Impact of lipoprotein(a) and fibrinogen on prognosis in patients with coronary artery disease: A retrospective cohort study
Abstract
BACKGROUND:
Despite the considerable progress made in preventative methods, medication, and interventional therapies, it remains evident that cardiovascular events (CVEs) continue to be the primary cause of both death and morbidity among individuals diagnosed with coronary artery disease (CAD).
OBJECTIVE:
To compare the connection between lipoprotein a (Lp[a]), fibrinogen (Fib), and both parameters combined with all-cause mortality to detect their value as prognostic biomarkers.
METHODS:
This is a retrospective study. Patients diagnosed with CAD between January 2007 and December 2020 at the Guangdong Provincial People’s Hospital (China) were involved in the study. 43,367 patients met the eligibility criteria. The Lp(a) and Fib levels were distributed into three tertile groups (low, medium, and high). All of the patients included in the study were followed up for all-cause mortality. Kaplan–Meier and Cox regression were performed to determine the relationship between Lp(a), Fib, and all-cause mortality. A concordance statistics model was developed to detect the impact of Fib and Lp(a) in terms of anticipating poor outcomes in patients with CAD.
RESULTS:
Throughout a median follow-up of 67.0 months, 6,883 (15.9%) patients died. Participants with high Lp(a) (above 27.60 mg/dL) levels had a significantly higher risk for all-cause mortality than individuals with low Lp(a) levels (below 11.13 mg/dL; adjusted hazard ratio [aHR] 1.219, 95% confidence interval [CI]: 1.141–1.304,
CONCLUSION:
High Lp(a) and Fib levels could be used as predictive biomarkers for all-cause mortality in individuals with CAD. The prediction accuracy for all-cause mortality improved after combining the two parameters.
1.Introduction
The prognosis of individuals with coronary artery disease (CAD) varies widely. Despite the considerable progress made in preventative methods, medication, and interventional therapies, it remains evident that cardiovascular events (CVEs) continue to be the primary cause of both death and morbidity among individuals diagnosed with CAD [1]. Accurate early risk stratification could facilitate the delivery of timely treatment for patients with CAD and may improve treatment outcomes in these cases. In recent years, researchers have identified serum biomarkers that are associated with an elevated risk of atherosclerotic events [2]. Many of these biomarkers, such as lipoprotein, C-reactive protein, and others, either alone or in combination, have been integrated into risk prediction models to assess whether their inclusion improves prediction accuracy [3]. However, there remains a lack of large-scale clinical studies on CAD-related biomarkers.
Lipoprotein a (Lp[a]) can increase atherosclerosis progression and promote blood clot formation while inhibiting the dissolution of blood clots (fibrinolysis). As a result, Lp(a) has been acknowledged as an independent atherosclerotic cardiovascular disorder risk factor [4, 5, 6]. Apolipoprotein a (Apo[a]) is a structural element of Lp(a) [7]. The fourth kringle of Apo(a) shares similarities with the plasminogen domain that binds with fibrin and ultimately interferes with the process of fibrinolysis [8]. Fibrinogen (Fib) is a glycoprotein present in the blood that has a vital function in the blood clotting mechanism. However, high levels of Fib can increase the risk of thrombus formation [9]. Numerous investigations have found a connection between raised Fib and Lp(a) levels and the risk of developing CAD [10, 11, 12, 13]. Moreover, some studies have revealed that when Fib and Lp(a) are considered in combination (rather than in isolation), they can enhance the predictive value for the occurrence of both stable CAD (SCAD) and acute coronary syndrome (ACS) [14, 15]. However, the relationship between these parameters and mortality remains unclear. To address this issue, in this investigation, we aimed to compare the connection between Lp(a), Fib, and both parameters combined with all-cause mortality to detect their value as prognostic biomarkers.
