Acute renal failure and cardiac arrhythmias associated with remdesivir use in patients with COVID-19 infections: Analysis using the US FDA adverse event reporting system
Abstract
BACKGROUND:
Recently, antivirals, including remdesivir, have been repurposed to treat COVID-19 infections. Initial concerns have been raised about the adverse renal and cardiac events associated with remdesivir.
OBJECTIVE:
This study aimed to analyse the adverse renal and cardiac events associated with remdesivir in patients with COVID-19 infections using the US FDA adverse event reporting system.
METHOD:
A case/non-case method was used to determine adverse drug events associated with remdesivir as the primary suspect drug between January 1, 2020, and November 11, 2021, for patients with COVID-19 infections. Cases were reports for remdesivir with ≥1 ADEs as preferred terms included in the Medical Dictionary of Regulatory Activities (MedDRA) system organ classes ‘Renal and urinary disorders’ or ‘cardiac’ disorders. To measure disproportionality in reporting of ADEs, frequentist approaches, including the proportional reporting ratio (PRR) and reporting odds ratio (ROR), were used. The empirical Bayesian Geometric Mean (EBGM) score and information component (IC) value were calculated using a Bayesian approach. A signal was defined as the lower limit of 95% confidence intervals of ROR ≥ 2, PRR ≥ 2, IC > 0, and EBGM > 1 for ADEs with ≥4 reports. Sensitivity analyses were undertaken by excluding reports for non-Covid indications and medications strongly associated with AKI and cardiac arrhythmias.
RESULTS:
In the main analysis for remdesivir use in patients with COVID-19 infections, we identified 315 adverse cardiac events comprising 31 different MeDRA PTs and 844 adverse renal events comprising 13 different MeDRA PTs. Regarding adverse renal events, disproportionality signals were noted for “renal failure” (ROR = 2.8 (2.03–3.86); EBGM = 1.92 (1.58–2.31), “acute kidney injury” (ROR = 16.11 (12.52–20.73); EBGM = 2.81 (2.57–3.07), “renal impairment” (ROR = 3.45 (2.68–4.45); EBGM = 2.02 (1.74–2.33). Regarding adverse cardiac events, strong disproportionality signals were noted for “electrocardiogram QT prolonged” (ROR = 6.45 (2.54–16.36); EBGM = 2.04 (1.65–2.51), “pulseless electrical activity” (ROR = 43.57 (13.64–139.20); EBGM = 2.44 (1.74–3.33), “sinus bradycardia” (ROR = 35.86 (11.16–115.26); EBGM = 2.82 (2.23–3.53), “ventricular tachycardia” (ROR = 8.73 (3.55–21.45); EBGM = 2.52 (1.89–3.31). The risk of AKI and cardiac arrythmias were confirmed by sensitivity analyses.
CONCLUSION:
This hypothesis-generating study identified AKI and cardiac arrhythmias associated with remdesivir use in patients with COVID-19 infections. The relationship between AKI and cardiac arrhythmias should be further investigated using registries or large clinical data to assess the impact of age, genetics, comorbidity, and the severity of Covid infections as potential confounders.
1.Introduction
The unexpected effects of COVID-19 (coronavirus, SARS CoV-2), an infectious disease caused by the SARS-CoV-2 virus, remain prevalent. In efforts to combat the spread of COVID-19, 50.3% of the global population has been vaccinated, and several therapeutics such as tocilizumab, remdesivir and casirivimab/imdevimab have been repurposed and approved as COVID-19 treatments [1–4]. In addition, a nirmatrelvir/ritonavir combination also leads the conversation inaccessible, at-home COVID-19 treatment options [5]. Despite this, the uncertainty surrounding COVID-19, intermittent surges of COVID-19 variants, and subsequent lack of protection against COVID-19 means that the demand for COVID-19 treatments is still as apparent as ever, and drug repurposing is a particularly interesting avenue in the fight against COVID-19 [6].
Drug repurposing involves identifying alternative uses and indications for existing drugs. For example, after gaining EMA and FDA approval in May 2020 and October 2020, respectively, remdesivir can be used to treat COVID-19 [4,7]. Therefore, as of January 2021, remdesivir is administered in COVID-19 patients with pneumonia requiring low-flow supplemental oxygen and COVID-19 in immunocompromised patients with pneumonia (under close medical supervision).
