Implementation of a robot-mediated upper limb rehabilitation protocol for a customized treatment after stroke: A retrospective analysis
Abstract
BACKGROUND:
Many authors have emphasized the need for individualized treatments in rehabilitation, but no tailored robotic rehabilitation protocol for stroke patients has been established yet.
OBJECTIVE:
To evaluate the effectiveness of a robot-mediated upper limb rehabilitation protocol based on clinical assessment for customized treatment of stroke patients.
METHODS:
Clinical data from 81 patients with subacute stroke, undergoing an upper limb robot-mediated rehabilitation, were analyzed retrospectively. 49 patients were treated using a customized robotic protocol (experimental group, EG) based on a clinically guided flowchart, while 32 were treated without it (control group, CG). Fugl-Meyer Assessment for Upper Extremity (FMA-UE), Motricity Index (MI), modified Barthel Index (mBI) and Numerical Rating Scale (NRS) measured before (T0) and after (T1) rehabilitation intervention were used as clinical outcomes.
RESULTS:
There was statistically significant improvement in both groups in terms of FMA-UE, MI, and mBI, while no change in NRS. Intergroup analysis showed significantly greater improvement of the FMA-UE (P = 0.002) and MI (P < 0.001) in the EG, compared with the CG.
CONCLUSION:
The implementation of our robotic protocol for customized treatment of stroke patients yielded greater recovery in upper limb motor function and strength over robotic treatment without a defined protocol.
1Introduction
According to the World Health Organization, stroke is the first cause of disability and the second leading cause of death worldwide (Truelsen et al., 2006). The most frequent consequences of a stroke are deficits in the limbs that are contralateral to the brain hemisphere damaged by the stroke (Poli et al., 2013). Injury to the brain’s motor centers can cause, especially in the upper limb (UL), neurological dysfunctions affecting strength, movement, sensory perception, and sensory-motor integration, resulting in decreased autonomy and a severe decline in quality of life (Nakayama et al., 1994). This UL disability makes it difficult for patients to perform basic daily duties such as eating, bathing, and dressing themselves (Prange et al., 2006; World Health Organization, 2007). In this context, promoting rehabilitation brings benefits in terms of motor and functional recovery, cognitive and psychological improvement, independence and early reintegration into social and domestic life.
Over the past decade, the use of robotic devices for the treatment of UL has taken on an important role in the field of neuro-rehabilitation (Mehrholz et al., 2015; Sivan et al., 2011). It is widely recognized that incorporating robot-assisted therapy into stroke rehabilitation plans is a beneficial supplement to conventional treatment. This addition aims to enhance therapy quantity and intensity, thereby fostering brain plasticity through complex yet controlled multisensory stimulation, while also standardizing treatment (Gueye et al., 2021). Another crucial aspect is the possibility offered by robotic devices to guarantee the execution of a “tailor-made” exercise for patients, by modulating various aspects of motor performance such as strength, speed and smoothness of gesture, level of assistance, resistance and range of motion. Nonetheless, very few studies described patient-specific indications to be implemented in the therapeutic choice of robotic program to guarantee better results (Bowen et al., 2016; Hebert et al., 2016; Winstein et al., 2016). None of them outlined (and proved the effectiveness of) a detailed protocol to implement in the clinical setting for robotic rehabilitation of the UL after stroke. Indeed, Morone et al. (2021), shedding light on the current state of evidence-based rehabilitation practices, emphasized a limitation in the practical applicability of the guidelines so far proposed. This is mainly ascribable to the wide variety of devices and systems employed and the different organizational models of rehabilitation facilities (Morone et al., 2020, 2021). The consequence is that there is still no agreement on the subjects’ specific characteristics that could benefit from robotic intervention (Calabrò et al., 2021; Morone et al., 2021).
This gap in literature leaves healthcare practitioners with limited information on how to provide and optimize robotic rehabilitation, hindering the implementation of standardized treatment strategies (Basteris et al., 2014). Indeed, physical therapists, once trained, choose the robots’ exergames in a subjective and unstructured manner. Therefore, a systematic reporting of the types of exercises, the choice of the robotic parameters and the rationale used for this selection is needed.
Considering the aforementioned, at our rehabilitation center, equipped with a set of four robotic and technological devices for UL treatment (Aprile et al., 2019), we developed a tailored robotic treatment protocol taking into account patients’ clinical characteristics. This protocol assists physical therapists in choosing the most suitable rehabilitation options from the available set, ensuring a personalized intervention.
Therefore, the objective of our study was to retrospectively assess the clinical outcomes of a UL robotic rehabilitation pathway that incorporates these four robotic devices, comparing the results obtained with and without the implementation of the personalized robotic treatment protocol based on patients’ clinical characteristics. By providing practical therapeutic indications for the selection of specific parameters of robotic treatment, we aimed to provide evidence to build structured protocols for UL rehabilitation after stroke, customized to the single patient’s profile.
2Methods
2.1Study design
We retrospectively analyzed clinical data of patients with sub-acute stroke admitted to our inpatient rehabilitation facility between May 2015 and March 2018. The study was conducted in accordance with the Declaration of Helsinki, and retrospectively approved by the Ethics Committee “Comitato Etico Lazio 1” (protocol number 1334/CE Lazio 1, 5 November 2020).
The clinical data analyzed here were collected on patients undergoing an UL robotic rehabilitation intervention, using a set of four robotic devices, provided with two different approaches: with and without the use of a flowchart aimed at providing a structured, customized robotic rehabilitation protocol (described below).
2.2Robotic setting
A study conducted at our rehabilitation center (Aprile et al., 2019) had identified a group of four specific devices for the overall UL treatment, arranged in accordance with a new organizational model (the RobotAREA). The RobotAREA is outfitted with two robots (MOTORE, Humanware, Italy, and Amadeo, Tyromotion, Austria), one electromechanical device (Diego, Tyromotion, Austria), and one sensor-based device (Pablo, Tyromotion, Austria). MOTORE is a planar robotic device used for elbow and scapulohumeral joint recovery. Amadeo is an end-effector device specifically designed for the sensorimotor recovery of the hand, fingers, and thumb. Diego is an electromechanical device used for unilateral or bilateral UL rehabilitation that provides arm gravity compensation. Pablo is a sensor-based device enabling UL rehabilitation through 3D movements of the upper body, arm, hand, and finger joints.
More details on the devices are reported in the Appendix.
