Gaze stability in young adults with previous concussion history
Abstract
BACKGROUND:
Disruption of visual-vestibular interaction after concussion can cause gaze instability with head movements. The long-term impact of concussion on gaze stability is unknown.
OBJECTIVE:
This cross-sectional comparative pilot study examined gaze stability in the chronic stage after concussion (greater than one year). A secondary objective was to examine the relationship between gaze stability and sleep.
METHODS:
Outcome measures included: 1. Gaze stability in logMAR (mean loss of dynamic visual acuity (DVA) in the yaw and pitch planes); 2. Pittsburgh Sleep Quality Index (PSQI); 3. Epworth Sleepiness Scale (ESS). Post-Concussion Symptom Scale (PCSS), time since injury, and number of concussions were collected for the people with concussion.
RESULTS:
The study sample included thirty-four adults (mean age 23.35±1.3 years). Seventeen had a history of 1–9 concussions, with a mean duration of 4.4±1.9 years since last concussion; and 17 were age and sex-matched controls. Mean pitch plane DVA loss was greater in the concussion group compared to the control group (p = 0.04). Participants with previous concussion had lower sleep quality based on the PSQI (p = 0.01) and increased daytime sleepiness based on the ESS (p = 0.01) compared to healthy controls. Mean DVA loss in the pitch plane was significantly correlated with the PSQI (r = 0.43, p = 0.01) and the ESS (r = 0.41, p = 0.02).
CONCLUSION:
Significant differences in dynamic visual acuity may be found in young adults long after a concussion, compared with those who have no concussion history. Furthermore, loss of dynamic visual acuity was associated with poorer sleep quality and higher daytime sleepiness.
1Introduction
Approximately 1.6 to 3.8 million concussions occur annually in youths participating in sports and recreational activities [7]. Most of the attention by the medical community has focused on the acute stage of concussion [3, 30, 37, 38], showing that symptom resolution and return to baseline neurocognitive status usually occurs within 7–10 days [31]. However, recent longitudinal studies have shown that symptoms can change, fluctuate, or persist for months after the initial injury [16, 22]. In one study, persistent post-concussion symptoms were seen in 20–48% of military personnel, while new symptoms presented in 28% of individuals up to 5 years later [22]. Symptoms like headaches, sleep disturbances, and difficulty concentrating have been shown to persist in more than 50% of people, one-year post concussion [11, 43].
The vestibular system has not received much attention in the chronic stage after a concussion, possibly because balance deficits appear to resolve quickly [29]. However, the vestibular system also plays an important role in maintaining a stable gaze during head movements [24]. The vestibular system through the vestibulo-ocular reflex (VOR) maintains gaze stability by inducing eye movements that are equal in magnitude and opposite in direction to head movements [40]. Dysfunction of the VOR leads to inability to hold images steady on the retina, resulting in blurred vision [40]. Deficits in visual-vestibular interaction after a concussion may occur due to peripheral vestibular end-organ damage, vestibulocochlear nerve injury, or due to axonal shearing in the central nervous system, including the brainstem and cerebellar pathways [21, 25, 27, 35, 45]. When gaze stability was examined in collegiate athletes who were 3 months to 9 years post-injury, higher asymmetry scores on the gaze stabilization test were found in the concussion group compared to healthy controls [17]. Collegiate athletes who were examined within 6 months of a concussion reported symptoms of dizziness or headache after rapid head movements (240 beats per minute), and demonstrated greater loss of dynamic visual acuity with horizontal head movement at speeds of 180 beats per minute when compared to age-matched controls [45]. Alshehri et al. performed the video head impulse test, a physiological measure of the VOR, in both young and older participants who were 4–6 months post-concussion. While they did not detect any deficits with VOR response in the yaw plane, participants had complaints of headache, dizziness and nausea after the testing was complete [1]. These studies show that vestibular-related symptoms may persist for months after a concussion, however, the presence of vestibular dysfunction, particularly gaze stability deficits, in the chronic stage of concussion is not clear.
Sleep disturbances are common after concussion, with as many as 30–70% of affected adults reporting inability to fall asleep or sleeping more often during the day [32, 44]. The relationship between sleep and vestibular function is bidirectional. Sleep deprivation has been shown to affect vestibular-ocular motor control [26]; while vestibular migraine has been shown to cause sleep disturbances [2]. Poor sleep quality is associated with decreased information processing [23, 42], which is particularly important in young adults. However, the relationship between gaze stability and sleep quality in young adults after a concussion has not been investigated.
To determine if gaze stability deficits are present in the chronic stage after a concussion, this study compared dynamic visual acuity in young adults >1-year post concussion to age-matched controls. We hypothesized that people with a previous concussion would demonstrate greater loss of visual acuity with head movement compared to controls. Our second objective was to examine the relationship between gaze stability deficits and sleep quality. Our hypothesis was that greater loss of dynamic visual acuity would be correlated with poor sleep quality.
2Methods
2.1Study design
This was a cross-sectional, comparative study using convenience sampling. This study was conducted at a mid-sized university, after receiving approval from the university’s Institutional Review Board.