Remdesivir, a therapeutic for COVID-19, is currently used on conditional recommendation, as there is “small net benefit or little difference between alternatives” [2]. Whilst remdesivir has been identified as a “beacon of hope” regarding COVID-19 [8], it is important to note the lack of safety data, specifically surrounding its use in COVID-19 and adverse drug events [3,4]. Given that remdesivir is a considered treatment for COVID-19, with plans to expand its use in COVID-19 patients, it is vital to investigate its safety profile in post-marketing data thoroughly. In doing so, research can help support the risk/benefit calculations of remdesivir use, as well as contribute to discussions surrounding its use, specifically whether there are special patient groups, i.e. renally impaired individuals, that it should or should not be administered to, and whether clinical monitoring, such as eGFR and ECG monitoring should become a requirement [9]. The safety of remdesivir is particularly important as it contains sulfobutyletherbeta-cyclodextrin (SBECD). This can accumulate in patients with kidney impairment, contributing to the hypothesis that remdesivir may be nephrotoxic [10].
RCTs and systematic reviews have been conducted to investigate the efficacy and safety of remdesivir [11]. However, a common theme amongst these studies is that they fail to delve deeper into the safety outcomes of remdesivir. Where they are noted, they do not investigate specific ADEs nor consider their consequences. For example, RCTs conducted by Beigel et al. and Spinner et al. showed that remdesivir shortened the time to recovery and was superior to standard care and mentioned incidence and a brief description of adverse events [12,13]. Similarly, Singh et al. and Ansems et al. noted that increased alanine transaminase (ALT), increased aspartate transaminase (AST), and decreased creatinine clearance were commonly reported with remdesivir use [14,15]. However, there is a significant lack of safety information and data surrounding all remdesivir adverse events.
This study analysed acute renal failure (ARF) and cardiac arrhythmia associated with remdesivir using the US FDA Adverse Events Reporting System (FAERS).
2.Methods
2.1.Ethical approval
The Ethical Implications of Research Activity Form to conduct this study was approved by the University of Bath.
2.2.Data source
A retrospective case/non-case study was conducted using the Elsevier PharmaPendium database. The use of PharmaPendium for drug safety research is described elsewhere [16]. The PharmaPendium database includes individual case safety reports (ICSRs) from the Food and Drug Administration adverse event reporting system (FAERS). The FAERS database includes spontaneous adverse event reporting systems submitted to the US Food and Drug Administration (FDA) that can be used to identify early signals of a potential drug-related safety issue [17]. This database includes reports that detail drugs and their associated AEs. They are reported by health professionals, consumers, and manufacturers, containing 15 million + adverse event reports, updated quarterly [16]. Each ICSR contains detailed information about the primary and secondary suspected drugs, concomitant drugs’ gender, age, reporter occupation, outcome, and event location.
2.3.Study design and participants
ICSRs for remdesivir, the primary suspect drug, were extracted from FAERS from January 1, 2020, to November 11, 2021. Cases were reports for remdesivir with at least one ADE included in the MedDRA SOC’ renal and urinary disorders’ or ‘cardiac disorders’. Non-cases were all other remaining reports for remdesivir. Our comparator group was other antivirals repurposed to treat Covid-19 infections, including tocilizumab, baricitinib and ribavirin.
An SMQ analysis was conducted on the 50 ADRs, providing preferred terms (PTs), subsequently mapped to SOC, HLGT and HLT. From this, 2 terms, “acute renal failure” (ARF) and “cardiac arrhythmias” and their corresponding PTs, were selected. These two terms were chosen as they contain previously identified PTs that may demonstrate high disproportionality safety signals, piquing researcher interest. This, paired with the idea that COVID-19 may also cause AKI (PT categorised under ARF), made ARF an interesting term worth investigating further.
2.4.Adverse drug reaction definition
The preferred terms (PTs) recommended in the Medical Dictionary of Regulatory Activities were used to define adverse events associated with remdesivir. Each PT belongs to the hierarchal system, linking to a higher-level term (HLT), higher-level group term (HLGT) and system organ class (SOC) [18].
2.5.Statistical analysis
The ratio of case/non-cases for ADEs associated with remdesivir was compared to the case/non-cases for all other repurposed drugs to treat Covid-19 infections for the same study period. Disproportionality analyses were used to generate the proportional reporting ratio (PRR) and reporting odds ratio (ROR). The information component (IC) and Empirical Bayesian Geometric Mean (EBGM) for each ADE were calculated using the Bayesian approach. A signal was determined if an ADE had ≥4 reports, and the lower limit of the 95% CI was ≥2 for ROR, ≥2 for PRR, >0 for IC value, and >1 for EBGM value. All analyses were performed using the statistical software ‘R’ (version 3.6.1) [19]. Disproportionality measures were calculated for all relevant PTs categorised under acute renal failure (ARF) and cardiac arrhythmias. The ROR and EBGM were calculated for all ARF and cardiac arrhythmias PTs. This data was then graphically illustrated as forest plots.
2.6.Sensitivity analyses
The initial de-duplicated dataset included remdesivir use for all indications. Our main analysis was a disproportionality analysis on data restricted for remdesivir for COVID listed as the primary indication. Therefore, it was important to exclude biases to test the robustness of the signal for AKI. Sensitivity analyses were conducted on (i) restricted data for COVID bias by removing all ICSR reports for Covid indication; (ii) excluding reports with drugs strongly associated with AKI and (concomitant drug bias); (iii) drugs associated with cardiac arrhythmias (concomitant drug bias).
