Sexual dimorphism in the response to dietary restriction in mice: A systematic review of the literature
Abstract
Background:
Dietary restriction (DR) is a widely used experimental intervention in aging research due to its consistent ability to extend lifespan in most species tested. DR is an all-encompassing term describing interventions that restrict some aspect of nutrition - from calorie amount to calorie type to timing of food intake - and yet share common functional endpoints including extended longevity, but also improvements in healthspan, or the time spent in good health, as well as metabolic fitness and stress resistance. Recent studies highlight the preponderance of sexual dimorphisms in the response to DR and argue for the importance of inclusion of both sexes in preclinical research.
OBJECTIVE:
We set out to perform a comprehensive assessment of documented health and lifespan outcomes of interventional DR studies in mice that display sexual dimorphism.
METHODS:
A systematic literature search was conducted according to the PRISMA statement to identify mouse DR studies in which both sexes were included using PubMed. The specific DR interventions examined included calorie restriction (CR), intermittent fasting (IF), protein restriction (PR) and methionine restriction (MetR), with experimental endpoints focused on lifespan and healthspan.
RESULTS:
Sexual dimorphism in the lifespan and healthspan effects of various DR regimens is a common finding in mice, with the magnitude and direction of dimorphic responses influenced by the specific dietary intervention as well as the strain of mouse used in the study.
CONCLUSIONS:
Despite the fact that preclinical lifespan and healthspan analyses in mice reveal sexual dimorphism in the response to DR, there is still a large gap in our understanding of how sex affects dietary outcomes. More preclinical research comparing both sexes in the same study with better attention to reporting metrics during peer review and in easily searchable text including title and abstract is required to further our understanding of the impact of sex on health and lifespan in response to DR in rodent studies.
1Introduction
The morbidity-mortality paradox, in which females tend to have worse health than males and yet live longer [1, 2], is an example of sexual dimorphism, the condition in which two sexes of the same species exhibit different characteristics unrelated to their sexual organs. Differences in size, fat metabolism and expression of drug metabolising enzymes are further examples of sexual dimorphism with potentially profound implications for biology and medicine. Nonetheless, the preponderance of both preclinical and clinical studies using only one sex has prevented a detailed accounting of which characteristics display sexual dimorphism, as well as an understanding of molecular mechanisms underlying these potentially important differences. Historically, a number of factors have precluded the use females in particular from research, including perceived increased variability amongst females due to effects of cycling sex hormones, as well as added cost of studies involving both sexes. It is important to note, that when we talk about sexual dimorphism in the context of animal studies, we consider sex a biological variable, defined genetically by XX or XY chromosomes [3].
Almost 30 years ago, the NIH recognized that excluding women from clinical research was “bad for women and bad for science” [4], and established the Office of Research on Women’s Health to address the issue in 1990. In 2016, the NIH mandated policies requiring applicants to include sex as a biological variable (SABV) in all preclinical studies, including those with primary-derived cells, or else to provide strong scientific justification for the use of only one sex based on rigorously defined exceptions that do not include cost considerations [4]. To further illustrate the importance of SABV, a cross-sectional study of C57BL/6Nia mice recently demonstrated that a number of parameters that showed an age-dependent decline in males were preserved in older female mice [5]. After controlling for multiple comparisons, lower percent body fat was associated with premature death but only among females; no health measures were significantly associated with premature death in males. This was true even for measures that differed among age groups [5].
In the context of aging research, several large-scale rodent studies [6–9] provide strong evidence of sexual dimorphism in lifespan and healthspan responses to one of the most heavily investigated anti-aging interventions, dietary restriction (DR). Defined as reduced food intake without malnutrition, DR describes a range of interventions that broadly impact the hallmarks of aging through pleiotropic mechanisms (for recent reviews, see [10–16]), resulting in extended longevity and improvement in markers of healthspan in most species tested to date.
Here we systematically review what is known about sexual dimorphisms in the lifespan and healthspan outcomes of dietary restriction interventions specifically in mice. To this end, we queried original research articles in the PubMed database describing experimental research measuring lifespan and/or healthspan outcomes in both male and female mice subject to various dietary restriction interventions, including calorie restriction (CR), intermittent fasting (IF), protein restriction (PR) and methionine restriction (MetR).
2Methodology
A systematic review of the literature was conducted according to the PRISMA statement [17] to identify publications reporting on mouse dietary restriction studies. PubMed was utilized as the search tool and database to screen the title, abstract and keywords of all articles (excluding reviews) using the search terms with Boolean operators as outlined in Table 1. All identified records were exported to Endnote (Endnote X9, Thomson Reuters, New York, USA), where authors removed duplicate records and irrelevant titles/abstracts and non-original research (re-analysis of previously published data, commentaries) (Supplementary Table 1). To ensure all relevant research was included, a manual review of the literature was also performed to ensure all possible research was included. The remaining potential records were then screened against the eligibility criteria as specified in Table 2, and eligible articles used as the basis for this systematic review.
Table 1
Search terms used in the systematic review. Search terms including Boolean operators and permutations used in the PubMed search with standard filter for English language. 284 articles were identified for further screening
| Search category | Search term | Boolean operator |
| Dietary intervention | Dietary restriction, diet restriction, calorie restriction, caloric restriction, intermittent fasting, alternate day fasting, every-other-day fasting, every other day fasting, EOD, methionine restriction, protein restriction, protein dilution, geometric framework | OR |
| AND | ||
| Experimental endpoint | Healthspan, lifespan, longevity, survival | OR |
| AND | ||
| Preclinical model | Mice, mouse | OR |
| AND | ||
| Sex | Male, female | AND |
| NOT | ||
| Review[Publication type] | ||
Table 2
Inclusion and exclusion criteria for the systematic review
| Inclusion criteria | Exclusion criteria |
| Studies involving mice | Non-English articles |
| Articles including both male and female sexes within the same study only | Non-original articles (i.e. review articles with or without systematic review or meta-analysis) |
| Studies including a form of dietary restriction limited to CR, MetR, or PR vs. an appropriate control diet | Studies involving a dietary intervention that was not CR/DR/PR/MetR |
| Must include a wildtype group | Studies including only one sex |
| Must include a healthspan and/or lifespan outcome, with lifespan outcome defined operationally as death due to natural causes or sacrifice due to aging-related morbidity | Studies involving rodents but not mice; studies with both rats and mice were included but only the mouse data was used |
| Publication date 1993 to December 2021 | Repeated publications on the same cohort to avoid publication bias |
| Must include a form of dietary restriction as the main intervention | Studies in which a drug or genetic intervention is the primary intervention, but with a dietary intervention as a control |
CR, calorie restriction; DR, dietary restriction; PR, protein restriction; MetR, methionine restriction.
The definitions of terms describing the different dietary/feeding paradigms covered in this systematic review are summarized in Table 3. Lifespan/healthspan data are presented in Table 4. Assessing healthspan in mice is limited by the lack of a gold standard definition of what measure(s) constitutes an improvement in healthspan. As recently reviewed [18, 19] there are a large number of assays available in mice which measure a wide range of physiological functions in mice, many of which are altered in aging [18]. For the purpose of this review, we limited our healthspan measures to changes in body composition/body weight, measures of insulin sensitivity and glucose tolerance, incidence of tumors/neoplasia and immunology parameters as these are well established to be altered with DR.
Table 3
Definition of terms describing the different feeding paradigms
| Feeding paradigm | Other names | Description |
| Ad libitum | Free access to food 24/7 | |
| Dietary restriction (DR) | An all-encompassing term describing interventions that restrict some aspect of nutrition | |
| Calorie restriction (CR) | Caloric restriction; intermittent fasting | Restriction of food availability by 10–60% calories relative to an AL fed control group; time spent in fasted state varies according to timing of food allotments, typically daily to thrice weekly |
| Intermittent fasting (IF) | Every-other-day fasting (EOD); alternate-day fasting (ADF); alternate-day feeding (ADF) | Food is withheld one or more days per week alternating with ad libitum feeding; can also be used to describe periods spent in the fasted state between food allotments in the context of calorie restriction |
| Protein restriction | Protein dilution | Diet with a reduced protein content, where protein is typically replaced by carbohydrates; commonly fed AL |
| Methionine restriction | Sulfur amino acid restriction | Reduced essential amino acid methionine, usually without the non-essential amino acid cysteine; commonly fed AL |
Red text describes regimens involving enforced restriction of either total calories or timing of food availability; green text describes regimens consisting of diets with altered macronutrient content but fed on an ad libitum basis.
3Discussion of the findings
3.1Patterns of inclusion of sex information in dietary restriction/aging publications over the past 29 years
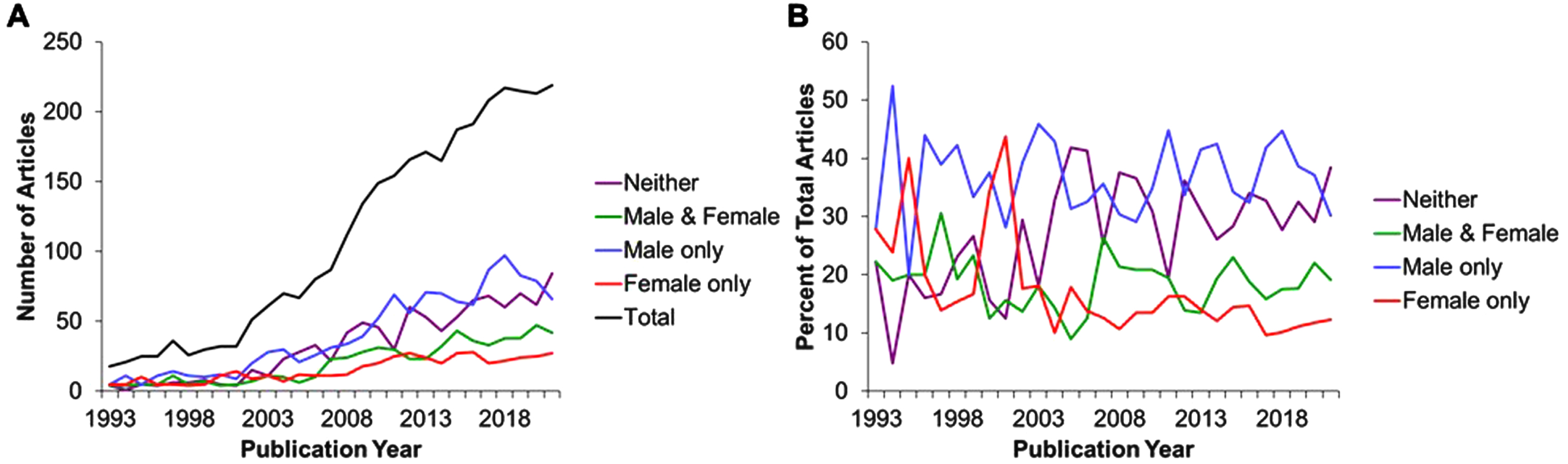
To gain insight into the trends in use and reporting sex of experimental animals in the preclinical literature regarding lifespan/healthspan benefits of DR, we modified the search criteria specifying sex (Table 1) to highlight studies reporting the use of only males (male NOT female), only females (female NOT male), or not reporting sex in the title, abstract or keywords (NOT (female OR male)) out of the total number of studies (sex term removed). Of the total number of 2809 articles (Fig. 1A, B) returned by searching without any sex criteria, we found 1016 articles reported males only (36.2%), 402 reported females only (14.3%), 514 articles reported both males and females(18.3%), while 877 reported neither male nor female sex (31.2%).
Thus, while a PubMed search of title, abstract and keywords but lacking the full text overestimates the number of papers that fail to report the sex of experimental animals by about one half (most of which actually used one sex), this is still a surprising number of papers and indicates a failure in the reporting of data as well as in the peer review process the preceded publication and citation in PubMed.
Finally, an analysis of these trends over the past almost 30 or so years (1993–2022), including 6 full years after the 2016 NIH preclinical mandate requiring applicants to include SABV, reveals an increase in the total number of papers in the field, but little change in the percentages over time with regard to sex reporting. Taken together, this brief survey indicates that in 2022 in this subfield, there remains a majority of papers that don’t consider both sexes, and a minority that either don’t report sex at all or don’t consider it important enough to include in the text searchable by PubMed. Allowing for the inherent limitations and bias of this type of basic search as a metric, it serves to suggests an ongoing lack of inclusion of females in DR research. These findings are supported by recent work investigating the inclusion of SABV in preclinical work since the implementation of this mandate [3, 20].
Fig. 1
Total numbers of publications (A) expressed as a percent of total (B) over the period from 1993 through 2021 : 2809 results.
3.2Sexual dimorphism in the extension of lifespan and healthspan by DR in rodents: Proof of principle in classic studies
Aging is a multifactorial process resulting in a progressive breakdown in tissue homeostasis leading to a decline in physiological reserve. The consequence of this inevitably is eventual death, but often includes an increased burden of chronic disease. The underlying cellular and molecular mechanisms are complex and poorly understood, but thought to be attributable to a number so-called “hallmarks of aging” including cellular senescence, mitochondrial dysfunction, stem cell exhaustion, deregulated nutrient sensing, loss of proteostasis, epigenetic alterations, telomere attrition, genomic instability and altered intercellular communications [21]. While the underlying mechanisms of the aging process are beyond the scope of this review, we direct the reader to several excellent reviews on the topic ([21–23]).
Dietary restriction (DR) is an all-encompassing term describing interventions that restrict some aspect of nutrition, but without deficiency of essential nutrient, that broadly impacts the hallmarks of aging, resulting in extension of lifespan and improvement in markers of healthspan in most species tested to date. Such interventions include calorie restriction (CR), involving reduced food intake (usually by 20–40% relative to ad libitum fed control animals on the same diet); intermittent fasting (IF), involving repeated enforced periods of fasting of different lengths, including every other day (EOD)/alternate day (AD) fasting, periodic fasting (PF) and time restricted feeding (TRF); and dietary dilution of specific macronutrients such as protein or essential amino acids such as methionine, but without enforced food restriction. These interventions, summarized in Table 3, will be discussed in further depth below.
The first report of the benefits of calorie restriction (CR) on lifespan in rodents can be traced back to 1935 when McCay, Crowell, and Maynard described how a severe reduction in calories after weaning retards growth and body size of albino male rats, while at the same time extending their lifespan relative to a control group given free access to food [24]. This work established the use of a dietary intervention to extend longevity and laid the groundwork to future use of this technique as a tool to interrogate the basis of the aging process itself.
