Investigation of musculoskeletal disorders, physical activity level, sleep quality, and fatigue in health professionals with and without a history of COVID-19
Abstract
BACKGROUND:
Healthcare workers belong to an occupational group that is at high risk during the coronavirus 2019 (COVID-19) pandemic. The increased workload of healthcare workers and the accompanying psychosocial stress caused by the pandemic can affect musculoskeletal system disorders, physical activity status, sleep quality, and fatigue in this group.
OBJECTIVE:
To investigate musculoskeletal system disorders, physical activity level, sleep quality, and fatigue in healthcare workers with and without a COVID-19.
METHODS:
A total of 200 healthcare professionals aged 18-65 years with and without a history of COVID-19 were in the study. Data were collected between January and March 2021. A “Preliminary Evaluation Form”, “Extended version of the Nordic Musculoskeletal System Questionnaire (NMQ-E)”, “the International Physical Activity Questionnaire-Short Form (IPAQ-SF)” and “the Pittsburgh Sleep Quality Index (PSQI) were used for data collection”.
RESULTS:
It was determined that musculoskeletal system disorders did not differ significantly between healthcare workers with and without a COVID-19 history (p > 0.05). It was found that the number of people with problems in the low-back region was higher in those with a COVID-19 history (p = 0.002). In the sleep duration component, the scores of those who did not have a COVID-19 history were found to be significantly higher than those who did (p = 0.10). In other comparisons, it was determined that there was no significant difference.
CONCLUSIONS:
It was found that the number of people with problems in the low-back region was higher in those with a COVID-19 history. Those without a COVID-19 history had higher scores in sleep duration parameter.
1Introduction
Coronavirus 2019 (COVID-19) was first identified as a new coronavirus on January 13, 2020, as a result of the analyses made in a group of cases that developed respiratory symptoms in the Wuhan Province of China in December 2019. COVID-19 was initially detected in those found in the seafood and animal market in this region. Later, it spread from person to person and to other provinces in the Hubei State, especially in the Province of Wuhan, and other states of China, and eventually other countries [1]. The World Health Organization (WHO) declared the COVID-19 outbreak a pandemic on March 11, 2020. [2]. People all around the world have been severely affected by this pandemic, and many lives have been lost. In this period, normal life has severely changed and people had to switch to working from home, and health workers had to use their work performance at a high level. It was reported that healthcare workers, the most affected occupational group, are disproportionately affected by fear of contracting COVID-19, work stress, social isolation, and discrimination [3].
Musculoskeletal disorders are defined as disorders that occur in the structures that make up the musculoskeletal system (muscles, bones, ligaments, tendons, and joints) [4]. It can also be defined as sprain and strain in the low-back, neck, shoulder, and knee, which are usually the affected body parts. In many people, these disorders are due to their occupation or work activities [5]. Healthcare workers are at high risk for work-related musculoskeletal disorders, and it has been reported that this is a common health problem [4].
Physical activity (PA) is defined as the consumption of energy during body movements performed by skeletal muscles. Physical activity has benefits on the musculoskeletal system, cardiovascular system, cognitive level, and sleep [6,7]. Public parks, gyms, sports facilities, and sports clubs were closed to contain the COVID-19 epidemic, and these restrictions caused a decrease in physical activity for many people. Healthcare workers have been under great stress in the COVID-19 period, which leads to poor sleep quality, short sleep duration, and acute sleep problems. Insomnia, fatigue, and burnout are common with this stress in healthcare workers during the COVID-19 outbreak [8]. Fatigue, one of the symptoms of COVID-19, may be a symptom of a physical illness, an underlying psychological problem, or both [9].
In COVID-19 studies, musculoskeletal symptoms have been defined among the initial findings and investigated only for the presence/absence of fatigue, myalgia, and arthralgia. There is insufficient information on the perseverance and severity of musculoskeletal disorders after COVID-19. It is important to investigate the effects on the musculoskeletal system and the changing physical activity status, especially in healthcare workers who are most affected by the virus.
In this study, it was aimed to investigate whether there is a difference in terms of musculoskeletal system disorders, physical activity level, sleep quality, and fatigue between healthcare workers with and without a history of COVID-19.
2Materials and methods
2.1Study design
This is a descriptive cross-sectional study. The population of the study consists of healthcare workers working in a city hospital or a university hospital. The sample of the study consists of healthcare workers who were actively working in these hospitals, met the inclusion criteria, and agreed to participate in the study (n = 200). For the samples of our study, the hospital management was contacted and the contact information of the participants in our study was reached by keeping the personal data confidential and confidential. The study was conducted in accordance with the Declaration of Helsinki. Ethics Committee Approval was obtained from Süleyman Demirel University Faculty of Medicine Clinical Research Ethics Committee (04/01/2021/72867572-050.01.04-559). Permissions were also obtained from the Isparta Provincial Health Directorate (15/01/2021/E-16657963-799) and Süleyman Demirel University Hospital (17/02/2021/E-26515734-605.01-21138). Participation in the study was on a voluntary basis and healthcare workers were informed about the study in writing before the study. Written informed consent was obtained from all participants before proceeding to the questionnaires.
2.2Participants
Healthcare workers between the ages of 18 and 65 with or without a history of COVID-19 were included in the study. Non-healthcare workers, those younger than 18 or older than 65, those who did not fill in the data collection forms and did not want to participate in the study, those with acute musculoskeletal disorders in different parts of the body, those with sleep disorders, those using psychoactive drugs, and professional athletes were excluded from the study.