Drugs associated with concomitant drug bias for AKI included trimethoprim, furosemide, metformin hydrochloride, diclofenac sodium, ibuprofen, vancomycin hydrochloride, naproxen, angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), calcium channel blockers, gentamicin, lithium, methotrexate, proton pump inhibitors (PPIs), statins, fibrates, colchicine, theophylline, or benzodiazepines.
Drugs for concomitant drug bias associated with cardiac arrhythmias included donepezil, neostigmine, physostigmine, pyridostigmine bupivacaine, propofol, adenosine, amiodarone disopyramide, dronedarone, flecainide, ivabradine, propafenone, quinidine, sotalol, thalidomide, citalopram/escitalopram, fluoxetine, clonidine, beta-blockers, diltiazem verapamil, digoxin, fingolimod, or dipyridamole [20].
3.Results
16,912 drug event pairs between January 2020 and November 2021, recorded in the FAERs database, were obtained for remdesivir and comparator drug groups. From this, 5174 AEs belonged to remdesivir. Of the 5174 AEs, 4621 had COVID-19 listed as the primary indication. The mean age of the patients within the 4621 reports for remdesivir was 62.2 years, and 38% of patients identified as female (Table 1). In addition, 1.3%, 7.4%, 75% and 14% of the reports were reported by consumers, health professionals, pharmacists, and physicians (Table 1). The main continent of origin was North America, with 89% of reports originating in the US. This is followed by 6.6% in Europe, 2.9% in Asia, and the remainder in South America, Australia, and Oceania. 29.8% (1378/4621) of the ICSRs were expedited (Table 1). Most patients administered remdesivir therapy were 75+ years old (Table 1). We also mapped remdesivir-associated renal and cardiac events at different MedDRA levels (Figs 1 and 2).
Table 1
Characteristics of the population included in the case reports
| Characteristic | Remdesivir, N = 4,621 |
| Age, median (IQR) | 65 (51, 75) |
| Unknown | 223 |
| Age range (%) | |
| <18 | 77 (1.7%) |
| 18–24 | 75 (1.6%) |
| 25–34 | 238 (5.2%) |
| 35–44 | 344 (7.4%) |
| 45–54 | 563 (12%) |
| 55–64 | 883 (19%) |
| 65–74 | 1048 (23%) |
| ≥ 75 | 1170 (25%) |
| Unknown | 223 (4.8%) |
| Gender, n (%) | |
| Female | 1,772 (38%) |
| Male | 2,793 (60%) |
| Unknown | 56 (1.2%) |
| Reporter occupation, n (%) | |
| Consumer | 61 (1.3%) |
| Health professional | 342 (7.4%) |
| Pharmacist | 3,477 (75%) |
| Physician | 625 (14%) |
| Unknown | 116 (2.5%) |
| Manufacturer, n (%) | |
| FDA-CTU | 3,179 (69%) |
| GILEAD | 1,442 (31%) |
| Report type, n (%) | |
| Direct | 3,179 (69%) |
| Expedited | 1,378 (30%) |
| Periodic | 64 (1.4%) |
| Outcomes, n (%) | |
| Death | 655 (14%) |
| Death, other | 347 (7.5%) |
| Disability | 11 (0.2%) |
| Hospitalization, other | 166 (3.6%) |
| Other | 1,607 (35%) |
| Unknown | 1,060 (23%) |
Fig. 1.
Mapping of remdesivir-associated renal events at different MeDRA levels: HLGT, high-level group term; HLT, high level term; SOC, system organ class. Orange boxes include terms that resulted in disproportionality signals; MeDRA: Medical Dictionary for Regulatory Activities.