2.2Participants
Individuals were recruited by word of mouth and flyers placed around the University. Participants were included if they were between 20 and 30 years of age and had a history of a physician-diagnosed concussion at least 1 year prior to the study. Participants were excluded if they had a diagnosed neurological problem, a diagnosis of inner ear disease, cancer with a history of chemotherapy, a history of diabetes, or if they worked night shifts. Exclusion criteria were based on evidence showing that chemotherapy and diabetes can independently affect the peripheral vestibular system [6, 15], while working night shift affects day time sleepiness [33]. Presence or absence of symptoms was not an exclusion criterion. Healthy controls were matched by age and gender and did not have a history of a diagnosed concussion.
2.3Study procedure
Participants completed a phone screen to verify inclusion/exclusion criteria. Once eligibility was verified, participants were scheduled for a testing session. They were instructed to wear or bring any corrective lenses for the testing session. Informed consent was completed prior to official study enrollment. Baseline data collected included demographics such as age and sex. Medical history (i.e., height, weight, number and year of concussions, and medication list) was also collected. A physical therapist (LD) conducted a clinical vestibular exam, which included testing of smooth pursuit, saccades, head thrust test, and the Dynamic Visual Acuity Test (DVAT). All participants completed the Pittsburgh Sleep Quality Index (PSQI), and the Epworth Sleepiness Scale (ESS). The concussion group also completed the Post-Concussion Symptom Scale (PCSS). Study procedures were completed in one testing session that lasted for an hour.
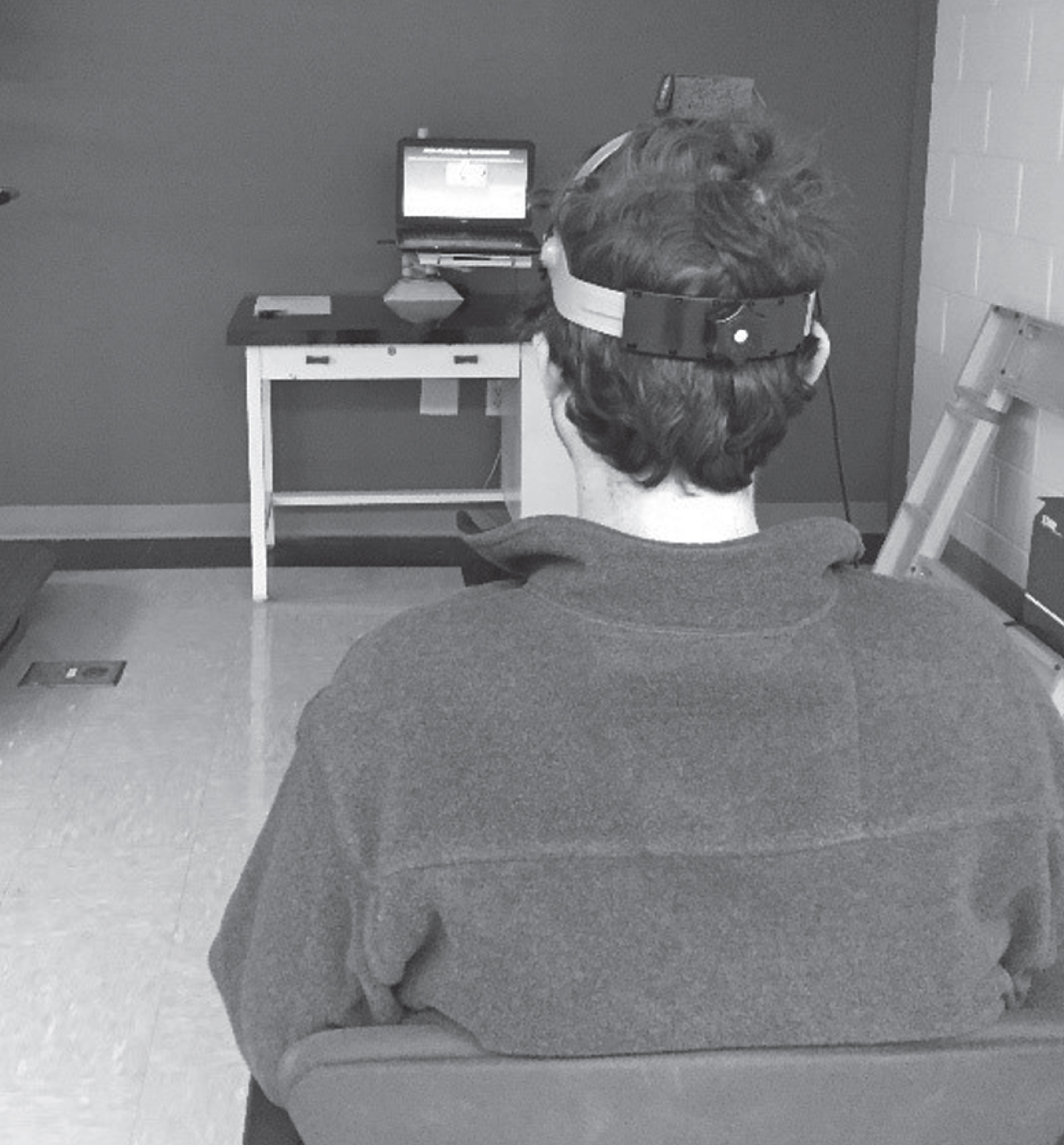
Static visual acuity, perception time, and DVA in the yaw and pitch planes were measured using the NeuroCom InVisiontrademark (NeuroCom International). The computerized DVA test is verified as a sensitive and specific assessment method for detecting peripheral vestibular impairment with good reliability (ICC r = 0.83–0.87) [10, 14]. Participants sat in a sturdy chair, in a room with controlled lighting, ten feet from the computer screen, wearing corrective eye wear as needed (Fig. 1). Testing commenced with the assessment of static visual acuity (SVA), that is baseline visual acuity was assessed with the head stationary. An optotype (the letter “E”) was presented on the computer screen and participants were instructed to identify the orientation of the E (up, down, right, left). Based on the Parameter Estimation of Sequential Test (PEST) algorithm, the smallest optotype that was correctly identified 60% of the time, was considered the static visual acuity in logMAR units. Optotype was set as 0.2 logMAR above the established SVA baseline for further testing.
