Global pandemic vaccine development, production and distribution challenges for the world population
Abstract
BACKGROUND:
The new type of virus (SARS-CoV-2 or COVID-19) from Coronaviridae family, discovered in 2019, caused a global pandemic with several massive lock-downs around the globe. Science and politicians became the center of world attention, receiving many questions without having clear answers. The hopes of many rested on vaccine development, which was done fast, facing novel challenges such as the massive production and distribution for several billions of people.
OBJECTIVE:
In this paper, the global reaction to the pandemic is reviewed along with some critical comments.
METHOD:
Different groups, including nations, took part in global lockdowns, while vaccine development was running in parallel without having enough capacity for some of the biggest medical demands in history. This review will bring together views from all interested groups in this pandemic crisis.
RESULTS:
The Western world waited too long (4 months), after the first case was confirmed in China, to introduce lock-down and safety measures. On the other side, vaccine development was done too fast to give clear long-term safety profiles of the medications developed. Due to the focus on development, it was overlooked that production and distribution of sterile products such as vaccines might have limitations globally. Usually when such limitations occur, power comes to the surface. Therefore, buyers who had power will get the vaccines they need first. However, we should recognize the economic impact that directly influenced healthcare funding. All of this will lead to post-crisis challenges, including depression, violence, suicide, migration, and many other social problems.
CONCLUSIONS:
The COVID-19 pandemic is a test for all of us, which many governments, industries and non-state actors are failing. It is a perfect “general probe” to detect some of the weaknesses of the current structure of global health. If politics and science do not work together to make a global production plan for vaccines and learn from this pandemic, then all of the lives lost were for nothing.
1.Introduction
One century after Spanish flu killed millions all over the globe [1–4], a newly discovered respiratory virus from the same family as some smaller epidemics from past 20 years shocked the world and spread quickly [5,6]. Amongst all viruses that are pathogenic for humans, respiratory viruses are the most dangerous due to their aggressiveness and their transmissivity. On the other side, the symptoms are very similar to those caused by the influenza virus and some bacterial infections. Overnight, coughing and sneezing in public became taboo [7,8].
The world’s eyes were on China, and somehow after SARS (2003), swine flu (2009), and Ebola (2014), everyone was expecting that COVID-19 would simply pass by eventually [2]. What actually happened was quite different. It infected millions. Suddenly, the virus was not just another flu, but a killing machine for those with chronic diseases, cancer, obesity, autoimmune diseases, AIDS or other infections, as well as the elderly. Looking back, we will try to answer several questions. Why were the global lock-downs 4 months too late? What could be done different next time? Which measures are most appropriate? Do we need a cure or vaccine? Do we have capabilities to produce and supply vaccines for global usage? There are many questions today with no simple solutions. However, what is clear is that the system failed to protect the global population. The WHO, governments, and scientists faced a huge challenge on the global level [8].
The best way to prevent infection and minimize symptoms and negative side effects is with a combination of a vaccine for prevention, and medications for treatment in cases of disease development. This paper is looking at different angles of how scientific society reacted during pandemic and how reality settled.
Global production of oral dosage forms (tablets capsules, powders, granules) is common. Capacities for this type of products are endless, while sterile production has limitations. No one ever produced any sterile product in such a short period of a time for a global market. Thinking about the vaccine regime, potential cold chain, or even production itself, nations did not have equal chances to benefit from preventative tools [9].
Finally, when immunizations are ready for powerful countries, the fight for accessibility begins. The ability to control the pricing, funding of research, distribution, and use of preventative measures become a proxy for a nation’s power.
In addition, when recovery hits the powerful, and differences forge a gap between those who would accept immunization, those who lost someone due to the pandemic, and those who refuse to be part of the fear (and also reject the vaccine itself), a real crisis might begin, similarly as it did in the past [10–13].
Overall, this article aims to put into perspective all the inputs that drive global vaccine development, production and distribution, and to review the outputs of that response with respect to preparing for future challenges, including:
The “proper” response time to a pandemic.
The position of the WHO.
Global vaccine production capacities.
General readiness of the health care system.
Post pandemic consequences.
2.Method
Analysis of historical data connected to epidemics, pandemics, vaccine development, flu vaccine production, distribution challenges, demographics, as well as health care differences, have been reviewed to suggest better procedures for potential future situations. Many articles, opinions and case studies have been reported and published during the COVID-19 pandemic. In this article, the analysis has three main components, connected to science and society: the start of pandemic, vaccine development including the supply process, and post pandemic challenges.
3.Results and discussion
3.1.Beginning of the pandemic
The COVID-19 virus was first identified in December 2019 in Wuhan, China. The World Health Organization declared a Public Health Emergency of International Concern only on 30 January 2020, and later declared a pandemic on 11 March 2020 [14]. As of the end of May 2021, almost 170 million cases have been confirmed, with more than 3.5 million deaths attributed to COVID-19, making it one of the deadliest pandemics in modern history [1]. The accuracy of such data has been questioned. For example, many medical doctors complained that hospitals abused the situation and reported every case of death as a COVID-19 caused case. Just as a reminder, populations with chronic diseases are the weakest. Therefore, if someone had cancer before being infected with COVID-19, once the person is infected, the virus will be more deadly. In that case, the patient’s cause of death might be counted as COVID-19. This is very sensitive topic, and without reliable data, it is not easy to evaluate the WHO’s response [15]. However, the big question is: If the pandemic could have been stopped, why it was not?