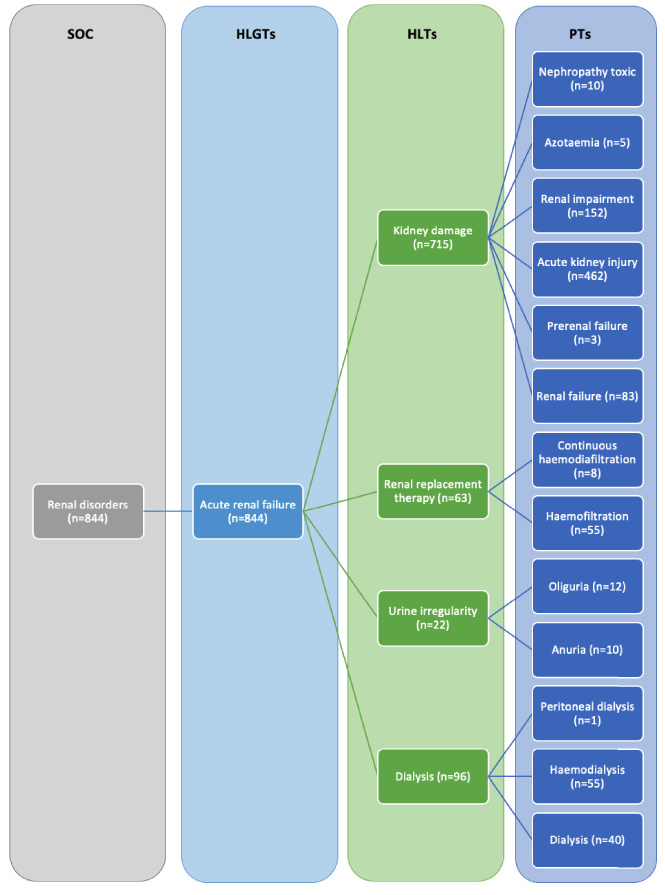
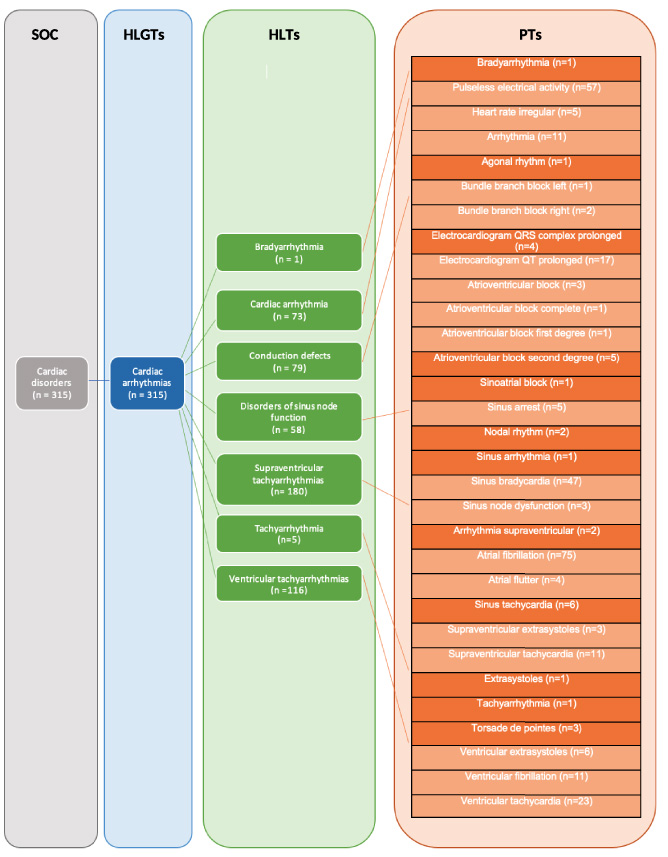
Fig. 2.
Mapping of remdesivir-associated cardiovascular events at different MeDRA levels: HLGT, high-level group term; HLT, high level term; SOC, system organ class. Light orange boxes include terms that resulted in disproportionality signals; MeDRA: Medical Dictionary for Regulatory Activities.
In the main analyses, strong disproportionality signals were noted for: “renal failure” (ROR = 2.8 (2.03–3.86); EBGM = 1.92 (1.58–2.31), “acute kidney injury” (ROR = 16.11 (12.52–20.73); EBGM = 2.81 (2.57–3.07), “renal impairment” (ROR = 3.45 (2.68–4.45); EBGM = 2.02 (1.74–2.33), “electrocardiogram QT prolonged” (ROR = 6.45 (2.54–16.36); EBGM = 2.04 (1.65–2.51), “pulseless electrical activity” (ROR = 43.57 (13.64–139.20); EBGM = 2.44 (1.74–3.33), “sinus bradycardia” (ROR = 35.86 (11.16–115.26); EBGM = 2.82 (2.23–3.53), “ventricular tachycardia” (ROR = 8.73 (3.55–21.45); EBGM = 2.52 (1.89–3.31).
(4621/5174) 89.3% of reported indications were COVID-19, with the remaining 10.7% of indications being: product used for the unknown indication (148/5174), pneumonia (4/5174), cardiac disorder (2/5174), hypertension (1/5174), wrong drug (1/5174), viral infection (1/5174), upper respiratory tract infection (1/5174), seizure (1/5174), respiratory failure (1/5174), off-label (1/5174), heart disease congenital (1/5174), acute respiratory failure (1/5174), or acute respiratory distress syndrome (1/5174).
