Extract identification and evaluation of the cytotoxic activity of Polygala fallax Hemsl in Heilongjiang ethnic medicine against tumors
Abstract
BACKGROUND:
Heilongjiang Province is a frontier province with distinctive characteristics, fertile land and rich products.
OBJECTIVE:
This study provides a new method for qualitatively studying flavonoids in traditional Chinese medicine and a new auxiliary means for identifying flavonoid isomers.
METHODS:
The flavonoids in Polygala fallax Hemsl were identified by ultra-performance liquid chromatography-photo-diode array (PDA)-quadrupole-electro- static field orbitrap mass spectrometry tandem by UV Spectrum, primary and secondary high-resolution mass spectrometry (MS
RESULTS:
The established QSRR model was used to verify the flavonoids identified from the Polygala fallax Hemsl.
CONCLUSION:
The structure of multiple Polygala fallax Hemsl has been identified using various spectral methods. The tumor cytotoxic activity of the isolated compounds was evaluated. This paper is of great significance for further elucidating the pharmacodynamic substance basis and further developing and utilizing Polygala fallax Hemsl.
1.Introduction
Heilongjiang Province is a frontier province with distinctive characteristics, fertile land, rich products, and a unique climate. The diagnosis, treatment methods, and techniques of ethnic minorities in the cold areas of northern China are distinctive and treasures in traditional Chinese medicine’s treasure house.
Yuan Zhi is a typical Chinese herb with definite healing properties, mainly produced in Shanxi, Shaanxi, Jilin, and Henan provinces. It has a long history of medicinal use and has been listed in the Shen Nong Materia Medica as a top-quality product [1]. The Chinese Pharmacopoeia specifies that the source of the herb Yuan Zhi is the dried root of Yuan Zhi or Oval Leaf Yuan Zhi. The clinical applications of Yuan Zhi are widespread, and its principal therapeutic component is a saponin-like substance [2, 3, 4]. Studies have shown that saponins have antibacterial and anti–inflammatory [5], antioxidant, antihypertensive, anticancer [6], anti-aging and other pharmacological effects [7], so how to effectively extract saponins from Polygala has become the focus of scientific research work.
1.1Polygala fallax Hemsl
Huanghua Yuanzhi (scientific name: Polygala fallax Hemsl.), a kind of plant in the Yuanzhi family, were born in a shady place near water in the valley forest of Jiangxi, Fujian, Hunan, Guangdong, Guangxi, and Yunnan [8, 9, 10, 11]. It has the effects of expelling wind and dampness, tonifying deficiency, relieving swelling, regulating menstruation, and promoting blood circulation. Indications: cold, rheumatic pain, tuberculosis, edema, postpartum weakness, irregular menstruation, traumatic injury [12, 13, 14, 15, 16, 17, 18].
1.2Flavonoids
Flavonoids are a class of secondary metabolites widely found in plants (including many herbs, vegetables, and fruits). They are derived from glucose through the mangiferin acid and the acetic acid-propanedioic to produce hydroxycinnamate and three acetic acid molecules, which are further synthesized into chalcone [19]. Flavonoids originally referred to a group of compounds whose primary parent nucleus was 2-phenylchromogenone. It is now generally used to refer to a class of components an essential C6-C3-C6 parent nucleus, i.e. a series of compounds in which two benzene rings (A and B rings, often with phenolic hydroxyl groups) are inter- connected by a central three carbon atom [20].
1.2.1Classification of flavonoids
Flavonoids are often classified into the following categories based on structural features such as the degree of oxidation of the intermediate 3-carbon chain, the position of the ring-ring linkage and whether the 3-carbon chain is cyclic or not [21]. According to the parent structure, 2-phenylchromanone, there are four active sites on the A ring, five active sites on the B ring and one active site at the C3 position, for a total of 10 positions that can be substituted by the active group, theoretically, 10 isomers can exist for flavonoid monosubstituted compounds. Polysubstituted flavonoids and their glycosides can have up to several dozen isomers. Although more than 9000 flavonoids have been identified, only 100 commercial standards are available for purchase, of which only a few dozen are glycosides, making it difficult to meet the demand for rapid, accurate, high-throughput qualitative identification of flavonoids [22].
