrus Disease-2019 (COVID-19) [1, 2]. This condition leads to the vessel occlusion in both arterial and venous districts [3, 4]. Although pulmonary embolism and deep venous thrombosis are the most described complication [5], some investigators had reported thrombotic localization also in the splanchnic venous district [6, 7].
In the present paper, we report the case of a patient with SARS-CoV-2 infection presenting with porto-mesenteric vein thrombosis, without pneumonia. In addition, we shortly review current literature supporting the possible role of COVID-19 as leading cause of splanchnic venous thrombosis.
2Case description
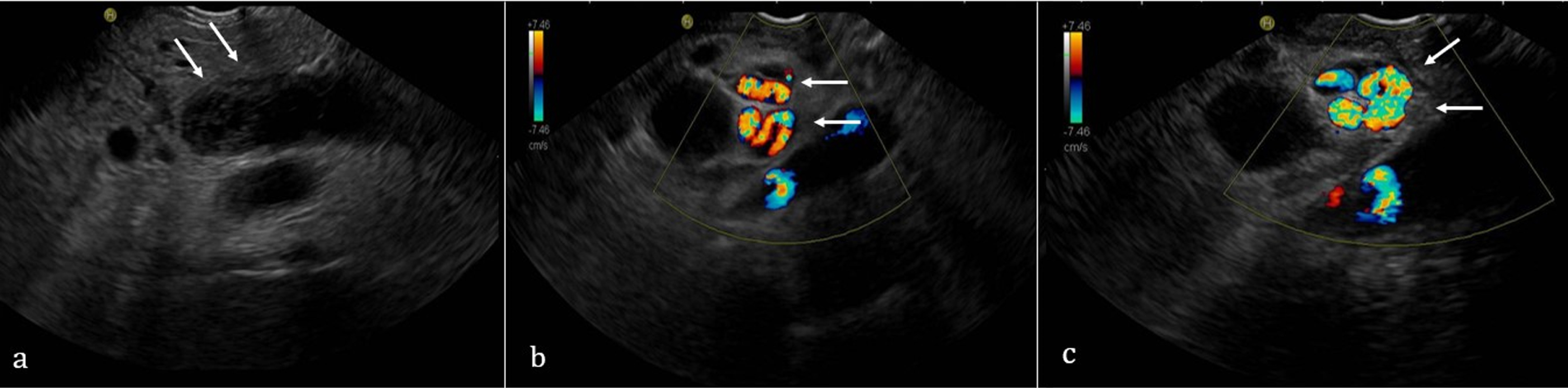
In October 2020, a 58-year-old Asian man came to the Emergency Department of our Hospital for the appearance of fever and abdominal pain. He not referred diarrhea or vomit, nor respiratory symptoms. His medical history was unremarkable. He did not take any medications and not referred history of smoking or alcohol consumption. He underwent to the nasopharyngeal swab screening for the RNA detection of SARS-CoV-2, which resulted positive and was admitted to the Internal Medicine Unit in the COVID-19 section. On admission, the patient was eupnoic, oxygen saturation was 99%on air room. Physical examination showed abdominal distention, with tenderness on deep palpation in the epigastric region. Laboratory data blood tests revealed normal leukocyte and lymphocytic count, PT and aPTT in normal range, D-Dimer 2.16 mg/L (n.v. < 0.55), Fibrinogen 669 mg/dL (n.v. 150–400), C-reactive protein 6.11 mg/dL (n.v. < 0.5), ALT 25 U/L, AST 27 U/L, serum bilirubin 2.57 mg/dL, Lipase 32 U/L and Creatinine 0.86 mg/dL. Blood gas analysis showed pH 7.41, pO2 85 mmHg, pCO2 42 mmHg and HCO3– 27 mmol/L, P/F ratio 404. High resolution computed tomography (CT) didn’t show ground glass aspect or other sign of interstitial pneumonia. Abdominal ultrasound revealed normal liver and spleen structure, but absent anterograde flow in the portal vein consistent with thrombosis (PVT) (Fig. 1). Contrast enhanced abdominal CT, confirmed the portal vein occlusion extended to superior mesenteric vein with collateral vessels, duodenal wall alterations (Fig. 2 a, b) and a cephalo-pancreatic inhomogeneity. Echo-endoscopy confirmed PVT with intrahepatic and peri-coledocic collateral vessels without pancreas alteration (Fig. 3). No esophageal varices or other pathological findings were observed at the esophagogastroduodenoscopy. Neoplastic biomarkers (CA-19.9 and CEA) were in the normal range; hepatitis C serology was negative, whereas hepatitis B markers were positive (HBs Ag 249 U/L, HBV DNA 35 UI/ml, Ab anti HBs 2 mUI/ml, Ab anti HBc positive) and indicated a state of “inactive HBV carrier”. Thrombophilia screening (including JAK 2 mutation and lupus anticoagulant) was negative. The patient started Enoxaparin (8000 IU bid) for one month and subsequently he switches to Rivaroxaban 20 mg/die. No specific therapy is initiated for SARS-CoV-2 infection given the absence of respiratory symptoms.
