Prognosis and enhancement of recovery in disorders of consciousness
Abstract
Disorders of consciousness after severe brain injury encompass conditions of coma, vegetative state/unresponsive wakefulness syndrome, and minimally conscious state. DoC clinical presentation pose perplexing challenges to medical professionals, researchers, and families alike. The outcome is uncertain in the first weeks to months after a brain injury, with families and medical providers often making important decisions that require certainty. Prognostication for individuals with these conditions has been the subject of intense scientific investigation that continues to strive for valid prognostic indicators and algorithms for predicting recovery of consciousness. This manuscript aims to provide an overview of the current clinical landscape surrounding prognosis and optimizing recovery in DoC and the current and future research that could improve prognostic accuracy after severe brain injury. Improved understanding of these factors will aid healthcare professionals in providing optimal care, fostering hope, and advocating for ethical practices in the management of individuals with DoC.
1Introduction
Disorders of consciousness (DoC) represent a complex spectrum of conditions that can present because of severe brain injury or dysfunction. The primary diagnostic behavioral phenotypes include coma, characterized by the complete absence of arousal and awareness; the vegetative state/unresponsive wakefulness syndrome (UWS), marked by wakefulness without awareness; and the minimally conscious state (MCS), exhibiting inconsistent evidence of purposeful behavior or conscious awareness (Giacino et al., 2022). See Table 1. Understanding DoC spectrum and prediction for recovery in individuals with severe brain injury has significant implications related to medical decision-making, withdrawal of life-sustaining therapies (WOLST), rehabilitation strategies, access to specialized post-acute care, and the support and education of patients’ families. For example, Thibaut et al. in 2020 found that patients with receptive or expressive language function (MCS+) have less severe short-term functional disability than those who do have language function (MCS-). They proposed that early recovery of language (MCS+) may prove to be a predictor of a more favorable long-term outcome (Thibaut et al., 2020).
Table 1
General behavioral phenotypes of DoC
| Behavior | Coma | VS/UWS | MCS | eMCS |
| Eye Opening | X | √ | √ | √ |
| Generalized Response | X | √ | X | X |
| Sleep Wake Cycles | X | √ | √ | √ |
| Localized Response | X | X | √ | √ |
| Visual Fixation | X | X | √ | √ |
| Visual Pursuit | X | X | √ | √ |
| Vocalization | X | X | √ | √ |
| Command Following | X | X | * | √ |
| Communication | X | X | * | √ |
| Functional Object Use | X | X | X | √ |
*Denotes intermittent, inconsistent in behavior.
Historically, DoC was often viewed as catastrophic/irreversible, with poor probabilities for meaningful recovery. However, over the past decade outcome data from large data sets have contradicted this pessimistic prognostic bias and have revealed findings that have reshaped our understanding of the human brain’s capacity to heal after severe injury. Evidence from large, multi-center databases supports a large percentage of patients experiencing DoC after severe brain injury, especially traumatic etiology, progress to recovering consciousness and achieve functional gains (Kowalski et al., 2021; McCrea et al., 2021). Neuroimaging, electrophysiology, and neurobehavioral assessments have provided improved valid modalities for assessing and monitoring the recovery trajectory and informing prognosis for DoC, even for patients with absent behavioral responses at bedside (Magnani et al., 2022).
Despite these advances, a significant gap remains in the ability to predict a patient’s probability of return of consciousness, in what timeframe, and the extent of functional recovery or severity and chronicity of disability. In the first weeks to months after a brain injury, outcome is generally not predictable with certainty (Fins, 2007; Giacino et al., 2018; F. M. Hammond, Katta-Charles, Russell, Zafonte, Claassen, Wagner, Puybasset, Egawa, Laureys, Diringer, Stevens, & the Curing Coma Campaign and its Contributing Members, 2021). Prognostication with acknowledgement of uncertainties is crucial to evidence-informed decision-making, timing of decisions, and supportive counseling for patient’s families or surrogates who are responsible for making necessary decisions related to continued care and recovery (F. M. Hammond, Katta-Charles, Russell, Zafonte, Claassen, Wagner, Puybasset, Egawa, Laureys, Diringer, Stevens, & Curing Coma Campaign and its Contributing Members, 2021; Kreitzer et al., 2023).
Prognosis with patients in DoC is also complicated by the lack of consensus of a good or favorable outcome. Perception of favorable outcome can also differ between professionals and the patient’s family. Prognostic and outcome studies are confounded with a “moving target” of recovery end points. Some studies evaluate recovery of consciousness and various studies evaluate outcome as recovery of function and a level of independence through various measures such as the Glasgow Outcome Scale-Extended (GOSE), Disability Rating Scale (DRS) and Functional Interdependence Measure (FIM). The GOSE is the most utilized outcome measure for TBI. However, much variability is found in dichotomizing the ordinal scale of 1–8 and what scores constitute “unfavorable” vs “favorable” outcome (Zuckerman et al., 2022).
Family interviews and surveys demonstrate greater variability of perception of “favorable outcome” from recovery of consciousness, ability to communicate to the ability to live independently and meaningfully engage in life roles (Fins, 2015; Kostick et al., 2021). Families’ view of outcome is also dependent upon what they feel their loved one would have wanted as a level of functioning, disability or quality of life. Conflicting view of favorable outcome between professionals and families can create tension and challenge prognostic counseling between professionals and families.
2Risks of nihilistic prognosis
Historically, patients experiencing DoC have received a universally poor prognosis related to recovery of consciousness and function. A study by Turgeon (2011) found that most deaths (70%) after severe TBI were associated with WOLST with approximately half of these deaths occurring within the first three days of injury (Turgeon et al., 2011), at a time when the outcome trajectory is uncertain. Data from the National Trauma Databank and CENTER-TBI database have found a similar temporal pattern regarding WOLST (DeMario et al., 2022; van Veen et al., 2021). These studies found significant variability in injury- and non-injury-related factors for individuals who died secondary to WOLST suggesting that decisions to withdraw care after severe brain injury may be impacted by individual physician practices, biases and perceptions regarding long-term outcomes. Furthermore, studies using clinical vignettes have indicated that clinician prognostications are often overly pessimistic (F. Hammond et al., 2009; Izzy et al., 2013).
These identified trends in WOLST after severe brain injury are concerning as recent outcome evidence exhibits that 12% of individuals with severe TBI (mean GCS 4.2) achieved a favorable outcome as defined by the Glasgow Outcome Scale-Extended (GOSE) within 2 weeks post-injury. That number quadrupled at 3 months post-injury, and 53% achieved a favorable outcome by 12 months (McCrea et al., 2021). Positive recovery trajectory was also identified within the TBI Model Systems data reporting that 82% of comatose patients recovered consciousness during inpatient rehabilitation (Kowalski et al., 2021). Among individuals not following commands at the time of TBI rehabilitation admission, the majority achieve independence in daily functional activities with improvements in functional independence continuing throughout at least the first decade after TBI (F. M. Hammond et al., 2019). Thus, it is probable that a significant percentage of individuals’ severe brain injury who die secondary to early WOLST could have progressed to recover consciousness and achieve a positive functional outcome. Unfortunately, the continued pervading nihilistic bias of medical professionals and the absence of valid early prognostic indicators and algorithms lead to a self-fulfilling prophecy of indefinite poor outcomes after severe brain injury.
3Current approaches to prognostication
Prognosis after severe brain injury begins at the time of injury, triage and interventions to stabilize the patient to sustain life. Etiology, Glasgow Coma Scale (GCS), structural pathology and location, presence of diffuse axonal injury, prolonged hypoxia, cerebral edema, intracranial hypertension, evidence of subcortical and brainstem herniation, absence of brainstem reflexes and pupillary reactivity are injury characteristics that many times are factors in the acute prognostication of future outcome for the patient (Souter et al., 2015). Individual patient characteristics also impact prognosis such as age at the time of injury (Gosseries et al., 2016; Izzy et al., 2013). A survey of diagnostic and prognostic considerations and practices revealed a multitude of variables that physicians and other professionals consider when providing a prognosis (Formisano et al., 2019; Izzy et al., 2013). Current TBI Best Practice Guidelines endorsed by the American College of Surgeons recommend a waiting period of a 72 hour minimum after severe TBI before consequential care decisions are considered regarding continuation of aggressive care or WOLST (Cryer & Manley, 2015; Souter et al., 2015). However, given the early uncertainty of prognosis and the potential for positive outcomes, the 2018 DoC Practice Guideline Recommendations supported by the American Academy of Neurology recommend deferring an indefinite poor prognosis for recovery until at least 28 days post-injury (Giacino et al., 2018). See Fig. 1.
Fig. 1
AAN DoC practice guideline recommendations related to prognosis.

Prognosis of recovery of consciousness and functional return is uncertain, inaccurate, and fraught with challenges within the acute days and weeks post-injury (van Veen et al., 2021). Various medications, medical comorbidities, complexities and complications can cloud the clinical presentation of severity of neurological injury. Available predictive tools and models are not accurate enough for precise patient-level outcome prediction in the acute setting with 70–80% positive predictive value at best (Brain Trauma Foundation, 2009; Geurts et al., 2014). While such variables cannot provide precise outcome predictions, validated models can help inform discussions of the range of possible outcomes.
Yet, the current healthcare systems, especially in the United States, utilize early prognosis to guide acute medical decisions due to pressures of shorter ICU lengths of stay and progression through the post-acute continuum of care if appropriate for the patient (Wijdicks & Hwang, 2021). Other pressures include a demand for organ donations with a lack of appreciation for potential meaningful outcomes after brain injury compounded by Medicare Conditions for Participation that require organ procurement notification requirements for “imminent death.” (Fins, 2012). In the case of a patient with severe, acute brain injury, it has been suggested that imminent death includes someone on mechanical ventilation in an intensive care unit or emergency department with a low GCS score. These criteria send a message of universally poor outcome to the healthcare team and family for patients with uncertain prognosis and potential for good recovery.
Brain injury medicine currently lacks the science and technology of brain injury biomarkers to guide precision medicine to develop acute prognostic algorithms that can confidently predict recovery of consciousness and return of functional abilities during these early days after injury. It is difficult to provide patient surrogates with meaningful information to guide decisions on continued care within the preferences of the patient, WOLST, and recommendations for discharge disposition after acute care. Acutely, clinicians are unable to accurately counsel families if their loved one will have long-term and life-long disability that will require indefinite 24-hour care or if their loved one will achieve “good” outcome. Since good outcomes are common among individuals with prolonged unconsciousness (Kowalski et al., 2021; McCrea et al., 2021), time (at least the first days –weeks) should be allowed for aggressive treatment, serial assessments, discussions between the clinical team and the family, shared decision-making, and alignment with what is known regarding the patient’s values and preferences.
Given the inaccuracies of early prognostication and the irreversible decisions often made based on such assumptions, it is more prudent to use time-delimited prognostication based on what is most certain at each time point rather than a more definitive rulings in indeterminate cases (Fins, 2013). Dr. Joseph Fins (2013) proposes a helpful analogy of tracking a hurricane across the ocean as the cone of uncertainty narrows over time, and recommends brain injury prognosis along a timeline post-injury mapped on to diagnostic milestones (such as, duration of comatose state, duration of VS, and time to reach MCS). Such phased prognostication should be based on what is known at each stage, informed by serial behavioral evaluation and other available markers (such as, imaging and neurophysiologic data) while acknowledging level of uncertainly.