There was 4 months between the first case and the global lockdown. In cases of a more pathogenic virus, this might have been far too late. The WHO was giving recommendations, but it had no power or authority to do anything more. Global lockdowns were needed.
Immediately after the first case was detected, the WHO and China should have localized the area until an investigation could be done properly. This should have happened in the first 2-3 weeks after virus was detected. In the case of global spread, which started already in January, all flights in and out of the country should have been stopped. Therefore, China should have been cut of the rest of the world for several weeks. After that, an immediate global lockdown should have been followed. Most of the Western world (EU, USA, CA) began lockdowns after 3 months (mid-March until mid-June 2020). That was absolutely needed at that point in time. On the other hand, that period was not used properly to prepare for the upcoming challenges. All of the faith was put on vaccine development. Before COVID-19, most of the vaccine development for SARS-Coronaviruses had failed. We have more than 30 years of HIV-1 and HIV-2, killing millions. Vaccines have never been developed to give more than 50% of efficacy to be approvable and safe for human massive use [9,16–18]. The fastest development of a vaccine in the past was 4.5 years, and the normal time required is 10 to 15 years. Risks are undertaken by an abbreviated version of the approval process, with pre-clinical and clinical development (Phase 1-3) taking about 12-24 months. Looking back to 2020, there was high uncertainty about the vaccine, and still today it is hard to believe that between the first COVID-19 case and the first Marketing Authorization for COVID-19 vaccine was less than 12 months [19].
I am still asking myself why politicians, scientists, and the WHO chose to “wait and see” during the first lockdown period. It was clear from the beginning that even in the best case scenario the world was going to go into massive crisis (economical, health, mental, etc.). Instead of investing money into the protection of the health care system, countries chose to lose money over a longer period. Sweden’s way of dealing with the pandemic was different and unacceptable. In my opinion, it was not the wrong way but it was not the complete way. Collective natural immunization in this case is fine, if the system is ready to protect the weakest, which was not the case in Sweden [20]. What should have been done differently?
First of all, the WHO should have reacted within 2 weeks in cases of new virus or bacterial infections. Regions where infections were detected should have been isolated for several weeks. In case of spreading, countries should be isolated and all means of public transportation should be stopped until new cases were not detected for 2-3 weeks. If global infections are already present, lockdowns for several months should be applicable as was done. All of this should have been happening already in January 2020.
During the lockdown, countries following the recommendations of the WHO should have been focusing on preparing to support the health care system. According to the WHO, COVID-19 has around a 2% mortality rate, which would be in the worst-case 156 million deaths (out of 7.8 billion) [14]. That number is comparable with Spanish flu a century ago which took a great toll on the global population in 4 waves over 2.5 years [1–3,21].
There was a need for simulations using historical data drawn from previous experiences with other respiratory pandemics to help prepare for the hospitalization of patients. Instead of driving countries into several lock-downs, losing jobs, reducing tax payments, increasing government help, getting into more debt, countries should have used lockdowns for building better hospital management.
Big facilities such as army dormitories, sports arenas, parts of schools and warehouses should have been modified for emergency use. Money should have been invested into additional hospital beds, respiratory equipment, medications, and protective accessories for medical workers. Recruitment of retired medical workers should have been done on a voluntarily basis. Students from medical, pharmaceutical, dental, and veterinarian science should have been taken into national health support institutions. In addition, medical students and army students could have been recruited to support public health needs. With all of that and lockdowns during the summer in the Northern hemisphere, countries would have been better prepared to support the additional needs of the community and to escape the needs for second and third lockdowns. At the time, we did not know if vaccine development would be successful or not, and we did not do anything. The world was putting all its efforts into a vaccine and potential cures with existing medications. However, the tested medications were not successful, and vaccine development needed time.
After a hot summer in the North, and the first high peak of COVID-19 infections in the South, the world was running into even more difficult periods from October 2020 until March 2021. Everyone was praying for a vaccine, while debates on social media became increasingly inflamed [22].
3.2.Vaccine development and global roll out
The smallpox vaccine was the first vaccine to be developed against a contagious disease in 1796 by the British doctor Edward Jenner, and that was one of the biggest discoveries in human history. Today there are more than 25 types of vaccines covering mostly viral and bacterial infections [23]. On a first glance, we would expect that science could develop any new vaccine against any pathogen if there was a global need for it.
However, this is not always the case. In addition, under normal conditions, the development of new vaccines can take 10 to 15 years [19]. Major obstacles can include constant changes of the microbial form, or the types of mechanism that microbes initiate after infection. We can look into few familiar typical cases. STIs are a significant problem today. There are 2 bacterial forms and 2 viruses that are dangerous for the human body. Only one of them has a vaccine. The others remain global challenges. The HPV vaccine was developed and modified several times in past two decades, while HIV still does not have, after almost three decades of development, any form of vaccine that is more than 50% efficient. In addition, syphilis and gonorrhea represent enormous challenges in the modern and developed world, because they are antibiotic resistant and change constantly.
Knowing that, the scientific world was skeptical that a COVID-19 vaccine would ever be available. And even if we had an effective vaccine, global populations had to be prepared for the limitations that would be connected to production capacities and the requirements and limitations of the global supply chain [9,19,21].
Searching the Web of Science between January 2020 and June 2021 using the keyword “pandemic,” you will get 62 thousand published works. Searching for “COVID,” an additional 111 thousand papers will be found for the same period. However, only 2 thousand manuscripts include clinical investigations for COVID-19 [24]. That gives a picture of the competition out there to get the vaccine developed first.