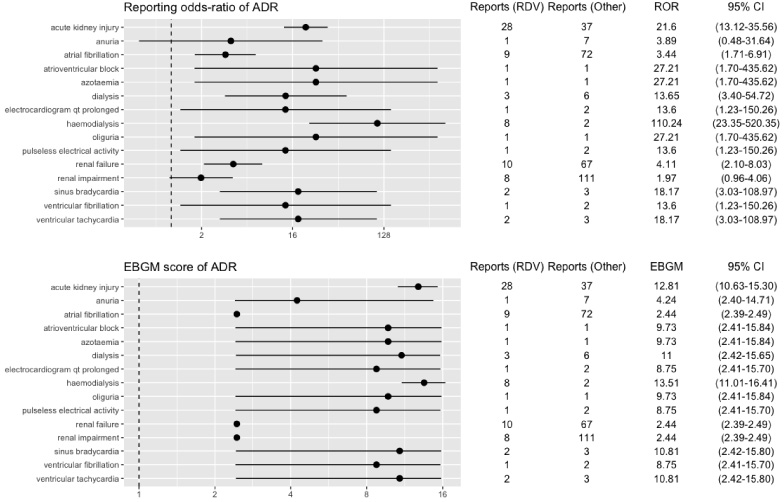
We found that important signals were retained in the sensitivity analysis conducted on restricted data for COVID bias by removing all ICSR reports for Covid indication (Table 2, Fig. 3). Similar results were reported in the sensitivity analysis conducted on restricted data for non-COVID bias data (Table 2, Fig. 4).
Table 2
Disproportionality analyses for remdesivir on renal and cardiac preferred terms
| Preferred term (PT) | Main analysis (ROR, 95% CI) | COVID bias (ROR, 95% CI) | Non-COVID bias (ROR, 95% CI) | Concomitant drug bias (ROR, 95% CI) |
| Renal failure | 2.80 (2.03–3.86) | 1.64 (0.84–3.18) | 3.46 (1.76–6.82) | 1.26 (0.83–1.91) |
| Acute kidney injury | 16.11 (12.52–20.73) | 2.73 (1.94–3.85) | 20.11 (11.82–34.20) | 12.47 (9.02–17.25) |
| Renal impairment | 3.45 (2.68–4.45) | 2.32 (1.33–4.03) | 1.75 (0.84–3.63) | 2.40 (1.71–3.35) |
| Atrial fibrillation | 2.20 (1.60–3.02) | 1.30 (0.68–2.47) | 2.60 (1.29–5.24) | 1.97 (1.35–2.87) |
| Atrioventricular block | 2.27 (0.46–11.25) | 0.23 (0.03–1.60) | 19.17 (1.20–306.94) | 2.62 (0.53–12.96) |
| Electrocardiogram QT prolonged | 6.45 (2.54–16.36) | 0.85 (0.28–2.56) | 9.59 (0.87–105.87) | 6.12 (2.35–15.93) |
| Pulseless electrical activity | 43.57 (13.64–139.20) | 3.95 (1.23–12.68) | N/A | 35.18 (10.88–113.78) |
| Sinus bradycardia | 35.86 (11.16–115.26) | 4.90 (1.18–20.25) | 38.41 (3.48–424.31) | 34.30 (10.59–111.02) |
| Ventricular fibrillation | 6.25 (1.99–19.64) | 0.75 (0.21–2.74) | 19.17 (1.20–306.94) | 5.24 (1.58–17.40) |
| Ventricular tachycardia | 8.73 (3.55–21.45) | 1.58 (0.47–5.32) | 12.80 (2.13–76.78) | 9.45 (3.50–25.46) |
Fig. 3.
RORs and EBGM scores with 95% CIs for PTs with remdesivir-associated renal and cardiac events based on COVID bias data. ADR, adverse drug reaction; CI, confidence interval; EBGM, Empirical Bayesian geometric mean; RDV, remdesivir; ROR, reporting odds ratio.
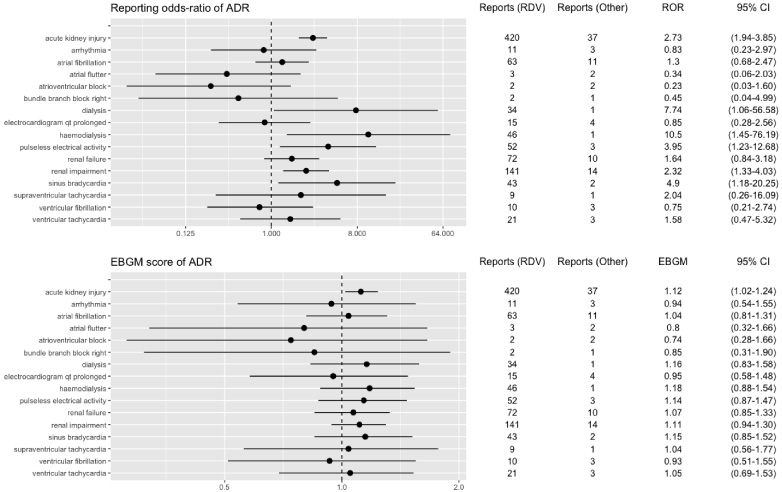
Fig. 4.