3.1Prognosis based on etiology of brain injury
Etiology is a significant factor after severe brain injury. There are various etiologies that can lead to acute and prolonged DoC presentation. These can be traumatic or non-traumatic etiologies. Prognosis is substantially dependent on causes of DoC and if the primary cause is readily reversible (Karpenko & Keegan, 2021). However, the most common etiologies leading to prolonged DoC that can challenge diagnosis and prognosis are severe TBI and hypoxic-ischemic brain injury (HIBI) after cardiac arrest (Bagnato et al., 2021; Giacino et al., 2022) and accurate prognostication relies on a thorough understanding of the underlying injury. Limitations in TBI data, including heterogeneity in injury severity, variability in documentation practices, and incomplete follow-up, can hinder accurate prognostication and impede the development of evidence-based guidelines (Roozenbeek et al., 2013).
Regardless of injury severity and heterogeneity of presentation, TBI etiology is associated with a more favorable prognosis and outcome (Avesani et al., 2018; Chiavaroli et al., 2016; Whyte et al., 2009). However, caution must be taken when applying these constructs of etiology and relation to recovery. There is a lack of longitudinal follow-up and data collection infrastructure for patients with non-traumatic brain injury. Those patients that die after HIBI, 40–60% occur due to WOLST and indefinite poor prognosis (Henson et al., 2022; May et al., 2019). A study by May (2019) utilized propensity matching of individuals with HIBI secondary to cardiac arrest and experienced WOLST versus those that did not. May and colleagues found that 21% of patients who did not experience WOLST achieved a good functional outcome (May et al., 2019). It remains unclear if individuals with non-traumatic brain injury are doomed to be in chronic VS after three months.
3.2Prognosis based on time since injury
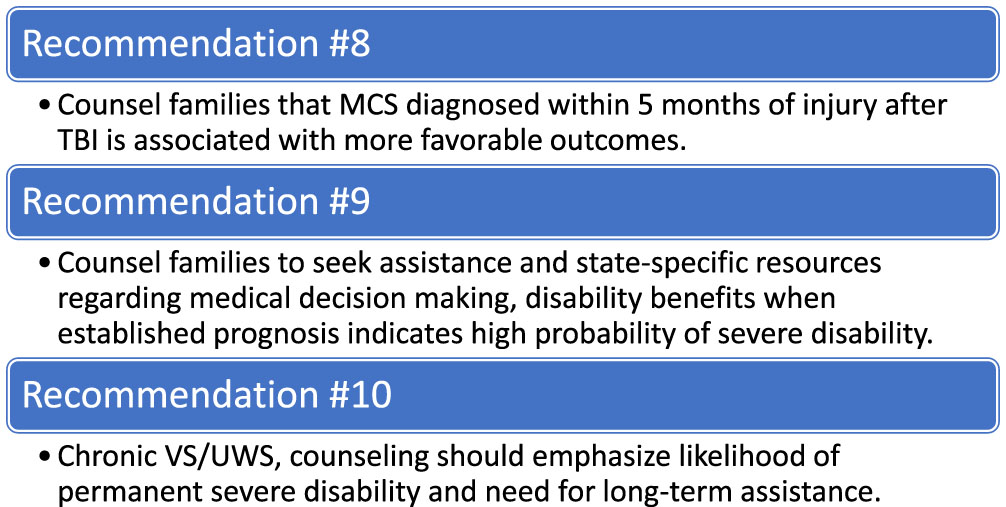
The AAN DoC Practice Guideline Recommendations address temporality from time of injury in relation to etiology of severe brain injury (Giacino et al., 2018). Emergence from VS/UWS is most likely during the first three months in injuries of non-traumatic etiology, while in traumatic etiology emergence is common throughout the first 12 months (Giacino et al., 2018). The Guidelines also concluded that the evidence did not clearly support or refute the prognostic value of time post-injury. Studies do support that the majority of individuals in prolonged traumatic VS/UWS will emerge in the first 6–12 months of injury. Combining data across studies examining time to emergence from prolonged VS/UWS of traumatic etiology have revealed 38% emergence within 3 months, 67% within 6 months and 78% within 12 months (Giacino et al., 2018). Furthermore, 20% of those in VS/UWS at 1 year after TBI or 6 months after non-traumatic brain injury, recovered responsiveness (Estraneo et al., 2010). Though late emergence is possible, severe functional impairment is expected among those with late emergence. The AAN guidelines recommend that as one remains in VS/UWS into the chronic phase (i.e., 3 months for non-traumatic and 12 months for TBI), prognostic counseling should emphasize the likelihood of permanent, severe disability (Giacino et al., 2018).
3.3Prognosis dependent upon diagnosis of level of consciousness
Prognosis is informed by accurate diagnosis of DoC (for example, if the person is in VS/UWS versus MCS or higher) and is critical for appropriate prognostication and treatment planning. As time passes post-injury, one’s diagnosed level of consciousness has prognostic value. For example, those with prolonged DoC (>28 days) with diagnosis of MCS within 5 months of injury have more likely prognosis for greater functional recovery compared to those remaining in VS/UWS (Giacino et al., 2018). Studies have reported high rates of misdiagnosis of up to 40% particularly in distinguishing between the vegetative state and the MCS (Schnakers et al., 2009). Additionally, one’s responsiveness may wax and wane or be artificially low due to a multitude of potential medical and physical confounding factors, further contributing to misdiagnosis. Misdiagnosis can have profound consequences, leading to inappropriate care, potential early WOLST and potential denial of specialized rehabilitation and post-acute care. Various assessment modalities are used to identify behavioral presentation of DoC and to monitor trajectory of recovery along the DoC spectrum. The GCS is the most widely utilized assessment for diagnosis of coma and DoC globally (Formisano et al., 2019; Helbok et al., 2022). However, recent investigation of the GCS sensitivity to accurately identify the behavioral nuances of diagnosing coma, VS/UWS, and MCS is poor (Bodien et al., 2021).
The Coma Recovery Scale-Revised (CRS-R) is a standardized neurobehavioral assessment with proven psychometric properties of validity, reliability and sensitivity in identifying a patient’s level of consciousness. The CRS-R is recommended for use within the AAN DoC Guideline Recommendations and European Academy of Neurology DoC Guidelines to inform diagnosis and prognosis (Giacino et al., 2018; Kondziella et al., 2020; Seel et al., 2010). The CRS-R has also been supported as a tool to inform prognosis by monitoring trajectory or recovery over time (Lucca et al., 2019). Unfortunately, the CRS-R has not been widely implemented within the ICU or acute care settings to assist in diagnosis and prognosis in DoC (Chaturvedi et al., 2021) thus impeding the ability to improve diagnostic accuracy. It is important to note that accurate diagnosis of DoC is also dependent upon multiple assessments overtime. DoC behavioral presentation is fluid and dependent upon changing patient and environmental factors throughout a 24-hour period. In order to achieve confidence that prognosis is accurately informed, it is imperative to ensure that valid assessments have been completed serially (Wannez et al., 2017; Yang et al., 2021).
3.4Prognosis through neuroimaging and electrophysiology
Advances in neuroimaging, electrophysiological studies and brain-computer interface (BCI) has fostered implementation of these technologies to improve clinical understanding of diagnosis and prognosis with DoC. Functional Magnetic Resonance Imaging (fMRI), electroencephalography (EEG), BCI, positron emission topography (PET) and transcranial magnetic stimulation (TMS) have allowed clinicians and researchers to identify emerging neural correlates and biomarkers to better inform diagnosis of DoC and thus improve accuracy of prognosis (Sanz, 2021). One of the most significant findings through use of EEG and fMRI has been the phenomenon of cognitive motor dissociation (CMD) also taxonomized as covert consciousness or covert awareness (Schiff, 2015; Schnakers et al., 2022). CMD is defined as a behaviorally unresponsive patient at the bedside, but through further assessment with fMRI or EEG using resting, passive or active paradigms (Gosseries et al., 2016; Sanz et al., 2021).
Resting and passive paradigms do not require participation of the patient and passive paradigms engage the patient in following commands or yes/no communication through mental imagery, these patients demonstrate through neural modulation (Gosseries et al., 2016; Owen et al., 2006; Schiff et al., 2005; Schnakers et al., 2022). Evidence reports that 14–17% of unresponsive patients demonstrate CMD through advanced technological assessment (Claassen et al., 2019; Edlow et al., 2017; Kondziella et al., 2016).
Further investigation of trajectory of recovery of patients who have been identified as presenting with CMD while in the ICU supports that these patients progress to eventually achieve consciousness and functional return at a higher rate than those patients who are unresponsive and CMD cannot be detected through advanced assessment (Edlow, Claassen; Pan et al., 2020). Detection of MCS and eMCS neuronal modulation through these neuroimaging paradigms is significant to prognosis as those patients identified as MCS present more preserved brain activity and therefore have a better prognosis (Gosseries, 2016). The compendium of evidence supporting the potential presence of CMD in unresponsive patients have led to including neuroimaging and electrophysiological modalities to assess and diagnose preserved consciousness when bedside behavioral assessment is ambiguous in recent DoC Practice Guidelines (Giacino et al., 2018; Kondziella, 2020).
The efficacy of neuroimaging and electrophysiological modalities as part of a multi-modal assessment approach in diagnosis and prognosis in DoC is well supported by published evidence. However, there continues to be limitations in wide-reaching adoption of these approaches due to a lack of feasibility and access to advanced technology such as fMRI, BCI, PET and TMS. Furthermore, clinical expertise is required to implement active paradigms and interpret results. There is an urgency in applying these advanced technologies at the bedside with DoC as unresponsive patients are at great risk of nihilistic prognosis and WOLST (Young et al., 2021).
3.5Other prognostic factors
Additional patient and injury characteristics are important to consider and are supported by past evidence to inform prognosis after severe brain injury. A detailed overview of these factors would require its own manuscript and many patient and injury characteristics are poorly researched and understood to provide significant confidence in prognosis based on a single or cohort of patient and injury factors. Injury severity, age and pre-injury health only account for <35% of outcome variability (Kals et al., 2022). Regardless, characteristics that have been consistently reported to impact prognosis is advanced age, history of premorbid conditions and disability, comorbid polytrauma, history of previous brain injury and level of education have all been found to be patient characteristics that can impact recovery and prognosis after severe brain injury (Mollayeva et al., 2019). A patient characteristic that we have yet to gain an understanding is the role individual genetics play in recovery from brain injury (Kals et al., 2022).
Injury characteristics and severity have been the primary drivers of prognosis after severe brain injury (MRC CRASH Trial Collaborators, 2008; Steyerberg et al., 2008). Evidence suggests injury characteristics that portend poorer prognosis and outcome are CT findings, specifically midline shift, herniation of structures such as the basal cisterns, comorbid cerebral infarction, subarachnoid hemorrhage, diffuse axonal injury, cerebral edema with increased intracerebral hypertension (Stevens & Sutter, 2013). Secondary complications, such as hydrocephalus, infections and respiratory failure have also been found to have significant consequence on prognosis. More frequent complications and medical setbacks, the less probability of a favorable outcome (Ganesh et al., 2013; Whyte & Nakase-Richardson, 2013). As mentioned earlier, there continues to be a lack of empirical evidence and thus poor understanding on the impact of personal genetic factors into recovery.