Fig. 1
Abdominal ultrasound showing portal vein thrombosis.
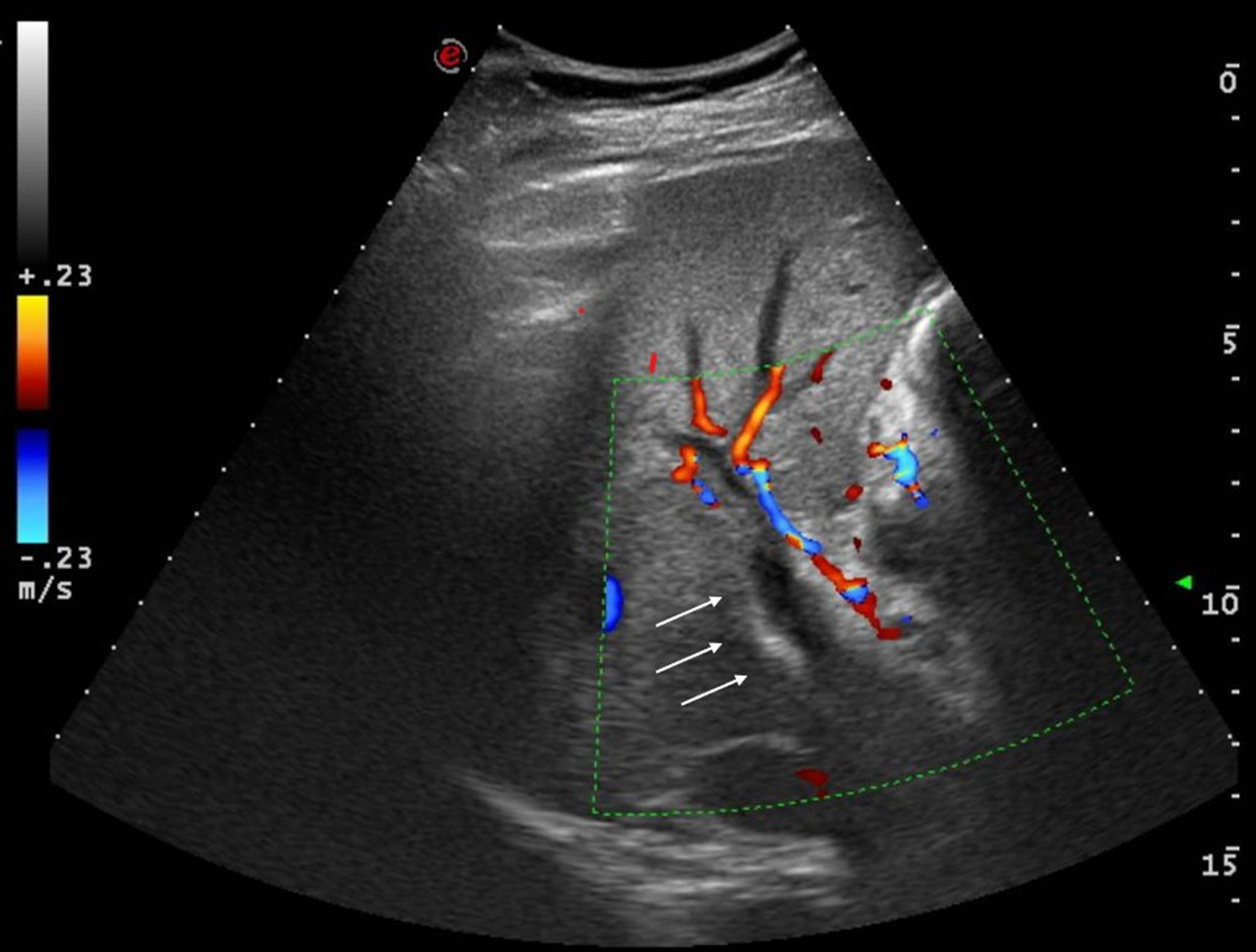
Fig. 2
Contrast enhanced computed tomography showing portal vein thrombosis (a) and collateral vessels (b).