Table 4
Assessment of sexual dimorphism in lifespan and healthspan effects of dietary restriction
| Mouse strain | Diet | Regimen | Median LS | Max LS | Lifespan sexual dimporphism? | Healthspan outcome | Healthspan sexual dimorphism? | Reference |
| Age-matched male and female C57BL/6J mice, 8 to 12 weeks of age (n = 9 to 15 per group) | Isocaloric, purified controlled diets (D11051801 and D11092301), were manufactured by Research Diets (New Brunswick, NJ). Semi-purified diets | 18% and 4% protein diets, 2–4 weeks | Not measured | Not measured | Not measured | Improved insulin sensitivity in M on PR diet, not in females; ↓ Plasma TG and CHOL in males on PR diet not females | Yes; effects are shown in males on PR diet but not in females; effects can be restored following OVX in females | [78] |
| C57BL/6J | 18% protein rodent diet (Harlan); naturally sourced diet | 30CR was started at 3 months of age | Not measured | Not measured | Not measured | No sexual dimorphism in the expression or response to CR for circadian clock genes Bmal1, Per1, Per2 and Per3. The expression of several clock genes: Cry1, Cry2, Rev-Erb α and Ror γγ was significantly different between males and females on both diets used. In addition, the effect of CR on the expression of Cry1, Rev-Erb α and Ror γ was sex-dependent | Yes | [79] |
| UM-HET3 mice | Purina 5058; naturally sourced diet | 40CR | Not measured | Not measured | Not measured | The number of GFAP-positive astrocytes in the hippocampus depended on sex (P = 0.002), but there were no significant effects of CR on microglia (P = 0.35) or number of astrocytes (P = 0.07) | Yes, depending on the outcome | [80] |
| Male and female C57Bl/6 and DBA/2 mice were purchased from Charles River | Not specified | 40CR started at 16 weeks age | Not measured | Not measured | Not measured | Significant increase in satellite cells in injured B6 CR M muscle (but females was NS) | Calorie restriction reduces muscle fibre size 7 days after muscle injury, but this is strain, sex and age-dependent | [75] |
| Ercc1-/- in a genetically uniform F1 C57BL6J/FVB hybrid background | AIN-93 G; semi-purified diet | 30CR (stepdown from 7–9 weeks age), fed just before the dark period | ↑in males from 10 to 35 weeks (250% extension; p < 0.0001) and females from 13 to 39 weeks (200% extension; p < 0.0001 | ↑in males from 14 to 46 weeks ↑in females from 19 to 49 weeks | No, both have increased LS with DR | Onset of tremors, imbalance, and paresis are dramatically postponed or even absent in Ercc1Δ/– and Xpg-/–mice under continuous and temporary DR regimes ↓FBG, insulin with DR | Healthspan parameters either only include males, or do not specify which sex | [81] |
| Xpg-/- mutant mice in a pure C57BL6J background | AIN-93 G; semi-purified diet | 30CR (stepdown from 7–9 weeks age), fed just before the dark period; lifelong or transient for only 6 weeks | ↑from 10 to 18 weeks with age (continuous DR); also increased with transient DR | ↑from 12 to 21 weeks with age (continuous DR); also increased with transient DR | Does not stratify between M &F | Not measured | Not measured | [81] |
| Ten -month old male and female C57BL/6J wildtype mice | Control diet (0.86% methionine) or MR diet containing 0.172% methionine | MetR or control diet for 8 weeks | Not measured | Not measured | Not measured | ↓FBG, no sex dimorphism | No | [82] |
| C57BL/6J mice | Harlan 18% protein diet; naturally sourced diet | AL, 20CR or 40CR | ↓ in Females on 40CR; ↑ in males on 20CR, 40CR, and females on 20CR | ↑ in both 20CR and 40CR for M&F | Yes | ↓ BW with CR dose-dependently ↓ tumor burden ↓ HOMA-IR | Yes, metric specific (i.e. insulin, change in fat mass, rectal temperature) | [8] |
| DBA/2J | Harlan 18% protein diet; naturally sourced diet | AL, 20CR or 40CR | ↑ in both M and F on 20CR and 40CR | ↑ in both M and F on 20CR and 40CR | No | ↓ BW with CR dose-dependently ↓ tumor burden ↓ HOMA-IR | No | [8] |
| C57BL/6 | Labofeed H (containing 60% carbohydrates, 30% proteins and 10% fat) | Every other day (EOD) feeding regiment alternating ad libitum feeding and fasting every other day food from 4 weeks age | Not measured | Not measured | Not measured | Significant ↓ in BW in EOD males; not in females. Significant ↓ in hepatocyte nuclear area with EOD independent of sex | BW = yes. Hepatocyte nuclear area = No | [83] |
| DBA/2J and C57BL/6J mice | Harlan 18% protein diet; naturally sourced diet | AL or 40CR, lifelong CR assessment at 18mo age | Not measured | Not measured | Not measured | Significant ↓ in FI in B6 males on CR; no significant effect in D2 males or in females | Yes | [35] |
| Male and female C57BL/6J | CF (0.84% methionine w/w) or MetR (0.12% methionine w/w) diets consisting of 14% kcal protein, 76% kcal carbohydrate, and 10% kcal fat | CF or MetR from 8–20 wks age | Not measured | Not measured | Not measured | In young mice, no sexual dimorphism on BW or body length; in old mice, sexual dimorphism; for femur length, no sexual dimorphism in either age group | Depends on age of mice | [84] |
| C57BL/6 mice | 7012 Teklad LM-450 | Ad libitium (AL), 40CR for 6mo | Not measured | Not measured | Not measured | Generally no sexual dimorphism in gene expression in liver of DR mice | Depends on gene | [85] |
| 4-week-old female and male C57Bl6 mice (6 animals/sex/group) | Not specified | EOD feeding for 9mo | Not measured | Not measured | Not measured | Sexual dimorphism in BW (M EOD have decreased BW, F do not). No sex differences in peripheral blood CBC-diff values | Depends on healthspan outcome | [86] |
| UM-HET3 mice | Not specified but based on other publications likely NIH-31; naturally sourced diet | 40DR | Not measured in this study | Not measured in this study | Not measured in this study | Significant ↓ in fasting insulin, IGF-1, FGF-21 with DR; no sex effects | No | [87] |
| Male and female Npy–/– and WT mice on a mixed 129S-Npytm1Rpa/J and 129S6/SvEvTac background | Charles River-LPF diet (Oriental Yeast Co. Ltd., Tsukuba, Japan) | 30CR from 12 weeks age fed 30 min before lights were turned off | ↑ 20.3% with DR in males, ↑36% with DR in F | ↑ with DR independent of sex, stronger effect in F | No, same direction (↑LS) of effect | ↓ BW in DR M, not in F. Respiratory quotient (RQ) and Energy expenditure (EE) were only analyzed in male mice | BW: Yes, sexual dimorphism | [88] |
| GHR-KO mice and their littermate controls | Lab Diet Formula 5001 (23 % protein, 4.5 % fat, 6 % fiber) (Nestlé Purina, St. Louis, MO, USA) | AL or 30CR from 28 weeks age fed in the AM | Not measured in this study | Not measured in this study | Not measured in this study | No sexual dimorphism in BW, wirehang test, inclining rod or inverted screen in DR mice | No | [89] |
| Ames dwarf (Prop1df/df) (Df) mice or their normal littermate controls [Prop1df/+(N)] | Not specified | AL or 30CR from 28 weeks age fed in the AM in middle-aged (∼ 70 –95 weeks-of-age) mice | Not measured in this study | Not measured in this study | Not measured in this study | ↓BW in CR independent of sex or GT ↓ strength for CR M compared to AL (WT only); no effect in females; significant ↓ in balance with CR in female WT, no effect in males | No effect on BW measures, but sexual dimorphism in strength measures with CR only in middle aged mice | [90] |
| Nestin-GFP reporter mouse line and C57BL/6 animals | Standard Purina Mills test diet; naturally sourced diet | AL or 40CR from 6mo age | Not measured in this study | Not measured in this study | Not measured in this study | CR increases both the total number of dividing cells and the number of dividing neural stem and progenitor cells in the DG of adult female mice | Yes | [91] |
| C57BL/6 (Harlan, Blackthorn UK) | Rodent pelleted chow (CRM (P); Special Diets Services, Witham, UK) | 40DR from 6mo age at 9:30am everyday | DR improved survival in both sexes, but the extension was significantly greater in females (P = 0.0163) | Survival is not completed at the time of publication | Yes, in females for mean lifespan | Tumor prevalence increased sharply in both AL sexes after 17 months of age; % of tumor-bearing mice was lower in males than in females over their whole remaining lifespan DR strongly reduced tumor prevalence in females. DR postponed tumor incidence but did not reduce the % of mice bearing neoplasms after 20 mo of age | No for BW | [41] |
| 129/SvJ mice | AIN-93M; semi-purified diet | AL (10CR) or 40CR from 8 weeks age | Not measured in this study | Not measured in this study | Not measured in this study | Long-term caloric restriction significantly depleted TAG stores in mice after 3 month CR. Sexual dimorphism in TAG turnover but only in 13mo male mice | Depends on measure | [92] |
| G93A mice (animal model of ALS) | NIH-31/NIA fortified diet; naturally sourced diet | AL or 40CR from 40 (?) days of age | Not directly assessed | Not directly assessed but the rate of rate of reaching endpoint in the CR mice (i.e., the hazard ratio) being 3.1-fold higher (95% CI: 2.6, 9.8) than the AL mice. The rate of reaching endpoint was 2.9-fold (95% CI: 2.1, 10.2) higher in the CR vs. AL females (P = 0.0001) and 4-fold (95% CI: 2.8, 34.2) higher in the CR vs. AL males (P = 0.0004) | No, CR shortened lifespan in both sexes | No sexual dimorphism in food intake, BW, body condition, ability to move, paw grip endurance. In both sexes CR worsened these parameters | No | [93] |
| 41 ILSXISS recombinant inbred (RI) mouse strains | Not specified | 2–5 months of age fed AL or 40DR | Not reported | Strain variation of mean lifespan in mice under DR was even greater, ranging six- to ten-fold: 217 to 1215 days in males and 113 to 1225 days in females. Effect of strain on lifespan was significant for both sexes under both feeding conditions (p < 1×10–6, ANOVA) | Yes | Not assessed in this study | Not assessed in this study | [7] |
| GHRKO and GHR WT mice | Lab Diet Formula 5001 (23% protein, 4.5% fat, 6% fiber) | Mice were either fed AL every day (AL group) or every other day (IF group) from 8–10 wks age | ↑in WT M on IF, not in WT F; no effect of IF in GHRKO | ↑in WT M on IF, not in WT F; no effect of IF in GHRKO | Sexual dimorphism in survival for WT-F-IF compared to WT-M-IF | IF reduces ITT-AUC for WT-M, but not females; no effect in GHRKOs; IF reduces BW in males (KO and WT), but only in KO females | Yes (and strain differences) | [45] |
| C 57BL/6J | TD.92051 for AL mice, TD.92173 for CR mice | AL or 40CR from 9 weeks age | Not measured in this study | Not measured in this study | Not measured in this study | No sexual dimorphism in response of CR to parasite infection or reproduction | No sexual dimprophism in the response to Heligmosomoides bakeri | [94] |
| G93A mice (ALS model) | Standard rodent diet (Harlan Teklad, Madison, Wisconsin; 22/5 rodent diet (W), product 8640) for AL mice. For CR mice, NIH-31/NIA fortified diet; naturally sourced diet | AL or 40% CR from 40 days until they lost 30% of their BW then they went to AL feeding (TCR, transient CR for 13-15 days) | ↓ in males, no effect in females | ↓ in males, no effect in females | Yes | Sexual dimorphism in body condition, ability to move (↓ in males), and pawgrip endurance (↑ in males) | Yes | [95] |
| GHRKO and WT mice | Lab Diet Formula 5001; naturally sourced diet | 30CR from 8 weeks age | ↑ in WT-F-CR vs. WT-F-AL; no effect in GHRKO or WT males | ↑ in WT-F-CR vs. WT-F-AL; ↑ in KO-F-CR vs. KO-F-AL; ↑ in WT-M-CR vs. AL; no effect in GHRKO males | Yes, depending on mean or maximum lifespan | ↓ BW improvements in insulin sensitivity | No | [96] |
| Ames Dwarf and normal littermates | Purina Lab Chow (Purina Mills, St. Louis, MO); naturally sourced diet | AL or 30CR | Not reported | Not reported | No, CR extends lifespan independent of sex (full curves not published) | ↓ BW with CR independent of sex or GT; ↑ total activity with CR | Yes, on fasting glucose –in normal males FBG ↓ with CR, in females NC; in KO mice, FBG ↓ with CR in males but ↑ in female KO on CR | [97] |
| C57BI/6NNia | NIH-31 or NIH-31 fortified if CR; naturally sourced diet | AL or 40CR starting at 14 weeks of age | ↑ with CR | ↑ with CR | No, but F have greater lifespan increase | ↓ BW with CR | No | [98] |
| DBA/2JNia | NIH-31 or NIH-31 fortified if CR; naturally sourced diet | AL or 40CR starting at 14 weeks of age | ↑ with CR | ↑ with CR | No, but F have greater lifespan increase | ↓ BW with CR (AL-F have different weight gain trajectory) | No | [98] |
| B6D2F1 | NIH-31 or NIH-31 fortified if CR; naturally sourced diet | AL or 40CR starting at 14 weeks of age | ↑ with CR | ↑ with CR | No, comparable increase in LS | ↓ BW with CR (AL-F have different weight gain trajectory) | No | [98] |
| B6C3F1 | NIH-31 or NIH-31 fortified if CR; naturally sourced diet | AL or 40CR starting at 14 weeks of age | ↑ with CR | ↑ with CR | No, comparable increase in LS | ↓ BW with CR | No | [98] |
| C57BL/6NNia | Emory morse diet | AL or 40CR starting at 14 weeks of age | ↑ with CR | ↑ with CR | No, but F have greater lifespan increase | ↓ BW with CR | No | [98] |
| UM-HET3 mice | Doesn’t specify but NIH-31; naturally sourced diet | AL or 40CR from 4mo age | Not measured in this study | Not measured in this study | Not measured in this study | CR retards age-related shifts in T-cell subsets CR ameloriates age related decrease in CD3 cell numbers, nut only in males | Yes, marker specific | [99] |
| C57BL/6 mice (NCTR) | NIH-31; naturally sourced diet | AL or 40CR starting at 4 wks of age | ↑ with CR | ↑ with CR | No, comparable LS extension between M&F | ↓ neoplasia with CR | Neoplasia: no sex difference but effect is stronger in F mice | [40] |
| B 6C3F1 mice (NCTR) | NIH-31; naturally sourced diet | AL or 40CR starting at 4 wks of age | ↑ with CR | ↑ with CR | No, but M have greater lifespan increase | ↓ cancer incidence: 25% and 17% lower for males and females respectively | No, but sex may influence type and incidence of cancer/neoplasm | [42] |
| C57BL/6 mice from NCTR | NIH-31 (super supplemented with vitamins for CR); naturally sourced diet | AL or 40CR from 14 weeks age | Not specified | ↑ with CR | No, but M have greater lifespan increase | Not reported | Not reported | [100] |
| 3-week old (NZB×NZW)F1 (B/W) hybrid mice | 22% protein 5% fat; 6% protein 5% fat; 22% protein 20% fat; 6% protein 20% fat | AL or 50DR | ↑ with DR for all diets when compared to the appropriate AL group; ↓ with low protein AL and high fat AL (F only) | ↑ with DR for all diets when compared to the appropriate AL group; ↑ in low protein, high fat, and low protein high fat AL groups | Only in median LS for females on LP-AL and HF-AL diets | ↓ BW with all DR diets | No | [101] |
| 3-week old DBA2/f mice | 22% protein 5% fat; 6% protein 5% fat | AL or 50DR | ↓ with DR normal protein diet; ↓DR on low protein diet; ↑ Low protein diet fed AL | ↓ with DR normal protein diet; ↓DR on low protein diet; ↑ Low protein diet fed AL | No | Not measured in this study | Not measured in this study | [101] |
| NZB mice | 22% case in as a source of protein (normal) and that designated II contained 6% protein (low) | Normal or low (6%) protein diet | Not measured in this study | Not measured in this study | Not measured in this study | ↓BW and BW gain over time with low protein diet; prevented thymic involution, splenomegaly and cell mediated immunity which develop with age | No | [76] |
| IRS1-/+ mice and controls | (Lab Diet 5053 Irradiated Pico Lab containing 3.41 kcal/g, 20.0% protein, 52.9% carbohydrates, 10.6% fat, 4.7% crude fiber, and 6.1% ash); naturally sourced diet | AL or 50CR from 3–12mo age | Not measured in this study | Not measured in this study | Not measured in this study | ↓Body temperature with CR, magnitude of change with CR is more at some times in females (but still in the same direction); female (F), but not male (M), Igf1r+/–mice display stronger hypothermic response to CR than their wildtype littermates | Only in female Igf1r+/- mice on CR, not in males or in wildtypes | [102] |
| C57Bl/6J | NIH-31-based diet (Lab Diet 5LG6); naturally sourced diet | AL or 40CR from 9 weeks age for 10 months | Not measured in this study | Not measured in this study | Not measured in this study | ↓BW, lean and fat mass with CR | No | [103] |
| ILSXISS strains | Not specified | AL or 40CR from 2–5mo age to 15–17mo age | Not measured in this study | Not measured in this study | Not measured in this study | Sexual dimorphism in the effect of DR on fat mass | Yes, maintenance of fat mass predicts survival response under CR; sex and strain specific | [32] |
| Bmal1-/- and WT C57BL/6J mice | 18% rodent diet (Harlan); naturally sourced diet | AL or 30CR from 3mo age to 5mo age | ↓ in Bmal1-/- mice ↑ in CR WT mice (not stratified by sex) | No change in Bmal1-/- mice ↑ in CR WT mice (not stratified by sex) | No | Insulin, IGF-1 and glucose measured | Not stratified by sex; no conclusion reached | [104] |
| C 57BL/6NNia mice | NIH-31, CR supplemented to same level of vitamins and minerals as AL group; naturally sourced diet | AL or 40CR | ↑ with CR | ↑ with CR | No | ↓BW with CR; ↓ incidence of dermatitis with CR; ↓ onset/incidence of many pathologies | No for BW or dermatitis; sex specific reductions in degenerations across organ systems such as eye (female), gallbladder (males) | [105] |
| Five-month-old male and female C57BL/6 mice | No specific details; assume semi-purified diet | 0.15% MetR or CD for one month | Not measured | Not measured | Not measured | ↓BW with MetR diet; ↓SAM/SAH methylation ratio in the liver in both sexes | Sex specific differences in gut microbiota | [106] |
| Male and female C57BL/6 mice | GFN diet based on AIN-93 G; semi-purified diet | A GFN diet from weaning | Not reported | Not reported | Not reported | ↓BW and fat mass with decreasing dietary protein, and in CR | Sex specific effects in the magnitude of different markers i.e. higher mTOR activation in females compared to males | [74] |
| Male and female RI line: ILS/ISS115/TejJ (115-IR) 6mo age | NIH-31; naturally sourced diet | AL, 10CR, 20CR, 40CR | ↑ with all levels of CR in females, NS effect in males on any CR dose | Trend to ↑ in CR females with 10CR and 40CR; ↑ 40CR in males only | Yes, depends on dose of CR | ↓BW and fat in CR independent of sex | No | [107] |
| Male and female RI line: ILS/ISS97/TejJ (97-RI) 6mo age | NIH-31; naturally sourced diet | AL, 40CR | ↑ with CR in females, NS in males with CR | ↑ with CR in females, trend (p = 0.061) in males with CR | Yes | ↓BW and fat in CR in males; ↓ or ↑ in BW and fat in CR females depending on time since CR was initiated | Yes, BW and fat changes in different directions depending on time in 40CR females | [107] |
| Male and female RI line: ILS/ISS98/TejJ (98-RI) 6mo age | NIH-31; naturally sourced diet | AL, 40CR | No significant difference | NS effect with CR in females, ↑ in males with CR | Yes | ↓ BW with CR independent of sex; ↑ Fat% with CR at all (female) or most (male) timepoints | Yes | [107] |
| Male and female RI line: ILS/ISS107/TejJ (107-RI) 6mo age | NIH-31; naturally sourced diet | AL, 40CR | ↑ with 40CR in females; ↓ with 40CR in males | NS effect in females on 40CR; significant ↓ in males on 40CR | Yes | ↓ BW with CR independent of sex; ↑ Fat% with CR at some timepoints (female) or ↓ Fat% at all (male) timepoints | Yes | [107] |
| Male and female C57BL/6J mice | NIH-31 irradiated; naturally sourced diet | Preweaning food restriction (by litter expansion), then AL for remaining 15mo of study | Not measured | Not measured | Not measured | ↓ BW, total fat (g) in those with preweaning food restriction | Sexually dimorphic response in fat depot response to preweaning food restriction | [108] |
| 16mo male and female mice C57BL/6Nia | Control (21% protein), low AA (7% protein, 67% restriction of all AAs compared to control), low BCAA (21% protein, 67% restriction of BCAAs compared to control) (Envigo, Madison WI); semi-purified diet | Ad libitum one of control, low AA or low BCAA diet | No effect | No effect | No | ↓ Frailty index score in males and females on low BCAA diet; ↓ cancer at necropsy in males on low BCAA diets, no effect in females; ↑ insulin sensitivity with males on low BCAA diet | Sexual dimorphism in frailty trajectories, cancer incidence at necropsy and insulin sensitivity in mice on low BCAA diets | [9] |
| Male and female mice C57BL/6Nia, from weaning | Control (21% protein), low AA (7% protein, 67% restriction of all AAs compared to control), low BCAA (21% protein, 67% restriction of BCAAs compared to control) (Envigo, Madison WI); semi-purified diet | Ad libitum one of control, low AA or low BCAA diet | Not reported | No effect in females on low BCAA diet; ↓ survival in females on low AA diet; ↑ survival on low AA and low BCAA diet in males; | Yes | ↓ Frailty index score in females on low BCAA diet; no effect on males; low AA frailty not reported; no effect of low BCAA diets on insulin sensitivity at 2.5mo age | Sexual dimorphism in frailty trajectories in mice on low BCAA diets | [9] |
| Male and female C57BL/6 mice were obtained between 60 and 65 weeks of age (Jackson Laboratory, Bar Harbor ME) | Rodent chow (18.6% protein, 44.2% carbohydrate, and 6.2% fat; Teklad Global Rodent Diet #2918, Envigo, Madison, WI). Naturally sourced diet | EOD fasting from 20 mo age | Not measured | Not measured | Not measured | Improved glucose tolerance and ↓ FBG in EOD fed mice | No | [46] |
| Male and female AKR/J mice (Jackson Laboratory #000648, Bar Harbor, ME) | Standard diet, high sucrose diet, western diet or 15CR; semi-purified diet | Ad libitum (SD, high sucrose and western diet) or 15CR | ↑ Survival for all female groups compared to SD, but only in sucrose males | ↑ Survival in high sucrose groups only | Yes, on median survival; no effect on maximal survival | ↑ Lean to fat ratio and ↓ Fat% in high sucrose and 15CR compared to AL in males | Yes, body composition measures | [109] |
| Male and female C57BL/6 mice from 10 weeks of age | 2018 Teklad Global 18% protein rodent diet (Envigo) base diet | Ad libitum, 30% CR, meal feeding (30% CR fed in 3 aliquots over the day), or diluted AL (AL food access with approx. 30% CR) | Only performed in males | Only performed in males | N/A | CR regimens improves glucose tolerance and insulin sensitivity in males independent of regimen, worse in females | Yes for glucose tolerance, insulin sensitivity and fat storage; no for insulin sensitivity; yes for body composition | [49] |
| Male and female DBA/2J mice from 10 weeks of age | 2018 Teklad Global 18% protein rodent diet (Envigo) base diet | Ad libitum, 30% CR, meal feeding (30% CR fed in 3 aliquots over the day), or diluted AL (AL food access with approx. 30% CR) | Not assessed | Not assessed | Not assessed | Improved glucose tolerance in both; insulin sensitivity only improved in females | Yes | [49] |
| Male and female UM-HET3 mice from 4-5 weeks age | Autoclaved LabDiet 5K54 (PMI Nutrition International, Brentwood, MO) | 66% –70% of the average amount eaten by ad-lib-fed mice of the same sex and age, fed once daily at 11:00 PM (light cycle 6AM–6PM) | Median LS not assessed; ↑ mean LS in DR groups | ↑ in DR groups | Effect of DR was sex dependent (p = 0.04, likelihood ratio chi-square test) | Not assessed | Not assessed | [110] |
| Male and female CByB6F1 mice from 4-5 weeks age | Autoclaved LabDiet 5K54 (PMI Nutrition International, Brentwood, MO) | 66–70% of the average amount eaten by ad-lib-fed mice of the same sex and age, fed once daily at 11:00 PM (light cycle 6AM–6PM) | Median LS not assessed; ↑ mean LS in DR groups | ↑ in DR groups | Effect of DR on maximum LS was sex dependent (p = 0.04, likelihood ratio chi-square test) | Not assessed | Not assessed | [110] |
AL, ad libitum; 10CR, 10% calorie restriction; 15CR, 15% calorie restriction; 20CR, 20% calorie restriction; 40CR, 40% calorie restriction; M, male; F, female; B6, C57BL/6; EM, emory morse; FI, frailty index; N/A, not applicable; LS, lifespan; BW, bodyweight; EOD, every other day; PR, protein restriction; SD, standard diet; FBG, fasting blood glucose; dietary restriction; LPD, low protein diet; DR-LPD, dietary restriction with the low protein diet (6% protein); DR-22P, dietary restriction using the 22% protein normal chow diet; LDLc, low density lipoprotein calculated; HDLc, high density lipoprotein calculated; ↓, decreased; ↑, increased; AL-HFD, ad libitum high fat diet; AL-CR-HFD, weight cycling with periods of AL HFD then HFD-calorie restriction; 20CR-HFD, high fat diet with 20% calorie restriction; 30CR-HFD, high fat diet with 30% calorie restriction; GFN, geometric framework; MF, meal feeding; SAM, S-adenosylmethionine; SAH, S-adenosylhomocysteine.