2.3Participants
In the analysis we included patients with UL impairment caused by an ischemic or hemorrhagic stroke who were admitted to our rehabilitation facility. We included subjects: (a) with a first event; (b) with age ranging from 18 to 85 years; (c) who performed between 25 and 30 UL robotic rehabilitation sessions; (d) for whom clinical outcomes before and after the intervention were available. We excluded chronic patients (i.e., time since the event is longer than 6 months). Subjects underwent 30 sessions of neurorehabilitation, lasting 45 min each, six times/week, focused on a UL robotic treatment using the set described above. In addition, patients performed daily conventional treatment aimed at trunk control, balance, and gait recovery.
2.4Clinical assessment
According to the evaluation protocol usually employed for patients with stroke in our facility, patients were clinically evaluated at baseline (T0) and at the end of the treatment (T1) using the Fugl-Meyer Assessment for Upper Extremity (FMA-UE) (Fugl-Meyer et al., 1975), the upper extremity Motricity Index (MI) (Bohannon, 1999), the modified Barthel Index (mBI) (Shah et al., 1989), and the Numerical Rating Scale of Pain (NRS) (Downie et al., 1978). The FMA-UE motor function domain consists of 22 items, producing an overall score that ranges from 0 (worst, complete flaccid paralysis) to 66 (best, normal function) (Woytowicz et al., 2017). This scale evaluates the severity of UL motor impairment. The MI specifically measures hand grasp, elbow flexion, and shoulder abduction (each scored 0–33, to a maximum possible total score of 100), and it is a reliable tool for assessing the strength of the paretic upper extremity after stroke. The mBI is a 10-item measure of physical disability used widely to assess behavior relating to activities of daily living, with scores ranging from 0 to 100. The NRS, an 11-point (0–10) numerical rating scale, measures the pain level perceived by the patient.
2.5Customized robot-mediated protocol development
The RobotAREA described above was available to a team of selected physical therapists who had received technical training on the robotic devices from the respective manufacturers. However, this training did not include a practical declination of those instructions concerning the patients’ symptomatic pictures, in terms of the exergame’s parameters choice.
In a first stage, physical therapists selected these parameters in an unstructured and subjective manner. We will refer to patients treated with this approach as Control Group (CG). During this initial stage, the team used its clinical expertise to identify four main clinical characteristics, or domains, outlining the UL patient’s condition, relevant to the therapeutic goal. These characteristics, selected from the International Classification of Functioning, Disability and Health (ICF) core set of categories of body functions for stroke (Geyh et al., 2004), were the range of motion (ROM), the strength, the spasticity, and the pain of the UL, and represent critical features.
In a second stage, by observing patients’ neuromotor responses to the daily treatment, the parameters of the four devices were gradually adjusted to the single patient’s progressive condition with an empirical method. The process specifically consisted in: i) daily clinical observation of each single subject to determine the critical domains that needed to be treated, ii) selection of the robot to use based on the patient’s current status concerning the four domains, iii) selection of exergame based on the therapeutic goal associated to the chosen clinical characteristic, iv) selection of the game’s parameters, and v) final clinical re-observation after treatment. The selection of the exergames’ parameters is based on the following principles.
• For pain: in contrast to pain fear and disuse syndrome (Leeuw et al., 2007), we encourage UL mobility by selecting reduced ROM movement with weight relief, avoiding strenuous exercises;
• For strength: considering the lower maximal voluntary force of the affected UL, we concentrated on adjusting the mode of assistance and the type of muscle tone control, without exacerbating pain or tone (Harris & Eng, 2010; Pollock et al., 2014);
• For ROM: given the negative correlation between ROM and pain (Anwer et al., 2018), we opted for adequate positioning and isometric/isotonic exercises, concerning the maximum tolerated joint excursion (Green et al., 2003);
• For spasticity: we adhered to appropriate UL positioning, passive mode exercises, and reduced ROM (Trompetto et al., 2014), without causing pain (Truini et al., 2013), as spasticity is a length- and speed-dependent phenomenon (Kamper et al., 2001; Lance, J.W., 1980).
This process of progressive empirical optimization of the robotic treatment, illustrated in Fig. 1, led to the creation of four flowcharts, one for each device of the RobotAREA. Based on the four clinical domains previously mentioned, these flowcharts indicated the optimal combination of robot parameters. The selection of parameters is based on the principles reported in the Appendix.
The therapists chose which principles to apply based on the presence of one or more primary aspects to be treated. Using these flowcharts as a guide, a structured rehabilitation protocol was created and tailored to each patient’s specific needs. This protocol was employed on a second group of patients, referred to as Experimental Group (EG). More details about the robotic treatment protocols are reported in the Appendix.
