Accommodative and pupillary dysfunctions in concussion/mild traumatic brain injury: A Review
Abstract
BACKGROUND:
Visual dysfunctions are common in individuals following concussion/mild traumatic brain injury (C/mTBI). Many deficits have been uncovered in their oculomotor system, such as in the pupil and accommodation.
OBJECTIVE:
To describe the static and dynamic abnormalities in the pupillary and accommodative systems in those with C/mTBI. This includes both diagnostic and therapeutic aspects, with emphasis on objectively-based test findings, as well as their basic and clinical ramifications.
METHODS:
PubMed, Google Scholar, and Semantic Scholar databases were searched from 1980–2020, using key words of accommodation, pupil, vision therapy, vision rehabilitation, and objective testing, for peer-reviewed papers, as well as related textbooks in the area, in those with C/mTBI.
RESULTS:
For both systems, most static and dynamic response parameters were abnormal: they were typically reduced, slowed, delayed, and/or more variable. Most of the abnormal accommodative parameters could be significantly improved with vision therapy.
CONCLUSIONS:
For both systems, most response parameters were abnormal, which could explain their visual symptoms and related problems. For accommodation, the improvements following vision therapy suggest the presence of considerable visual system plasticity, even in older adults with chronic brain injury.
1Introduction
The area of concussion/mild traumatic brain injury (C/mTBI) came to the world forefront with convergence of two events: the Iraq/Afghanistan wars and the sports concussion “epidemic” (Ciuffreda et al., 2016; Ciuffreda et al., 2021). The related medical problems presented in these individuals having C/mTBI cut across the spectrum: sensory, motor, perceptual, cognitive, language, attentional, physical, physiological, and/or behavioral (Zasler et al., 2021). More specifically, the diagnosis of C/mTBI produced a constellation of vision problems of a sensory (e.g., reduced contrast sensitivity), motor (e.g., slowed vergence), and/or perceptual (e.g., impaired figure-ground discrimination) nature (Suchoff et al., 2001; Suter & Harvey, 2011).
With regard to the oculomotor system, a treasure trove of abnormalities has been uncovered within the near triad and its response synkinesis: vergence, accommodation, and the pupil (Suchoff et al., 2001; Greenwald et al., 2012; Ciuffreda et al., 2021). In this review, the focus will be on the last two systems, including both the static (i.e., steady-state) and dynamic (i.e., transient) aspects, and along with their basic science and clinical implications.
2Method
PubMed, Google Scholar, and Semantic Scholar databases were searched from 1980–2020, using key words of accommodation, pupil, vision therapy, vision rehabilitation, and objective testing, for peer-reviewed papers, as well as related textbooks in the area, in those with C/mTBI.
3PUPIL
3.1Function, components, and mechanism
The pupil of the eye is approximately circular in shape and is defined anatomically by an aperture centered in the colored iris. It serves three functions (Ciuffreda, 2006; Kardon, 2003). Reduction in its diameter: (1) minimizes the adverse effects of the eye’s inherent optical aberrations on the quality of the retinal image, (2) assists in the process of ocular accommodation by increasing the eye’s depth-of-focus to minimize the amount of accommodation necessary to attain high-quality, focused retinal imagery, and (3) decreases the amount of light entering the eye to assist in visual comfort, that is the pupillary light reflex (PLR). It is the last aspect that will be considered for further discussion.
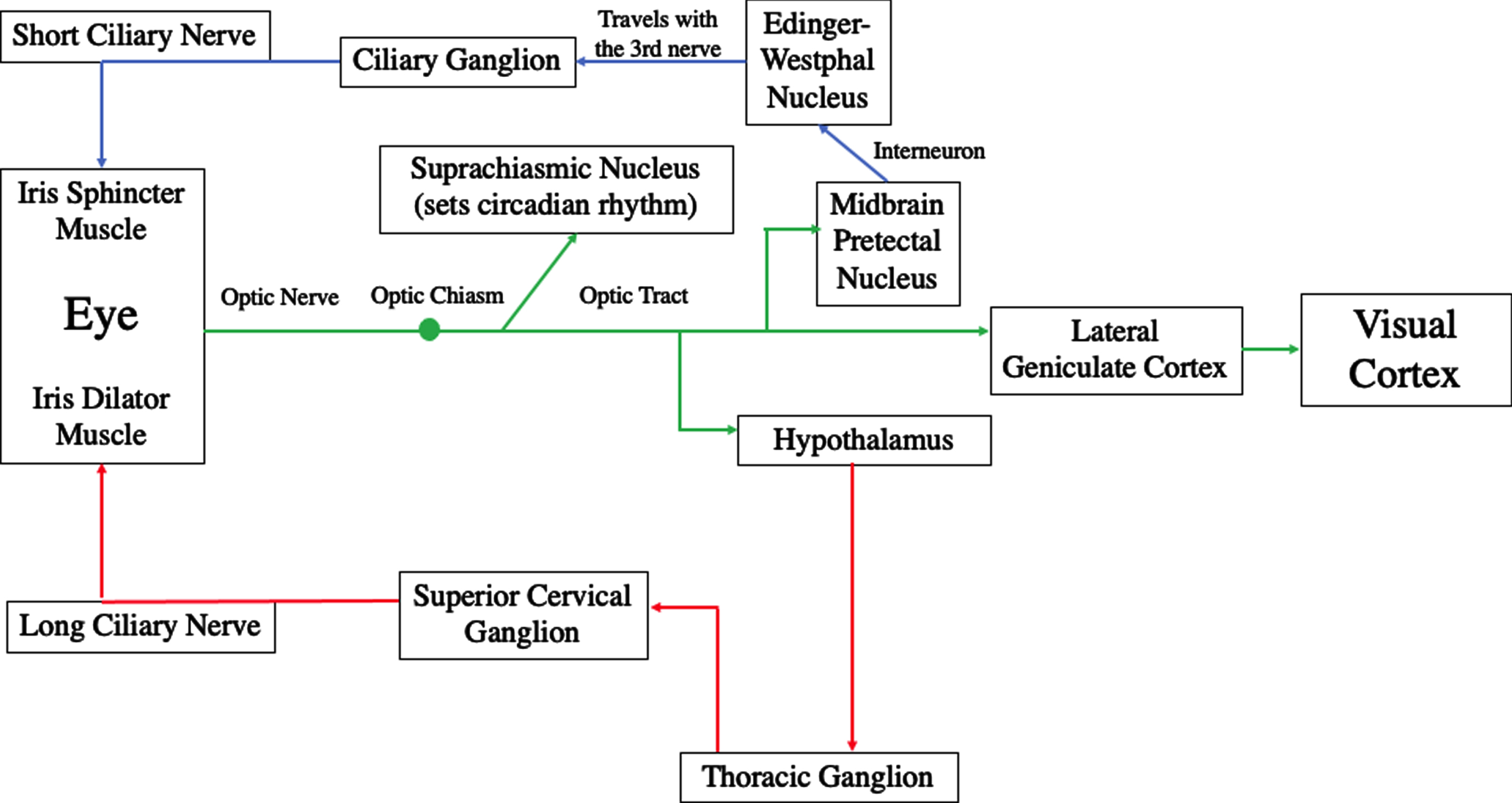
The pupillary system is controlled by the parasympathetic and sympathetic systems per the autonomic nervous system (Kardon, 2003; Adoni & McNeet, 2007). Pupillary constriction is produced by the parasympathetic system with the neurotransmitter acetylcholine to activate the sphincter muscle of the iris, whereas pupillary dilation is produced by the sympathetic system with the neurotransmitter norepinephrine to activate the dilator muscle of the iris. Once light enters the pupil, it impinges on three important retinal elements: the cones, rods, and intrinsically-photosensitive retinal ganglion cells (ipRGCs). All three participate in the PLR, with the rods and cones primarily controlling the rapid constriction phase lasting less than one second, and the ipRGCs primarily controlling the remaining slow redilation phase including the late sustained, steady-state component lasting up to five seconds. Regarding the pathway of the parasympathetic system, the optic nerve fibers go to the midbrain’s pretectal nucleus, and then to the left and right halves of the Edinger-Westphal nucleus, with this fiber split responsible for the consensual pupillary response, and also located within the trauma-susceptible midbrain (Rucker et al., 2019); then onto the ciliary ganglion via the III nerve, and from there via the short ciliary nerve to the sphincter muscle of the iris for pupillary constriction. Regarding the pathway of the sympathetic system, the optic nerve fibers go to the hypothalamus and then descend onto the cervicothoraic level (C7-T2); followed by the superior cervical ganglion, and finally via the long ciliary nerve to the dilator muscle of the iris for pupillary dilation (Troung, 2016) (See Fig. 1).
Fig. 1
Proposed mechanisms of photosensitivity. Reprinted with permission from Troung, 2016.
3.2Clinical assessment of the pupil
Assessment of the pupil provides critical information regarding the integrity of the early, afferent visual pathways (Kardon, 2003). The basic evaluation involves a penlight to assess the PLR, and a sharp object such as a pencil tip to assess the near response to an accommodative stimulus. Each pupil is separately stimulated, and both the direct and consensual responses are carefully observed and compared. The pupils should be equal, round, regular, and react to light and accommodative stimuli (PERRRLA), in a brisk manner. In the case of blunt eye/orbital trauma secondary to a more global head trauma, however, the pupil on the affected side might exhibit an irregular shape, and be accompanied by an asymmetric, slowed dynamic response. Such an abnormal response might require referral to either a neuro-ophthalmologist or neurologist for additional clinical and medical laboratory testing. In emergency room hospital settings, use of objectively-based, infrared pupillometry is becoming more common for quantitative dynamic pupillary assessment (Ciuffreda et al., 2017). It allows for the detection of more subtle deficits, such as a pathway delay (i.e., increased latency/reaction time), with automated parameter analysis that can be input into the electronic medical record.
