The impact of HIV on the risk of COVID-19 death among hospitalized patients
Abstract
BACKGROUND:
Little is known about the association between Human Immunodeficiency Virus (HIV) infection and risk of death among hospitalized COVID-19 patients. We aimed to investigate this association using a multicenter study.
MATERIAL AND METHODS:
This multicenter study was conducted using the registry database of Coronavirus Control Operations Headquarter from March 21, 2021 to January 18, 2020 in the province of Tehran, Iran. The interest outcome was COVID-19 death among hospitalized patients living with and without HIV. The Cox regression models with robust standard error were used to estimate the association between HIV infection and risk of COVID-19 death. The subgroup and interaction analysis were also performed in this study.
RESULTS:
326052 patients with COVID-19 were included in the study, of whom 127 (0.04%) were living with HIV. COVID-19 patients with HIV were more likely to be female, older, and to have symptoms such as fever, muscular pain, dyspnea and cough. The death proportion due to COVID-19 was 18 (14.17%) and 21595 (6.63%) among HIV and non-HIV patients, respectively. Patients living with HIV had lower mean survival time compared to those without HIV (26.49 vs. 15.31 days,
CONCLUSION:
We found no strong evidence of association between HIV infection and higher risk of COVID-19 death among hospitalized patients. To determine the true impact of HIV on the risk of COVID-19 death, factors such as age, comorbidities, hospital ward, viral load, CD4 count, and antiretroviral treatment should be considered.
1.Introduction
Since December 2019, SARS-CoV-2 (SARS-CoV-2) has become a health emergency. It has caused more than 346 million infections and over 5.5 million deaths as of 22 January 2022 [1]. Several factors have been reported as predictors of severe outcomes and death, including older age, being male, and comorbidities such as hypertension, diabetes, and those involving immune-suppression like Human Immunodeficiency Virus (HIV) [2, 3].
Overall, immunocompromised patients have a poorer prognosis when it comes to serious infections. Prior respiratory virus epidemics, particularly seasonal influenza, were associated with poor outcomes due to HIV [4, 5].
HIV patients who are not receiving HIV treatment or who have low CD4 cell counts are at an increased risk of severe illness from COVID-19, according to the Centers for Disease Control and Prevention (CDC) [6]. The prevalence of older age, hypertension, diabetes, chronic kidney disease, and smoking have been found to be higher among COVID-19 patients with HIV. Furthermore, these patients are more likely to suffer cardiac injury and myocarditis than COVID-19 patients without HIV [7, 8, 9]. The severity of COVID-19 may be reduced in HIV positive patients in two ways: 1) antiretroviral therapy of HIV may decrease the severity of SARS-CoV-2, and 2) a suppressed immune system may be protective against SARS-CoV-2-induced cytokine storms [10]. The relation between HIV and COVID-19 is not clear. Some studies indicated no elevated risk of poor outcomes and mortality among HIV patients due to SARS-CoV-2 [11, 12], whereas limited studies have reported increased risks of hospitalization and mortality among COVID-19 patients with diagnosed HIV [7]. According to a population-based cohort study conducted in South Africa, HIV infection is associated with a doubled mortality risk among patients with COVID-19 Similarly, a study from Madrid, Spain reported a high prevalence of life-threatening illnesses among HIV positive individuals who were infected with COVID-19 [13]. Additionally, some factors such as older age, diabetes, renal and pulmonary disorders of HIV patients, worsened their prognosis following COVID-19 [14].
Iran has been among the most prone countries to this novel virus with over 132000 deaths as of 22 January 2022 [1]. In addition, Tehran has been an epicenter for both COVID-19 and HIV/AIDS epidemics, so it has become a good choice to investigate the association of these two epidemics [15]. To the best of our knowledge, there is a lack of established data regarding the HIV and COVID-19 pandemic in Iran. In this multicenter study, we aimed to evaluate the impact of HIV infection on the risk of COVID-19 death in Tehran, Iran.
2.Material and methods
2.1Data source/study participants
This multicenter study was conducted using the registry database of Coronavirus Control Operations Headquarter from March 21, 2021 to January 18, 2020 in the province of Tehran which is located in the north-central region of Iran. In 2016, the last national census conducted in Iran showed that 13,267,637 people lived in this province, and 8,693,706 lived in urban areas. The major epicenter of COVID-19 is in Tehran, which is the most populous area of the country [15].
The Iranian nation’s central registry for novel coronavirus diseases was established in March 2020. All suspected, probable, and confirmed cases of COVID-19 were prospectively recorded on the national registry of COVID-19 database following WHO definition guidelines [16].
2.2Inclusion/exclusion criteria
The study included all patients with COVID-19 who visited COVID-19 designated healthcare facilities in the province of Tehran between March 2021 and January 2022.
2.3Study variables and outcome
The outcome of interest was COVID-19 death among hospitalized patients living with and without HIV. Primary predictor was HIV status based on medical record in the database registration. The database did not include information about antiretroviral therapy, viral load, CD4 counts, or AIDS status. Demographic variables (age, sex, smoking, and nationality), sign and symptoms (vomit, diarrhea, anorexia, paralysis, fever, seizure, muscular pain, chest pain, abdominal pain, respiratory distress, nausea, headache, cough, vertigo, skin lesion, loss of taste and anosmia), comorbidities (heart disease, asthma, neurological disease, hypertension, hematologic disease, liver disease, kidney disease, diabetes, cancer, immunodeficiency, and other chronic disease), drug abuse, Computed Tomography (CT) results, and wards were included in this study as other variables.
The censored cases were considered patients who were discharged or lost to follow-up. The survival time was defined as the time between admission of patients and when they died or were discharged.
2.4Statistical analyses
2.4.1Descriptive analysis
Descriptive statistics were presented using frequency (percentage) and median (interquartile range [IQR]) for categorical and continuous variables, respectively. The 95% confidence interval (CI) for proportions was calculated using Newcombe methods [17].