2.3Data collection
Data were collected between January 2021 and March 2021 with “Google Forms” by sharing a link to the data collection form via email or WhatsApp. To avoid duplication of responses, the link did not accept multiple responses from the same participant. After participating in the study, the participants were asked to provide information about their participation via e-mail. The answers were checked one by one and the missing information was requested to be completed and sent again. The accuracy of the data was thus ensured.
Demographic and baseline characteristics (including gender, age, height, weight, education level, marital status, occupation, employment status, night-shift status, and comorbidities), smoking, alcohol use, and initial symptoms at hospitalization (musculoskeletal and others) were questioned. In addition, the status of being admitted to the intensive care unit, the time elapsed after COVID-19, the status of working in the COVID-19 service, the type of exposure to COVID-19 patients, and the duration of care for COVID-19 patients were also questioned.
The Preliminary Evaluation Form, the extended version of the Nordic Musculoskeletal Questionnaire (NMQ-E), the International Physical Activity Questionnaire-Short Form (IPAQ-SF), the Pittsburgh Sleep Quality Index (PSQI), and the Fatigue Severity Scale (FSS) were used for data collection. It took approximately 20-25 minutes to respond to the data.
2.4Nordic Musculoskeletal Questionnaire-Extended (NMQ-E)
The Turkish translation and adaptation study of the NMQ-E was performed by Alaca et al. It is an assessment tool used for the evaluation of musculoskeletal disorders. The NMQ-E assesses the presence, onset, incidence, results, and effects of musculoskeletal pain in 9 zones of the body, including neck, shoulder, back, elbow, hand/wrist, waist, hip/thigh, knee, and foot/ankle. In this questionnaire, the age of the pain onset, hospitalization due to pain, changing the place of work, and having pain in the last year, last month, and then-current day are questioned. In addition, it questions whether the then-current pain affects the individual’s work/home life, seeking medical care from a doctor/physiotherapist, taking medication, and getting a sick leave. It can be administered as a self-report questionnaire or by interviewing the person [10].
2.5International Physical Activity Questionnaire-Short Form (IPAQ-SF)
The scale was developed by Craig et al. and the Turkish validity and reliability study of the questionnaire was performed by Öztürk in 2005. The questionnaire consists of 7 items. It takes “physical activities performed at least 10 minutes at a time” as a criterion for the assessment. It provides information on the time spent in walking, moderate-to-intense activities, and intense activities. The time spent sitting is a separate question. The total score includes the sum of time (minutes) by frequency (days) of walking, moderate-to-intense activity, and intense activity. As a result of the calculations, a score is obtained in MET-minutes. One MET-minute is calculated by multiplying the minute of activity with the MET score. MET-minute scores were determined based on the weight-calorie values of a 60-kilogram person. Kilocalories are calculated as MET-min x (person’s body weight in kg / 60 kilograms). Standard MET values are as follows: Walking: 3.3 MET; moderate physical activity: 4.0 METs; intense physical activity: 8.0 METs; and seating: 1.5 MET. The total score is obtained by adding walking, moderate, and intense physical activity MET-min/week scores, and classified according to cut-off points. This classification is as follows: <600 MET-min/week physically inactive; 600-3000 MET-min/week low-level physical activity; and >3000 MET-min/week adequate physical activity [11,12].
2.6Pittsburgh Sleep Quality Index (PSQI)
The PSQI was developed by Buysse et al. and the Turkish validity and reliability study of the index was conducted by Ağargün et al. in 1996. The PSQI has been widely used in many studies. It provides a quantitative measure of sleep quality. The scale consists of 24 items and evaluates sleep quality belonging to the previous 4 weeks. The first 19 questions allow self-evaluation. The last 5 questions are answered by the partner or a roommate of the individual and these questions are not included in the calculation. The scale consists of 7 components and each component is scored between 0 and 3. These components are subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbance, use of sleeping pills, and daytime dysfunction. The sum of these seven component scores gives the total index score, which ranges from 0 to 21. A high total score indicates poor sleep quality. The scale has a cut-off point and is 5 for the total PSQI score [13,14].
2.7Fatigue Severity Scale (FSS)
The FSS was developed by Krupp et al. and its Turkish validity and reliability study was performed by Armutlu et al. The scale used to determine the state of fatigue consists of 9 items assessing only the previous week. The highest score that can be obtained from the scale is 63 and the total score is found by the average of 9 questions. A score of 4 and above indicates pathological fatigue [15,16].
2.8Data analysis
The data obtained in the study were analyzed with the SPSS 22.0 program. Characteristics were analyzed with descriptive statistics. The mean and standard deviation are presented for continuous variables and frequencies and percentages are presented for categorical variables. Chi-square analysis was used for categorical data, frequency distributions of demographic variables, and categorical comparisons. Chi-square Fisher’s exact test was used if the expected value was less than 5 in 2x2 dimensional tables. For the two-group mean comparison, the independent sample t-test was used for normally distributed data, and the Mann-Whitney U test was used for the data that did not show normal distribution. The level of significance in the analyses was determined as 95% (p < 0.05). The power analysis and sample level of the study were calculated based on the study of Wang et al. According to this study, it can be seen that recruiting n = 74 healthcare workers in our study can represent the study with 90% power (it is predicted that values of 0.70 and above are valid and 0.80 will be quite sufficient in studies) [17].
3Results
The study was completed with the health workers (n = 200) who voluntarily agreed to participate in the study, and the findings obtained from the study are outlines below.
The height and weight of those with and without a history of COVID-19, and the number of days elapsed after COVID-19 for those with a history of COVID-19 are presented in Table 1.