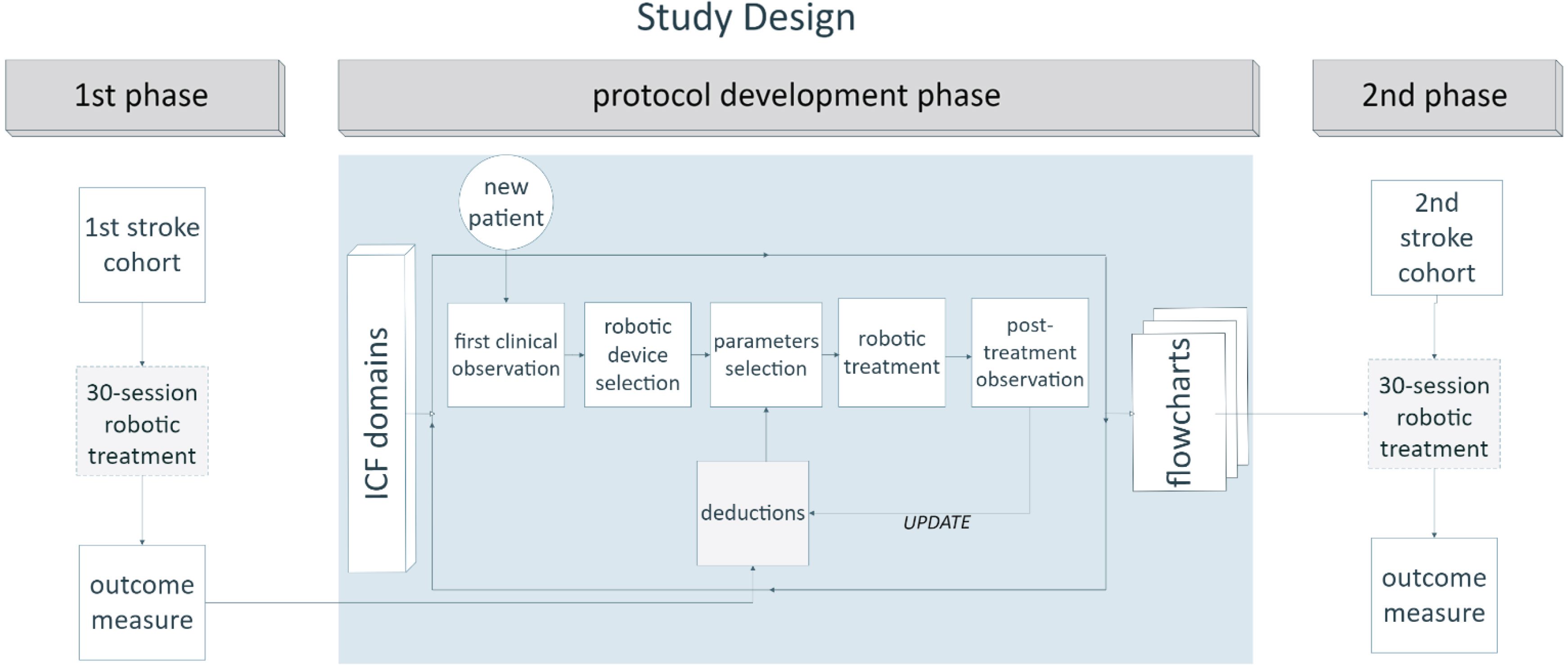
Fig. 1
Diagram of study design. Representation of the strategic phases of the work. In the initial phase, the first cohort of stroke patients underwent a 30-session robotic treatment without any defined, structured protocol. An intermediate phase consisted of protocol development based on an empirical method, starting from a daily evaluation of individual patients (within a new, intermediate group of patients) before and after treatment, and progressing to the construction of flowcharts with practical indications about parameter selection for each robot. Each intermediate evaluation helped to update therapists’ deductions regarding the optimal parameter selection. Treatment was tailored to the specific patient’s condition and was based on the evaluation of his/her ICF domains, namely range of motion, strength, spasticity, and pain. Finally, a new cohort of patients underwent a robotic treatment based on the implemented protocol. Data from the first (control) and the last (experimental) group of patients were included in our retrospective analysis. See the text for more details.
2.6Statistical analyses
Statistical analyses on the data collected from the sample described above were performed using a commercial statistical software (SPSS version 28, IBM, New York). Given the non-normality of the data distribution resulting from the Shapiro-Wilk test, we employed non-parametric statistical tests. First, for an intragroup analysis, we used the non-parametric Wilcoxon test to compare the data collected at T0 to those at T1 separately for the CG and the EG, i.e., to identify significant changes in outcome measures between the baseline and the end of the treatment in each group. Then, for an intergroup analysis, we used the non-parametric Mann-Whitney test to compare these changes from baseline (T1-T0) between the two groups. The statistical level of significance has been set to α < 0.05 for all statistical tests.
3RESULTS
3.1Sample
According to the inclusion criteria reported above, data from 81 patients were included in the analysis: 32 patients belonging to the CG (without implementation of the customized protocol) and 49 belonging to the EG (with implementation of the customized protocol). Demographics and clinical characteristics of the sample in the two analyzed groups are reported in Table 1 using descriptive statistics, with numerical data expressed as the mean (SD) or cases (percentages). P values reported in the table refer to Mann-Whitney U tests and Fisher Exact tests, respectively for mean and cases. The two groups were comparable in terms of age, sex, time since stroke (days), affected side, and type of stroke, while the time since stroke and the mBI were higher in the CG.
Table 1
Demographics and clinical characteristics of the sample in the two analyzed groups. Data are mean (SD) or n (%)
| Control | Experimental | P | |
| Group | Group | ||
| (N = 32) | (N = 49) | ||
| Age (years) | 65.9 (10.3) | 64.8 (11.6) | 0.698 |
| Sex | |||
| Man | 24 (75%) | 29 (59%) | 0.110 |
| Woman | 8 (25%) | 20 (41%) | |
| Time since stroke (days) | 113.5 (41.2) | 92.6 (41.6) | 0.025 |
| Affected side | |||
| Right | 14 (44%) | 24 (49%) | 0.408 |
| Left | 18 (56%) | 25 (51%) | |
| Type of stroke | 0.525 | ||
| ischemic | 19 (59%) | 30 (61%) | |
| haemorrhagic | 13 (41%) | 19 (39%) | |
| Fugl Meyer Assessment | 20.3 (19.3) | 25.2 (15.8) | 0.022 |
| Motricity Index | 40.0 (22.7) | 39.8 (26.2) | 0.907 |
| Barthel Index | 44.8 (21.4) | 35.7 (22.0) | 0.041 |
| NRS | 3.1 (3.0) | 3.8 (3.4) | 0.303 |
| Modified Ashworth Scale | |||
| Shoulder | 0.5 (0.7) | 0.3 (0.6) | 0.107 |
| Elbow | 1.0 (0.8) | 0.7 (0.9) | 0.059 |
| wrist | 0.9 (0.9) | 0.6 (0.8) | 0.249 |
3.2Intragroup analysis
Intragroup analysis showed significant improvement after treatment in both groups in the FMA-UE (CG: P < 0.001; EG: P < 0.001), in the MI (CG: P = 0.002; EG: P < 0.001) and in the mBI (CG: P < 0.001; EG: P < 0.001). There was no evidence of changes in the NRS (CG: P = 0.733; EG: P = 0.806). Figure 2 shows the mean values and standard deviations in the investigated outcome measures at T0 and T1 for the CG and the EG.
Fig. 2
Mean values and standard deviations of the measures of the Fugl-Meyer Assessment for Upper Extremity (FMA-UE), the Motricity Index (MI), the Numerical Rating Scale of Pain (NRS), and the modified Barthel Index (mBI) in the two groups (CG in red, EG in green). P values, referring to statistically significant values of the mean change from baseline (T1-T0) in each group, are reported in asterisks notation: *P < 0.05; **P < 0.01, ***P < 0.001.