2.Methods
2.1Study design and participants
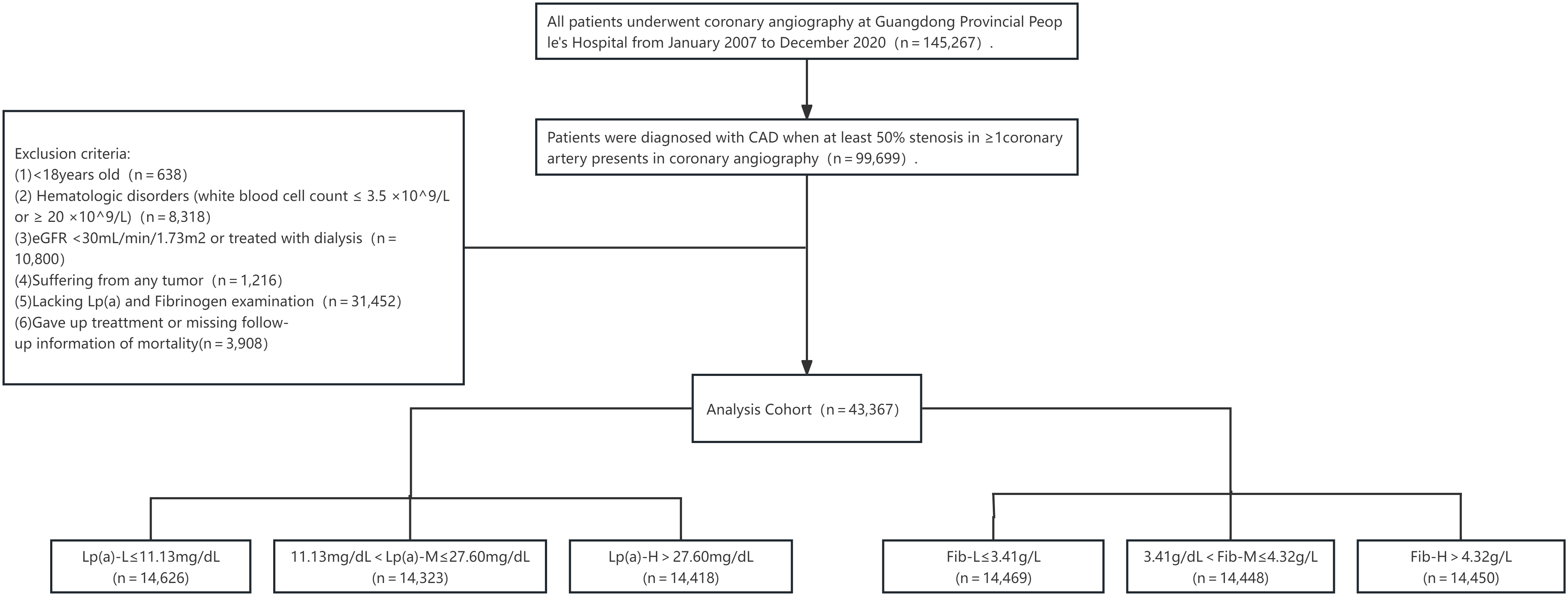
This investigation depended on information obtained from the Cardiorenal Improvement study (Clinicaltrials.gov NCT04407936). Patients who underwent coronary angiography (CAG) at Guangdong Provincial People’s Hospital (China) between January 2007 and December 2020 were eligible for this study.
Inclusion criteria: (1) aged above 18 years; (2) received a diagnosis of CAD based on the 10th Revision Codes of the International Classification of Diseases (ICD-10; I20.xx–I25.xx, I50.00001, and I91.40001); (3) the standard used for diagnosing CAD was a coronary artery stenosis degree exceeding 50%.
Exclusion criteria: (1) patients with blood diseases (white blood cell count
Figure 1.
Study flowchart.
2.2Ethical considerations
This investigation was authorised by the Ethics Committee of Guangdong Provincial People’s Hospital and followed the principles set out in the Declaration of Helsinki.
2.2.1Grouping
Grouping was conducted based on the data characteristics of this study. All of the patients were divided into three subgroups according to the tertiles of plasma Lp(a) levels as follows: low (L), medium (M), and high (H). The Lp(a)-L levels were 11.13 mg/dL or less; Lp(a)-M levels were between 11.13 and 27.60 mg/dL; and Lp(a)-H levels were above 27.60 mg/dL. Similarly, the participants were distributed into 3 additional groups according to the tertiles of plasma Fib levels as follows: Fib-L, consisting of levels rated 3.41 g/L or less; Fib-M, consisting of Fib levels between 3.41 and 4.32 g/L; and Fib-H, reflecting Fib levels above 4.32 g/L.
To test the link between Fib and Lp(a) with all-cause mortality, the patients were categorised into 9 groups as follows: Group 1: Lp(a)-L
2.2.2Basic information
Basic patient information was obtained from the computerised medical managing system of the Guangdong Provincial People’s Hospital, which included demographic features, comorbidities, lab investigations, and drugs prescribed upon discharge. The comorbidities extracted from the medical records included a history of arterial fibrillation (AF), chronic kidney disease (CKD), congestive heart failure (CHF), percutaneous coronary intervention, diabetes mellitus (DM), and acute myocardial infarction (AMI). Chronic kidney disease is characterised by an eGFR that falls below 60 ml/min/1.73 m2 [18] All other comorbidities were defined using the diagnostic codes as determined by the ICD-10.
2.2.3Lipoprotein a and fibrinogen measurement
Measurements of patients’ Lp(a) and Fib levels were taken on admission to the hospital via a blood sample. The Lp(a) concentration was determined using an immunoturbidimetry chemistry analyser (AU5800 Analyzer, Beckman Coulter, Brea, California), while the Fib concentration was measured via quantitative latex turbidimetric test using a CA-7000 automatic coagulation analyser (Sysmex Corporation, Kobe, Japan).
2.3Endpoint definitions
The main endpoint was the incidence of all-cause mortality, which was described as any death that took place between the participant’s enrolment in the study and the follow-up period’s conclusion on 31 December 2023. The median follow-up for patients was 67.0 months (the 25–75 percentile is 41.2–99.8 months). The follow-up records were obtained by qualified nurses during outpatient or telephone consultations and recorded by research assistants according to the ICD-10 nomenclature.