However, recent advances in funding and scientific collaboration, specifically the Genetic Association In Neurotrauma (GAIN) Consortium are leading efforts to investigate genetic impact on TBI outcomes (Kals et al., 2022). There also is a growing body of knowledge identifying various brain injury biomarkers and endotypes and investigating their impact on prognosis and outcome (Kondziella & Stevens, 2022).
4Communication of prognosis
Despite the inaccuracies and uncertainties, communication about prognosis is important and helps address several goals. Families must be provided the information needed to engage in shared decision-making. The unknown is scary. Information about their loved one, helps establish expectations, promotes adjustment and coping, and facilitates planning. However, the limited data on brain injury prognostic communication suggests we are not doing well with providing this important information. A survey of 117 significant others and 149 people with TBI (n = 130; 50 mild, 12 moderate, and 68 severe) found that 54% were not provided enough information about TBI with approximately 50% indicating they were not told about brain injury symptoms, what recovery to expect, treatment goals, updates on recovery, education materials, or needed rehab services (Biester et al., 2016).
There is abundant literature about communicating prognosis in terminal conditions, such as cancer, that involves progressive deterioration. However, limited evidence exists to guide communicating prognosis after severe brain injury, which starts with coma and progresses to a range of outcomes including death, VS/UWS, varying degrees of disability, and resumption of life as before. Given that the starting point is coma, maybe it isn’t surprising that physician prognoses tend to be inaccurate and overly pessimistic (F. Hammond et al., 2009; Izzy et al., 2013). Brain injury prognostication should not be a one-time discussion. A series of discussions should take place over time with the prognosis updated based on the most current evaluations and information available. With each discussion, the level of uncertainty of the outcome must be acknowledged. Per the AAN evidence-based guidelines, statements suggesting a universally poor prognosis must be avoided in family/surrogate communications during the first 28 days (Giacino et al., 2018). Prognostic statements should be evidence-based and specify the predictors used, time period for the application of predictors, time period for outcome, the specific outcome, level of precision, communication of the diagnosed level of responsiveness, and the prognosis (Giacino et al., 2020) (the range of possible outcomes). Unsubstantiated pessimism must be avoided. The communication should be accurate and understandable.
As nearly half of all Americans lack the capacity to understand and make appropriate health decisions (health literacy), it is important to employ strategies to enhance understanding of the information presented (Institute of Medicine (US) Committee on Health Literacy, 2004). Thus, evidence-based health communication techniques should be employed, such as communication that is patient-centered, clear, confirms understanding, and reinforces the information (Sudore & Schillinger, 2009). Start with assessing what the family already knows and wants to know. Use plain language that avoids jargon and short sentences. Use a pace they can follow and communicate a limited number of points. Ask what questions they have. Check their understanding using teach-back technique, asking them to describe what they heard so you can make sure you explained things clearly. Consider supplementing the communication with educational materials that incorporate graphs or pictures and is written at a 5th-grade level. This conversation should occur in a quiet and comfortable location absent interruptions. Track their emotions and respond with empathy.
The communication should foster hope given the uncertainties and the evidence for possible good outcomes. Strategies to facilitate hope and coping include: emphasizing what can be done, pointing out available support, reassuring that you/system will be there throughout the trajectory (or, spell out alternatives if unavailable), exploring the patient/family goals, identifying areas where control can be fostered, respecting patient’s wishes, discussing coping strategies, and recognizing the spectrum of hope (hope for full resolution while accepting effects of injury) (Clayer, 2007).
There are a number of considerations when incorporating statistics in the communication. Many may not find statistics helpful. So, avoid quoting statistics unless you ask first. When using statistics, it is important to also first know what outcome the family is interested in knowing about. Simply and correctly conveying a statistic requires full knowledge of the study that the statistic is based on and making sure it is applicable to the clinical situation, considering, for example, the study population, the outcome measure, and the follow-up window. Did the study report confidence intervals? Were the study findings on mortality impacted by WOLST? How would you translate poor recovery or severe disability, and are you interpreting it correctly? Should the findings of a six-month outcome study be used to when the family may want to know about long-term outcome? When quoting statistics, it is helpful to double-frame with positive and negative outcomes. An example of double framing is “6 out of 10 people with this type of injury are independent in self-care one year after injury; that is, less than 4 in 10 require some assistance with self-care.” Avoid average outcomes, as the average does not convey the possible range of outcomes.
Rather than using statistics, you might ask if it would be helpful for you to talk about best and worst case scenarios. Best case/worst case scenarios have been advocated in shared decision-making to help families consider how they might experience each scenario and the potential downstream consequences (Kruser et al., 2015). The best/worst/most likely case determination should be evidence-based, correctly translated, and tempered by the level of certainty. Remember terms like “Good Recovery” will mean something different to a family member, and possibly to the clinician, from the actual study measure definition. See Fig. 2.
Fig. 2
AAN DoC practice guidelines related to prognostic counseling recommendations.
5Prognosis and access to specialized post-acute care and rehabilitation
The acknowledgement of a large percentage of DoC patients eventually achieving consciousness and a level of functional recovery supports the need for access to specialized rehabilitation to support optimal outcomes. A compendium of evidence have demonstrated the need and benefit of specialized post-acute rehabilitation for individuals in DoC (Giacino et al., 2022, 2018, 2020; Giacino & Trott, 2004; Hux, 2019; Kowalski et al., 2021; Mackay et al., 1992; Pignat et al., 2015; Roberts & Greenwood, 2019; Seel et al., 2013; Tepas et al., 2009; Weaver et al., 2022).
Access to rehabilitation services is dependent upon prognosis of severe brain injury. When clinical teams apply a poor prognosis and nilhistic bias a patient may be labeled as not appropriate for referral to rehabilitation. This can limit access to rehabilitation services within the ICU as well as in post-acute care. When clinical teams apply a poor prognosis or counseling families toward WOLST, rehabilitation services may be deemed as futile. Contrarily, there is evidence supporting the initiation of early rehabilitation even within the ICU settings for individuals with severe brain injury and DoC (Bartolo et al., 2016; Moyer et al., 2021; Roth et al., 2013; Yen et al., 2022).
There is a misnomer in accessing rehabilitation services that the patient must be able to participate in a rehabilitation program. This is a barrier to rehabilitation inert to the DoC population. Strong advocacy from the clinical team is required to overcome the barriers for patients in DoC in accessing rehabilitation. Specialized rehabilitation programs serving patients and families experiencing DoC have published recommendations guiding services and practices for post-acute care settings that can be followed to ensure quality interdisciplinary care for the individual in DoC (Giacino et al., 2020).
6Enhancing recovery in DoC
Neuroplasticity refers to the brain’s ability to reorganize and adapt its structure and function in response to injury or new experiences. In the context of severe brain injury, understanding the neuroplastic changes in severe brain injury has implications for prognosis and predicting recovery outcomes. Studies have demonstrated that severe brain injury can induce significant neuroplastic changes in various regions of the brain. For example, functional magnetic resonance imaging (fMRI) studies have revealed functional reorganization in the motor cortex following severe traumatic brain injury (TBI). The intact areas surrounding the lesion site can undergo functional reorganization and compensate for the damaged regions, leading to improvements in motor function (Grefkes & Fink, 2020). Structural neuroimaging studies using diffusion tensor imaging (DTI) have shown that severe brain injury can lead to white matter reorganization and the formation of new neural connections, facilitating functional recovery (Armstrong et al., 2016).
Research has also demonstrated that individuals with severe brain injury can exhibit neuroplasticity in cognitive domains. Functional and structural changes have been observed in the frontal and parietal regions involved in attention, memory, and executive functions. These changes are associated with improvements in cognitive abilities over time (Bonnelle et al., 2011). The mechanisms underlying neuroplasticity in severe brain injury are complex and involve various cellular and molecular processes. Studies have shown that neuroplastic changes following brain injury involve synaptic remodeling, neurogenesis, axonal sprouting, and changes in neurotransmitter systems (Murphy & Corbett, 2009). These processes contribute to the rewiring of neural circuits, enabling functional recovery.
These changes contribute to the recovery of motor and cognitive functions. Recognizing and harnessing the neuroplasticity of the brain through appropriate rehabilitation interventions can optimize recovery outcomes for individuals with DoC. As mentioned previously, evidence demonstrates the efficacy of specialized rehabilitation. However, there continues to be a lack of evidence through quantitative research methodologies such as controlled trials to investigate the efficacy of specific rehabilitation interventions such as intensive mobilization, sensory stimulation, and use of assistive technology. Furthermore, what studies do exist have poor reporting of dosing and specifics of interventions (Murtaugh et al., 2023). Thus, more robust research utilizing quality methodological approaches is crucial to identify what rehabilitations are most effective for patients with DoC to improve recovery.
6.1Promote recovery of consciousness through pharmacotherapy
Medications are commonly used to enhance cognitive recovery following brain injury. While there is no definitive pharmacological intervention for cognitive restoration, certain medications have been studied for their potential benefits. See Table 2.
Table 2
Level of evidence of commonly used neurostimulants after TBI (Plantier et al., 2016)
| Medication | Article | Level of Evidence |
| Sertraline | Lee et al., 2005 | Level 2, Grade B |
| Methayler et al., (2001) | Level 2, Grade B | |
| Methylphenidate | Lee et al., 2005 | Level 2, Grade B |
| Amantadine | Hammond et al., 2014 | Level 1, Grade A |
Plantier, D., Luauté, J., & SOFMER group (2016). Drugs for behavior disorders after traumatic brain injury: Systematic review and expert consensus leading to French recommendations for good practice. Annals of Physical and Rehabilitation Medicine, 59(1), 42–57. https://doi.org/10.1016/j.rehab.2015.10.003.
Cholinesterase inhibitors, such as donepezil (Aricept) and rivastigmine (Exelon), are commonly prescribed for the treatment of cognitive impairment in Alzheimer’s disease. These medications work by inhibiting the breakdown of acetylcholine, a neurotransmitter involved in memory and attention processes. Some studies have explored their use in brain injury patients to enhance cognitive recovery (Walker et al., 2004; Whelan et al., 2000).
Methylphenidate (Ritalin), a central nervous system stimulant enhances dopamine and norepinephrine activity, which may improve attention and alertness (Barra et al., 2022). As responses to methylphenidate can vary, monitoring for side effects in the acute setting, such as increased heart rate and blood pressure, is warranted.
Amantadine is FDA-approved as an antiviral and anti-Parkinson’s medication that acts as a dopamine agonist. It has been used to enhance cognitive recovery in individuals with brain injury. Findings of a randomized, controlled trial by Giacino and Whyte et al. suggests that amantadine 100 mg –200 mg twice daily likely hastens functional recovery among inpatients 4 –16 weeks post-TBI with MCS or VS/UWS (Barra et al., 2022; Giacino et al., 2018; Giacino et al., 2012). Adequate renal clearance is needed to eliminate this medication that cannot be dialyzed.
The antidepressant medications Selective Serotonin Reuptake Inhibitors (SSRIs) and Serotonin-Norepinephrine Reuptake Inhibitors (SNRIs) are frequently prescribed to improve mood regulation and enhance participation in rehabilitation efforts (Hicks et al., 2021).
