Oral manifestations of COVID-19: A review
Abstract
An important indicator of general health, well-being, and quality of life is oral health. The SARS-CoV-2 virus, which has been discovered to have a number of adverse effects. One of the earliest tissue areas to become infected by the virus and undergo alterations is the oral cavity. Oral manifestations included ulcer, erosion, bulla, vesicle, pustule, fissured or depapillated tongue, macule, papule, plaque, pigmentation, halitosis, white patches, haemorrhagic crust, necrosis, petechiae, swelling, erythema, and spontaneous bleeding. The tongue (38%), labial mucosa (26%), and palate (22%) were the three most typical sites of involvement. Aphthous stomatitis, herpetiform lesions, candidiasis, vasculitis, mucositis, drug eruption, necrotizing periodontal disease, angina bullosa-like, angular cheilitis, atypical sweet syndrome, and Melkerson-Rosenthal syndrome were suggested diagnoses for the lesions. In 68% of instances, oral lesions were symptomatic. There were almost equally as many oral lesions in both sexes (49% female and 51% male). More extensive and severe oral lesions were present in patients who were older and who had COVID-19 diseases that were more severe. The most significant risk factors for the development of oral lesions in COVID-19 patients include poor oral hygiene, opportunistic infections, stress, immunosuppression, vasculitis, and hyper-inflammatory response. It is crucial to identify any changes in the mucosa in COVID-19 patients and administer assertive treatment to prevent complications. Patients should also try to maintain adequate oral hygiene throughout the course of the illness to prevent the colonisation of opportunistic microorganisms and to prevent complications both orally and systemically.
1Introduction
According to the World Health Organization (WHO) situation report on Corona Virus Disease 19 (COVID 19), which was released on October 12, 2020, there have been 37,109,851 cases of COVID 19 worldwide, including 10,70,355 fatalities [1]. On January 30, 2020, the WHO declared the condition a Public Health Emergency of International Concern. In November 2019, the illness began as pneumonia of unknown origin in Wuhan of China. It quickly spread to millions of people around the world, causing potentially fatal complications in both healthy and medically compromised people with a propensity for sick and elderly people with weakened immune systems. The current pandemic of coronavirus infection in humans has caused severe mortality and morbidity. Coronaviruses were only known to cause infections in animals before these occurrences. Data from the World Health Organization (WHO) show that by October 19, 2021, there will have been over 240 million confirmed cases reported globally, and there will have been over 4.8 million deaths [2]. Associated with both confirmed and suspected COVID-19 patients there are various oral complications. The World Health Organization has now identified taste disorders as one of these signs of COVID-19. It has also been demonstrated that COVID-19 is associated with oral mucosal lesions. Improper medication use, lowered immunity, vascular damage, local and systemic inflammation, and a lack of oral hygiene during COVID 19 treatment can all lead to oral complications. For COVID-19 patients, the oral signs, and symptoms, such as taste disturbances, ecchymosis, candidiasis, traumatic ulcers, HSV-1 infection, geographic tongue, thrush, etc., should receive more attention during the course of treatment. For COVID-19 patients, a multidisciplinary approach to oral healthcare and clinical oral examinations will be advantageous for the course of treatment and recovery [3].