2.4Statistical analysis
The patients were subsequently divided into the all-mortality and alive groups based on their survival status at the completion of the follow-up period. Whether the variables showed normal distribution was evaluated using visual (histograms, probability curves) and analytical (Kolmogorov–Smirnov or Shapiro–Wilk tests) methods [19]. The means and standard deviation were utilised to summarise the normally distributed continuous variables, which were analysed employing a
Kaplan–Meier curves were employed to examine patient prognoses, and Cox proportional hazards analysis was conducted to test the connection between Fib and/or Lp(a) in the all-cause mortality among individuals with CAD. A concordance statistics (C-statistic) model was developed to detect the impact of Fib and Lp(a) administration on the original model (based on age, gender, DM, CKD, smoking, hypertension, and lipid levels) as it related to expecting poor outcomes in patients with CAD. The analyses of all data were conducted using version 4.3.2 of the R software program and the riskRegression R package [20]. All of the statistical tests conducted were two-tailed, and
3.Results
3.1Baseline characteristics
Typically, 14,5267 patients experienced CAG during the data collection period, 99,699 of whom were diagnosed with CAD. Following the exclusion of cases that did not meet the eligibility criteria of this investigation, 43,367 remained (Fig. 1). The mean participant age was 62.67
Table 1
Baseline features of the patients
| Characteristic* | Total, ( | Death, ( | Survival, ( | ||||
|---|---|---|---|---|---|---|---|
| Demographic features | |||||||
| Age, years, mean (SD) | 62.67 | (10.59) | 66.39 | (10.70) | 61.97 | (10.42) | |
| Female, | 10,100 | (23.3) | 1,459 | (21.2) | 8,641 | (23.7) | |
| Medical history | |||||||
| Hypertension, | 24,092 | (55.6) | 3,883 | (56.4) | 20,209 | (55.4) | 0.1203 |
| CHF, | 5,250 | (12.1) | 1,447 | (21.0) | 3,803 | (10.4) | |
| CKD, | 7,833 | (18.1) | 2,136 | (31.0) | 5,697 | (15.6) | |
| Stroke, | 2,363 | (5.4) | 513 | (7.5) | 1,850 | (5.1) | |
| PCI, | 32,575 | (75.1) | 5,085 | (73.9) | 27,490 | (75.3) | 0.0101 |
| AMI, | 7,788 | (18.0) | 1,471 | (21.4) | 6,317 | (17.3) | |
| Atrial fibrillation, | 1685 | (3.9) | 428 | (6.2) | 1,257 | (3.4) | |
| DM, | 14,450 | (33.3) | 2,497 | (36.3) | 11,953 | (32.8) | |
| Laboratory tests | |||||||
| HbA1c, %, mean (SD) | 6.51 | (1.38) | 6.67 | (1.49) | 6.48 | (1.36) | |
| CHOL, mmol/L, mean (SD) | 4.49 | (1.21) | 4.45 | (1.17) | 4.50 | (1.21) | 0.0006 |
| LDLC, mmol/L, mean (SD) | 2.80 | (0.96) | 2.74 | (0.95) | 2.81 | (0.96) | |
| HDLC, mmol/L, mean (SD) | 0.99 | (0.25) | 0.99 | (0.27) | 0.99 | (0.25) | |
| TRIG, mmol/L, mean (SD) | 1.68 | (1.24) | 1.53 | (1.05) | 1.70 | (1.27) | |
| Lipoprotein(a), mg/dL, mean (SD) | 29.47 | (31.97) | 32.16 | (34.20) | 28.96 | (31.51) | |
| APOA, g/L, mean (SD) | 1.11 | (0.25) | 1.12 | (0.25) | 0.83 | (0.23) | |
| APOB, g/L, mean (SD) | 0.85 | (0.24) | 0.85 | (0.24) | 0.66 | (0.27) | |
| Fibrinogen,g/L, mean (SD) | 4.08 | (1.25) | 4.30 | (1.38) | 4.04 | (1.22) | |
| eGFR, mL/min/1.73 m2, mean (SD) | 79.94 | (23.58) | 71.79 | (22.84) | 81.48 | (23.41) | |
| Medications | |||||||
| Statins, | 40886 | (94.3) | 6396 | (92.9) | 34490 | (94.5) | 0.8611 |
| Dual antiplatelet drugs, | 34322 | (79.1) | 5429 | (78.9) | 28893 | (79.2) | 0.0619 |
| ACEI/ARB, | 30987 | (71.5) | 5054 | (73.4) | 25933 | (71.1) | 0.0812 |
| | 34910 | (80.5) | 5411 | (78.6) | 29499 | (80.9) | 0.0748 |
*The mean value (standard deviation) were utilized to summarize the normally distributed continuous variables, which were analyzed employing the
Throughout a median follow-up time of 67.0 months (the 25–75 percentile is 41.2–99.8 months), 6,883 patients died. The patients in the all-cause mortality group were older (
3.2Impact of Lp(a) and Fib on all-cause mortality incidence
The occurrence of all-cause mortality was the lowest in the Lp(a)-L group (13.35%), followed by the Lp(a)-M (14.65%) and Lp(a)-H (19.65%) (
Figure 2.