Zolpidem, a hypnotic GABA-A receptor inhibitor commonly used for sleep induction, has been documented to have a paradoxical effect among 5% of individuals with DoC (Whyte et al., 2014). Research is needed to better understand the mechanism by which zolpidem has this effect on enhancing consciousness and how and when it could be used to enhance function.
6.2Rehabilitation enhancing recovery
Patients with DoC as a result of severe brain injury, often face a prolonged stay in the ICU, where they are at risk of developing complications such as muscle weakness, contractures, pressure ulcers, and respiratory (Banerjee et al., 2011; Desai et al., 2011). Immobility and prolonged bed rest can exacerbate these complications and delay recovery. Early mobilization and rehabilitation interventions, including passive range of motion exercises, sitting on the edge of the bed, standing, and walking, aim to mitigate the adverse effects of immobility and promote functional (Bartolo et al., 2017; Naess et al., 2020; Roth et al., 2013) recovery.
Implementing early mobilization in the ICU requires a multidisciplinary approach involving healthcare providers, including physicians, nurses, physical therapists, and occupational therapists, among others (Greiss et al., 2016; Naess et al., 2020). Numerous studies have highlighted the benefits of early mobilization in patients with DoC in the ICU. A systematic review by Lang et al. (2020) analyzed multiple studies involving critically ill patients and demonstrated that early mobilization interventions were associated with a reduction in ICU-acquired weakness, shorter duration of mechanical ventilation, shorter ICU and hospital stays, and improved functional outcomes. While specific data on the effects of early mobilization in patients with DoC are limited, anecdotal evidence and case reports suggest that early mobilization can promote improvements in arousal, consciousness, and functional recovery in some individuals.
Individuals with DoC have continued medical needs that warrant continued physician and nursing management in an inpatient rehabilitation setting. Discharge disposition that has specialized interdisciplinary approach and expertise to meet the medical, functional, and neurobehavioral needs of the patient to enhance continued recovery of consciousness and function is crucial. A growing body of evidence suggests that patients with severe brain injury who have access to specialized post-acute rehabilitation services have a higher probability of achieving functional independence and ongoing improvement in long-term outcome (Giacino et al., 2018; Kowalski et al., 2021). As mentioned earlier, several studies have emphasized the importance of rehabilitation in DoC. A systematic review by Weaver (2023) analyzed the existing literature on the effectiveness of rehabilitation interventions in patients with DoC and found evidence supporting the positive impact of various rehabilitation interventions (Weaver et al., 2023). The review highlighted the potential benefits of various rehabilitation approaches, including physical therapy, occupational therapy, speech therapy, and cognitive rehabilitation. It emphasized the need for tailored and individualized rehabilitation programs to address the specific needs and goals of each patient (Weaver et al., 2023).
6.3Impedance to recovery
Medical issues can significantly impact the recovery process in individuals with brain injury/DoC. Awareness, identification, and management of these medical issues are crucial for optimizing outcomes. Medical factors can also influence assessment accuracy, which can influence diagnosis and lead to false prognoses or delays in recovery. Recognizing the factors influencing accurate assessment is vital for avoiding false prognoses and ensuring appropriate interventions.
Medical complications impeding recovery can be difficult to detect, with many conditions presenting simply as failure to improve or a plateau in recovery. A high index of suspicion is needed as well as a plan for routine screening to detect some of the conditions. Some of the common medical culprits impeding recovery and masking responsiveness include occult seizure, post-traumatic hydrocephalus, hygromas, endocrine dysfunction, neurostorming, severe spasticity, sleep/wake-cycle disruption, iatrogenic sedation and infections (Whyte et al., 2013). See Table 3.
Table 3
Medical conditions potentially impeding recovery of consciousness
| Seizures | Hydrocephalus | Hygroma |
| Neurological post-operative complications | Neuroendocrine abnormalities | Paroxysmal sympathetic hyperactivity |
| Sleep/Wake cycle disturbance | Sedating medications | Infections |
Seizures commonly occur following brain injury and can have detrimental effects on recovery. They can exacerbate brain damage, impede the restoration of consciousness, and hinder functional improvements (Vespa et al., 1999). Early detection, appropriate management, and seizure control strategies are crucial for optimizing recovery outcomes.
Hydrocephalus, characterized by the accumulation of cerebrospinal fluid, is a frequent complication following brain injury. It can lead to increased intracranial pressure, causing further brain damage and hindering recovery. Timely diagnosis, monitoring, and appropriate interventions, such as shunt placement or endoscopic third ventriculostomy, are essential to optimize recovery outcomes (Arnts et al., 2020; Ganesh et al., 2013).
Hygromas and postoperative complications. Hygromas, collections of cerebrospinal fluid, can develop because of surgical interventions following brain injury. These complications can exert pressure on brain tissue, hinder recovery, and lead to additional neurological deficits. Early detection, prompt intervention, and appropriate management, such as drainage or shunt placement, are necessary to minimize their impact on recovery (Signorelli et al., 2020).
Neuroendocrine abnormalities. Brain injury can disrupt the normal functioning of the neuroendocrine system, leading to hormonal imbalances. These abnormalities can significantly impact recovery by affecting arousal, cognition, and overall neurological functioning (Javed et al., 2015). Laboratory screening should be considered in cases of DoC. Close monitoring of hormone levels and appropriate hormone replacement therapies may be necessary to mitigate the impact of neuroendocrine abnormalities on recovery.
Paroxysmal sympathetic hyperactivity, also termed “neurostorming”, a sudden and severe autonomic dysfunction observed in individuals with severe brain injury, can result in fluctuations in vital signs, increased muscle tone, and impaired recovery. Prompt recognition, appropriate monitoring, and supportive management are essential in minimizing the impact of neurostorming on the recovery process (Meyfroidt et al., 2017).
Spasticity, characterized by increased muscle tone and involuntary contractions, can prevent response to assessments and impede rehabilitation efforts and functional recovery. Multidisciplinary interventions, including physical therapy, medication, botulinum toxin injections and intrathecal baclofen therapy, are crucial for managing spasticity and enhancing recovery outcomes (Thibaut et al., 2018; Zhang et al., 2021).
Disruption of sleep/wake cycle with excessive daytime sleepiness and/or profound sleep deprivation is common in acute TBI. The assessment of sleep/wake cycles and promotion of restorative sleep is important in the recovery from severe brain injury. Sleep medications are sometimes prescribed to improve sleep quality and regulate sleep-wake cycles. While medications to promote sleep may be helpful, the prescriber should avoid agents with anticholinergic properties, common among sleeping aids. Monitoring for side effects is warranted (Cacciatore et al., 2021).
Medications commonly used in the management of acute DoC, such as sedatives, anesthetics, and antiepileptic drugs, can influence recovery. Inappropriate medication use, over-sedation, and iatrogenic factors can delay emergence from DoC and impair functional recovery. Careful medication management, individualized treatment plans, and minimizing iatrogenic factors are essential for optimizing recovery outcomes (Strens et al., 2004).
7Future advances in prognostic confidence in DoC
The future of neuroprognostication holds great promise, with ongoing efforts to refine existing prognostic tools and develop novel techniques to promote precision medicine to improve accuracy and confidence in prognosis for recovery of consciousness and functional outcome. The concept of precision medicine, tailored to the individual characteristics and underlying pathophysiology, holds promise in improving outcomes for individuals with DoC. By identifying specific endotypes, subtypes of DoC based on distinct biological mechanisms, treatment approaches can be customized to target the underlying pathology and maximize recovery potential. A study by Bodien et al. (2017) highlighted the potential of precision medicine in predicting outcomes and guiding treatment decisions in individuals with severe brain injury. Incorporating advanced neuroimaging modalities, such as functional MRI and EEG, alongside clinical assessments, can provide a more comprehensive understanding of the brain’s functional integrity and potential for recovery (Bodien et al., 2017). Research by Vanhaudenhuyse et al. (2010) demonstrated the utility of functional MRI in predicting outcomes in patients with DoC, highlighting its potential as a prognostic tool (Vanhaudenhuyse et al., 2010). To improve accuracy in diagnosis, several factors need to be addressed. These include the standardization of diagnostic criteria and definition of DoC (Helbok et al., 2022), the implementation of multi-modal consciousness assessment, and increased awareness and training among healthcare professionals involved in the care of individuals with DoC.
The emphasis on improving diagnosis and prognosis in DoC care has driven the recent development of multiple international entities focused on facilitating collaboration of experts to engage in scientific discovery, advocacy, knowledge translation and implementation of quality DoC care. The Curing Coma campaign is an example of this effort has emerged as a dynamic force for raising awareness and promoting research in the field of DoC. Focusing on understanding the underlying mechanisms of consciousness and developing innovative interventions, this campaign has the potential to revolutionize prognostication, treatment strategies, and patient outcomes (Mainali et al., 2022; Provencio et al., 2020).
Realizing these prognostic advancements and improving outcomes for individuals with DoC requires cultural changes. There is a need for increased awareness and understanding of DoC among the general public, healthcare professionals, and policymakers. This includes promoting ethical discussions surrounding treatment decisions, fostering open communication with families, and ensuring access to comprehensive rehabilitation services. The work of Fins & Bernat (2018) emphasized the importance of societal attitudes and cultural shifts in providing appropriate care and support for individuals with DoC (Fins & Bernat, 2018).
8Conclusion
Prognosis and recovery in DoC have long been areas of intense research and clinical interest due to the need for improved accuracy of predicting outcomes. Advancements in neuroprognostication techniques and a deeper understanding of the factors influencing accurate diagnosis have provided valuable insights into the potential outcomes and recovery trajectories for individuals with DoC. However, there is considerable uncertainty and inaccuracies in applying this information to the individual patient in the acute setting. Consequently, the current approach to prognosis should be humility knowing the incredible level of uncertainty in the early days and weeks post-injury.
Positive outcomes among individuals with prolonged unconsciousness are well documented. Thus, early WOLST can be a self-fulfilling prophecy of poor outcome and eliminate the possibility of recovery. In indeterminate cases, aggressive treatment should be provided, pending further assessment, discussions between the clinical team and the family, shared decision-making, and alignment with what is known regarding the patient’s values and preferences. The AAN evidence-based guidelines (Giacino et al., 2018) recommend providers avoid statements indicating a universally poor prognosis when communicating with families during the first 28-days post-brain injury. Instead, it is recommended that providers use a time-delimited approach that acknowledges uncertainty and is informed by data available along the post-injury course.
Neuroprognostication has been, and will continue to be, complex and challenging for care providers and clinical teams. Comprehension of the various factors that can positively or negatively affect recovery of consciousness and functional recovery is required to inform prognosis. Furthermore, we now have evidence-based recommendations that can guide practice approach to diagnosis and prognosis in DoC, including recommendations to advocate and support access to specialized rehabilitation to support recovery. Finally, continued research infrastructure and focus on precision medicine, DoC endotypes and genomics will expand our accuracy and confidence in providing prognosis to families in the future.
Conflict of interest
The authors do not have any conflict of interest to disclose related to this manuscript.