1.1Structure and classification
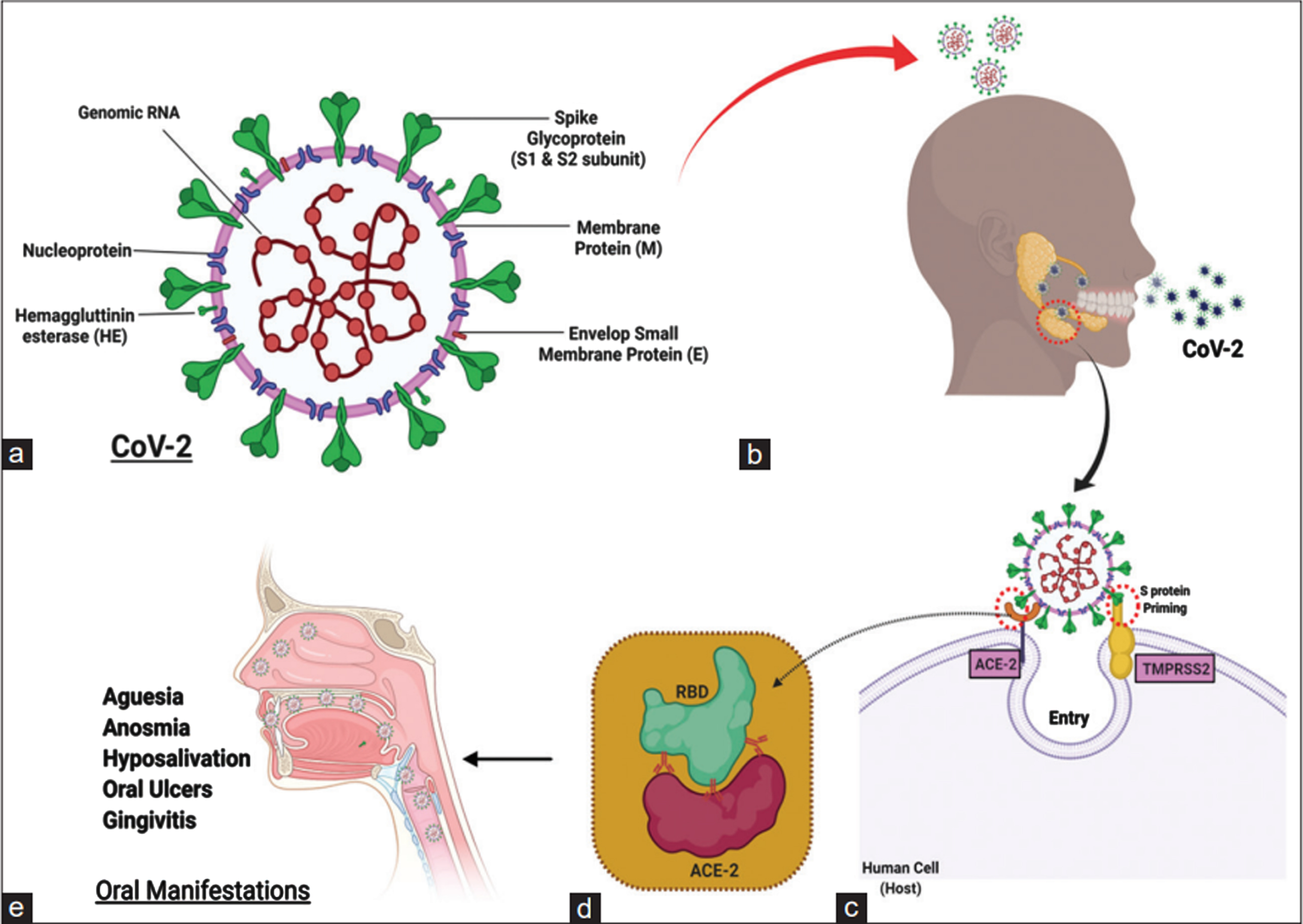
The word “coronavirus” comes from the Latin word CORONA, which means “Crown,” because, when viewed under an electron microscope, it has a structure that resembles a Crown with Multiple Spikes [4]. The first coronaviruses were found in poultry in the early 1930 s [5], and the first human coronaviruses were found in the 1960 s [6]. With a genome size of roughly 26 to 32 kilobases and multiple spikes on its surface, the new coronavirus, known as SARS CoV 2, is an enveloped, positive sense single stranded RNA virus that belongs to the beta coronavirus genus. SARS CoV 2 is a zoonotic coronavirus that is similar to the coronaviruses that cause SARS and MERS. The most likely natural reservoir is the Chinese horseshoe bat (Rhinolophus sinicus), and its genome is very similar to the coronavirus of bat origin RaTG13 [7, 8]. SARS CoV (SARS S) primarily uses endosomal membrane fusion or plasma membrane fusion to enter host target cells. In the binding of receptors, membrane fusion, and virus entry, the S protein (a glycoprotein) of the SARS CoV plays a minor role [9]. The S protein binds to the cellular receptor angiotensin converting enzyme 2 (ACE2) to start the SARS CoV entry [10]. Additionally, the cellular serine protease 2 TMPRSS2 is used for S protein priming (Fig. 1). Another isoform of CoV, namely CoV 2, has been identified as the primary virulent form in the current outbreak. According to a recent study, ACE2 and TMPRSS2 are required for SARS CoV 2 (SARS S 2) cell entry [10, 11]. In conclusion, SARS CoV 2 uses the ACE2 SARS CoV receptor ACE2 for entry in the host cell and TMPRSS2 primes the spike protein of SARS–CoV–2 [12]. A study was done to determine whether SARS-CoV2 and viruses that cause MERS and H1N1 influenza are related. Additional research was also conducted to comprehend the mutations that take place in the SARS-CoV2 sequences throughout the propagation of the disease and connected them to the functional domains of proteins. The resulting phylogenetic tree showed that the H1N1 and MERS viruses are distantly related, but SARS-CoV2 is closely related to both. The mutation study of six of the ten SARS-CoV2 proteins reveals that there were more than 50-point mutations in the sequences from 34 different countries. It’s interesting to note that during the analysis, four proteins remained unchanged. Therefore, when developing the diagnostics or treatments for this disease, these four proteins may be taken into account [13].
Fig. 1
Detail structure of coronavirus detailed coronavirus structure (a) SARS-CoV-2 transmission via aerosols and respiratory droplets (b). Spike glycoproteins are bound by Angiotensin-converting Enzyme-2 (ACE2) to initiate SARS-CoV-2 entry into host target cells. Additionally, spike proteins are primed with serine protease (c and d). Relationship between RBD and ACE2 (d). Oral SARS-CoV-2 Manifestations (all or none may occur separately or simultaneously) (e).