2.4.2Survival and mortality analysis
The Cox regression model with the robust standard error estimates was used to study the association between HIV infection and COVID-19 mortality. Initial analyses were unadjusted, followed by adjustments for sex and age, comorbidities, wards, cancer, and partial pressure of oxygen (PO2)
2.4.3Subgroup and interaction analyses
Subgroup analyses were performed to evaluate the impact of HIV on the risk of COVID-19 death in different levels of age (
2.4.4Sensitivity analyses
The propensity score matching as an alternative method was used was to estimate the HIV impact on the risk of COVID-19 death controlling same variables as our adjusted model, including sex and age, comorbidities, wards, cancer, and PO2
All analyses were performed by R (version 4.1.2) and SPSS (version 26). P-values less than 0.05 were regarded as statistically significant.
3.Results
3.1Patients characteristics by HIV
326052 patients with COVID-19 were included in the study, of whom 127 (0.04%) were living with HIV. The median age of COVID-19 patients with HIV (58 years) was higher than those without HIV (50 years). Compared with those without HIV; higher proportions were observed in patients older than 60 years in the HIV group. COVID-19 patients with HIV were more likely to be women (52.76%), while a higher proportion of those without HIV were men (50.17%). Fever (35.43%), muscular pain (41.73%), dyspnea (43.31%), and cough (55.12%) were the most common signs and symptoms among HIV group. In comparison with those without HIV, patients with HIV had higher heart disease (8.66% vs. 7.22%), asthma (1.57% vs. 0.73%), neurological disease (1.57% vs. 0.49%), liver disease (4.72% vs. 0.31%), kidney disease (5.51% vs. 0.99%), and diabetes (28.35% vs. 8.42%). The proportion of COVID-19 patients with HIV who were admitted to the ICU was higher than non-HIV patients (27.56% vs. 12.60%; Table 1).
Table 1
Patient characteristics and demographics by HIV status
| HIV negative | HIV positive | ||||
|---|---|---|---|---|---|
| Total (No. %) | 325925 | (99.96) | 127 | (0.04) | – |
| Sex (No.,%) | 0.510 | ||||
| Women | 162421 | (49.83) | 67 | (52.76) | |
| Men | 163504 | (50.17) | 60 | (47.24) | |
| Age | 0.082 | ||||
| | 98634 | (30.26) | 26 | (20.47) | |
| 40–49 | 58549 | (17.96) | 21 | (16.54) | |
| 50–59 | 60472 | (18.55) | 22 | (17.32) | |
| 60–69 | 54482 | (16.72) | 28 | (22.05) | |
| 70–79 | 32288 | (9.91) | 17 | (13.39) | |
| 80–89 | 18013 | (5.53) | 10 | (7.87) | |
| | 3487 | (1.07) | 3 | (2.36) | |
| Median (IQR) | 50.0 | (37.0, 64.0) | 58.0 | (41.0, 69.0) | |
| Nationality (No.,%) | 0.640 | ||||
| Iranian | 10422 | (3.20) | 3 | (2.36) | |
| Non-Iranian | 315503 | (96.80) | 124 | (97.64) | |
| Sign and Symptoms (No.%) | |||||
| Vomit | 11831 | (3.63) | 5 | (3.94) | 0.853 |
| Diarrhea | 9839 | (3.02) | 5 | (3.94) | 0.597 |
| Anorexia | 30485 | (9.35) | 12 | (9.45) | 1.000 |
| Paralysis | 335 | (0.10) | 0 | (0.00) | 1.000 |
| Fever | 125395 | (38.47) | 45 | (35.43) | 0.524 |
| Seizure | 1078 | (0.33) | 1 | (0.79) | 0.344 |
| Muscular pain | 121504 | (37.28) | 53 | (41.73) | 0.313 |
| Chest pain | 8766 | (2.69) | 6 | (4.72) | 0.160 |
| Abdominal pain | 7805 | (2.39) | 7 | (5.51) | 0.034 |
| Dyspnea | 129051 | (39.60) | 55 | (43.31) | 0.414 |
| Nausea | 22088 | (6.78) | 12 | (9.45) | 0.286 |
| Headache | 34675 | (10.64) | 21 | (16.54) | 0.042 |
| Cough | 188263 | (57.76) | 70 | (55.12) | 0.590 |
| Vertigo | 10560 | (3.24) | 11 | (8.66) | 0.003 |
| Skin lesion | 315 | (0.10) | 1 | (0.79) | 0.116 |
| loss of taste | 5602 | (1.72) | 4 | (3.15) | 0.286 |
| Anosmia | 9384 | (2.88) | 4 | (3.15) | 1.000 |
| Comorbidities (No.%) | |||||
| Heart disease | 23544 | (7.22) | 11 | (8.66) | 0.606 |
| Asthma | 2387 | (0.73) | 2 | (1.57) | 0.239 |
| Neurological disease | 1607 | (0.49) | 2 | (1.57) | 0.130 |
| Hypertension | 31737 | (9.74) | 5 | (3.94) | 0.034 |
| Hematologic diseases | 1042 | (0.32) | 0 | (0.00) | 1.000 |
| Liver disease | 1025 | (0.31) | 6 | (4.72) | |
| Kidney disease | 3234 | (0.99) | 7 | (5.51) | |
| Diabetes | 27445 | (8.42) | 36 | (28.35) | |
| Other chronic diseases | 12513 | (3.84) | 7 | (5.51) | 0.347 |
| Immunodeficiency | 634 | (0.19) | 3 | (2.36) | 0.002 |
| No. of comorbidities (No.%) | |||||
| 0 | 255813 | (78.49) | – | ||
| 1 | 43093 | (13.22) | 69 | (54.33) | |
| 2 | 20034 | (6.15) | 44 | (34.65) | |
| 3 | 6029 | (1.85) | 7 | (5.51) | |