As a reminder, the development of vaccines in the past began with specific parts of the microbe, or an inactivated microbe, or some of the specific products of the microbe (toxin, protein, enzymes). Modern development also used so-called “gen” therapy to make the human body produce or create molecules that will assist in stimulating immune responses unique for the targeted microbe. If this were simple, we would have developed vaccines for SARS (2003), Swine Flu (2009), and Ebola (2014) [3,9,19]. For most people it is still a mystery as to why the scientific community does not develop vaccines against these viruses, but for COVID-19 the need was so sure.
What exactly happened in the first part of 2020? As usual, many scientific groups were using real time patients in hospitals to experiment with existing medications [25,26]. At the same time, many false information was spread on social media creating a masse [22]. It was difficult to be exposed to all that contradictory information from different sources and evaluate it rationally. Although one medication was taken seriously for complete phase III clinical study after it seemed capable of reducing symptoms and speeding up recovery during and after COVID-19 infection. However, in a few months, the outcome was negative for potential approval of using hydroxychloroquine against COVID-19 [26]. At the same time, groups of scientists were working on isolating plasma [25] from the blood of people who had survived COVID-19 infections with significant amounts of antibodies in the blood several weeks after a complete recovery. Unfortunately, this method did not produce useful results.
In parallel, different scientific communities were quietly developing vaccines. By the end of 2020, about 65 clinical studies were running in different phases (I-III) trying to get a vaccine with an efficacy above 50% in comparison to placebo (control) groups [27,28]. At the time of writing, more than 350 trials have been conducted [28].
In the first phase, a lot of information and analysis had to be done to learn about the virus. After that, a profile of specific parts of the virus had to be explored. Accordingly, types of vaccines had to be developed and tested on small laboratory scales concerning stability, safety, and efficacy. Some of the selected formulations with added stabilization agents and stimulators have required investigations on animals. Finally, when selected formulations got promising results, first trials got into the Phase I clinical study in humans. Phase I and Phase II studies are all about safety on smaller and extended populations. Under normal circumstances, it would take 4–6 years to cover those two phases before we go into the third and final stage of development. In the case of the COVID-19 vaccine, this period of several years was reduced to only a few months. Therefore, we moved forward with minimal data, not knowing medium- and long- term side effects. Overall, the final stage of development took 2–3 months instead of 2–3 years [9,19]. In the middle of that period, some companies started publishing intermediate results (50% of subjects from the study) to create buzz for economic investment. This kind of science losses the meaning it might have had for every young kid raised to believe that “science does not have borders and brings humankind together!”
Overnight, we heard about American/German vaccines, Russian vaccines, Chinese vaccines, Oxford British vaccines, more Chinese vaccines, Cuban vaccines, and so on. It is disappointing that vaccines came with nationalities [27–29]. Politicians began positioning for supplies and tried to get the lowest possible price. One happy consequence was that small start-up companies were able to partner with bigger players to invest in clinical studies and production. However, even this was not managed well. Vaccines should not be stamped with nationalities or any other political device. I am sure that most researchers that developed COVID-19 vaccines are Indians or Chinese, no matter which part of the world they are in. Therefore, it is unfair to name vaccines according to the place where they were developed, or, according to who paid for their development. This was definitely the beginning of the big global conflict that started end of 2020, and separated nations in 2021. Some of the factors to be discussed in this paper relate to limited global capacities for production, transparency on the pricing, supply chain issues, distribution challenges, regulatory approvals, global coverage, political interests, financial interests, as well as how countries are finding ways to get vaccines depends on population size, power, price, and other factors.
Production of the vaccine starts with the production of an active principle, which is carrying the main component for its effective use. This all has to be done under good manufacturing practices and has to be sterile. All other components which are called excipients, have to be sterilized and made available for massive scale production. The next step in production is equipment and space. Again, in that manner, globally sterile production is much more limited in comparison to solid dosage forms production. All pre-conditions have to be covered to be able to produce safe and sterile products in this space, which is according to international standards as well as a quality of air. Having said that, we are narrowing down opportunities for future support of the production of any new vaccine [9,19]. In most cases, production sites which are used for vaccine production are also sites where injections, infusions, implants, and other sterile products can be produced. Therefore, for established pharmaceutical companies to be able to support huge demands for vaccine related to pandemic, some choices about priority have to be made. In addition, sometimes for a particular product, we need a dedicated piece of equipment (which luckily was not the case for any of the COVID-19 vaccines) and the normal time to get it up and running would be 6–12 months.
To understand the issue of global capacities, we can look into the production of seasonal flu vaccines. Two decades ago, flu vaccines had a globally dedicated capacity of about a few hundred million. With the support of the WHO, 18 countries have been involved in building up capacities to the level of 1.5 billion doses (annual) by 2019. However, due to low acceptance of the vaccine as well as low efficacy (10–60% depending on the year) [21], on average over the past decade, 150-200 million doses have been globally administrated on the annual base. Using this model and capacities in 18 production sites which are located on all continents, except Africa, moderate annual production could reach 4 billion doses and the best case for optimization will reach a bit more than 8 billion doses. Therefore, the global platform for massive production of vaccine (flu) is in place. However, it was not discussed to be used in COVID-19 vaccine roll out [9,19]. Thus, global capacities existed, but not well used.