Table 1
Height, weight of groups and number of days after COVID-19
| COVID-19 | ||
| Those with a history of COVID-19 | Those without a history of COVID-19 | |
| (n = 100) | (n = 100) | |
| X±SD | X±SD | |
| Height (cm) | 167.19±7.20 | 167.42±7.42 |
| Weight (kg) | 68.01±11.97 | 64.91±11.43 |
| Number of days elapsed since COVID-19 (X±SD) | 42.15±43.74 | |
X: Mean, SD: Standard deviation.
The comparison of the sociodemographic data of the participants is given in Table 2. Of the healthcare workers with a history of COVID-19, 71% were women and 53% were in an age range of 25-34 years. Of those without a history of COVID-19, 69% were women and 49% were between the ages of 25 and 34 years (Table 2).
Table 2
Comparison of sociodemographic data of groups with and without a COVID-19 history
| Those with a history of COVID-19 | Those without a history of COVID-19 | p | ||
| (n = 100) | (n = 100) | |||
| n(%) | n(%) | |||
| Sex | Female | 71 (71) | 69 (69) | .758 |
| Male | 29 (29) | 31 (31) | ||
| Age | 18–24 years | 21 (21) | 29 (29) | .413 |
| 25–34 years | 53 (53) | 49 (49) | ||
| 35–54 years | 26 (26) | 22 (22) | ||
| Education level | High school | 17 (17) | 8 (8) | .057 |
| Associate degree | 12 (12) | 21 (21) | ||
| University | 61 (61) | 52 (52) | ||
| Post-graduate | 10 (10) | 19 (19) | ||
| Job tenure | Less than 1 year | 11 (11) | 17 (17) | .307 |
| 1–5 years | 43 (43) | 46 (46) | ||
| Over 5 years | 46 (46) | 37 (37) | ||
| Working in shift | 77 (77) | 70 (70) | .252 | |
| Smokers | 36 (36) | 43 (43) | .311 | |
| Alcohol consumption | 4 (4) | 18 (18) | .003* | |
| Working at a COVID-19 clinic | 65 (65) | 53 (53) | .084 | |
| Contact with COVID-19 patients | 50 (50) | 35 (35) | .145 | |
| Profession | Health technician | 24 (24) | 24 (24) | .339 |
| Physiotherapist | 4 (4) | 9 (9) | ||
| Nurse | 50 (50) | 35 (35) | ||
| Midwife | 9 (9) | 9 (9) | ||
| Physician | 13 (13) | 19 (19) | ||
Chi-square analysis *p < 0.05. Fisher’s exact test *p < 0.05.
According to the analyses, it was found that gender, age, marital status, education level, income level, place of work, and profession <s>d< /s>id not differ significantly between the groups (p > 0.05) (Table 2).
It was determined that alcohol use in participants without a history of COVID-19 was statistically significantly higher than in those with (p = 0.002). In the other comparisons, it was determined that the data did not differ statistically significantly between groups (p > 0.05) (Fig. 1, Table 2).
Fig. 1
COVID-19 symptoms percentage chart.
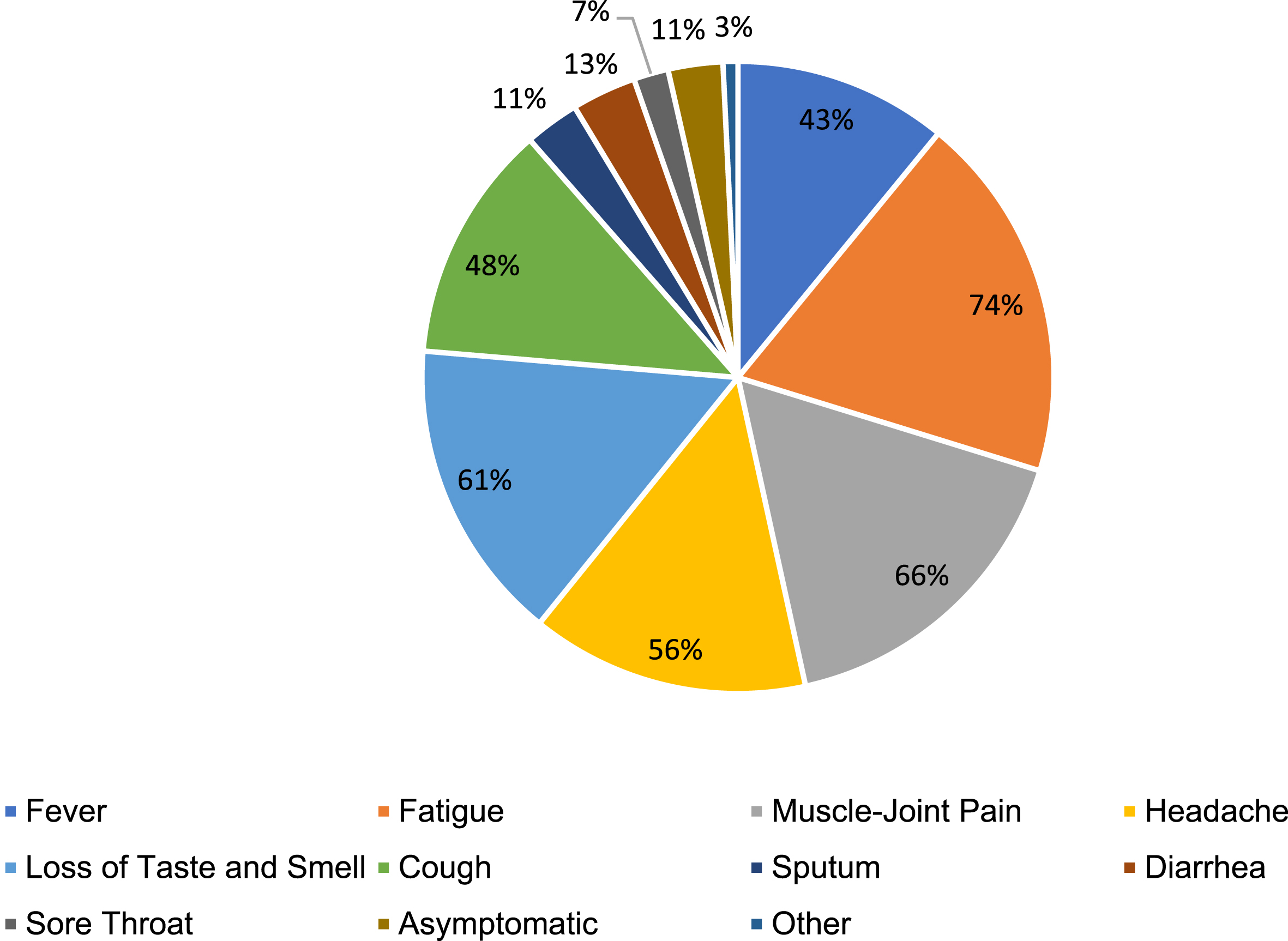
According to the analyses performed, it was determined that there was no significant difference between groups in terms of the presence of chronic disease (p = 0.276) and weekly exposure time to COVID-19 patients (p = 0.431) (See Table 3).