| | 956 | (0.29) | 7 | (5.51) | |
| Cancer (No.%) | 0.008 | ||||
| No | 322752 | (99.03) | 122 | (96.06) | |
| Yes | 3173 | (0.97) | 5 | (3.94) | |
| Pregnancy (No.%) | 1.000 | ||||
| No | 323851 | (99.36) | 126 | (99.21) | |
| Yes | 2074 | (0.64) | 1 | (0.79) | |
|
Table 1, continued | |||||
|---|---|---|---|---|---|
| HIV negative | HIV positive | ||||
| Smoking (No.%) | |||||
| No | 321955 | (98.78) | 114 | (89.76) | |
| Yes | 3970 | (1.22) | 13 | (10.24) | |
| Drug abuse (No.%) | |||||
| No | 324105 | (99.44) | 121 | (95.28) | |
| Yes | 1820 | (0.56) | 6 | (4.72) | |
| CT Scan (No.%) | 0.270 | ||||
| Negative | 87270 | (26.78) | 40 | (31.50) | |
| Positive | 238655 | (73.22) | 87 | (68.50) | |
| Wards (No.%) | |||||
| Non-ICU treated | 284867 | (87.40) | 92 | (72.44) | |
| ICU-treated | 41058 | (12.60) | 35 | (27.56) | |
| PO2 | 0.859 | ||||
| No | 168587 | (51.73) | 67 | (52.76) | |
| Yes | 157338 | (48.27) | 60 | (47.24) | |
| Death status (No.%) | 0.002 | ||||
| Survived | 304330 | (93.37) | 109 | (85.83) | |
| Deceased | 21595 | (6.63) | 18 | (14.17) | |
| Hospitalization days. median (IQR) | 4 | (2, 7) | 5 | (3, 8) | |
Note: The exact Pearson-Chi square was used to evaluate the association between HIV and variables. Abbreviation: Effect size (EF), intensive care unit (ICU), interquartile range (IQR), human immunodeficiency virus (HIV).
3.2Mortality and survival
Of 127 COVID-19 patients with HIV, 18 (14.17%) were deceased. The death percentage was 6.63% among HIV negative patients with COVID-19. Among COVID-19 patients with HIV who died, higher death percentage were found among those age 60 or older (66.67%, 95% CI: 41.15–85.64), those with diabetes (44.44%, 95% CI: 22.40–68.65), and those in ICU (66.67%, 95% CI: 41.15–85.64). Hospitalization days were higher in HIV positive patients who died from COVID-19 compared to HIV negative patients (8 vs. 6; Table 2). Among the deceased patients with HIV, the mean survival time was 15.31 (95% CI: 13.14, 17.49), while it was 26.49 (95% CI: 25.90, 27.08) days for the deceased without HIV (See Supplementary Table 1, Additional File 1). The Kaplan-Meier survival curve showed a significant difference in survival rates between HIV positive and negative patients with COVID-19 (
Table 2
Characteristics among deceased patients with COVID-19 by HIV status
| HIV negative ( | HIV positive ( | ||||
|---|---|---|---|---|---|
| N | Percentage (95% CI) | N | Percentage (95% CI) | ||
| Sex (No.,%) | 1.000 | ||||
| Women | 9468 | 43.84 (43.18, 44.51) | 8 | 44.44 (22.40, 68.65) | |
| Men | 12127 | 56.16 (55.49, 56.82) | 10 | 55.56 (31.35, 77.60) | |
| Age | 0.798 | ||||
| | 6442 | 29.83 (29.22, 30.45) | 6 | 33.33 (14.36, 58.85) | |
| | 15153 | 70.17 (69.55, 70.78) | 12 | 66.67 (41.15, 85.64) | |
| Common Comorbidities (No.%) | |||||
| Heart disease | 3482 | 16.12 (15.64, 16.62) | 0 | 0.00 (0.00, 21.88) | 0.099 |
| Hypertension | 4145 | 19.19 (18.67, 19.73) | 1 | 5.56 (0.29, 29.37) | 0.228 |
| Liver disease | 145 | 0.67 (0.57, 0.79) | 2 | 11.11 (1.95, 36.07) | 0.007 |
| Kidney disease | 665 | 3.08 (2.85, 3.32) | 3 | 16.67 (4.41, 42.26) | 0.017 |
| Diabetes | 3639 | 16.85 (16.36, 17.36) | 8 | 44.44 (22.40, 68.65) | 0.006 |
| No. of comorbidities (No.%) | 0.017 | ||||
| 0 | 13258 | 61.39 (60.74, 62.04) | 6 | 33.33 (14.36, 58.85) | |
| | 8337 | 38.61 (37.96, 39.26) | 12 | 66.67 (41.15, 85.64) | |
| Cancer (No.%) | 0.086 | ||||
| No | 21004 | 97.26 (97.03, 97.47) | 16 | 88.89 (63.93, 98.05) | |
| Yes | 591 | 2.74 (2.53, 2.97) | 2 | 11.11 (1.95, 36.07) | |
| Smoking (No.%) | 1.000 | ||||
| No | 21290 | 98.59 (98.42, 98.74) | 18 | 100.00 (78.12, 100.00) | |
| Yes | 305 | 1.41 (1.26, 1.58) | 0 | 0.00 (0.00, 21.88) | |
| Wards (No.%) | 0.637 | ||||
| Non-ICU treated | 8644 | 40.03 (39.37, 40.69) | 6 | 33.33 (14.36, 58.85) | |
| ICU treated | 12951 | 59.97 (59.31, 60.63) | 12 | 66.67 (41.15, 85.64) | |
| PO2 | 0.116 | ||||
| No | 17722 | 82.07 (81.55, 82.57) | 12 | 66.67 (41.15, 85.64) | |
| Yes | 3873 | 17.93 (17.43, 18.45) | 6 | 33.33 (14.36, 58.85) | |
| Hospitalization days. median (IQR) | – | 6 (2, 11) | – | 8 (5, 15.75) | 0.074 |
Note: The Exact Pearson-Chi square was used to evaluate the association between HIV and categorical variables. Exact Mann-Whiney U test was used to examine the relation between numeric variable and HIV. Abbreviation: Effect size (EF), intensive care unit (ICU), human immunodeficiency virus (HIV), confidence interval (CI).