3.3Afferent pupillary defect
One of the few gross pupillary abnormalities that may be found in the clinic patient with mTBI is an afferent pupillary defect (APD). In a retrospective, clinical study of 160 patients medically diagnosed as having mTBI, APD was found in 1.9% (Rutner et al., 2006). Basically, APD refers to one pupil being much less responsive to direct light stimulation (i.e., PLR) than that observed in the fellow eye (Kardon, 2003). It reflects unilateral, peripheral nerve damage in the pathway (e.g., traumatic optic neuropathy). APD can be readily quantified in the clinic using a penlight and a graded neutral density (ND) filter bar, which is based on a non-linear logarithmic scale (e.g., ND = 0.3 = 50% light transmittance, ND = 1.0 = 10% light transmittance). The penlight is shone into one pupil, and then rapidly into the other. Both the direct and consensual responses should be brisk and relatively equal. However, if one pupil appears to “dilate” with direct stimulation, which really represents reduced or less constriction in the affected eye, the ND bar is then introduced over the normal eye. The test is repeated with rapid alternation of the light, and with progressive increase in the ND filter density, until the responses of the eyes are “balanced”/equal. This endpoint ND magnitude represents the neurosensory “depth” of the deficit. In essence, one is “creating” an APD in the normal eye until inter-pupillary response equality is elicited.
3.4Objectively-based pupillography
Technological advances to assess pupillary dynamics in humans over the past fifty years have been remarkable. In the 1970s, such instrumentation was limited to the research laboratory, was large and cumbersome, and was expensive. However, with the revolution in electronics miniaturization, pupillographic recording can now be found embedded into electrodiagnostic systems (e.g., Diagnosys) and even in an iPhone (e.g., Brightlamp). A portable, monocular, hand-held, and clinically-friendly system is the Neuroptics PLR-200 (See Fig. 2), which the two authors have used extensively in the testing of both normal individuals and in those with C/mTBI (Thiagarajan & Ciuffreda, 2015).
Fig. 2
Neuroptics pupillometer and subject alignment during testing. Reprinted with permission from Thiagarajan & Ciuffreda, 2015.

It provides an automated, quantitative analysis of the key parameters (e.g., peak constriction velocity, latency), as well as a 5-second, high-quality, video recording of the response, with excellent reliability and repeatability. A smaller and much less expensive device has been introduced using an iPhone and special phone app. In a small pilot study (Nichols & Schulman, 2020), this system was reported to yield repeatable findings in two normal individuals. It produced a good record of the 10-second sustained pupillary response, its main parameter of specialized interest in the study. In a more recent study (McKay et al., 2020), this iPhone version was compared with the Neuroptics system shown in Fig. 2. The iPhone results were not found to be very repeatable, except for baseline pupillary diameter, when compared with the Neuroptics system. Thus, at present, it appears that the iPhone system may be good to quantify objectively baseline pupillary diameter in the clinic, an important entity, as well as for the specialized sustained pupillary response. However, further improvements are needed for its more general clinical use.
3.5Objectively-based pupillometry in C/mTBI
3.5.1Early studies in adults
There were two groups that performed the earliest studies in individuals with C/mBTI, with both using the Neuroptics PLD-200 monocular system described earlier. The Capo-Aponte group performed two investigations. In one (Capo-Aponte et al., 2018), they compared 100 adults diagnosed with acute C/mTBI (< 72 hours post-injury) to 100 adult, age-matched controls, all in the military. Pupillary parameters included: maximum and minimum diameter (mm), percent constriction, constriction latency (msec), average and maximum constriction velocity (mm/sec), average dilation velocity (mm/sec), and the 75% redilation recovery time (sec) (T75). Three parameters were significantly different between the groups: average constriction and dilation velocities were slower, and the T75 recovery time was longer, in those with brain injury. In another study (Capo-Aponte et al., 2013), they tested 20 adults with blast-induced mTBI in the subacute phase (7–35 days post-injury) and compared them with 20 age-matched controls, all in the military. Four parameters were significantly different: latency was increased, average constriction and dilation velocities were slower, and T75 was longer, in those with blast injury. Two years later, the Ciuffreda group tested 17 adults with chronic mTBI and compared them with 15 age-matched controls (Thiagarajan & Ciuffreda, 2015). Five parameters were significantly different: latency trended to be longer/delayed, constriction amplitude was smaller, and all velocities were slower/lower in the mTBI group as compared to the controls. In all three studies, the investigators proposed that these parameters might serve as objective biomarkers for C/mTBI in the specific groups tested. Interestingly, while there were some different significant parameters in each group, there were also commonalities: both average constriction and dilation velocities were significantly slower/lower in all three investigations denoting overall slowed dynamics of the pupillary system. Hence, these two parameters could be used for simplicity in the testing of adults with mTBI in all three phases: acute, subacute, and chronic.
3.5.2Later studies in adults
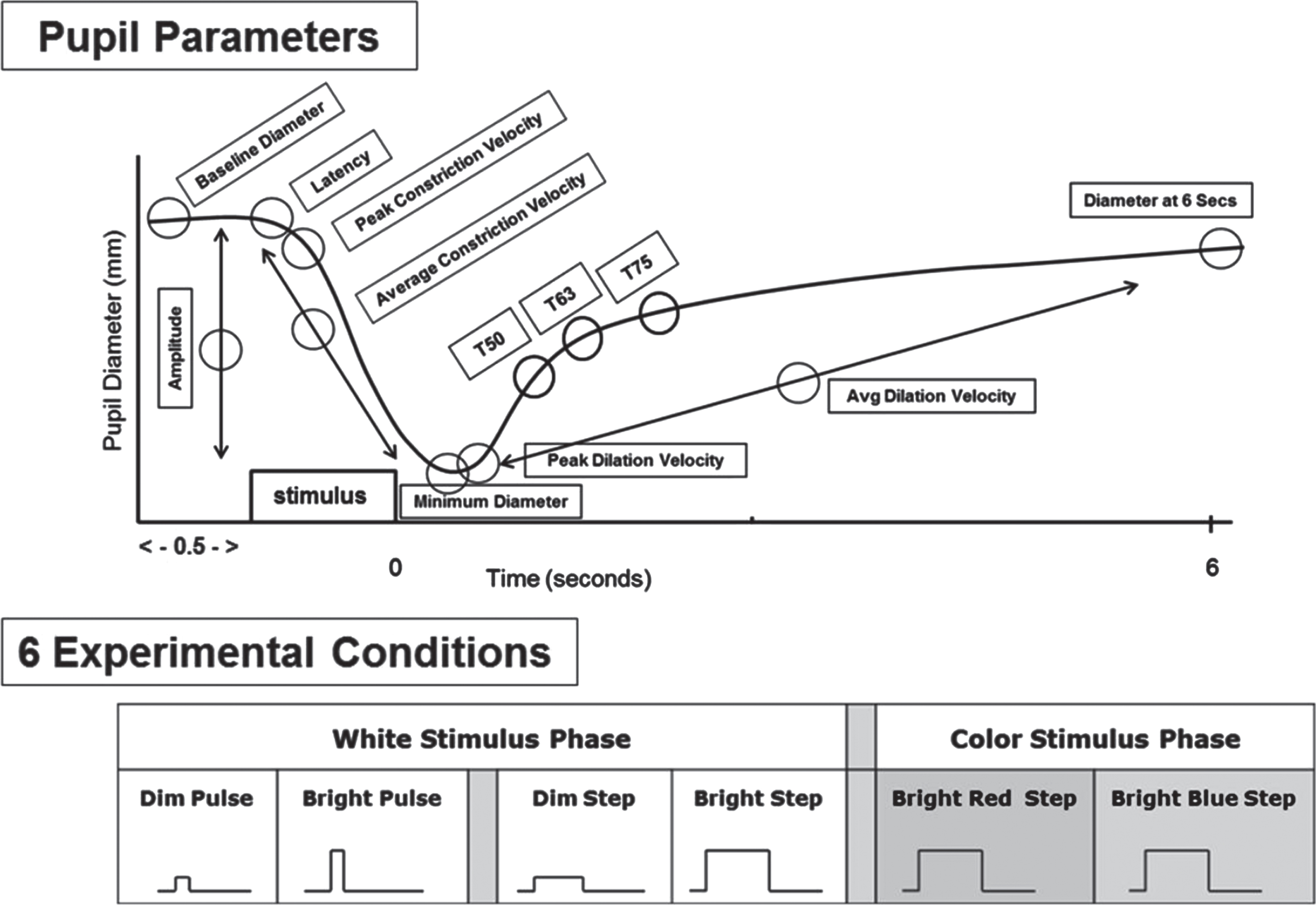
More recently, a series of studies was performed in the Ciuffreda laboratory (Ciuffreda et al., 2017). These differed in several respects from those previously performed. First, a binocular, infrared pupillometer was used (Neuroptics DP-2000). Second, the light stimuli could be custom-designed and were not fixed (Fig. 3). These included dim (4 lux) and bright (251 lux) pulses (100msec) and steps (1000 msec), as well as bright (251 lux) chromatic red (628 nm) and blue (463 nm) steps (1000msec). Third, two additional pupillary parameters were incorporated: maximum dilation velocity (mm/sec) and the 6-second, post-stimulus pupil diameter (mm) (6PSPD). Subjects were adults aged 21–60 years, with 32 having medically-diagnosed, chronic mTBI and 40 visually-normal, aged-matched controls. None had afferent pupillary defect (APD), and none were taking any drugs or medications that might influence the pupillary response. There were several interesting findings (Truong & Ciuffreda, 2016a): 1) For all 6 test conditions, the majority (5 or more) of the nine pupillary parameters significantly differed between the two groups: responses were delayed and slowed, with smaller baseline and final pupillary diameters in those with mTBI versus the normal cohort; 2) For the bright red step condition, 8 out of 9 parameters were different between groups as described above: only constriction amplitude was not; 3) For the bright white pulse stimulus, which approximated that used in the earlier monocular studies, 6 out of 9 parameters were again significantly different: only peak constriction velocity, average dilation velocity, and constriction amplitude were not; 4) Constriction latency was significantly slowed and different between the two groups for all but the white step condition. Thus, as before, there were some parameters that were test condition dependent. However, again, a commonality across test conditions was the parameter of average constriction velocity. There was no significant interocular response asymmetry difference either within or between the two groups (< 4%) (Truong & Ciuffreda, 2016b).
Fig. 3
Top: Schematic representation of a pupil response profile and the associated pupil parameters assessed as indicated by the open circles. The prestimulus time is 0.5 seconds, and the post-stimulus time is 6.0 seconds. Bottom: Schematic representation of the six experimental test stimulus conditions. The x-axis represents the relative time, and the y-axis represents the relative stimulus intensity. Dim = 4 lux, Bright = 251 lux, Pulse = 100ms, and Step = 1,000ms. Figure adapted with permission from Troung, 2016.