RORs and EBGM scores with 95% CIs for PTs with remdesivir-associated renal and cardiac events based on non-COVID bias data. ADR, adverse drug reaction; CI, confidence interval; EBGM, Empirical Bayesian geometric mean; RDV, remdesivir; ROR, reporting odds ratio.
4.Discussion
The analysis of 16,912 FDA spontaneous reports of repurposed drugs for COVID-19 uncovered novel signals for remdesivir linking it to AKI and cardiac arrhythmias.
4.1.Acute kidney injury
The relatively strong disproportionality signals for AKI associated with remdesivir in our main analyses are confirmed by sensitivity analyses. However, the observed disproportionality signals for AKI are inconsistent with findings from other clinical studies, including a systematic review conducted by Xu et al., who concluded that AKI presence in patients with COVID-19 is not induced remdesivir but rather by COVID [21]. Similarly, a study by Adamsick et al. suggested that severe COVID-19 infections lead to AKI in up to 20–40% of critically ill patients [22]. A study conducted by Chan et al. concluded that AKI is common in patients hospitalised with COVID-19 [23]. The EMA highlighted AKI as a potential safety signal of remdesivir in February 2021 but have since concluded that there is no evidence to indicate that the reported kidney problems are associated with remdesivir. Similarly, NICE COVID-19 rapid guidelines note that AKI is common in patients with COVID-19. It also mentions that treatments for COVID-19 may increase the risk of AKI [3]. Further evidence is required to understand the risk of AKI associated with remdesivir as our analyses did not control for potential confounders, including age, ethnicity, dose, and comorbidity.
However, our findings align with two retrospective studies conducted on ICSRs reported in Vigibase and a preliminary FAERS study [13,24]. We found the reporting for the remdesivir-AKI combination is significantly higher in COVID bias data (n = 420) than non-COVID bias data (n = 28). However, the ROR of the remdesivir-AKI combination in non-COVID bias data (ROR = 20.1) still demonstrates a relatively strong signal. In addition, we uncovered several renal ADEs (predisposed to AKI, i.e., renal failure, azotaemia, renal impairment) with relatively high disproportionality signals. Further, it supports the plausibility that there may be an association between remdesivir and COVID-19, possibly mediated by a sulfobutyletherbeta-cyclodextrin moiety of remdesivir accumulates in renal impairment and is nephrotoxic. The current guidance from the manufacturer is to avoid remdesivir in patients with an eGFR of less than 30 ml/min [25].
4.2.Cardiac arrhythmias
Analyses of high-level MedDRA terms (Fig. 2) suggest that remdesivir therapy is associated with sinus bradycardia [ROR = 35.86 (11.16–115.26); EBGM = 2.82 (2.23–3.53)]. These findings are consistent with a recent case study by Day et al. and a case report by Ching et al. that concluded sinus bradycardia is likely associated with remdesivir therapy [26,27]. Therefore, prescribers should be aware of these serious ADEs with remdesivir therapy. Even though causality cannot be determined by disproportionality analysis alone, it is worth noting that sinus bradycardia does reverse upon discontinuation of remdesivir, as shown by the study conducted by Attena et al. [28]. Furthermore, signals for ventricular arrhythmias and pulseless electrical activity should also be discussed.
4.3.Strengths and limitations
However, there are limitations to this study. Firstly, this study uses data solely from the FAERS database. Whilst this is advantageous as it provides a very large quantity and variety of reports on a somewhat global scale, it is worth mentioning that the data in this database is primarily of US origin. In addition to this, the data is unrepresentative of the global population, and the findings cannot be extrapolated to other populations. Furthermore, ADEs can be reported by health professionals, pharmacists, consumers, manufacturers, or physicians, and hence there are often variations in how ADEs are described and categorised.
An example is haemodialysis, which found high disproportionality in reporting haemodialysis. However, upon closer inspection, haemodialysis itself is not an ADE. The term haemodialysis, used throughout the FAERS database, likely relates to a consequence of AKI, as documented by the MedDRA hierarchy. As a result, all ICSRs with haemodialysis listed as an ADE will not be correctly considered AKI unless closely examined. Similarly, two identical terms, e.g., hypertension and high blood pressure, can be listed as two different ADEs, despite being the same.
Due to the potential under-reporting, ICSR reports submitted to FAERS are voluntary and may not reflect all ADRs. In addition, this study analyses spontaneous reports but does not determine the causality between a drug and an adverse drug event. Therefore, the impact of potential confounders, including age, gender, and comorbidities, was not assessed. However, we considered concomitant drugs administered with remdesivir to mitigate concomitant bias (statistical analyses conducted on data that exclude drugs that may cause AKI or sinus bradycardia). In addition, the FAERS database does not include patient conditions (excluding indication). Therefore, it was not possible to assess whether patient medical conditions, specifically those that cause or potentiate AKI or sinus bradycardia, were present, whether this affects this study’s results, and how the subsequent information is contextualised.