Figure 1.
The Kaplan-Meier survival curve of patients with HIV positive and HIV negative.
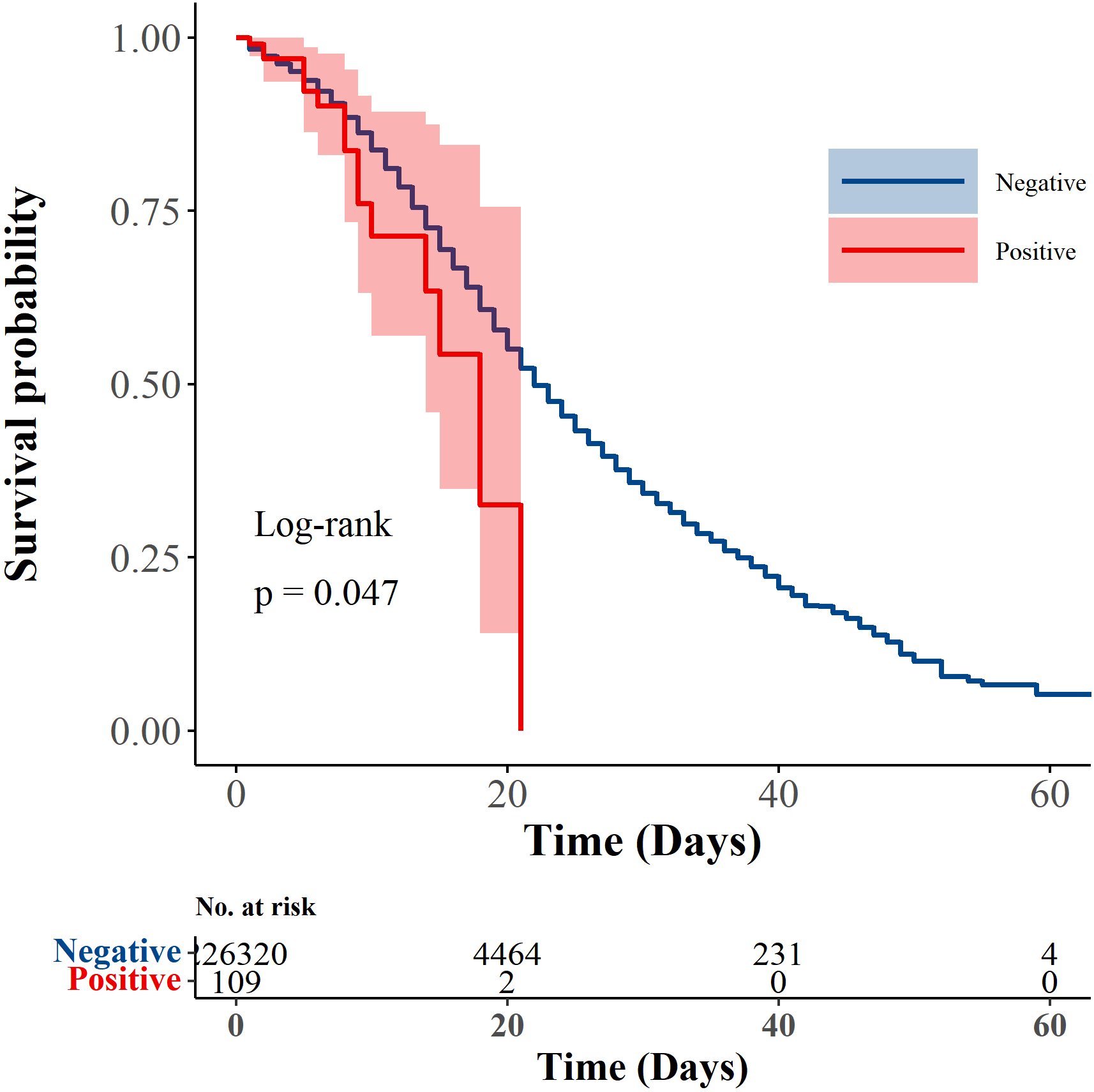
Table 3
The impact of HIV on the HR of patients with COVID-19 after matching confounders
| Model | Description | HR (95% CI) | |
|---|---|---|---|
| 3 | sex, comorbidity, cancer, PO2 | 1.50 (1.09, 2.06) | 0.012 |
| 4 | Model 3 | 1.49 (0.99, 2.24) | 0.057 |
| 5 | Model 4 | 1.30 (0.94, 1.81) | 0.118 |
Abbreviation: Hazard ratio (HR), human immunodeficiency virus (HIV), confidence interval (CI), partial pressure of oxygen (PO2).