References
1 | Armstrong, R. C. , Mierzwa, A. J. , Marion, C. M. , Sullivan, G. M. ((2016) ) White matter involvement after TBI: Clues to axon and myelin repair capacity. Experimental Neurology 275 Pt 3: , 328–333. https://doi.org/10.1016/j.expneurol.2015.02.011 |
2 | Arnts, H. , van Erp, W. S. , Sanz, L. R. D. , Lavrijsen, J. C. M. , Schuurman, R. , Laureys, S. , Vandertop, W. P. , van den Munckhof, P. ((2020) ) The Dilemma of Hydrocephalus in Prolonged Disorders of Consciousness. Journal of Neurotrauma 37: (20), 2150–2156. https://doi.org/10.1089/neu.2020.7129 |
3 | Avesani, R. , Dambruoso, F. , Scandola, M. , Formisano, R. , De Tanti, A. , Ferro, S. , Smania, N. , Roncari, L. , Rossato, E. ((2018) ) Epidemiological and clinical characteristics of patients in a vegetative state in Italian rehabilitation units. What about outcome? Functional Neurology 33: (2), 97–103. |
4 | Bagnato, S. , D’Ippolito, M. E. , Boccagni, C. , De Tanti, A. , Lucca, L. F. , Nardone, A. , Salucci, P. , Fiorilla, T. , Pingue, V. , Gennaro, S. , Ursino, M. , Colombo, V. , Barone, T. , Rubino, F. , Andriolo, M. ((2021) ) Sustained Axonal Degeneration in Prolonged Disorders of Consciousness. Brain Sciences 11: (8), 1068. https://doi.org/10.3390/brainsci11081068 |
5 | Banerjee, A. , Girard, T. D. , Pandharipande, P. ((2011) ) The complex interplay between delirium, sedation, and early mobility during critical illness: Applications in the trauma unit. Current Opinion in Anaesthesiology 24: (2), 195–201. https://doi.org/10.1097/ACO.0b013e3283445382 |
6 | Barra, M. E. , Edlow, B. L. , Brophy, G. M. ((2022) ) Pharmacologic Therapies to Promote Recovery of Consciousness. Seminars in Neurology 42: (3), 335–347. https://doi.org/10.1055/s-0042-1755271 |
7 | Bartolo, M. , Bargellesi, S. , Castioni, C. A. , Bonaiuti, D. ((2016) ) Early rehabilitation for severe acquired brain injury in intensive care unit: Multicenter observational study. EUROPEAN JOURNAL OF PHYSICAL AND REHABILITATION MEDICINE 52: (1), 11. |
8 | Bartolo, M. , Bargellesi, S. , Castioni, C. A. , Intiso, D. , Fontana, A. , Copetti, M. , Scarponi, F. , Bonaiuti, D. , Intensive Care and Neurorehabilitation Italian Study Group ((2017) ) Mobilization in early rehabilitation in intensive care unit patients with severe acquired brain injury: An observational study. Journal of Rehabilitation Medicine 49: (9), 715–722. https://doi.org/10.2340/16501977-2269 |
9 | Biester, R. C. , Krych, D. , Schmidt, M. J. , Parrott, D. , Katz, D. I. , Abate, M. , Hirshson, C. I. ((2016) ) Individuals With Traumatic Brain Injury and Their Significant Others’ Perceptions of Information Given About the Nature and Possible Consequences of Brain Injury: Analysis of a National Survey.; quiz E. Professional Case Management 21: (1), 23–33quiz E3-4. https://doi.org/10.1097/NCM.0000000000000121 |
10 | Bodien, Y. G. , Barra, A. , Temkin, N. R. , Barber, J. , Foreman, B. , Vassar, M. , Robertson, C. , Taylor, S. R. , Markowitz, A. J. , Manley, G. T. , Giacino, J. T. , Edlow, B. L. , the TRACK-TBI Investigators Badjatia, N. , Duhaime, A.-C. , Ferguson, A. R. , Gaudette E. PhD , Gopinath, S. , Keene, C. D. , Zafonte, R. ((2021) ) Diagnosing Level of Consciousness: The Limits of the Glasgow Coma Scale Total Score. Journal of Neurotrauma 38: (23), 3295–3305. https://doi.org/10.1089/neu.2021.0199 |
11 | Bodien, Y. G. , Giacino, J. T. , Edlow, B. L. ((2017) ) Functional MRI Motor Imagery Tasks to Detect Command Following in Traumatic Disorders of Consciousness. Frontiers in Neurology 8: , 688. https://doi.org/10.3389/fneur.2017.00688 |
12 | Bonnelle, V. , Leech, R. , Kinnunen, K. M. , Ham, T. E. , Beckmann, C. F. , De Boissezon, X. , Greenwood, R. J. , Sharp, D. J. ((2011) ) Default mode network connectivity predicts sustained attention deficits after traumatic brain injury. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 31: (38), 13442–13451. https://doi.org/10.1523/JNEUROSCI.1163-11.2011 |
13 | Brain Trauma Foundation: EARLY INDICATORS OF PROGNOSIS IN SEVERE TRAUMATIC BRAIN INJURY. (2009). Brain Trauma Foundation. https://braintrauma.org/coma/guidelines/prognosis |
14 | Cacciatore, M. , Magnani, F. G. , Leonardi, M. , Rossi Sebastiano, D. , Sattin, D. ((2021) ) Sleep Treatments in Disorders of Consciousness: A Systematic Review. Diagnostics (Basel, Switzerland) 12: (1), 88. https://doi.org/10.3390/diagnostics12010088 |
15 | Chaturvedi, J. , Mudgal, S. K. , Venkataram, T. , Gupta, P. , Goyal, N. , Jain, G. , Sharma, A. K. , Sharma, S. K. , Bendok, B. R. ((2021) ) Coma recovery scale: Key clinical tool ignored enough in disorders of consciousness. Surgical Neurology International 12: , 93. https://doi.org/10.25259/SNI_935_2020 |
16 | Chiavaroli, F. , Derraik, J. G. B. , Zani, G. , Lavezzi, S. , Chiavaroli, V. , Sherwin, E. , Basaglia, N. ((2016) ) Epidemiology and clinical outcomes in a multicentre regional cohort of patients with severe acquired brain injury. Disability and Rehabilitation 38: (20), 2038–2046. https://doi.org/10.3109/09638288.2015.1111439 |
17 | Claassen, J. , Doyle, K. , Matory, A. , Couch, C. , Burger, K. M. , Velazquez, A. , Okonkwo, J. U. , King, J.-R. , Park, S. , Agarwal, S. , Roh, D. , Megjhani, M. , Eliseyev, A. , Connolly, E. S. , Rohaut, B. ((2019) ) Detection of Brain Activation in Unresponsive Patients with Acute Brain Injury. New England Journal of Medicine 380: (26), 2497–2505. https://doi.org/10.1056/NEJMoa1812757 |
18 | Clayer, M. T. ((2007) ) Clinical practice guidelines for communicating prognosis and end-of-life issues with adults in the advanced stages of a life-limiting illness, and their caregivers. The Medical Journal of Australia 187: (8), 478. https://doi.org/10.5694/j.1326-5377.2007.tb01374.x |
19 | Cryer, G. , Manley, G. (2015). ACS/TQIP Best Practices in the Management of Traumatic Brain Injury. American College of Surgeons. https://www.facs.org/media/mkej5u3b/tbiguidelines.pdf |
20 | DeMario, B. S. , Stanley, S. P. , Truong, E. I. , Ladhani, H. A. , Brown, L. R. , Ho, V. P. , Kelly, M. L. ((2022) ) Predictors forWithdrawal of Life-Sustaining Therapies in Patients With TraumaticBrain Injury: A Retrospective Trauma Quality Improvement ProgramDatabase Study. e-e. Neurosurgery 91: (2), 50. https://doi.org/10.1227/neu.0000000000002020 |
21 | Desai, S. V. , Law, T. J. , Needham, D. M. ((2011) ) Long-termcomplications of critical care. Critical CareMedicine 39: (2), 371–379. https://doi.org/10.1097/CCM.0b013e3181fd66e5 |
22 | Edlow, B. L. , Chatelle, C. , Spencer, C. A. , Chu, C. J. , Bodien, Y. G. , O’Connor, K. L. , Hirschberg, R. E. , Hochberg, L. R. , Giacino, J. T. , Rosenthal, E. S. , Wu, O. ((2017) ) Early detection of consciousness in patients with acute severe traumatic brain injury. Brain 140: (9), 2399–2414. https://doi.org/10.1093/brain/awx176 |
23 | Estraneo, A. , Moretta, P. , Loreto, V. , Lanzillo, B. , Santoro, L. , Trojano, L. ((2010) ) Late recovery after traumatic, anoxic, or hemorrhagic long-lasting vegetative state. Neurology 75: (3), 239–245. https://doi.org/10.1212/WNL.0b013e3181e8e8cc |
24 | Fins, J. J. (2007). Ethics of clinical decision making and communication with surrogates. In Posner, C. Saper, N. D. Schiff,&F. Plum (Eds.), Plum and Posner’s diagnosis of stupor and coma (4th ed.). Oxford University Press. |
25 | Fins, J. J. ((2012) ) Severe Brain Injury and Organ Solicitation: A Call for Temperance. AMA Journal ofEthics 14: (3), 221–226. https://doi.org/10.1001/virtualmentor.2012.14.3.stas1-1203 |
26 | Fins, J. J. ((2013) ) Disorders of Consciousness and Disordered Care: Families, Caregivers, and Narratives of Necessity. Archives of Physical Medicine and Rehabilitation 94: (10), 1934–1939. https://doi.org/10.1016/j.apmr.2012.12.028 |
27 | Fins, J. J. (2015). Rights come to mind: Brain injury, ethics, and the struggle for consciousness. Cambridge University Press. |
28 | Fins, J. J. , Bernat, J. L. ((2018) ) Ethical, palliative, and policy considerations in disorders of consciousness. Neurology 91: (10), 471–475. https://doi.org/10.1212/WNL.0000000000005927 |
29 | Formisano, R. , Giustini, M. , Aloisi, M. , Contrada, M. , Schnakers, C. , Zasler, N. , Estraneo, A. ((2019) ) An International survey on diagnostic and prognostic protocols in patients with disorder of consciousness. Brain Injury 33: (8), 974–984. https://doi.org/10.1080/02699052.2019.1622785 |
30 | Ganesh, S. , Guernon, A. , Chalcraft, L. , Harton, B. , Smith, B. , Louise-Bender Pape, T. ((2013) ) Medical Comorbidities in Disorders of Consciousness Patients and Their Association With Functional Outcomes. Archives of Physical Medicine and Rehabilitation 94: (10), e3. https://doi.org/10.1016/j.apmr.2012.12.026 |
31 | Geurts, M. , Macleod, M. R. , Van Thiel, G.J.M.W. , Van Gijn, J. , Kappelle, L. J. , Van Der Worp, H. B. ((2014) ) End-of-life decisions in patients with severe acute brain injury. The Lancet Neurology 13: (5), 515–524. https://doi.org/10.1016/S1474-4422(14)70030-4 |