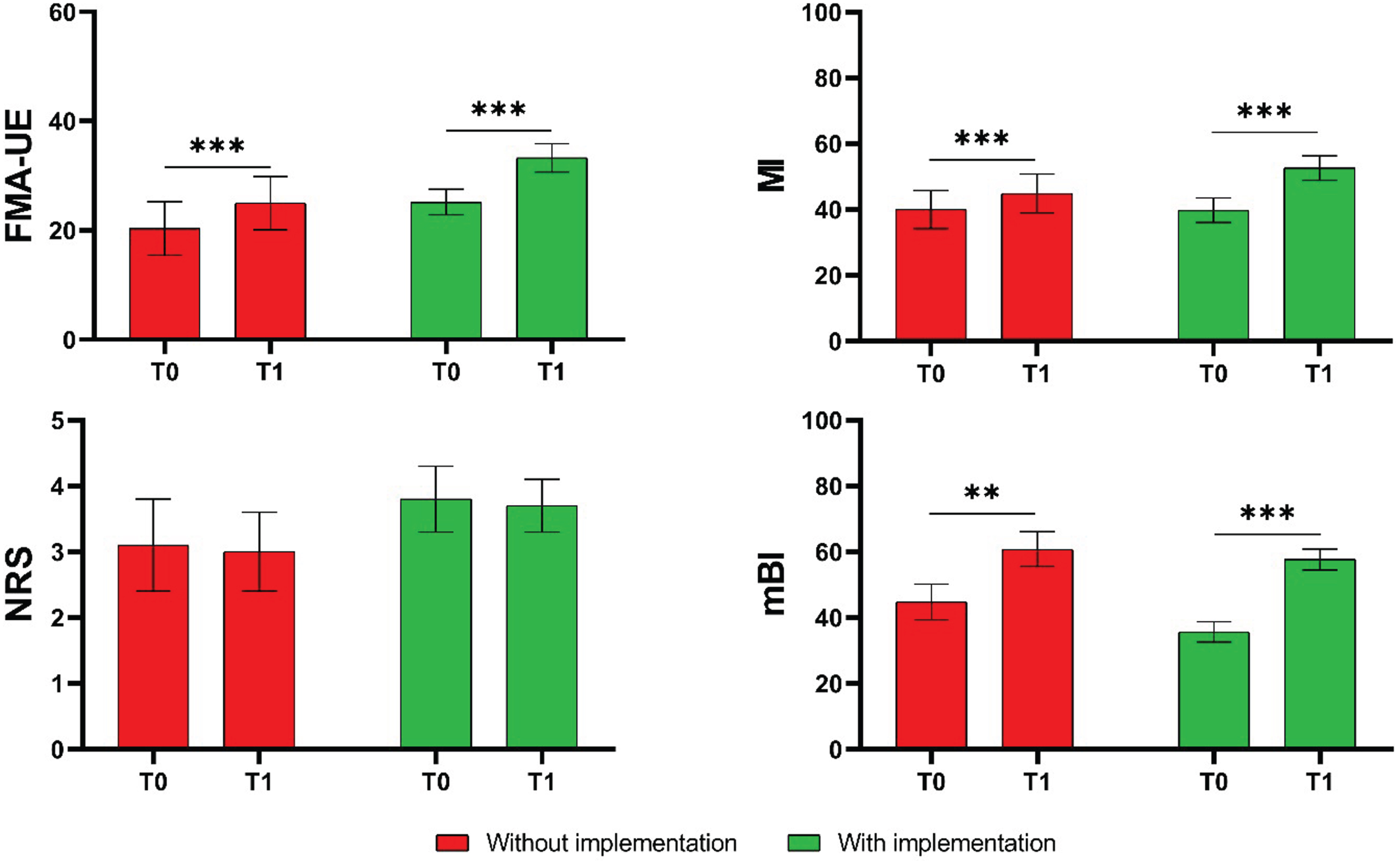
Table 2 reports the changes from baseline of the chosen outcome measures (ΔFMA-UE, ΔMI, ΔmBI and ΔNRS) for the two groups. N = 12 patients (37.5%) in the CG, and n = 36 patients (73.5%) in the EG exceeded the cutoff of 5 in the FMA-UE measure considered as the minimal clinically important difference (MCID) for improvement.
Table 2
Mean deltas of improvement (i.e., changes from baseline) in the measures of the Fugl-Meyer Assessment for Upper Extremity (FMA-UE), the Motricity Index (MI), the Numerical Rating Scale of Pain (NRS), and the modified Barthel Index (mBI) in the two groups
| Control Group | Experimental Group | |
| (n = 32) | (n = 49) | |
| Mean (SD) | Mean (SD) | |
| ΔFMA-UE | 4.75 (6.11) | 8.16 (5.76) |
| ΔMI | 4.88 (8.54) | 12.74 (11.46) |
| ΔmBI | 16.0 (12.83) | 22.06 (16.03) |
| ΔNRS | –0.06 (2.58) | –0.08 (3.24) |
3.3Intergroup analysis
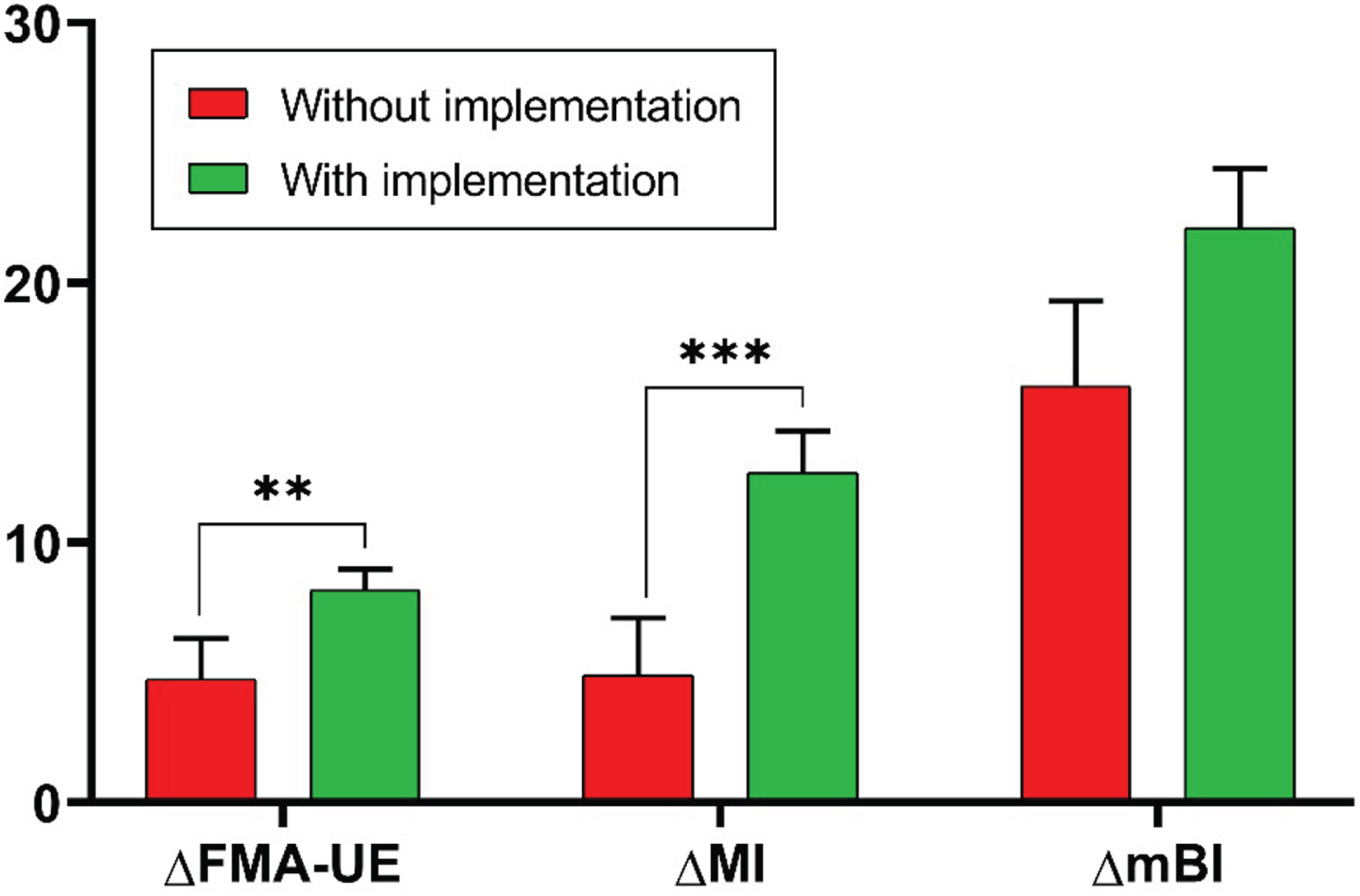
Considering the outcome measures that showed significant improvements with rehabilitation in both groups, namely the FMA-UE, the MI, and the mBI, we analyzed the intergroup differences of the changes from baseline in these measures. This analysis showed a statistically significant difference in ΔFMA-UE (P = 0.002) and ΔMI (P < 0.001), indicative of a greater recovery in motor function and UL strength in the EG with the implementation of the customized protocol, compared with the CG without this implementation. There was no significant intergroup difference in the ΔmBI. Figure 3 shows the changes from baseline of the FMA-UE, the MI, and the mBI between the two groups.
Fig. 3
Changes from baseline obtained in the Fugl-Meyer Assessment for Upper Extremity (ΔFMA-UE), in the Motricity Index (ΔMI), and in the modified Barthel Index (ΔmBI) in the two groups (CG in red, EG in green). P values, referring to statistically significant values of the intergroup differences of change from baseline, are reported in asterisks notation: *P < 0.05; **P < 0.01, ***P<0.001.
4DISCUSSION
This retrospective study aimed to compare the effects of a robotic rehabilitation pathway, using a set of four robotic devices, with and without the implementation of a structured, customized UL robotic treatment protocol based on clinical characteristics. The protocol was built on clinical evidence gained through therapists’ expertise and recommended the ideal combination of robot exergame’s parameters for each current patient’s condition. Our results demonstrate that this implementation, allowing the type and mode of intervention to be defined in a “tailor-made” fashion, provides an overall better recovery in the UL motor function compared with robotic treatment without structured protocols.