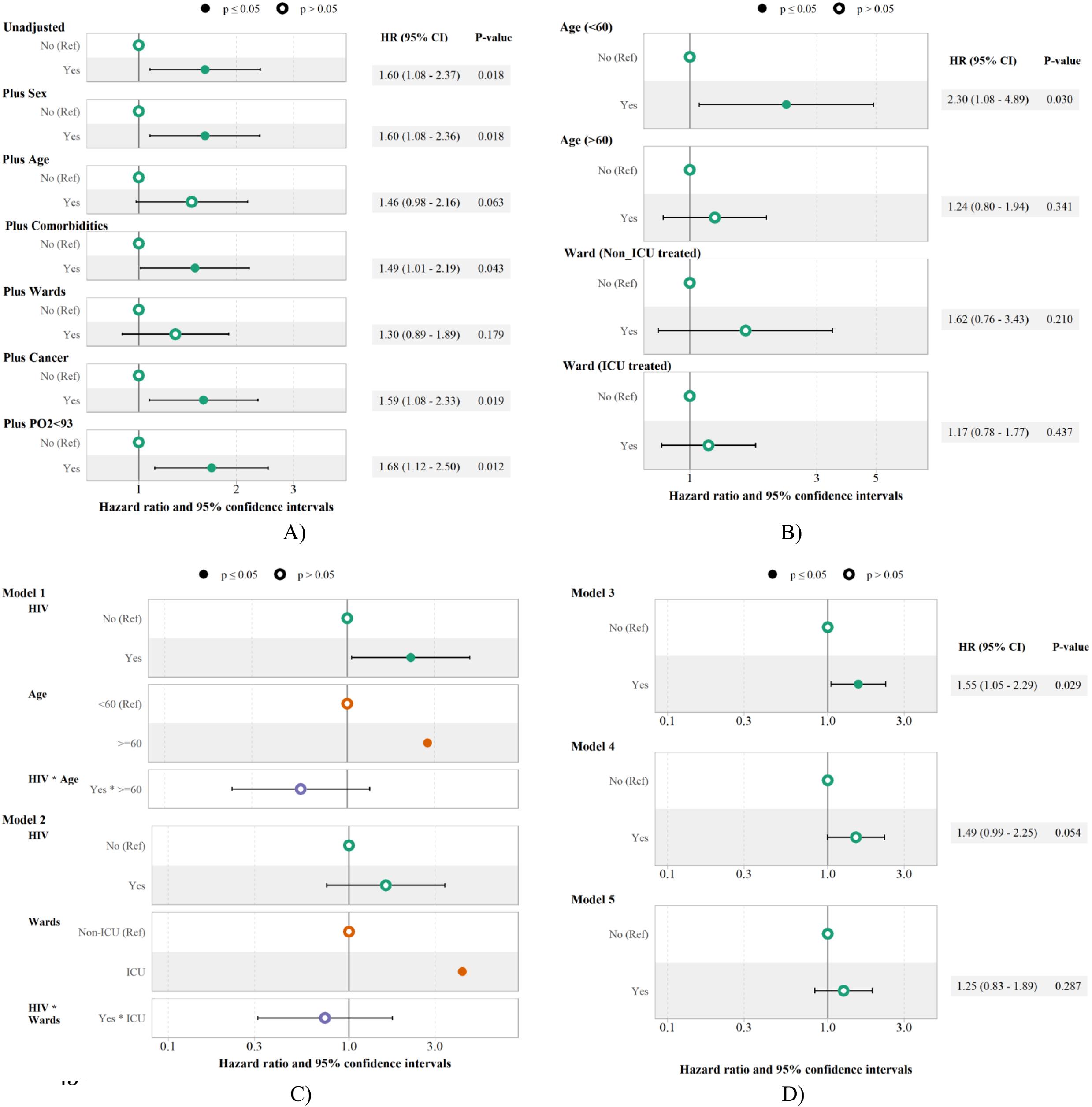
Crude risk of COVID-19 death was higher in patients with HIV than in those without HIV (HR: 1.60, 95% CI: 1.08–2.37). Compared to non-HIV patients, higher risk of COVID-19 death was observed among HIV patients after adjusting for sex (1.60, 1.08–2.36), comorbidities (1.49, 1.01–2.19), cancer (1.59, 1.08–2.33), and PO2
Figure 2.
The unadjusted and adjusted HR and 95% CI of death among hospitalized COVID-19 patients with HIV. A) The unadjusted and adjusted Cox regression models, B) The subgroup analysis of HIV impact on the death by age and wards, C) The interaction analysis of HIV-Age (Model 1) and HIV ward (Model2), D) The impact of HIV on the death adjusting for sex, comorbidity, cancer, PO2
3.3Subgroup and interaction analysis
The HIV impact on the risk of death among COVID-19 patients was presented by subgroup of age and wards. HIV was significantly associated with a 2.30 (1.08–4.89) times higher risk of COVID-19 death among patients younger than 60, while no significant association was observed between HIV and risk of COVID-19 death among patients older than 60, with the HR of 1.24 (0.80–1.94). The impact of HIV on COVID-19 death was not significant among both ICU and non-ICU patients, with the HR of 1.62 (0.76–3.43 ) and 1.17 (0.78–1.77), respectively (Fig. 2-B).
In the next step, the interaction effect of HIV in two Cox models (model 1: HIV
As shown in Fig. 2-D, HIV was associated with higher risk of COVID-19 death after adjusting for sex, comorbidity, cancer, PO2
3.4Sensitivity analysis
As an alternative, sensitivity analysis was performed with the propensity score matching method to adjust for confounders, and significant association between HIV and higher risk of COVID-19 death was found (model 3: HR 1,50, 1.09–2.06). HIV remained unassociated with risk of COVID-19 death in model 2 (HR: 1.49, 0.99–2.25) and model 3 (HR: 1.25, 0.83–1.89) using propensity score matching (Table 3).