The first study to consider intermittent fasting as a more translatable approach also used rats of both sexes, and was the first to uncouple reduced animal size from longevity [25]. In this study, fasting for 1 day in 3 increased the life span of males and females 20% and 15%, respectively, and proved more effective than 1 in 4 or every other day fasting. Classic studies in subsequent decades on the nutritional basis [26] and physiological effects [2, 27] of DR in rats were notable in their exclusive focus on the male sex. Nonetheless, studies in which both male and female rats were included highlight important sexual dimorphisms in multiple physiological responses to DR including, but not limited to, plasma glucose and fructosamine levels, plasma triglyceride and cholesterol levels [28], and number and onset of tumors [29].
Beginning in the 1940s, researchers were studying the effects of food restriction on tumor development using mouse models. This led to the description of a number of different phenotypes modulated by DR such as body composition, insulin sensitivity and immune functions; all of which are now recognized as hallmarks of the DR response [8]. From these investigations, the mouse emerged as an important tool to study aging and age-related diseases due to many factors not limited to their physiological similarity to humans, the ease of maintaining and breeding them in the laboratory, and the availability of many inbred strains [30]. In recent years, the mouse has become the model for testing interventions for improving health and lifespan. Below we describe those studies which have an experimental design that includes both male and female mice to address modulation of lifespan and/or healthspan by DR; these studies are summarized in Table 4. It is important to note that in addition to sexual dimorphism, strain differences in the response to DR were also observed [5, 8, 31, 32], urging a cautionary approach to general translation of these findings.
3.3Sex differences in CR-mediated lifespan and healthspan improvements
Traditionally, the success of DR interventions against aging have been based on their ability to increase mean and/or maximal lifespan. Over the past decade, an additional emphasis has been placed upon the ability of such interventions to improve healthspan independent of their ability to increase lifespan. In humans, healthspan can be defined as the length of adult life during which a person maintains the capacity to perform all routine activities of daily living (dressing, bathing, eating, toileting, transferring) as well as instrumental activities of daily living (finances, shopping, transportation, food preparation, managing medications, using the telephone) [18]. In mice, although a comparable accepted definition is still lacking, healthspan could be defined as the period of life under conditions of ideal husbandry in which the mouse is able to move around, feed itself, and care for itself, for example with grooming [18]. To standardize quantification of these measures in mice a number of indices have been developed to measure mouse frailty [33, 34]. These tools are analogous to human frailty such as the Fried frailty index and the Rockwood deficit accumulation index. These include both observational and functional deficit assessments and have been validated against a number of pre-clinical outcomes. In one study, lifelong 40% CR significantly reduced frailty in male, but not female C57BL/6 mice when compared to their AL counterparts [35]. There was no effect of 40% CR on reducing frailty in DBA/2J mice, another inbred strain [35]. When started late in life, 6 months of MetR is sufficient to reduce frailty [36] in male C57BL/6Nia mice. Female data has not been reported. In another study, frailty index predicted mortality in female, but not male, 3x Tg Alzheimer’s mice [37]. Considering the relevance of these findings to lifespan/healthspan uncoupling, the underlying mechanisms of sexual dimorphism require further investigation.
One of the hallmark features of CR is the ability to delay the onset and incidence of cancer in animal models. Indeed, before McCay connected growth restriction via CR to longevity, the influence of food restriction on the growth of transplanted and spontaneous tumors was known [38]. Interestingly, there is contrasting evidence with some suggesting that the number of tumors in mice does not appear to be sex-specific [39, 40], while others do report a sexually dimorphic effect in the number and type of tumors [41, 42]. Whether this also holds true to other healthspan and ‘hallmark features of CR’ requires further investigations (Table 4).
Certainly, daily reduction in caloric intake is not the only means to achieve such beneficial outcomes. EOD feeding, ADF, IF and other fasting paradigms also demonstrate improvements in many physiological domains that overlap with CR, including lifespan extension [43, 44]. In recent years, intermittent fasting type diets have gained increased traction as they intersperse fasting with non-fasting days and potentially seem more applicable than a daily 40% CR. In this paradigm, short periods of intense energy restriction (75–100% reduced caloric intake on fasting days) followed by ‘normal’ eating on non-fasting days. A study in Growth Hormone Receptor Knock-Out (GHRKO) mice showed that IF (EOD feeding) increased lifespan in WT mice compared to control fed mice in males but not females. They also demonstrated that GHRKO mice do not respond to IF [45]. A study of late-lie IF (EOD feeding) in C57BL/6 mice (21 mo age) reported attenuation of some hallmarks of CR including improved glucose tolerance, restoration of metabolic flexibility and decreased frailty [46] in both sexes. Lifespan however was not measured. As the majority of the studies published do not include females or do not include a lifespan outcome, we exclude them from the scope of this systematic review on sexual dimorphism and refer the reader to several excellent reviews [44, 47] on this topic.
While the underlying molecular mechanisms regarding the beneficial effects of CR on improved lifespan and healthspan have been under investigation for many years, there has been less work done to disentangle the question of whether fasting time or calories is more important for the physiological benefits. Recently, fasting time has been reported to be positively associated with the effects on survival and reduced disease incidence in mice [48]. The findings in this study were recently built upon by others using a diluted AL paradigm where food is diluted with indigestible cellulose, but provided AL, leading to an approximate 30% restriction [49]. When compared with classical 30% CR and meal feeding (30% CR fed across the day in three allotments), the authors found that fasting is necessary for the CR-induced improvements in frailty and lifespan in male mice [49].
3.4Sex differences in the methionine restriction -mediated life- and health-span improvements
One of the major challenges in translating CR to humans is quite simply that most humans would find it incredibly difficult to reduce daily caloric intake by 20–40%, especially for the significant portion of their life required to increase longevity (if this is even possible in humans). To this end, interventions allowing for DR benefits without actual food restriction represent an attractive alternative. In the early 90’s Orentriech and colleagues reported that a reduction in a single amino acid, methionine, resulted in a 30% increase in lifespan of male Fischer 344 rats [50]. Notably, rats were able to eat as much as they wanted of a diet with 0.17% w/w (compared to a control diet of 0.86% methionine). On a technical note, it is important to clarify that when we use a methionine restricted diet, it is a diet restricted in sulfur amino acids (SAA) since the non-essential sulfur amino acid cysteine is absent in MetR diets. This lack of cysteine is required for the MetR phenotypes as it has been shown that cysteine blocks the effects of MetR [51]. Interestingly, the metabolic phenotype of MetR can be obtained with diets that are deprived of other amino acids such as leucine [52], although none have yet shown the same lifespan extension. Recent work has demonstrated that diets low in isoleucine or valine recapitulate the metabolic phenotype seen with MetR [53], however their effects on lifespan were not tested in this study.
The initial study describing how a MetR diet (0.1–0.15%) can increase lifespan in mice was published in 2005. When female mice were fed the MetR diet from 6 weeks of age, this resulted in an increase in maximal lifespan of 9.2% (estimated from the survival curves at the time of publication) [54]. Subsequent studies have shown that this can be replicated in males (but not shown in females) when started at 12 months of age [55]. Interestingly the metabolic phenotype of MetR is dose-dependent with there being a threshold level of methionine that abrogates the beneficial effects; however this has only been reported in males and not females [56]. Given the differential response in C57BL/6 females to CR [8] it would be incredibly interesting to see if this was also true for females on MetR. Benefits of MetR that are not sexually dimorphic (at least as currently described in wildtype mice in the literature) include reductions in bodyweight, fat mass and oxidative stress coupled with improvements in insulin sensitivity as well as changes in circulating insulin, glucose, leptin, adiponectin, IGF-1 and FGF-21. It is important to note that a number of these benefits of MetR overlap with CR despite ad libitum access to food.
Studies have also demonstrated the applicability of MetR as a treatment for different progeria syndromes including Hutchinson-Gilford progeria syndrome (HGPS) [57] and Cockayne syndrome [58]. Importantly, MetR was able to extend median lifespan in both male and female HGPS mice and had a lower mortality rate [57]. In Cockayne syndrome mice, MetR extends lifespan and improves healthspan parameters; although the study includes both male and female mice (personal communication), this study is limited in that the authors do not differentiate between male and female mice [58]. More importantly, the healthspan of these mice was improved significantly with MetR as evidenced by an amelioration of the loss of bone structure and lack of grooming behaviors, as well as improvements in aortic and skeletal muscle fibrosis [57]. This is interesting given that generally MetR is thought to reduce bone mass [59]. A recent study of young and old male and female mice noted that bone morphology is altered in an age and sex specific manner, with MetR mice having reduced bone mass. However after correcting for body size, MetR mice had no impairment in biomechanical properties [60]. This points to the role of sex steroids in the hormonal regulation of bone morphology in response to MetR [60]. Indeed, short term studies (up to 5 weeks) have demonstrated a sexual dimorphism in hormonal responses to MetR in young mice [61].
In 6-week-old male and female mice preconditioned with a western diet (WD) for 12 weeks before being switched to a WD deficient in methionine (or staying on WD) there is no sexual dimorphism in the physiological response to the MR diet in terms of bodyweight, food intake, insulin resistance/glucose homeostasis or energy expenditure [61]. However, sexual dimorphism was present in terms of plasma FGF21 levels with only males having increased levels despite increased levels of liver transcript in both sexes. Interestingly UCP1 expression was increased in gonadal WAT of MR fed male mice but not females. This suggests that in females, increased energy expenditure occurs via a FGF21/UCP1 independent mechanism [61]. Growth hormone has been implicated in the mechanistic response of MetR but studies have not investigated the sexual dimorphism (or lack thereof) [62] in these mice. Furthermore, there are sexual dimorphic tissue specific metabolic responses in Snell dwarf vs control mice fed a MetR diet have been observed. Hepatic hypotaurine being 3-fold higher in normal males versus females, a difference that was not seen in the Snell dwarf mice where the hypotaurine concentration in both sexes was comparable to the lower value found in normal females [63]. While the molecular mechanism underlying these sex differences is not known, it is plausible to attribute these differences at least in part to sex hormones. Indeed, estrogen removal in animals or menopause in women is associated with metabolic disturbances including hepatic triglyceride accumulation and decreased insulin sensitivity [64].
3.5Alterations in macronutrient contents/ratios and impact on health and lifespan
Another method to achieve some of the beneficial effects of CR without reducing caloric intake is to alter either the ratio of protein:carbohydrate:fat (P:F:C) content in the diet (termed the geometric framework GF set of diets), or by modifying a specific component such as protein (i.e. low protein diets). Short or long-term reduced protein intake is associated with many beneficial effects including metabolic outcomes [65, 66], reduced surgical complications [67, 68] and improved lifespan [69]. The commonality in these dietary interventions is that they induce a phenotype which overlaps with CR to some extent and affect CR-related pathways.
The Geometric Framework for Nutrition (GFN) is a model that was developed to investigate how nutrients, other dietary constituents and their interactions influence physiology and health. The GFN model has been used to demonstrate how organisms across many taxa possess nutrient-specific appetites, select foods, control food intake and utilize ingested nutrients to attain their intake, growth and maintenance requirements [70]. The initial study in mice examined over 25 different diets in C57BL/6 mice found that the main determinant of lifespan is carbohydrate:protein ratio and is independent of calorie intake [69]. The authors used AL diets of normal, medium and low energy density (termed caloric dilution) to ask the question if amount and/or type of nutrient is important for health outcomes in mice [69]. It is important to note that the authors do not use CR in the traditional sense, rather energy dilution in that while the mice ingested less calories at the lower energy density foods, they eat almost twice as much food mass (available ad libitum to them). Although both males and females are included in the study, the authors collapse the sexes and do not present sex specific health outcomes which is a limitation of this elaborate study. They do however present one analyses for survival using Cox regression analyses to show that the hazard ratio is generally lower for females, but there is not a sex-diet interaction apparent [69]. We would encourage the authors to present data stratified by sex even if it is included as supplemental material.
Follow up studies to this lifespan paper have investigated the effect of P:C ratio on various outcomes and found sex dependent effects of diet on fertility [71], and skin structure [72]. Interestingly prolonged fertility correlating with increased lifespan is also a feature seen in CR mice [73]. A paper published from the same group investigated a protein titration across metabolic and cognitive outcomes compared to traditional CR (20% reduction in daily calories compared to control diet, 19% protein amounts), where they present sex as an outcome variable. This study noted sex specific differences (some variables higher in males compared to females and vice versa) in insulin, cholesterol, adiponectin and bodyweight/composition however the general diet trends (i.e. elevated circulating FGF-21 with low protein) were consistent across males and females [74] suggesting that at least in the metabolic/longevity sense reducing protein content does not appear to have any sexual dimorphic effects. Interestingly, when the authors examined cognitive function, females generally performed better on Morris water maze and novel object recognition tests than males did, however the effect of diet is hard to tease out in this study. Sexual dimorphism was observed in the hippocampal gene expression of nutrient sensing pathways SIRT1, mTOR and PGC-1a with overlap in so-called pro-longevity genes/pathways between dietary interventions of CR and LPHC. These are consistent with other studies which have reported sexual dimorphism in nutrient sensing pathways [8, 61, 75] with different dietary interventions.