3.5.3Studies in adolescents with C/mTBI
There have been two recent investigations in adolescents diagnosed with C/mTBI using the same Neuroptics, monocular pupillometer as described earlier. The findings were surprising when compared with the aforementioned ones in adults. In the first performed by Master et al. (2020), they prospectively tested 98 athletes aged 12–18 years medically diagnosed within 28 days of concussive injury, thus in the acute/subacute phases combined, with comparison to 134 age-matched controls. The concussed group exhibited significantly faster dynamics (e.g., average constriction velocity) for nearly all parameters, as compared with the control group, along with a larger baseline diameter: the opposite of that found in the aforementioned adult studies. The results could not be explained by an autonomic deficit of either the parasympathetic or sympathetic systems: one cannot have a larger baseline pupillary diameter and faster constriction/dilation dynamics. However, the presence of a larger baseline diameter alone in these younger individuals could explain the results. With a larger diameter, more light would enter the eye. This would result in a bigger response amplitude, and by necessity a larger average/peak velocity, per the “main sequence” neurological relation: the larger the response amplitude, the greater the velocity (Ciuffreda et al., 2017). These unexpected findings were confirmed in a retrospective study in 92 pediatric patients aged 7–17 years, which included those in all three phases of medically diagnosed injury combined (Hsu et al., 2021). There was some commonality in the two studies: average and maximum constriction velocities, and average dilation velocity, were significantly faster in the concussed individuals.
3.6Pupillometry findings in those with C/mTBI and photosensitivity (PS)
A common visual symptom in those with C/mTBI is PS (Truong et al., 2014). It is important to confirm this objectively, for example in legal cases claiming related visual disability. One study has done so using infrared pupillometry (Truong & Ciuffreda, 2016c). In an adult group of 32 individuals having medically diagnosed mTBI, 65% (n = 22) reported to have PS. Responses were compared to those with C/mTBI but without PS. The objective findings were consistent with the perceived symptom. Six parameters were significantly different: maximum and minimum diameters were larger, and the redilation aspects (i.e., maximum redilation velocity and T75/T50) were faster, with a larger final pupillary diameter (6SPSD), in those with PS versus their non-PS cohort. Thus, the initial and final pupillary diameters were larger, along with decreased constriction time (i.e., faster redilation), in those with PS. More light could enter the eye throughout all phases of the response, thus likely a factor in their visual symptom of photosensitivity.
3.7Treatment for photosensitivity
Photosensitivity (PS) is reported to be present in approximately 10% of the general population and in at least 50% of those with C/mTBI (Truong et al., 2014). While treatment for PS is limited, it is simple and effective (Truong et al., 2014). The first is use of a wide brim hat (e.g., a baseball cap) to reduce the luminous intensity from above, such as the bright sky outdoors or brightly-illuminated indoor situations (Kapoor & Ciuffreda, 2002). Regarding the latter, a secondary positive effect may also occur: the critical flicker fusion (CFF) frequency value in these patients is frequently remarkably high (e.g., 58 Hertz, Hz) (Chang et al., 2007), which approximates the inherent flicker (i.e., 60 Hz) of fluorescent illumination. Thus, the brim would prevent this offensive flicker from over stimulating the patient’s visual system, in particular the abnormal magnocellular pathway that is involved in motion perception (Chang et al., 2007). The second approach involves the prescription of a low-density achromatic tint (e.g., 20% light reduction, neutral gray) for indoor and/or outdoor usage, which will not distort their color perception. The recommendation is to prescribe the least dense tint that the patient finds to be satisfactory. In a retrospective study in patients with PS and C/mTBI (Truong et al., 2014), it was found that in those who reported some reduction in their visual sensation of PS over the long-term (i.e., 1 year), most had less dense tints; those who did not typically had denser tints, which is logical. For example, if a patient is prescribed an 80% tint, only 20% of the light enters the eye. This has been speculated to be insufficient to allow for long-term, neural, visual light adaptation to occur (Troung et al., 2014). Third, and lastly, a potential approach is the use of uniform field, chromatic phototherapy (i.e., syntonics) (Stern, 2011), which remains controversial. A clinical trial is warranted to assess its efficacy in individuals with C/mTBI and PS.
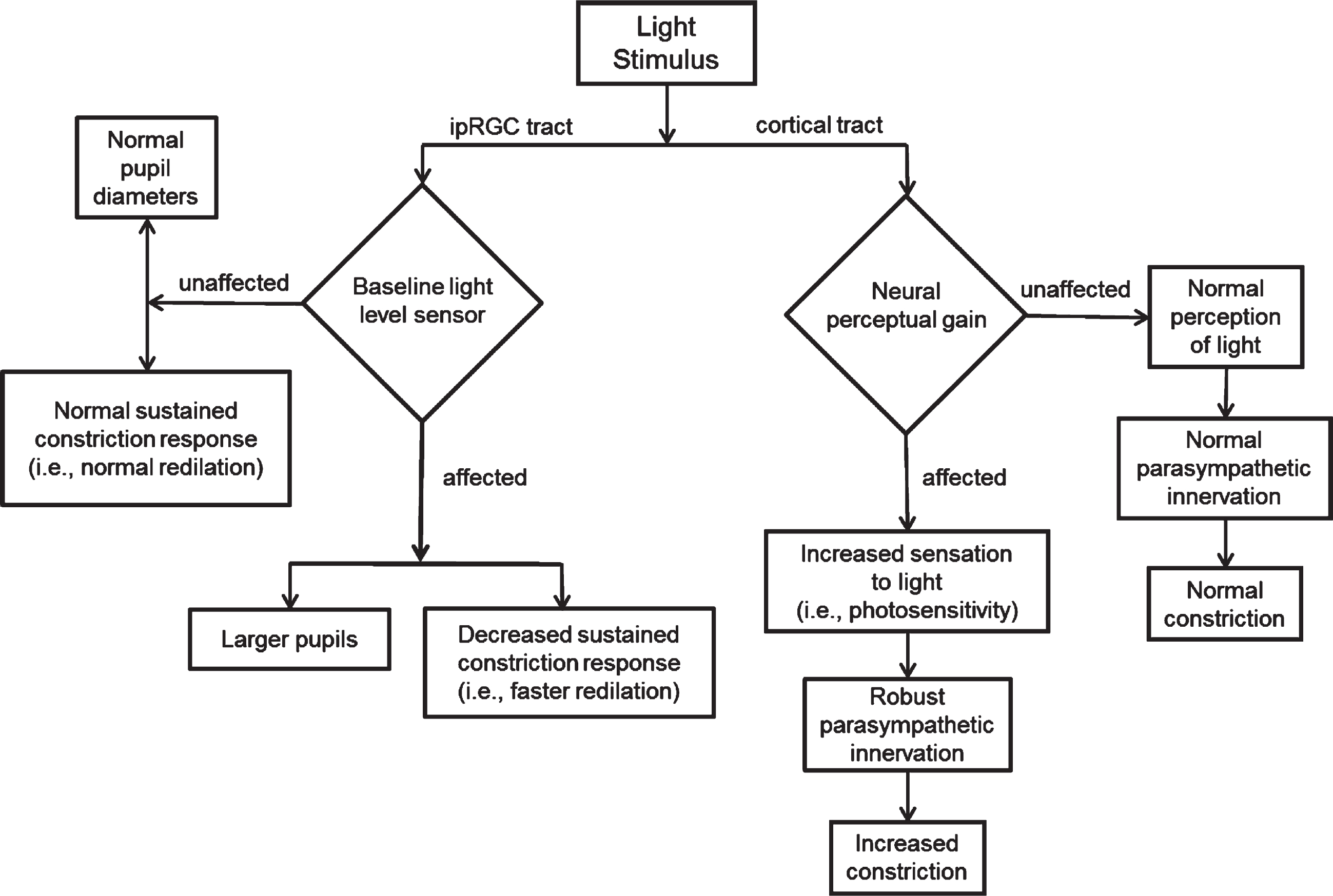
Based on the above findings, a conceptual model was developed to depict the laboratory findings in those with mTBI and PS (Fig. 4). Basically, per the assumption of damage to the ipRGC tract in those with C/mTBI and PS, leading to its lack of robustness to assess ambient light levels, it was hypothesized to cause abnormality of baseline light sensing (Fig. 4 - left). This resulted in larger baseline and final pupillary diameters, with faster redilation to the final steady-state level (6PSPD). Hence, more light would enter the eye throughout the response resulting, at least in part, to the symptom of PS. This overall response was speculated to be due to an imbalance between the parasympathetic and the sympathetic systems. A more complex and physiologically-based neural circuit for PS has been proposed by Digre and Brennan (2012). In contrast, in the normal control subjects with PS, a different scenario was proposed involving different parameters (Fig. 4 –right). There were significant differences in pupillary parameters in those with versus without PS. In those with PS, maximum and average constriction velocity were faster, constriction amplitude was greater, and redilation was slower (T50). The presence of a cortical abnormality involving neuro-perceptual gain was proposed. This too would lead to increased light sensitivity, but now with a more robust parasympathetic response to effectively modulate the overall constriction dynamics to combat the PS.
Fig. 4
Proposed possible mechanisms of photosensitivity based on Troung’s (2016) findings. Reprinted with permission from Troung, 2016.
3.8Refractive error influence on pupillary dynamics
The area of pupillary dynamics in the C/mTBI literature suggests an important role in its diagnosis (Truong et al., 2018). One important clinical factor that may exert an influence on its overall responsivity is refractive error. This has been investigated over the past decade with respect to baseline pupillary diameter only, with equivocal findings. Hence, this was more fully investigated in both normal and those with C/mTBI over a range of white light stimulus conditions (i.e., dim and bright pulses and steps) for the nine basic pupillary parameters described earlier (Ciuffreda et al., 2017) (Fig. 3). The response profiles were fit with either linear or curvilinear (i.e., Gaussian) mathematical expressions. The refractive range (spherical equivalent) tested was from -9D to +2D. The findings were interesting. Most test conditions revealed some degree of refractive error dependence in both groups, and the majority (75%) were best fit using a Gaussian profile with myopic apices in the range of –2.3D to –4.9D. Refractive errors exceeding approximately –5D to +1D exhibited reduced parameter values relative to those having less refractive error, with values being lower by up to 20%. Thus, any individual outside this refractive range would likely show lower than normal values, and this would have to be considered in the differential diagnosis. However, the parameter of latency with its linear response profile revealed the least amount of refractive error dependence, which makes it a good general candidate to assist in the pupillary-based diagnosis. Furthermore, response latency was excellent in the differential diagnosis of normal versus C/mTBI for the two dim light conditions. Myopes had the largest baseline pupillary diameter. The authors speculated that biomechanical and/or pharmacological aspects were involved in the aforementioned refractive error dependence.