Kaplan-Meier curves for the cumulative hazard in Fib, Lp(a), and combined groups. (a) Lp(a) groups, (b) Fib groups, (c) combined groups.
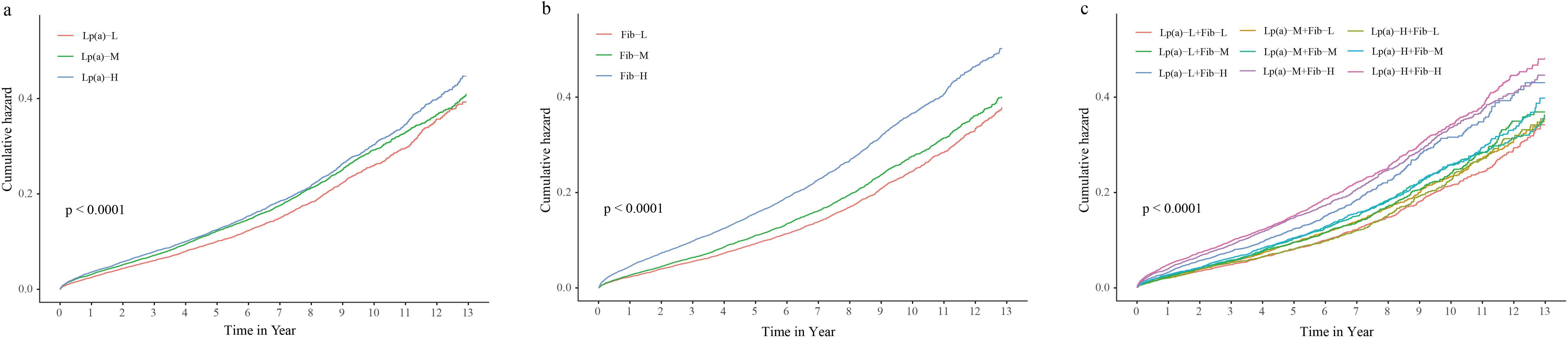
The univariate Cox regression models revealed that the Lp(a)-M and Lp(a)-H groups indicated a 1.133 and 1.203-fold greater risk, respectively, of all-cause mortality, compared with the Lp(a)-L and (Lp(a)-M groups (hazard ratio [HR]; 95% confidence interval [CI]: 1.133 [1.067–1.202],
3.3Interrelationship of Lp(a), Fib Levels and all-cause mortality
The all-cause mortality was the lowest in the Lp(a)-L and Fib-L models (12.54%), followed by Lp(a)-L
3.4Constructing the risk prediction model
The C-statistic of the original model was 0.643 (95% CI: 0.635–0.651). The administration of Lp(a) significantly enhanced the C-statistic by 0.008 (
4.Discussion
The impact of Fib and Lp(a) levels on mortality risk in individuals with CAD remains unclear. Accordingly, we conducted a large retrospective cohort study to assess the influence of Lp(a) and Fib on mortality risk and developed a novel prediction model for patients with CAD. To achieve this aim, we first explored the Lp(a) and Fib baseline levels’ effect on adverse medical outcomes following CAD. Our results indicated that raised Fib and Lp(a) concentrations were significantly linked with an enhanced risk of developing long-term adverse events, even after adjusting the confounding risk factors. Subsequently,
Table 2
Models of Cox regression for Lp(a) and Fib categories with all-cause mortality
| Items | Univariate cox regression | Multivariate cox regression | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||||||||
| Categorical variable | Tertile/ range | HR | 95%CI | HR | 95%CI | HR | 95%CI | |||
| Lp(a) categories | Lp(a)L ( | Reference | ||||||||
| Lp(a)-M (11.13–27.60) | 1.133 | 1.067–1.202 | 1.108 | 1.044–1.176 | 0.001 | 1.117 | 1.044–1.196 | 0.001 | ||
| Lp(a)-H ( | 1.203 | 1.134–1.275 | 1.207 | 1.138–1.28 | 1.219 | 1.141–1.304 | ||||
| Fibrinogen categories | Fib-L ( | Reference | ||||||||
| Fib-M (3.41–4.32) | 1.129 | 1.061–1.201 | 1.086 | 1.02–1.155 | 0.009 | 1.072 | 0.998–1.151 | 0.057 | ||
| Fib-H ( | 1.548 | 1.461–1.64 | 1.487 | 1.403–1.575 | 1.415 | 1.323–1.514 | ||||
| Combined categories | Lp(a)-L | Reference | ||||||||
| Lp(a)-L | 1.107 | 0.999–1.228 | 0.054 | 1.087 | 0.980–1.205 | 0.115 | 1.089 | 0.966–1.227 | 0.164 | |
| Lp(a)-L | 1.047 | 0.936–1.171 | 0.421 | 1.092 | 0.976–1.222 | 0.128 | 1.123 | 0.987–1.277 | 0.078 | |
| Lp(a)-M | 1.136 | 1.025–1.259 | 0.015 | 1.096 | 0.989–1.215 | 0.081 | 1.084 | 0.963–1.220 | 0.184 | |
| Lp(a)-M | 1.182 | 1.069–1.308 | 0.001 | 1.135 | 1.026–1.255 | 0.014 | 1.134 | 1.057–1.336 | 0.033 | |
| Lp(a)-M | 1.226 | 1.108–1.357 | 1.195 | 1.080–1.323 | 0.001 | 1.188 | 1.057–1.336 | 0.004 | ||
| Lp(a)-H | 1.467 | 1.317–1.635 | 1.447 | 1.299–1.613 | 1.351 | 1.192–1.531 | ||||
| Lp(a)-H | 1.611 | 1.467–1.770 | 1.531 | 1.394–1.682 | 1.472 | 1.321–1.640 | ||||
| Lp(a)-H | 1.702 | 1.558–1.859 | 1.645 | 1.506–1.797 | 1.592 | 1.437–1.763 | ||||
Model 1, Unadjusted model. Model 2, Adjusted for age and gender. Model 3, Hyperlipemia, diabetes mellitus, chronic kidney disease, smoking, hypertension, and medications such as drug Statins.