Fig.1
Experimental set-up for dynamic visual acuity testing.
For perception time testing (PTT), the optotype “E” appeared for varying time periods in milliseconds. Based on the PEST algorithm, the minimum time required to correctly identify the optotype was collected as the perception time. The NeuroCom InVision software uses a variable optotype display time, linked to the subject’s PTT score and the selected head velocity for the test. If an individual had a PTT score within 40msec, the display window was set between 40–75 msec. For this study, participants had to obtain a PTT score ≤60 msec. Based on the SVA and PTT values, the algorithm presented the optotype for the DVAT.
Table 1
Descriptive Statistics
| Concussion (N = 17) | Controls (N = 17) | p value | |
| Age (years) | 23.35±1.3 | 23.94±2.5 | p = 0.39 |
| BMI (kg/m2) | 24.5±3.7 | 23.1±3.3 | p = 0.25 |
| PSQI score | 6.3±4.3 | 2.8±2.1 | p = 0.01* |
| Epworth Sleepiness Scale | 8.8±3.8 | 5.5±3.0 | p = 0.01* |
Independent samples t-test were used to compare groups. Significant differences are indicated by an *. BMI- body mass index; PSQI-Pittsburgh Sleep Quality Index.
Table 2
Difference between concussion and control groups in measures of gaze stability
| Concussion | Controls | p value | Effect size | |
| Visual acuity | –0.20±0.08 | –0.21±0.06 | 0.71 | 0.1 |
| Perception Time (milliseconds) | 22.35±6.6 | 21.76±3.9 | 0.76 | 0.1 |
| Mean DVA loss yaw plane (logMAR) | 0.18±0.1 | 0.13±0.06 | 0.06 | 0.6 |
| Mean DVA loss pitch plane (logMAR) | 0.17±0.13 | 0.09±0.07 | 0.04* | 0.8 |
Independent sample t-tests compared differences between groups. Significant differences are denoted by *. DVA – dynamic visual acuity.
The computerized DVAT measured the difference between SVA and DVA when head movements were performed in the yaw and pitch planes. Participants wore a headband with a 3-axis integrating gyro (InterSense Inertia Cube2 ®, Engineering Systems Technology, Kaiserslautern, Germany) to determine rotational head velocity in the yaw and pitch planes (Fig. 1). Participants had to generate rotational head movements to at least 20 degrees from midline in each direction. Once the participant’s head velocity achieved a minimum of 85 deg/sec (range 85–120 deg/sec) in the yaw plane or 60 deg/sec (range 60–85 deg/sec) in the pitch plane, the optotype “E” appeared in the center of the screen. Based on the PEST algorithm, DVA was recorded as the size of the smallest optotype that the participant could identify while rotating the head at or above the minimum velocity. The outcome variables were the mean DVA loss in the right and left direction in the yaw plane, and mean DVA loss in the upward and downward direction in the pitch plane in logMAR. Higher logMAR values indicate greater loss of DVA. All participants completed practice trials to ensure that they understood the testing protocol.
All participants completed two questionnaires to assess sleep quality and daytime sleepiness: The Pittsburgh Sleep Quality Index (PSQI) and the Epworth Sleepiness Scale (ESS). The PSQI is a 9-item questionnaire to assess sleep quality over the past month, specifically examining latency, duration, habitual sleep efficiency, sleep disturbances, use of sleeping medications, and daytime dysfunction. A global PSQI score ranges from 0 to 21, with a score greater than 5 (sensitivity 89.6% and specificity 86.5%), indicating poor sleep quality requiring management by a healthcare provider [4]. The ESS is an 8-question survey used for measuring a person’s “average sleep propensity” across a range of activities in their daily life. It has strong test-retest reliability (ICC = 0.81–0.93), internal consistency (Cronbach’s alpha = 0.73–0.9), and external criterion validity. In general, ESS scores of 0–5 indicate lower normal daytime sleepiness; 6–10 indicate higher normal daytime sleepiness; 11–12 indicate mild excessive daytime sleepiness; 13–15 indicate moderate excessive daytime sleepiness; and 16–24 indicate severe excessive daytime sleepiness [18].
The PCSS was used to assess post-concussion symptoms in the group with concussion only. This scale contains 22 self-reported symptoms (e.g. dizziness, fogginess, headache) rated on a 6-point Likert scale with 0 indicating “none” and 6 indicating “severe”. The maximum possible PCSS score is 132 [25, 39].
3Statistical analysis
Descriptive statistics (mean, standard deviation) were used to examine participant demographics. Between-group differences in the concussion and control group were determined by independent sample t-tests. Measures of between-group effect sizes were calculated using Cohen’s d (0.2 = small effect, 0.5 = medium effect, and >0.8 = large effect). Since this was a pilot study, no corrections were applied for multiple comparisons, and an α level of 0.05 was used. Correlations between gaze stability measures, PSQI, and ESS were tested using Spearman’s correlations due to inequality of variance. All analyses were conducted using SPSS for Windows version 25.0 (SPSS Inc., Chicago, USA).