1.2.2Methods for the characterization of flavonoids
The existing methods for the characterization of flavonoids include: wave spectrometry [23], UV spectrometry [24], thin-layer chromatography [25], High-Performance Liquid Chromatography (HPLC) [26], Ultra-Performance Liquid Chromatography-Mass Spectrometry (UPLC-MS) [27], UPLC-MS combined with database method [28], HPLC-MS combined with mass spectrometry cleavage law method, etc. [29]. The wave-spectrum analysis method is the gold standard for identifying unknown compounds. However, the method requires access to the pure product of the unknown compound, and the extraction, separation and purification process is cumbersome, lengthy, and costly. The UV spectroscopy method shows poor specificity, while thin layer chromatography shows low sensitivity and separation efficiency. Although HPLC and UPLC improve the sensitivity, specificity and speed of analysis of flavonoids, it requires the use of standards and are prone to false positives due to the presence of overlapping peaks. The liquid mass spectrometry, particularly ultra-performance liquid chromatography- high-resolution mass spectrometry (UPLC-HRMS), with its advantages of high sensitivity, selectivity, and throughput, as well as the continuous improvement of the mass spectrometry database and the increasing clarity of mass spectrometry cleavage patterns, has become an effective analytical tool for the qualitative analysis of flavonoids and other polar natural products. We conducted a compositional analysis of the extracts of Wolfsbane based on the HPLC-TOF-MS technique combined with a database. We identified 22 flavonoid components, presumed to be cleaved mainly by losing fragments such as CO and H
1.2.3Cytotoxic and antitumor drugs
Cytotoxicity is a simple cell-killing event caused by cells or chemicals that do not depend on the cell death mechanism of apoptosis or necrosis. Sometimes it is necessary to test the cytotoxicity of specific substances.
Platinum antineoplastic drugs are the most important branch of metal drugs, and are the most widely used metal chemotherapy drugs in clinical practice. Platinum is currently the only approved metal anti-tumor drug. Cisplatin is cytotoxic with anti-proliferative activity and its pharmacological target is usually considered to be DNA. In the high chloride environment of plasma (
In this paper, UPLC-PAD-Q-Orbitrap-MS technology was used to identify the flavonoids in Scutellaria baicalensis, combined with the database, mass spectrometry , and literature data, and the existing commercial standards of flavonoids were used to construct a mathematical model to realize the accurate and rapid identification of unknown flavonoids and their isomers. It provides a new method for qualitatively studying flavonoids in traditional Chinese medicine and a new auxiliary means for identifying flavonoid isomers.
2.Instruments, reagents and materials
2.1Instrument
Dionex Ultimate 3000 Ultra High-Performance Liquid Chromatograph, Thermo Corporation, USA; Thermo Scientific Q Exactive Series Mass Spectrometer, Thermo Corporation, USA; Data analysis software, Thermo Scientific Xcalibur Workstation, Compound Discover 3.2; Data analysis and processing software, Matlab R2010a.
2.2Reagents and materials
Polygala fallax Hemsl, Southern Medicinal Materials Co, LTD; Methanol (chromato-graphic pure), acetonitrile (chromatographic pure).
2.3Test Methodology
2.3.1Preparation of the sample solution of Polygala fallax Hemsl
0.6 g of the dried powder of Polygala fallax Hemsl was measured into a 50 mL centrifuge tube. The dried powder was put under Ultrasonic treatment at 50
2.3.2Analysis of the constituents of Polygala fallax Hemsl
After sample injection, the compounds were initially screened against UV absorption peaks using a Thermo Scientific Xcalibur workstation. We fit the molecular ion data of MS1 to get the compound formula and set the allowable calculation error of the compound sum to 5 ppm. Compound Discoverer 3.2 software was combined with mzVault, mzCloud, and ChemSpider databases were also used to perform compositional analysis of the sample feed results to screen for flavonoids in Polygala fallax Hemsl. It recorded retention time (tR), compound name (name), mass-to-charge ratio (MCR), mass error (error), and secondary fragment ion (MS2 fragment ion).