1.2Transmission of SARS CoV–2
It is believed that SARS CoV 2 is first transmitted from bats to humans in or near Wuhan, China, and then from one person to another. Direct person-to-person transmission happens when an infected person coughs or sneezes and creates respiratory droplets that are inadvertently inhaled by another person. Within a 6-foot radius, this kind of transfer happens between the closed contacts. Another way to spread the virus is by touching contaminated surfaces with your hands and then putting your mouth, nose, or eyes close afterward [14]. Viral particles created during coughing or sneezing settle to the surface and cause contamination. According to research by Kampf et al., the human coronavirus can survive for up to 9 days at room temperature on inanimate objects like metal, glass, or plastic. Additionally, self-inoculation from contaminated dry surfaces results in further transmission to the mouth, eye, and nose [15, 16]. Recent studies have found the virus in faecal samples and suggest that the virus can also be transmitted through the faecal oral route [17, 18]. The typical method of transmission is through direct contact with the symptomatic patient, but asymptomatic people can also spread the disease. A few authors have proposed that the SARS CoV 2 can also spread through the air [19].
1.3Clinical manifestations of COVID–19
The COVID 19 symptoms were divided into three general categories by WHO. Fever, a dry cough, and fatigue are the most typical signs. Body aches and pains, a sore throat, diarrhoea, conjunctivitis, headaches, loss of taste or smell, a skin rash, and discoloration of the fingers and toes are less common symptoms. Breathing problems or shortness of breath, chest pressure or pain, and loss of speech or movement are the severe symptoms [1, 20]. Clinical symptoms can range from being completely asymptomatic to experiencing mild flu-like symptoms to having a severe respiratory illness. People with comorbid conditions like diabetes, hypertension, and ischemic heart disease experience more severe symptoms. The pathogenesis and pathophysiology of COVID 19 infection are greatly influenced by ACE2. ACE inhibitors are some antihypertensive medications, and this causes an increased risk of infection by SARS–CoV–2 [21].
1.4Effect of SARS–CoV–2 on blood cells
In COVID-19 patients, increased estimated blood viscosity (EBV), which is calculated from haematocrit and globulins, is linked to thrombotic problems, organ failure, and higher mortality. EBV does not consider fibrinogen or cellular connections, despite being instructive. Use in vitro blood samples from patients to examine whether they have altered whole blood viscosity (WBV) when assessed at both high and low shear rates. A cross-sectional study of 58 patients has concluded that acute and convalescent patients, hyperviscosity offers a potential explanation for the thrombotic risk. Important ramifications for thromboprophylaxis result from these findings [22]. There is mounting evidence that COVID-19 also affects the endothelium system in addition to the lungs. Recent research has demonstrated that this can result in impaired microcirculation and, as a result, functional issues with all inner organs. Endothelial dysfunction, widespread inflammation, and complement components may all work together to contribute to the overall pro-coagulative condition seen in COVID-19 individuals, which can result in both venular and arteriolar occlusions [23].
1.5Effect of SARS–CoV–2 on haemostasis
In a study, dynamic changes in coagulation, haematological, and biochemical markers were examined for their relationships to COVID-19 patient mortality. It involved all COVID-19 patients who had severe respiratory failure and were critically sick. It was found that in critically ill COVID-19 patients, monitoring anomalies in coagulation, haematological, and biochemical markers as well as their dynamical changes may help with care and mortality prediction [24]. The coagulation profile of COVID-19 patients who had an acute stroke and its relationship to patients’ outcomes were reported in a study. The baseline D-dimer value and its peak value during hospitalisation were significantly greater in patients who had a poor outcome, demonstrating that the D-dimer can be a predictor of death in COVID-19 patients with acute stroke [25].
1.6Effect of SARS–CoV–2 on thrombotic processes in the whole body together with microcirculatory disorders
Contrast enhanced sonography (CEUS), in the hands of skilled examiners, provides the opportunity to study dynamic microcirculatory abnormalities in real time dynamically without any risk for the thyroid gland or kidneys, even in severe progressive disease bedside. The first experiences with abdominal CEUS tests are given based on severe COVID-19 infections. A narrowing of the organ-supplying arteries, as well as a delayed capillary filling of vessels close to the capsule, a regional reduced parenchymal perfusion, or an inflammatory hyperemia with capillary hypercirculation, can all be seen on CEUS in the stage of an imminent organ failure with significantly reduced kidney and liver function. It is feasible to dynamically monitor the arterial and venous blood flow in the mesentery and rapidly rule out organ infarction [26]. Use of contrast enhanced ultrasound (CEUS) was also done in severe cases of COVID-19 infection to assess pulmonary changes near the pleura. CEUS opens up new possibilities for bedside monitoring of pleural reactive inflammatory or peripheral thrombus embolism in severe cases of COVID-19 infection [27].
A poorly understood thrombotic consequence of COVID-19 are abdominal thromboses. A study was done to present abdominal multimodality ultrasound imaging results for the evaluation of thrombotic lesions in hospitalised COVID-19 patients. It showed that hospitalised COVID-19 patients can experience thromboembolic consequences. The early and precise diagnosis of these issues can be achieved with multimodality ultrasound tests [28].