As expected, our intragroup analysis showed significant improvements in both groups in terms of UL motor function (FMA-UE), strength (MI), and ability to perform activities of daily living (mBI). These results are coherent with recent literature (Aprile et al., 2020; Bertani et al., 2017; Mehrholz et al., 2015) showing that the use of robotic devices has a positive impact on stroke patients’ recovery of arm function. In addition, Mehrholz et al. (2020) underlined the fact that variables like stroke severity and time since acute event do not influence the effects of employing robotic devices for UL rehabilitation. Despite variations in specific methodologies, the consistent outcomes across studies emphasize the reliability of these results and corroborate the use of robotic rehabilitation for UL recovery in stroke.
The outcome measure referred to pain (NRS) presented a tendency to decrease in both groups in our study, even though it did not reach statistically significant difference values. This result confirms the non-negative influence of robotic treatment on patients’ pain (Aprile et al., 2021; Huang et al., 2022). Indeed, while pain has a negative impact on the patient’s activities of daily living in addition to inhibiting the affected UL motor recovery (Bayley et al., 2012), a recent meta-analysis (Rogers et al., 2019) demonstrated that robot-assisted therapy can enhance the patient’s quality of life by lowering pain and recovering the damaged limb’s motor function.
When comparing the changes from baseline due to the rehabilitation between the two groups, we found greater improvement after robotic rehabilitation in FMA-UE and MI scales, meaning a better recovery in UL motor function and strength, in the group undergoing rehabilitation with the structured customized UL robotic treatment protocol implementation. These results prove, for the first time, that a patient-tailored treatment protocol is beneficial to improve UL function in people with subacute stroke. Notably, Bayley et al. (2012) have already highlighted the importance of assessing patient outcomes for prioritizing therapeutic activities in a treatment protocol. Previous attempts to produce comprehensive guidelines failed in clarifying not only the optimal dosage in terms of timing, frequency and number of repetitions (Calabrò et al., 2021), but also the subjects’ characteristics that could benefit from the robotic treatment (Calabrò et al., 2021; Morone et al., 2021). We therefore address the need for a more granular approach in guideline recommendations with our robotic-tailored protocol. A granular approach acknowledges the importance of matching the right device to the unique needs of the patient, taking into account his/her daily state, the prominent aspects of their motor deficiency, the location of the impairment (proximal or distal), and the expected clinical outcomes. Similarly, this strategy allows for a detailed determination of the most effective intervention mode in terms of robots’ exergame parameters, considering factors like patient’s clinical characteristics (such as those discussed here) and specific rehabilitation goals.