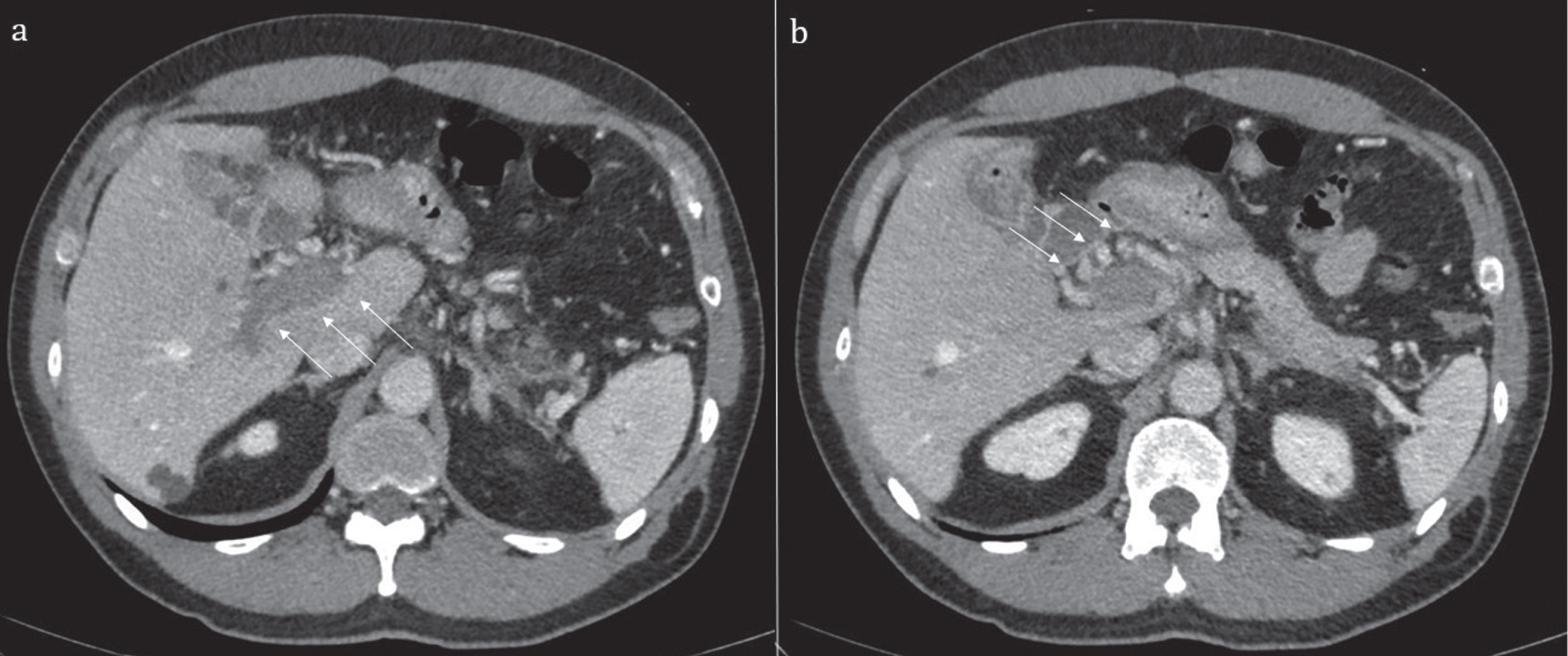
Fig. 3
Echo-endoscopy showing portal vein thrombosis (a) and collateral vessels (b and c).
Three months later, the patient came to our attention for an ambulatory follow up visit. He referred well-being and good adherence at the anticoagulant therapy. He underwent contrast enhanced ultrasound (CEUS) of abdomen showing portal cavernoma with a normal blood flow; patency of the right, left and intrahepatic portal branches; peri-gastric collateral vessels. We decided to continue the anticoagulant therapy for another three months.