32 | Giacino, J. , J. T. , Katz, D. I. , Schiff, N. D. , Whyte, J. , Ashman, E. J. , Ashwal, S. , Barbano, R. , Hammond, F. M. , Laureys, S. , Ling, G. S. F. , Nakase-Richardson, R. , Seel, R. T. , Yablon, S. , Getchius, T. S. D. , Gronseth, G. S. , Armstrong, M. J. ((2018) ) Practice Guideline Update Recommendations Summary: Disorders of Consciousness. Archives of Physical Medicine and Rehabilitation 99: (9), 1699–1709. https://doi.org/10.1016/j.apmr.2018.07.001 |
33 | Giacino, J. , J. T. , Whyte, J. , Nakase-Richardson, R. , Katz, D. I. , Arciniegas, D. B. , Blum, S. , Day, K. , Greenwald, B. D. , Hammond, F. M. , Pape, T. B. , Rosenbaum, A. , Seel, R. T. , Weintraub, A. , Yablon, S. , Zafonte, R. D. , Zasler, N. ((2020) ) Minimum Competency Recommendations for Programs That Provide Rehabilitation Services for Persons With Disorders of Consciousness: A Position Statement of the American Congress of Rehabilitation Medicine and the National Institute on Disability, Independent Living and Rehabilitation Research Traumatic Brain Injury Model Systems. Archives of Physical Medicine and Rehabilitation 101: (6), 1072–1089. https://doi.org/10.1016/j.apmr.2020.01.013 |
34 | Giacino, J. , Katz, D. I. , Schiff, N. D. , Bodien, Y. (2022). Assessment and rehabilitative management of individuals with disorders of consciousness. In N. D. Zasler, D. I. Katz, R. D. Zafonte, & D. B. Arciniegas (Eds.), Brain Injury Medicine: Principles and Practice (third, pp. 447-461). Demos Medical Publishing. |
35 | Giacino, J. T. , Katz, D. I. , Schiff, N. D. , Whyte, J. , Ashman, E. J. , Ashwal, S. , Barbano, R. , Hammond, F. M. , Laureys, S. , Ling, G. S. F. , Nakase-Richardson, R. , Seel, R. T. , Yablon, S. , Getchius, T. S. D. , Gronseth, G. S. , Armstrong, M. J. ((2018) ) Comprehensive systematic review update summary: Disorders of consciousness: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Neurology 91: (10), 461–470. https://doi.org/10.1212/WNL.0000000000005928 |
36 | Giacino, J. T. , Trott, C. T. ((2004) ) Rehabilitative management of patients with disorders of consciousness: Grand rounds. The Journal of Head Trauma Rehabilitation 19: (3), 254–265. https://doi.org/10.1097/00001199-200405000-00006 |
37 | Giacino, J. T. , Whyte, J. , Bagiella, E. , Kalmar, K. , Childs, N. , Khademi, A. , Eifert, B. , Long, D. , Katz, D. I. , Cho, S. , Yablon, S. A. , Luther, M. , Hammond, F. M. , Nordenbo, A. , Novak, P. , Mercer, W. , Maurer-Karattup, P. , Sherer, M. ((2012) ) Placebo-controlled trial of amantadine for severe traumatic brain injury. The New England Journal of Medicine 366: (9), 819–826. https://doi.org/10.1056/NEJMoa1102609 |
38 | Gosseries, O. , Pistoia, F. , Charland-Verville, V. , Carolei, A. , Sacco, S. , Laureys, S. ((2016) ) The Role ofNeuroimaging Techniques in Establishing Diagnosis, Prognosis and Therapy in Disorders of Consciousness. TheOpen Neuroimaging Journal 10: (1), 52–68. https://doi.org/10.2174/1874440001610010052 |
39 | Grefkes, C. , Fink, G. R. ((2020) ) Recovery from stroke: Current concepts and future perspectives. Neurological Research and Practice 2: , 17. https://doi.org/10.1186/s42466-020-00060-6 |
40 | Greiss, C. , Yonclas, P. P. , Jasey, N. , Lequerica, A. , Ward, I. , Chiaravalloti, N. , Felix, G. , Dabaghian, L. , Livingston, D. H. ((2016) ) Presence of a dedicated trauma center physiatrist improves functional outcomes following traumatic brain injury. The Journal of Trauma and Acute Care Surgery 80: (1), 70–75. https://doi.org/10.1097/TA.0000000000000890 |
41 | Hammond, F. M. , Giacino, J. T. , Nakase Richardson, R. , Sherer, M. , Zafonte, R. D. , Whyte, J. , Arciniegas, D. B. , Tang, X. ((2019) ) Disorders of Consciousness due to Traumatic Brain Injury: Functional Status Ten Years Post-Injury. Journal of Neurotrauma 36: (7), 1136–1146. https://doi.org/10.1089/neu.2018.5954 |
42 | Hammond, F. M. , Katta-Charles, S. , Russell, M. B. , Zafonte, R. D. , Claassen, J. , Wagner, A. K. , Puybasset, L. , Egawa, S. , Laureys, S. , Diringer, M. , Stevens, R. D. , Curing Coma Campaign and its Contributing Members. ((2021) ) Research Needs for Prognostic Modeling and Trajectory Analysis in Patients with Disorders of Consciousness. Neurocritical Care 35: (Suppl 1), 55–67. https://doi.org/10.1007/s12028-021-01289-y |
43 | Hammond, F. M. , Katta-Charles, S. , Russell, M. B. , Zafonte, R. D. , Claassen, J. , Wagner, A. K. , Puybasset, L. , Egawa, S. , Laureys, S. , Diringer, M. , Stevens, R. D. the Curing Coma Campaign and its Contributing Members. ((2021) ) Research Needs for Prognostic Modeling and Trajectory Analysis in Patients with Disorders of Consciousness. Neurocritical Care 35: (S1), 55–67. https://doi.org/10.1007/s12028-021-01289-y |
44 | Hammond, F. , Natarajan, S. , Toomer, A. , Huynh, T. , Norton, J. (2009). Accuracy of predicting 6- month outcome at 48 hours following severe traumatic brain injury: A pilot study. Poster Presentation. |
45 | Helbok, R. , Rass, V. , Beghi, E. , Bodien, Y. G. , Citerio, G. , Giacino, J. T. , Kondziella, D. , Mayer, S. A. , Menon, D. , Sharshar, T. , Stevens, R. D. , Ulmer, H. , Venkatasubba Rao, C. P. , Vespa, P. , McNett, M. , Frontera, J. the Curing Coma Campaign and its Contributing Members. ((2022) ) The Curing Coma Campaign International Survey on ComaEpidemiology, Evaluation, and Therapy (COME TOGETHER. Neurocritical Care 37: (1), 47–59. https://doi.org/10.1007/s12028-021-01425-8 |
46 | Henson, T. , Rawanduzy, C. , Salazar, M. , Sebastian, A. , Weber, H. , Al-Mufti, F. , Mayer, S. A. ((2022) ) Outcomeand prognostication after cardiac arrest. Annals of the New York Academy of Sciences 1508: (1), 23–34. https://doi.org/10.1111/nyas.14699 |
47 | Hicks, A. J. , Clay, F. J. , James, A. C. , Hopwood, M. , Ponsford, J. L. ((2021) ) Effectiveness of pharmacotherapy for depression after traumatic brain injury in adults: An umbrella review protocol. JBI Evidence Synthesis 19: (7), 1720–1734. https://doi.org/10.11124/JBIES-20-00363 |
48 | Hux, K. ((2019) ) Post-acute rehabilitation effects on functional outcome and discharge disposition of people with severe traumatic brain injury. Brain Injury 33: (10), 1332–1340. https://doi.org/10.1080/02699052.2019.1641745 |
49 | Institute of Medicine (US) Committee on Health Literacy. (2004). Health Literacy: A Prescription to End Confusion (L. Nielsen-Bohlman, A. M. Panzer, & D. A. Kindig, Eds.). National Academies Press (US). http://www.ncbi.nlm.nih.gov/books/NBK216032/ |
50 | Izzy, S. , Compton, R. , Carandang, R. , Hall, W. , Muehlschlegel, S. ((2013) ) Self-Fulfilling Prophecies Through Withdrawal of Care: Do They Exist in Traumatic Brain Injury, Too? Neurocritical Care 19: (3), 347–363. https://doi.org/10.1007/s12028-013-9925-z |
51 | Javed, Z. , Qamar, U. , Sathyapalan, T. ((2015) ) Pituitary and/or hypothalamic dysfunction following moderate to severe traumatic brain injury: Current perspectives. Indian Journal of Endocrinology and Metabolism 19: (6), 753–763. https://doi.org/10.4103/2230-8210.167561 |
52 | Kals, M. , Kunzmann, K. , Parodi, L. , Radmanesh, F. , Wilson, L. , Izzy, S. , Anderson, C. D. , Puccio, A. M. , Okonkwo, D. O. , Temkin, N. , Steyerberg, E. W. , Stein, M. B. , Manley, G. T. , Maas, A. I. R. , Richardson, S. , Diaz-Arrastia, R. , Palotie, A. , Ripatti, S. , Rosand, J. , Zafonte , R. ((2022) ) A genome-wide association study of outcome from traumatic brain injury. EBioMedicine 77: , 103933. https://doi.org/10.1016/j.ebiom.2022.103933 |
53 | Karpenko, A. , Keegan, J. ((2021) ) Diagnosis of Coma. Emergency Medicine Clinics of North America 39: (1), 155–172. https://doi.org/10.1016/j.emc.2020.09.009 |
54 | Kondziella, D. , Bender, A. , Diserens, K. , van Erp, W. , Estraneo, A. , Formisano, R. , Laureys, S. , Naccache, L. , Ozturk, S. , Rohaut, B. , Sitt, J. D. , Stender, J. , Tiainen, M. , Rossetti, A. O. , Gosseries, O. , Chatelle, C. the EAN Panel on Coma, Disorders of Consciousness. ((2020) ) European Academy of Neurology guideline on the diagnosis of coma and other disorders of consciousness. European Journal of Neurology 27: (5), 741–756. https://doi.org/10.1111/ene.14151 |
55 | Kondziella, D. , Friberg, C. K. , Frokjaer, V. G. , Fabricius, M. , Møller, K. ((2016) ) Preserved consciousness in vegetative and minimal conscious states: Systematic review and meta-analysis. Journal of Neurology, Neurosurgery & Psychiatry 87: (5), 485–492. https://doi.org/10.1136/jnnp-2015-310958 |
56 | Kondziella, D. , Stevens, R. D. ((2022) ) Classifying Disorders of Consciousness: Past, Present, and Future. Seminars in Neurology 42: (03), 239–248. https://doi.org/10.1055/a-1883-1021 |
57 | Kostick, K. M. , Halm, A. , O’Brien, K. , Kothari, S. , Blumenthal-Barby, J. S. ((2021) ) Conceptualizations of consciousness and continuation of care among family members and health professionals caring for patients in a minimally conscious state. Disability and Rehabilitation 43: (16), 2285–2294. https://doi.org/10.1080/09638288.2019.1697383 |
58 | Kowalski, R. G. , Hammond, F. M. , Weintraub, A. H. , Nakase-Richardson, R. , Zafonte, R. D. , Whyte, J. , Giacino, J. T. ((2021) ) Recovery of Consciousness and Functional Outcome in Moderateand Severe Traumatic Brain Injury. JAMA Neurology 78: (5), 548. https://doi.org/10.1001/jamaneurol.2021.0084 |