With traditional B-mode and elastography, the small intestinal morphology was described. Additionally, utilising CEUS and color-coded perfusion parameters, the dynamic impacts of small bowel micro vascularization related to COVID-19 were assessed. Investigations were conducted on 13 patients who had severe acute respiratory distress syndrome (ARDS) caused by COVID-19. In conclusion, it was demonstrated that the small bowel may also be a critical interaction location in COVID-19 [29].
1.7Oral manifestations of COVID-19
An ulcer, erosion, bulla, vesicle, pustule, fissured or depapillated tongue, macule, papule, plaque, pigmentation, halitosis, whitish areas, hemorrhagic crust, necrosis, petechiae, swelling, erythema, and spontaneous bleeding were among the oral manifestations. The tongue (38 %), labial mucosa (26 %), palate (22%), gingiva (8 %), buccal mucosa (5 %), oropharynx (4 %), and tonsil were the most frequently affected areas (1%). Aphthous stomatitis, herpetiform lesions, candidiasis, vasculitis, mucositis, drug eruption, necrotizing periodontal disease, angina bullosa-like, angular cheilitis, atypical sweet syndrome, and Melkerson-Rosenthal syndrome were suggested diagnoses for the lesions. In 68 % of cases, oral lesions were symptomatic—painful, burning, or pruritic. Both genders had nearly equal rates of oral lesions (49 % female, 51 % male) The interval between the onset of systemic symptoms and the onset of oral lesions ranged from 4 days before to 12 weeks after. Systemic symptoms first appeared in three cases before oral lesions, and in four cases, oral and systemic symptoms simultaneously manifested. Kawasaki-like lesions had the longest latency period. Oral lesions usually disappeared 3 to 28 days after they first appeared. Depending on the aetiology, various treatments such as artificial saliva, chlorohexidine mouthwash, nystatin, oral fluconazole, topical or systemic corticosteroids, systemic antibiotics, systemic acyclovir, and photo biomodulation therapy (PBMT) were recommended for oral lesions [30].
1.8Aphthous-like lesions
shallow ulcers with erythematous halos and yellow-white pseudo membranes. In one patient, oral lesions and systemic symptoms both developed at the same time, and in other patients, the latency period ranged from 2 to 10 days. Two patients had positive PCR results for the herpes simplex virus, and one patient had a positive history of recurrent aphthous stomatitis (RAS) (HSV). Younger patients with mild infections frequently had aphthous-like lesions without necrosis, whereas older patients with immunosuppression and severe infections frequently had aphthous-like lesions with necrosis and hemorrhagic crusts. After 5 to 15 days, lesions were healed [31]. The improvement of the systemic disease coincided with the regression of oral lesions. Increased levels of tumour necrosis factor (TNF)- can cause neutrophils to migrate to the oral mucosa and form lesions that resemble aphthous. Other potential causes for the development of such lesions in COVID-19 patients include stress and immunosuppression brought on by the infection [30, 32].
1.9Herpetiform/zosteriform lesions
Multiple painful, unilateral, round, yellowish-gray ulcers with an erythematous rim on the keratinized and nonkeratinized mucosae were the initial symptoms of herpetiform lesions. These lesions showed up before, at the same time as, or after systemic symptoms. After healing from herpetiform lesions, geographic tongue manifested in one instance. The development of secondary herpetic gingivostomatitis has been attributed to stress and immunosuppression brought on by COVID-19 [30].
1.10Ulcer and erosion
On the tongue, hard palate, and labial mucosa, ulcerative or erosive lesions manifested as painful lesions with irregular borders. Lesions started to appear 4 to 7 days after the onset of systemic symptoms in some cases, and they recovered 5 to 21 days later in others. HSV-1 and HSV-2 PCR tests were negative in two instances. Different causes for the development of ulcerative and erosive lesions have been proposed, including drug eruption (to an NSAID in one case), vasculitis, or secondary thrombotic vasculopathy to COVID-19 [33–37].
1.11White/red plaques
On the dorsum of the tongue, gingiva, and palate of patients with confirmed or suspected COVID-19, white and red patches or plaques have been reported. White or red patches or plaques may be caused by candidiasis as a result of prolonged antibiotic therapy, a decline in general health, or a failure to practise good oral hygiene [30, 32].
1.12EM-like lesions
In patients with cutaneous target lesions in the extremities, EM-like lesions manifested as blisters, desquamative gingivitis, erythematous macules, erosions, and painful cheilitis with haemorrhagic crust. Lesions developed 7–24 days after the onset of systemic symptoms and disappeared in 2–4 weeks [30].
1.13Angina bullosa-like lesions
In two confirmed cases of COVID-19, angina bullosa-like lesions on the tongue and hard palate appeared as asymptomatic erythematous purple blisters without spontaneous bleeding [38].
1.14Melkerson-Rosenthal syndrome
A 51-year-old woman was described as presenting with malaise, unilateral lip swelling, a fissured tongue, and right facial paralysis. She had previously experienced spontaneous healing of her Melkersson-Rosenthal syndrome four years prior, with no relapse. Computed tomography (CT) scan results revealed ground-glass opacities in both lungs, and laboratory oratory data indicated an elevated level of CRP. After receiving treatment for COVID-19 disease, the patient was fully recovered [39].