3Discussion
In the present paper, we described the case of a patient diagnosed with SARS-CoV-2 infection presenting with portal vein thrombosis but without pneumonia. COVID-19 typically presents with flu-like symptoms such as cough and sore throat but it can progress to interstitial pneumonia, bronchitis, and acute respiratory distress syndrome. Besides pulmonary complications, COVID-19 is characterized by a prothrombotic milieu leading to thrombosis in various vascular district [1]. The exact mechanisms of these disorders are not fully elucidated, but it seems that COVID-induced coagulopathy might be the consequence of a complex interplay between systemic inflammation, thrombosis, and endothelial dysfunction. The combination of these factors might be at the basis of the pro-coagulative state observed during SARS-CoV-2 infection [8, 9]. Pulmonary embolism and deep venous thrombosis are the most described thrombotic complications. A recent meta-analysis found a cumulative in-hospital rate of acute pulmonary embolism in general wards of 14.7%. Higher rates were described in intensive care unit patients, with a pooled cumulative incidence rate of 23.4%[5].
Accumulating evidence from clinical case reports suggested that thrombotic manifestation might also develop into unusual sites such as portal and mesenteric vein [6]. Usually, PVT is observed in advanced chronic liver disease (cirrhosis, liver malignancy), whereas in normal liver, PVT is mainly the consequence of the combination between local and systemic prothrombotic factors such as abdominal inflammatory processes, thrombophilia, and JAK 2 positive mielo-proliferative neoplasm [10]. The gastrointestinal system is involved in the COVID-19 often in association with respiratory complications; indeed SARS-CoV-2 can infect intestinal epithelial cells through the angiotensin-converting enzyme 2 receptors. Furthermore, SARS-CoV-2 RNA has been detected in feces of infected patients [11]. Abdominal pain, nausea, vomiting, diarrhea and elevated transaminases are the commonest signs. Of note, in rare cases the gastrointestinal involvement in COVID-19 is characterized by thrombotic complications in both arterial and venous splanchnic district [6, 7]. Below, we reviewed current literature about this topic, focalizing on thrombotic complications in the venous district.
Table 1 summarizes all available cases of porto-mesenteric vein thrombosis in the course of COVID-19. Although a definite conclusion cannot to be drawn, these evidences allow us to make some considerations. First, splanchnic thrombosis doesn’t seem to have specific gender or age predilection. Males are slightly more affected than females, whereas age ranges from 22 to 79 years with a median of 46 years. Second, unspecific symptoms such as abdominal pain (with or without fever) and vomit are the commonest modalities of presentation, but some patients experienced severe gastrointestinal complications. Third, portal vein represents the more involved site of thrombosis, sometimes isolated and in other cases associated with mesenteric veins thrombosis (7 isolated PVT, 6 PVT with mesenteric veins, 3 only mesenteric veins). Only a few studies performed the thrombophilia screening (including JAK2 mutation) and, except for the lupus anticoagulant positivity in two cases, none other alteration were found. The clinical consequences of thrombosis as well as treatment are heterogeneous. The most not developed signs of gastro-intestinal ischemia so that they could effectively managed with conservative therapy (heparin, warfarin or new oral anticoagulants). In more severe cases, patients need emergent surgical intervention since they developed gastro-intestinal ischemia. In these last cases patients had the worse outcome, with prolonged hospitalization or death.