we discovered that high Lp(a) and Fib levels could increase the risk of developing all-cause mortality 1.219 and 1.415-fold, respectively. However, patients in the Lp(a)-H and Fib-H group had a 1.592-fold greater all-cause mortality risk. In addition, the predictive model based on both Lp(a) and Fib promoted the prognostic performance for adverse events by 0.009.
Table 3
C-statistic of Lp(a) and Fib categories for anticipating all-cause mortality
| Models | C-statistic (95% CI) | ||
|---|---|---|---|
| Original model | 0.643 (0.635–0.651) | – | |
| Original model | 0.651 (0.643–0.660) | 0.008 (0.008–0.009) | |
| Original model | 0.645 (0.636–0.653) | 0.002 (0.001–0.002) | |
| Original model | 0.652 (0.644–0.660) | 0.009 (0.009–0.009) |
The original model included age, gender, diabetes mellitus, chronic kidney disease, smoking, hypertension, and hyperlipidemia as risk factors.
Although several studies evaluated the Fib and Lp(a) effect on the risk of developing main adverse cardiovascular and cerebrovascular events (MACCE) in individuals with CAD, the findings remain controversial. Some investigations revealed the vital function of Fib and/or Lp(a) in promoting cardiovascular disorder development [21, 22, 23, 24, 25] and CVEs [26, 27, 28, 29, 30, 31] The ‘PROCAM’ study showed that, compared with healthy men with low low-density lipoprotein (LDL) and Fib levels, patients with high LDL and Fib levels had a 6.1-fold raised risk of developing coronary conditions like sudden cardiac mortality, as well as fatal and nonfatal AMI. Additionally, Fib was also an independent CAD risk factor (
The present investigation successfully identified raised levels of Lp(a) and Fib as significant risk factors that are associated with the occurrence of long-term adverse outcomes, even after the adjustment of confounders. The effect of inflammation on the development and advancement of atherosclerosis is crucial since it may involve plaque instability and the progression of cardiovascular disorders [36]. In this regard, Lp(a) can impact the anti-inflammatory pathway, trigger vasodilation mediated by nitric oxide, and alter the balance of procoagulant and anticoagulant agents within blood vessel walls [37]. It also participates in the construction of atheromatous plaques, which may cause ischemic and stenosis events. High Fib levels (above 3.5 g/L) have been associated with several inflammatory diseases, including CAD [38, 39]. Additionally, Lp(a) exhibits a significant resemblance to plasminogen and exerts anti-fibrinolytic impacts through its Apo[a] constituent [6]. A crucial component of the fibrinolytic system, Fib adds to the complexity of this relationship. Elevated levels of Lp(a) and Fib can potentially disrupt the inflammatory and coagulation pathways, thereby increasing the risk of CVEs [40].
To date, very few investigations have evaluated the influence of Lp(a) and Fib on prognosis in individuals with CAD. The Quebec Cardiovascular Study showed that high Fib and Lp(a) levels can significantly increase the CAD risk in men who are free of clinical CAD [41]. However, this study exclusively assessed the susceptibility to CAD in an ostensibly healthy male population. In contrast, our investigation encompassed both male and female patients diagnosed with CAD, revealing a heightened risk of all-cause mortality associated with elevated levels of Lp(a) and Fib-H. Caiyan Cui et al. found that patients with ACS had an elevated risk of developing MACCE, all-cause mortality, nonfatal myocardial infarction and stroke, as well as revascularisation if their Fib levels exceeded 3.08 g/L and their Lp(a) levels were above 300 mg/L [13]. The Lp(a)-and-Fib-based model further enhanced the prediction accuracy for developing adverse events in these patients [14]. Similarly, Yan Zhang et al. showed that combining Fib and Lp(a) can promote the prediction of recurrent CVEs in angiographically proven stable individuals with CAD [15]. Consistent with these studies, we also found that the incorporation of Fib and Lp(a) levels could improve the all-cause mortality prediction. However, compared to previous studies, our research had a larger patient cohort, a longer follow-up time, and different endpoints. In addition, we also included patients with SCAD and ACS.