1.15Atypical sweet syndrome
A 61-year-old woman was reported to have come in with symptoms including fever, fatigue, arthralgia, myalgia, several erythematous nodules on the scalp, trunk, and extremities, as well as minor aphthous ulcers on the hard palate and buccal mucosa. COVID-19 RT-PCR results were positive. An erythema nodosum-like sweet syndrome was supported by the results of a skin biopsy, which revealed diffuse neutrophilic infiltration in the upper dermis along with granulomatous infiltration in the lower dermis and subcutaneous area [40].
1.16Kawasaki-like disease
Patients with COVID-19 who had Kawasaki-like disease developed oral lesions such as cheilitis, glossitis, and an erythematous and swollen tongue (red strawberry tongue) (Kawa-COVID). In contrast to the direct effects of the virus on the skin and oral mucosa, the prolonged latency between the onset of systemic symptoms (respiratory or gastrointestinal) and the onset of oral or cutaneous symptoms may be caused by a delayed immune system hyperactivation response and a secondary release of acute inflammatory cytokines [30].
1.17Necrotizing periodontal disease
A 35-year-old woman who had oral lesions, submandibular lymphadenopathy, halitosis, and fever was reported to be suspect for COVID-19. A painful, diffusely erythematous, oedematous gingiva with necrosis of inter-papillary areas was among the oral lesions. The suggested diagnosis was COVID 19 and bacterial coinfections, particularly prevotella intermedia, which caused necrotizing periodontal disease. After 5 days, the lesions healed [41].
1.18Vesicles and pustules
9-year-old women who had diarrhoea, abdominal pain, weakness, and an erythematous papular exanthema in her mouth and on her acromion has been reported. Vesicular eruptions and erosions on the tongue and buccal mucosa were among the oral lesions. A COVID-19 PCR test came out positive. After one week, lesions healed. Another case described a 51-year-old man who had a fever, dry cough, lethargy, dysgeusia, anosmia, and a positive COVID-19 serology. After 10 days, the oropharynx and hard palate both developed extensive erythema, along with petechiae and pustules on the border of the soft palate. Enanthema caused by COVID-19 was the indicated diagnosis, and the lesions cleared up within a few days [42].
1.19Petechiae
A few studies have noted Petechiae on the oropharynx, palate, and lower lip mucosa. Patients with petechiae had shorter latency times than those with petechiae and macular lesions combined. Possible causes of petechiae have included thrombocytopenia brought on by COVID-19 infection or the prescribed medication [30].
1.20Nonspecific lesions (mucositis)
Several studies have described erythematous-violaceous macules, patches, papules, and plaques on the tongue, lip mucosa, hard palate, and oropharynx. Mucositis in COVID-19 patients may be brought on by thrombotic vasculopathy, vasculitis, or hypersensitivity related to the virus. The potential causes of mucositis in COVID-19 include vasculitis, thrombotic vasculopathy, and mucosal hypersensitivity secondary to COVID-19 [30].
1.21Post inflammatory pigmentation
A 40-year-old woman was reported to have pigmentation in her attached and interpapillary gingiva. Post-inflammatory pigmentations are caused by elevated levels of inflammatory cytokines, such as interleukin-1 [IL-1], tumour necrosis factor [TNF]-, and arachidonic acid metabolites (prostaglandins), which are produced as a byproduct of stem cell factor (SCF) and basic-fibroblast growth factor from basal layer keratinocytes [43].
1.22Loose teeth
In some patients, symptoms like tooth loss, sensitive gums, or grey or brittle teeth may be present. It might occur as a result of the SARS-ability CoV-2’s to irritate the gums and cause a sudden tooth loss [44].
1.23Taste disorders
In COVID-19, taste disorders are frequent. Taste disorders were more common in Europe, North America, and the Middle East among COVID-19 positive patients and less common in Asia. Taste disorders are now accepted as a diagnostic criterion for COVID-19 in the majority of regions due to their high incidence and some degree of presentation in the early stages of the disease. The inability to accurately perceive chemical stimuli, such as taste and spiciness, is known as a taste disorder. The three main categories of taste disorders are: total loss of taste (ageusia), diminished intensity of taste (hypogeusia), and distorted taste (dysgeusia). A relatively easy-to-use but subjective questionnaire can be used to diagnose taste disorders. Alternative methods include chemical gustometry. Patients with COVID-19 and those Alternative methods include chemical gustometry. Both COVID-19 patients and those with the acute cold can have taste disorders, but those with the disease have a difficulty telling the difference between bitter and sweet flavors while those with the acute cold have a similar ability to tell the difference between sour and salty flavors. We can distinguish COVID-19 patients from people with the acute cold using taste strips as well [3].