4Accommodation
4.1Accommodative function, components, and mechanism
The process to increase the dioptric power of the crystalline lens to focus upon an object of interest precisely and maintain a high resolution foveal retinal image is defined as ocular accommodation. An effort to focus on a near object not only triggers accommodation, but also neurologically stimulates convergence and pupillary constriction, known as the tightly coupled “near-triad” (Myers & Stark, 1990; Loewenfeld & Lowenstein, 1993). Contraction of the ciliary muscle releases the resting zonular tension surrounding the lens equator. This process involves multiple combined mechanisms: an increase in the anterior/posterior surface curvatures of the lens combined with increased axial thickness and decreased lens diameter (Ciuffreda, 2006). The overall accommodative response is produced by the non-linear interaction of the 4 major components of accommodation (Heath, 1956): 1) blur-driven, 2) vergence-driven (disparity), 3) proximity-driven, and 4) tonic innervation reflecting the baseline parasympathetic neural input to the ciliary muscle (Gilmartin, 1986). Under normal binocular viewing conditions, blur and disparity are the major stimuli to drive the accommodative response (Ciuffreda, 2006). However, a higher-order voluntary mechanism has been also shown to influence the accommodative response (Richter et al., 2000). Following a latency of ∼400 msec, and similar to the other related oculomotor system (i.e.,vergence) (Semmlow et al. 1993), the net accommodative response displays a dual-mode behavior: it is comprised of an initial, open-loop, pre-programmed, fast response followed by the final, feed-back controlled, fine-tuning, slow response (Hung & Ciuffreda, 1988). While the dynamic response is measured during the actual change in accommodation, the steady-state response is measured after the desired amplitude has just been attained. The duration of this combined response is approximately 1 second. Studies that evaluated dynamic characteristics of accommodation have shown that the peak velocity of accommodation is amplitude dependent, that is, the higher the amplitude, the higher the peak velocity (Schinder et al., 1984; Ciuffreda & Kruger, 1988).
4.2Neurological control and correlates of accommodation
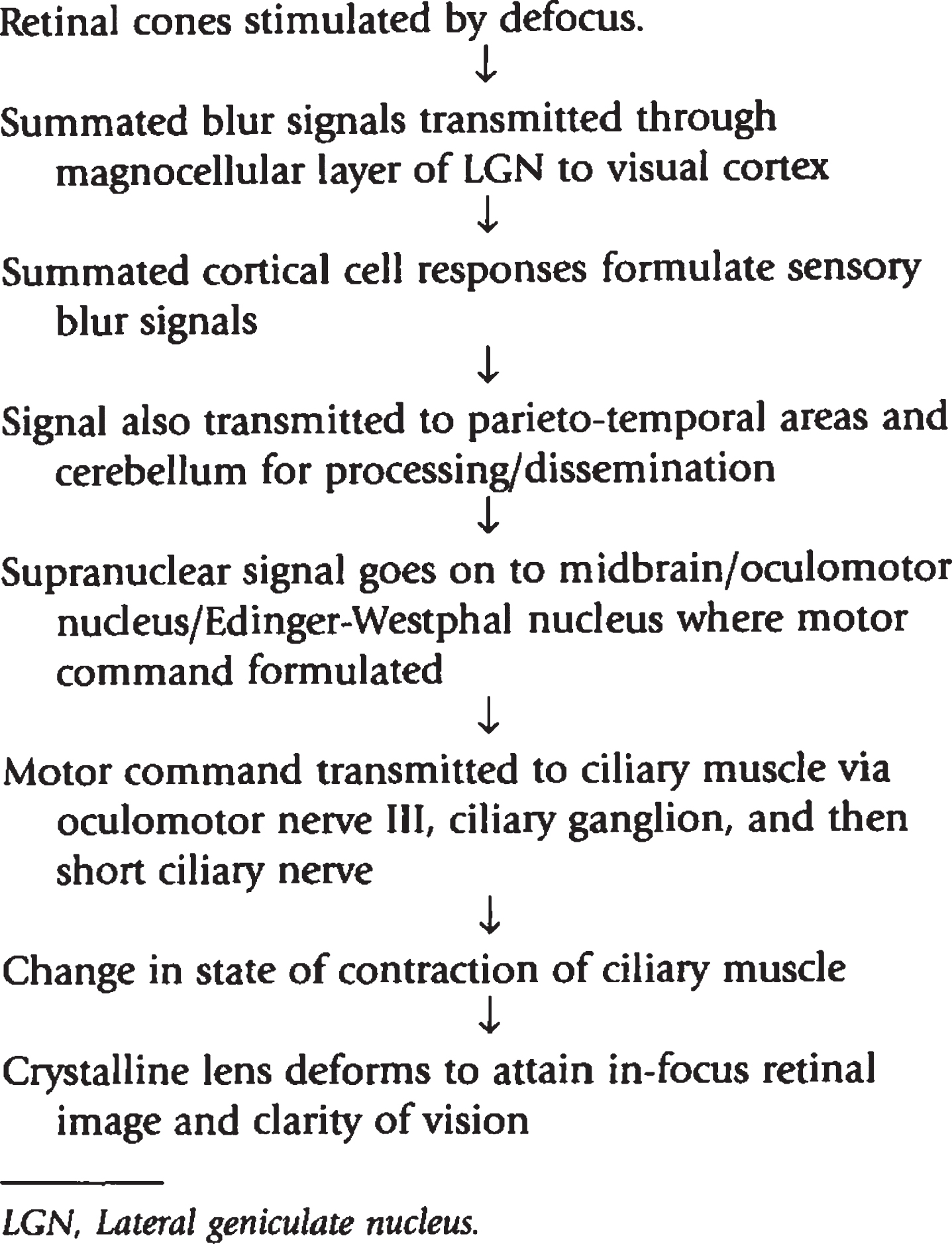
Based on various neurophysiological and anatomical experiments, the neural network of accommodation is known to be extensive (Richter et al., 2004; Lv et al., 2020; May et al., 2016 & 2019; Ciuffreda, 2006; Snell & Lemp, 1998; Ohtsuka et al., 2002; Ohtsuka & Sawa, 1997). The possible pathway is as follows (Fig. 5): The blur signal via the retinal ganglion cells exits the eye through the optic nerve, which decussates partially at the optic chiasm, and ascends via the optic tract to synapse at the lateral geniculate nucleus (LGN). Two distinct optic radiation pathways that leave the LGN have been identified: one travelling laterally and inferiorly through the temporal area and forming Meyer’s loop, and the other traveling more superiorly through the parietal area. The dual pathways of the optic radiations converge and synapse at the calcarine fissure of the occipital lobe, which comprises the primary visual cortex (V1/Brodmann area 17/ striate cortex). Several projections arise from the striate cortex, including the secondary visual cortex, the superior colliculus, and the LGN. The secondary visual cortices integrate blur information from the striate cortex, other cortical areas, and the thalamus, thus combining information from the two halves of the visual field through the corpus collosum, and then relaying it to other areas involving higher levels of visual perception in the parietal and temporal areas of brain. The posterior parietal cortex (PPC), which is a secondary visual cortex, is connected to the frontal eye fields (FEF), and the FEF sends projections via the internal capsule to the main oculomotor nucleus, as well as the parasympathetic accessory oculomotor nucleus (i.e., Edinger-Westphal nucleus). Additionally, the PPC has descending projections to the rostral superior colliculus (SC). The rostral SC projects to the Edinger-Westphal nucleus via the primary shorter route through the pretectum, and also a secondary longer side route through the nucleus reticularis tegmenti pontis (pons), cerebellar cortex, and cerebellar nuclei. Studies have also shown a significant role of the cerebellar vermis, and the cortices surrounding the right superior temporal sulcus and inferior temporal gyrus regions to be actively involved in blur information processing (Richter et al., 2004; Lv et al. 2020).
Fig. 5
Sensory and motor pathway of blur-driven accommodation. Reprinted with permission from Borish, 2006.
In response to this blur input, the parasympathetic pathway commences with a motor signal generated at the Edinger-Westphal nucleus. This is located slightly posterior to the main oculomotor nucleus in the midbrain at the level of the SC. This system provides the primary rapid (∼1sec) drive to the accommodative system for all distances and directions. Preganglionic parasympathetic fibers pass through the red nucleus and travel along with the oculomotor nerve towards the orbit. These fibers branch with the inferior division of the oculomotor nerve going toward the inferior oblique muscle. Prior to reaching the inferior oblique muscle, the parasympathetic fibers follow a short, thick branch to synapse in the ciliary ganglion. From there, postganglionic fibers follow the short ciliary nerves to innervate the ciliary muscle, and hence produces alteration of lens shape and increase in the dioptric power in a time-optimal manner.
In contrast, the slower-acting (10–40 sec) sympathetic system involves a more circuitous route originating from the hypothalamus and travelling down the spinal cord to the lower cervical and upper thoracic segments, where it synapses in the lateral horn. The second-order (i.e., preganglionic) neurons leave the spinal cord via the ventral roots of C8, T1, and T2 to enter the sympathetic chain and synapse within the superior cervical ganglion. The third-order (i.e., postganglionic) neurons follow the carotid plexus, and then enter the orbit either independently or with the first division of the trigeminal nerve. Some fibers also lead directly to the ciliary muscle via the long ciliary nerves, while others pass through the ciliary ganglion, without synapsing, before entering the eye via either the short or long ciliary nerves, thus decreasing lens power at distance. The sympathetic system is activated at all distances and directions during sustained accommodation.
Since the accommodative afferent and efferent neural pathways are quite extensive, any injury causing shearing of axons to the multitude of brain and contiguous structures may adversely impact upon the accommodative system. As commonly associated with the rotational acceleration of the head following a blunt trauma, injuries involving the midbrain area which house accommodation-related neurons could result in an accommodative dysfunction (Ciuffreda et al., 2007; Rucker et al., 2019), as will be described in detail later.