Let us turn to some other missteps. Because clinical studies have been done very fast, developers and producers of vaccines were initially suggesting 2 doses within a 3 week period. Globally we have around 7.8 billion people. Those below the age of 15 are 26% of population [30], leaving 5.8 billion vaccine-eligible people. If each were given 2 doses within 3 weeks [14], and a third dose after 6 months, we would need 11.6 billion doses to be produced in one year. This is currently impossible. Even if only 50% of people were interested in getting vaccinated, that would also not be covered by the supply available in 2020. The two biggest suppliers (Pfizer and Sinopharm) announced that after some improvements in 2022 they would be able to produce 5.5 billion doses (2.5 billion by Pfizer and 3.0 billion by Sinopharm) [31,32]. With the rest of the vaccine producers we could get to the sufficient number of vaccines by the end of 2022, 2 years after first vaccine was approved.
To make matters worse, most of the vaccines require cold storage and transport (called the cold-chain). Products have to be kept at the same controlled temperature [9,19]. Once a product is mixed and packed in primary packaging (mostly sterile glass vials), it has to be put on the refrigerated conditions (+2 to +8°C, or a temperature below 0°C). It must be transported from where it is produced to a distributing warehouse. From a warehouse, it goes to trucks, which take it to other warehouses for distribution, airplanes, cargo ships or trains. Once at its destination country, it goes to a custom warehouse, and then a distributor warehouse, and sometimes a local quality testing and release has to be done. Finally, smaller minivans take the vaccines to their point of application, where medical stuff administer the vaccine to patients. One wrong step anywhere in this chain and vaccines can be inactivated or worse, impurities can be created which have potentially harmful effects. There are so many options for mistakes. Knowing that not all countries are equally developed and not all people involved in the transportation process are equally trained for it, any extreme storage conditions can be difficult to manage (such as −70 to −80°C). Most of the existing vaccines (there are around 25 at the time of writing) are stored under refrigerated conditions (+2 to +8°C) [14,29]. Today only the Sinopharm COVID-19 virus-inactivated vaccine has a sticker on the vial that changes color in case of temperature deviation [14]. A simplified diagram of distribution is shown in Fig. 1.
Fig. 1.
Simulation of cold-chain distribution.
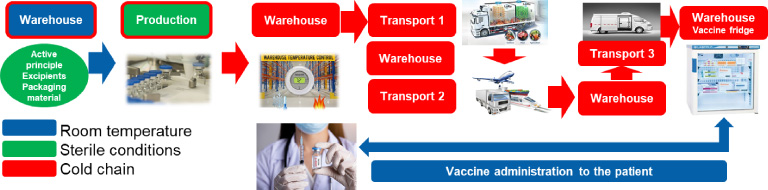
Another challenge is the global approval process, which enables targeted discrimination. Every country has different regulation for medical products [9,19]. The FDA and the EMA have been approving vaccines coming from the Global North and not from the Global South or Russia. The WHO recommended all the vaccines that the FDA and the EMA approved, while only on May 7th 2021 did the WHO give approval for the Sinopharm (Chinese) vaccine. Approval was issued after a systematic audit of data from developmental and clinical studies, as well as after an inspection of production facilities [14]. This approval gave some hope for global access to approved vaccines for countries who did not have access to vaccines produced by the companies approved by the FDA and the EMA. I do not want to judge FDA and EMA, since we do not know level of transparencies from developers that did not get approval yet in EU and USA or their intention to get those two approvals. However, WHO approval of Sinopharm vaccine is confirmation that some of the processes could be done faster also in some of the medical agencies.
Global coverage, political and financial interest are another source of disappointment [16,33,34]. The EU was proud to announce that over 300 million doses were secured at a very low price from Western suppliers during 2021 [29]. The USA gave significant funding for vaccine development and flexed its political power by paying more for the same vaccines, in contrast to the EU, which was trying to pay the least amount possible. Some smaller countries were put off by this situation and decided to do their own national phase approval process. Hungary, for example, was part of the EMA evaluation for all submitted vaccines, but they also did their own separate national phase process. Switzerland and Serbia have similar population sizes: around 8 million inhabitants. Switzerland’s GDP is 15 times higher than Serbia’s. The same medication in Switzerland will cost on average 5-10 times more than it does in Serbia. Both countries are not part of the EMA CP (centralized procedure) or DCP (de-centralized procedure), but they both have only national regulatory process according to local legislation. By the end of May 2021, Switzerland had approved 2 suppliers and 17.5% of population was fully vaccinated. In the same period, Serbia had approved 4 options, and 28.3% of the population was fully vaccinated [14,29]. Lessons learned here are important for potential future pandemics: we must match power, size, and pricing with supply opportunities.
Vaccines for non-COVID diseases range in price from 3 to 250 USD per dose. The seasonal flu vaccine has more or less similar a cost structure compared to COVID-19 vaccines: both have production costs between 1.5-3.0 USD [9,19]. Variance in cost depends on the workforce in the country of vaccine production. Currently, seasonal flu shots are sold for 3-30 USD (underdeveloped markets versus in the USA) [35]. Unofficial information has been shared, according to which different producers charge 4 to 40 USD for COVID-19 vaccines, with the same suppliers having different Net Selling Prices for different countries [14,29]. For example, the same vaccine can be sold to Serbia for 15 USD per dose, to EU countries for 4 USD per dose and to the USA for 30 USD per dose. A simple question receives a simple answer: Who will get the most? For low price doses, agreements were signed with suppliers who have limited capacities. At the same time, the USA negotiated with the same suppliers, and as usual, they were prepared to pay more. Serbia knew its own capabilities and being small and outside of the European Union, it was all about pricing and political willingness to play on several fronts. Taking all of this under consideration, size does matter in combination with power and flexibility. Switzerland has the right size (being smaller, it is easier to manage in a time of crisis), and enough money and power, but it does not have flexibility when it comes to how to source the vaccine. Health should have priority over everything else, unfortunately, in this case it was money and power that won the day.
