Lessons learned from the COVID-19 pandemic: Improving initial investigations with the implementation of a COVID-19 blood request panel
Abstract
BACKGROUND:
During the COVID-19 pandemic, the Hillingdon Hospitals NHS Foundation Trust produced trust guidelines for the initial blood investigation of COVID-19 inpatients. However, insufficient education meant inconsistent adherence to this guidance.
OBJECTIVE:
To examine whether the implementation of a COVID-19 blood request panel improves adherence to local trust guidelines.
METHOD:
Between March and April 2020, initial blood investigations performed for positive COVID-19 cases were compared to guidelines. Results were presented locally and a COVID-19 panel was added to the electronic system that provided prompts for appropriate investigations. A re-audit between May and June 2020 was conducted to assess adherence post-intervention.
RESULTS:
383 patients were identified in the initial audit cohort and a sample of 20 patients were re-audited. Adherence to Full Blood Count, Urea and Electrolytes, C-reactive Protein and Liver Function Tests increased to 100% from 99.7% (p = 0.8), 99.2% (p = 0.69), 98.7% (p = 0.61), and 96.6% (p = 0.4) respectively. Coagulation screen adherence increased to 90% from 72.8% (p = 0.09). Appropriate requesting of D dimers increased to 50% from 19.9% (p = 0.001). Inappropriate troponin requesting decreased to 26.3% from 38.9% (p = 0.23).
CONCLUSION:
A user-friendly COVID-19 panel of investigations resulted in improved adherence to guidelines. Clear communication and education are essential to help alleviate uncertainty during a pandemic.
1.Background
At the beginning of the COVID-19 pandemic, there was a lack of national evidence-based risk scores and local guidelines for investigation, thus a quality improvement project (QIP) was conducted to improve this. At The Hillingdon Hospitals NHS Foundation Trust (THH), local guidelines on appropriate investigations for patients with COVID-19 were introduced (Fig. 1).
Fig. 1.
Local THH guidelines for the management of inpatients with COVID-19 (March 2020).
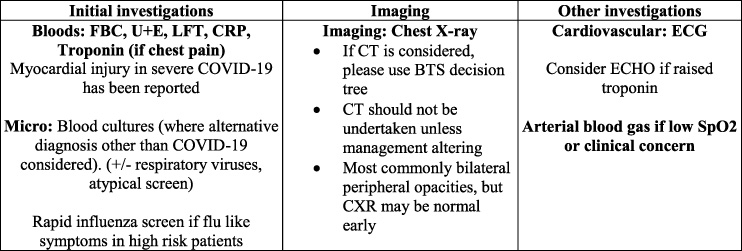
The COVID-19 pandemic triggered a high output of new evidence and changing opinion in clinical practice [1]. In the initial guidance, there were no recommendations to perform a coagulation or D-dimer sample for these patients (Fig. 1), as there was initially a lack of understanding that the disease promotes a pro-thrombotic state [2].
Care bundles exist in medicine and have been associated with improving initial investigations and management for patients, leading to better outcomes [3]. With respect to COVID-19, there is evidence that certain biomarkers are associated with worse prognosis [4]. Patients with severe acute phase reactants, such as raised C-Reactive Protein (CRP) and fibrinogen, are more likely to have COVID-19-associated hypercoagulability [5]. Moreover, prolonged Prothrombin Time (PT) and Activated Partial Thromboplastin Time (APTT) are associated with higher mortality [6]. Further, patients with severe COVID-19 may develop a hyper inflammatory response, which is linked to Acute Respiratory Distress Syndrome (ARDS) and multiorgan failure [2]. Given these crucial associations, the authors aimed to design a COVID-19 blood investigation panel to help improve the adherence of these requests to local guidelines.
2.Methods
An initial audit was undertaken to determine the number of blood investigations being performed on this patient cohort, and the proportion of patients presenting with chest pain and shortness of breath (SOB) with subsequent troponin and D-dimer performed under each respective symptom. The research team focused on patients who were seen and or admitted following attendance to the Emergency Department (ED) at the Hillingdon Hospital. A retrospective review of all adult patients with a positive SARS-CoV-2 Reverse Transcription Polymerase Chain Reaction (SARS-CoV-2 RT-PCR) test, between 16th March and 14th April 2020, was performed. These results were then compared to the trust’s COVID-19 guidelines.
The results from this audit were then discussed in a large multidisciplinary team involving medical and ED healthcare professionals involved in the care of this patient cohort. The results and recommendations were distributed to the target audience through the trust communications email.
The intervention consisted of a COVID-19 blood request panel on admission – including Full Blood Count (FBC), Urea and Electrolytes (U+Es), Liver Function Tests (LFTs), CRP, and a coagulation screen – alongside two prompts asking about chest pain and SOB, additionally requesting a troponin and D-dimer if these symptoms were present. In addition to disseminating trust-wide emails, education was facilitated through producing informative clinical decision support posters to display throughout the trust, and the creation of new COVID-19 investigation guidelines.
Implementing the intervention required cooperation across multiple trust departments, followed by discussion with the Information Technology (IT) department to create the panel. Healthcare staff were encouraged to contact the QIP leads with any questions or feedback, either in person or through email.