4.Discussion
In this large multi-center study using data from Coronavirus Control Operations Headquarters in Iran, we found that the risk of COVID-19 death in patients with HIV was about twice as likely as for those without HIV. Among HIV patients who died of COVID-19, diabetes mellitus and liver disease were significantly more common than other comorbidities. The mean and median survival times were considerably lower among HIV patients compared with non-HIV patients. The crude risk of COVID-19 death was significantly higher among HIV patients. The risk of COVID-19 death was about 50% higher among people with HIV compared to those without HIV after adjusting for sex, comorbidities, cancer, and PO2
In the current study, we found that patients who died from COVID-19 and were living with HIV had a lower chance of survival than non-HIV infected individuals. It is likely to reflect an increased mortality risk for those with HIV, regardless of whether they are infected with COVID-19. One possible explanation is the poor quality of the Iranian surveillance system for communicable diseases. Accordingly, the mean survival time for HIV patients was about 248 months, and approximately one in four patients died in Iran [18]. Although previous published articles supported this idea in many countries, there are differences in the survival rates of HIV patients [19]. This might be attributed to differed coverage of antiretroviral therapy (ART) program initiatives, access to medical services, prevalence of other comorbidities, and duration of the study period [18]. The low survival rate among HIV patients may be partly explained by several established reasons. According to a study by Pourcher et al., HIV-infected persons had a greater prevalence of comorbidities and a higher mortality rate. Moreover, they had a two-fold higher hospitalization rate and a four-fold higher healthcare cost than those without HIV [20]. Some evidence declared that lifestyle factors such as smoking and drug abuse, exposure to co-pathogens (e.g., hepatitis C), side effects of highly active antiretroviral therapy (HAART) (e.g., cardiac injury), presence of HIV-related diseases, immunosuppression, acute bacterial infection, flare up of latent opportunistic superinfections, and immune reconstitution syndromes were the main causes of death from HIV [21, 22]. Furthermore, the impact of older age, sex, race, fibrinogen level, C-reactive protein (CRP) level, being illiterate, bedridden, family-related risk behaviors (e.g. drug abuse, cigarette smoking, excessive alcohol consumption), and the second-line life-saving ART regimen had been examined by a number of studies which revealed that they were associated with high mortality among HIV infected individuals [23, 24, 25, 26]. Notably, some researchers have provided an indication that people living with HIV on ARTs had high rates of cardiovascular diseases, sexually transmitted diseases, mental health conditions, neoplasms, diabetes, obesity, and chronic respiratory disease [23]. Moreover, low baseline CD4 counts (CD4
Our findings have confirmed that survival time among COVID-19 patients with HIV was shorter than for those without HIV. However, it was observed that the risk of COVID-19 death varied when confounders were adjusted for versus when they were not. Several factors seem to be associated with a higher risk of COVID-19 death among HIV positive patients [2]. For example, people with HIV may be more susceptible to either cardiac injury or myocarditis of COVID-19 infection, which results in higher mortality or post-acute sequelae of COVID-19 than those without HIV [7]. Nevertheless, little is known about whether HIV infection is associated with worse outcomes among COVID-19 infected patients. In concordance with our findings, prior study conducted in Zambia have demonstrated that HIV individuals hospitalized for COVID-19 were more likely to develop or die due to COVID-19 compared to those without HIV [29]. But they did not provide a clear explanation about how HIV infection might influence the risk of COVID-19 death, which is a critical issue. A study from the UK and South Africa declared that HIV doubled the mortality risk of COVID-19 [2, 28]. According to the study by James M et al. HIV patients without viral suppression and those with lower CD4 counts have higher rates of COVID-19 severity, hospitalization, and ICU admission [6]. Other studies found that CD4 levels less than 200 cells/mm3 were significantly related to decreased survival among COVID-19 inpatients with diagnosed HIV [30, 31] In contrast, some research suggested that HIV infection did not affect or even decreased mortality from COVID-19, due to a reduced immune response or partial impact of antiretroviral therapy against SARS-CoV2 [7, 32, 33]. Thus, it may be beneficial and crucial to address the possible causes of this association in order to reduce the risk of COVID-19 death among those living with HIV.
In the current study, it was found that age was one of the main factors affecting the risk of COVID-19 death in HIV patients. The results of other published papers were conflicting. Based on a study by Durstenfeld et al. the effect of HIV on COVID-19 death was negligible before and after age adjustment [7]. In contrast, Bhaskaran et al. showed that the effect of HIV on the risk of COVID-19 death became significant once age was taken into account. Accordingly, the reason was that HIV group was younger than the non-HIV group in their study. Thereby, younger age was associated with a lower risk of COVID-19 death. As they compared HIV and non-HIV patients without accounting for age, the younger age of HIV patients (which means a lower risk) effectively cancelled out the higher risk associated with HIV, leaving an HR of around 1. In contrast, when they adjusted for age, they were able to eliminate the age effect from the comparison between HIV and non-HIV. Therefore, they were now effectively saying “what is the association between HIV and COVID-19 death, among people of a similar age?”. The true (positive) association between HIV and COVID-19 death thus emerged [2]. The present study found a significant impact of HIV on the risk of COVID-19, but after adjusting for age, the effect of HIV on death risk was no longer significant. One reason for the difference in the above results is the fact that none of these studies considered important and effective variables that affect HIV patient survival, as mentioned earlier. Therefore, the results may have been different if these variables were adjusted along with other variables such as age.
The study’s strength is the use of a large dataset that includes more than 326052 hospitalized COVID-19 patients across Tehran province hospitals and designated COVID-19 healthcare facilities. In addition, the number of comorbidities was taken into account in the study in order to adjust the impact of HIV on the risk of COVID-19 death. Moreover, the Cox proportional regression model with robust estimator errors was employed in this study to determine the more accurate significance of the relationship between variables and the risk of death from COVID-19. The subgroup analysis and effect modification were conducted using covariates of age and ward. Furthermore, the propensity score matching method was utilized in this study, which generally adjusts confounders in a different manner, so performing such sensitivity analyses could provide reassurance that the results are not influenced by any specific method. In contrast, this study has some limitations. The database did not include information about antiretroviral therapy, viral load, CD4 counts, AIDS status, and laboratory results. Moreover, a prospective cohort study using high-quality and reliable data is needed to estimate the survival rate of patients. Our study used retrospective cohort data collected by the Coronavirus Control Operations Headquarters, thus, the quality of the registered data may be questionable. Additionally, COVID-19 disease severity was not specified in these data, and therefore a subgroup analysis for the effect of HIV on the death risk could not be conducted for different degrees of severity. The association between COVID-19 death and HIV infection was evaluated in hospitalized patients; therefore, results may differ from studies that included both hospitalized and non-hospitalized patients. There were relatively few deaths among COVID-19 patients with HIV, reducing the power of Cox regression models to adjust more than one variable in order to evaluate the impact of HIV on the risk of COVID-19 death.