Although the GFN is a relatively new tool, the idea of altering macronutrient content in the diet to improve health outcomes has been around for a number of decades. Low protein diets (LPD) have been in use since the early 1970s (in mice) as a therapeutic intervention for autoimmunity and longevity. In NZB mice fed a normal (22%) or low protein (6% from casein) diet, the LPD abrogated thymic involution, and prevented development of splenomegaly. Furthermore it was able to maintained the cell-mediated immunities, antibody-producing capacity and immune functions which are known to decline with age in these mice in a sex independent manner [76]. At 24 months of age, 9.1% of females on the LPD diet were still alive compared to 0% of mice in the other three groups [76] which is consistent with the studies by Solon-Biet et al. [69] showing low protein:carbohydrate ratio is associated with better longevity. In a subsequent study, authors compared both normal and low protein diets with and without 50% DR [77]. Mice on the LPD showed decreased bodyweight, however there was no sexual dimorphism observed [77]. Interestingly, the additive effect of 50% DR on the LPD was detrimental to lifespan in both male and female DBA2/f mice, with the effect being more pronounced in the median lifespan of male DBA2/f mice [77]. Notably, this effect was strain specific as when the same regimen was repeated in F1 offspring of the NZB×NZW F1 strain, DR had a profound sex independent effect of increasing median lifespan in both a normal and LPD of 30–42%. LPD+DR also increased maximum lifespan, although the effect was not as pronounced (7% increase in LPD-DR vs 42% increase) as the effect of a normal 22% protein diet [77]. Again, the differences were not sex dependent. The authors note that in this study no advantage was found to result from lowering the calories in addition to lowering the protein intake, and calorie restriction per se did not favor prolonged survival, but only in the DBA2/f mice. Clearly, there are strain specific effects which require further investigation.
4Conclusions
Recent studies have illustrated the ability of sex to impact health and lifespan outcomes in mouse studies. However, with only 21.4% of studies including both sexes, it is apparent that there is still far to go. Here we present a systematic review of the literature on how sexual dimorphism may be modulated in response to different dietary restriction and feeding paradigms. These data illustrate the importance of including both sexes when considering translational approaches of these interventions to humans and highlights the potential of leveraging such differences to provide novel insights into the pathophysiology of the aging process itself.
Acknowledgments
This manuscript is dedicated in loving memory of Dr. Jay Mitchell. We apologize to the authors whose work is not included due to space limits.
Funding
This study was supported by the National Institute on Aging, 5R01DK090629 to J.R.M., and P01AG055369 to S.J.M.
Conflicts of interest
The authors declare no conflicts of interest.
Supplementary material
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/NHA-220162.
References
[1] | Kulminski AM , Culminskaya IV , Ukraintseva SV , Arbeev KG , Land KC , Yashin AI . Sex-Specific Health Deterioration and Mortality: The Morbidity-Mortality Paradox over Age and Time. Experimental Gerontology. (2008) ;43: (12):1052–7. |
[2] | Maeda H , Gleiser CA , Masoro EJ , Murata I , McMahan CA , Yu BP . Nutritional influences on aging of Fischer 344 rats: II. Pathology. Journal of Gerontology. (1985) ;40: (6):671–88. |
[3] | Carmody C , Duesing CG , Kane AE , Mitchell SJ . Perspective: Is Sex as a Biological Variable Still Being Ignored in Pre-Clinical Aging Research? J Gerontol A Biol Sci Med Sci. 2022. |
[4] | Clayton JA , Collins FS . Policy: NIH to balance sex in cell and animal studies. Nature. (2014) ;509: (7500):282–3. |
[5] | Fischer KE , Hoffman JM , Sloane LB , Gelfond JAL , Soto VY , Richardson AG , et al. A cross-sectional study of male and female C57BL/6Nia mice suggests lifespan and healthspan are not necessarily correlated. Aging. (2016) ;8: (10):2370–91. |
[6] | Garratt M , Nakagawa S , Simons MJP . Life-span Extension With Reduced Somatotrophic Signaling: Moderation of Aging Effect by Signal Type, Sex, and Experimental Cohort. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences. (2017) ;72: (12):1620–6. |
[7] | Liao CY , Rikke BA , Johnson TE , Diaz V , Nelson JF . Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell. (2010) ;9: (1):92–5. |
[8] | Mitchell SJ , Madrigal-Matute J , Scheibye-Knudsen M , Fang E , Aon M , Gonzalez-Reyes JA , et al. Effects of Sex, Strain, and Energy Intake on Hallmarks of Aging in Mice. Cell Metab. (2016) ;23: (6):1093–112. |
[9] | Richardson NE , Konon EN , Schuster HS , Mitchell AT , Boyle C , Rodgers AC , et al. Lifelong restriction of dietary branched-chain amino acids has sex-specific benefits for frailty and lifespan in mice. Nat Aging. (2021) ;1: (1):73–86. |
[10] | Fontana L . Interventions to promote cardiometabolic health and slow cardiovascular ageing. Nature reviews Cardiology. (2018) ;15: (9):566–77. |
[11] | Green CL , Lamming DW . Regulation of metabolic health by essential dietary amino acids. Mech Ageing Dev. 2018. |
[12] | Hanjani NA , Vafa M . Protein Restriction, Epigenetic Diet, Intermittent Fasting as New Approaches for Preventing Age-associated Diseases. International Journal of Preventive Medicine. (2018) ;9: , 58. |
[13] | Ables GP , Johnson JE . Pleiotropic responses to methionine restriction. Experimental Gerontology. (2017) ;94: , 83–8. |
[14] | Golbidi S , Daiber A , Korac B , Li H , Essop MF , Laher I . Health Benefits of Fasting and Caloric Restriction. Current Diabetes reports. (2017) ;17: (12):123. |
[15] | Picca A , Pesce V , Lezza AMS . Does eating less make you live longer and better? An update on calorie restriction. Clinical Interventions in Aging. (2017) ;12: , 1887–902. |
[16] | Green CL , Lamming DW , Fontana L . Molecular mechanisms of dietary restriction promoting health and longevity. Nature Reviews Molecular Cell Biology. (2022) ;23: (1):56–73. |
[17] | Moher D , Shamseer L , Clarke M , Ghersi D , Liberati A , Petticrew M , et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) statement. Systematic Reviews. (2015) ;4: , 1. |
[18] | Richardson A , Fischer KE , Speakman JR , de Cabo R , Mitchell SJ , Peterson CA , et al. Measures of Healthspan as Indices of Aging in Mice-A Recommendation. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences. (2016) ;71: (4):427–30. |
[19] | Bellantuono I , de Cabo R , Ehninger D , Di Germanio C , Lawrie A , Miller J , et al. A toolbox for the longitudinal assessment of healthspan in aging mice. Nat Protoc. (2020) ;15: (2):540–74. |
[20] | Shansky RM , Murphy AZ . Considering sex as a biological variable will require a global shift in science culture. Nature Neuroscience. (2021) ;24: (4):457–64. |
[21] | López-Otín C , Blasco MA , Partridge L , Serrano M , Kroemer G . The Hallmarks of Aging. Cell. (2013) ;153: (6):1194–217. |
[22] | Fontana L , Partridge L . Promoting health and longevity through diet: from model organisms to humans. Cell. (2015) ;161: (1):106–18. |
[23] | Pan H , Finkel T . Key proteins and pathways that regulate lifespan. The Journal of Biological Chemistry. (2017) ;292: (16):6452–60. |
[24] | McCay CM , Crowell MF , Maynard LA . The effect of retarded growth upon the length of life span and upon the ultimate body size. Nutrition (Burbank, Los Angeles County, Calif).155-71; discussion. (1989) ;5: (3):72. |
[25] | Carlson AJ , Hoelzel F . Apparent prolongation of the life span of rats by intermittent fasting. The Journal of Nutrition. (1946) ;31: , 363–75. |
[26] | Ross MH . Length of life and nutrition in the rat. The Journal of Nutrition. (1961) ;75: , 197–210. |
[27] | Yu BP , Masoro EJ , McMahan CA . Nutritional influences on aging of Fischer 344 rats: I. Physical, metabolic, and longevity characteristics. Journal of Gerontology. (1985) ;40: (6):657–70. |
[28] | Van Liew JB , Davis PJ , Davis FB , Bernardis LL , Deziel MR , Marinucci LN , et al. Effects of aging, diet, and sex on plasma glucose, fructosamine, and lipid concentrations in barrier-raised Fischer 344 rats. Journal of Gerontology.B. (1993) ;48: (5):184–90. |
[29] | Lipman RD , Dallal GE , Bronson RT . Effects of genotype and diet on age-related lesions in ad libitum fed and calorie-restricted F344, BN, and BNF3F1 rats. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences.B. (1999) ;54: (11):478–91. |
[30] | Perlman RL . Mouse models of human disease: An evolutionary perspective. Evolution, Medicine, and Public Health. (2016) ;2016: 1 170–6. |
[31] | Liao C-Y , Rikke BA , Johnson TE , Diaz V , Nelson JF . Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell. (2010) ;9: (1):92–5. |
[32] | Liao CY , Rikke BA , Johnson TE , Gelfond JA , Diaz V , Nelson JF . Fat maintenance is a predictor of the murine lifespan response to dietary restriction. Aging Cell. (2011) ;10: (4):629–39. |
[33] | Whitehead JC , Hildebrand BA , Sun M , Rockwood MR , Rose RA , Rockwood K , et al. A clinical frailty index in aging mice: comparisons with frailty index data in humans. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences. (2014) ;69: (6):621–32. |
[34] | Liu H , Graber TG , Ferguson-Stegall L , Thompson LV . Clinically relevant frailty index for mice. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences. (2014) ;69: (12):1485–91. |
[35] | Kane AE , Hilmer SN , Boyer D , Gavin K , Nines D , Howlett SE , et al. Impact of Longevity Interventions on a Validated Mouse Clinical Frailty Index. J Gerontol A Biol Sci Med Sci. (2016) ;71: (3):333–9. |
[36] | Schultz MB , Kane AE , Mitchell SJ , MacArthur MR , Warner E , Vogel DS , et al. Age and life expectancy clocks based on machine learning analysis of mouse frailty. Nat Commun. (2020) ;11: (1):4618. |
[37] | Kane AE , Shin S , Wong AA , Fertan E , Faustova NS , Howlett SE , et al. Sex Differences in Healthspan Predict Lifespan in the 3xTg-AD Mouse Model of Alzheimer’s Disease. Frontiers in Aging Neuroscience. (2018) ;10: :172. |
[38] | Rous P . THE INFLUENCE OF DIET ON TRANSPLANTED AND SPONTANEOUS MOUSE TUMORS. The Journal of experimental medicine. (1914) ;20: (5):433–51. |
[39] | Tucker MJ . The effect of long-term food restriction on tumours in rodents. International Journal of Cancer. (1979) ;23: (6):803–7. |
[40] | Blackwell BN , Bucci TJ , Hart RW , Turturro A . Longevity, body weight, and neoplasia in ad libitum-fed and diet-restricted C57BL6 mice fed NIH-31 open formula diet. Toxicol Pathol. (1995) ;23: (5):570–82. |
[41] | Cameron KM , Miwa S , Walker C , von Zglinicki T . Male mice retain a metabolic memory of improved glucose tolerance induced during adult onset, short-term dietary restriction. Longev Healthspan. (2012) ;1: , 3. |
[42] | Sheldon WG , Bucci TJ , Hart RW , Turturro A . Age-related neoplasia in a lifetime study of ad libitum-fed and food-restricted B6C3F1 mice. Toxicol Pathol. (1995) ;23: (4):458–76. |
[43] | Xie K , Neff F , Markert A , Rozman J , Aguilar-Pimentel JA , Amarie OV , et al. Every-other-day feeding extends lifespan but fails to delay many symptoms of aging in mice. Nature Communications. (2017) ;8: (1):155. |
[44] | Mattson MP , Longo VD , Harvie M . Impact of intermittent fasting on health and disease processes. Ageing Research Reviews. (2017) ;39: , 46–58. |
[45] | Arum O , Bonkowski MS , Rocha JS , Bartke A . The growth hormonereceptor gene-disrupted mouse fails to respond to an intermittentfasting diet. Aging Cell. (2009) ;8: (6):756–60. |
[46] | Henderson YO , Bithi N , Link C , Yang J , Schugar R , Llarena N , et al. Late-life intermittent fasting decreases aging-related frailty and increases renal hydrogen sulfide production in a sexually dimorphic manner. Geroscience. (2021) ;43: (4):1527–54. |
[47] | Longo VD , Panda S . Fasting, Circadian Rhythms, and Time-Restricted Feeding in Healthy Lifespan. Cell Metabolism. (2016) ;23: (6):1048–59. |
[48] | Mitchell SJ , Bernier M , Mattison JA , Aon MA , Kaiser TA , Anson RM , et al. Daily Fasting Improves Health and Survival in Male Mice Independent of Diet Composition and Calories. Cell Metab.221-8.e. (2019) ;29: (1):3. |
[49] | Pak HH , Haws SA , Green CL , Koller M , Lavarias MT , Richardson NE , et al. Fasting drives the metabolic, molecular and geroprotective effects of a calorie-restricted diet in mice. Nature Metabolism. (2021) ;3: (10):1327–41. |
[50] | Orentreich N , Matias JR , DeFelice A , Zimmerman JA . Low methionine ingestion by rats extends life span. The Journal of Nutrition. (1993) ;123: (2):269–74. |
[51] | Wanders D , Stone KP , Forney LA , Cortez CC , Dille KN , Simon J , et al. Role of GCN2-Independent Signaling Through a Noncanonical PERK/NRF2 Pathway in the Physiological Responses to Dietary Methionine Restriction. Diabetes. (2016) ;65: (6):1499–510. |
[52] | Lees EK , Banks R , Cook C , Hill S , Morrice N , Grant L , et al. Direct comparison of methionine restriction with leucine restriction on the metabolic health of C57BL/6J mice. Scientific Reports. (2017) ;7: (1):9977. |
[53] | Yu D , Richardson NE , Green CL , Spicer AB , Murphy ME , Flores V , et al. The adverse metabolic effects of branched-chain amino acids are mediated by isoleucine and valine. Cell Metabolism.905-22.e. (2021) ;33: (5):6. |
[54] | Miller RA , Buehner G , Chang Y , Harper JM , Sigler R , Smith-Wheelock M . Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging cell. (2005) ;4: (3):119–25. |
[55] | Sun L , Sadighi Akha AA , Miller RA , Harper JM . Life-Span Extension in Mice by Preweaning Food Restriction and by Methionine Restriction in Middle Age. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 64A. (2009) )7 711–22. |
[56] | Forney LA , Wanders D , Stone KP , Pierse A , Gettys TW . Concentration-dependent linkage of dietary methionine restriction to the components of its metabolic phenotype. Obesity. (2017) ;25: (4):730–8. |
[57] | Barcena C , Quiros PM , Durand S , Mayoral P , Rodriguez F , Caravia XM , et al. Methionine Restriction Extends Lifespan in Progeroid Mice and Alters Lipid and Bile Acid Metabolism. Cell Reports. (2018) ;24: (9):2392–403. |
[58] | Brace LE , Vose SC , Vargas DF , Zhao S , Wang XP , Mitchell JR . Lifespan extension by dietary intervention in a mouse model of Cockayne syndrome uncouples early postnatal development from segmental progeria . Aging Cell. (2013) ;12: (6):1144–7. |
[59] | Ables GP , Perrone CE , Orentreich D , Orentreich N . Methionine-restricted C57BL/6J mice are resistant to diet-induced obesity and insulin resistance but have low bone density. PloS One. (2012) ;7: (12):e51357. |
[60] | Ouattara A , Cooke D , Gopalakrishnan R , Huang T-h , Ables GP . Methionine restriction alters bone morphology and affects osteoblast differentiation. Bone Reports. (2016) ;5: , 33–42. |
[61] | Yu D , Yang SE , Miller BR , Wisinski JA , Sherman DS , Brinkman JA , et al. Short-term methionine deprivation improves metabolic health via sexually dimorphic, mTORC1-independent mechanisms. FASEB journal : Official publication of the Federation of American Societies for Experimental Biology. 2018:fj201701211R. |
[62] | Brown-Borg HM , Rakoczy SG , Wonderlich JA , Rojanathammanee L , Kopchick JJ , Armstrong V , et al. Growth hormone signaling is necessary for lifespan extension by dietary methionine. Aging Cell. (2014) ;13: (6):1019–27. |