As a scientist, pharmacist, end user, patient, global citizen, all this can easily guide me into confusion as being part of global population.
Now, how should things be done differently in the future?
Following the paradigm of season flu vaccines, the WHO should drive any new vaccine development for emergency use in pandemic situations [9]. All scientific groups should be in contact with the WHO during development prior to phase III clinical trials. Once vaccines pass Phase I and Phase II minimum safety requirements, international boards of scientists and experts should choose a maximum of 3–4 options (formulations) for global Phase III clinical studies [19]. All formulations should undergo the same study protocols and the one with the best (the highest) efficacy should be chosen for mass production. Accordingly, technology and production processes should be established and shared amongst the 18 countries that already have seasonal flu vaccine production in place. With that, up to 8 billion doses could be delivered within a one-year period [19]. Of course, the question is what and how to settle financial matters. One possibility is that all companies that have been part of a successful evaluation process after the Phase II study can claim the total costs for development, which will be afterwards covered by sales profit. Royalty (profit) sharing plans can be implemented. Normal mark up for production sites is 10–15%, on top of which 20–25% can be given to the developer responsible for the best formulation. An additional 10% can be used to cover of the costs for others that have been in Phase I and Phase II studies but whose formulations were not chosen as the most promising. An illustration of the costs sharing and profit sharing model is shown in the Fig. 2.
Fig. 2.
Simulation of a cost-sharing and profit-sharing model for unified global vaccine development.
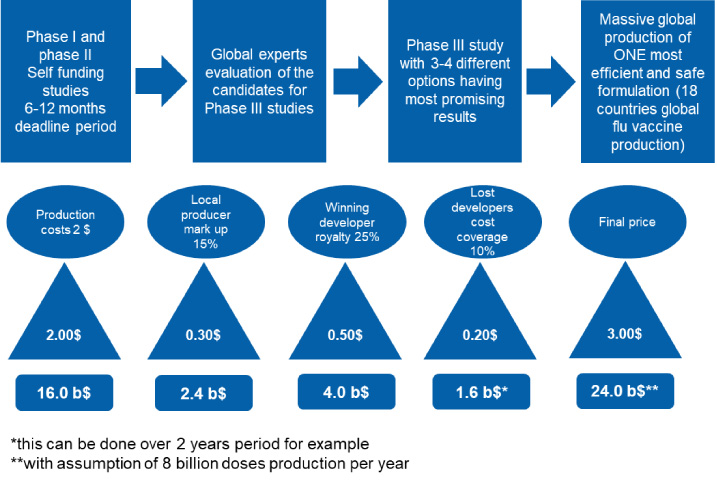
In such an arrangement, a regulatory expert group could be established by the WHO, with half of the representatives coming from the scientific side and without any conflicts of interest, and half of the representatives coming from highly regulated countries such as CA, USA, EU, CN, JP, AU, RU and BR. This group of representatives would evaluate the scientific data, as well as inspect the clinical facilities used in the Phase III study and all production sites, which are part of global seasonal flu vaccine production plan. With such an approach, I as a consumer would not have to worry about different prices, supply issues, availability, quality, travel restrictions, political games, technology or other factors. It is all about health. Definitely, global oversite like this should be created for clinical safety and pharmacovigilance for future pandemics [36].
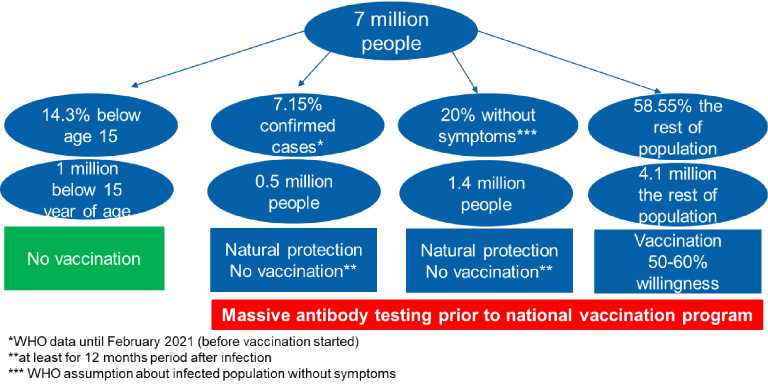
The immunization process can also be improved. Figure 3. provides an example in the case of Serbia. It is standard to begin immunization with a critical population subset (e.g., those with compromised immune systems, the elderly, medical workers, and front-line workers). This could be improved with pre-vaccination screening. By a mandatory act, the entire population should be tested for antibodies within one-month period prior to vaccination. Everyone who recovered from a COVID-19 infection and has still antibodies should be able to get confirmation from the government, so that they only require one shot of the vaccine, or even to be released from the first 6-12 months of the vaccination drive (also in all countries globally). People younger than 16 (or in some, 18) should be excluded. With this, in a relatively short time, a sufficient immunization level would be reached, without the need for financial awards or punishment measures. Neither of those two options are in alignment with human rights. As some groups do not believe in the benefits of vaccines, this should be respectfully accepted [37].