Another limitation of SRS is under-reporting. The system solely relies on individuals (health professionals, pharmacists, physicians, and consumers) reporting adverse drug events voluntarily. With ∼10% of serious ADRs being reported and less than 1% of suspected serious ADRs being reported, it is worth noting that this is an extremely small minority of ADRs being reported [29]. This means that the ADEs in the database only make up a small amount of all the potential ADEs associated with that drug. Further analyses in different settings and with different patient populations are warranted to corroborate our study findings.
Nevertheless, the findings from this study can inform appropriate clinical practice surrounding remdesivir use. For example, it may be worth assessing and monitoring the patient’s eGFR following initiation of remdesivir. These findings may also highlight the need for dose adjustments for patients with renal impairment.
Contrastingly, there are also strengths to this study. We mitigated concomitant drug bias and indication bias to assess the robustness of the findings. Sensitivity analyses completed on ICSRs that excluded the concomitant use of drugs that can potentiate AKI or cardiac arrhythmias contribute to this study’s strength.
5.Conclusion
The risk of sinus bradycardia is likely associated with remdesivir use in patients with COVID-19 infections. The risk of acute kidney injury is more likely associated with COVID-19 rather than remdesivir use in patients with COVID-19 infections. Even though this study could not determine causality, it is worth monitoring sinus bradycardia and AKI and, where appropriate, determining renal and cardiac function before initiating and monitoring remdesivir therapy. While there is a plethora of small RCTs and observational studies on the efficacy and safety of remdesivir, there is still a notable lack of research into specific adverse events associated with remdesivir and its risks and potential consequences. Therefore, findings from analyses of post-marketing data may be useful as temporary advice until appropriate and sufficient data on its safety is made available.
Author contributions
Study concept and design: Lisajo Orogun and Prasad S. Nishtala; Statistical analysis: Prasad S. Nishtala, Te-yuan Chyou; Interpretation of data: All authors; Drafting of the manuscript: Lisajo Orogun and Prasad S. Nishtala; Critical revision of the manuscript for important intellectual content: All authors; Study supervision: Prasad S. Nishtala.
Conflict of interest
None of the authors declare any conflict of interest.
Data sharing
All relevant raw data for this study is available from the corresponding author upon reasonable request.
Ethics statement
The study was approved by the Departments Ethics Officer of the University of Bath. The study used publicly available data for its analysis.
Informed consent
Formal consent is not required for this type of study.
Funding
No funding was received to conduct this study.
References
[1] | Mathieu E, Ritchie H, Rodés-Guirao L, Appel C, Giattino C, Hasell J “Coronavirus Pandemic (COVID-19”. 2020. Published online at OurWorldInData.org. [updated 2023 May 8]. Available from: ‘https://ourworldindata.org/coronavirus’. |
[2] | European Medicines Agency. Paxlovid for the treatment of COVID-19-EMEA/H/A-5(3)/1513 [Internet]. 2021 [updated 2022 Jan 2]. Available from: https://www.ema.europa.eu/en/documents/referral/paxlovid-pf-07321332-ritonavir-covid-19-article-53-procedure-conditions-use-conditions-distribution_en.pdf. |
[3] | National Institute for Health and Care Excellence. COVID-19 rapid guideline: Managing COVID-19 [Internet]. V19. 2021 [updated 2022 Jan 2]. Available from: https://app.magicapp.org/\#/guideline/L4Qb5n/section/LAJvRn. |
[4] | Bellino S. COVID-19 treatments approved in the European Union and clinical recommendations for the management of non-hospitalized and hospitalized patients. Ann Med. (2022) ;54: (1):2856–60. |
[5] | Shah MM, Joyce B, Plumb ID, Sahakian S, Feldstein LR, Barkley E Paxlovid associated with decreased hospitalization rate among adults with COVID-19 - United States, April–September 2022. Am J Transplant. (2023) ;23: (1):150–5. |
[6] | Oliver JC, Silva EN, Soares LM, Scodeler GC, Santos AS, Corsetti PP Different drug approaches to COVID-19 treatment worldwide: an update of new drugs and drugs repositioning to fight against the novel coronavirus. Ther Adv Vaccines Immunother. (2022) ;10: :25151355221144845. |