4Results
Thirty-four individuals completed the study (See Table 1 for participant demographics); 17 in the concussion group (5 males, 12 females) and 17 age and sex-matched healthy controls. All participants were students at the university and most of them held part-time jobs either on or off campus. Concussions were either sports-related or due to motor vehicle accidents. Participants with a previous concussion had between 1 to 9 diagnosed concussions, of which 59% (n = 10) had between 1 to 3 concussions, 35% (n = 6) had between 4 to 6 concussions, and 6% (n = 1) had 9 concussions. The mean duration between the last concussion and testing was 4.4±1.8 years (range: 2–7 years). The mean PCSS score in the concussion group was 16.6±18.8 (range: 0–61). The most common complaints identified were headaches and difficulty concentrating (52.9%); drowsiness (47%); fatigue, trouble falling asleep, sensitivity to light, and difficulty remembering (41%). Clinical examination showed no abnormalities in smooth pursuit, saccades, and head thrust in all participants.
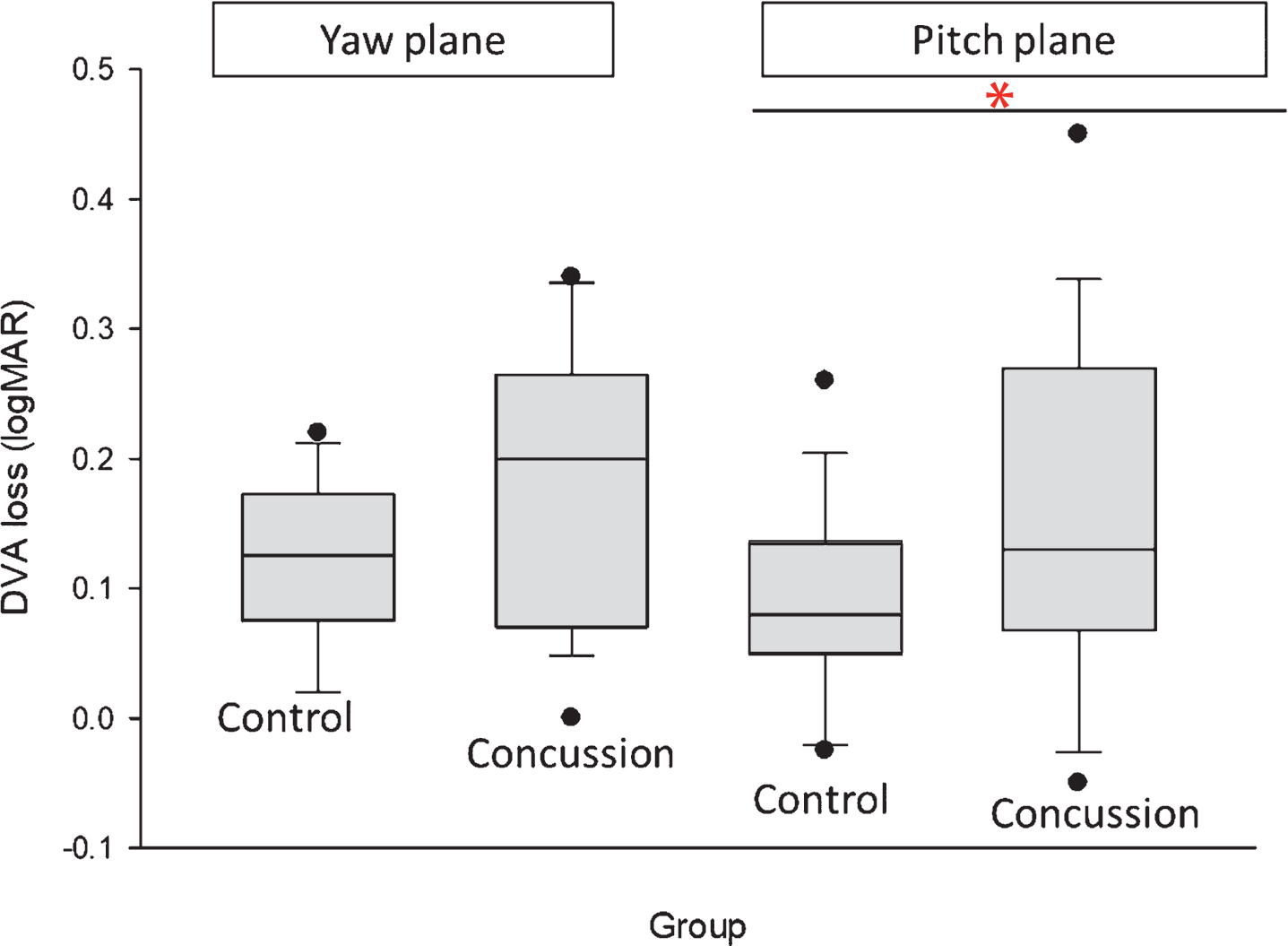
All participants had PTT scores of 40msec or less. Mean DVA loss in the pitch plane was significantly different between the concussion and control group (p = 0.04) (Table 2 and Fig. 2), while DVA loss in the yaw plane between groups was not significant (p = 0.06).