Table 1
Summary of current reports describing splanchnic vein thrombosis in patients with COVID-19
| Authors | Age/gender | Symptoms | Medical history | Pulmonary involvement | Thrombosis localization | Thrombophilia screening | Management/outcome |
| Alemán et al. [12] | 44/M | Abdominopelvic pain | None | N/A | Superior mesenteric vein, splenic and portal vein | N/A | Anticoagulant therapy/Discharged |
| Norsa et al. [13] | 62/M | Abdominal pain and bilious vomiting | Obesity, arterial hypertension, diabetes mellitus type 2 | None | Inferior vena cava and superior mesenteric vein | N/A | Small intestine resection/Death |
| De Barry et al. [7] | 79/F | Fever, abdominal pain, diarrhea | None | Diffuse GGO associated with consolidation | Right-portal vein, superior mesenteric vein | N/A | Necrotic ileum and right colon resection/Death |
| Filho et al. [14] | 33/M | Severe low back pain radiating to the hypogastric region | Obesity | Peripheral GGO | Inferior mesenteric vein | N/A | Oral anticoagulation/Discharged |
| Ignat et al. [15] | 28/F | Abdominal pain and vomiting | None | None | Superior mesenteric and portal vein | Essential thrombocythemia | Oral anticoagulation and bowel resection/Discharged |
| Olson et al. [16] | 51/M | N/A | N/A | Multifocal peripheral infiltrates | Portal vein thrombosis and gas | N/A | Gastric ischemia |
| Fan et al. [17] | 30/M | Abdominal pain and bilious vomiting | None | Bilateral basal pneumonia | Superior mesenteric vein | Lupus anticoagulant positivity | Twice-daily enoxaparin and resection of the affected small bowel loop/Discharged |
| Rodriguez-Nakamura et al. [18] | 42/F | Colic abdominal pain | Extreme obesity and a ventriculoperitoneal shunt due to a partially resected craniopharyngioma | Mild atypical pneumonia | Portal and mesenteric veins | N/A | Loop resection, entero-enteral manual anastomosis, partial omentectomy/Death |
| La Mura et al. [19] | 72/M | Fever, jaundice, and obnubilation | Parkinson disease, anxious-depressive syndrome, and mild vascular dementia | None | Left portal vein and the right portal vein with its branches for VIII and V segments | Von Willebrand factor abnormality | Enoxaparin administration/Discharged |
| Ofosu et al. [20] | 55/M | Fever, shortness of breath, and altered mental status | Hyperlipidemia | Midzone ground glass opacities | Right anterior and posterior divisions of the right portal vein | N/A | Apixaban 5 mg BID/Discharged |
| Abeysekera et al. [21] | 42/F | Fever and a dry cough, right hypochondrial pain | Chronic hepatitis B, previous trauma-related splenectomy | Bilateral, patchy, GGO with basal predominance | Portal and mid-superior mesenteric vein | Negative | Apixaban 5 mg BID/Discharged |
| Low et al. [22] | 51/M | Hematemesis | Lower extremity deep vein thrombosis | N/A | Right and left portal veins | N/A | Nasogastric decompression and intravenous heparin |
| Jafari et al. [23] | 26/M | Respiratory distress and fatigue and subsequent abdominal pain | Asthma | Multifocal patchy consolidations and bilateral pleural effusion | Portal vein | N/A | Heparin infusion/Discharged |
| Borazjani et al. [24] | 23/M | Dyspnea. GI bleeding associated with abdominal pain | Asthma | Bilateral peripheral and peri-broncho-vascular patchy GGO | Portal vein | Negative | Heparin infusion/Discharged |
| Franco-Moreno et al. [25] | 27/M | Severe colic abdominal pain | None | Bilateral consolidations with GGO | Portal vein | Negative | Heparin administration/Discharged |
| Pang et al. [26] | 30/M | Abdominal pain and vomiting | None | Lower zone airspace opacities | Superior mesenteric vein | N/A | Heparin administration/Discharged |
| Del Hoyo et al. [27] | 61/F | Acute abdominal pain and vomiting | Type 2 diabetes mellitus | Bi-basal atelectasis | Right hepatic vein and spleno-portal axis | Lupus anticoagulant antibodies positivity | Heparin administration/Death |
The mechanism of this unusual localization is difficult to understand and far to the aim of this paper. However, we can propose some mechanistic hypotheses. In a recent paper, Sonzogni et al. described the liver histological findings in COVID-19, such as: microvascular alterations, increased number of portal vein branches associated with lumen dilatation, partial or complete luminal thrombosis of the portal and sinusoidal vessels, and fibrosis of portal tract [28]. Liver and pulmonary microvascular alterations were also described by Jung et al, through the use of CEUS, in patients with severe COVID-19 [29, 30]. On these bases, we can speculate that systemic inflammation and local microvascular alterations could favor thrombotic complications in main venous districts.
In our clinical case, an interesting point needing to be highlighted is the absence of any pulmonary involvement. Indeed, the patient didn’t need supplemental oxygen therapy; furthermore, the chest CT not showed typical alterations. In our opinion this represents an important finding since it demonstrates that COVID-19-induced hypercoagulability (and possibly endothelial damage as well) can occur in all vascular districts, regardless of respiratory involvement and its severity. SARS-CoV-2 has a broad spectrum of organotropism beyond the respiratory tract, and it is possible than in some subjects the endothelium-tropism is more pronounced.