4.1Limitations
Our investigation has several restrictions that must be acknowledged. While it is vital to note that this study was observational and conducted within a single centre, which restricted our capacity to draw direct causal effects, the study nonetheless benefitted from a substantial sample size and a lengthy follow-up period. Therefore, the present research still provides a robust representation of patients with CAD in southern China. Additionally, Fib and Lp(a) levels were obtained only at the starting point and may have altered over time. Therefore, further research is necessary to examine the long-term effect of Lp(a) and Fib levels on prognosis.
5.Conclusion
High Lp(a) and Fib levels can significantly increase the all-cause mortality risk in individuals with CAD. However, the combined use of both parameters improved the prediction for all-cause mortality, even after accounting for confounders. Our Lp(a) and Fib model can be used to stratify patients with CAD based on the mortality risk, thereby optimising the monitoring of high-risk patients. However, further longitudinal investigations are essential to confirm the validity of the model.
Ethical approval
The research conducted with human subjects underwent a thorough evaluation and received approval from the Ethics Committee of Guangdong Provincial People’s Hospital (No. GDREC2019555H(R1)).
Data availability
The authors will offer the raw data that supports their findings upon receiving an appropriate request.
Funding
No financial support was received for the research, authorship, or publication of this manuscript.
Author contributions
JL had full accessibility to data and was responsible for its integrity and the precision of the data analysis. DKL, DDL, XYH created the concept and design for the study. YYZ, DHH, ZLL data management. DKL, DDL drafted the manuscript. JL and JYC revised the article. Each author made significant contributions to the collection, analysis, and interpretation of the data. The final version was confirmed by all authors.
Acknowledgments
The authors express their gratitude to the staff and individuals who were involved in this research for their significant contributions.
Conflict of interest
None of the authors have any personal, financial, commercial, or academic conflicts of interest to report.
References
[1] | Tsao CW, Aday AW, Almarzooq ZI, et al. Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association. Circulation. (2023) Feb 21; 147: (8): e93-e621. doi: 10.1161/CIR.0000000000001123. |
[2] | Della Corte V, Todaro F, Cataldi M, Tuttolomondo A. Atherosclerosis and Its Related Laboratory Biomarkers. Int J Mol Sci. (2023) Oct 24; 24: (21): 15546. doi: 10.3390/ijms242115546. |
[3] | Freitas IA, Lima NA, Silva GBD Jr., Castro RL Jr., Patel P, Lima CCV, Lino DODC. Novel biomarkers in the prognosis of patients with atherosclerotic coronary artery disease. Rev Port Cardiol (Engl Ed). (2020) Nov; 39: (11): 667-672. English, Portuguese. doi: 10.1016/j.repc.2020.05.010. Epub 2020 Oct 24. PMID: 33239161. |
[4] | Ferretti G, Bacchetti T, Johnston TP, et al. Lipoprotein(a): A missing culprit in the management of athero-thrombosis? J Cell Physiol. (2018) Apr; 233: (4): 2966-2981. doi: 10.1002/jcp.26050. |
[5] | Gragnano F, Calabrò P. Role of dual lipid-lowering therapy in coronary atherosclerosis regression: Evidence from recent studies. Atherosclerosis. (2018) Feb; 269: : 219-228. doi: 10.1016/j.atherosclerosis.2018.01.012. |
[6] | From the American Association of Neurological Surgeons (AANS), American Society of Neuroradiology (ASNR), Cardiovascular and Interventional Radiology Society of Europe (CIRSE), et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int J Stroke. (2018) Aug; 13: (6): 612-632. doi: 10.1177/1747493018778713. |
[7] | Boffa MB. Beyond fibrinolysis: The confounding role of Lp(a) in thrombosis. Atherosclerosis. (2022) May; 349: : 72-81. doi: 10.1016/j.atherosclerosis.2022.04.009. |
[8] | Saeedi R, Frohlich J. Lipoprotein (a), an independent cardiovascular risk marker. Clin Diabetes Endocrinol. (2016) Mar 31; 2: : 7. doi: 10.1186/s40842-016-0024-x. |
[9] | Shabani M, Bakhshi H, Ostovaneh MR, et al. Temporal change in inflammatory biomarkers and risk of cardiovascular events: the Multi-ethnic Study of Atherosclerosis. ESC Heart Fail. (2021) Oct; 8: (5): 3769-3782. doi: 10.1002/ehf2.13445. |