3.Experimental results and discussion
3.1UHPLC-PAD identification results
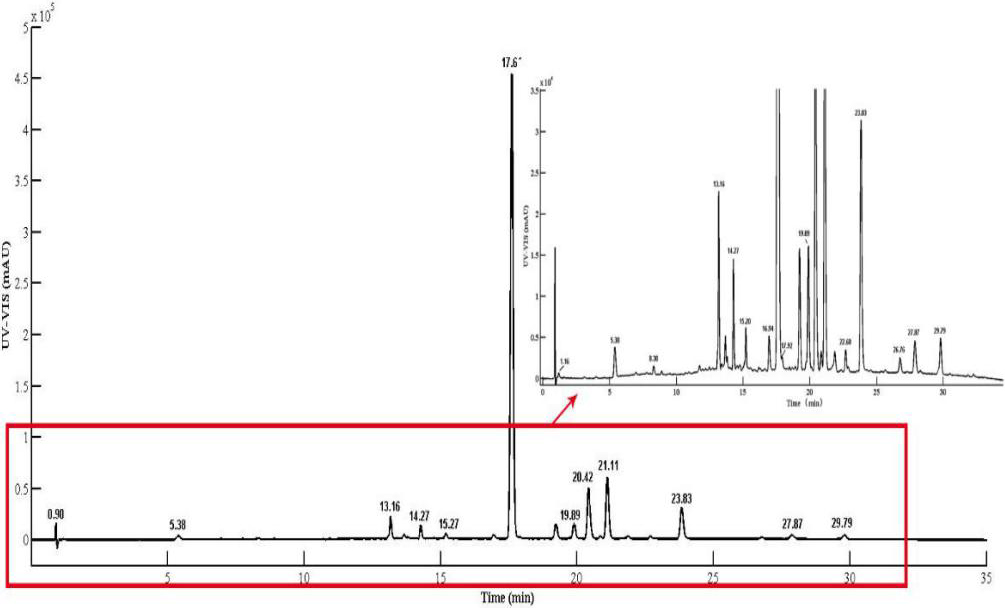
The chromatogram obtained at 268 nm using PAD as the detector is shown in Fig. 1 for the extracts of Polygala fallax Hemsl. As can be seen from Fig. 1, 18 peaks with peak heights more fabulous than 1000 mAU were obtained under the chromatographic conditions. 18 UV-visible spectra of PAD were obtained using the instrument’s software, and 12 peaks corresponded to the UV absorption characteristics of flavonoids. Most of the flavonoid components had two maximum UV absorptions in the wavelength range of 230
Figure 1.
The chromatography diagram of baicaleol extract obtained at 310 nm using the PAD as a detector.
3.2UHPLC-HRMS identification results
This paper uses UHPLC-HRMS/MS technology to analyze the error of multi-galaxies and the identification of the chemical composition of the extracts of Polygala fallax Hemsl. The method can separate complex mixtures efficiently while providing precise molecular masses of the compounds to be identified, which yields the elemental composition of the compounds to be identified. According to the structural characteristics of flavonoids, the basic elemental composition of most flavonoid components is C, H and O. Common flavonoids should have a molecular unsaturation (RDB) of at least 11 (Dihydroflavone not less than 10), with a hydrogenated positive ion of no less than 10.5 (Dihydro-flavone not less than 9.5) and a reduced hydrogenated negative ion of no less than 11.5 (Dihydroflavone not less than 10.5). The preliminary determination of flavonoids meets the above conditions. A total of 154 peaks with peak intensities greater than 1.50E
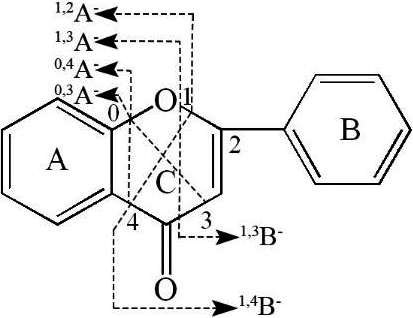
Figure 2.