4.3Accommodative dysfunction in mTBI
Due to the complex coup-contrecoup nature and pervasiveness of the brain insult, TBI results in a myriad of visual dysfunctions, including accommodative function (Leslie, 2001; Zost, 2001; Green et al., 2010; Thiagarajan & Ciuffreda., 2014). Since the accommodative afferent and efferent neural pathways are quite extensive, any injury causing shearing of axons to the multitude of brain and contiguous structures may adversely impact upon the accommodative system. Anatomically, the midbrain region that houses neurons related to accommodation is very vulnerable to the damage caused by the cranial rotational acceleration forces and neck flexure acting upon the head following a blunt trauma (Rucker et al., 2019). As a result, accommodative dysfunction is a common sequela (Ciuffreda et al., 2007). In addition to direct head trauma, considering the sympathetic neural network of accommodation especially involving the cervical region, whiplash injuries in the absence of direct head insult could also affect accommodation markedly (Ciuffreda, 2006; Ciuffreda et al., 2001). Accommodative abnormality in mTBI is well-established in the literature through both clinically-based case series and laboratory studies. However, few investigated the impact of mTBI on accommodative function in a comprehensive manner involving both static and dynamic aspects.
4.4Clinical aspects of accommodation dysfunction
Earlier clinical studies used accommodative amplitude (i.e., the maximum amount of accommodation exerted with maximum effort) as the only diagnostic criterion to classify accommodative dysfunction in this population. Based on Duane’s (1922) age-normed curve, several studies reported accommodative insufficiency (i.e., reduced amplitude) in approximately 10–33% of the mild TBI (mTBI) population (Al-Qurainy, 1995; Gianutsos et al., 1988; Suchoff et al., 1999; Ciuffreda et al., 2007; Green et al., 2010). While the aforementioned studies were performed in the civilian population, similar findings have been reported in active duty war fighters following head trauma (Goodrich et al., 2007; Brahm et al., 2009; Stelmack et al., 2009; Capo-Aponte et al., 2012). In addition, reports also suggest approximately 18–33% of whiplash patients exhibiting reduced accommodative amplitude (Roca, 1972; Burke et al., 1992; Brown, 2003), which agrees with the previously stated prevalence in the more traditionally categorized patients with mTBI. An interesting case study from Harrison (1987) reported a twenty-year-old male patient with TBI who exhibited a persistent inability to accommodate in one eye three years after the injury, thus suggesting a more peripheral defect (e.g., ciliary ganglion and/or ciliary nerve). While this patient initially demonstrated a reduced accommodative convergence-to-accommodation (AC/A) ratio (1.33:1), the ratio normalized (3:1) without treatment eighteen months post-injury. A more recent retrospective study confirmed the above findings. Ciuffreda et al. (2007) reviewed 160 individuals with mTBI and found 41% of them under the age of 40 years to have accommodative insufficiency.
While accommodative insufficiency is quite common in this population, accommodative excess has also been reported in patients with mTBI, but less frequently (Leslie, 2001). In a sample of 161 patients with medically diagnosed mTBI, approximately 20% reported distance blur that was corrected with minus lenses (“pseudomyopia”), with their cycloplegic refraction eliciting either emmetropia, low hyperopia, or significantly less myopia (Kowal, 1992). However, more recently, in a retrospective study with a similar number of patients with medically-diagnosed mTBI (N = 160), Ciuffreda et al. (2007) reported only 4% of the population demonstrating accommodative excess. Lastly, there have also been several case studies reporting the rare but significant development of persistent bilateral accommodative spasm in individuals following head trauma (Bohlmann & France, 1987; Monteira et al., 2003; Chan & Trobe, 2002). Reports showed that the condition persisted for 7–10 years or even more, despite long term use of cycloplegic eye drops, such as atropine, to attempt to reduce the accommodative spasm. Since these studies showed accommodative spasm bilaterally, it is suggestive of a central defect. For example, MRI findings of one patient revealed lesions involving the subcortical white matter consisting of left temporal lobe areas, periventricular region, cerebellar vermis, and dorsal pons which are actively involved in accommodation. Interestingly, no lesions were detected in the mid-brain (Monteiro et al., 2003).
Of the clinical accommodative measures studied in this population, accommodative facility is the least investigated parameter. It indirectly assesses the overall accommodative dynamic characteristics that incorporates the important parameter of peak velocity of the response. Thus, an individual with a slowed accommodative response to either positive and/or negative flipper lenses (e.g., ±2.00D) would exhibit reduced facility (i.e., reduced number of cycles in one minute for a given flipper lens power). Accommodative infacility in the brain-injured population has been reported in relatively few studies (Ciuffreda et al., 2007; Scheiman & Gallaway, 2001). This occurs either alone or in conjunction with accommodative insufficiency or accommodative excess (Leslie, 2001). In contrast, the results of Capo-Aponte et al. (2012) did not find reduced accommodative facility in 20 war fighters with mTBI when compared to non-mTBI cohort when tested with±2.00D flippers. Accommodative infacility (under both monocular and binocular conditions) was also not found by Green et al. (2010) in 12 patients with mTBI when compared with age-matched normal individuals when tested with lower powered±1.00D flipper lenses. However, when a fatigue-paradigm was introduced, in which the subjects were asked to perform binocular accommodative flipper for a continuous 3-minute duration, the accommodative flipper testing rate significantly reduced in the mTBI population as compared to the normal individuals, indicating a clinically significant fatigue effect in the mTBI population.
In addition to significantly reduced accommodative amplitude, Green et al. (2010) also found that 50% (6/12) of the subjects with mTBI demonstrated an abnormal stimulus AC/A ratio, negative relative accommodation (NRA), and positive relative accommodation (PRA). However, their data did not show significant differences for other basic parameters such as tonic accommodation and the slope of accommodative stimulus-response (AS/R) function when compared with the age-matched normal population.
4.5Laboratory-based accommodative dynamics in mTBI
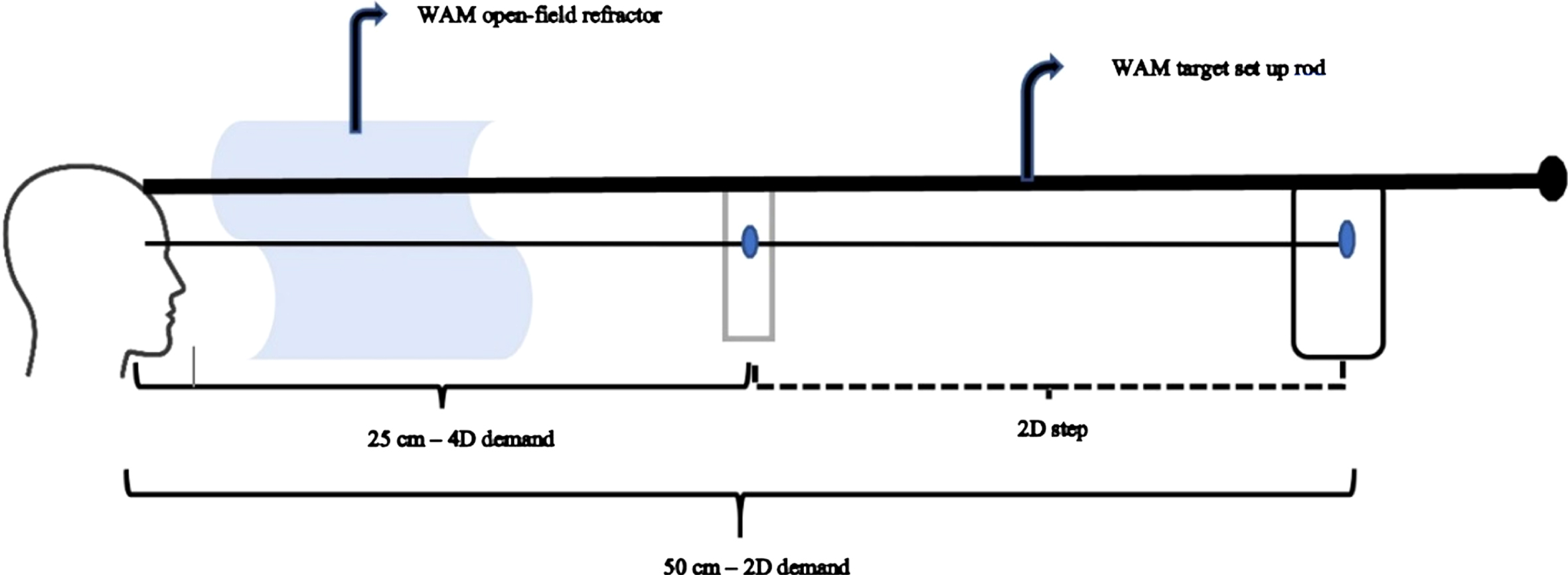
It is evident from the previous section that there has been numerous clinically-based research that studied accommodative function in TBI. However, laboratory-based investigations to evaluate and quantify the detailed dynamics of accommodation are sparse. Two major studies assessed accommodative dynamics to a rapid step change in accommodation in those with chronic mTBI (Green, 2009; Green et al., 2010; Thiagarajan & Ciuffreda, 2014). The commercially-available WAM 5500 objective, infrared, open-field autorefractor (Grand Seiko; Hiroshima, Japan) with a sampling rate of 5Hz was used. Targets were positioned at 50cm (2D stimulus) and 25cm (4D stimulus) and presented along the midline. These stimuli fell within the subject’s linear zone of accommodative responsivity. Subjects monocularly viewed (with the fellow eye completely occluded) a line of high contrast, black 20/30 Snellen letters having a luminance of 36 cd/m2 positioned at 2D that were on a white background, and a high contrast 20/60 word with a luminance of 36 cd/m2 at 4D on a transparent background; hence, the subject could see the far target through the transparent background. Subjects were encouraged to maintain the target in focus at all times. When instructed, the subject changed focus as rapidly as possible between the two stimuli. Subjects fixated and focused upon each stimulus for approximately 6–8 secs to reach steady-state, before switching to the other stimulus. A total of 15–20 responses were acquired and analyzed. A continuous measure of dynamic accommodative responses were recorded during the test period of 2–3 mins. See Fig. 6 for test arrangement.
Fig. 6
target arrangement with WAM 5500 autorefractor used for measuring accommodative dynamics for 2D step (increasing and decreasing) responses. Subjects switched their monocular focus between the 50cm (2D) and 25cm (4D) targets upon examiner’s verbal instruction in an increasing (50cm to 25cm) and decreasing (25cm to 50cm) step manner.
Green et al.’s study (2010) investigated a range of dynamic parameters of accommodation in 12 visually-symptomatic individuals with mTBI (mean age: 31 years; 6 months –13 years following TBI) and 10 visually-normal, age-matched control individuals (mean age: 27 years). See Table 1 for mean values of objective measures obtained in both groups. A dynamic accommodative response trace in an individual with mTBI (bottom trace) as compared to the age-matched normal individual (top trace) is shown in Fig. 7. The subject with mTBI in this figure would be categorized as having moderate to severe accommodative dysfunction with significantly slowed dynamics along with increased steady-state response variability that explains his ill-sustained accommodation.