Table 3
COVID-19 comparison of the data of groups with and without a COVID-19 history
| Those with a history of COVID-19 | Those without a history of COVID-19 | p | ||
| (n = 100) | (n = 100) | |||
| n (%) | n (%) | |||
| Chronic disease | No | 87(87) | 86(86) | .236 |
| Lung disease | 4(4) | 3(3) | ||
| Heart disease | 0(0) | 4(4) | ||
| Diabetes mellitus | 5(5) | 2(2) | ||
| Other | 4(4) | 5(5) | ||
| Weekly exposure time to COVID-19 patients | 40 hours | 29(29) | 29(29) | .276 |
| 41–48 hours | 26(26) | 43(43) | ||
| 49–56 hours | 30(30) | 27(27) | ||
| 56 hours or more | 15(15) | 1(1) |
Chi-square analysis *p < 0.05.
It was determined in the inter-group comparison that there was a significant difference in the sleep duration component. The sleep duration scores of those without a history of COVID-19 were found to be significantly higher (p = 0.010). In other comparisons, it was determined that there was no significant difference (Table 4).
Table 4
Comparison of scale scores of participants with and without COVID-19 history
| Those with a history of COVID-19 | Those without a history of COVID-19 | Ta/Zb | p | ||
| X±SD | X±SD | ||||
| FSS | 5.08±1.37 | 4.84±1.42 | 1.192a | .235 | |
| IPAQ-SF | Walking (MET/weeks) | 427.00±336.13 | 436.95±295.53 | –.501b | .616 |
| Moderate physical activity (MET/ weeks) | 417.77±282.92 | 560.00±332.56 | –1.038b | .299 | |
| Severe physical activity (MET/weeks) | 730.66±838.89 | 1181.53±1158.46 | –1.426b | .154 | |
| PSQI | Subjective sleep quality | 1.47±.67 | 1.430±.55 | .458a | .647 |
| Sleep latency | 2.980±.20 | 2.97±.22 | .334a | .739 | |
| Sleep time | 1.06±.63 | 1.29±.62 | –2.587b | .010* | |
| Habitual sleep activity | .13±.41 | .25±.64 | –1.567b | .119 | |
| Sleeping disorder | 1.50±.68 | 1.46±.59 | .440a | .660 | |
| Use of sleeping pills | .20±.66 | .06±.31 | 1.902a | .059 | |
| Daytime dysfunction | 1.48±.99 | 1.47±.92 | .073a | .942 | |
| PSQI total | 8.82±2.61 | 8.93±2.25 | –.319b | .750 | |
aIndependent sample t-test, bMann-Whitney U test (Z score), *p < 0.05; IPAQ-SF: International Physical Activity Questionnaire-Short Form; PSQI: Pittsburgh Sleep Quality Index; FSS: Fatigue Severity Scale.
When the differences in musculoskeletal problems history (12 months, 1 month, and then-current day) responses were analyzed according to the status of having a history of COVID-19, it was found that the number of those who had problems in the lower-back region then-current day was higher in those with a history of COVID-19 (p = 0.002) (Table 5).