[63] | Vitvitsky V , Martinov M , Ataullakhanov F , Miller RA , Banerjee R . Sulfur-based redox alterations in long-lived Snell dwarf mice. Mech Ageing Dev. (2013) ;134: (7-8):321–30. |
[64] | Garcia-Carrizo F , Priego T , Szostaczuk N , Palou A , Picó C . Sexual Dimorphism in the Age-Induced Insulin Resistance, Liver Steatosis, and Adipose Tissue Function in Rats. Frontiers in Physiology. (2017) ;8: , 445. |
[65] | MacArthur MR , Mitchell SJ , Treviño-Villarreal JH , Grondin Y , Reynolds JS , Kip P , et al. Total protein, not amino acid composition, differs in plant-based versus omnivorous dietary patterns and determines metabolic health effects in mice. Cell Metab. 2021. |
[66] | Green CL , Pak HH , Richardson NE , Flores V , Yu D , Tomasiewicz JL , et al. Sex and genetic background define the metabolic, physiologic,and molecular response to protein restriction. Cell Metabolism. (2022) ;34: (2):209–26.e5. |
[67] | Trocha K , Kip P , MacArthur MR , Mitchell SJ , Longchamp A , Treviño-Villarreal JH , et al. Preoperative Protein or Methionine Restriction Preserves Wound Healing and Reduces Hyperglycemia. J Surg Res. (2019) ;235: , 216–22. |
[68] | Trocha KM , Kip P , Tao M , MacArthur MR , Treviño-Villarreal JH , Longchamp A , et al. Short-term preoperative protein restriction attenuates vein graft disease via induction of cystathionine γ-lyase. Cardiovasc Res. (2020) ;116: (2):416–28. |
[69] | Solon-Biet SM , McMahon AC , Ballard JW , Ruohonen K , Wu LE , Cogger VC , et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metabolism. (2014) ;19: (3):418–30. |
[70] | Simpson SJ , Le Couteur DG , James DE , George J , Gunton JE , Solon-Biet SM , et al. The Geometric Framework for Nutrition as a tool in precision medicine. Nutrition and Healthy Aging. (2017) ;4: (3):217–26. |
[71] | Solon-Biet SM , Walters KA , Simanainen UK , McMahon AC , Ruohonen K , Ballard JW , et al. Macronutrient balance, reproductive function, and lifespan in aging mice. Proceedings of the National Academy of Sciences of the United States of America. (2015) ;112: (11):3481–6. |
[72] | Hew J , Solon-Biet SM , McMahon AC , Ruohonen K , Raubenheimer D , Ballard JW , et al. The Effects of Dietary Macronutrient Balance on Skin Structure in Aging Male and Female Mice. PloS One.e. (2016) ;11: (11):0166175. |
[73] | Selesniemi K , Lee H-J , Tilly JL . Moderate caloric restriction initiated in rodents during adulthood sustains function of the female reproductive axis into advanced chronological age. Aging Cell. (2008) ;7: (5):622–9. |
[74] | Wahl D , Solon-Biet SM , Wang QP , Wali JA , Pulpitel T , Clark X , et al. Comparing the Effects of Low-Protein and High-Carbohydrate Diets and Caloric Restriction on Brain Aging in Mice. Cell Re-43.e. (2234) ;25: (8):6. |
[75] | Boldrin L , Ross JA , Whitmore C , Doreste B , Beaver C , Eddaoudi A , et al. The effect of calorie restriction on mouse skeletal muscle is sex, strain and time-dependent. Sci Rep. (2017) ;7: (1):5160. |
[76] | Fernandes G , Yunis EJ , Good RA . Influence of protein restriction on immune functions in NZB mice. J Immunol. (1976) ;116: (3):782–90. |
[77] | Fernandes G , Yunis EJ , Good RA . Influence of diet on survival of mice. Proceedings of the National Academy of Sciences of the United States of America. (1976) ;73: (4):1279–83. |
[78] | Larson KR , Russo KA , Fang Y , Mohajerani N , Goodson ML , Ryan KK . Sex Differences in the Hormonal and Metabolic Response to Dietary Protein Dilution. Endocrinology. (2017) ;158: (10):3477–87. |
[79] | Astafev AA , Patel SA , Kondratov RV . Calorie restriction effects on circadian rhythms in gene expression are sex dependent. Sci Rep. (2017) ;7: (1):9716. |
[80] | Sadagurski M , Cady G , Miller RA . Anti-aging drugs reduce hypothalamic inflammation in a sex-specific manner. Aging Cell. (2017) ;16: (4):652–60. |
[81] | Vermeij WP , Dolle ME , Reiling E , Jaarsma D , Payan-Gomez C , Bombardieri CR , et al. Restricted diet delays accelerated ageing and genomic stress in DNA-repair-deficient mice. Nature. . (2016) ;537: (7620):427–31. |
[82] | Grant L , Lees EK , Forney LA , Mody N , Gettys T , Brown PA , et al. Methionine restriction improves renal insulin signalling in aged kidneys. Mech Ageing Dev. (2016) ;157: , 35–43. |
[83] | Piotrowska K , Tarnowski M , Zgutka K , Pawlik A . Gender Differences in Response to Prolonged Every-Other-Day Feeding on the Proliferation and Apoptosis of Hepatocytes in Mice. Nutrients. (2016) ;8: (3):176. |
[84] | Ouattara A , Cooke D , Gopalakrishnan R , Huang TH , Ables GP . Methionine restriction alters bone morphology and affects osteoblast differentiation. Bone Rep. (2016) ;5: , 33–42. |
[85] | Yu Z , Sunchu B , Fok WC , Alshaikh N , Perez VI . Gene expression in the liver of female, but not male mice treated with rapamycin resembles changes observed under dietary restriction. Springerplus. (2015) ;4: , 174. |
[86] | Grymula K , Piotrowska K , Sluczanowska-Glabowska S , Mierzejewska K , Tarnowski M , Tkacz M , et al. Positive effects of prolonged caloric restriction on the population of very small embryonic-like stem cells - hematopoietic and ovarian implications. J Ovarian Res. (2014) ;7: , 68. |
[87] | Miller RA , Harrison DE , Astle CM , Fernandez E , Flurkey K , Han M , et al. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell. (2014) ;13: (3):468–77. |
[88] | Chiba T , Tamashiro Y , Park D , Kusudo T , Fujie R , Komatsu T , et al. A key role for neuropeptide Y in lifespan extension and cancer suppression via dietary restriction. Sci Rep. (2014) ;4: , 4517. |
[89] | Arum O , Rickman DJ , Kopchick JJ , Bartke A . The slow-aging growth hormone receptor/binding protein gene-disrupted (GHR-KO) mouse is protected from aging-resultant neuromusculoskeletal frailty. Age (Dordr). (2014) ;36: (1):117–27. |
[90] | Arum O , Rasche ZA , Rickman DJ , Bartke A . Prevention of neuromusculoskeletal frailty in slow-aging ames dwarf mice: longitudinal investigation of interaction of longevity genes and caloric restriction. PLoS One. (2013) ;8: (10):e72255. |
[91] | Park JH , Glass Z , Sayed K , Michurina TV , Lazutkin A , Mineyeva O , et al. Calorie restriction alleviates the age-related decrease in neural progenitor cell division in the aging brain. Eur J Neurosci. (2013) ;37: (12):1987–93. |
[92] | Banke NH , Yan L , Pound KM , Dhar S , Reinhardt H , De Lorenzo MS , et al. Sexual dimorphism in cardiac triacylglyceride dynamics in mice on long term caloric restriction. J Mol Cell Cardiol. (2012) ;52: (3):733–40. |
[93] | Patel BP , Safdar A , Raha S , Tarnopolsky MA , Hamadeh MJ . Caloric restriction shortens lifespan through an increase in lipid peroxidation, inflammation and apoptosis in the G93A mouse, an animal model of ALS. PLoS One. (2010) ;5: (2):e9386. |
[94] | Kristan DM . Chronic calorie restriction increases susceptibility of laboratory mice (Mus musculus) to a primary intestinal parasite infection. Aging Cell. (2007) ;6: (6):817–25. |
[95] | Rocha JS , Bonkowski MS , de Franca LR , Bartke A . Effects of mild calorie restriction on reproduction, plasma parameters and hepatic gene expression in mice with altered GH/IGF-I axis. Mech Ageing Dev. (2007) ;128: (4):317–31. |
[96] | Bonkowski MS , Rocha JS , Masternak MM , Al Regaiey KA , Bartke A . Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc Natl Acad Sci U S A. (2006) ;103: (20):7901–5. |
[97] | Mattison JA , Wright C , Bronson RT , Roth GS , Ingram DK , Bartke A . Studies of aging in ames dwarf mice: Effects of caloric restriction. J Am Aging Assoc. (2000) ;23: (1):9–16. |
[98] | Turturro A , Witt WW , Lewis S , Hass BS , Lipman RD , Hart RW . Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci.B. (1999) ;54: (11):492–501. |
[99] | Miller RA . Age-related changes in T cell surface markers: a longitudinal analysis in genetically heterogeneous mice. Mech Ageing Dev. (1997) ;96: (1-3):181–96. |
[100] | Peng S , Tilley R , Srivastava V , Hart R , Busbee D . Mitogen-activation of spleen cells in aged animals is potentiated by dietary restriction: a preliminary report. Mech Ageing Dev. (1990) ;52: (1):71–8. |
[101] | Fernandes G , Yunis EJ , Good RA . Influence of diet on survival of mice. Proc Natl Acad Sci U S A. (1976) ;73: (4):1279–83. |
[102] | Cintron-Colon R , Sanchez-Alavez M , Nguyen W , Mori S , Gonzalez-Rivera R , Lien T , et al. Insulin-like growth factor 1 receptor regulateshypothermia during calorie restriction. Proc Natl Acad Sci U S A. (2017) ;114: (36):9731–6. |
[103] | Gibbs VK , Brewer RA , Miyasaki ND , Patki A , Smith DL , Jr . Sex-dependent Differences in Liver and Gut Metabolomic Profiles With Acarbose and Calorie Restriction in C57BL/6 Mice. J Gerontol A Biol Sci Med Sci. (2018) ;73: (2):157–65. |
[104] | Patel SA , Chaudhari A , Gupta R , Velingkaar N , Kondratov RV . Circadian clocks govern calorie restriction-mediated life span extension through BMAL1- and IGF-1-dependent mechanisms. Faseb j. (2016) ;30: (4):1634–42. |
[105] | Turturro A , Duffy P , Hass B , Kodell R , Hart R . Survival characteristics and age-adjusted disease incidences in C57BL/6 mice fed a commonly used cereal-based diet modulated by dietary restriction. J Gerontol A Biol Sci Med Sci. (2002) ;57: (11):B379–89. |
[106] | Wallis KF , Melnyk SB , Miousse IR . Sex-Specific Effects of Dietary Methionine Restriction on the Intestinal Microbiome. Nutrients. (2020) ;12: (3). |
[107] | Unnikrishnan A , Matyi S , Garrett K , Ranjo-Bishop M , Allison DB , Ejima K , et al. Reevaluation of the effect of dietary restriction on different recombinant inbred lines of male and female mice. Aging Cell. (2021) ;20: (11):e13500. |
[108] | Kurup K , Mann SN , Jackson J , Matyi S , Ranjo-Bishop M , Freeman WM , et al. Litter expansion alters metabolic homeostasis in a sex specific manner. PLoS One. (2021) ;16: (9):e0237199. |
[109] | Palliyaguru DL , Rudderow AL , Sossong AM , Lewis KN , Younts C , Pearson KJ , et al. Perinatal diet influences health and survival in a mouse model of leukemia. Geroscience. (2020) ;42: (4):1147–55. |
[110] | Flurkey K , Astle CM , Harrison DE . Life extension by diet restriction and N-acetyl-L-cysteine in genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. (2010) ;65: (12):1275–84. |
[111] | Formoso-Rafferty N , Cervantes I , Sanchez JP , Gutierrez JP , Bodin L . Effect of feed restriction on the environmental variability of birth weight in divergently selected lines of mice. Genet Sel Evol. (2019) ;51: (1):27. |
[112] | Assali DR , Hsu CT , Gunapala KM , Aguayo A , McBurney M , Steele AD . Food anticipatory activity on a calorie-restricted diet is independent of Sirt1. PLoS One. (2018) ;13: (6):e0199586. |
[113] | Muller C , Zidek LM , Ackermann T , de Jong T , Liu P , Kliche V , et al. Reduced expression of C/EBPbeta-LIP extends health and lifespan in mice. Elife. 2018;7 |
[114] | Bielas J , Herbst A , Widjaja K , Hui J , Aiken JM , McKenzie D , et al. Long term rapamycin treatment improves mitochondrial DNA quality in aging mice. Exp Gerontol. (2018) ;106: , 125–31. |
[115] | Dommerholt MB , Dionne DA , Hutchinson DF , Kruit JK , Johnson JD . Metabolic effects of short-term caloric restriction in mice with reduced insulin gene dosage. J Endocrinol. (2018) ;237: (1):59–71. |
[116] | Martinez-Lopez N , Tarabra E , Toledo M , Garcia-Macia M , Sahu S , Coletto L , et al. System-wide Benefits of Intermeal Fasting byAutophagy. Cell Metab. (2017) ;26: (6):856–71.e5. |
[117] | Majtan T , Hulkova H , Park I , Krijt J , Kozich V , Bublil EM , et al. Enzyme replacement prevents neonatal death, liver damage, and osteoporosis in murine homocystinuria. Faseb j. (2017) ;31: (12):5495–506. |
[118] | Charles KN , Li MD , Engin F , Arruda AP , Inouye K , Hotamisligil GS . Uncoupling of Metabolic Health from Longevity through Genetic Alteration of Adipose Tissue Lipid-Binding Proteins. Cell Rep. (2017) ;21: (2):393–402. |
[119] | Zainabadi K , Liu CJ , Caldwell ALM , Guarente L . SIRT1 is a positive regulator of in vivo bone mass and a therapeutic target for osteoporosis. PLoS One. (2017) ;12: (9):e0185236. |
[120] | Maegawa S , Lu Y , Tahara T , Lee JT , Madzo J , Liang S , et al. Caloric restriction delays age-related methylation drift. Nat Commun. (2017) ;8: (1):539. |
[121] | Fujii N , Narita T , Okita N , Kobayashi M , Furuta Y , Chujo Y , et al. Sterol regulatory element-binding protein-1c orchestrates metabolic remodeling of white adipose tissue by caloric restriction. Aging Cell. (2017) ;16: (3):508–17. |
[122] | Wang T , Tsui B , Kreisberg JF , Robertson NA , Gross AM , Yu MK , et al. Epigenetic aging signatures in mice livers are slowed by dwarfism, calorie restriction and rapamycin treatment. Genome Biol. (2017) ;18: (1):57. |
[123] | Cole JJ , Robertson NA , Rather MI , Thomson JP , McBryan T , Sproul D , et al. Diverse interventions that extend mouse lifespan suppress shared age-associated epigenetic changes at critical gene regulatory regions. Genome Biol. (2017) ;18: (1):58. |
[124] | Pietrocola F , Demont Y , Castoldi F , Enot D , Durand S , Semeraro M , et al. Metabolic effects of fasting on human and mouse blood in vivo . Autophagy. (2017) ;13: (3):567–78. |
[125] | Shen J , Landis GN , Tower J . Multiple Metazoan Life-span Interventions Exhibit a Sex-specific Strehler-Mildvan Inverse Relationship Between Initial Mortality Rate and Age-dependent Mortality Rate Acceleration. J Gerontol A Biol Sci Med Sci. (2017) ;72: (1):44–53. |
[126] | Moreno CL , Yang L , Dacks PA , Isoda F , Deursen JM , Mobbs CV . Role of Hypothalamic Creb-Binding Protein in Obesity and Molecular Reprogramming of Metabolic Substrates. PLoS One. (2016) ;11: (11):e0166381. |
[127] | Mani BK , Osborne-Lawrence S , Vijayaraghavan P , Hepler C , Zigman JM . beta1-Adrenergic receptor deficiency in ghrelin-expressing cells causes hypoglycemia in susceptible individuals. J Clin Invest. (2016) ;126: (9):3467–78. |
[128] | Dance A . Live fast, die young. Nature. (2016) ;535: (7612):453–5. |
[129] | Gonzalez PN , Gasperowicz M , Barbeito-Andres J , Klenin N , Cross JC , Hallgrimsson B . Chronic Protein Restriction in Mice Impacts Placental Function and Maternal Body Weight before Fetal Growth. PLoS One. (2016) ;11: (3):e0152227. |
[130] | Koopman JJ , van Heemst D , van Bodegom D , Bonkowski MS , Sun LY , Bartke A . Measuring aging rates of mice subjected to caloric restriction and genetic disruption of growth hormone signaling. Aging (Albany NY). (2016) ;8: (3):539–46. |
[131] | Victoria B , Dhahbi JM , Nunez Lopez YO , Spinel L , Atamna H , Spindler SR , et al. Circulating microRNA signature of genotype-by-age interactions in the long-lived Ames dwarf mouse. Aging Cell. (2015) ;14: (6):1055–66. |
[132] | Walsh ME , Sloane LB , Fischer KE , Austad SN , Richardson A , Van Remmen H . Use of Nerve Conduction Velocity to Assess Peripheral Nerve Health in Aging Mice. J Gerontol A Biol Sci Med Sci. (2015) ;70: (11):1312–9. |
[133] | Mitchell SE , Delville C , Konstantopedos P , Hurst J , Derous D , Green C , et al. The effects of graded levels of calorie restriction: II. Impact of short term calorie and protein restriction on circulating hormone levels, glucose homeostasis and oxidative stress in male C57BL/6 mice. Oncotarget. (2015) ;6: (27):23213–37. |
[134] | Herbas MS , Shichiri M , Ishida N , Kume A , Hagihara Y , Yoshida Y , et al. Probucol-Induced alpha-Tocopherol Deficiency Protects Mice against Malaria Infection. PLoS One. (2015) ;10: (8):e0136014. |