Table 1
Dynamic accommodative parameters in the mTBI and normal groups
| Dynamic parameters | mTBI | Normal |
| PV –Inc.step (D/sec) | 5.1±0.6* | 8.0±0.4 |
| PV –Dec.step (D/sec) | 6.1±0.5* | 8.0±0.4 |
| TC –Inc.step (msec) | 430±39* | 271±11 |
| TC –Dec.step (msec) | 337±17* | 245±9 |
| SS variability –Inc.step (D) | 0.16±0.02 | 0.15±0.01 |
| SS variability –Dec.step (D) | 0.12±0.01 | 0.13±0.01 |
| Response amplitude –Inc.step (D) | 1.62±0.12 | 1.83±0.08 |
| Response amplitude –Dec.step (D) | 1.56±0.08 | 1.59±0.06 |
Symbols: PV = peak velocity (highest value in the velocity profile during the duration of response), TC = time constant (time taken to reach 63% of final response), Inc.step=increasing accommodative response from 2D to 4D, dec.step = decreasing accommodative response from 4D to 2D, SS = steady-state response (measured during steady fixation at a particular target), * = significantly different from normal. Reprinted with permission from Green, 2009
Fig. 7
Dynamic accommodative trace in a normal subject (a) and an individual with mTBI (b) showing slowed dynamic trajectory (red arrow) in the latter subject. Blue dotted line indicates step change in the stimulus between the two target distances. Reprinted with permission from Green, 2009.
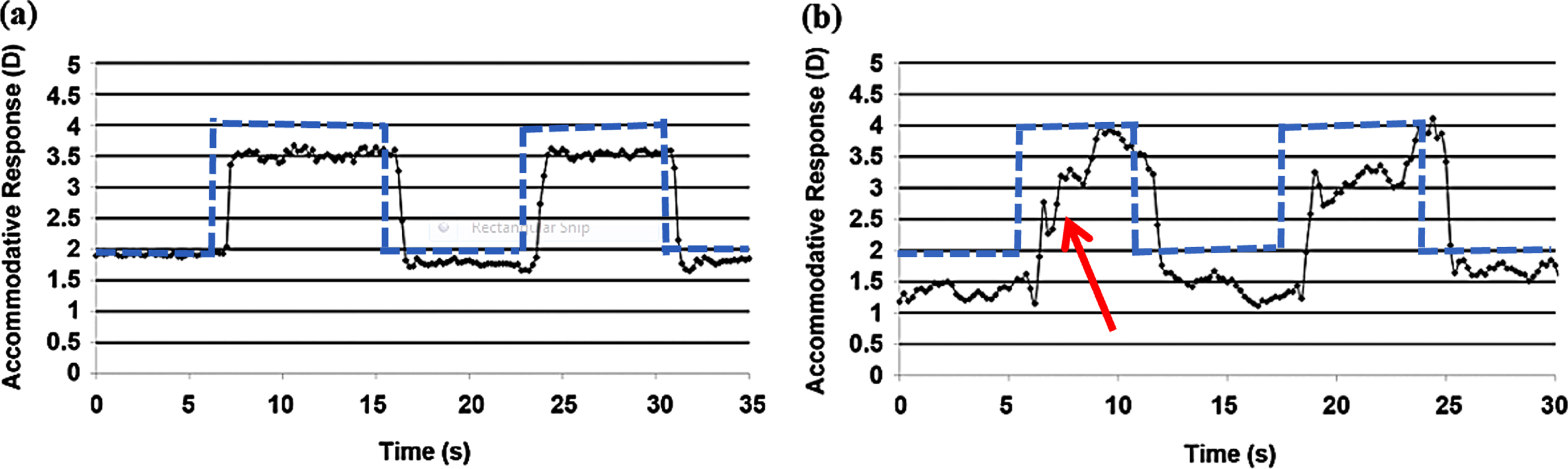
The study found significantly slowed accommodative dynamic characteristics in the mTBI population. Peak velocity for both increasing and decreasing step changes in accommodation was significantly reduced. In addition, it was associated with a prolonged time constant in both directions. No significant difference was observed for either the accommodative response amplitude or the steady-state variability between the two groups. In other words, those with mTBI were able to acquire the required amplitude; however, they exhibited a slowed trajectory and prolonged response duration. Once they attained the target amplitude, individuals with mTBI were able to sustain the response during the measured window of time. Similar to Green et al. (2010), Thiagarajan & Ciuffreda (2014) showed similar, abnormal first-order dynamic characteristics in the mTBI population at baseline prior to the oculomotor rehabilitation intervention. In both studies, the steady-state responses were only recorded for 6–8 secs, and the variability of these responses, once they attained the required amplitude (both at the 2D & 4D levels), did not a show significant difference between the two groups. However, if the responses were recorded for a longer period of time, it is likely that the mTBI group would exhibit increased steady-state response variability compared to the normal group, which could explain their symptom of intermittent blur during sustained near fixation, such as reading.
4.6Vision therapy/vision rehabilitation for accommodative dysfunction
While diagnostic clinical studies and case reports on accommodative dysfunction in mTBI are frequently reported in the literature (Leslie, 2001), investigations on efficacy of vision therapy on accommodative deficits in this population are sparse. For example, in 3 cases with mTBI (1 child, 2 young-adults), Scheiman and Gallaway (2001) assessed accommodative insufficiency before and after combined office-based with home-based vision therapy. On average, therapy sessions ranged from 11–45. Patients had a clinically significant improvement in accommodative amplitude that approached normalcy post-training. Two of the three subjects that initially had reduced or absent accommodative facility markedly improved approaching normalcy. Likewise, Ciuffreda et al. (2008) reported similar improvement in accommodative amplitude in 33 subjects who primarily complained of near blur. Their study had specific training paradigms that included monocular predictable step training to improve rapid reflex accommodative facility, monocular predictable ramp training to improve slow accommodative tracking ability, and monocular stationary target training to improve accommodative stability and sustainability (Hung & Ciuffreda, 1988). All training was performed in free space using loose lenses for 2 to 8 months for a total of 10–30 sessions. Improvement was found in 90% of the patients. However, these two studies evaluated clinical measures of accommodation only.
More recently, a placebo-controlled, clinical trial using an interventional cross-over design, that assessed accommodation, vergence, versional eye movements, subjective attention, and visual symptoms before and after oculomotor rehabilitation, was performed (Thiagarajan, 2012; Thiagarajan & Ciuffreda, 2014). To negate undesirable inter-subject variability, each subject acted as their own control with such a design. Twelve adult subjects (8 females, 4 males) between the ages of 23 and 33 yrs (mean±standard deviation [SD]: 29±3 yrs) with medically documented chronic mTBI and near work-related visual symptoms, such as blur, and having an injury onset of > 1 yr (1–10 years postinjury), participated in this study. Subjects in their acute/subacute phase of mTBI were excluded to avoid possible contamination from any natural recovery (Chen et al., 2010). In the 15-week study duration, during phase 1 therapy, each subject received 6 weeks of either conventional oculomotor training (OMT) or the placebo training (SHAM therapy), and then crossed-over to the phase 2 training of either true or SHAM training. The evaluative procedures (clinical, laboratory-based measures, subjective visual attention, and nearvision symptom scale) were recorded before and after both training sessions (i.e., during weeks 1, 8, 15). See Tables 2 & 3 for the list of parameters assessed. All clinical parameters were assessed using standardized clinical techniques (Borish, 2006). Like in Green et al. (2010), the laboratory-based, first-order accommodative dynamics to 2 D increasing and decreasing step responses were obtained using the commercially-available WAM 5500 objective, infrared, open-field autorefractor and the same paradigm. A subjective correlate of visual attention was assessed using the Visual Search and Attention Test (VSAT) (Trenerry et al., 1990). Individual symptoms related to near work were rated by the subjects using the Convergence Insufficiency Symptom Survey (CISS) (Rouse et al., 2004 CITT).
Table 2
Mean (±1SEM) values of laboratory-based objective accommodative parameters for monocular steps of accommodation before (baseline) and after true oculomotor training (post-OMT)
| Dynamic parameter | Baseline | Post-OMT | P value |
| Inc- Peak velocity (D/sec) | 4.5(0.6) | 5.8(0.6) | < 0.01 |
| Dec- Peak velocity (D/sec) | 4.2(0.7) | 5.6(0.6) | < 0.01 |
| Inc- Time constant (millisec) | 499(47) | 362(31) | < 0.01 |
| Dec- Time constant (millisec) | 589(99) | 412(75) | < 0.01 |
| Inc- Steady-state response level (4D stimulus) | 3.42(0.1)* | 3.46(0.1) | 0.59 |
| Dec- Steady-state response level (2D stimulus) | 1.74(0.08)* | 1.79(0.07) | 0.54 |
| Inc- Steady-state variability (D) | 0.14(0.02)* | 0.11(0.009) | 0.21 |
| Dec- Steady-state variability (D) | 0.11(0.01)* | 0.10(0.009) | 0.74 |
| Inc- Response amplitude (2D step) | 1.94(0.13)* | 1.91(0.08) | 0.67 |
| Dec- Response amplitude (2D step) | 1.88(0.10)* | 1.83(0.08) | 0.46 |
Inc- increasing step; Dec- decreasing step. BOLD, italicized –statistically significant. * –normal at baseline. Reprinted with permission from Thiagarajan, 2012
At each 6-week training phase, a total of 12 sessions of in-office training was performed (2 sessions/week, 45mins/session). There was no home training. During each training session, all three oculomotor components, namely, accommodation, vergence, and versional eye movements were trained for a total of 9 hours (Thiagarajan, 2012; Thiagarajan et al., 2014). Each component was trained for a total of 3 hours. For the purpose of this review, however, only the accommodation training results are discussed. At baseline, all subjects manifested at least one clinical sign of accommodative dysfunction, i.e., a reduced near point of accommodation and/or reduced accommodative facility. During the accommodative training phase, both amplitude and facility were trained at 40cm using various magnitudes of positive/negative lenses in a repetitive manner. Based on the subject’s task performance, difficulty was altered by increasing the dioptric power of the lens. During the placebo training phase, either a plano powered clear or colored lens that did not stimulate blur-driven accommodation was used monocularly and binocularly, while the subject either read a text paragraph or watched a cartoon movie at 40cm on a computer screen, similar to that performed for the OMT. Several key parameters of static and dynamic accommodation improved significantly following only 3 hours of accommodative training that spanned across 6 weeks (Thiagarajan, 2012; Thiagarajan & Ciuffreda, 2014). In contrast, none of the parameters showed any significant improvement following the placebo training, and hence will not be further discussed here.