Table 5
Comparison of musculoskeletal problems according to the NMQ-E and the status of having a COVID-19 history
| Musculoskeletal problems | Musculoskeletal problems | Musculoskeletal problems | |||||||
| 12 months | 4 weeks | then-currently | |||||||
| COVID-19 history status | p | COVID-19 history status | p | COVID-19 history status | p | ||||
| Those with a history of COVID-19 | Those without a history of COVID-19 | Those with a history of COVID-19 | Those without a history of COVID-19 | Those with a history of COVID-19 | Those without a history of COVID-19 | ||||
| n (%)* | n (%)* | n (%)* | n (%)* | n (%)* | n (%)* | ||||
| Neck | 37 (74) | 47 (77) | .709 | 34 (92) | 39 (83) | .229 | 20 (54) | 26 (55) | .908 |
| Shoulder | 32 (84) | 27 (68) | .086 | 26 (81) | 26 (96) | .075 | 18 (56) | 14 (52) | .735 |
| Back | 50 (77) | 58 (82) | .492 | 49 (98) | 53 (91) | .134 | 38 (76) | 37 (64) | .170 |
| Elbow | 3 (33) | 5 (63) | .229 | 3 (100) | 5 (100) | – | 3 (100) | 1 (20) | .054 |
| Hand | 12 (67) | 11 (68) | .897 | 11 (92) | 9 (82) | .484 | 5 (42) | 4 (36) | .795 |
| Waist | 61 (84) | 54 (81) | .647 | 59 (97) | 52 (96) | .901 | 50 (82) | 30 (56) | .002* |
| Hip | 6 (75) | 7 (64) | .599 | 4 (67) | 6 (86) | .416 | 2 (33) | 3 (43) | .725 |
| Knee | 16 (57) | 22 (65) | .543 | 15 (94) | 21 (91) | .778 | 4 (25) | 13 (57) | .052 |
| Foot | 4 (67) | 7 (78) | .634 | 4 (100) | 6 (86) | .428 | 2 (50) | 4 (57) | .819 |
Chi-square analysis *p < 0.05. *Number and percentage of those answering the scale as “Yes”. NMQ-E: Nordic Musculoskeletal Questionnaire-Extended.
4Discussion
The main results of our study investigating musculoskeletal disorders, physical activity level, sleep quality and fatigue in healthcare workers after COVID-19. It has been found that the number of people who have problems in the low-back region today is higher in those who have had COVID-19. In the sleep duration parameter, it was found that the scores of those who did not have COVID-19 were significantly higher than those who did.
Musculoskeletal disorders are common in healthcare workers. These disorders can be caused by the following factors: staying in the same position for a long time, working in shifts, performing repetitive movements, lifting and transferring patients, anxiety, and work stress [18]. Although it has been reported that healthcare workers are at high risk for COVID-19, it is stated that several factors cause anxiety and stress in healthcare professionals, such as the high risk of contracting COVID-19, probability of transmitting the virus to their families, and having long working hours [19,20]. These anxiety and stress situations can lead to musculoskeletal disorders in healthcare workers who continue to work in a hospital environment, whether or not they had a COVID-19 history. It was reported that one of the symptoms of COVID-19 is musculoskeletal findings (myalgia and arthralgia) [21]. Having had COVID-19 may reveal musculoskeletal disorders. When the literature is examined, there are studies examining musculoskeletal system disorders in healthcare workers in the COVID-19 pandemic [22-25]. Arca et al. In their study, they reported that musculoskeletal pain was mostly experienced in the neck (73.4%) and back (61.4%) regions of healthcare workers in the COVID-19 pandemic, and this situation was more common in those who were in contact with COVID-19 patients working in the front line [22]. In a study conducted on doctors, it was shown that during the post-COVID-19 lockdown period, doctors experienced pain mostly in the lumbar region and its prevalence was high [23]. In another study, it was stated that the COVID-19 pandemic changed the approach of rehabilitation services to patients and increased the prevalence of work-related musculoskeletal disorders among physiotherapists [24]. As a result of the study examining the prevalence of musculoskeletal disorders in healthcare workers in the COVID-19 pandemic, it was determined that COVID-19 brought an excessive workload in healthcare professionals and this resulted in work-related musculoskeletal disorders [25]. In our study, unlike these studies in the literature, musculoskeletal disorders were examined in patients with and without COVID-19, and it was determined that there was no difference in terms of musculoskeletal disorders in healthcare workers who had and did not have COVID-19. This result shows that musculoskeletal system disorders do not exist in healthcare workers, even though they do not have COVID-19, and it shows that COVID-19 transmission does not make a difference. On the other hand, when the musculoskeletal problems history (12 months, 4 weeks, today) responses were compared between the groups, it was seen that the number of those who had instant problems in the lumbar region was higher in those who had COVID-19. The reason for this situation was thought to be due to the sequelae of COVID-19 in individuals and the continuation of their working life before the recovery process after the disease was fully realized.
It was found in the present study that healthcare workers with a history of COVID-19 had the most problems in the waist, back, and neck regions, in this order, and that health workers with no COVID-19 history had the most problems in the back, waist, and neck regions, in this order. Karaaslan et al. found that the most common musculoskeletal symptoms of the recovered COVID-19 patients aged 18-70 years were back pain, low back pain, and neck pain [26]. In a study conducted in university teaching staff during the COVID-19 pandemic, the highest prevalence of musculoskeletal disorders was found in the lower back, neck, and knee regions, in this order, compared to the pre-COVID-19 period [27]. The data we obtained in our study and the results of the studies in the literature show similarities, and in our study, it was additionally found that the majority of those who did not have a COVID-19 history had problems in the back region [28]. It shows us that the low back region, which is among the symptoms of the musculoskeletal system, is the region most affected by this virus in those with a history of COVID-19.
It is thought that musculoskeletal disorders in healthcare workers have increased due to the increase in intensive care patients and the increase in the number of patients in need of care during the pandemic process. Considering that most of the healthcare professionals participating in our study were working in shifts, it is thought that the increase in the number of patients per healthcare worker in a shift, the increase in the care needs of the patients, and the inability to sufficiently rest due to busy working hours are effective factors in the increase of musculoskeletal system disorders.