Fig. 3.
Immunization process simulation during the pandemic on the country level, using demographic data from Serbia. With 2 million people vaccinated (ca. 30%) and 4 million doses, a country can reach sufficient immunization and get out of pandemic measurements.
The COVID-19 pandemic was potentially less damaging in comparison to what a future pandemic might do. We must learn and change to survive.
3.3.Post pandemic challenges
At the moment of writing, the world is already 1.5 years into pandemic mode. For some of the countries with great power and privileges to have secured a sufficient vaccine supply, pandemic measurements (travel bans, lockdowns, etc.) are beginning to loosen. However, we are still far from the world we used to have.
COVID-19 is going to be around in this or another form for a long time, and we are going to have to learn how to live with it. The strongest and healthiest will find a way to manage; however, we are all much weaker now than we were 2 years ago. Lack of social interactions and physical distancing measures caused a decline in mental health measures [38]. Suicide, depression, anxiety, violence, crime, and divorce are just some of the normal human reactions connected to any type of crisis [15,38–44]. Besides that, an important challenge concerns tensions between groups willing to get vaccinated and those that are not. I mean, it is a valid point if you are between 18 and 50 years old, and without any chronical conditions, why would you be interested to get vaccine at all. On top of this, natural immunization has been completely ignored in favour of the “artificial” way. People who had recovered from one or even two COVID-19 infections are not recognized as equal to those who are vaccinated. All of these political movements drive massive public protests [15]. And human rights can be jeopardized. In the coming months, governments will begin to stop supporting the unemployed, and accordingly less money will be circulating. With higher a unemployment rate, less people will be able to pay for health insurance. With less money in the health care system, less money will go to the pharmaceutical companies. This can then affect the acquisition of supply for active principals, raw materials and packaging components. Overall, we face a dark cycle [45].
By the second half of 2020, the number of patients visiting oncology doctors and other non COVID-19 related therapeutic area medical doctors created a critical mass of new CNS, cardiovascular, oncology and other patients. This is a crisis not easily managed [46], and it also contributes to mental health concerns. Depression is a defining mental health factor during and long after any crisis. Repeatedly, researches have reported that during a crisis of any type there is a significant jump of new cases. After a crisis, things may worsen still due to unhappiness, unemployment, and bereavement. Financial limitations drive a deeper level of depression, with no light at the end of the tunnel [15,38–44,47,48].
Beside our minds, also our bodies are weaker than before. We are wearing masks for a long time. Isolation of normal flora around us is making our immune system much weaker. On top of this, most people had to stay at home for long periods without sports or physical activities, which can contribute to problems with obesity and the resulting cardiovascular issues, diabetes, muscular and respiratory complications [42].
Lucky, those who remain employed and are strong enough to fight against depression, and are able to get vaccinated might be able to plan some nice vacations. But even this will not be the same. Travel conditions have changed dramatically. Ships, airplanes and trains have not been used for a long time. As with every other machine, when it is not in use, parts are damaged. Companies will follow regulations and work using minimum safety checks, but technical problems will become a regular part of many trip stories, and some of them could be fatal. We could expect much more air crashes in the future as well as some other accidents related to more than one year of transportation and tourism being shut down [47,48].
Although unemployment will increase, there will be significant need for certain professions globally. Many countries lost frontline workers, with medical professions affected severely. Due to inappropriate approach, and not sufficient preparation at the beginning of pandemic, significant number of medical stuff was lost in the first few months of pandemic. Economic and employment migration will solve shortages in some countries, but worsen those in others, from pure or less developed countries to the ones with better economic footprints. It does not matter how and where this will be, a gap in medical workers will be a challenge that faces most of the global population. Underdeveloped countries will face general migrations, which will cause additional problems in targeted countries [42].
All of this is just the tip of an iceberg of challenges, in front of us, and global vaccine production will not be able to help the world recover as soon as needed. We are lucky that vaccines were developed and worked well. Without this, the crisis would be longer and population would be on the edge of mental survival.
Hopefully, politicians and scientists are working on different scenarios and lessons learned to protect the world against COVID-19 mutations (which might not be covered by existing vaccines) as well as against any other future pandemics, since this time we failed at the beginning but we are recovering at the half way point, with the availability of vaccines [7,8].
Just as a reminder, beside all upcoming post pandemic issues, we have to be also aware that this pandemic exposed global weak points in healthcare, transportation and other systems, which could be taken advantage of in contexts of global conflicts, for example, using biological weapons. Therefore, UN and WHO should be revealing all of these issues, with all nations, to protect humanity.
4.Conclusions
COVID-19 drove the world into a global pandemic situation before healthcare and political systems could react sufficiently. The WHO proved to be just a single element in a larger decision-making process, lacking as it does sufficient power to enforce recommendations. The current set up between the WHO and its state members gives only recommendations, which every country at the end decides to follow or not. Pandemic emergency plans must now be developed and revised, including provisions for global vaccine production capacities. Powers must be given to the WHO or the UN, or any other organization (existing or new) to make sure that production footprints can be assessed and defined without any privileges for powerful groups or specific industries. Intellectual property and exclusivity have to be addressed in specific crisis such as pandemics.
As of today, 17 COVID-19 vaccines have been developed and 13 marketed, all clearly driven by commercial interest [28,46]. Accordingly, some governments are building local facilities for future COVID-19 vaccine producing using business models, to have better coverage and security, but also supply independence [49–51].