Fig.2
Differences between groups in dynamic visual acuity loss in the yaw and pitch planes. DVA loss is measured in logMAR values. Significant differences between groups is indicated by * (p = 0.04), DVA – dynamic visual acuity.
The concussion group had lower sleep quality based on the PSQI (p = 0.01) and increased daytime sleepiness based on the ESS (p = 0.01) compared to healthy controls (Table 1). Component scores on the PSQI that were significantly different between groups included sleep quality (p = 0.02), sleep latency (p = 0.03), habitual sleep efficiency (p = 0.03), and sleep dysfunction (p = 0.04).
4.1Correlations between gaze stability and sleep
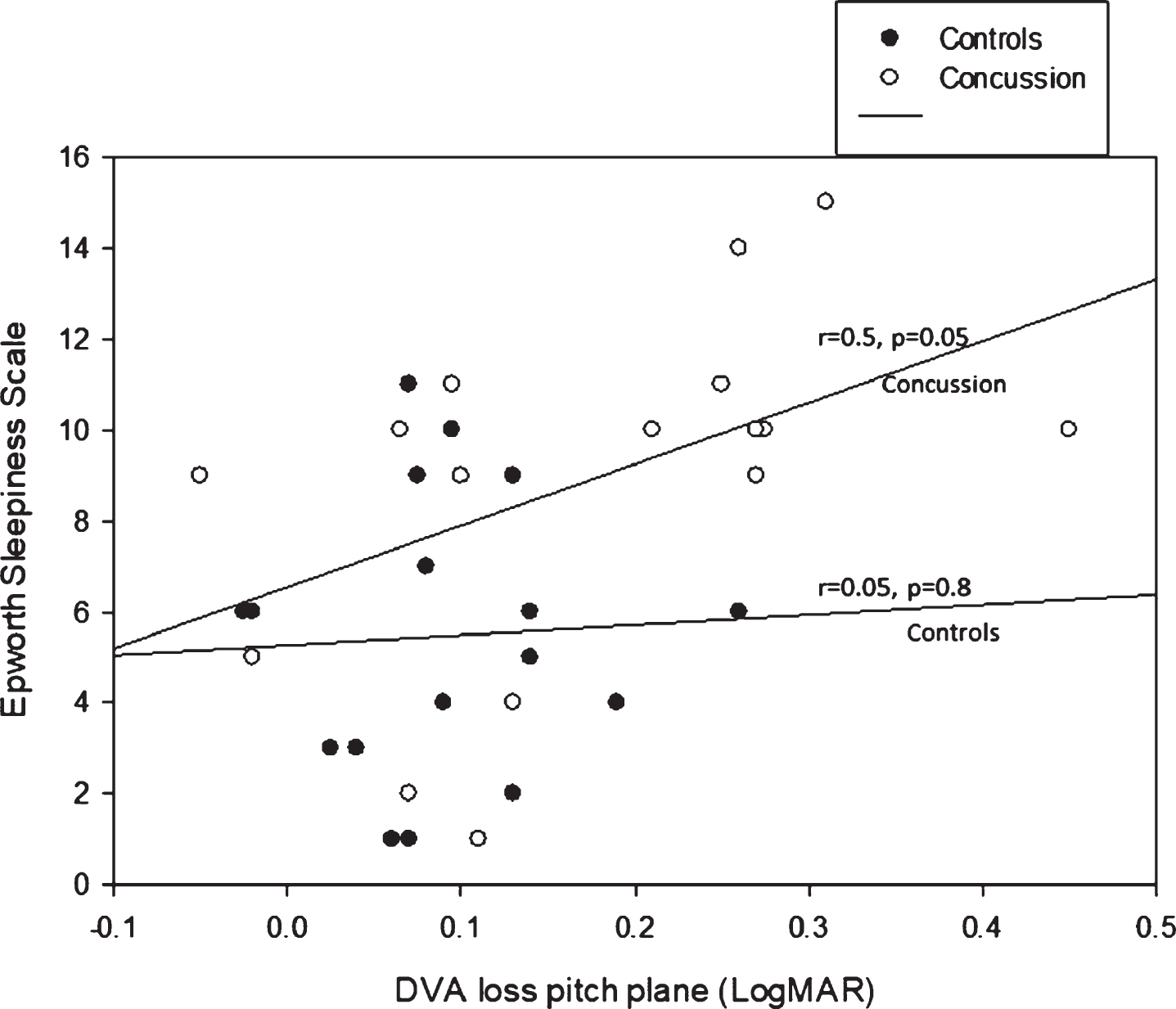
Mean DVA loss in the pitch plane was correlated with the PSQI (r = 0.45, p = 0.01) and the ESS (r = 0.44, p = 0.01) (Fig. 3 shows the correlations for the concussion and control group separately).
5Discussion
Fig.3
Correlations between DVA loss in the pitch plane and the Epworth Sleepiness Scale.