The major strength of the present work is the wealth of clinical information available. Indeed, we adopted a comprehensive diagnostic work-up allowing us to exclude the most common causes of portal thrombosis (such as hepatic cirrhosis, neoplastic diseases, thrombophilia disorder). So, we can affirm with reasonable certainty that SARS-CoV-2 infection is the main cause of portal thrombosis in our patient.
In conclusion, we described the case of COVID-19 related portal vein thrombosis in a patient free of respiratory involvement. In our opinion, this manuscript contains two main messages. First, it highlights the multifaceted modalities of presentation of COVID-19, beyond interstitial pneumonia. Second, it invites the clinicians to maintain a high index of suspicion for thrombosis in patients diagnosed with SARS-CoV-2 infection manifesting gastrointestinal symptoms. An appropriate diagnostic work-up could allow to obtain an early diagnosis and consequently improve the clinical outcome of patients.
Acknowledgments
None.
Conflict of interest
None declared.
References
[1] | Abou-Ismail MY , Diamond A , Kapoor S , Arafah Y , Nayak L . The hypercoagulable state in COVID-19: Incidence, pathophysiology, and management. Thromb Res (2020) ;194: :101–15. |
[2] | Amgalan A , Othman M . J Exploring possible mechanisms for COVID-19 induced thrombocytopenia: Unanswered questions. Thromb Haemost. (2020) ;18: :1514–6. |
[3] | Schulman S . Coronavirus Disease Prothrombotic Factors, and Venous Thromboembolism. Semin Thromb Hemost. (2020) ;46: :772–6. |
[4] | Alzoughool F , Alanagreh L , Abumweis S , Atoum M . Cerebrovascular comorbidity, high blood levels of C-reactive protein and D-dimer are associated with disease outcomes in COVID-19 patients. ClinHemorheol Microcirc. (2021) ;77: :311–322. |
[5] | Roncon L , Zuin M , Barco S , Valerio L , Zuliani G , Zonzin P , et al. Incidence of acute pulmonary embolism in COVID-19 patients: Systematic review and meta-analysis. Eur J Intern Med. 2020: :S0953–6205(20)30349-6. |
[6] | Singh B , Kaur P , Maroules M . Splanchnic vein thrombosis in COVID- A review of literature. Dig Liver Dis. (2020) ;52: :1407–9. |
[7] | de Barry O , Mekki A , Diffre C , Seror M , El Hajjam M , Carlier RY . Arterial and venous abdominal thrombosis in a 79-year-old woman with COVID-19 pneumonia. Radiol Case Rep. (2020) ;15: :1054–7. |
[8] | Jung F , Krüger-Genge A , Franke RP , Hufert F , Küpper JH . COVID-19 and the endothelium. Clin Hemorheol Microcirc. (2020) ;75: :7–11. |
[9] | Varga Z , Flammer AJ , Steiger P , Haberecker M , Andermatt R , Zinkernagel AS , Mehra MR , Schuepbach RA , Ruschitzka F , Holger Moch H . Endothelial cell infection and endotheliitis in COVID-19. The Lancet. 2020. |
[10] | Cruz-Ramón V , Chinchilla-López P , Ramírez-Pérez O , Aguilar-Olivos NE , Alva-López LF , Fajardo-Ordoñez E , et al. Thrombosis of the Portal Venous System in Cirrhotic vs. Non-Cirrhotic Patients. Ann Hepatol. (2018) ;17: :476–81. |
[11] | Wong SH , Lui RN , Sung JJ . Covid-19 and the digestive system. J Gastroenterol Hepatol. (2020) ;35: :744–8. |
[12] | Alemán W , Cevallos LC . Subacute mesenteric venous thrombosis secondary to COVID- A late thrombotic complication in a nonsevere patient. Radiol Case Rep. (2021) ;16: :899–902. |
[13] | Norsa L , Valle C , Morotti D , Bonaffini PA , Indriolo A , Sonzogni A . Intestinal ischemia in the COVID-19 era. Dig Liver Dis. (2020) ;52: :1090–1. |