5.Conclusion
In this large multicenter study, COVID-19 patients with HIV were more likely to die, regardless of age and ward. The risk of COVID-19 death was relatively low after adjusting for age and ward. In order to determine the true impact of HIV on the risk of COVID-19 death, it is necessary to take into account factors such as age, comorbidities, hospital ward, viral load, CD4 count, and antiretroviral treatment information.
Abbreviations
ART: Antiretroviral Therapy
CDC: Centers for Disease Control and Prevention
CI: Confidence Interval
CRP: C-Reactive Protein
HAART: Highly Active Antiretroviral Therapy
HIV: Human Immunodeficiency Virus
HR: Hazard Ratio
ICU: Intensive Care Unit
IQR: Interquartile Range
PO2: Partial Pressure of Oxygen
SARS-CoV-2: Severe Acute Respiratory Syndrome
Coronavirus 2
WHO: World Health Organization
Authors’ contributions
Each named author has substantially contributed to conducting the research and drafting this manuscript. Conceptualization, M.A.L.; methodology, M.A.L.; software, M.A.L.; formal analysis, M.A. and M.A.L.; investigation, M.A., M.A.L. and P.S.P., A.Z., R.V., G.M.; resources, G.H., A.Z. and R.V.; responsible for data collection, G.H., A.Z.; data curation, G.H., A.Z., R.V.; writing-original draft preparation, M.A.L, M.A., N.T., P.S.P.; writing-review and editing, M.A.L, M.A., N.T., P.S.P.; visualization, M.A.L.; supervision, R.V. and G.M.; All authors have read and agreed to the published version of the manuscript.
Funding
No financial support was received for this research.
Availability of data and materials
It is possible to obtain the data that supported the findings of this study from the Coronavirus Control Operations Headquarter in Tehran, however there are restrictions on access to these data, which were used under license for this study, and thus are not publicly available. Requests regarding the data may be made to the senior author.
Ethics approval and consent to participate
Shahid Beheshti University of Medical Sciences Ethics Committee approved the study with a waiver of informed consent (Reference number: IR.SBMU. RETECH.REC.1400.473). All data were de-identified prior to analysis.
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
The authors thank all participants, reviewers, and editors for their helpful comments.
References
[1] | W. Covid, Coronavirus Pandemic: Reported Cases and Deaths by Country, Territory, or Conveyance. In. |
[2] | K. Bhaskaran, C.T. Rentsch, B. MacKenna, A. Schultze, A. Mehrkar, C.J. Bates, R.M. Eggo, C.E. Morton, S.C. Bacon and P. Inglesby, HIV infection and COVID-19 death: a population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform, The Lancet HIV 8: (1) ((2021) ), e24–e32. |
[3] | E.J. Williamson, A.J. Walker, K. Bhaskaran, S. Bacon, C. Bates, C.E. Morton, H.J. Curtis, A. Mehrkar, D. Evans and P. Inglesby, OpenSAFELY: factors associated with COVID-19 death in 17 million patients, Nature 584: (7821) ((2020) ), 430. |
[4] | S. Kenmoe, J.J. Bigna, A.F. Modiyingi, M.S. Ndangang, P.A. Ngoupo, F.B.N. Simo, S. Tchatchouang, E. Temfack and R. Njouom, Case fatality rate and viral aetiologies of acute respiratory tract infections in HIV positive and negative people in Africa: The VARIAFRICA-HIV systematic review and meta-analysis, Journal of Clinical Virology 117: ((2019) ), 96–102. |
[5] | K.M. Neuzil, G.W. Reed, E.F. Mitchel, Jr. and M.R. Griffin, Influenza-associated morbidity and mortality in young and middle-aged women, Jama 281: (10) ((1999) ), 901–907. |
[6] | J.M. Tesoriero, C.-A.E. Swain, J.L. Pierce, L. Zamboni and M. Wu, D.R. Holtgrave, C.J. Gonzalez, T. Udo, J.E. Morne and R. Hart-Malloy, COVID-19 outcomes among persons living with or without diagnosed HIV infection in New York State, JAMA Network Open 4: (2) ((2021) ), e2037069–e2037069. |
[7] | M.S. Durstenfeld, K. Sun, Y. Ma, F. Rodriguez, E.A. Secemsky, R.V. Parikh and P.Y. Hsue, Impact of HIV Infection on COVID-19 Outcomes Among Hospitalized Adults in the US, medRxiv 2021. |
[8] | Y.B. Hadi, S.F. Naqvi, J.T. Kupec and A.R. Sarwari, Characteristics and outcomes of COVID-19 in patients with HIV: a multicentre research network study, Aids 34: (13) ((2020) ), F3–F8. |
[9] | Risk factors for coronavirus disease 2019 (COVID-19) death in a population cohort study from the Western Cape Province, South Africa, Clinical Infectious Diseases 73: (7) ((2021) ), e2005–e2015. |
[10] | B. Cao, Y. Wang, D. Wen, W. Liu, J. Wang, G. Fan, L. Ruan, B. Song, Y. Cai and M. Wei, A trial of lopinavir – ritonavir in adults hospitalized with severe COVID-19, New England Journal of Medicine ((2020) ). |
[11] | C. Gervasoni, P. Meraviglia, A. Riva, A. Giacomelli, L. Oreni, D. Minisci, C. Atzori, A. Ridolfo, D. Cattaneo, Clinical features and outcomes of HIV patients with coronavirus disease 2019, Clinical Infectious Diseases ((2020) ). |
[12] | K. Sigel, T. Swartz, E. Golden, I. Paranjpe, S. Somani, F. Richter, J.K. De Freitas, R. Miotto, S. Zhao and P. Polak, COVID-19 and people with HIV infection: outcomes for hospitalized patients in New York City, Clinical Infectious Diseases ((2020) ). |