1.24Macroglossia disease
The incidence of macroglossia is relatively small. One (0.5 percent) of 210 patients with severe COVID-19 who were admitted to intensive care units in 14 clinical studies later developed macroglossia, according to a review of the patients. Macroglossia is a clinical diagnosis with a complex etiology. Before the COVID-19 outbreak, there had been a few reports of macroglossia cases. The natural extension of the tongue past the tooth or alveolar crest is the clinical definition of macroglossia. True macroglossia and relative macroglossia are two different pathological subtypes of macroglossia. True macroglossia has clear histopathological abnormalities, whereas relative macroglossia may have corresponding clinical symptoms but has a normal histological structure. Infection or keratinized tongue plaques are two problems that some patients with macroglossia may have. Congenital lingual vein malformation or lymphatic vascular malformation, which are more prevalent in young children, are the most common causes of macroglossia in non-COVID-19 patients, whereas macroglossia caused by COVID-19 is more prevalent in adults, the majority of patients have been on ventilators [3].
2Discussion
The first case of the coronavirus illness (COVID 19), which is brought on by the SARS CoV2 coronavirus, was discovered in Wuhan, China in December 2019. The illness became a pandemic and had an impact on people’s lives all across the world. Millions of people died as a result of it worldwide [13]. A mild illness characterized by fever, exhaustion, myalgia, and a dry cough may appear in SARS-CoV-2 infected individuals. Nevertheless, 14% of people will experience symptoms that necessitate hospitalization with oxygen support, and 5% need intensive care. Contrarily, between 20 and 75 percent of infected people in the general population do not exhibit any symptoms, making it difficult to diagnose and treat the illness. Considering that SARS-CoV2 can spread through fomites, which are salivary droplets, the oral cavity is one of the main entry points for the virus. The gingival sulcus is a well-known niche where inflammatory molecules and enzymes build up and encourage the colonisation of microorganisms, supporting the hypothesis that the oral cavity may act as a virus reservoir and it might potentially serve as a reservoir for SARS-CoV-2. As a result, the oral cavity mucosa is among the first tissues to come into contact with the virus and may experience some changes.
In the early stages of COVID-19 (less than 14 days after infection), we are able to identify patients who developed symptoms primarily related to alteration in flavour perception, dysgeusia, and a burning mouth; as the disease advanced, the patients primarily displayed ulcerative lesions or Candida albicans. These data coincide with studies made by other research centres that mention that dysgeusia is the most common symptom with an 80% presence among subjects infected with SARS-CoV-2, as well as the presence of ulcerative lesions in 65% of the population, which are located mainly on the tongue, palate, lip, and cheek, followed by C albicans in 22.7% of cases. Ageusia and hypogeusia are two examples of the various types of taste disturbances that are referred to as dysgeusia. Numerous mechanisms have been proposed as potential explanations for the etiology of taste loss during COVID-19; neurotropism is one such theory. This damage could happen either by directly crossing the olfactory epithelium through the cribriform plate to reach the central nervous system, which has high expression of ACE2 in endothelial cells and neurons.
In contrast, Sakaguchi et al found that taste cells and taste buds in stratified squamous epithelium of the tongue were positive for ACE2, transmembrane protease serine protease 2, which may be the primary cause of the loss of taste perception. Different etiologies, such as infections, immunosuppressive conditions, trauma, or neoplasms, can result in ulcerative lesions. Findings had indicated that moderate to severe COVID-19 was the main site for the occurrence of ulcerative lesions, and the presence of ulcers was associated with herpes in the studies that were reviewed. This virus has the property of remaining dormant in the trigeminal ganglion or gasser ganglion after the initial infection. Reactivation will largely depend on immunosuppression periods, so it is not surprising that after contracting SARS-CoV-2, the patient develops painful ulcerative lesions as a result of a relapse of the herpes virus. This is in line with the early COVID-19 symptom of a burning mouth.
Ulcers in COVID-19 patients arise from the disease’s inherent immunosuppression, as a side effect of oxygen supplementation therapy, or as a result of the cytokine storm. Finally, it was found that C albicans was the second most typical lesion. It does not harm the host in an immunocompetent state because it is a component of the human microbiome, but it can cause systemic infections as well as infections of the mucous membranes and superficial skin when the immune system is compromised.
As a result, this injury is not thought to be directly linked to SARS-CoV-2 but rather a secondary effect of COVID-19. One area that should not be neglected is oral hygiene, and it is crucial to motivate the patient to practice good hygiene habits. Recent research has demonstrated that patients with bad oral hygiene make their COVID-19 symptoms worse. In contrast, patients who maintained good oral hygiene saw a significant reduction in COVID-19 symptoms.
3Conclusion
The illness COVID-19 has a number of adverse effects. The most frequent oral symptoms of COVID-19 disease include aphthous-like lesions, herpetiform lesions, candidiasis, and oral lesions of Kawasaki-like disease. Dysgeusia, which manifests in the early days of infection, is the primary SARS-CoV-2-related oral symptom. The most significant risk factors for the development of oral lesions in COVID-19 patients include poor oral hygiene, opportunistic infections, stress, underlying diseases (diabetes mellitus, immunosuppression), trauma (secondary to intubation), vascular compromise, and hyper-inflammatory response. It’s important to distinguish between lesions or other changes that are truly caused by SARS-CoV-2 and those that the patient already possessed. It is crucial to identify any changes in the mucosa in COVID-19 patients and administer assertive treatment to prevent complications. Patients should also try to maintain adequate oral hygiene throughout the course of the disease to prevent the colonisation of opportunistic microorganisms and to prevent oral and systemic complications.