[14] | Filho AC , da Silva Cunha B . Inferior mesenteric vein thrombosis and COVID-19. Rev Soc Bras Med Trop. (2020) ;53: :e20200412. |
[15] | Ignat M , Philouze G , Aussenac-Belle L , Faucher V , Collange O , Mutter D , et al. Small bowel ischemia and SARS-CoV-2 infection: an underdiagnosed distinct clinical entity. Surgery. (2020) ;168: :14–6. |
[16] | Olson MC , Lubner MG , Menias CO , Mellnick VM , Gettle LM , Kim DH , et al. RadioGraphics Update: Venous Thrombosis and Hypercoagulability in the Abdomen and Pelvis-Findings in COVID-19. Radiographics. (2020) ;40: :E24–E28. |
[17] | Fan BE , Chang CCR , Teo CHY , Yap ES . COVID-19 Coagulopathy with Superior Mesenteric Vein Thrombosis Complicated by an Ischaemic Bowel. Hamostaseologie. (2020) ;40: :592–3. |
[18] | Rodriguez-Nakamura RM , Gonzalez-Calatayud M , Martinez AR . Acute mesenteric thrombosis in two patients with COVID-19. Two cases report and literature review. Int J Surg Case Rep. (2020) ;76: :409–14. |
[19] | La Mura V , Artoni A , Martinelli I , Rossio R , Gualtierotti R , Ghigliazza G , et al. Acute Portal Vein Thrombosis in SARS-CoV-2 Infection: A Case Report. Am J Gastroenterol. (2020) ;115: :1140–2. |
[20] | Ofosu A , Ramai D , Novikov A , Sushma V . Portal Vein Thrombosis in a Patient With COVID-19. Am J Gastroenterol. (2020) ;115: :1545–6. |
[21] | Abeysekera KWM , Karteszi H , Clark A , Gordon FH . Spontaneous portomesenteric thrombosis in a non-cirrhotic patient with SARS-CoV-2 infection. BMJ Case Rep. (2020) ;13: :e238906. |
[22] | Low SW , Swanson KL , McCain JD , Sen A , Kawashima A , Pasha SF . Gastric ischemia and portal vein thrombosis in a COVID-19-infected patient. Endoscopy. (2020) ;52: :E465–E466. |
[23] | Jafari SH , Naseri R , Khalili N , Haseli S , Bahmani M . Portal vein thrombosis associated with COVID- points to consider. BJR Case Rep. (2020) ;6: :20200089. |
[24] | Borazjani R , Seraj SR , Fallahi MJ , Rahmanian Z . Acute portal vein thrombosis secondary to COVID- a case report. BMC Gastroenterol. (2020) ;20: :386. |
[25] | Franco-Moreno A , Piniella-Ruiz E , Montoya-Adarraga J , Ballano-Franco C , Alvarez-Miguel F , Peinado-Martinez C , et al. Portal vein thrombosis in a patient with COVID-19. Thromb Res. (2020) ;194: :150–2. |
[26] | Pang JHQ , Tang JH , Eugene-Fan B , Lee CL , Low JK . A Peculiar Case of Small Bowel Stricture in a Coronavirus Disease Patient with Congenital Adhesion Band and Superior Mesenteric Vein Thrombosis. Ann Vasc Surg. (2021) ;70: :286–9. |
[27] | Del Hoyo J , López-Muñoz P , Fernández-de la Varga M , Garrido-Marín A , Valero-Pérez E , Prieto M , et al. Hepatobiliary and Pancreatic: A fatal case of extensive splanchnic vein thrombosis in a patient with Covid-19. J Gastroenterol Hepatol. (2020) ;35: :1853. |
[28] | Sonzogni A , Previtali G , Seghezzi M , Grazia Alessio M , Gianatti A , Licini L , et al. Liver histopathology in severe COVID 19 respiratory failure is suggestive of vascular alterations. Liver Int. (2020) ;40: :2110–6. |
[29] | Jung EM , Stroszczynski C , Jung F . Contrast enhanced ultrasound (CEUS) to assess pleural pulmonal changes in severe COVID-19 infection: First results. Clin Hemorheol Microcirc. (2020) ;75: :19–26. |
[30] | Jung EM , Stroszczynski C , Jung F . Contrast enhanced ultrasonography (CEUS) to detect abdominal microcirculatory disorders in severe cases of COVID-19 infection: First experience. Clin Hemorheol Microcirc. (2020) ;74: :353–61. |