[135] | Shimokawa I , Komatsu T , Hayashi N , Kim SE , Kawata T , Park S , et al. The life-extending effect of dietary restriction requires Foxo3 in mice. Aging Cell. (2015) ;14: (4):707–9. |
[136] | Hou C , Amunugama K . On the complex relationship between energy expenditure and longevity: Reconciling the contradictory empirical results with a simple theoretical model. Mech Ageing Dev. (2015) ;149: , 50–64. |
[137] | Aveleira CA , Botelho M , Carmo-Silva S , Pascoal JF , Ferreira-Marques M , Nobrega C , et al. Neuropeptide Y stimulates autophagy in hypothalamic neurons. Proc Natl Acad Sci U S A.E. (2015) ;112: (13):1642–51. |
[138] | Youm YH , Nguyen KY , Grant RW , Goldberg EL , Bodogai M , Kim D , et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. (2015) ;21: (3):263–9. |
[139] | Hine C , Harputlugil E , Zhang Y , Ruckenstuhl C , Lee BC , Brace L , et al. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell. (2015) ;160: (1-2):132–44. |
[140] | Li W , Li X , Miller RA . ATF4 activity: a common feature shared by many kinds of slow-aging mice. Aging Cell. (2014) ;13: (6):1012–8. |
[141] | Ramsey JJ , Tran D , Giorgio M , Griffey SM , Koehne A , Laing ST , et al. The influence of Shc proteins on life span in mice. J Gerontol A Biol Sci Med Sci. (2014) ;69: (10):1177–85. |
[142] | Arum O , Saleh JK , Boparai RK , Kopchick JJ , Khardori RK , Bartke A . Preservation of blood glucose homeostasis in slow-senescing somatotrophism-deficient mice subjected to intermittent fasting begun at middle or old age. Age (Dordr). (2014) ;36: (3):9651. |
[143] | Sadagurski M , Landeryou T , Blandino-Rosano M , Cady G , Elghazi L , Meister D , et al. Long-lived crowded-litter mice exhibit lasting effects on insulin sensitivity and energy homeostasis. Am J Physiol Endocrinol Metab.E. (2014) ;306: (11):1305–14. |
[144] | Rieger J , Bahr O , Maurer GD , Hattingen E , Franz K , Brucker D , et al. ERGO: a pilot study of ketogenic diet in recurrent glioblastoma. IntJ Oncol. (2014) ;44: (6):1843–52. |
[145] | Belluscio LM , Berardino BG , Ferroni NM , Ceruti JM , Canepa ET . Early protein malnutrition negatively impacts physical growth and neurological reflexes and evokes anxiety and depressive-like behaviors. Physiol Behav. (2014) ;129: , 237–54. |
[146] | Weimer S , Priebs J , Kuhlow D , Groth M , Priebe S , Mansfeld J , et al. D-Glucosamine supplementation extends life span of nematodes and of ageing mice. Nat Commun. (2014) ;5: , 3563. |
[147] | Colman RJ , Beasley TM , Kemnitz JW , Johnson SC , Weindruch R , Anderson RM . Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat Commun. (2014) ;5: , 3557. |
[148] | Cai W , Uribarri J , Zhu L , Chen X , Swamy S , Zhao Z , et al. Oral glycotoxins are a modifiable cause of dementia and the metabolic syndrome in mice and humans. Proc Natl Acad Sci U S A. (2014) ;111: (13):4940–5. |
[149] | Levine ME , Suarez JA , Brandhorst S , Balasubramanian P , Cheng CW , Madia F , et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. (2014) ;19: (3):407–17. |
[150] | Westbrook R , Bonkowski MS , Arum O , Strader AD , Bartke A . Metabolic alterations due to caloric restriction and every other day feeding in normal and growth hormone receptor knockout mice. J Gerontol A Biol Sci Med Sci. (2014) ;69: (1):25–33. |
[151] | Brown-Borg HM , Rakoczy S . Metabolic adaptations to short-term every-other-day feeding in long-living Ames dwarf mice. Exp Gerontol. (2013) ;48: (9):905–19. |
[152] | Jove M , Ayala V , Ramirez-Nunez O , Naudi A , Cabre R , Spickett CM , et al. Specific lipidome signatures in central nervous system from methionine-restricted mice. J Proteome Res. (2013) ;12: (6):2679–89. |
[153] | Sandri M , Barberi L , Bijlsma AY , Blaauw B , Dyar KA , Milan G , et al. Signalling pathways regulating muscle mass in ageing skeletal muscle: the role of the IGF1-Akt-mTOR-FoxO pathway. Biogerontology. (2013) ;14: (3):303–23. |
[154] | Graff J , Kahn M , Samiei A , Gao J , Ota KT , Rei D , et al. A dietary regimen of caloric restriction or pharmacological activation of SIRT1 to delay the onset of neurodegeneration. J Neurosci. (2013) ;33: (21):8951–60. |
[155] | Huffman DM , Augenlicht LH , Zhang X , Lofrese JJ , Atzmon G , Chamberland JP , et al. Abdominal obesity, independent from caloric intake, accounts for the development of intestinal tumors in ApcN/+) female mice. Cancer Prev Res (Phila). (2013) ;6: (3):177–87. |
[156] | Ignatenko NA , Gerner EW . Get the fat out! Cancer Prev Res (Phila) ((2013) ;6: (3):161–4. |
[157] | Livi CB , Hardman RL , Christy BA , Dodds SG , Jones D , Williams C , et al. Rapamycin extends life span of Rb1+/- mice by inhibiting neuroendocrine tumors. Aging (Albany NY). (2013) ;5: (2):100–10. |
[158] | Lapierre LR , De Magalhaes Filho CD , McQuary PR , Chu CC , Visvikis O , Chang JT , et al. The TFEB orthologue HLH-30 regulates autophagy and modulates longevity in Caenorhabditis elegans. Nat Commun. (2013) ;4: , 2267. |
[159] | Fahlstrom A , Zeberg H , Ulfhake B . Changes in behaviors of male C57BL/6J mice across adult life span and effects of dietary restriction. Age (Dordr). (2012) ;34: (6):1435–52. |
[160] | Kenyon C . Could a hormone point the way to life extension? Elife. (2012) ;1: , e00286. |
[161] | Zhang Y , Xie Y , Berglund ED , Coate KC , He TT , Katafuchi T , et al. The starvation hormone, fibroblast growth factor-21, extendslifespan in mice. Elife. (2012) ;1: , e00065. |
[162] | Comas M , Toshkov I , Kuropatwinski KK , Chernova OB , Polinsky A , Blagosklonny MV , et al. New nanoformulation of rapamycin Rapatar extends lifespan in homozygous p53-/- mice by delaying carcinogenesis. Aging (Albany NY). (2012) ;4: (10):715–22. |
[163] | Steinbaugh MJ , Sun LY , Bartke A , Miller RA . Activation of genes involved in xenobiotic metabolism is a shared signature of mouse models with extended lifespan. Am J Physiol Endocrinol Metab.E. (2012) ;303: (4):488–95. |
[164] | Yilmaz OH , Katajisto P , Lamming DW , Gultekin Y , Bauer-Rowe KE , Sengupta S , et al. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature. (2012) ;486: (7404):490–5. |
[165] | Gesing A , Masternak MM , Wang F , Karbownik-Lewinska M , Bartke A . Deletion of growth hormone receptor gene but not visceral fatremoval decreases expression of apoptosis-related genes in thekidney-potential mechanism of lifespan extension. Age (Dordr). (2012) ;34: (2):295–304. |
[166] | Aires DJ , Rockwell G , Wang T , Frontera J , Wick J , Wang W , et al. Potentiation of dietary restriction-induced lifespan extension by polyphenols. Biochim Biophys Acta. (2012) ;1822: (4). 522–6. |
[167] | Lamming DW , Ye L , Katajisto P , Goncalves MD , Saitoh M , Stevens DM , et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. (2012) ;335: (6076).1638–43. |
[168] | McCaskey SJ , Rondini EA , Langohr IM , Fenton JI . Differential effects of energy balance on experimentally-induced colitis. World J Gastroenterol. (2012) ;18: (7):627–36. |
[169] | Soare A , Cangemi R , Omodei D , Holloszy JO , Fontana L . Long-term calorie restriction, but not endurance exercise, lowers core body temperature in humans. Aging (Albany NY). (2011) ;3: (4):374–9. |
[170] | Bruss MD , Thompson AC , Aggarwal I , Khambatta CF , Hellerstein MK . The effects of physiological adaptations to calorie restriction on global cell proliferation rates. Am J Physiol Endocrinol Metab.E. (2011) ;300: (4):735–45. |
[171] | Wang Q , Huang J , Zhang X , Wu B , Liu X , Shen Z . The spatial association of gene expression evolves from synchrony to asynchrony and stochasticity with age. PLoS One. (2011) ;6: (9):e24076. |
[172] | Hernandez-Corbacho MJ , Jenkins RW , Clarke CJ , Hannun YA , Obeid LM , Snider AJ , et al. Accumulation of long-chain glycosphingolipids during aging is prevented by caloric restriction. PLoS One. (2011) ;6: (6):e20411. |
[173] | Anisimov VN , Piskunova TS , Popovich IG , Zabezhinski MA , Tyndyk ML , Egormin PA , et al. Gender differences in metformin effect on aging, life span and spontaneous tumorigenesis in 129/Sv mice. Aging (Albany NY). (2010) ;2: (12):945–58. |
[174] | Shelton LM , Huysentruyt LC , Seyfried TN . Glutamine targeting inhibits systemic metastasis in the VM-M3 murine tumor model. Int J Cancer. (2010) ;127: (10):2478–85. |
[175] | Rikke BA , Liao CY , McQueen MB , Nelson JF , Johnson TE . Genetic dissection of dietary restriction in mice supports the metabolic efficiency model of life extension. Exp Gerontol. (2010) ;45: (9):691–701. |
[176] | Dietrich MO , Antunes C , Geliang G , Liu ZW , Borok E , Nie Y , et al. Agrp neurons mediate Sirt1’s action on the melanocortin system and energy balance: roles for Sirt1 in neuronal firing and synaptic plasticity. J Neurosci. (2010) ;30: (35):11815–25. |
[177] | Satoh A , Brace CS , Ben-Josef G , West T , Wozniak DF , Holtzman DM , et al. SIRT1 promotes the central adaptive response to diet restriction through activation of the dorsomedial and lateral nuclei of the hypothalamus. J Neurosci. (2010) ;30: (30):10220–32. |
[178] | Austad S . Recent advances in vertebrate aging research Aging Cell ((2010) ;9: (3):297–303. |
[179] | Mattson MP . Genes and behavior interact to determine mortality in mice when food is scarce and competition fierce. Aging Cell. (2010) ;9: (3):448–9; discussion 50-2. |
[180] | Manuel-Apolinar L , Zarate A , Rocha L , Hernandez M . Fetal malnutrition affects hypothalamic leptin receptor expression after birth in male mice. Arch Med Res. (2010) ;41: (4):240–5. |
[181] | Tocchetti A , Soppo CB , Zani F , Bianchi F , Gagliani MC , Pozzi B , et al. Loss of the actin remodeler Eps8 causes intestinal defects and improved metabolic status in mice. PLoS One. (2010) ;5: (3):e9468. |
[182] | Chen JH , Tarry-Adkins JL , Heppolette CA , Palmer DB , Ozanne SE . Early-life nutrition influences thymic growth in male mice that may be related to the regulation of longevity. Clin Sci (Lond). (2009) ;118: (6):429–38. |
[183] | Vlassara H , Uribarri J , Ferrucci L , Cai W , Torreggiani M , Post JB , et al. Identifying advanced glycation end products as a major sourceof oxidants in aging: implications for the management and/orprevention of reduced renal function in elderly persons. SeminNephrol. (2009) ;29: (6):594–603. |
[184] | Selman C , Tullet JM , Wieser D , Irvine E , Lingard SJ , Choudhury AI , et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. . (2009) ;326: (5949):140–4. |
[185] | Kaeberlein M , Kapahi P . Cell signaling. Aging is RSKy business. Science. . (2009) ;326: (5949):55–6. |
[186] | Partadiredja G , Worrall S , Bedi KS . Early life undernutrition alters the level of reduced glutathione but not the activity levels of reactive oxygen species enzymes or lipid peroxidation in the mouse forebrain. Brain Res. (2009) ;1285: 22–9. |
[187] | Zou S , Carey JR , Liedo P , Ingram DK , Muller HG , Wang JL , et al. The prolongevity effect of resveratrol depends on dietary composition and calorie intake in a tephritid fruit fly. Exp Gerontol. (2009) ;44: (6-7):472–6. |
[188] | Covington MD , Arrington DD , Schnellmann RG . Calpain 10 is required for cell viability and is decreased in the aging kidney. Am J Physiol Renal Physiol. (2009) ;296: (3):F478–86. |
[189] | Chiba T , Inoue D , Mizuno A , Komatsu T , Fujita S , Kubota H , et al. Identification and characterization of an insulin receptor substrate 4-interacting protein in rat brain: implications for longevity. Neurobiol Aging. (2009) ;30: (3):474–82 . |
[190] | Bokov AF , Ko D , Richardson A . The effect of gonadectomy and estradiol on sensitivity to oxidative stress. Endocr Res. (2009) ;34: (1-2):43–58. |
[191] | Estep PW , 3rd, Warner JB , Bulyk ML . Short-term calorie restriction in male mice feminizes gene expression and alters key regulators of conserved aging regulatory pathways. PLoS One. (2009) ;4: (4):e5242. |
[192] | Chen JH , Martin-Gronert MS , Tarry-Adkins J , Ozanne SE . Maternal protein restriction affects postnatal growth and the expression of key proteins involved in lifespan regulation in mice. PLoS One. (2009) ;4: (3):e4950. |
[193] | Lanza IR , Short DK , Short KR , Raghavakaimal S , Basu R , Joyner MJ , et al. Endurance exercise as a countermeasure for aging. Diabetes. (2008) ;57: (11):2933–42. |
[194] | Selesniemi K , Lee HJ , Tilly JL . Moderate caloric restriction initiated in rodents during adulthood sustains function of the female reproductive axis into advanced chronological age. Aging Cell. (2008) ;7: (5):622–9. |
[195] | Cai W , He JC , Zhu L , Chen X , Zheng F , Striker GE , et al. Oral glycotoxins determine the effects of calorie restriction on oxidant stress, age-related diseases, and lifespan. Am J Pathol. (2008) ;173: (2):327–36. |
[196] | Sharov AA , Falco G , Piao Y , Poosala S , Becker KG , Zonderman AB , et al. Effects of aging and calorie restriction on the global gene expression profiles of mouse testis and ovary. BMC Biol. (2008) ;6: , 24. |
[197] | Swindell WR . Comparative analysis of microarray data identifies common responses to caloric restriction among mouse tissues. Mech Ageing Dev. (2008) ;129: (3):138–53. |
[198] | Ertl RP , Chen J , Astle CM , Duffy TM , Harrison DE . Effects of dietary restriction on hematopoietic stem-cell aging are genetically regulated. Blood. (2008) ;111: (3):1709–16. |
[199] | Swindell WR . Gene expression profiling of long-lived dwarf mice: longevity-associated genes and relationships with diet, gender and aging. BMC Genomics. (2007) ;8: , 353. |
[200] | Hirabayashi Y , Inoue T . Implications of hemopoietic progenitor cell kinetics and experimental leukemogenesis: Relevance to Gompertzean mortality as possible hematotoxicological endpoint. Exp Hematol. (2007) ;35: (4 Suppl 1):125–33. |
[201] | Burger JM , Hwangbo DS , Corby-Harris V , Promislow DE . The functional costs and benefits of dietary restriction in Drosophila. Aging Cell. (2007) ;6: (1):63–71. |
[202] | Al-Regaiey KA , Masternak MM , Bonkowski MS , Panici JA , Kopchick JJ , Bartke A . Effects of caloric restriction and growth hormone resistance on insulin-related intermediates in the skeletal muscle. J Gerontol A Biol Sci Med Sci. (2007) ;62: (1):18–26. |
[203] | Conti B , Sanchez-Alavez M , Winsky-Sommerer R , Morale MC , Lucero J , Brownell S , et al. Transgenic mice with a reduced core body temperature have an increased life span. Science. . (2006) ;314: (5800):825–8. |
[204] | Hernandez-Valencia M , Patti ME . A thin phenotype is protective for impaired glucose tolerance and related to low birth weight in mice. Arch Med Res. (2006) ;37: (7):813–7. |
[205] | Harper JM , Salmon AB , Chang Y , Bonkowski M , Bartke A , Miller RA . Stress resistance and aging: influence of genes and nutrition. Mech Ageing Dev. (2006) ;127: (8):687–94. |
[206] | Wijnhoven SW , Beems RB , Roodbergen M , van den Berg J , Lohman PH , Diderich K , et al. Accelerated aging pathology in ad libitum fed Xpd(TTD) mice is accompanied by features suggestive of caloric restriction. DNA Repair (Amst). (2005) ;4: (11):1314–24. |
[207] | Selby PB , Earhart VS , Raymer GD . The influence of dominant lethal mutations on litter size and body weight and the consequent impact on transgenerational carcinogenesis. Mutat Res. (2005) ;578: (1-2):382–94. |
[208] | Hamadeh MJ , Rodriguez MC , Kaczor JJ , Tarnopolsky MA . Caloric restriction transiently improves motor performance but hastens clinical onset of disease in the Cu/Zn-superoxide dismutase mutant G93A mouse. Muscle Nerve. (2005) ;31: (2):214–20. |