4.7Therapeutic effects on accommodative dynamic behavior
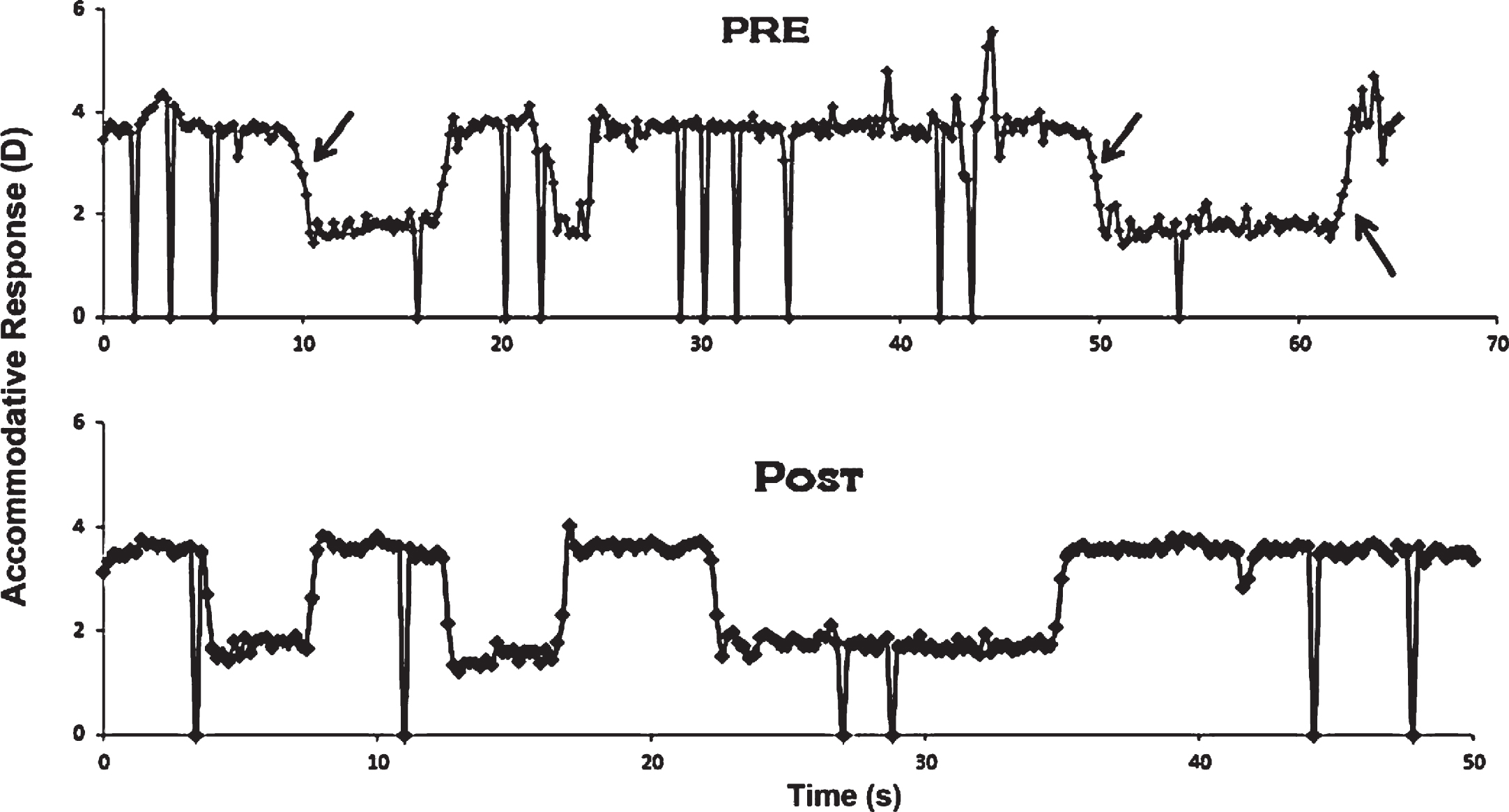
Figure 8 presents unedited accommodative 2 D, objective step response traces in a typical subject with mTBI before and after OMT (Thiagarajan & Ciuffreda, 2014). Similar to the Green et al. (2010) findings, at baseline, the dynamic trajectory for both increasing and decreasing steps of accommodation (Schnider et al., 1984; Ciuffreda & Kruger, 1988) exhibited slowed responsivity as evident from the reduced peak velocity and related prolonged time constant. The group mean peak velocity (∼4.4 D/s) at baseline in those with mTBI was ∼40 percent less than that found in the normal individuals (8 D/s) for the same stimulus amplitude (i.e., 2 D) from the literature for both increasing and decreasing steps of accommodation. However, following OMT, there was a significant increase in peak velocity by ∼30 percent from the baseline value for both increasing and decreasing steps of accommodation, although it had not normalized. The response amplitudes and steady-state variability were normal at baseline, and hence did not show any significant difference following therapy. This was confirmed by the normal accommodative stimulus-response gain found in the study. However, subjects now attained their SS response level more rapidly. Concomitantly, the time constant exhibited a correlated and significant decrease. This effect was true for both increasing and decreasing steps of accommodation, as expected. See Table 2 for a range of dynamic parameters assessed.
Fig. 8
Unedited monocular step accommodative response traces as a function of time in a typical mTBI subject before (pre) and after (post) accommodative training. Large deflections represent blinks. Arrows denote slowed dynamic trajectory. D = diopter. Reprinted with permission from Thiagarajan, 2012.
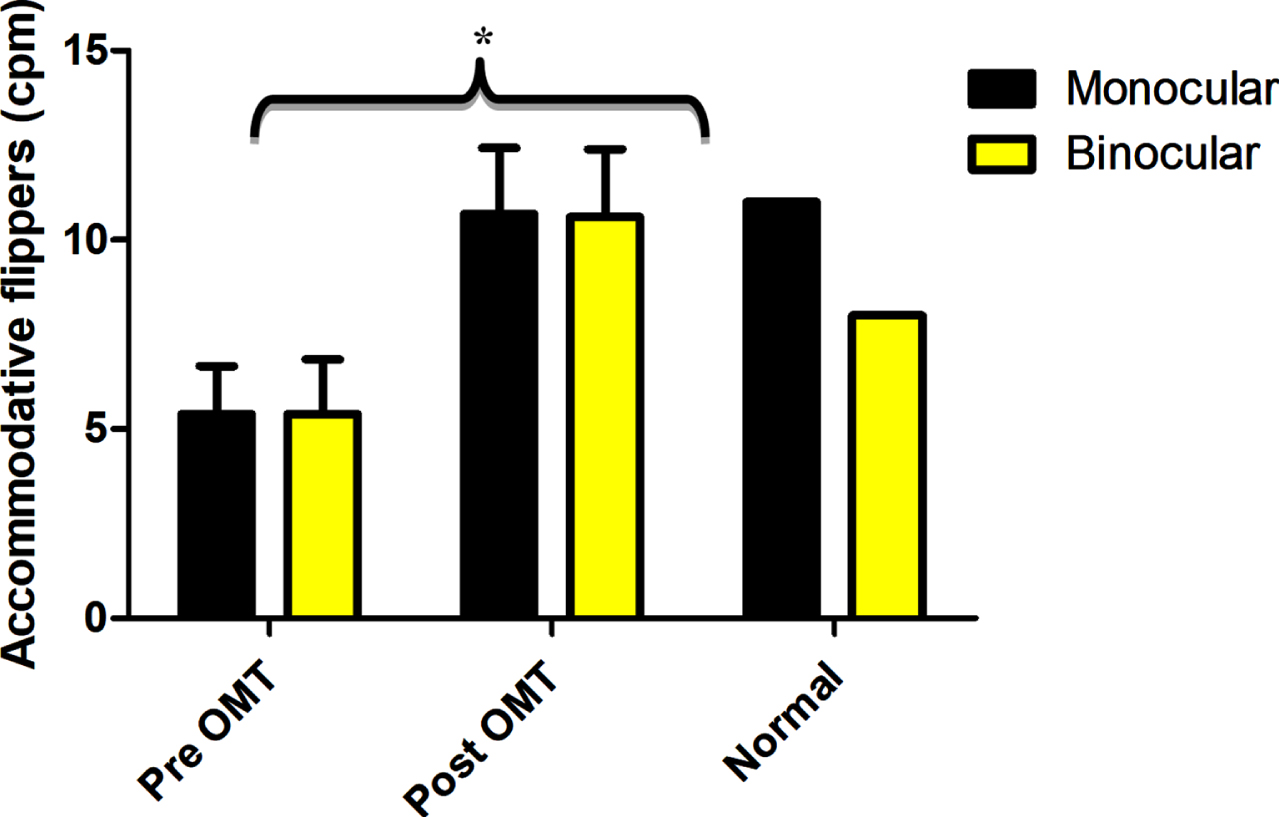
The clinical analogue of the objective dynamic accommodative measure is the accommodative flipper facility. The mean monocular and binocular cycles for a±2.00 D flipper in the subjects with mTBI were close to 40% less than the normal value at baseline. This improved remarkably and significantly following training and normalized. See Fig. 9.
Fig. 9
Mean accommodative facility before (Pre OMT) and after (Post OMT) training in mTBI in comparison to expected clinic norm for monocular and binocular accommodative facilities. Error bars indicate +1SEM. * - significantly increased from baseline. cpm –cycles per minute. Reprinted with permission from Thiagarajan, 2012.