The C loop cleavage diagram of the flavonoid compound Subsequently, secondary mass spectral fragments of flavonoids often show.
3.3HRMS/MS identification results
Figure 2 shows the primary cleavage of the mother nucleus of flavonoids. First, the B ring is detached, the carbon-carbon bond on the C ring of a flavonoid is broken by the retro Diels-Alder (RDA) reaction to form a pair of complementary ions A
Subsequently, secondary mass spectral fragments of flavonoids often show fragments characteristic of the benzene ring. These fragments with mass –to -charge ratios of 39, 51, 65, 77, and 78. In addition, flavonoids often show neutral loss of 18 (H
Figure 3.
MS
![MS2 diagram of [M-H]-m/z 303.05096.](https://content.iospress.com:443/media/thc/2023/31-S1/thc-31-S1-thc236050/thc-31-thc236050-g003.jpg)
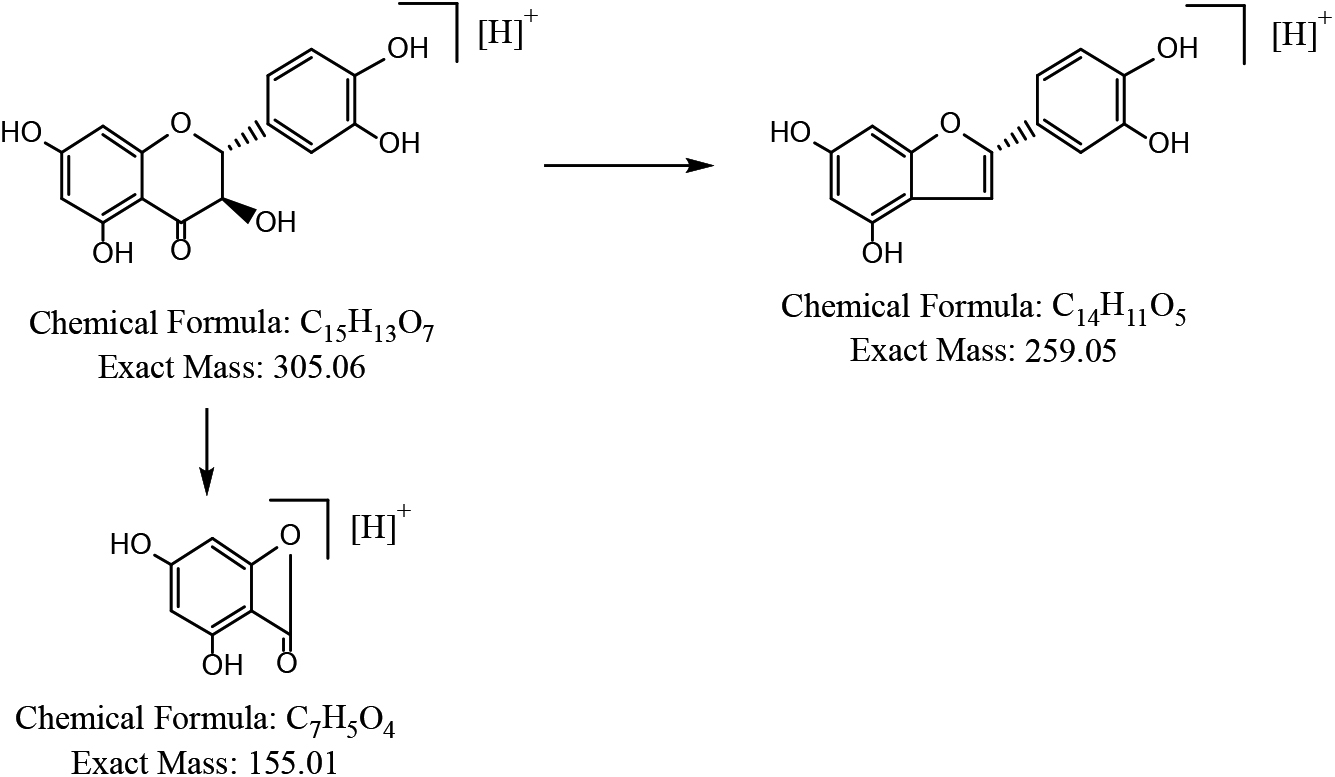
3.3.1Identification of (2R, 3R) –dihydroquercetin
In the negative ion detection mode of ESI-MS, the ionic formula is C
In summary, the secondary mass spectra of the above compounds in the positive and negative ion modes and the cleavage pattern of the compound are consistent with the cleavage pattern of flavonoids. Therefore, the compound can be presumed to be a flavonoid. The compound was also identified by database search as (2R, 3R) -Taxifolin, (2R, 3R) -dihydroquercetin. The cleavage pattern is shown in Fig. 4.
