An old entity, a new trigger: Post COVID-19 vaccine pityriasis rubra pilaris
Abstract
BACKGROUND:
Pityriasis rubra pilaris (PRP) is a rare, acquired, chronic papulosquamous dermatosis which can occur in all ages. PRP can be associated with infection, autoimmunity, drugs and malignancies, and can be idiopathic.
OBJECTIVE:
PRP following vaccination has been rarely described in the literature. To the best of our knowledge, we report the first case of PRP two weeks following COVID-19 vaccination (Covishield).
CASE REPORT:
A 72-year-old male presented to the outpatient dermatology department at All India Institute of Medical Sciences – Bhopal with minimally pruritic superficial plaques since one week. The patient was vaccinated against COVID-19 with Covishield two weeks earlier. The lesions developed as erythematous scaly follicular papules and plaques over axilla that rapidly spread to the trunk in the following weeks and involved palms and soles as well as thickening and fissuring. The clinical features suggested PRP. The histopathology showed epidermal acanthosis with hypergranulosis alternating with parakeratosis and orthokeratosis with broad rete ridges with follicular plugging. The patient had started taking topical corticosteroids and emollients, which proved effective. There was no recurrence after receiving a second dose on follow-up.
CONCLUSION:
In patients presenting with new onset PRP in this COVID-19 era, the possibility of vaccine as a trigger should be taken into consideration, and further dosing should be carefully monitored in view of possible recurrence.
1.Introduction
Pityriasis rubra pilaris (PRP) is a rare, acquired, chronic papulosquamous dermatosis which can occur in all ages. The exact etiology and pathogenesis of the disease is unknown despite the multitude of associations with the disease. Associations with bacterial and viral infections, aberrant Vitamin A metabolism, genetic mutations, autoimmune disorders and malignancies have been reported [1]. Disease occurrence after vaccination has been rarely reported. We describe a case of PRP occurring after COVID vaccination.
2.Case report
A 72-year-old male presented to the outpatient dermatology department at All India Institute of Medical Sciences – Bhopal with a two-week history of erythematous scaly papulosquamous plaques on the face, trunk and limbs with palmoplantar thickening and fissuring. On examination, the patient had ill-defined thin, dusky erythematous follicular papules and plaques affecting the face, and extensors more than flexors of the upper and lower limbs and trunk (Fig, 1a,b). The palms and soles were affected by diffuse hyperkeratosis with fissuring and well-defined erythematous plaques extending from the sole to involve the lateral border of the feet (Fig. 1c). The Grattage test was positive and Auspitz sign was negative. The patient had no personal or family history of skin disease. He neither had preceding upper respiratory tract infection, persistent fever associated with weight loss or recent introduction of a drug. He had a known case of hypertension controlled by medications for 8 years. He received his first dose of Covishield three weeks before the development of the lesions. Histological examination from plaques on the limbs and trunk showed hyperkeratosis with alternating parakeratosis and orthokeratosis with acanthosis and broad rete ridges and mild perivascular and periadnexal inflammatory infiltrate in the dermis (Fig. 2). The chest X-ray was normal. A full blood count including white blood cell count, platelet count, liver and renal function tests, lipid profile, urine routine microscopy and thyroid hormones were within normal limits. Serology for human immunodeficiency virus, hepatitis B and C was negative. The reverse transcription polymerase chain reaction (RT-PCR) for COVID-19 was negative. These findings point to a post-vaccination aetiology of PRP.
Fig. 1.
(a) Bilaterally symmetric erythematous scaly follicular papules and plaques present over back with islands of sparing. (b) Bilaterally symmetric ill-defined erythematous scaly follicular papules and plaques over extensors of forearms with lesions involving palms. (c) Scaling and fissuring with plantar keratoderma.
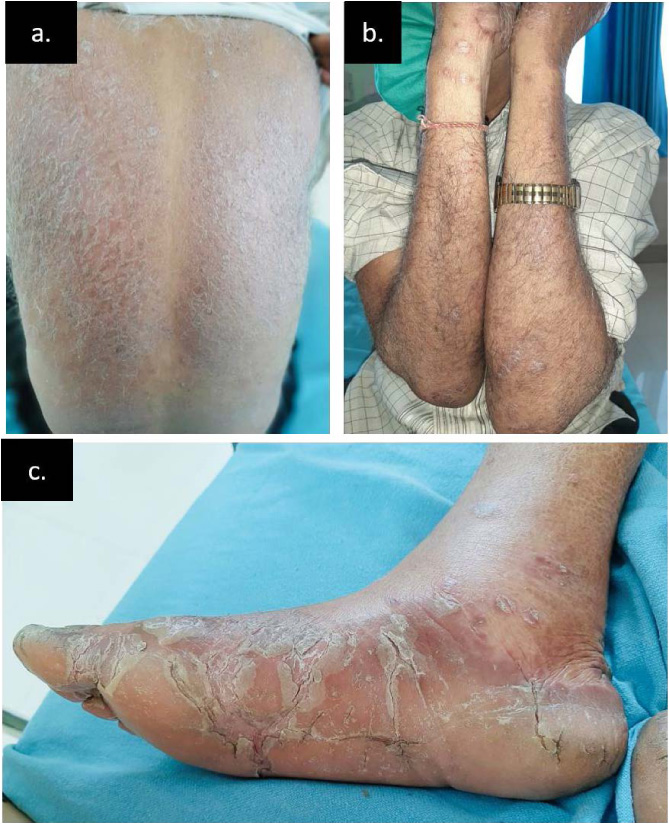
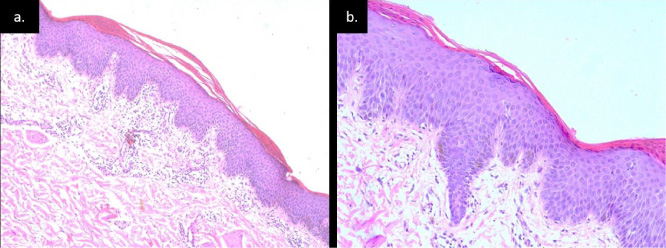
Fig. 2.