The primary finding of our analysis was therefore the clinical benefit of establishing customized protocols for UL robotic rehabilitation in stroke patients. It is noteworthy that this effort was made possible by deploying the model of the RobotAREA developed by Aprile et al. (2019). Each device in the RobotAREA acts on different UL joints and on different plans of movement ensuring the patient receives comprehensive rehabilitation with account of his/her clinical needs. The present investigation complements the strategic device selection with a customization of treatments designed in the framework of this rehabilitation model, to amplify the clinical impact of the proposed robotic intervention. Future research should hint at potential advancements in its clinical feasibility, but also delve deeper into the economic advantages and long-term outcomes of these interventions, paving the way for more comprehensive and accessible stroke rehabilitation strategies.
4.1Limitations
The main limitation of our study is its retrospective nature. It will be crucial to conduct future studies to examine the application of customized treatment protocols on a larger sample of patients, in various settings and rehabilitation facilities with prospective trials.
The analysis referred specifically to the RobotAREA devices mentioned above. Our study should be intended as a reference model of robotic treatment, enabling healthcare practitioners to easily make their deductions on the ideal robotic intervention. We believe that our general approach based on common ICF domain evaluation is easily applicable to different rehabilitation scenarios by adapting the logic of our flowcharts to the robotic setting in use, at least for the same categories of robot and electromechanical devices employed here.
In our analysis, we focused purely on motor and functional measures of the post-stroke patient’s UL. It would be of great significance to explore the cognitive sphere, which has been extensively studied in the literature in terms of attention, procedural memory, short-term memory, hemi-inattention, and so on (Chen et al., 2013; Mullick et al., 2015; Rogers et al., 2019). Indeed, patients must use cognitive strategies while performing exergames during robotic therapy, as proven in a recent meta-analysis (Mullick et al., 2015; Nie et al., 2022; Xiao et al., 2022).
5Conclusion
The implementation of tailor-made treatment protocols, organized in flowcharts, within a rehabilitation setting of four robotic devices, significantly improved UL motor function in subjects with subacute stroke. To the best of our knowledge, this is the first study implementing a customized robotic protocol for UL treatment in stroke based on structured information about the patient’s clinical state.
Acknowledgments
The authors have no acknowledgments.
Conflict of interest
The authors declare no conflict of interest.
Ethics statement
The study was conducted in accordance with the Declaration of Helsinki, and retrospectively approved by the Ethics Committee “Comitato Etico Lazio 1” (protocol number 1334/CE Lazio 1, 5 November 2020).
Informed consent
All participants involved in this research study have provided written informed consent.
Funding
This study was partially supported by the Italian Ministry of Health (Ricerca Corrente).
Supplementary material
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/NRE-230367.
References
1 | Anwer, S. , Alghadir, A. H. , Al-Eisa, E. S. , Iqbal, Z. A. ((2018) ) The relationships between shoulder pain, range of motion, and disability in patients with shoulder dysfunction. Journal of Back and Musculoskeletal Rehabilitation, 31: (1), 163–167. https://doi.org/10.3233/BMR-169762 |
2 | Aprile, I. , Germanotta, M. , Cruciani, A. , Loreti, S. , Pecchioli, C. , Cecchi, F. , Montesano, A. , Galeri, S. , Diverio, M. , Falsini, C. , Speranza, G. , Langone, E. , Papadopoulou, D. , Padua, L. , Carrozza, M. C. , FDG Robotic Rehabilitation Group ((2020) ) Upper Limb Robotic Rehabilitation After Stroke: A Multicenter, Randomized Clinical Trial. Journal of Neurologic Physical Therapy: JNPT, 44: (1), 3–14. https://doi.org/10.1097/NPT.0000000000000295. |
3 | Aprile, I. , Germanotta, M. , Cruciani, A. , Pecchioli, C. , Loreti, S. , Papadopoulou, D. , Montesano, A. , Galeri, S. , Diverio, M. , Falsini, C. , Speranza, G. , Langone, E. , Carrozza, M. , Cecchi, F. ((2021) ) Poststroke shoulder pain in subacute patients and its correlation with upper limb recovery after robotic or conventional treatment: A secondary analysis of a multicenter randomized controlled trial. International Journal of Stroke, 16: (4), 396–405. https://doi.org/10.1177/1747493020937192 |
4 | Aprile, I. , Pecchioli, C. , Loreti, S. , Cruciani, A. , Padua, L. , Germanotta, M. ((2019) ) Improving the Efficiency of Robot-Mediated Rehabilitation by Using a New Organizational Model: An Observational Feasibility Study in an Italian Rehabilitation Center. Articolo. Applied Sciences, 9: (24), 24. https://doi.org/10.3390/app9245357 |
5 | Basteris, A. , Nijenhuis, S. M. , Stienen, A. H. , Buurke, J. H. , Prange, G. B. , Amirabdollahian, F. ((2014) ) Training modalities in robot-mediated upper limb rehabilitation in stroke: A framework for classification based on a systematic review. Journal of NeuroEngineering and Rehabilitation, 11: (1), 111. https://doi.org/10.1186/1743-0003-11-111 |