59 | Kreitzer, N. , Murtaugh, B. , Creutzfeldt, C. , Fins, J. J. , Manley, G. , Sarwal, A. , Dangayach, N. ((2023) ) Prognostic humility and ethical dilemmas after severe brain injury: Summary, recommendations, and qualitative analysis of Curing Coma Campaign virtual event proceedings. Frontiers in Human Neuroscience 17: , 1128656. https://doi.org/10.3389/fnhum.2023.1128656 |
60 | Kruser, J. M. , Nabozny, M. J. , Steffens, N. M. , Brasel, K. J. , Campbell, T. C. , Gaines, M. E. , Schwarze, M. L. ((2015) ) Best Case/Worst Case”: Qualitative Evaluation of a Novel Communication Tool for Difficult in-the-Moment Surgical Decisions. Journal of the American Geriatrics Society 63: (9), 1805–1811. https://doi.org/10.1111/jgs.13615 |
61 | Lang, J. K. , Paykel, M. S. , Haines, K. J. , Hodgson, C. L. ((2020) ) Clinical Practice Guidelines for Early Mobilization in the ICU: ASystematic Review. Critical Care Medicine 48: (11), e1121–e1128. https://doi.org/10.1097/CCM.0000000000004574 |
62 | Lucca, L. F. , Lofaro, D. , Pignolo, L. , Leto, E. , Ursino, M. , Cortese, M. D. , Conforti, D. , Tonin, P. , Cerasa, A. ((2019) ) Outcome prediction in disorders of consciousness: The role of coma recovery scale revised. BMC Neurology 19: (1), 68. https://doi.org/10.1186/s12883-019-1293-7 |
63 | Mackay, L. E. , Bernstein, B. A. , Chapman, P. E. , Morgan, A. S. , Milazzo, L. S. ((1992) ) Early intervention in severe head injury: Long-term benefits of a formalized program. Archives of Physical Medicine and Rehabilitation 73: (7), 635–641. |
64 | Magnani, F. G. , Barbadoro, F. , Cacciatore, M. , Leonardi, M. ((2022) ) The importance of instrumental assessment in disorders of consciousness: A comparison between American, European, and UK International recommendations. Critical Care 26: (1), 245. https://doi.org/10.1186/s13054-022-04119-5 |
65 | Mainali, S. , Aiyagari, V. , Alexander, S. , Bodien, Y. , Boerwinkle, V. , Boly, M. , Brown, E. , Brown, J. , Claassen, J. , Edlow, B. L. , Fink, E. L. , Fins, J. J. , Foreman, B. , Frontera, J. , Geocadin, R. G. , Giacino, J. , Gilmore, E. J. , Gosseries, O. , Hammond, F. the Curing Coma Campaign collaborators. ((2022) ) Proceedings of the Second Curing Coma Campaign NIH Symposium: Challenging the Future of Research for Coma and Disorders of Consciousness. Neurocritical Care 37: (1), 326–350. https://doi.org/10.1007/s12028-022-01505-3 |
66 | May, T. L. , Ruthazer, R. , Riker, R. R. , Friberg, H. , Patel, N. , Soreide, E. , Hand, R. , Stammet, P. , Dupont, A. , Hirsch, K. G. , Agarwal, S. , Wanscher, M. J. , Dankiewicz, J. , Nielsen, N. , Seder, D. B. , Kent, D. M. ((2019) ) Early withdrawal of life support after resuscitation from cardiac arrest is common and may result in additional deaths. Resuscitation 139: , 308–313. https://doi.org/10.1016/j.resuscitation.2019.02.031 |
67 | McCrea, M. A. , Giacino, J. T. , Barber, J. , Temkin, N. R. , Nelson, L. D. , Levin, H. S. , Dikmen, S. , Stein, M. , Bodien, Y. G. , Boase, K. , Taylor, S. R. , Vassar, M. , Mukherjee, P. , Robertson, C. , Diaz-Arrastia, R. , Okonkwo, D. O. , Markowitz, A. J. , Manley, G. T. TRACK-TBI Investigators Zafonte R. ((2021) ) Functional Outcomes Over the First Year After Moderate to Severe Traumatic Brain Injury in the Prospective, Longitudinal TRACK-TBI Study. JAMA Neurology 78: (8), 982–992. https://doi.org/10.1001/jamaneurol.2021.2043 |
68 | Meyfroidt, G. , Baguley, I. J. , Menon, D. K. ((2017) ) Paroxysmal sympathetic hyperactivity: The storm after acute brain injury. The Lancet Neurology 16: (9), 721–729. https://doi.org/10.1016/S1474-4422(17)30259-4 |
69 | Mollayeva, T. , Mollayeva, S. , Pacheco, N. , D’Souza, A. , Colantonio, A. ((2019) ) The course and prognostic factors of cognitive outcomes after traumatic brain injury: A systematic review and meta-analysis. Neuroscience and Biobehavioral Reviews 99: , 198–250. https://doi.org/10.1016/j.neubiorev.2019.01.011 |
70 | Moyer, M. T. , Hinkle, J. L. , Mendez, J. D. ((2021) ) An Integrative Review: Early Mobilization of Patients With External Ventriculostomy Drains in the Neurological Intensive Care Unit. Journal of Neuroscience Nursing 53: (5), 220–224. https://doi.org/10.1097/JNN.0000000000000609 |
71 | MRC CRASH Trial Collaborators. ((2008) ) Predicting outcome after traumatic brain injury: Practical prognostic models based on large cohort of international patients. BMJ 336: (7641), 425–429. https://doi.org/10.1136/bmj.39461.643438.25 |
72 | Murphy, T. H. , Corbett, D. ((2009) ) Plasticity during stroke recovery: From synapse to behaviour. Nature Reviews. Neuroscience 10: (12), 861–872. https://doi.org/10.1038/nrn2735 |
73 | Murtaugh, B. , Morrissey, A.-M. , Fager, S. , Knight, H. , Rushing, J. , Weaver, J. A. (2023). The role of the rehabilitation therapist in treating persons with DoC: An umbrella review. Manuscript Submitted for Publication. NeuroRehabilitation. |
74 | Naess, H. L. , Vikane, E. , Wehling, E. I. , Skouen, J. S. , Bell, R. F. , Johnsen, L. G. ((2020) ) Effect of Early Interdisciplinary Rehabilitation for Trauma Patients: A Systematic Review. Archives of Rehabilitation Research and Clinical Translation 2: (4), 100070. https://doi.org/10.1016/j.arrct.2020.100070 |
75 | Owen, A. M. , Coleman, M. R. , Boly, M. , Davis, M. H. , Laureys, S. , Pickard, J. D. ((2006) ) Detecting awareness in the vegetative state. Science (New York, N.Y.) 313: (5792), 1402. https://doi.org/10.1126/science.1130197 |
76 | Pignat, J.-M. , Jöhr, J. , Diserens, K. ((2015) ) From disorders of consciousness to early neurorehabilitation using assistive technologies in patients with severe brain damage. Current Opinion in Neurology 28: (6), 587–594. https://doi.org/10.1097/WCO.0000000000000264 |
77 | Plantier, D. , Luauté, J. SOFMER group ((2016) ) Drugs forbehavior disorders after traumatic brain injury: Systematic reviewand expert consensus leading to French recommendations for goodpractice. Annals of Physical and Rehabilitation Medicine 59: (1), 42–57. https://doi.org/10.1016/j.rehab.2015.10.003 |
78 | Provencio, J. J. , Hemphill, J. C. , Claassen, J. , Edlow, B. L. , Helbok, R. , Vespa, P. M. , Diringer, M. N. , Polizzotto, L. , Shutter L. , Suarez, J. I. , Stevens, R. D. , Hanley, D. F. , Akbari, Y. , Bleck,T. P. , Boly, M. , Foreman, B. , Giacino, J. T. , Hartings, J. A. , Human, T. Neurocritical Care Society Curing Coma Campaign. ((2020) ) The Curing Coma Campaign: Framing Initial ScientificChallenges-Proceedings of the First Curing Coma Campaign ScientificAdvisory Council Meeting. Neurocritical Care 33: (1), 1–12. https://doi.org/10.1007/s12028-020-01028-9 |
79 | Roberts, H. , Greenwood, N. ((2019) ) Speech and language therapy best practice for patients in prolonged disorders of consciousness: A modified Delphi study.. International Journal of Language & Communication Disorders 54: (5), 841–854. https://doi.org/10.1111/1460-6984.12489 |
80 | Roozenbeek, B. , Maas, A. I. R. , Menon, D. K. ((2013) ) Changing patterns in the epidemiology of traumatic brain injury. Nature Reviews. Neurology 9: (4), 231–236. https://doi.org/10.1038/nrneurol.2013.22 |
81 | Roth, C. , Stitz, H. , Kalhout, A. , Kleffmann, J. , Deinsberger, W. , Ferbert, A. ((2013) ) Effect of Early Physiotherapy on IntracranialPressure and Cerebral Perfusion Pressure. Neurocritical Care 18: (1), 33–38. https://doi.org/10.1007/s12028-012-9799-5 |
82 | Sanz, L. R. D. , Thibaut, A. , Edlow, B. L. , Laureys, S. , Gosseries, O. ((2021) ) Update on neuroimaging in disorders of consciousness. Current Opinion in Neurology 34: (4), 488–496. https://doi.org/10.1097/WCO.0000000000000951 |
83 | Schiff, N. D. ((2015) ) Cognitive Motor Dissociation Following Severe Brain Injuries. JAMA Neurology 72: (12), 1413–1415. https://doi.org/10.1001/jamaneurol.2015.2899 |
84 | Schiff, N. D. , Rodriguez-Moreno, D. , Kamal, A. , Kim, K. H. S. , Giacino, J. T. , Plum, F. , Hirsch, J. ((2005) ) FMRI revealslarge-scale network activation in minimally conscious patients. Neurology 64: (3), 514–523. https://doi.org/10.1212/01.WNL.0000150883.10285.44 |
85 | Schnakers, C. , Bauer, C. , Formisano, R. , Noé, E. , Llorens, R. , Lejeune, N. , Farisco, M. , Teixeira, L. , Morrissey, A.-M. , De Marco, S. , Veeramuthu, V. , Ilina, K. , Edlow, B. L. , Gosseries, O. , Zandalasini, M. , De Bellis, F. , Thibaut, A. , Estraneo, A. ((2022) ) What names for covert awareness? A systematic review. Frontiersin Human Neuroscience 16: , 971315. https://doi.org/10.3389/fnhum.2022.971315 |
86 | Schnakers, C. , Vanhaudenhuyse, A. , Giacino, J. , Ventura, M. , Boly, M. , Majerus, S. , Moonen, G. , Laureys, S. ((2009) ) Diagnostic accuracy of the vegetative and minimally conscious state: Clinical consensus versus standardized neurobehavioral assessment. BMC Neurology 9: (1), 35. https://doi.org/10.1186/1471-2377-9-35 |
87 | Seel, R. T. , Douglas, J. , Dennison, A. C. , Heaner, S. , Farris, K. , Rogers, C. ((2013) ) Specialized Early Treatment for Persons With Disorders of Consciousness: Program Components and Outcomes. Archives of Physical Medicine and Rehabilitation 94: (10), 1908–1923. https://doi.org/10.1016/j.apmr.2012.11.052 |