Science and politics must learn its lessons, including developing the following points. Time is ticking! [52].
Global system has to be focused on following topics:
Faster pandemic responses and better advance planning
Greater power and accountability for WHO representatives and bodies
A centralized vaccine development program
Increased global capacity evaluation and plans for massive vaccination drives and/or other medication production, distribution and supply
Global health care plans for the fast establishment of recruitment of medical personnel and mobile hospitals
The future is in our hands!
Conflict of interest
None to report.
References
[1] | Pergolizzi JV, LeQuang JA, Taylor R, Wollmuth C, Nalamachu M, Varrassi G Four pandemics: Lessons learned, lessons lost. Signa Vitae. (2021) ;17: (1):1–5. |
[2] | Luca L, Baroiu L, Ciubara AB, Anghel R, Bulgaru Iliescu AI, Anghel L Covid-19 and the Spanish Flu. From suffering to re-silience. Broad Research in Artificial Intelligence and Neuroscience. (2020) ;11: (3):1–7. |
[3] | Piret J, Boivin G. Pandemics throughout history. Front Microbiol. (2021) ;11: :631736. |
[4] | Basco S, Deomenech J, Roses JR. The redistibutive effects of pandemic: Evidence on the Spanish flu. J World Dev. (2021) ;141: :105389. |
[5] | Chan S, Chu J, Zhang Y, Nadarajah S. Count regression model for Covid-19. Physica A. (2021) ;563: :125460. |
[6] | Fathi-Kazerooni S, Rojas-Cessa R, Dong Z, Umpaichitra V. Correlation of subway turnstile entries and Covid-19 incidence and deaths in New York City. Inf Dis Mod. (2021) ;6: :183–94. |
[7] | Gumel AB, Iboi EA, Ngonghala CN, Elbasha EH. A primer on using mathematics to understand COVID-19 dynamics: Modeling, analysis and simulations. Inf Dis Modell. (2021) ;6: :148–68. |
[8] | Kim K, Yoo S, Lee S, Lee D, Lee KH. Network analysis to identify the risk of epidemic spreading. Appl Sci. (2021) ;11: :2997. |
[9] | Sparrow E, Wood JG, Chadwick C, Newall AT, Torvalsden S, Moen A Global production capacity of seasonal and pandemic influenza vaccines in 2019. Vaccine. (2021) ;39: :512–20. |
[10] | Fourie J, Jayes J. Health inequality and the 1918 influenza in South Africa. J World Dev. (2021) ;141: :105407. |
[11] | Abildgren K. Archival big data and the Spanish Flu in Copenhagen. Info Disco Del. (2021) . doi:10.1108/IDD-11-2020-0142. |
[12] | Novaes A. The end has (not yet) come: The 1918 Spanish Flu and the COVID-19 pandemic in Brazilian seventh-day adventist bulletin. Studies in World Christianity. (2021) ;27: :26–47. |
[13] | Gardner K. Decision-making amongst the community in coventry during the 1918–19 Spanish flu pandemic. Midland History. (2021) ;46: :101–118. |
[14] | WHO. World Health Organization. [Available from: https://www.who.int]. |
[15] | Aassve A, Alfani G, Gandolfi F, Le Moglie M. Epidemics and trust: The case of the Spanish Flu. Health Economics. (2021) ;30: :840–57. |
[16] | Dunkley A. Perspective on COVID-19. Int J Risk Saf Med. (2020) ;31: (4):183–91. |
[17] | Adami EA, Chavez Rico SL, Akamatsu MA, Miyaki C, Raw I, de Oliveira D H7N9 pandemic preparedness: A large-scale production of a split inactivated vaccine. Biochem Biophys Res Commun. (2021) ;19: (545):145–9. |
[18] | Bandi H, Bertsimas D. Optimizing influenza vaccine composition: A machine learning approach. Naval Research Logistics. (2021) . doi:10.1002/nav.21974. |
[19] | Sharma O, Sultan AA, Ding H, Triggle CR. A review of the progress and challanges of developing a vaccine for Covid-19. Front Immunol. (2020) ;11: :585354. |
[20] | Claeson M, Hanson S. Covid-19 and the Swedish Enigma. The Lancet. (2020) ;10271: (397):259–61. |
[21] | Mackowiak PA. Prior pandemics. Looking to the past for insight into the COVID-19 pandemic. J Comm Hospital Internal Med Perspect. (2021) ;11: (2):163–70. |
[22] | Landi S, Costantini A, Fasan M, Bonazzi M. Public engagement and dialogic accounting through social media during COVID-19 crisis: A missed opportunity? Accounting, Auditing & Accountability J. doi:10.1108/AAAJ-08-2020-4884. |
[23] | Wikipedia - Vaccine. [Available from: https://en.wikipedia.org/wiki/Vaccine]. |
[24] | Web of Science. [Available from: https://www.webofknowledge.com]. |
[25] | Alzoughool F, Alanagreh L. Coronavirus drugs: Using plasma from recovered patients as a treatment for COVID-19. Int J Risk Saf Med. (2020) ;31: (2):47–51. |
[26] | Alanagreh L, Alzoughool F, Atoum M. Risk of using hydroxychloroquine as a treatment of COVID-19. Int J Risk Saf Med. (2020) ;31: (3):111–6. |
[27] | The New York Times. The Coronavirus Outbreak. Corona Vaccine Tracker. [Available from: https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html\#vector]. |
[28] | Covid-19 Vaccine Tracker. Global overview of candidates, trials and approvals. [Available from: https://covid19.trackvaccines.org/vaccines/]. |