[10] | Gencer B, Mach F. Potential of Lipoprotein(a)-Lowering Strategies in Treating Coronary Artery Disease. Drugs. (2020) Feb; 80: (3): 229-239. doi: 10.1007/s40265-019-01243-5. PMID: 31916186. |
[11] | Cho SMJ, Koyama S, Honigberg MC, Surakka I, Haidermota S, Ganesh S, Patel AP, Bhattacharya R, Lee H, Kim HC, Natarajan P. Genetic, sociodemographic, lifestyle, and clinical risk factors of recurrent coronary artery disease events: a population-based cohort study. Eur Heart J. (2023) Sep 21; 44: (36): 3456-3465. doi: 10.1093/eurheartj/ehad380. |
[12] | Su H, Cao Y, Chen Q, Ye T, Cui C, Chen X, Yang S, Qi L, Long Y, Xiong S, Cai L. The association between fibrinogen levels and severity of coronary artery disease and long-term prognosis following percutaneous coronary intervention in patients with type 2 diabetes mellitus. Front Endocrinol (Lausanne). (2023) Nov 29; 14: : 1287855. doi: 10.3389/fendo.2023.1287855. |
[13] | Kryczka KE, Kruk M, Demkow M, Lubiszewska B. Fibrinogen and a Triad of Thrombosis, Inflammation, and the Renin-Angiotensin System in Premature Coronary Artery Disease in Women: A New Insight into Sex-Related Differences in the Pathogenesis of the Disease. Biomolecules. (2021) Jul 15; 11: (7): 1036. doi: 10.3390/biom11071036. PMID: 34356659; PMCID: PMC8301902. |
[14] | Cui CY, Ye T, Cheng LC, et al. Lipoprotein a Combined with Fibrinogen as an Independent Predictor of Long-Term Prognosis in Patients with Acute Coronary Syndrome: A Multi-Center Retrospective Study. J Cardiovasc Dev Dis. (2022) Sep 23; 9: (10): 322. doi: 10.3390/jcdd9100322. |
[15] | Wang H, Wu P, Jiang D, Zhang H, Zhang J, Zong Y, Han Y. Relationship between serum homocysteine, fibrinogen, lipoprotein-a level, and peripheral arterial disease: a dose-response meta-analysis. Eur J Med Res. (2022) Nov 21; 27: (1): 261. doi: 10.1186/s40001-022-00870-1. PMID: 36411481; PMCID: PMC9677707. |
[16] | Lin J, Reilly MP, Terembula K, et al. Plasma lipoprotein(a) levels are associated with mild renal impairment in type 2 diabetics independent of albuminuria. PLoS One. (2014) Dec 9; 9: (12): e114397. doi: 10.1371/journal.pone.0114397. |
[17] | Zhang Y, Jin JL, Cao YX, Liu HH, Zhang HW, Guo YL, Wu NQ, Gao Y, Hua Q, Li YF, Xu RX, Cui CJ, Liu G, Dong Q, Sun J, Li JJ. Prognostic utility of lipoprotein(a) combined with fibrinogen in patients with stable coronary artery disease: a prospective, large cohort study. J Transl Med. (2020) Oct 1; 18: (1): 373. doi: 10.1186/s12967-020-02546-y. |
[18] | Roth D, Bloom RD, Molnar MZ, et al. KDOQI US Commentary on the 2018 KDIGO Clinical Practice Guideline for the Prevention, Diagnosis, Evaluation, and Treatment of Hepatitis C. Am J Kidney Dis. (2020) May; 75: (5): 665-683. doi: 10.1053/j.ajkd.2019.12.016. |
[19] | Guler A, Turkmen I, Atmaca S, Karakurt H, Kahraman S, Aydin S, Sevinc S, Tukenmez Karakurt S, Turkvatan Cansever A, Erturk M, Babur Guler G. Influence of cardiac biomarkers on predicting significant coronary artery disease in hypertrophic cardiomyopathy patients. Heart Vessels. (2023) Nov; 38: (11): 1329-1336. doi: 10.1007/s00380-023-02287-0. |
[20] | Newman JD, Anthopolos R, Ruggles KV, Cornwell M, Reynolds HR, Bangalore S, Mavromatis K, Held C, Wallentin L, Kullo IJ, McManus B, Newby LKK, Rosenberg Y, Hochman JS, Maron DJ, Berger JS, ISCHEMIA Biorepository Research Group. Biomarkers and cardiovascular events in patients with stable coronary disease in the ISCHEMIA Trials. Am Heart J. (2023) Dec; 266: : 61-73. doi: 10.1016/j.ahj.2023.08.007. |
[21] | Gilliland TC, Liu Y, Mohebi R, et al. Lipoprotein(a), Oxidized Phospholipids, and Coronary Artery Disease Severity and Outcomes. J Am Coll Cardiol. (2023) May 9; 81: (18): 1780-1792. doi: 10.1016/j.jacc.2023.02.050. |
[22] | Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, et al. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. (2009) Jun 10; 301: (22): 2331-9. doi: 10.1001/jama.2009.801. |
[23] | Li JJ, Ma CS, Zhao D, et al. Lipoprotein(a) and Cardiovascular Disease in Chinese Population: A Beijing Heart Society Expert Scientific Statement. JACC Asia. (2022) Nov 15; 2: (6): 653-665. doi: 10.1016/j.jacasi.2022.08.015. |