[209] | Boylston WH , Gerstner A , DeFord JH , Madsen M , Flurkey K , Harrison DE , et al. Altered cholesterologenic and lipogenic transcriptional profile in livers of aging Snell dwarf (Pit1dw/dwJ) mice. Aging Cell. (2004) ;3: (5):283–96. |
[210] | Bauer JH , Goupil S , Garber GB , Helfand SL . An accelerated assay for the identification of lifespan-extending interventions in Drosophila melanogaster. Proc Natl Acad Sci U S A. (2004) ;101: (35):12980–5. |
[211] | Masternak MM , Al-Regaiey K , Bonkowski MS , Panici J , Sun L , Wang J , et al. Divergent effects of caloric restriction on gene expression in normal and long-lived mice. J Gerontol A Biol Sci Med Sci. (2004) ;59: (8):784–8. |
[212] | Tsuchiya T , Dhahbi JM , Cui X , Mote PL , Bartke A , Spindler SR . . Additive regulation of hepatic gene expression by dwarfism and caloric restriction. Physiol Genomics. (2004) ;17: (3):307–15. |
[213] | Gybina AA , Prohaska JR . Increased rat brain cytochrome c correlates with degree of perinatal copper deficiency rather than apoptosis. J Nutr. (2003) ;133: (11):3361–8. |
[214] | Ikeno Y , Bronson RT , Hubbard GB , Lee S , Bartke A . Delayed occurrence of fatal neoplastic diseases in ames dwarf mice: correlation to extended longevity. J Gerontol A Biol Sci Med Sci. (2003) ;58: (4):291–6. |
[215] | Forster MJ , Morris P , Sohal RS . Genotype and age influence the effect of caloric intake on mortality in mice. Faseb j. (2003) ;17: (6):690–2. |
[216] | Combs TP , Berg AH , Rajala MW , Klebanov S , Iyengar P , Jimenez-Chillaron JC , et al. Sexual differentiation, pregnancy, calorie restriction, and aging affect the adipocyte-specific secretory protein adiponectin. Diabetes. (2003) ;52: (2):268–76. |
[217] | Bluher M , Kahn BB , Kahn CR . Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. (2003) ;299: (5606):572–4. |
[218] | Miller RA , Harper JM , Galecki A , Burke DT . Big mice die young: earlylife body weight predicts longevity in genetically heterogeneousmice. Aging Cell. (2002) ;1: (1):22–9. |
[219] | Hauck SJ , Aaron JM , Wright C , Kopchick JJ , Bartke A . Antioxidant enzymes, free-radical damage, and response to paraquat in liver and kidney of long-living growth hormone receptor/binding protein gene-disrupted mice. Horm Metab Res. (2002) ;34: (9):481–6. |
[220] | Rollo CD . Growth negatively impacts the life span of mammals. Evol Dev. (2002) ;4: (1):55–61. |
[221] | Greene AE , Todorova MT , McGowan R , Seyfried TN . Caloric restriction inhibits seizure susceptibility in epileptic EL mice by reducing blood glucose. Epilepsia. (2001) ;42: (11):1371–8. |
[222] | Hamilton ML , Van Remmen H , Drake JA , Yang H , Guo ZM , Kewitt K , et al. Does oxidative damage to DNA increase with age? Proc Natl Acad Sci U S A ((2001) ;98: (18):10469–74. |
[223] | Pompei F , Polkanov M , Wilson R . Age distribution of cancer in mice: the incidence turnover at old age. Toxicol Ind Health. (2001) ;17: (1):7–16. |
[224] | Ramsey JJ , Colman RJ , Binkley NC , Christensen JD , Gresl TA , Kemnitz JW , et al. Dietary restriction and aging in rhesus monkeys: the University of Wisconsin study. Exp Gerontol. (2000) ;35: (9-10):1131–49. |
[225] | Burks DJ , Font de Mora J , Schubert M , Withers DJ , Myers MG , Towery HH , et al. IRS-2 pathways integrate female reproduction and energy homeostasis. Nature. (2000) ;407: (6802):377–82. |
[226] | Scrofano MM , Shang F , Nowell TR Jr , Gong X , Smith DE , Kelliher M , et al. Calorie restriction, stress and the ubiquitin-dependent pathway in mouse livers. Mech Ageing Dev. (1998) ;105: (3):273–90. |
[227] | Scrofano MM , Shang F , Nowell TR Jr . Gong X , Smith DE , Kelliher M , et al. Aging, calorie restriction and ubiquitin-dependent proteolysis in the livers of Emory mice. Mech Ageing Dev. (1998) ;101: (3):277–96. |
[228] | Gong X , Shang F , Obin M , Palmer H , Scrofano MM , Jahngen-Hodge J , et al. Antioxidant enzyme activities in lens, liver and kidney of calorie restricted Emory mice. Mech Ageing Dev. (1997) ;99: (3):181–92. |
[229] | Sell DR , Monnier VM . Age-related association of tail tendon break time with tissue pentosidine in DBA/2 vs C57BL/6 mice: the effect of dietary restriction. J Gerontol A Biol Sci Med Sci. (1997) ;52: (5):B277–84. |
[230] | Effect of Dietary Restriction on Toxicology and Carcinogenesis Studies in F344/N Rats and B6C3F1 Mice. Natl Toxicol Program Tech Rep Ser. (1997) ;460: , 1–414. |
[231] | Stoll S , Hafner U , Kranzlin B , Muller WE . Chronic treatment of Syrian hamsters with low-dose selegiline increases life span in females but not males. Neurobiol Aging. (1997) ;18: (2):205–11. |
[232] | Duffy PH , Leakey JE , Pipkin JL , Turturro A , Hart RW . The physiologic, neurologic, and behavioral effects of caloric restriction related to aging, disease, and environmental factors. Environ Res. (1997) ;73: (1-2):242–8. |
[233] | Sprott RL . Diet and calorie restriction. Exp Gerontol. (1997) ;32: (1-2):205–14. |
[234] | Miller RA , Bookstein F , Van der Meulen J , Engle S , Kim J , Mullins L , et al. Candidate biomarkers of aging: age-sensitive indices of immune and muscle function covary in genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. (1997) ;52: (1):B39–47. |
[235] | Koizumi A , Wada Y , Tuskada M , Kayo T , Naruse M , Horiuchi K , et al. A tumor preventive effect of dietary restriction is antagonized by a high housing temperature through deprivation of torpor. Mech Ageing Dev. (1996) ;92: (1):67–82. |
[236] | Willott JF , Erway LC , Archer JR , Harrison DE . Genetics of age-related hearing loss in miceII. Strain differences and effects of caloric restriction on cochlear pathology and evoked response thresholds. Hear Res. (1995) ;88: (1-2):143–55. |
[237] | Rich JN , Elion GB , Wellner D , Colvin OM , Groothuis DR , Hilton JH , et al. The effect of L-amino acid oxidase on activity of melphalan against an intracranial xenograft. Cancer Chemother Pharmacol. (1995) ;36: (5):379–84. |
[238] | Spindler SR , Grizzle JM , Walford RL , Mote PL . Aging and restriction of dietary calories increases insulin receptor mRNA, and aging increases glucocorticoid receptor mRNA in the liver of female C3B10RF1 mice. J Gerontol. (1991) ;46: (6):B233–7. |
[239] | Turturro A , Hart RW . Longevity-assurance mechanisms and caloric restriction. Ann N Y Acad Sci. (1991) ;621: , 363–72. |
[240] | Ogura M , Ogura H , Ikehara S , Dao ML , Good RA . Decrease by chronic energy intake restriction of cellular proliferation in the intestinal epithelium and lymphoid organs in autoimmunity-prone mice. Proc Natl Acad Sci U S A. (1989) ;86: (15):5918–22. |
[241] | Watson J , Godfrey D , Stimson WH , Belch JJ , Sturrock RD . The therapeutic effects of dietary fatty acid supplementation in the autoimmune disease of the MRL-mp-lpr/lpr mouse. Int J Immunopharmacol. (1988) ;10: (4):467–71. |
[242] | Giovanella BC , Shepard RC , Stehlin JS , Venditti JM , Abbott BJ . Calorie restriction: effect on growth of human tumors heterotransplanted in nude mice. J Natl Cancer Inst. (1982) ;68: (2):249–57. |
[243] | Zamiri MJ . Effects of reduced food intake on reproduction in mice. Aust J Biol Sci. (1978) ;31: (6):629–39. |
[244] | Fernandes G , Friend P , Yunis EJ , Good RA . Influence of dietary restriction on immunologic function and renal disease in (NZB×NZW) F1 mice. Proc Natl Acad Sci U S A. (1978) ;75: (3):1500–4. |
[245] | Gerbase-DeLima M , Liu RK , Cheney KE , Mickey R , Walford RL . Immune function and survival in a long-lived mouse strain subjected to undernutrition. Gerontologia. (1975) ;21: (4):184–202. |
[246] | Newell BL , Kechris K , McQueen MB , Johnson TE . Genetic analysis of a murine QTL for diet restriction on chromosome 15. Age (Dordr). (2015) ;37: (1):9740. |
[247] | Petkovich DA , Podolskiy DI , Lobanov AV , Lee SG , Miller RA , Gladyshev VN . Using DNA Methylation Profiling to Evaluate Biological Age and Longevity Interventions. Cell Metab. (2017) ;25: (4)::954–60.e6. |
[248] | Spindler SR , Mote PL , Flegal JM , Teter B . Influence on longevity of blueberry, cinnamon, green and black tea, pomegranate, sesame, curcumin, morin, pycnogenol, quercetin, and taxifolin fed iso-calorically to long-lived, F1 hybrid mice. Rejuvenation Res. (2013) ;16: (2):143–51. |
[249] | Bourzac K . Interventions: Live long and prosper. Nature. (2012) ;492: (7427):S18–20. |
[250] | Bronson RT , Lipman RD , Harrison DE . Age-related gliosis in the white matter of mice. Brain Res. (1993) ;609: (1-2):124–8. |
[251] | Hahn O , Stubbs TM , Reik W , Gronke S , Beyer A , Partridge L . Hepatic gene body hypermethylation is a shared epigenetic signature of murine longevity. PLoS Genet. (2018) ;14: (11):e1007766. |
[252] | Hamadeh MJ , Tarnopolsky MA . Transient caloric restriction in early adulthood hastens disease endpoint in male, but not female, Cu/Zn-SOD mutant G93A mice. Muscle Nerve. (2006) ;34: (6):709–19. |
[253] | Hauck SJ , Hunter WS , Danilovich N , Kopchick JJ , Bartke A . Reduced levels of thyroid hormones, insulin, and glucose, and lower body core temperature in the growth hormone receptor/binding protein knockout mouse. Exp Biol Med (Maywood). (2001) ;226: (6):552–8. |
[254] | Pendergrass WR , Li Y , Jiang D , Fei RG , Wolf NS . Caloric restriction: conservation of cellular replicative capacity in vitro accompanies life-span extension in mice. Exp Cell Res. (1995) ;217: (2):309–16. |
[255] | Scudellari M . Ageing research: Blood to blood. Nature. . (2015) ;517: (7535):426–9. |
[256] | Tuna BG , Atalay PB , Altunbek M , Kalkan BM , Dogan S . Effects of Chronic and Intermittent Calorie Restriction on Adropin Levels in Breast Cancer. Nutr Cancer. (2017) ;69: (7):1003–10. |
[257] | Wang Z , Komatsu T , Ohata Y , Watanabe Y , Yuan Y , Yoshii Y , et al. Effects of rikkunshito supplementation on resistance to oxidative stress and lifespan in mice. Geriatr Gerontol Int. (2020) ;20: (3):238–47. |
[258] | Baldauf C , Sondhi M , Shin BC , Ko YE , Ye X , Lee KW , et al. Murine maternal dietary restriction affects neural Humanin expression and cellular profile. J Neurosci Res. (2020) ;98: (5):902–20. |
[259] | Natarajan N , Vujic A , Das J , Wang AC , Phu KK , Kiehm SH , et al. Effect of dietary fat and sucrose consumption on cardiac fibrosis in mice and rhesus monkeys. JCI Insight. (2019) ;4: (18). |
[260] | Tyshkovskiy A , Bozaykut P , Borodinova AA , Gerashchenko MV , Ables GP , Garratt M , et al. Identification and Application of Gene Expression Signatures Associated with Lifespan Extension. Cell Metab. (2019) ;30: (3):573–93.e8. |
[261] | Komljenovic A , Li H , Sorrentino V , Kutalik Z , Auwerx J , Robinson-Rechavi M . Cross-species functional modules link proteostasis to human normal aging. PLoS Comput Biol. (2019) ;15: (7):e1007162. |
[262] | Bartke A , Evans TR , Musters CJM . Anti-aging interventions affect lifespan variability in sex, strain, diet and drug dependent fashion. Aging (Albany NY). (2019) ;11: (12):4066–74. |
[263] | Smith BJ , Miller RA , Ericsson AC , Harrison DC , Strong R , Schmidt TM . Changes in the gut microbiome and fermentation products concurrent with enhanced longevity in acarbose-treated mice. BMC Microbiol. (2019) ;19: (1):130. |
[264] | Ma X , Mani K , Liu H , Kovacs A , Murphy JT , Foroughi L , et al. Transcription Factor EB Activation Rescues Advanced αB-Crystallin Mutation-Induced Cardiomyopathy by Normalizing Desmin Localization. J Am Heart Assoc. (2019) ;8: (4):e010866. |
[265] | Mukherjee P , Augur ZM , Li M , Hill C , Greenwood B , Domin MA , et al. Therapeutic benefit of combining calorie-restricted ketogenic diet and glutamine targeting in late-stage experimental glioblastoma. Commun Biol. (2019) ;2: , 200. |
[266] | Apple DM , Mahesula S , Fonseca RS , Zhu C , Kokovay E . Calorie restriction protects neural stem cells from age-related deficits in the subventricular zone. Aging (Albany NY). (2019) ;11: (1):115–26. |
[267] | Chaturvedi S , Bertino JR . . Methods to Study the Role of Methionine-Restricted Diet and Methioninase in Cancer Growth Control. Methods Mol Biol. (2019) ;1866: 1–12. |
[268] | Gokarn R , Solon-Biet SM , Cogger VC , Cooney GJ , Wahl D , McMahon AC , et al. Long-term Dietary Macronutrients and Hepatic Gene Expression in Aging Mice. J Gerontol A Biol Sci Med Sci. (2018) ;73: (12):1618–25. |
[269] | Smith DL Jr , Yang Y , Nagy TR , Patki A , Vasselli JR , Zhang Y , et al. Weight Cycling Increases Longevity Compared with Sustained Obesity in Mice. Obesity (Silver Spring). (2018) ;26: (11):1733–9. |
[270] | Thompson MJ , Chwiałkowska K , Rubbi L , Lusis AJ , Davis RC , Srivastava A , et al. A multi-tissue full lifespan epigenetic clockfor mice. Aging (Albany NY). (2018) ;10: (10):2832–54. |
[271] | Walters RO , Arias E , Diaz A , Burgos ES , Guan F , Tiano S , et al. Sarcosine Is Uniquely Modulated by Aging and Dietary Restriction in Rodents and Humans. Cell Rep. (2018) ;25: (3):663–76.e6. |
[272] | Wang J , Chen X , Osland J , Gerber SJ , Luan C , Delfino K , et al. Deletion of Nrip1 Extends Female Mice Longevity, Increases Autophagy, and Delays Cell Senescence. J Gerontol A Biol Sci Med Sci. (2018) ;73: (7):882–92. |
[273] | Masoumy EP , Sawyer AA , Sharma S , Patel JA , Gordon PMK , Regnault TRH , et al. The lifelong impact of fetal growth restriction on cardiac development. Pediatr Res. (2018) ;84: (4):537–44. |
[274] | Abdulmahdi W , Rabadi MM , Jules E , Marghani Y , Marji N , Leung J , et al. Kidney dysfunction in the low-birth weight murine adult: implications of oxidative stress. Am J Physiol Renal Physiol.-. (2018) ;315: (3):F583–f94. |
[275] | Spadaro O , Youm Y , Shchukina I , Ryu S , Sidorov S , Ravussin A , et al. Caloric restriction in humans reveals immunometabolic regulators of health span. Science. (2022) ;375: (6581):671–7. |
[276] | Greig JA , Jennis M , Dandekar A , Chorazeczewski JK , Smith MK , Ashley SN , et al. Muscle-directed AAV gene therapy rescues the maple syrup urine disease phenotype in a mouse model. Molecular Genetics and Metabolism. (2021) ;134: (1):139–46. |
[277] | Tang X , Li G , Shi L , Su F , Qian M , Liu Z , et al. Combined intermittent fasting and ERK inhibition enhance the anti-tumor effects of chemotherapy via the GSK3β-SIRT7 axis. Nat Commun. (2021) ;12: (1):5058. |
[278] | Joseph A , Chen H , Anagnostopoulos G , Montégut L , Lafarge A , Motiño O , et al. Effects of acyl-coenzyme A binding protein(ACBP)/diazepam-binding inhibitor (DBI) on body mass index. CellDeath Dis. (2021) ;12: (6):599. |
[279] | Hoyer-Allo KJR , Späth MR , Hanssen R , Johnsen M , Brodesser S , Kaufmann K , et al. Modulation of Endocannabinoids by Caloric Restriction Is Conserved in Mice but Is Not Required for Protection from Acute Kidney Injury. Int J Mol Sci. (2021) ;22: (11). |
[280] | Hettinger ZR , Confides AL , Vanderklish PW , Sidhom S , Masternak MM , Dupont-Versteegden EE . Skeletal muscle RBM3 expression is associated with extended lifespan in Ames Dwarf and calorie restricted mice. Exp Gerontol. (2021) ;146: , 111214. |
[281] | Plummer JD , Postnikoff SD , Tyler JK , Johnson JE . Selenium supplementation inhibits IGF-1 signaling and confers methionine restriction-like healthspan benefits to mice. Elife. 2021;10. |
[282] | Côrtes LS , Silveira HS , Lupi LA , de Mello Santos T , Cavariani MM , Domeniconi RF , et al. Maternal protein restriction impairs nutrition and ovarian histomorphometry without changing p38MAPK and PI3K-AKT-mTOR signaling in adult rat ovaries. Life Sci. (2021) ;264: , 118693. |
[283] | Zanini BM , Andrade KRS , Pradiee J , Veiga GB , Garcia DN , Mondadori RG , et al. Calorie restriction during gestation affects ovarian reserve in offspring in the mouse. Reprod Fertil Dev. (2020) ;32: (18):1338–49. |