[29] | Bloomberg’s Covid-19 Vaccine Tracker [Available from: https://www.bloomberg.com/graphics/covid-vaccine-tracker-global-distribution/]. |
[30] | Statista. Proportion of selected age groups of world population in 2020, by region [Available from: https://www.statista.com/statistics/265759/world-population-by-age-and-region/]. |
[31] | China/Society. China’s Sinopharm to raise vaccine production to 3 billion per year. [Available from: https://www.globaltimes.cn/page/202103/1217366.shtml]. |
[32] | BioPharma-Reporter. Pfizer and BioNTech ramp up Covid 19 vaccine production to 2.5 billion doses. [Available from: https://www.biopharma-reporter.com/Article/2021/03/31/Pfizer-and-BioNTech-ramp-up-COVID-19-vaccine-production-to-2.5-billion-doses\#:~:text=FREE%20newsletter%20Subscribe-,Pfizer%20and%20BioNTech%20ramp%20up%20COVID%2D19,production%20to%202.5%20billion%20doses&text=Pfizer%20and%20BioNTech%20expect%20to,of%20production%20in%20Marburg%2C%20Germany]. |
[33] | Bottari C. Some reflections on organizational profiles in Italy in the time of COVID-19. Int J Risk Saf Med. (2020) ;31: (3):117–9. |
[34] | Cioffi A, Rinaldi R. COVID-19 and healthcare-associated infections. Int J Risk Saf Med. (2020) ;31: (4):181–2. |
[35] | Orenstein AW, Mootrey G, Pazol K, Hinman RA. Financing immunization of adults in the United States. Clinical Pharmacol Therapeutics. (2008) ;82: (6):764–8. |
[36] | Nord M, Ysander M, Sullivan T, Patel M. Practical consideration for creating a strategic and proactive clinical safety and pharmacovigilance organization for the future. Int J Risk Saf Med. (2021) ;32: (1):1–16. |
[37] | Breggin PR. Moving past the vaccine/autism controversy – to examine potential vaccine neurological harms. Int J Risk Saf Med. (2021) ;32: (1):25–39. |
[38] | Stack S, Rockett IRH. Social distancing predicts suicide rates: Analysis of the 1918 flu pandemic in 43 large cities, research note. Suicide Life Threat Behav. (2021) ;00: :1–3. |
[39] | Simou E, Koutsogeorgou E.. Effects of the economic crises on health and healthcare in Greece in the literature from 2009 to 2013: A systemic review. Health Policy. (2014) ;115: :111–119. |
[40] | Suciu MC, Stan C, Nagel-Piciorus L, Imbrisca C. The post-crisis healthcare system; Effects of the economic crisis in Romania. Theoretical and Applied Economics. (2012) ;5: (570):157–168. |
[41] | Banks J, Karjalainen H, Propper C. Recessions and health: The long-term health consequences of responses to coronavirus. The Institute for Fiscal Studies. (2020) , IFS Briefing Note BN281. |
[42] | Arora A. The next three epochs: Health system challenges amidst and beyond the COVID-19 era. Int J Health Plann Mgmt. (2021) ;1–4. |
[43] | Coelho IL, Sousa-Uva M, Pina N, Marques S, Matias-Dias C, Rodrigues AP. Economic crisis in Portugal: Trajectory of the incidence of depression and correlation with unemployment. Acta Med Port. (2021) ;34: (4):278–82. |
[44] | Cam HH, Top FU, Ayyildiz TK. Impact of the COVID-19 pandemic on mental health and health-related quality of life among university students in Turkey. Current Psychol. (2021) . doi:10.1007/s12144-021-01674-y. |
[45] | Enriquez-Fernandez S, del Castillo-Rodriguez C. Healthcare risk management during the SARS-CoV-2 virus pandemic in the European Union: The guaranteed access to medicines. Int J Risk Saf Med. (2021) ;32: (1):1–10. |
[46] | IQVIA European Thought Leadership 22/04/2021 (report) [Available from: https://www.iqvia.com/insights/the-iqvia-institute/reports]. |
[47] | Grech V, Grech P, Fabri S. A risk balancing act – Tourism competition using health leverage in the COVID-19 era. Int J Risk Saf Med. (2020) ;31: (3):121–30. |
[48] | Jin X, Zhao Y, Song W, Zhao T. Save for safe: Effect of COVID-19 pandemic on consumers’ saving and spending behavior in China. Front Psychol. (2021) ;12: 636859. |
[49] | Reuters. Brazil eyes July for full local production of AstraZeneca vaccine. [Available from: https://www.reuters.com/article/us-health-coronavirus-brazil-astrazeneca-idUSKBN2B72IU]. |
[50] | Global Times. Brazil trusts Chinese-made vaccine; hopes stronger ties unaffected by US political sphere: Ambassador. [Available from: https://www.globaltimes.cn/page/202104/1222276.shtml]. |
[51] | Reuters. Serbia plans to start manufacturing Sinopharm, Sputnik shots. [Available from: https://www.reuters.com/article/us-health-coronavirus-serbia-vaccine-idUSKBN2B32V2]. |
[52] | Responsible life science police between private and public funding. Rade Injac: Global pandemic vaccine development, studies, production, distribution, pricing, acceptance, funding for 7.8 billion people! [Available from: https://www.youtube.com/watch?v=IP6I_SF2RO0]. |