Figure 4.
Secondary fragmentation of dihydroquercetin in positive ion mode.
3.3.2Identification of quercetin
In the negative ion detection mode of ESI-MS, the compound excimer ion [M-H]
Figure 5.
MS
![MS2 diagram of [M-H]-m/z 301.03531.](https://content.iospress.com:443/media/thc/2023/31-S1/thc-31-S1-thc236050/thc-31-thc236050-g005.jpg)
Zhang’s group [32] used high-resolution mass spectrometry, a new strategy for the structural characterization of potential new phthalide compounds which was proposed by isomer structure predictions combined with a QSRR analysis using phthalide compounds in Chuanxiong. The collected raw HRMS data were preliminarily screened by an in-house database; the MS/MS fragmentation patterns of the analogous compounds were summarized; the reported phthalide compounds were identified, and the structures of the isomers were reasonably predicted. The retention times of the phthalide isomers in Chuanxiong were well predicted by the QSRR model combined with reasonable structure predictions. A total of 81 peaks were detected from Chuanxiong and assigned to reasonable structures, and 26 potential new phthalide compounds were structurally characterized.
Li’s group [33] used high-resolution mass spectrometry combining with high performance liquid chromatography to study phthalates, presenting a retention time (tR) prediction model based on quantitative structure-retention relationship (QSRR). This model can predict the retention time of a given structure of phthalates including isomers. The results of this study showed that the developed QSRR model could be a useful tool to predict the retention times of unknown metabolites of phthalates and their alternatives in future non-targeted screening analysis.
Therefore, the QSRR model has a broad prospect in the field of analyzing unknown compounds. Based on this method, this study applied it to the analysis of flavonoids in Scutellaria baicalensis.
In this study, flavonoids were first analyzed in the extracts of Polygala fallax Hemsl by a UV detector. According to the structural characteristics of flavonoids, 12 peaks containing flavonoids were initially screened, but the identification of flavonoids could not be made accurately by UV absorption judgment alone. Only a preliminary judgment could be made that the compound might be a flavonoid, and using UV detection alone was a large error in the judgment of the compound. In order to increase the accuracy of judging flavonoids, we use UPLC-HRMS to obtain high -resolution mass spectrometry extracted ion chromatograms after linear gradient injection of baicalin alcohol extract. 154 peaks with peaks higher than 1.50E
Table 1
IC
| The sample | A549 | Hela | MCF7 |
|---|---|---|---|
| 1 | |||
| 2 | 99.2 | ||
| 3 | 87.2 | 95.5 | |
| Cisplatin | 15.8 | 15.6 | 16.3 |
1: quercetin; 2: (2R, 3R) – dihydroquercetin; 3: baicaleol extract.
3.4Results of tumour cytotoxic activity assay
The cytotoxic activity of the compounds against three human-derived tumor cell lines, lung cancer cell line A549, cervical cancer cell line Hela and breast cancer cell line MCF7, was determined by MTT assay with cisplatin as a positive control. Table 1 shows the ICso values of cisplatin and these compounds. The results indicate that the extracts of Flos Farinaceae(2R, 3R)-dihydroquercetin, and quercetin compounds showed potent inhibitory activity against the human-derived tumor cells tested.