[24] | Cao YX, Liu HH, Sun D, et al. The different relations of PCSK9 and Lp(a) to the presence and severity of atherosclerotic lesions in patients with familial hypercholesterolemia. Atherosclerosis. (2018) Oct; 277: : 7-14. doi: 10.1016/j.atherosclerosis.2018.07.030. |
[25] | Cui Z, Zhao G, Liu X. Blood fibrinogen level as a biomarker of adverse outcomes in patients with coronary artery disease: A systematic review and meta-analysis. Medicine (Baltimore). (2022) Aug 19; 101: (33): e30117. doi: 10.1097/MD.0000000000030117. |
[26] | Nestel PJ, Barnes EH, Tonkin AM, et al. Plasma lipoprotein(a) concentration predicts future coronary and cardiovascular events in patients with stable coronary heart disease. Arterioscler Thromb Vasc Biol. (2013) Dec; 33: (12): 2902-8. doi: 10.1161/ATVBAHA.113.302479. |
[27] | Suwa S, Ogita M, Miyauchi K, et al. Impact of Lipoprotein (a) on Long-Term Outcomes in Patients with Coronary Artery Disease Treated with Statin After a First Percutaneous Coronary Intervention. J Atheroscler Thromb. (2017) Nov 1; 24: (11): 1125-1131. doi: 10.5551/jat.38794. |
[28] | Tsimikas S. A Test in Context: Lipoprotein(a): Diagnosis, Prognosis, Controversies, and Emerging Therapies. J Am Coll Cardiol. (2017) Feb 14; 69: (6): 692-711. doi: 10.1016/j.jacc.2016.11.042. |
[29] | O’Donoghue ML, Morrow DA, Tsimikas S, et al. Lipoprotein(a) for risk assessment in patients with established coronary artery disease. J Am Coll Cardiol. (2014) Feb 18; 63: (6): 520-7. doi: 10.1016/j.jacc.2013.09.042. |
[30] | Jin JL, Cao YX, Zhang HW, et al. Lipoprotein(a) and Cardiovascular Outcomes in Patients With Coronary Artery Disease and Prediabetes or Diabetes. Diabetes Care. (2019) Jul; 42: (7): 1312-1318. doi: 10.2337/dc19-0274. |
[31] | O’Donoghue ML, Fazio S, Giugliano RP, et al. Lipoprotein(a), PCSK9 Inhibition, and Cardiovascular Risk. Circulation. (2019) Mar 19; 139: (12): 1483-1492. doi: 10.1161/CIRCULATIONAHA.118.037184. |
[32] | Kris-Etherton PM, Stewart PW, Ginsberg HN, et al. The Type and Amount of Dietary Fat Affect Plasma Factor VIIc, Fibrinogen, and PAI-1 in Healthy Individuals and Individuals at High Cardiovascular Disease Risk: 2 Randomized Controlled Trials. J Nutr. (2020) Aug 1; 150: (8): 2089-2100. doi: 10.1093/jn/nxaa137. |
[33] | Kwon SW, Lee BK, Hong BK, et al. Prognostic significance of elevated lipoprotein(a) in coronary artery revascularization patients. Int J Cardiol. (2013) Sep 1; 167: (5): 1990-4. doi: 10.1016/j.ijcard.2012.05.007. |
[34] | Schwartz GG, Ballantyne CM, Barter PJ, et al. Association of Lipoprotein(a) With Risk of Recurrent Ischemic Events Following Acute Coronary Syndrome: Analysis of the dal-Outcomes Randomized Clinical Trial. JAMA Cardiol. (2018) Feb 1; 3: (2): 164-168. doi: 10.1001/jamacardio.2017.3833. |
[35] | Moissl AP, Delgado GE, Krämer BK, et al. Gender- and subgroup-specific sensitivity analysis of alcohol consumption and mortality in the Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Data Brief. (2022) Jan 26; 41: : 107873. doi: 10.1016/j.dib.2022.107873. |
[36] | Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. (2012) Sep; 32: (9): 2045-51. doi: 10.1161/ATVBAHA.108.179705. |
[37] | Enas EA, Chacko V, Senthilkumar A, et al. Elevated lipoprotein(a)–a genetic risk factor for premature vascular disease in people with and without standard risk factors: a review. Dis Mon. (2006) Jan; 52: (1): 5-50. doi: 10.1016/j.disamonth.2006.01.002. |
[38] | Surma S, Banach M. Fibrinogen and Atherosclerotic Cardiovascular Diseases-Review of the Literature and Clinical Studies. Int J Mol Sci. (2021) Dec 24; 23: (1): 193. doi: 10.3390/ijms23010193. |
[39] | Davalos D, Akassoglou K. Fibrinogen as a key regulator of inflammation in disease. Semin Immunopathol. (2012) Jan; 34: (1): 43-62. doi: 10.1007/s00281-011-0290-8. |
[40] | Li H, Zhang P, Yuan S, Tian H, Tian D, Liu M. Modeling analysis of the relationship between atherosclerosis and related inflammatory factors. Saudi J Biol Sci. (2017) Dec; 24: (8): 1803-1809. doi: 10.1016/j.sjbs.2017.11.016. Epub 2017 Nov 17. |
[41] | Cantin B, Després JP, Lamarche B, et al. Association of fibrinogen and lipoprotein(a) as a coronary heart disease risk factor in men (The Quebec Cardiovascular Study). Am J Cardiol. (2002) Mar 15; 89: (6): 662-6. doi: 10.1016/s0002-9149(01)02336-0. |