(a) Epidermis shows hyperkeratosis alternating orthokeratosis and parakeratosis with broad rete ridges dermis showing mild perivascular and periadnexal infiltrate (H&E stain, 10X). (b) Epidermis shows hyperkeratosis with alternating parakeratosis and orthokeratosis hypergranulosis (H&E stain, 40X).
Treatment was started on topical, high-potency corticosteroids and emollients which proved effective with no recurrence following the second dose of Covishield, which was given six weeks from the first dose.
3.Discussion
PRP is an uncommon inflammatory dermatosis characteristically presenting as follicular papules and palmoplantar keratoderma. Initially believed to be a variant of psoriasis, it was later described as a distinct entity by Alphonse Devergie in 1856. It affects all ages and races and has a bimodal peak of age distribution with cases being more common in the paediatric age group [1]. Griffith classified PRP into five types with the sixth subtype associated with HIV being added later by Miralles et al. Type I is the most common of all variants with the best prognosis in terms of patients attaining remission within three years of the onset of the disease. It is associated with many infections, both bacterial and viral, in particular with HIV. It is postulated that the infectious superantigens triggered the disease and treatment with antimicrobials and antiretrovirals has led to clinical improvement in these cases [1].
To the best of our knowledge only four cases of PRP have been reported following vaccination. The trigger mechanism of post-vaccination PRP may be immunological or infectious. Previous reports of disease occurrence have been associated with diphtheria-pertussis-tetanus, anti-influenza, measles-mumps-rubella, and the oral polio vaccine [2]. Cases described had lesions two to three weeks after receiving the vaccine of which two were in children under 2 years and the other two were in adult females. In the fourth case a 19-month-old child received the DPT vaccine and oral polio vaccine as a part of routine immunisation and two weeks later developed lesions that resolved in topical therapy in five months [2]. No data was provided on any of the case reports on the recurrence of subsequent vaccination. We report the fifth case of post-vaccination PRP in a 72-year-old male following the administration of Covishield and no recurrence following the second dose.
Covishield (ChAdOx1 nCoV-19 (AZD1222)) is a recombinant vaccine comprising of a replication-deficient simian adenoviral vector that expresses the full-length SARS-CoV-2 spike protein. These viral vectored vaccines are highly immunogenic, have favourable safety profiles and can induce strong immune responses in older adults and immunocompromised individuals [3]. In preclinical trials Covishield has shown to induce Th-1 cells and produce a robust B cell activation and proliferation with production of neutralising anti-IgA and IgG antibodies post-vaccination [3]. It is administered as two doses four to twelve weeks apart in individuals above 18 years and may prevent the vaccines from developing disease. The most common side effect is pain, swelling and redness at the injection site. A small number of people develop fever, vomiting, flu-like symptoms, nausea, chills, joint pain or muscle ache with anaphylaxis being rare [4]. PRP is a new addition to the adverse events following the vaccine with the remote possibility of a recurrence or flare on subsequent vaccination. It is difficult to fully ascertain the cause of the disease following vaccination. A thorough workup and evaluation has led us to believe that this is a potential trigger for the disease.
Conflict of interest
None to report.
References
[1] | Wang D, Cui-Lian Chong V, Chong W, Oon HH. A review on pityriasis rubra pilaris. American Journal of Clinical Dermatology. (2018) ;19: (3):377–90. |
[2] | Mohamed M, Belhadjali H, Hammedi F, Meriem C, Zili J. Pityriasis rubra pilaris occurring after vaccination with diphtheria-pertussis-tetanus and oral poliovirus vaccines. Indian Journal of Dermatology, Venereology, and Leprology. (2015) ;81: (6):618–20. |
[3] | Ewer KJ, Barrett JR, Belij-Rammerstorfer S, Sharpe H, Makinson R T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222 vaccine in a phase 1/2 clinical trial. Nature Medicine. (2021) ;27: :270–8. |
[4] | Serum Institute of India, Fact sheet for vaccine recipient: ChAdOx1 nCoV-19 corona virus vaccine (recombinant) – Covishield. 2021. Accessed from: https://www.seruminstitute.com/product_covishield.php. |