According to the analyses performed to determine the differences in the IPAQ-SF, PSQI subscale and FSS obtained from the study in groups with and without a COVID-19 history, it was found that there was a significant difference in the sleep duration component. It was determined that the sleep duration scores of those who did not have a COVID-19 history were significantly higher than those who had a COVID-19 history. It was determined that there was no significant difference in other sleep parameters. It is thought that healthcare workers with no COVID-19 history had better sleep times due to the lack of negative effects of COVID-19. In addition, it can be thought that the sleep time of healthcare workers who had a COVID-19 history decreased due to musculoskeletal disorders.
A significant reduction in total weekly energy expenditure has been reported in the literature during the COVID-19 quarantine [29]. Parallel to this situation, Kua et al. In their study, they found that there was a significant decrease in the frequency, duration and intensity of physical activity in healthcare workers during the COVID-19 pandemic compared to pre-quarantine [30]. The results of the study show that MET scores are not high in healthcare workers, which is consistent with studies in the literature [31,32]. It is thought that healthcare workers who are at risk of COVID-19 infection due to their working environment may face increased susceptibility to diseases due to weak anti-viral defenses caused by long breaks from physical activity. This situation reveals the importance of physical activity during the pandemic process.
In the meta-analysis conducted to analyze the existing evidence on the prevalence of depression, anxiety, and insomnia among healthcare professionals during the COVID-19 epidemic, the prevalence of insomnia in healthcare professionals was reported to be 38.9%. According to the results of this meta-analysis, almost 4 out of 10 healthcare workers were reported to have difficulty in sleeping and/or had insomnia [33].
Poor sleep quality was reported in approximately 75% of healthcare workers during the COVID-19 outbreak [34]. In a study, it was determined that health workers had poor sleep quality and were in a poor psychological state during the COVID-19 outbreak [35]. The results of our study, in line with these studies, revealed that health workers had poor sleep quality if they had a COVID-19 history. However, unlike the results of our study, in the meta-analysis of Xia et al., it was reported that sleep disorders are higher in front-line healthcare workers with a history of COVID-19 compared to healthcare workers who do not work on the front-line and did not have a COVID-19 history [36].
In our study, the average FSS score of healthcare workers with a history of COVID-19 was found to be 5. Similar to our study, Zou et al. found that the fatigue severity scores of frontline healthcare workers in the COVID-19 pandemic were≥5 [8].
Healthcare workers belong to an occupational group among which work-related musculoskeletal disorders are common. There may be several underlying factors such as working in shifts, long working hours, having insufficient rest, and work-related stress conditions. In the COVID-19 pandemic, the workload of healthcare professionals increased even more, sleep patterns were disrupted with irregular shifts, and psychosocial stress due to many factors increased as a result of the pandemic process. These factors can increase the musculoskeletal system complaints that may normally present. In this study, it was found that those who had COVID-19 had complaints in the low-back region and that their sleep duration was reduced. This study showed us that back problems were experienced then recently after COVID.
In line with these results, approaches to complaints in the low-back region of healthcare workers can be utilized during the COVID pandemic. Sleep quality can be improved by reducing shift hours for healthcare workers. The pandemic process causes physical inactivity in health workers, as in the whole population. To prevent this, healthcare professionals should be directed to aerobic activities such as walking and swimming because aerobic activities will help the person to cope with stress, and they will be beneficial for musculoskeletal disorders and sleep problems. Healthcare professionals need to be aware of the risk of experiencing musculoskeletal pain and receive training to avoid or cope with their pain. The workload of healthcare workers should be reduced and they should be provided opportunities to work in an ergonomically appropriate environment.
The limitations of our study are as follows. There is a need for a larger sample group in future studies because participation in the study is on a voluntary basis, so it does not show the results of those who did not participate. This study reflects the results of the data collected in a 3-month period and there is a need for studies conducted over a longer period of time. In addition, it is not known the extent of the COVID-19 effects as there is no pre-COVID-19 data.
5Conclusion
It was found that the number of people who had problems in the low-back region was higher in those with a COVID-19 history and that the sleep duration parameter scores of those without a COVID-19 history were significantly higher. There was no difference between groups in other scale scores. Similar outcomes are seen in musculoskeletal system disorders, physical activity, sleep quality, and fatigue in healthcare workers in both groups, regardless of the COVID-19 history. This shows that during the COVID-19 pandemic, health workers are highly affected whether they had a COVID-19 history.
Ethical approval
Ethical approval was obtained from the Süleyman Demirel University Faculty of Medicine Clinical Research Ethics Committee (04/01/2021/72867572-050.01.04-559).
Informed consent
Participants were informed about the study and their consent was obtained.
Conflict of interest
The authors have no conflicts of interest.
Acknowledgments
The authors have no acknowledgements.
Funding
No financial support was received.