The primary purpose of this study was to determine if gaze stability deficits are present more than one year after a concussion. We found significant differences in DVA loss in the pitch plane between the concussed group (mean logMAR 0.17±0.13) and the control group (mean logMAR 0.09±0.07). Kaufman et al. examined gaze stability in high school and college football players using the DVAT and found no differences in DVA loss in either the pitch or yaw planes between those who had suffered concussions and controls [19]. However, all the subjects in their study were reported to be asymptomatic. Although the presence or absence of current symptoms were not exclusion criteria for our study, most participants in the concussion group reported symptoms on the PCSS (16.6±18.8; range 0–61). In a study examining athletes within 6 months of a concussion, Wright et al. found significant differences in DVAT in the yaw plane at speeds of 180 beats/minute. The group with concussion had a significantly higher decline in gaze stability with head movement [mean DVA loss for concussion group 2.43±0.43 compared to controls 1.45±0.19 (p = 0.03)]. Although participants were similar in age in both studies, the main difference was that DVAT was conducted in standing and was not computerized in the Wright et al. study [45]. These studies show that gaze stability deficits in the concussion population may be influenced by factors such as presence of symptoms, time since injury, as well as the equipment and testing protocol used.
Gaze stability is a function of the vestibular system and the visual “following” system [9, 13] and has combined input from the vestibulo-ocular reflex (VOR), the cervico-ocular reflex (COR), optokinetic reflex, and pursuit and saccadic eye movements [8, 41]. Because the vestibular and visual systems play an important role in calibrating eye and head movements, disruption in either the vestibular system, visual system, or pathways that integrate both systems can result in retinal slippage during head movements. As little as 2–4 degrees per second of retinal slip is enough to degrade visual acuity [36]. Our study found a significant difference between the concussion and control groups on the DVAT. The mean DVA loss in the concussion group in the yaw plane (logMAR 0.18±0.1) and in the pitch plane (0.17±0.13) were higher than controls; however, all groups were below the clinical cut-off of 0.2 logMAR. There is limited research examining gaze stability in young adults between 20 to 30 years of age who have had previous concussion. More work is needed to examine the relationship between gaze stability metrics in this age group with performance of visual scanning during dynamic activity.
Our secondary objective was to examine the relationship between gaze stability and sleep deficits. Our hypothesis was that persistent gaze stability deficits would be correlated with poor sleep quality. We saw a moderate correlation between gaze stability measures and sleep in the concussion group, that is 41% of participants reported difficulty falling asleep and 47% had increased daytime sleepiness. Sleep disturbances have been described as one of the most debilitating symptoms post-concussion [28]. A study by Tkachenko et al. examined people 2 days-2 years after a concussion injury with the Sports Concussion Assessment Tool-3 (SCAT-3), showing that sleep problems like drowsiness and difficulty falling asleep became worse with time [43]. In people with chronic symptoms after a concussion, insomnia was reported by 65.3% of patients and sleep disturbances were present in 23.7% at least five years post-injury [12].
The vestibular system is known to synchronize circadian rhythms [2]. In animal models, the vestibular system through the otoliths was shown to influence the induction of sleep based on the amount of daily activity [5]. Astronauts are known to have decrease in sleep time due to the gravity eliminated effect on the otoliths [34], and severe sleep disorders are seen in people with central vestibular conditions, such as vestibular migraine [20]. Since neuroplasticity occurs during sleep, vestibular compensation may be influenced by the quality of sleep [2], which may hasten recovery following vestibular damage. This study was not designed to study the causal relationship between a stable gaze and sleep; however, we have shown correlations between visual-vestibular deficits in the chronic stage (>1 year after last concussion) and sleep dysfunction. Addressing vestibular deficits through vestibular rehabilitation and promoting healthy sleep habits after a concussion may help to mitigate chronic issues.
5.1Limitations
We acknowledge that a major limitation of this study is the small sample size. Because recruitment into the concussion group was based on verbal report of a physician diagnosed concussion, we may have had some participants in the control group with concussions which may not have been diagnosed by a physician. The PCSS was used to identify current symptoms in the concussion group; however, we did not have the control group complete the PCSS. Having a comparison between the 2 groups would be meaningful because symptoms on the PCSS including fatigue, anxiety, and drowsiness are commonly seen in students regardless of concussion history. All testing was conducted during the same time of day, in the mornings between 9-noon; however, some participants were tested close to scheduled exams during the semester which may have affected their responses on the PSQI. We used slower speeds when testing the DVA with a velocity range between 85–120 deg/sec for the horizontal DVA and 60–85 deg/sec for the vertical DVA compared to other laboratories. It is possible that slow speeds would allow inclusion of the oculomotor system to help with gaze stability rather than isolating VOR function. We used a convenience sample to enroll participants from one university setting; hence, the results may not be generalizable to other populations. It is possible that the students who did participate may have done so because they had symptoms.
5.2Future directions
Studies examining the relationship between DVA and specific symptoms on the PCSS like headaches would further elucidate the role of vestibular dysfunction in persistent symptoms.
6Conclusion
Among young adults, significant differences in DVA loss were found in the chronic stage after concussion, compared to those without a concussion history. Higher loss of dynamic visual acuity was correlated with poor sleep quality and higher daytime sleepiness. Future studies are needed to examine the benefit of vestibular rehabilitation and early sleep education in this young adult population with concussion.
Acknowledgments
The authors would like to thank Martha Fagan, Miranda Mularoni and Julia Lakin for assistance with participant testing. The authors appreciate the participants who volunteered time to contribute to this study.