[7] | US Food and Drug Administration (FDA). Coronavirus (COVID-19) Update: FDA Authorizes First Oral Antiviral for Treatment of COVID-19 [Internet]. 2021 [updated 2022 Jan 2]. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-oral-antiviral-treatment-covid-19. |
[8] | Nili A, Farbod A, Neishabouri A, Mozafarihashjin M, Tavakolpour S, Mahmoudi H. Remdesivir: a beacon of hope from Ebola virus disease to COVID-19. Rev Med Virol. (2020) ;30: (6):1–13. |
[9] | British National Formulary. Remdesivir [Internet]. 2021 [updated 2022 Jan 4]. Available from: https://bnf.nice.org.uk/drug/remdesivir.html. |
[10] | Luke DR, Wood ND, Tomaszewski KE, Damle B. Pharmacokinetics of sulfobutylether-𝛽-cyclodextrin (SBECD) in subjects on hemodialysis. Nephrol Dial Transplant. (2012) ;27: (3):1207–12. |
[11] | Grundeis F, Ansems K, Dahms K, Thieme V, Metzendorf MI, Skoetz N Remdesivir for the treatment of COVID-19. Cochrane Database Syst Rev. (2023) ;1: (1):Cd014962. |
[12] | Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC Remdesivir for the Treatment of Covid-19 – Final Report. N Engl J Med. (2020) ;383: (19):1813–26. |
[13] | Spinner CD, Gottlieb RL, Criner GJ, Arribas López JR, Cattelan AM, Soriano Viladomiu A Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. (2020) ;324: (11):1048–57. |
[14] | Singh A, Kamath A. Assessment of adverse events associated with remdesivir use for coronavirus disease 2019 using real-world data. Expert Opin Drug Saf. (2021) ;20: (12):1559–64. |
[15] | Ansems K, Grundeis F, Dahms K, Mikolajewska A, Thieme V, Piechotta V Remdesivir for the treatment of COVID-19. Cochrane Database Syst Rev. (2021) ;8: (8):Cd014962. |
[16] | Clark M, Steger-Hartmann T. A big data approach to the concordance of the toxicity of pharmaceuticals in animals and humans. Regul Toxicol Pharmacol. (2018) ;96: :94–105. |
[17] | US Food and Drug Administration (FDA). FDA Adverse Event Reporting System (FAERS): Latest Quarterly Data Files. FDA. 2023 [updated 2023 Feb 8]. Available from: https://www.fda.gov/drugs/fda-adverse-event-reporting-system-faers/faers-data-files-quarterly. |
[18] | Introductory Guide to MedDRA Version 25.1. McLean, Virginia [updated 2012 September]; [updated 2022 September 1]. Available from: http://www.meddra.org/how-to-use/support-documentation. |
[19] | R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available from: https://www.R-project.org/. |
[20] | Nishtala PS, Gill S, Chyou TY. Analysis of the US FDA adverse event reporting system to identify adverse cardiac events associated with hydroxychloroquine in older adults. Pharmacoepidemiol Drug Saf. (2020) ;29: (12):1689–95. |
[21] | Xu Z, Tang Y, Huang Q, Fu S, Li X, Lin B Systematic review and subgroup analysis of the incidence of acute kidney injury (AKI) in patients with COVID-19. BMC Nephrol. (2021) ;22: (1):52. |
[22] | Adamsick ML, Gandhi RG, Bidell MR, Elshaboury RH, Bhattacharyya RP, Kim AY Remdesivir in patients with acute or chronic kidney disease and COVID-19. J Am Soc Nephrol. (2020) ;31: (7):1384–6. |
[23] | Chan L, Chaudhary K, Saha A, Chauhan K, Vaid A, Zhao S AKI in hospitalized patients with COVID-19. J Am Soc Nephrol. (2021) ;32: (1):151–60. |
[24] | Charan J, Kaur RJ, Bhardwaj P, Haque M, Sharma P, Misra S Rapid review of suspected adverse drug events due to remdesivir in the WHO database; findings and implications. Expert Rev Clin Pharmacol. (2021) ;14: (1):95–103. |
[25] | Electronic Medicines Compendium (EMC). Veklury 100 mg powder for concentrate for solution for infusion. Electronic Medicines Compendium (EMC). 2022 [updated Feb 8]. Available from: https://www.medicines.org.uk/emc/product/11597/smpc\#gref. |
[26] | Day LB, Abdel-Qadir H, Fralick M. Bradycardia associated with remdesivir therapy for COVID-19 in a 59-year-old man. CMAJ. (2021) ;193: (17):E612-e5. |
[27] | Ching PR, Lee C. Remdesivir-associated bradycardia. BMJ Case Rep. (2021) ;14: (9):e245289. |
[28] | Attena E, Albani S, Maraolo AE, Mollica M, De Rosa A, Pisapia R Remdesivir-induced Bradycardia in COVID-19: a single center prospective study. Circ Arrhythm Electrophysiol. (2021) ;14: (7):e009811. |
[29] | Goldman SA. Limitations and strengths of spontaneous reports data. Clin Ther. (1998) ;20: (Suppl C):C40–4. |