4.Conclusion
This paper uses an ultra-performance liquid phase, diode array detector, and high-resolution quadrupole orbital trap mass spectrometer to detect and analyze the methanol extract of Scutellaria baicalensis Georgi. A total of 12 chromatograms containing flavonoid compounds were analyzed using PAD spectroscopy. More than 90 compounds were tentatively identified as possible flavonoids by high-resolution primary mass spectrometry combining elemental composition and unsaturation analysis. The secondary mass spectrometry data combined with the mass spectro- metric cleavage patterns of the flavonoids identified more than 40 of them as flavonoids, and the identified flavonoids were verified. Cleavage mechanisms were analyzed using the CD software Mass front. Finally, two flavonoid isomers were identified and validated using the quantitative parameters c in the QSRR model, namely baicalin. The experimental results of this thesis show that the method we have used to identify flavonoids is systematic, comprehensive, accurate, and reliable and can be used to identify and analyze flavonoid compounds in other traditional Chinese medicines, natural products, or foods.
Conflict of interest
None to report.
Funding
This work was financially supported by the 2022 National Social Science Foundation: Research on Traditional Phenological Calendar of Ethnic Minorities along the Heilongjiang Coast (Project No. 22BMZ074).
References
[1] | Liu L, Feng WH, Liu XQ, Liang YH, Li C, Wang ZM. Research progress on Polygalae Radix. China journal of Chinese Materia Medica. (2021) ; 46: (22): 5744-5759. doi: 10.19540/j.cnki.cjcmm.20210518.601. |
[2] | Liu JF, Liu AL, Mao FY, Zhao YS, Cao Z, Cen NN, et al. Determination of the active ingredients and biopotency in Polygala tenuifolia Willd. and the ecological factors that influence them. Industrial Crops and Products. (2019) ; 134: : 113-123. doi: 10.1016/j.indcrop.2019.03.074. |
[3] | Liang HL, Liu BY, Wu C, Zhang XJ, Wang ML, Huang XY, et al. Effects of light intensity on the growth of Polygala fallax Hemsl. (Polygalaceae). Frontiers in Plant Science. (2022) ; 13: : 985628. doi: 10.3389/fpls.2022.985628. |
[4] | Yang BB, Li XJ, Yu K, Jiang XL, Wang L, Li F, et al. Sugar easters and xanthones from the roots of Polygala tenuifolia Willd and their cytoprotective activity. Fitoterapia. (2022) ; 161: : 105256. doi: 10.1016/j.fitote.2022.105256. |
[5] | Lacaille-Dubois MA, Delaude C, Mitaine-Offer AC. A review on the phyto-pharmacological studies of the genus Polygala. Journal of Ethnopharmacology. (2020) ; 249: : 112417. doi: 10.1016/j.jep.2019.112417. |
[6] | Xin T, Zhang FB, Jiang QY, Chen CH, Huang DY, Li YJ. et al. Extraction, purification and antitumor activity ofawater-soluble polysaccharide from the roots of Polygala tenuifolia. Carbohydrate Polymers. (2012) ; 90: (2): 1127-1131. doi: 10.1016/j.carbpol.2012.06.058. |
[7] | Lacaille-Dubois M, Delaude C, Mitaine-Offer A. A review on the phyto-pharmacological studies of the genus Polygala. Journal of Ethnopharmacology. (2020) ; 249: (C): 112417. doi: 10.1016/j.jep.2019.112417. |
[8] | Chen Z, Yang Y, Han Y, Wang XJ. Neuroprotective Effects and Mechanisms of Senegenin, an Effective Compound Originated from the Roots of Polygala Tenuifolia. Frontiers in Pharmacology. (2022) ; 13: . doi: 10.3389/fphar.2022.937333. |