References
[1] | Alshehri M.M , Sparto P.J , Furman J.M , Fedor S , Mucha A , Henry L.C , Whitney S.L , The usefulness of the video head impulse test in children and adults post-concussion, J Vestib Res 26: ((2016) ), 439–446. |
[2] | Besnard S , Tighilet B , Chabbert C , Hitier M , Toulouse J , Le Gall A , Machado M.L and Smith P.F , The balance of sleep: Role of the vestibular sensory system, Sleep Med Rev 42: ((2018) ), 220–228. |
[3] | Broglio S.P , Guskiewicz K.M , Sell T.C , Lephart S.M , No acute changes in postural control after soccer heading, Br J Sports Med 38: ((2004) ), 561–567. |
[4] | Buysse D.J , Reynolds C.F 3rd , Monk T.H , Berman S.R and Kupfer D.J , The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research, Psychiatry Res 28: ((1989) ), 193–213. |
[5] | Ciriello J , Caverson M.M , Hypothalamic orexin-A (hypocretin-1) neuronal projections to the vestibular complex and cerebellum in the rat, Brain Res 1579: ((2014) ), 20–34. |
[6] | D’Silva L.J , Staecker H , Lin J , Maddux C , Ferraro J , Dai H , Kluding P.M , Otolith Dysfunction in Persons With Both Diabetes and Benign Paroxysmal Positional Vertigo, Otol Neurotol 38: ((2017) ), 379–385. |
[7] | Daneshvar D.H , Nowinski C.J , McKee A.C , Cantu R.C , The epidemiology of sport-related concussion, Clin Sports Med 30: ((2011) ), 1–17, vii. |
[8] | Della Santina C.C , Cremer P.D , Carey J.P and Minor L.B , Comparison of head thrust test with head autorotation test reveals that the vestibulo-ocular reflex is enhanced during voluntary head movements, Arch Otolaryngol Head Neck Surg 128: ((2002) ), 1044–1054. |
[9] | Demer J.L , Evaluation of vestibular and visual oculomotor function, Otolaryngol Head Neck Surg 112: ((1995) ), 16–35. |
[10] | Demer J.L , Honrubia V , Baloh R.W , Dynamic visual acuity: a test for oscillopsia and vestibulo-ocular reflex function, Am J Otol 15: ((1994) ), 340–347. |
[11] | Dikmen S , Machamer J , Fann J.R , Temkin N.R , Rates of symptom reporting following traumatic brain injury, J Int Neuropsychol Soc 16: ((2010) ), 401–411. |
[12] | Duclos C , Dumont M , Wiseman-Hakes C , Arbour C , Mongrain V , Gaudreault P.O , Khoury S , Lavigne G , Desautels A , Gosselin N , Sleep and wake disturbances following traumatic brain injury, Pathol Biol (Paris) 62: ((2014) ), 252–261. |
[13] | Herdman S.J , Hall C.D , Schubert M.C , Das V.E , Tusa R.J , Recovery of dynamic visual acuity in bilateral vestibular hypofunction, Arch Otolaryngol Head Neck Surg 133: ((2007) ), 383–389. |
[14] | Herdman S.J , Tusa R.J , Blatt P , Suzuki A , Venuto P.J , Roberts D , Computerized dynamic visual acuity test in the assessment of vestibular deficits, Am J Otol 19: ((1998) ), 790–796. |
[15] | Hile E.S , Fitzgerald G.K , Studenski S.A , Persistent mobility disability after neurotoxic chemotherapy, Phys Ther 90: ((2010) ), 1649–1657. |
[16] | Hiploylee C , Dufort P.A , Davis H.S , Wennberg R.A , Tartaglia M.C , Mikulis D , Hazrati L.N , Tator C.H , Longitudinal Study of Postconcussion Syndrome: Not Everyone Recovers, J Neurotrauma ((2016) ). |
[17] | Honaker J.A , Criter R.E , Patterson J.N , Jones S.M , Gaze Stabilization Test Asymmetry Score as an Indicator of Previous Concussion in a Cohort of Collegiate Football Players, Clin J Sport Med 25: ((2015) ), 361–366. |
[18] | Johns M.W , A new method for measuring daytime sleepiness: the Epworth sleepiness scale, Sleep ((1991) ) 14: , 540–545. |
[19] | Kaufman D.R , Puckett M.J , Smith M.J , Wilson K.S , Cheema R , Landers M.R , Test-retest reliability and responsiveness of gaze stability and dynamic visual acuity in high school and college football players, Phys Ther Sport 15: ((2014) ), 181–188. |
[20] | Kim S.K , Kim J.H , Jeon S.S , Hong S.M , Relationship between sleep quality and dizziness, PLoS One ((2018) ) 13: , e0192705. |
[21] | Kisilevski V , Podoshin L , Ben-David J , Soustiel J.F , Teszler C.B , Hafner H , Chistyakov A , Results of otovestibular tests in mild head injuries, Int Tinnitus J 7: ((2001) ), 118–121. |
[22] | Lange R.T , Brickell T.A , Ivins B , Vanderploeg R.D , French L.M , Variable, not always persistent, postconcussion symptoms after mild TBI in U.S. military service members: a five-year cross-sectional outcome study, J Neurotrauma 30: ((2013) ), 958–969. |
[23] | Larson E.B , Sleep disturbance and cognition in people with TBI, NeuroRehabilitation 43: ((2018) ), 297–306. |
[24] | Leigh R.J , Brandt T , A reevaluation of the vestibulo-ocular reflex: new ideas of its purpose, properties, neural substrate, and disorders, Neurology 43: ((1993) ), 1288–1295. |