Conflict of interest
The authors have no conflict of interest to report.
References
[1] | World Health Organization Coronavirus Disease (covid–19) Pandemic.2020. 2. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al (2020). |
[2] | Zhu N , Zhang D , Wang W , Li X , Yang B , Song J , et al. A novel coronavirus from patients with pneumonia in china, 2019. N Engl J Med (2020) ; 382: : 727–33. |
[3] | Zhou X , Dong J , Guo Q , Li M , Li Y , Cheng L , Ren B The Oral Complications of COVID-19. Front Mol Biosci (2022) ; 8: : 803785. |
[4] | Lan J , Ge J , Yu J , Shan S , Zhou H , Fan S , et al. Structure of the sars–cov–2 spike receptor–binding domain bound to the ace2 receptor. Nature (2020) ; 581: : 215–20. |
[5] | Beach J , Schalm O A filterable virus, distinct from that of laryngotracheitis, the cause of a respiratory disease of chicks. Poul Sci (1936) ; 15: : 199–206. |
[6] | Tyrrell D , Bynoe M Cultivation of a novel type of common–cold virus in organ cultures. Br Med J (1965) ; 1: : 1467. |
[7] | Mackenzie JS , Smith DW Covid- A novel zoonotic disease caused by a coronavirus from China: What we know and what we don’t. Microbiol Aust (2020) ; 41: : 45–50. |
[8] | Zhou P , Yang X-L , Wang X-G , Hu B , Zhang L , Zhang W , et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature (2020) ; 579: : 270–73. |
[9] | Choudhary S , Malik YS , Tomar S , Tomar S Identification of sars–cov–2 cell entry inhibitors by drug repurposing using in silico structure–based virtual screening approach. Front Immunol. (2020) ; 11: : 1664. doi: 10.3389/fimmu.2020.01664. |
[10] | Patel AB , Verma A , Covid-19 and angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: What is the evidence? JAMA (2020) ; 323: : 1769–70. |
[11] | Ou X , Liu Y , Lei X , Li P , Mi D , Ren L , et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun (2020) ; 11: : 1620. |
[12] | Hoffmann M , Kleine-Weber H , Schroeder S , Krüger N , Herrler T , Erichsen S , et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. (2020) ; 181: : 271–80.e8. |
[13] | Kaushik A , Kasana S , Verma P , Singh AK , Point mutation of COVID-19 proteins: A study on noval corona virus (nCov) correlation with MERS and H1N1 viruses and in silico investigation of nCoV proteins for future applications. Journal of Cellular Biotechnology (2021) ; 7: : 85–98. |
[14] | Bi Q , Wu Y , Mei S , Ye C , Zou X , Zhang Z , et al. Epidemiology and transmission of covid-19 in 391 cases and of their close contacts in shenzhen, china: A retrospective cohort study. Lancet Infect Dis (2020) ; 20: : 911–9. |
[15] | Kampf G , Todt D , Pfaender S , Steinmann E , Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect (2020) ; 104: : 246–51. |
[16] | Van Doremalen N , Bushmaker T , Morris DH , Holbrook MG , Gamble A , Williamson BN , et al. Aerosol and surface stability of sars-cov-2 as compared with sars-cov-1. N Engl J Med (2020) ; 382: : 1564–67. |
[17] | Gu J , Han B , Wang J Covid- Gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology (2020) ; 158: : 1518–19. |
[18] | Lee I-C , Huo T-I , Huang Y-H , Gastrointestinal and liver manifestations in patients with covid-19. J Chin Med Assoc (2020) ; 83: : 521–23. |
[19] | Morawska L , Cao J , Airborne transmission of sars-cov- The world should face the reality. Environ Int (2020) ; 139: : 105730. |
[20] | Kar S , Covid- A brief clinical overview. J Geriatr Care Res (2020) ; 7: : 74–8. |
[21] | Tignanelli CJ , Ingraham NE , Sparks MA , Reilkoff R , Bezdicek T , Benson B , et al. Antihypertensive drugs and risk of covid-19 Lancet Respir Med (2020) ; 8: : e30–1. |
[22] | Shaik A , Chen Q , Mar P , Kim H , Mejia P , Pacheco H , Goonewardena SN , Cho DJ , Rosenson RS , Blood hyperviscosity in acute and recent COVID-19 infection. Clin Hemorheol Microcirc (2022) ; 82: (2), 149–55. |
[23] | Jung F , Krüger-Genge A , Franke RP , Hufert F , Küpper J-H , COVID-19 and the endothelium. Clin Hemorheol Microcirc (2020) ; 75: : 7–11. |