References
[1] | Sağlık Bakanlığı T.C. . Covid-19 (SARS-CoV2 Enfeksiyonu) Rehberi. 2020 [cited 2021 Oct 4]; Available from: https://www.tahud.org.tr/file/ac3d7f7f-752f-4f4f-97d4-3ea943204c8d/COVID-19_Rehberi-6-12.04.2020.pdf |
[2] | World Health Organization (WHO), Director-General's opening remarks at the media. 2021. [Internet]. [cited 2021 Oct 5]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019?gclid=CjwKCAjwzOqKBhAWEiwArQGwaOofYWx80bZZPHWEkAIHMHpLeOStMIWnbzlW4OoT1UBLU_mUnXd |
[3] | Bozdağ F , Ergün N . Psychological Resilience of Healthcare Professionals During COVID-19 Pandemic. Psychological Reports [Internet]. 2020 Oct 13 [cited 2021 Oct 5];0033294120965477. Available from: https://journals.sagepub.com/doi/full/10.1177/0033294120965477 |
[4] | Sirisawasd S , Taptagaporn S , Boonshuyar C , Earde P . Interventions commonly used to prevent work-related musculoskeletal disorders among healthcare workers. Journal of Health Research. (2018) ;32: (5):371–83. |
[5] | Oranye NO , Bennett J . Prevalence of work-related musculoskeletal and non-musculoskeletal injuries in health care workers: the implications for work disability management. Ergonomics. (2018) ;61: (3):355–66. |
[6] | Ong JL , Lau TY , Massar SAA , Chong ZT , Ng BKL , Koek D , et al. COVID-19-related mobility reduction: heterogenous effects on sleep and physical activity rhythms. Sleep [Internet]. 2021 Feb 12 [cited 2021 Oct 5];44(2):1–13. Available from: https://academic.oup.com/sleep/article/44/2/zsaa179/5904453 |
[7] | Shahidi SH , Stewart Williams J , Hassani F . Physical activity during COVID-19 quarantine. Acta Paediatrica, International Journal of Paediatrics [Internet]. 2020 Oct 1 [cited 2021 Oct 5];109(10):2147–8. Available from: https://pubmed.ncbi.nlm.nih.gov/32557827/ |
[8] | Zou X , Liu S , Li J , Chen W , Ye J , Yang Y , et al. Factors Associated With Healthcare Workers’ Insomnia Symptoms and Fatigue in the Fight Against COVID-19, and the Role of Organizational Support. Frontiers in Psychiatry. (2021) ;12: :356. |
[9] | Morgul E , Bener A , Atak M , Akyel S , Aktaş S , Bhugra D , et al. COVID-19 pandemic and psychological fatigue in Turkey. International Journal of Social Psychiatry. (2021) ;67: (2):128–35. |
[10] | Alaca N , Safran EE , Karamanlargil Aİ , TimucinE. Translation and cross-cultural adaptation of the extended version of the Nordic musculoskeletal questionnaire into Turkish. Journal of Musculoskeletal Neuronal Interactions [Internet]. 2019 Dec 1 [cited 2021 Oct 5];19(4):472–81. Available from: /pmc/articles/PMC6944807/ |
[11] | Craig CL , Marshall AL , Sjöström M , Bauman AE , Booth ML , Ainsworth BE , et al. International physical activity questionnaire: 12-Country reliability and validity. Medicine and Science in Sports and Exercise [Internet]. 2003 Aug 1 [cited 2021 Oct 5];35(8):1381–95. Available from: https://pubmed.ncbi.nlm.nih.gov/12900694/ |
[12] | Genç A , Şener Ü , Karabacak H , Üçok K . Kadın ve Erkek Genç Erişkinler Arasında Fiziksel Aktivite ve Yaşam Kalitesi Farklılıklarının Araştırılması. Kocatepe Tıp Dergisi. (2011) ;12: (3):145–50. |
[13] | Buysse DJ , Reynolds CF , Monk TH , Berman SR , Kupfer DJ . The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Research. (1989) ;28: (2):193–213. |
[14] | Ağargün MY KH. Pittsburgh uyku kalitesi indeksinin gecerligi ve guvenirligi. TPD [Internet]. 1996 [cited 2021 Oct 5];7(2):107–15. Available from: https://ci.nii.ac.jp/naid/20001210233 |
[15] | Krupp LB , Larocca NG , Muir Nash J , Steinberg AD . The fatigue severity scale: Application to patients with multiple sclerosis and systemic lupus erythematosus. Archives of Neurology [Internet]. 1989 [cited 2021 Oct 5];46(10):1121–3. Available from: https://pubmed.ncbi.nlm.nih.gov/2803071/ |
[16] | Armutlu K , Cetisli Korkmaz N , Keser I , Sumbuloglu V , Irem Akbiyik D , Guney Z , et al. The validity and reliability of the Fatigue Severity Scale in Turkish multiple sclerosis patients. International Journal of Rehabilitation Research. (2007) ;30: (1):81–5. |
[17] | Wang X , Liu W , Zhao J , Lu Y , Yu C , Hu S , et al. Clinical characteristics of 80 hospitalized frontline medical workers infected with COVID-19 in Wuhan, China. Journal of Hospital Infection. (2020) Jul 1;105: (3):399–403. |
[18] | Del Campo MT , Romo PE , de la Hoz RE , Villamor JM , Mahíllo-Fernández I . Anxiety and depression predict musculoskeletal disorders in health care workers. Archives of Environmental and Occupational Health [Internet]. 2017 Jan 2 [cited 2021 Oct 5];72(1):39–44. Available from: https://www.tandfonline.com/doi/abs/10.1080/19338244.2016.1154002 |
[19] | Shanafelt T , Ripp J , Trockel M . Understanding and Addressing Sources of Anxiety among Health Care Professionals during the COVID-19 Pandemic. JAMA - Journal of the American Medical Association [Internet]. 2020 Jun 2 [cited 2021 Oct 5];323(21):2133–4. Available from: https://pubmed.ncbi.nlm.nih.gov/32259193/ |