6 | Bayley, M. T. , Hurdowar, A. , Richards, C. L. , Korner-Bitensky, N. , Wood-Dauphinee, S. , Eng, J. J. , McKay-Lyons, M. , Harrison, E. , Teasell, R. , Harrison, M. , Graham, I. D. ((2012) ) Barriers to implementation of stroke rehabilitation evidence: Findings from a multi-site pilot project. Disability and Rehabilitation, 34: (19), 1633–1638. https://doi.org/10.3109/09638288.2012.656790 |
7 | Bertani, R. , Melegari, C. , De Cola, M. C. , Bramanti, A. , Bramanti, P. , Calabrò, R. S. ((2017) ) Effects of robot-assisted upper limb rehabilitation in stroke patients: A systematic review with meta-analysis. Neurological Sciences, 38: (9), 1561–1569. https://doi.org/10.1007/s10072-017-2995-5 |
8 | Bohannon, R. ((1999) ) Motricity Index Scores are Valid Indicators of Paretic Upper Extremity Strength Following Stroke. Journal of Physical Therapy Science –J PHYS THER SCI, 11: , 59–61. https://doi.org/10.1589/jpts.11.59 |
9 | Bowen, A. , James, M. , Young, G. (2016). Royal College of Physicians 2016 National clinical guideline for stroke [Report]. RCP. https://pearl.plymouth.ac.uk/handle/10026.1/10488 |
10 | Calabrò, R. S. , Sorrentino, G. , Cassio, A. , Mazzoli, D. , Andrenelli, E. , Bizzarini, E. , Campanini, I. , Carmignano, S. M. , Cerulli, S. , Chisari, C. , Colombo, V. , Dalise, S. , Fundarò, C. , Gazzotti, V. , Mazzoleni, D. , Mazzucchelli, M. , Melegari, C. , Merlo, A. , Stampacchia, G. , . . . Italian Consensus Conference on Robotics in Neurorehabilitation (CICERONE). ((2021) ) Robotic-assisted gait rehabilitation following stroke: A systematic review of current guidelines and practical clinical recommendations. European Journal of Physical and Rehabilitation Medicine, 57: (3), 460–471. https://doi.org/10.23736/S1973-9087.21.06887-8 |
11 | Chen, C. , Leys, D. , Esquenazi, A. ((2013) ) The interaction between neuropsychological and motor deficits in patients after stroke. Neurology, 80: (3 Supplement 2), S27–S34. https://doi.org/10.1212/WNL.0b013e3182762569 |
12 | Downie, W. W. , Leatham, P. A. , Rhind, V. M. , Wright, V. , Branco, J. A. , Anderson, J. A. ((1978) ) Studies with pain rating scales. Annals of the Rheumatic Diseases, 37: (4), 378–381. https://doi.org/10.1136/ard.37.4.378 |
13 | Fugl-Meyer, A. R. , Jääskö, L. , Leyman, I. , Olsson, S. , Steglind, S. ((1975) ) The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scandinavian Journal of Rehabilitation Medicine, 7: (1), 13–31. |
14 | Geyh, S. , Cieza, A. , Schouten, J. , Dickson, H. , Frommelt, P. , Omar, Z. , Kostanjsek, N. , Ring, H. , Stucki, G. ((2004) ) ICF Core Sets for stroke. Journal of Rehabilitation Medicine, 44: (Suppl), 135–141. https://doi.org/10.1080/16501960410016776 |
15 | Green, S. , Buchbinder, R. , Hetrick, S. E. ((2003) ) Physiotherapy interventions for shoulder pain. Cochrane Database of Systematic Reviews, 2003: (2), CD004258. https://doi.org/10.1002/14651858.CD004258 |
16 | Gueye, T. , Dedkova, M. , Rogalewicz, V. , Grunerova-Lippertova, M. , Angerova, Y. ((2021) ) Early post-stroke rehabilitation for upper limb motor function using virtual reality and exoskeleton: Equally efficient in older patients. Neurologia I Neurochirurgia Polska, 55: (1), 91–96. https://doi.org/10.5603/PJNNS.a2020.0096 |
17 | Harris, J. E. , Eng, J. J. ((2010) ) Strength training improves upper-limb function in individuals with stroke: A meta-analysis. Stroke, 41: (1), 136–140. https://doi.org/10.1161/STROKEAHA.109.567438 |
18 | Hebert, D. , Lindsay, M. P. , McIntyre, A. , Kirton, A. , Rumney, P. G. , Bagg, S. , Bayley, M. , Dowlatshahi, D. , Dukelow, S. , Garnhum, M. , Glasser, E. , Halabi, M.-L. , Kang, E. , MacKay-Lyons, M. , Martino, R. , Rochette, A. , Rowe, S. , Salbach, N. , Semenko, B. , . . . Teasell, R. ((2016) ) Canadian stroke best practice recommendations: Stroke rehabilitation practice guidelines, update 2015. International Journal of Stroke: Official Journal of the International Stroke Society, 11: (4), 459–484. https://doi.org/10.1177/1747493016643553 |
19 | Huang, J. , Ji, J.-R. , Liang, C. , Zhang, Y.-Z. , Sun, H.-C. , Yan, Y.-H. , Xing, X.-B. ((2022) ) Effects of physical therapy-based rehabilitation on recovery of upper limb motor function after stroke in adults: A systematic review and meta-analysis of randomized controlled trials. Annals of Palliative Medicine, 11: (2), Articolo 2. https://doi.org/10.21037/apm-21-3710 |
20 | Kamper, D. G. , Schmit, B. D. , Rymer, W. Z. ((2001) ) Effect of muscle biomechanics on the quantification of spasticity. Annals of Biomedical Engineering, 29: (12), 1122–1134. https://doi.org/10.1114/1.1424918 |
21 | Lance, J.W. (1980). Symposium synopsis. Spasticity: Disordered motor control – ScienceOpen. https://www.scienceopen.com/document?vid=9a711532-d33b-4ecb-92ac-c11496a65f5b |
22 | Leeuw, M. , Goossens, M. E. J. B. , Linton, S. J. , Crombez, G. , Boersma, K. , Vlaeyen, J. W. S. ((2007) ) The Fear-Avoidance Model of Musculoskeletal Pain: Current State of Scientific Evidence. Journal of Behavioral Medicine, 30: (1), 77–94. https://doi.org/10.1007/s10865-006-9085-0 |
23 | Mehrholz, J. , Pohl, M. , Platz, T. , Kugler, J. , Elsner, B. ((2015) ) Electromechanical and robot-assisted arm training for improving activities of daily living, arm function, and arm muscle strength after stroke. The Cochrane Database of Systematic Reviews, 2015: (11), CD006876. https://doi.org/10.1002/14651858.CD006876.pub4 |
24 | Mehrholz, J. , Thomas, S. , Kugler, J. , Pohl, M. , Elsner, B. ((2020) ) Electromechanical-assisted training for walking after stroke. Cochrane Database of Systematic Reviews, 5: (5), CD006185. https://doi.org/10.1002/14651858.CD006185.pub5 |
25 | Morone, G. , Cocchi, I. , Paolucci, S. , Iosa, M. ((2020) ) Robot-assisted therapy for arm recovery for stroke patients: State of the art and clinical implication. Expert Review of Medical Devices, 17: (3), 223–233. https://doi.org/10.1080/17434440.2020.1733408 |