[24] | Zlojutro B , Jandric M , Momcicevic D , Dragic S , Kovacevic T , Djajic V , Stojiljkovic M , Skrbic R , Djuric D , Kovacevic P , Dynamic changes in coagulation, hematological and biochemical parameters as predictors of mortality in critically ill COVID-19 patients: A prospective observational study. Clin Hemorheol Microcirc. 2022 Nov 7. doi: 10.3233/CH-221583. |
[25] | Rasyid A , Riyanto DL , Harris S , Kurniawan M , Mesiano T , Hidayat R , Wiyarta E , Association of coagulation factors profile with clinical outcome in patient with COVID-19 and acute stroke: A second wave cohort study. Clin Hemorheol Microcirc (2022) ; 82: (4), 371–7. |
[26] | Jung EM , Stroszczynski C , Jung F , Contrast enhanced ultrasonography (CEUS) to detect abdominal microcirculatory disorders in severe cases of COVID-19 infection: First experience. Clin Hemorheol Microcirc (2020) ; 74: : 353–61. |
[27] | Jung EM , Stroszczynski C , Jung F , Contrast enhanced ultrasound (CEUS) to assess pleural pulmonal changes in severe COVID-19 infection: First results. Clin Hemorheol Microcirc (2020) ; 75: : 19–26. |
[28] | Dong Y , Qiu Y , Cao J , Fan P , Wang WP , Fleischmann J , Jung EM , Ultrasound features of abdominal thrombosis in COVID 19 patients. Clin Hemorheol Microcirc. (2022) ; 82: (3), 239–48. |
[29] | Tews HC , Kandulski A , Schmid S , Schlosser S , Schirner S , Putz FJ , Cosma L , Gülow K , Müller M , Jung EM , Multimodal ultrasound imaging with conventional B-mode, elastography, and parametric analysis of contrast-enhanced ultrasound (CEUS): A novel approach to assess small bowel manifestation in severe COVID-19 disease. Clin Hemorheol Microcirc (2022) ; 82: (4), 341–360. |
[30] | Iranmanesh B , Khalili M , Amiri R , Zartab H , Aflatoonian M , Oral manifestations of COVID-19 disease: A review article. Dermatologic Therapy. (2021) ; 34: : e14578. |
[31] | Brandao TB , Gueiros LA , Melo TS , et al. Oral lesions in patients with SARS-CoV-2 infection: could the oral cavity be a target organ? Oral Surg Oral Med Oral Pathol Oral Radiol 2020. |
[32] | dos Santos JA , Normando AG , da Silva RL , et al. Oral mucosal lesions in a COVID-19 patient: new signs or secondary manifestations? Int J Infect Dis (2020) ; 9: . |
[33] | Chaux-Bodard AG , Deneuve S , Desoutter A , Oral manifestation of Covid-19 as an inaugural symptom? J Oral Med Oral Surg. (2020) ; 26: (2), 18. |
[34] | Soares CD , de Carvalho RA , de Carvalho KA , de Carvalho MG , de Almeida OP , Letter to editor: oral lesions in a patient with Covid-19. Med Oral Patol Oral Cir Bucal. (2020) ; 25: (4), e563. |
[35] | Sakaida T , Isao T , Matsubara A , Nakamura M , Morita A , Unique skin manifestations of COVID-19: is drug eruption specific to COVID-19? J Dermatol Sci. 2020. |
[36] | Ciccarese G , Drago F , Boatti M , Porro A , Muzic SI , Parodi A , Oral erosions and petechiae during SARS-CoV-2 infection. J Med Virol. 2020. 18. Ansari R, Gheitani M, Heidari F, Heidari F. Oral cavity lesions as a manifestation of the novel virus (COVID-19): a letter-to-editor. Oral Dis. 2020. |
[37] | Singh C , Tay J , Shoqirat N , Skin and mucosal damage in patients diagnosed with COVID- a case report. J Wound Ostomy Continence Nurs (2020) ; 47: (5), 435–8. |
[38] | Cruz Tapia RO , Peraza Labrador AJ , Guimaraes DM , Matos Valdez LH , Oral mucosal lesions in patients with SARS-CoV-2 infection Report of four cases. Are they a true sign of COVID-19 disease? Spec Care Dentist (2020) ; 40: (6), 555–60. |
[39] | Taslıdere B , Mehmetaj L , Özcan AB , Gülen B , Taslıdere N , Melkersson-Rosenthal syndrome induced by COVID-19 a case report. Am J Emerg Med. 2020. |
[40] | Taskın B , Vural S , Altug E , et al. COVID-19 presenting withatypical Sweet’s syndrome. J Eur Acad Dermatol Venereol (2020) ; 34: : e534–e535. |
[41] | Patel J , Woolley J Necrotizing periodontal disease: oral manifestation of COVID-19. Oral Dis 2020. |
[42] | Cebeci Kahraman F , ÇaS kurlu H Mucosal involvement in a COVID19-positive patient: a case report. Dermatol Ther. (2009) ; 14: (6), E272–7. |
[43] | Corchuelo J , Ulloa FC Oral manifestations in a patient with a history of asymptomatic COVID-19. Case Report Int J Infect Dis 2020. |
[44] | Abdalla S , Zwaid S (2021). COVID-19 Cause Missing Teeth. |