[9] | Pastore JFB. Revision of the ‘Polygala herbiola group’ (Polygalaceae): a new species and a new variety. Kew Bulletin. (2022) ; 77: (1): 221-232. doi: 10.1007/s12225-022-10009-4. |
[10] | Martinez A, Pastore JBF. Two New Species of Polygala (Polygalaceae) Endemic to the Phytogeographic Province of La Payunia, in Argentina. Systematic Botany. (2022) ; 47: (1): 232-241. doi: 10.1600/036364422X16442668423545. |
[11] | Cheng YG, Tan JY, Li JL, Wang SH, Liu KL, Wang JM, et al. Chemical constituents from the aerial part of Polygala tenuifolia. Natural Product Research. (2022) ; 36: (21): 5449-5454. doi: 10.1080/14786419.2021.2013838. |
[12] | Mane MP, Patil RS. Magdum AB, Kakade SS, Patil DN, Nimbalkar MS. Chemo-profiling by UPLC-QTOF MS analysis and in vitro assessment of Anti-inflammatory activity of Field Milkwort (Polygala arvensis Willd.). South African Journal of Botany. (2022) ; 149: : 49-59. doi: 10.1016/j.sajb.2022.05.043. |
[13] | Xiang W, Zhang GD, Li FY, Wang TL, Suo TC, Wang CH, Li Z, Zhu Y. Chemical Constituents from the Roots of Polygala arillata and Their Anti-Inflammatory Activities. Journal of Chemistry. (2019) ; 2019: : 8079619. doi: 10.1155/2019/8079619. |
[14] | Jiang N, Wei SS, Zhang YW, He WL, Pei HY, Huang H, et al. Protective Effects and Mechanism of Radix Polygalae Against Neurological Diseases as Well as Effective Substance. Frontiers in Psychiatry. (2021) ; 12: : 688703. doi: 10.3389/fpsyt.2021.688703. |
[15] | Yu SA, Dong XD, Ji HY, Yu J, Liu AJ. Antitumor activity and immunomodulation mechanism of a novel polysaccharide extracted from Polygala tenuifolia Willd evaluated by S180 cells and S180 tumor-bearing mice. International Journal of Biological Macromolecules. (2021) ; 192: : 546-556. doi: 10.1016/j.ijbiomac.2021.10.025. |
[16] | Nguyen MV, Han JW, Dang LQ, Ryu SM, Lee DH, Kim H, et al. Clerodane Diterpenoids Identified from Polyalthia longifolia Showing Antifungal Activity against Plant Pathogens. Journal of Agricultural and Food Chemistry. (2021) ; 69: (36): 10527-10535. doi: 10.1021/acs.jafc.1c02200. |
[17] | Zeng W, Wu AG, Zhou XG, Khan I, Zhang RL, Lo HH, et al. Saponins isolated from Radix polygalae extent lifespan by modulating complement C3 and gut microbiota. Pharmacological Research. (2021) ; 170: : 105697. doi: 10.1016/j.phrs.2021.105697. |
[18] | Son SR, Yoon YS, Hong JP, Kim JM, Lee KT, Jang DS. Chemical Constituents of the Roots of Polygala tenuifolia and Their Anti- Inflammatory Effects. Plants. (2022) ; 11: (23): 3307. doi: 10.3390/plants11233307. |
[19] | Wei MX, Yu JY, Liu XX, Li XQ, Zhang MW, Yang PW, Yang JH. Synthesis of artemisinin-piperazine-furan ether hybrids and evaluation of in vitro cytotoxic activity. European Journal of Medicinal Chemistry. (2021) ; 215: : 113295. doi: 10.1016/j.ejmech.2021.113295. |
[20] | Taleghani A, Tayarani-Najaran Z. Potent Cytotoxic Natural Flavonoids: The Limits of Perspective. Current Pharmaceutical Design. (2018) ; 24: (46): 5555-5579. doi: 10.2174/1381612825666190222142537. |
[21] | Babu BV, Konduru NK, Nakanishi W, et al. Experimental and theoretical advances in functional understanding of flavonoids as anti-tumor agents. Anti-Cancer Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Cancer Agents). (2013) ; 13: (2): 307-332. doi: 10.2174/1871520611313020017. |