The improvement was measured by comparing the proportion of tests conducted pre and post-intervention. Targeted management was measured by comparing the proportion of patients with chest pain and SOB and whether they had an initial troponin and D-dimer respectively. A re-audit of a second cohort was performed between 15th May and 14th June 2020. These findings were presented locally and shared via trust communications emails.
Statistical analysis for significance of difference between pre- and post-interventional outcomes was done using Chi-Square test without Yate’s correction.
3.Results
The initial audit identified 383 patients from this time period. As seen in Fig. 2, a large proportion of blood investigations were not performed for this cohort. It was hypothesised that the novelty of the disease and reduced awareness of the disease pathophysiology resulted in clinicians being unsure as to which initial investigations to request.
Fig. 2.
Adherence to local trust guidelines on initial blood requests including initial D-dimer and Troponin requests, dependent on patient’s presenting symptoms, pre- and post-intervention.
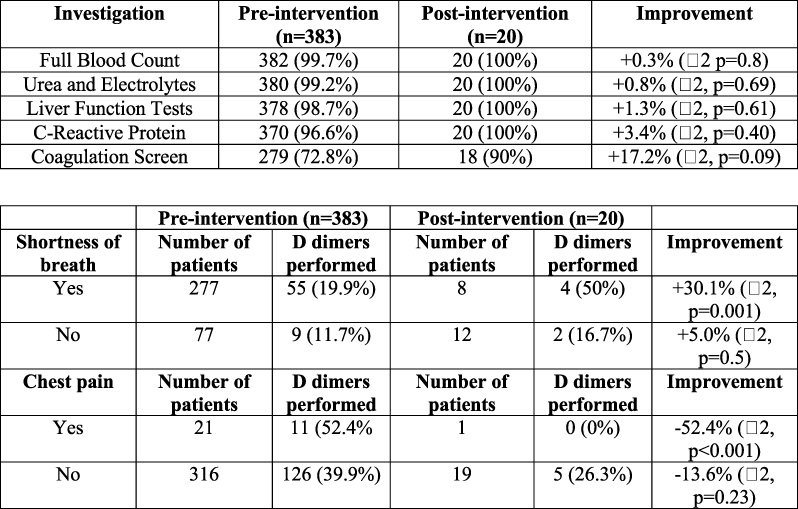
The re-audit identified 20 patients. Although not statistically significant, there was a global improvement in FBC, U+E, LFTs, CRP and coagulation screen requests for these patients (Fig. 2). Furthermore, there was a significant improvement in D-dimer being appropriately requested (p = 0.001). There was also an improvement in troponin being inappropriately requested (p = 0.23). However, there was a decrease in troponin requests being appropriately performed and an increase in D-dimer requests being inappropriately requested.
4.Conclusion
Initially, doctors’ knowledge on COVID-19 investigations was sparse. Our intervention resulted in better adherence to the updated guidelines and tailoring of care to each patient. Although there was significance in the improvement in D dimer bloods being appropriately requested, the authors acknowledge that the small sample size may result in a type II error and so further prospective audit is required to reduce this chance in future. A particular challenge also was finding the most effective method of educating clinicians given the range of departments and constraints of rotas. This challenge may partially explain why troponin and D dimers were not appropriately requested despite education being implemented; in future creating teaching sessions at the medical Grand Round which are engaging, simple and relevant may have the most impact in improving these parameters.
In the future, we would consider further (a) a plan for educating clinicians, and (b) shortening the timeline for initial data collection in order to assess the effect of implementation in real terms. Dissemination and consistency of information throughout departments within the hospital must be prompt in the future.
Bundling of COVID-19 investigations could result in better-targeted management of patients’ care and early recognition of the severity of symptoms by facilitating the development of risk scores. The approach of active implementation of evidence-based medicine is important in the context of audit of patient outcomes during any pandemic, disaster, or new emerging medical threat as part of the development of guidelines in an efficient, timely, and effective manner.
Conflict of interest
None to report.
References
[1] | Gupta R, Misra A. Contentious issues and evolving concepts in the clinical presentation and management of patients with COVID-19 infection with reference to use of therapeutic and other drugs used in Co-morbid diseases (Hypertension, diabetes etc.). Diabetes Metab Syndr. (2020) ;14: (3):251–4. |
[2] | Jose RJ, Manuel A. COVID-19 cytokine storm: The interplay between inflammation and coagulation. Lancet Respir Med. (2020) ;8: (6):e46–7. |
[3] | Milano PK, Desai SA, Eiting EA, Hofmann EF, Lam CN, Menchine M. Sepsis bundle adherence is associated with improved survival in severe sepsis or septic shock. West J Emerg Med. (2018) ;19: (5):774–81. |
[4] | Perez-Guzman PN, Daunt A, Mukherjee S, Crook P, Forlano R, Kont MD Clinical characteristics and predictors of outcomes of hospitalized patients with COVID-19 in a multi-ethnic London NHS Trust: A retrospective cohort study. Clin Infect Dis. (2021) ;73: (11):e4047–57. |
[5] | Maier CL, Truong AD, Auld SC, Polly DM, Tanksley CL, Duncan A. COVID-19-associated hyperviscosity: A link between inflammation and thrombophilia? Lancet. (2020) ;395: (10239):1758–9. |
[6] | Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. (2020) ;18: (4):844–7. |