[25] | Lovell M.R , Iverson G.L , Collins M.W , Podell K , Johnston K.M , Pardini D , Pardini J , Norwig J , Maroon J.C , Measurement of symptoms following sports-related concussion: reliability and normative data for the post-concussion scale, Appl Neuropsychol 13: ((2006) ), 166–174. |
[26] | Maruta J , Heaton K.J , Maule A.L , Ghajar J , Predictive visual tracking: specificity in mild traumatic brain injury and sleep deprivation, Mil Med 179: ((2014) ), 619–625. |
[27] | Maskell F , Chiarelli P , Isles R , Dizziness after traumatic brain injury: overview and measurement in the clinical setting, Brain Inj 20: ((2006) ), 293–305. |
[28] | Mathias J.L , Alvaro P.K , Prevalence of sleep disturbances, disorders, and problems following traumatic brain injury: a meta-analysis, Sleep Med 13: ((2012) ), 898–905. |
[29] | Mathiasen R , Hogrefe C , Harland K , Peterson A , Smoot M.K , Longitudinal Improvement in Balance Error Scoring System Scores among NCAA Division-I Football Athletes, J Neurotrauma 35: ((2018) ), 691–694. |
[30] | McCrea M , Guskiewicz K.M , Marshall S.W , Barr W , Randolph C , Cantu R.C , Onate J.A , Yang J , Kelly J.P , Acute effects and recovery time following concussion in collegiate football players: the NCAA Concussion Study, JAMA 290: ((2003) ), 2556–2563. |
[31] | McCrory P , Meeuwisse W.H , Aubry M , Cantu R.C , Dvorak J , Echemendia R.J , Engebretsen L , Johnston K , Kutcher J.S , Raftery M , Sills A , Benson B.W , Davis G.A , Ellenbogen R , Guskiewicz K.M , Herring S.A , Iverson G.L , Jordan B.D , Kissick J , McCrea M , McIntosh A.S , Maddocks D , Makdissi M , Purcell L , Putukian M , Schneider K , Tator C.H , Turner M , Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport, Zurich, November , J Athl Train 48: ((2013) ), 554–575. |
[32] | Minen M.T , Boubour A , Walia H , Barr W , Post-Concussive Syndrome: a Focus on Post-Traumatic Headache and Related Cognitive, Psychiatric, and Sleep Issues, Curr Neurol Neurosci Rep ((2016) ) 16: , 100. |
[33] | Narciso F.V , Barela J.A , Aguiar S.A , Carvalho A.N , Tufik S , de M.T , Mello, Effects of Shift Work on the Postural and Psychomotor Performance of Night Workers, PLoS One ((2016) ) 11: , e0151609. |
[34] | Pandi-Perumal S.R , Gonfalone A.A , Sleep in space as a new medical frontier: the challenge of preserving normal sleep in the abnormal environment of space missions, Sleep Sci 9: ((2016) ), 1–4. |
[35] | Prins M , Greco T , Alexander D , Giza C.C , The pathophysiology of traumatic brain injury at a glance, Dis Model Mech 6: ((2013) ), 1307–1315. |
[36] | Pritcher M.R , Whitney S.L , Marchetti G.F , Furman J.M , The influence of age and vestibular disorders on gaze stabilization: a pilot study, Otol Neurotol 29: ((2008) ), 982–988. |
[37] | Raikes A.C , Schaefer S.Y , Sleep Quantity and Quality during Acute Concussion: A Pilot Study, Sleep 39: ((2016) ), 2141–2147. |
[38] | Riemann B.L , Guskiewicz K.M , Effects of mild head injury on postural stability as measured through clinical balance testing, J Athl Train 35: ((2000) ), 19–25. |
[39] | Sandel N.K , Lovell M.R , Kegel N.E , Collins M.W , Kontos A.P , The relationship of symptoms and neurocognitive performance to perceived recovery from sports-related concussion among adolescent athletes, Appl Neuropsychol Child 2: ((2013) ), 64–69. |
[40] | Schubert M.C , Minor L.B , Vestibulo-ocular physiology underlying vestibular hypofunction, Phys Ther 84: ((2004) ), 373–385. |
[41] | Schubert M.C , Zee D.S , Saccade and vestibular ocular motor adaptation, Restor Neurol Neurosci 28: ((2010) ), 9–18. |
[42] | Sufrinko A , Johnson E.W , Henry L.C , The influence of sleep duration and sleep-related symptoms on baseline neurocognitive performance among male and female high school athletes, Neuropsychology ((2016) ) 30: , 484–491. |
[43] | Tkachenko N , Singh K , Hasanaj L , Serrano L , Kothare S.V , Sleep Disorders Associated With Mild Traumatic Brain Injury Using Sport Concussion Assessment Tool 3, Pediatr Neurol ((2016) ) 57: , 46–50.e41. |
[44] | Wickwire E.M , Williams S.G , Roth T , Capaldi V.F , Jaffe M , Moline M , Motamedi G.K , Morgan G.W , Mysliwiec V , Germain A , Pazdan R.M , Ferziger R , Balkin T.J , MacDonald M.E , Macek T.A , Yochelson M.R , Scharf S.M , Lettieri C.J , Sleep, Sleep Disorders, and Mild Traumatic Brain Injury. What We Know and What We Need to Know: Findings from a National Working Group, Neurotherapeutics 13: ((2016) ), 403–417. |
[45] | Wright W.G , Tierney R.T , McDevitt J , Visual-vestibular processing deficits in mild traumatic brain injury, J Vestib Res ((2017) ) {27: , 27–37. |