[20] | Aisa T , Diviney D , Thomas J , Al Qadheeb N , Abdelbaky M , Afify H , et al. Stress level assessment among health care workers involved in the management of critically ill COVID-19 patients. Irish Journal of Medical Science [Internet]. 2021 Jul 31 [cited 2021 Dec 11];1–7. Available from: https://link.springer.com/article/10.1007/s11845-021-02721-0 |
[21] | Hoong CWS , Amin MNME , Tan TC , Lee JE . Viral arthralgia a new manifestation of COVID-19 infection? A cohort study of COVID-19-associated musculoskeletal symptoms. International Journal of Infectious Diseases. (2021) Mar 1;104: :363–9. |
[22] | Arca M , Dönmezdil S , Durmaz ED . The effect of the COVID-19 Pandemic on anxiety, depression, and musculoskeletal system complaints in healthcare workers. Work. (2021) Jan 1;69: (1):47–54. |
[23] | AlOmar RS. Levels of Physical Activity and Prevalence of Musculoskeletal Disorders Among Physicians in Saudi Arabia Post COVID-19 Lockdown: An Epidemiological Cross-Sectional Analysis: https://doi.org/101177/21501327211040359 [Internet]. 2021 Aug 19 [cited 2022 Sep 7];12. Available from: https://journals.sagepub.com/doi/full/10.1177/21501327211040359 |
[24] | Kakaraparthi VN , Vishwanathan K . Increased prevalence of work-related musculoskeletal disorders among physiotherapists during the COVID-19 pandemic: A Commentary. Work. (2022) Jan 1;72: (4):1191–3. |
[25] | Alzeyadi1 AA , Elsiddig2 AI , Maani , Khan3 A , Alkhaldi4 SA , Amani , et al. Prevalence of musculoskeletal disorders among health care workers during covid-19 pandemic in the western region of Saudi Arabia. (2022) ;26(ms104e2106):2321–7359. |
[26] | Karaarslan F , Demircioğlu Güneri F , Kardeş S . Postdischarge rheumatic and musculoskeletal symptoms following hospitalization for COVID- prospective follow-up by phone interviews. Rheumatology International [Internet]. 2021 May 12 [cited 2021 Oct 25];41(7):1263–71. Available from: https://link.springer.com/article/10.1007/s00296-021-04882-8 |
[27] | Jafari-Nodoushan A , Bagheri G , Mosavi Nodoushan fatemesadat. Effect of COVID-19 virus on Prevalence of Musculoskeletal Disorders of Faculty Members of Yazd University. Iranian Journal of Ergonomics [Internet]. 2020 Oct 10 [cited 2021 Oct 25];8(3):1–12. Available from: http://journal.iehfs.ir/article-1-745-en.html |
[28] | Özdemir BY. Investigation of Low Back Pain in the White-Collar Population Working From Home Due to the COVID-19 Pandemic. Fiziksel Tıp ve Rehabilitasyon Bilimleri Dergisi. (2021) ;24: (2):135–42. |
[29] | Giustino V , Parroco AM , Gennaro A , Musumeci G , Palma A , Battaglia G . Physical activity levels and related energy expenditure during COVID-19 quarantine among the sicilian active population: A cross-sectional online survey study. Sustainability (Switzerland) [Internet]. 2020 May 26 [cited 2021 Oct 5];12(11):4356. Available from: https://www.mdpi.com/2071-1050/12/11/4356/htm |
[30] | Kua Z , Hamzah F , Tan PT , Ong LJ , Tan B , Huang Z . Physical activity levels and mental health burden of healthcare workers during COVID-19 lockdown. Stress and Health [Internet]. 2022 Feb 1 [cited 2022 Sep 8];38(1):171–9. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/smi.3078 |
[31] | Gjaka M , Feka K , Bianco A , Tishukaj F , Giustino V , Parroco AM , et al. The effect of covid-19 lockdown measures on physical activity levels and sedentary behaviour in a relatively young population living in kosovo. Journal of Clinical Medicine [Internet]. 2021 Feb 14 [cited 2021 Oct 5];10(4):1–15. Available from: https://europepmc.org/articles/PMC7918337 |
[32] | McCarthy H , Potts HWW , Fisher A . Physical activity behavior before, during, and after COVID-19 restrictions: Longitudinal smartphone-tracking study of adults in the United Kingdom. Journal of Medical Internet Research [Internet]. 2021 Feb 3 [cited 2021 Oct 5];23(2):e23701. Available from: https://www.jmir.org/2021/2/e23701 |
[33] | Pappa S , Ntella V , Giannakas T , Giannakoulis VG , Papoutsi E , Katsaounou P . Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: A systematic review and meta-analysis [Internet]. Vol. 88, Brain, Behavior, and Immunity. Brain Behav Immun; 2020 [cited 2021 Oct 5]. p. 901–7. Available from: https://pubmed.ncbi.nlm.nih.gov/32437915/ |
[34] | Jahrami H , BaHammam AS , AlGahtani H , Ebrahim A , Faris MAI , AlEid K , et al. The examination of sleep quality for frontline healthcare workers during the outbreak of COVID-19. Sleep and Breathing [Internet]. 2021 Mar 1 [cited 2021 Oct 5];25(1):503–11. Available from: https://pubmed.ncbi.nlm.nih.gov/32592021/ |
[35] | Du J , Dong L , Wang T , Yuan C , Fu R , Zhang L , et al. Psychological symptoms among frontline healthcare workers during COVID-19 outbreak in Wuhan [Internet]. Vol. 67, General Hospital Psychiatry. Elsevier Inc.; 2020 [cited 2021 Oct 5]. p. 144–5. Available from: https://europepmc.org/articles/PMC7194721 |
[36] | Xia L , Chen C , Liu Z , Luo X , Guo C , Liu Z , et al Prevalence of Sleep Disturbances and Sleep Quality in Chinese Healthcare Workers During the COVID-19 Pandemic: A Systematic Review and Meta-Analysis. Vol. 12, Frontiers in Psychiatry. Frontiers; 2021. p. 149. |