[284] | Bruens L , Ellenbroek SIJ , Suijkerbuijk SJE , Azkanaz M , Hale AJ , Toonen P , et al. Calorie Restriction Increases the Number of Competing Stem Cells and Decreases Mutation Retention in the Intestine. Cell Reports. (2020) ;32: (3):107937. |
[285] | Aon MA , Bernier M , Mitchell SJ , Di Germanio C , Mattison JA , Ehrlich MR , et al. Untangling Determinants of Enhanced Health and Lifespan through a Multi-omics Approach in Mice. Cell Metab. (2020) ;32: (1):100–16.e4. |
[286] | Wiesenborn DS , Gálvez EJC , Spinel L , Victoria B , Allen B , Schneider A , et al. The Role of Ames Dwarfism and CalorieRestriction on Gut Microbiota. J Gerontol A Biol Sci Med Sci. (2020) ;75: (7):e1–e8. |
[287] | Huminiecki L , Atanasov AG , Horbańczuk J . Etiology ofatherosclerosis informs choice of animal models and tissues forinitial functional genomic studies of resveratrol. Pharmacol Res. (2020) ;156: , 104598. |
[288] | Belsky DW , Caspi A , Arseneault L , Baccarelli A , Corcoran DL , Gao X , et al. Corcoran DL, Gao X, et al. Quantification of the pace of biological aging in humans through a blood test, the DunedinPoAm DNA methylation algorithm. Elife. 2020;9. |
[289] | Dal Magro BM , Stone V , Klein CP , Maurmann RM , Saccomori AB , Dos Santos BG , et al. Developmental programming: intrauterine caloric restriction promotes upregulation of mitochondrial sirtuin with mild effects on oxidative parameters in the ovaries and testes of offspring. Reprod Fertil Dev. (2020) ;32: (8):763–73. |
[290] | Solon-Biet SM , Cogger VC , Pulpitel T , Wahl D , Clark X , Bagley E , et al. Branched chain amino acids impact health and lifespan indirectly via amino acid balance and appetite control. Nat Metab. (2019) ;1: (5):532–45. |
[291] | Gotardo AT , Dipe VV , Hueza IM , Górniak SL . Maternal feed restriction during pregnancy in Wistar rats: Evaluation of offspring using classical and immunoteratology protocols. Hum Exp Toxicol. (2017) ;36: (6):603–15. |
[292] | Hehar H , Mychasiuk R . The use of telomere length as a predictive biomarker for injury prognosis in juvenile rats following a concussion/mild traumatic brain injury. Neurobiol Dis. (2016) ;87: , 11–8. |
[293] | Hehar H , Ma I , Mychasiuk R . Effects of Metabolic Programming on Juvenile Play Behavior and Gene Expression in the Prefrontal Cortex of Rats. Dev Neurosci. (2016) ;38: (2):96–104. |
[294] | Salvatierra CS , Reis SR , Pessoa AF , De Souza LM , Stoppiglia LF , Veloso RV , et al. Short-term low-protein diet during pregnancy alters islet area and protein content of phosphatidylinositol 3-kinase pathway in rats. An Acad Bras Cienc. (2015) ;87: (2):1007–18. |
[295] | Araminaite V , Zalgeviciene V , Simkunaite-Rizgeliene R , Stukas R , Kaminskas A , Tutkuviene J . Maternal caloric restriction prior topregnancy increases the body weight of the second-generation maleoffspring and shortens their longevity in rats. Tohoku J Exp Med. (2014) ;234: (1):41–50. |
[296] | Grymula K , Piotrowska K , Słuczanowska-Gł ąbowska S , Mierzejewska K , Tarnowski M , Tkacz M , et al. Positive effects ofprolonged caloric restriction on the population of very smallembryonic-like stem cells - hematopoietic and ovarian implications. J Ovarian Res. (2014) ;7: , 68. |
[297] | Yu W , Zhou HF , Lin RB , Fu YC , Wang W . Short-term calorie restrictionactivates SIRT1-4 and -7 in cardiomyocytes in vivo andin vitro . Mol Med Rep. (2014) ;9: (4):1218–24. |
[298] | Marissal-Arvy N , Duron E , Parmentier F , Zizzari P , Mormède P , Epelbaum J . QTLs influencing IGF-1 levels in a LOU/CxFischer 344F2 rat population. Tracks towards the metabolic theory of Ageing. Growth Horm IGF Res. (2013) ;23: (6):220–8. |
[299] | Laaksonen KS , Nevalainen TO , Haasio K , Kasanen IH , Nieminen PA , Voipio HM . Food and water intake, growth, and adiposity of Sprague-Dawley rats with diet board for 24 months. Lab Anim. (2013) ;47: (4):245–56. |
[300] | Mercken EM , Crosby SD , Lamming DW , JeBailey L , Krzysik-Walker S , Villareal DT , et al. Calorie restriction in humans inhibits the PI3K/AKT pathway and induces a younger transcription profile. Aging Cell. (2013) ;12: (4):645–51. |
[301] | Smith CM , Mayer JA , Duncan ID . Autophagy promotes oligodendrocyte survival and function following dysmyelination in a long-lived myelin mutant. J Neurosci. (2013) ;33: (18):8088–100. |
[302] | Nascimento E , Guzman-Quevedo O , Delacourt N , da Silva Aragão R , Perez-Garcia G , de Souza SL , et al. Long-lasting effect of perinatal exposure to L-tryptophan on circadian clock of primary cell lines established from male offspring born from mothers fed on dietary protein restriction. PLoS One. (2013) ;8: (2):e56231. |
[303] | Canale CI , Huchard E , Perret M , Henry PY . Reproductive resilience to food shortage in a small heterothermic primate. PLoS One. (2012) ;7: (7):e41477. |
[304] | Uban KA , Sliwowska JH , Lieblich S , Ellis LA , Yu WK , Weinberg J , et al. Prenatal alcohol exposure reduces the proportion of newly produced neurons and glia in the dentate gyrus of the hippocampus in female rats. Horm Behav. (2010) ;58: (5):835–43. |
[305] | Yamaza H , Komatsu T , Wakita S , Kijogi C , Park S , Hayashi H , et al. FoxO1 is involved in the antineoplastic effect of calorie restriction. Aging Cell. (2010) ;9: (3):372–82. |
[306] | Peng CH , Chang YL , Kao CL , Tseng LM , Wu CC , Chen YC , et al. SirT1–a sensor for monitoring self-renewal and aging process in retinal stem cells. Sensors (Basel). (2010) ;10: (6):6172–94. |
[307] | Bonorden MJ , Rogozina OP , Kluczny CM , Grossmann ME , Grande JP , Lokshin A , et al. Cross-sectional analysis of intermittent versus chronic caloric restriction in the TRAMP mouse. Prostate. (2009) ;69: (3):317–26. |
[308] | Valle A , Silvestri E , Moreno M , Chambery A , Oliver J , Roca P , et al. Combined effect of gender and caloric restriction on liver proteomic expression profile. J Proteome Res. (2008) ;7: (7):2872–81. |
[309] | Martin-Gronert MS , Tarry-Adkins JL , Cripps RL , Chen JH , Ozanne SE . Maternal protein restriction leads to early life alterations in the expression of key molecules involved in the aging process in rat offspring. Am J Physiol Regul Integr Comp Physiol.R. (2008) ;294: (2):494–500. |
[310] | Altun M , Bergman E , Edström E , Johnson H , Ulfhake B . Behavioral impairments of the aging rat. Physiol Behav. (2007) ;92: (5):911–23. |
[311] | Shelley P , Tarry-Adkins J , Martin-Gronert M , Poston L , Heales S , Clark J , et al. Rapid neonatal weight gain in rats results in a renal ubiquinone (CoQ) deficiency associated with premature death. Mech Ageing Dev. (2007) ;128: (11-12):681–7. |
[312] | Martin B , Pearson M , Kebejian L , Golden E , Keselman A , Bender M , et al. Sex-dependent metabolic, neuroendocrine, and cognitive responses to dietary energy restriction and excess. Endocrinology. (2007) ;148: (9):4318–33. |
[313] | Tarry-Adkins JL , Joles JA , Chen JH , Martin-Gronert MS , van der Giezen DM , Goldschmeding R , et al. Protein restriction in lactation confers nephroprotective effects in the male rat and is associated with increased antioxidant expression. Am J Physiol Regul Integr Comp Physiol.R. (2007) ;293: (3):1259–66. |
[314] | Valle A , Guevara R , García-Palmer FJ , Roca P , Oliver J . Sexual dimorphism in liver mitochondrial oxidative capacity is conserved under caloric restriction conditions. Am J Physiol Cell Physiol.C. (2007) ;293: (4):1302–8. |
[315] | Pires KM , Aguila MB , Mandarim-de-Lacerda CA . Early renal structure alteration in rat offspring from dams fed low protein diet. Life Sci. (2006) ;79: (22):2128–34. |
[316] | Petry CJ , Jennings BJ , James LA , Hales CN , Ozanne SE . Suckling a protein-restricted rat dam leads to diminished albuminuria in her male offspring in adult life: a longitudinal study. BMC Nephrol. (2006) ;7: , 14. |
[317] | Langley-Evans SC , Sculley DV . The association between birthweight and longevity in the rat is complex and modulated by maternal protein intake during fetal life. FEBS Lett. (2006) ;580: (17):4150–3. |
[318] | Valle A , Català-Niell A , Colom B , García-Palmer FJ , Oliver J , Roca P . Sex-related differences in energy balance in response tocaloric restriction. Am J Physiol Endocrinol Metab. (2005) ;289: (1):E15–22. |
[319] | Langley-Evans SC , Sculley DV . Programming of hepatic antioxidant capacity and oxidative injury in the ageing rat. Mech Ageing Dev. (2005) ;126: (6-7):804–12. |
[320] | Vasselli JR , Weindruch R , Heymsfield SB , Pi-Sunyer FX , Boozer CN , Yi N , et al. Intentional weight loss reduces mortality rate in a rodent model of dietary obesity. Obes Res. (2005) ;13: (4):693–702. |
[321] | Keenan KP , Hoe CM , Mixson L , McCoy CL , Coleman JB , Mattson BA , et al. Diabesity: a polygenic model of dietary-induced obesity from ad libitum overfeeding of Sprague-Dawley rats and its modulation by moderate and marked dietary restriction. Toxicol Pathol. (2005) ;33: (6):650–74. |
[322] | Wang C , Weindruch R , Fernández JR , Coffey CS , Patel P , Allison DB . Caloric restriction and body weight independently affectlongevity in Wistar rats. Int J Obes Relat Metab Disord. (2004) ;28: (3):357–62. |
[323] | Cooney GT , Holcroft J , de Boer JG . The effect of dietary restriction on PhIP-induced mutation in the distal colon and B[a]P- and ENU-induced mutation in the liver of the rat. Nutr Cancer. (2004) ;50: (1):63–70. |
[324] | Teillet L , Gouraud S , Corman B . Does food restriction increase life span in lean rats? . J Nutr Health Aging. (2004) ;8: (4):213–8. |
[325] | Petruska JM , Haushalter TM , Scott A , Davis TE . Diet restriction in rat toxicity studies: automated gravimetric dispensing equipment for allocating daily rations of powdered rodent diet into pouches and 7-day feeders. Contemp Top Lab Anim Sci. (2001) ;40: (5):37–43. |
[326] | Aihie Sayer A , Dunn R , Langley-Evans S , Cooper C . Prenatal exposure to a maternal low protein diet shortens life span in rats. Gerontology. (2001) ;47: (1):9–14. |
[327] | Keenan KP , Coleman JB , McCoy CL , Hoe CM , Soper KA , Laroque P . Chronic nephropathy in ad libitum overfed Sprague-Dawley rats and its early attenuation by increasing degrees of dietary (caloric) restriction to control growth. Toxicol Pathol. (2000) ;28: (6):788–98. |
[328] | Hubert MF , Laroque P , Gillet JP , Keenan KP . The effects of diet, ad Libitum feeding, and moderate and severe dietary restriction on body weight, survival, clinical pathology parameters, and cause of death in control Sprague-Dawley rats. Toxicol Sci. (2000) ;58: (1):195–207. |
[329] | Honda S , Nemoto K , Mae T , Kinjoh K , Kyogoku M , Kawamura H , et al. Mice with early onset of death (EOD) due to lupus glomerulonephritis. Clin Exp Immunol. (1999) ;116: (1):153–63. |
[330] | Laroque P , Keenan KP , Soper KA , Dorian C , Gerin G , Hoe CM , et al. Effect of early body weight and moderate dietary restriction on the survival of the Sprague-Dawley rat. Exp Toxicol Pathol. (1997) ;49: (6):459–65. |
[331] | Solomon HM , Wier PJ , Fish CJ , Hart TK , Johnson CM , Posobiec LM , et al. Spontaneous and induced alterations in the cardiac membranous ventricular septum of fetal, weanling, and adult rats. Teratology. (1997) ;55: (3):185–94. |
[332] | Keenan KP , Soper KA , Smith PF , Ballam GC , Clark RL . Diet, overfeeding, and moderate dietary restriction in control Sprague-Dawley rats: I. Effects on spontaneous neoplasms. Toxicol Pathol. (1995) ;23: (3):269–86. |
[333] | Keenan KP , Soper KA , Hertzog PR , Gumprecht LA , Smith PF , Mattson BA , et al. Diet, overfeeding, and moderate dietary restriction in control Sprague-Dawley rats: II. Effects on age-related proliferative and degenerative lesions. Toxicol Pathol. (1995) ;23: (3):287–302. |
[334] | Roe FJ , Lee PN , Conybeare G , Kelly D , Matter B , Prentice D , et al. The Biosure Study: influence of composition of diet and food consumption on longevity, degenerative diseases and neoplasia in Wistar rats studied for up to 30 months post weaning. Food Chem Toxicol. (1995) ;33: (Suppl 1):1s–100s. |
[335] | Höger H , Gialamas J , Adamiker D . Reduced tumour incidence in mice with inherited seborrhoeic dermatitis. Lab Anim. (1994) ;28: (4):340–6. |
[336] | Mizutani H , Engelman RW , Kinjoh K , Kurata Y , Ikehara S , Matsuzawa Y , et al. Calorie restriction prevents the occlusive coronary vascular disease of autoimmune (NZW×BXSB)F1 mice. Proc Natl Acad Sci U S A. (1994) ;91: (10):4402–6. |
[337] | Keenan KP , Smith PF , Hertzog P , Soper K , Ballam GC , Clark RL . The effects of overfeeding and dietary restriction on Sprague-Dawley rat survival and early pathology biomarkers of aging. Toxicol Pathol. (1994) ;22: (3):300–15. |
[338] | Gumprecht LA , Long CR , Soper KA , Smith PF , Haschek-Hock WM , Keenan KP . The early effects of dietary restriction on the pathogenesis of chronic renal disease in Sprague-Dawley rats at 12 months. Toxicol Pathol. (1993) ;21: (6):528–37. |
[339] | Salmon GK , Leslie G , Roe FJ , Lee PN . Influence of food intake and sexual segregation on longevity, organ weights and the incidence of non-neoplastic and neoplastic diseases in rats. Food Chem Toxicol. (1990) ;28: (1):39–48. |
[340] | Chatterjee B , Roy AK . Changes in hepatic androgen sensitivity and gene expression during aging. J Steroid Biochem Mol Biol. (1990) ;37: (3):437–45. |
[341] | Berg BN , Simms HS . Nutrition, onset of disease, and longevity in the rat. Can Med Assoc J. (1965) ;93: (17):911–3. |
[342] | Hsueh AM , Simonson M , Chow BF , Hanson HM . The importance of the period of dietary restriction of the dam on behavior and growth in the rat. J Nutr. (1974) ;104: (1):37–46. |
[343] | Pond WG , Wu JF . Mature body weight and life span of male and female progeny of primiparous rats fed a low protein or adequate diet throughout pregnancy. J Nutr. (1981) ;111: (11):1949–54. |
[344] | Anantharaman K . Energy/protein interrelation in experimental food restriction. Experientia Suppl. (1983) ;44: , 157–70. |
[345] | Anderson DJ , Watson AL , Yunis EJ . Environmental and genetic factors that influence immunity and longevity in mice. Basic Life Sci. (1985) ;35: , 231–40. |
[346] | Groziak SM , Kirksey A . Effects of maternal dietary restriction in vitamin B-6 on neocortex development in rats: B-6 vitamer concentrations, volume and cell estimates. J Nutr. (1987) ;117: (6):1045–52. |
[347] | Olmedillas Del Moral M , Fröhlich N , Figarella K , Mojtahedi N , Garaschuk O . Effect of Caloric Restriction on the in vivo Functional Properties of Aging Microglia. Frontiers in Immunology. (2020) ;11: , 750. |
[348] | Hill CM , Albarado DC , Coco LG , Spann RA , Khan MS , Qualls-Creekmore E , et al. FGF21 is required for protein restriction to extend lifespan and improve metabolic health in male mice. Nat Commun. (2022) ;13: (1):1897. |
[349] | Arriola Apelo SI , Lin A , Brinkman JA , Meyer E , Morrison M , Tomasiewicz JL , et al. Morrison M, Tomasiewicz JL, et al. Ovariectomy uncouples lifespan from metabolic health and reveals a sex-hormone-dependent role of hepatic mTORC2 in aging. Elife. 2020;9. |
[350] | Fontana L , Cummings NE , Arriola Apelo SI , Neuman JC , Kasza I , Schmidt BA , et al. Decreased Consumption of Branched-Chain Amino Acids Improves Metabolic Health. Cell Rep. (2016) ;16: (2):520–30. |
[351] | Lamming DW , Mihaylova MM , Katajisto P , Baar EL , Yilmaz OH , Hutchins A , et al. Depletion of Rictor, an essential protein component of mTORC2, decreases male lifespan. Aging Cell. (2014) ;13: (5):911–7. |