[22] | Gacche RN, Shegokar HD, Gond DS, Yang ZZ, Jadhav AD. Evaluation of selected flavonoids as antiangiogenic, anticancer, and radical scavenging agents: an experimental and in silico analysis. Cell Biochemistry and Biophysics. (2011) ; 61: (3): 651-663. doi: 10.1007/s12013-011-9251-z. |
[23] | Kontogianni VG, Primikyri A, Sakka M, Gerothanassis IP. Simultaneous determination of artemisinin and its analogs and flavonoids in Artemisia annua crude extracts with the use of NMR spectroscopy. Magnetic Resonance in Chemistry. (2020) ; 58: (3): 232-244. doi: 10.1002/mrc.4971. |
[24] | Atef A, Al-Amier HA, Ibrahim TA. Comparative study of the flavonoids of some Verbena species cultivated in Egypt by using high-performance liquid chroma-tography coupled with ultraviolet spectroscopy and atmospheric pressure chemical ionization mass spectrometry. Journal of Chromatography A, (2010) ; 1217: (41): 6388-6393. doi: 10.1016/j.chroma.2010.08.025. |
[25] | Pobłocka-Olech L, Głód D, Żebrowska ME, Sznitowska M, Krauze-Baranowska M. TLC determination of flavonoids from different cultivars of Allium cepa and Allium ascalonicum. Acta Pharmaceutica. (2016) ; 66: (4): 543-554. doi: 10.1515/acph-2016-0038. |
[26] | Zhao XS, Zhang SH, Liu D, Yang MH, Wei JH. Analysis of flavonoids in dalbergia odorifera by ultra-performance liquid chromatography with tandem mass spectrometry. Molecules. (2020) ; 25: (2): 389. doi: 10.3390/molecules25020389. |
[27] | Lei ZT, Sumner BW, Bhatia A, Sarma SJ, Sumner LW. UHPLC-MS Analyses of Plant Flavonoids. Current Protocols in Plant Biology. (2019) ; 4: (1): e20085. doi: 10.1002/cppb.20085. |
[28] | Sheng ZL, Jiang YM, Liu JM, Yang B. UHPLC-MS/MS analysis on flavonoids composition in Astragalus membranaceus and their antioxidant activity. Antioxidants. (2021) ; 10: (11): 1852. doi: 10.3390/antiox10111852. |
[29] | Li SN, Liu S, Liu ZQ, Liu CM, Song FG, Pi ZF. Bioactivity screening, extraction, and separation of lactate dehydrogenase inhibitors from Polygala tenuifolia Willd. based on a hyphenated strategy. Journal of Separation Science. (2017) ; 40: (6): 1385-1395. doi: 10.1002/jssc.201601216. |
[30] | Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nature Reviews Drug Discovery. (2005) ; 4: (4): 307-320. doi: 10.1038/nrd1691. |
[31] | Timerbaev AR, Hartinger CG, Aleksenko SS, Keppler BK. Interactions of antitumor metallodrugs with serum proteins: advances in characterization using modern analytical methodology. Chemical Reviews. (2006) ; 106: (6): 2224-2248. doi: 10.1021/cr040704h. |
[32] | Zhang QQ, Huo MQ, Zhang YL, Qiao YJ, Gao XY. A strategy to improve the identification reliability of the chemical constituents by high-resolution mass spectrometry-based isomer structure prediction combined with a quantitative structure retention relationship analysis: Phthalide compounds in Chuanxiong as a test case. Journal of Chromatography A. (2018) ; 1552: : 17-28. doi: 10.1016/j.chroma.2018.03.055. |
[33] | Meshref S, Li Y, Feng YL. Prediction of liquid chromatographic retention time using quantitative structure-retention relationships to assist non-targeted identification of unknown metabolites of phthalates in human urine with high-resolution mass spectrometry. Journal of Chromatography A. (2020) ; 1634: : 461691. doi: 10.1016/j.chroma.2020.461691. |