[13] | P. Vizcarra, M.J. Pérez-Elías, C. Quereda, A. Moreno, M.J. Vivancos, F. Dronda, J.L. Casado, S. Moreno, M.J. Pérez-Elías and J. Fortún, Description of COVID-19 in HIV-infected individuals: a single-centre, prospective cohort, The lancet HIV 7: (8) ((2020) ), e554–e564. |
[14] | D. De Francesco, S.O. Verboeket, J. Underwood, E. Bagkeris, F.W. Wit, P.W. Mallon, A. Winston, P. Reiss and C.A. Sabin, Patterns of co-occurring comorbidities in people living with HIV, In: Open forum infectious diseases: 2018: Oxford University Press US; (2018) : ofy272. |
[15] | Population and Housing Censuses [https://www.amar.org.ir/english/Population-and-Housing-Censuses]. |
[16] | WHO COVID-19 Case definition [https://www.who.int/publications/i/item/WHO-2019-nCoV-Surveillance_Case_Definition-2020.2]. |
[17] | Newcombe RG: Two-sided confidence intervals for the single proportion: comparison of seven methods, Stat Med 17: (8) ((1998) ), 857–872. |
[18] | M. Akbari, M. Fararouei, A.A. Haghdoost, M.M. Gouya and P.A. Kazerooni, Survival and associated factors among people living with HIV/AIDS: A 30-year national survey in Iran, Journal of Research in Medical Sciences: The Official Journal of Isfahan University of Medical Sciences 24: ((2019) ). |
[19] | F. Nigussie, A. Alamer, Z. Mengistu and E. Tachbele, Survival and Predictors of Mortality Among Adult HIV/AIDS Patients Initiating Highly Active Antiretroviral Therapy in Debre-Berhan Referral Hospital, Amhara, Ethiopia: A Retrospective Study, HIV/AIDS (Auckland, NZ) 12: ((2020) ), 757. |
[20] | V. Pourcher, J. Gourmelen, I. Bureau and S. Bouee, Comorbidities in people living with HIV: An epidemiologic and economic analysis using a claims database in France, Plos One 15: (12) ((2020) ), e0243529. |
[21] | J. Chakravarty, N.K. Tiwary, S.R. Prasad, S. Shukla, A. Tiwari, R.N. Mishra and S. Sundar, Determinants of survival in adult HIV patients on antiretroviral therapy in Eastern Uttar Pradesh: a prospective study, The Indian Journal of Medical Research 140: (4) ((2014) ), 491. |
[22] | N. Obel, L.H. Omland, G. Kronborg, C.S. Larsen, C. Pedersen, G. Pedersen, H.T. Sørensen and J. Gerstoft, Impact of non-HIV and HIV risk factors on survival in HIV-infected patients on HAART: a population-based nationwide cohort study, PloS One 6: (7) ((2011) ), e22698. |
[23] | K. Varshney, P. Ghosh, H. Stiles and R. Iriowen, Risk Factors for COVID-19 Mortality Among People Living with HIV: A Scoping Review, AIDS and Behavior, (2022) , pp. 1–10. |
[24] | H. Refera and E. Wencheko, Survival of HIV-TB co-infected adult patients under ART in Ambo Referral Hospital, Ethiopia, Ethiopian Journal of Health Development 27: (2) ((2013) ), 88–93. |
[25] | Z.Z. Aung, Y.M. Saw, T.N. Saw, N. Oo, H.N.N. Aye, S. Aung, H.N. Oo, S.M. Cho, M. Khaing and T. Kariya, Survival rate and mortality risk factors among TB-HIV co-infected patients at an HIV-specialist hospital in Myanmar: A 12-year retrospective follow-up study, International Journal of Infectious Diseases 80: ((2019) ), 10–15. |
[26] | P.C. Tien, A.I. Choi, A.R. Zolopa, C. Benson, R. Scherzer, P. Bacchetti, M. Shlipak and C. Grunfeld, Inflammation and mortality in HIV-infected adults: analysis of the FRAM study cohort, Journal of acquired immune deficiency syndromes (1999) 55: (3) ((2010) ), 316. |
[27] | N. Karimi, M. Safari, M. Mirzaei, A. Kassaeian, G. Roshanaei and T. Omidi, Determining the Factors Affecting the Survival of HIV Patients: Comparison of Cox Model and the Random Survival Forest Method, Disease and Diagnosis 8: (2) ((2019) ), 124–129. |
[28] | P. Tamma, S. Aitken, R.A. Bonomo, A.J. Mathers, D. Van Duin and C.J. Clancy, OUP accepted manuscript, Clin Infect Dis ((2020) ). |
[29] | D. Chanda, P.A. Minchella, D. Kampamba, M. Itoh, J.Z. Hines, S. Fwoloshi, M.A. Boyd, K. Hamusonde, L. Chirwa and K. Nikoi, COVID-19 Severity and COVID-19 – Associated Deaths Among Hospitalized Patients with HIV Infection-Zambia, March–December 2020, Morbidity and Mortality Weekly Report 70: (22) ((2021) ), 807. |
[30] | M. Davies, HIV and risk of COVID-19 death: a population cohort study from the Western Cape Province, South Africa, (2020) . |
[31] | J. Del Amo, R. Polo and S. Moreno, Incidence and severity of COVID-19 in HIV-positive persons receiving antiretroviral therapy: a cohort study [Published online ahead of print June 26, 2020], Ann Intern Med. |
[32] | E.A. Meyerowitz, A.Y. Kim, K.L. Ard, N. Basgoz, J.T. Chu, R.M. Hurtado, C.K. Lee, W. He, T. Minukas and S. Nelson, Disproportionate burden of coronavirus disease 2019 among racial minorities and those in congregate settings among a large cohort of people with HIV, AIDS (London, England) 34: (12) ((2020) ), 1781. |
[33] | L. Park, C. Rentsch, K. Sigel, M. Rodriguez-Barradas, S. Brown and M. Goetz, COVID-19 in the largest US HIV cohort, AIDS 2020: ((2020) ), 23rd. |