88 | Seel, R. T. , Sherer, M. , Whyte, J. , Katz, D. I. , Giacino, J. T. , Rosenbaum, A. M. , Hammond, F. M. , Kalmar, K. , Pape, T. L.-B. , Zafonte, R. , Biester, R. C. , Kaelin, D. , Kean, J. , Zasler, N. ((2010) ) Assessment Scales for Disorders of Consciousness: Evidence-Based Recommendations for Clinical Practice and Research. Archives of Physical Medicine and Rehabilitation 91: (12), 1795–1813. https://doi.org/10.1016/j.apmr.2010.07.218 |
89 | Signorelli, F. , Ioannoni, E. , Olivi, A. , Montano, N. ((2020) ) Factors involved in the development of subdural hygroma after decompressive craniectomy for traumatic brain injury. A systematic review and meta-analysis. Journal of Clinical Neuroscience: Official Journal of the Neurosurgical Society of Australasia 78: , 273–276. https://doi.org/10.1016/j.jocn.2020.05.033 |
90 | Souter, M. J. , Blissitt, P. A. , Blosser, S. , Bonomo, J. , Greer, D. , Jichici, D. , Mahanes, D. , Marcolini, E. G. , Miller, C. , Sangha, K. , Yeager, S. ((2015) ) Recommendations for the Critical Care Management of Devastating Brain Injury: Prognostication, Psychosocial, and Ethical Management: A Position Statement for Healthcare Professionals from the Neurocritical Care Society. Neurocritical Care 23: (1), 4–13. https://doi.org/10.1007/s12028-015-0137-6 |
91 | Stevens, R. D. , Sutter, R. ((2013) ) Prognosis in severe brain injury. Critical Care Medicine 41: (4), 1104–1123. https://doi.org/10.1097/CCM.0b013e318287ee79 |
92 | Steyerberg, E. W. , Mushkudiani, N. , Perel, P. , Butcher, I. , Lu, J. , McHugh, G. S. , Murray, G. D. , Marmarou, A. , Roberts, I. , Habbema, J. D. F. , Maas, A. I. R. ((2008) ) Predicting Outcome after Traumatic Brain Injury: Development and International Validation of Prognostic Scores Based on Admission Characteristics. PLoS Medicine 5: (8), e165. https://doi.org/10.1371/journal.pmed.0050165 |
93 | Strens, L. H. A. , Mazibrada, G. , Duncan, J. S. , Greenwood, R. ((2004) ) Misdiagnosing the vegetative state after severe brain injury: The influence of medication. Brain Injury 18: (2), 213–218. https://doi.org/10.1080/0269905031000149533 |
94 | Sudore, R. L. , Schillinger, D. ((2009) ) Interventions to Improve Care for Patients with Limited Health Literacy. Journal of Clinical Outcomes Management: JCOM 16: (1), 20–29. |
95 | Tepas, J. J. , Leaphart, C. L. , Pieper, P. , Beaulieu, C. L. , Spierre, L. R. , Tuten, J. D. , Celso, B. G. ((2009) ) The effect of delay in rehabilitation on outcome of severe traumatic brain injury. Journal of Pediatric Surgery 44: (2), 368–372. https://doi.org/10.1016/j.jpedsurg.2008.10.089 |
96 | Thibaut, A. , Bodien, Y. G. , Laureys, S. , Giacino, J. T. ((2020) ) Minimally conscious state “plus”: Diagnostic criteria and relation to functional recovery. Journal of Neurology 267: (5), 1245–1254. https://doi.org/10.1007/s00415-019-09628-y |
97 | Thibaut, A. , Wannez, S. , Deltombe, T. , Martens, G. , Laureys, S. , Chatelle, C. ((2018) ) Physical therapy in patients with disorders of consciousness: Impact on spasticity and muscle contracture. NeuroRehabilitation 42: (2), 199–205. https://doi.org/10.3233/NRE-172229 |
98 | Turgeon, A. F. , Lauzier, F. , Simard, J.-F. , Scales, D. C. , Burns, K. E. A. , Moore, L. , Zygun, D. A. , Bernard, F. , Meade, M. O. , Dung, T. C. , Ratnapalan, M. , Todd, S. , Harlock, J. , Fergusson, D. A. ((2011) ) Mortality associated with withdrawal of life-sustaining therapy for patients with severe traumatic brain injury: A Canadian multicentre cohort study. Canadian Medical Association Journal 183: (14), 1581–1588. https://doi.org/10.1503/cmaj.101786 |
99 | van Veen, E. , van der Jagt, M. , Citerio, G. , Stocchetti, N. , Gommers, D. , Burdorf, A. , Menon, D. K. , Maas, A. I. R. , Kompanje, E. J. O. , Lingsma, H. F. , CENTER-TBI investigators and participants. ((2021) ) Occurrence and timing of withdrawal of life-sustaining measures in traumatic brain injury patients: A CENTER-TBI study. Intensive Care Medicine 47: (10), 1115–1129. https://doi.org/10.1007/s00134-021-06484-1 |
100 | Vanhaudenhuyse, A. , Noirhomme, Q. , Tshibanda, L. J.-F. , Bruno, M.-A. , Boveroux, P. , Schnakers, C. , Soddu, A. , Perlbarg, V. , Ledoux, D. , Brichant, J.-F. , Moonen, G. , Maquet, P. , Greicius, M. D. , Laureys, S. , Boly, M. ((2010) ) Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients. Brain: A Journal of Neurology 133: (Pt 1), 161–171. https://doi.org/10.1093/brain/awp313 |
101 | Vespa, P. M. , Nuwer, M. R. , Nenov, V. , Ronne-Engstrom, E. , Hovda, D. A. , Bergsneider, M. , Kelly, D. F. , Martin, N. A. , Becker, D. P. ((1999) ) Increased incidence and impact of nonconvulsive and convulsive seizures after traumatic brain injury as detected by continuous electroencephalographic monitoring. Journal of Neurosurgery 91: (5), 750–760. https://doi.org/10.3171/jns.1999.91.5.0750 |
102 | Walker, W. , Seel, R. , Gibellato, M. , Lew, H. , Cornis-Pop, M. , Jena, T. , Silver, T. ((2004) ) The effects of Donepezil on traumatic brain injury acute rehabilitation outcomes. Brain Injury 18: (8), 739–750. https://doi.org/10.1080/02699050310001646224 |
103 | Wannez, S. , Heine, L. , Thonnard, M. , Gosseries, O. , Laureys, S. Coma Science Group collaborators. ((2017) ) The repetition of behavioral assessments in diagnosis of disorders of consciousness: Repeated CRS-R Assessments for Diagnosis in DOC. Annals of Neurology 81: (6), 883–889. https://doi.org/10.1002/ana.24962 |
104 | Weaver, J. A. , Watters, K. , Cogan, A. M. (2022). Interventions Facilitating Recovery of Consciousness Following Traumatic Brain Injury: A Systematic Review. OTJR: Occupation, Participation and Health, 153944922211177. https://doi.org/10.1177/15394492221117779 |
105 | Weaver, J. A. , Watters, K. , Cogan, A. M. ((2023) ) Interventions Facilitating Recovery of Consciousness Following Traumatic Brain Injury: A Systematic Review. OTJR: Occupation, Participation and Health 43: (2), 322–336. https://doi.org/10.1177/15394492221117779 |
106 | Whelan, F. J. , Walker, M. S. , Schultz, S. K. ((2000) ) Donepezil in the treatment of cognitive dysfunction associated with traumatic brain injury. Annals of Clinical Psychiatry: Official Journal of the American Academy of Clinical Psychiatrists 12: (3), 131–135. https://doi.org/10.1023/a:1009008816928 |
107 | Whyte, J. , Gosseries, O. , Chervoneva, I. , DiPasquale, M. C. , Giacino, J. , Kalmar, K. , Katz, D. I. , Novak, P. , Long, D. , Childs, N. , Mercer, W. , Maurer, P. , Eifert, B. (2009). Predictors of short-term outcome in brain-injured patients with disorders of consciousness. In Progress in Brain Research (Vol. 177, pp. 63-72). Elsevier. https://doi.org/10.1016/S0079-6123(09)17706-3 |
108 | Whyte, J. , Nakase-Richardson, R. ((2013) ) Disorders of Consciousness: Outcomes, Comorbidities, and Care Needs. Archives of Physical Medicine and Rehabilitation 94: (10), 1851–1854. https://doi.org/10.1016/j.apmr.2013.07.003 |
109 | Whyte, J. , Nordenbo, A. M. , Kalmar, K. , Merges, B. , Bagiella, E. , Chang, H. , Yablon, S. , Cho, S. , Hammond, F. , Khademi, A. , Giacino, J. ((2013) ) Medical complications during inpatient rehabilitation among patients with traumatic disorders of consciousness. Archives of Physical Medicine and Rehabilitation 94: (10), 1877–1883. https://doi.org/10.1016/j.apmr.2012.12.027 |
110 | Whyte, J. , Rajan, R. , Rosenbaum, A. , Katz, D. , Kalmar, K. , Seel, R. , Greenwald, B. , Zafonte, R. , Demarest, D. , Brunner, R. , Kaelin, D. ((2014) ) Zolpidem and restoration of consciousness.. American Journal of Physical Medicine & Rehabilitation 93: (2), 101–113. https://doi.org/10.1097/PHM.0000000000000069 |
111 | Wijdicks, E. F. M. , Hwang, D. Y. ((2021) ) Predicting Coma Trajectories: The Impact of Bias and Noise on Shared Decisions. Neurocritical Care 35: (2), 291–296. https://doi.org/10.1007/s12028-021-01324-y |
112 | Yang, H. , Ye, C. , Liu, X. , Sun, L. , Wang, A. , Wang, J. , Hu, N. , Hu, X. , Gosseries, O. , Laureys, S. , Di, H. , Fang, J. ((2021) ) Estimating the Minimal Number of Repeated Examinations for Random Responsiveness With the Coma Recovery Scale— Revised as an Example. Frontiers in Integrative Neuroscience 15: , 685627. https://doi.org/10.3389/fnint.2021.685627 |
113 | Yen, H.-C. , Han, Y.-Y. , Hsiao, W.-L. , Hsu, P.-M. , Pan, G.-S. , Li, M.-H. , Chen, W.-S. , Chuang, H.-J. ((2022) ) Functional mobility effects of progressive early mobilization protocol on people with moderate-to-severe traumatic brain injury: A pre-post intervention study. NeuroRehabilitation 51: (2), 303–313. https://doi.org/10.3233/NRE-220023 |
114 | Young, M. J. , Bodien, Y. G. , Giacino, J. T. , Fins, J. J. , Truog, R. D. , Hochberg, L. R. , Edlow, B. L. ((2021) ) The neuroethics of disorders of consciousness: A brief history of evolving ideas. Brain 144: (11), 3291–3310. https://doi.org/10.1093/brain/awab290 |
115 | Zhang, B. , Karri, J. , O’Brien, K. , DiTommaso, C. , Kothari, S. , Li, S. ((2021) ) Spasticity Management in Persons with Disorders of Consciousness. PM&R 13: (7), 657–665. https://doi.org/10.1002/pmrj.12458 |
116 | Zuckerman, D. A. , Giacino, J. T. , Bodien, Y. G. ((2022) ) Traumatic Brain Injury: What Is a Favorable Outcome? Journal of Neurotrauma 39: (13-14), 1010–1012. https://doi.org/10.1089/neu.2021.0356 |