26 | Morone, G. , Palomba, A. , Martino Cinnera, A. , Agostini, M. , Aprile, I. , Arienti, C. , Paci, M. , Casanova, E. , Marino, D. , Rosa, LA G. , Bressi, F. , Sterzi, S. , Gandolfi, M. , Giansanti, D. , Perrero, L. , Battistini, A. , Miccinilli, S. , Filoni, S. , Sicari, M. , . . . < <CICERONE> >Italian Consensus Conference on Robotic in Neurorehabilitation. ((2021) ) Systematic review of guidelines to identify recommendations for upper limb robotic rehabilitation after stroke. European Journal of Physical and Rehabilitation Medicine, 57: (2), 238–245. https://doi.org/10.23736/S1973-9087.21.06625-9 |
27 | Mullick, A. A. , Subramanian, S. K. , Levin, M. F. ((2015) ) Emerging evidence of the association between cognitive deficits and arm motor recovery after stroke: A meta-analysis. Restorative Neurology and Neuroscience, 33: (3), 389–403. https://doi.org/10.3233/RNN-150510 |
28 | Nakayama, H. , Jørgensen, H. S. , Raaschou, H. O. , Olsen, T. S. ((1994) ) Recovery of upper extremity function in stroke patients: The Copenhagen Stroke Study. Archives of Physical Medicine and Rehabilitation, 75: (4), 394–398. https://doi.org/10.1016/0003-9993(94)90161-9 |
29 | Nie, P. , Liu, F. , Lin, S. , Guo, J. , Chen, X. , Chen, S. , Yu, L. , Lin, R. ((2022) ) The effects of computer-assisted cognitive rehabilitation on cognitive impairment after stroke: A systematic review and meta-analysis. Journal of Clinical Nursing, 31: (9–10), 1136–1148. https://doi.org/10.1111/jocn.16030 |
30 | Poli, P. , Morone, G. , Rosati, G. , Masiero, S. ((2013) ) Robotic technologies and rehabilitation: New tools for stroke patients’ therapy. BioMed Research International, 2013: , 153872. https://doi.org/10.1155/2013/153872 |
31 | Pollock, A. , Farmer, S. E. , Brady, M. C. , Langhorne, P. , Mead, G. E. , Mehrholz, J. , van Wijck, F. ((2014) ) Interventions for improving upper limb function after stroke. The Cochrane Database of Systematic Reviews, 2014: (11), CD010820. https://doi.org/10.1002/14651858.CD010820.pub2 |
32 | Prange, G. B. , Jannink, M. J. A. , Groothuis-Oudshoorn, C. G. M. , Hermens, H. J. , Ijzerman, M. J. ((2006) ) Systematic review of the effect of robot-aided therapy on recovery of the hemiparetic arm after stroke. Journal of Rehabilitation Research and Development, 43: (2), 171–184. https://doi.org/10.1682/jrrd.2005.04.0076 |
33 | Rogers, J. M. , Duckworth, J. , Middleton, S. , Steenbergen, B. , Wilson, P. H. ((2019) ) Elements virtual rehabilitation improves motor, cognitive, and functional outcomes in adult stroke: Evidence from a randomized controlled pilot study. Journal of NeuroEngineering and Rehabilitation, 16: (1), 56. https://doi.org/10.1186/s12984-019-0531-y |
34 | Shah, S. , Vanclay, F. , Cooper, B. ((1989) ) Improving the sensitivity of the Barthel Index for stroke rehabilitation. Journal of Clinical Epidemiology, 42: (8), 703–709. https://doi.org/10.1016/0895-4356(89)90065-6 |
35 | Sivan, M. , O’Connor, R. J. , Makower, S. , Levesley, M. , Bhakta, B. ((2011) ) Systematic review of outcome measures used in the evaluation of robot-assisted upper limb exercise in stroke. Journal of Rehabilitation Medicine, 43: (3), 181–189. https://doi.org/10.2340/16501977-0674 |
36 | Trompetto, C. , Marinelli, L. , Mori, L. , Pelosin, E. , Currà, A. , Molfetta, L. , Abbruzzese, G. ((2014) ) Pathophysiology of Spasticity: Implications for Neurorehabilitation. BioMed Research International, 2014: , 354906. https://doi.org/10.1155/2014/354906 |
37 | Truelsen, T. , Piechowski-Jóźwiak, B. , Bonita, R. , Mathers, C. , Bogousslavsky, J. , Boysen, G. ((2006) ) Stroke incidence and prevalence in Europe: A review of available data. European Journal of Neurology, 13: (6), 581–598. https://doi.org/10.1111/j.1468-1331.2006.01138.x |
38 | Truini, A. , Barbanti, P. , Pozzilli, C. , Cruccu, G. ((2013) ) A mechanism-based classification of pain in multiple sclerosis. Journal of Neurology, 260: (2), 351–367. https://doi.org/10.1007/s00415-012-6579-2 |
39 | Winstein, C. J. , Stein, J. , Arena, R. , Bates, B. , Cherney, L. R. , Cramer, S. C. , Deruyter, F. , Eng, J. J. , Fisher, B. , Harvey, R. L. , Lang, C. E. , MacKay-Lyons, M. , Ottenbacher, K. J. , Pugh, S. , Reeves, M. J. , Richards, L. G. , Stiers, W. , Zorowitz, R. D. , American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Quality of Care and Outcomes Research. ((2016) ) Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke, 47: (6), e98–e169. https://doi.org/10.1161/STR.0000000000000098 |
40 | World Health Organization. (2007). International classification of functioning, disability and health: Children and youth version: ICF-CY. World Health Organization. https://iris.who.int/handle/10665/43737 |
41 | Woytowicz, E. J. , Rietschel, J. C. , Goodman, R. N. , Conroy, S. S. , Sorkin, J. D. , Whitall, J. , McCombe Waller, S. ((2017) ) Determining Levels of Upper Extremity Movement Impairment by Applying a Cluster Analysis to the Fugl-Meyer Assessment of the Upper Extremity in Chronic Stroke. Archives of Physical Medicine and Rehabilitation, 98: (3), 456–462. https://doi.org/10.1016/j.apmr.2016.06.023 |
42 | Xiao, Z. , Wang, Z. , Song, G. , Zhong, Y. , Zhang, W. ((2022) ) Rehabilitation efficacy comparison of virtual reality technology and computer-assisted cognitive rehabilitation in patients with post-stroke cognitive impairment: A network meta-analysis. Journal of Clinical Neuroscience, 103: , 85–91. https://doi.org/10.1016/j.jocn.2022.07.005 |