4.8Training effects on static measures of accommodation
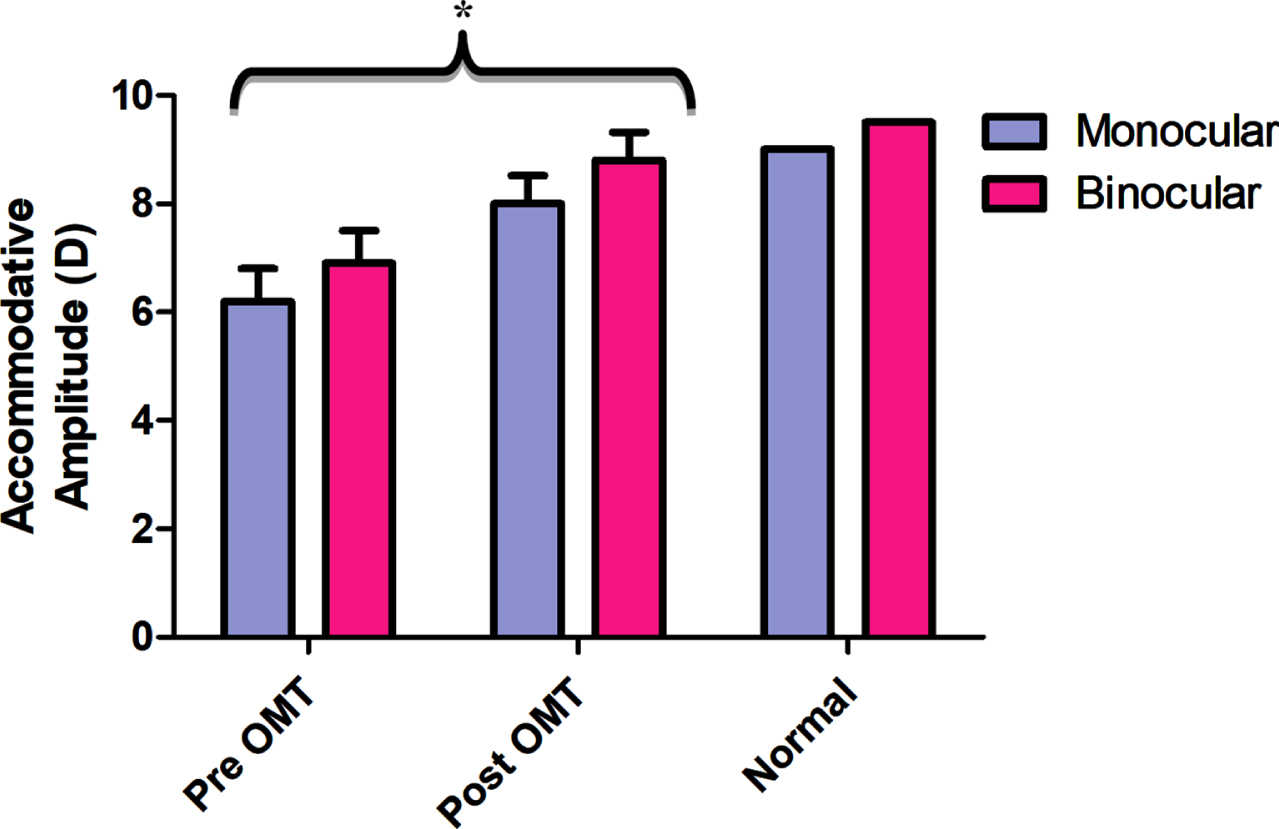
The main diagnostic parameter for assessing accommodative function in the clinic is the near point of accommodation (NPA) (i.e., the maximum amplitude of accommodation). It is the most frequently found abnormal accommodative parameter in the mTBI population (Ciuffreda et al., 2007, 2008; Green et al. 2010; Thiagarajan & Ciuffreda, 2014). On average, individuals with mTBI demonstrate approximately a 30% reduced amplitude both monocularly and binocularly as compared to Duane’s age-matched normative value (Duane, 1912). Following accommodative training, Thiagarajan & Ciuffreda (2014) showed that the monocular and binocular accommodative amplitudes significantly improved, with amplitudes reaching around 90% of the expected age normative value (See Fig. 10). In addition, they found that the increased accommodative amplitude was correlated with decreased near-vision symptoms per the CISS score, along with increased subjective attention as evident from the VSAT percentile scores (Thiagarajan & Ciuffreda, 2014; Thiagarajan, 2012). Training did not influence the relative accommodative amplitudes (NRA and PRA), as these values were normal prior to therapy, and hence no changes were expected following accommodative training. While this study did not measure the lag/lead of accommodation using dynamic retinoscopy, one can expect a normal lag of accommodation given the normal response amplitudes and steady-state variability (within the measured window of time) in this population.
Fig. 10
Mean accommodative amplitude before (Pre OMT) and after (Post OMT) training in mTBI in comparison to Duane’s age-matched normal values for monocular and binocular accommodation. Error bars indicate +1SEM. * - significantly increased from baseline. Reprinted with permission from Thiagarajan, 2012.
Thus, these findings from Thiagarajan & Ciuffreda (2014), along with the aforementioned clinical case series (Scheiman & Gallaway, 2001) and retrospective analysis (Ciuffreda et al., 2008), support the idea that targeted, specific, repetitive, programmed therapy procedures can remediate a range of accommodative disorders occurring as a consequence of an mTBI (Ciuffreda, 2002). Symptoms were ameliorated along with concurrent normalization of clinical signs, as well as improvement in subjective visual attention. In addition, Thiagarajan & Ciuffreda (2015) evaluated persistence of training effects in 8 subjects with mTBI who participated in the cross-over trial (Thiagarajan, 2012; Thiagarajan & Ciuffreda, 2014). Amplitudes of accommodation and accommodative facilities were remeasured at 3 months and 6 months following the completion of the OMT training. Results showed that both the parameters persisted during this time period, and furthermore they were not significantly different from the immediate post-OMT visit, hence demonstrating persistence of training effects up to 6 months following training. However, future studies are warranted to assess for longer periods of persistence (i.e., post 1 year or 5 years), and this information could be used to indicate the need for future booster and/or maintenance therapy to remain symptom-free.
4.9Accommodative deficits and their remediation: proposed neurophysiological mechanisms
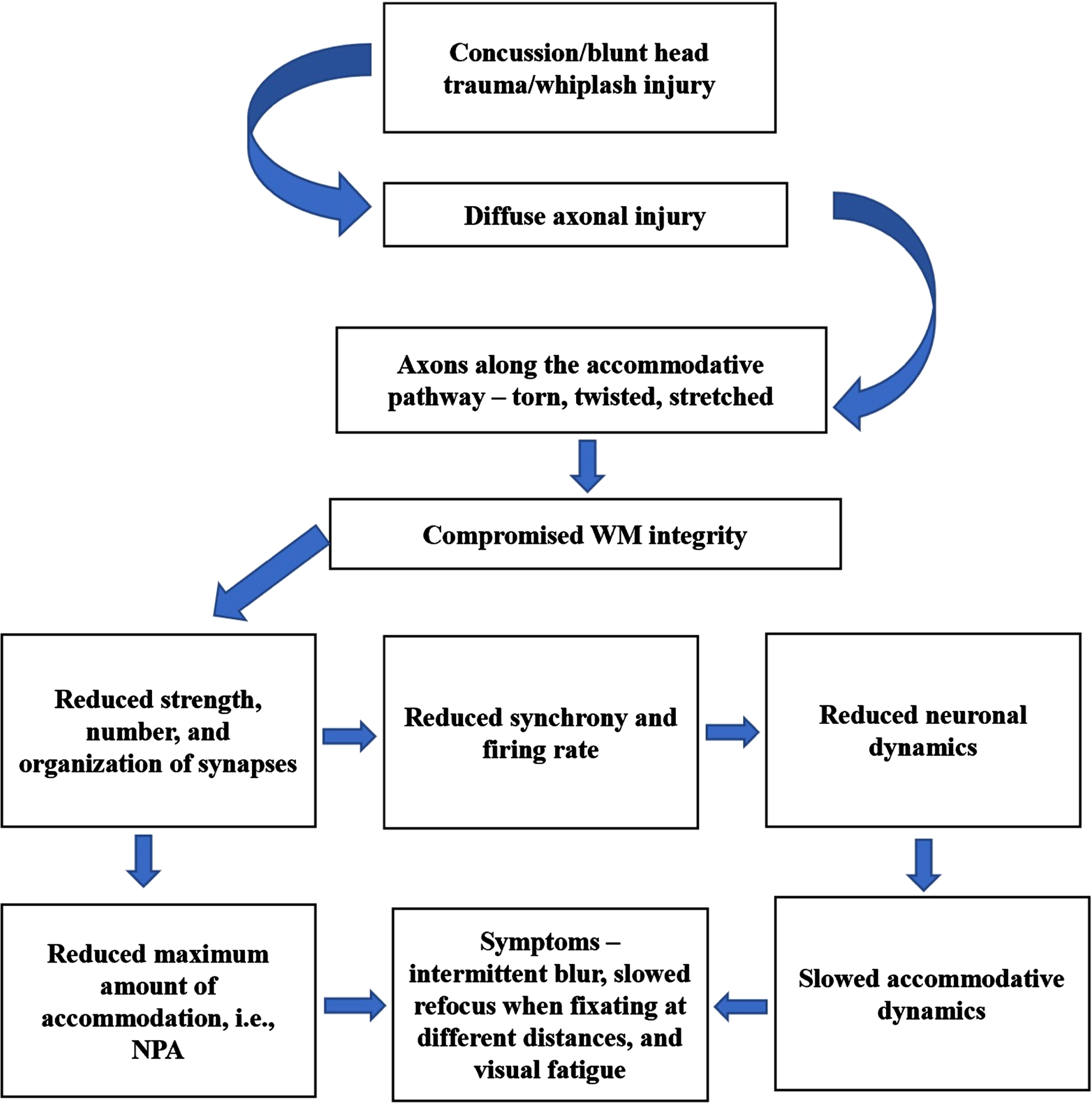
Following head trauma, diffuse axonal injury causing shearing of axons results in compromised white-matter integrity causing slower conduction of nerve impulses (Bazarian et al., 2007; Bigler, 2007). As a result, the strength, number, and organization of synapses along the accommodative pathway are likely reduced. This in turn can directly adversely affect the accommodative amplitude as found in the mTBI population. In addition, reduced synchrony of firing rate can impact on the overall neuronal dynamics causing slowed accommodative responsivity, thus likely also producing near-vision symptoms (Thiagarajan, 2012; Thiagarajan & Ciuffreda, 2014). Vision therapy acts as a relearning process, in which functional recovery of the system being trained regains its automaticity through repeated synaptic stimulation and increased synaptic strength (Hebb, 1949), also known as, long-term potentiation (Citri & Malenka, 2008; Johnston, 2009). Remapping and reconfiguration of neural circuits both within and across relevant brain regions appears to play a significant role in the recovery process. Functional improvement during visual neurorehabilitation is believed to occur through 3 key neural strategies (Warraich & Kleim, 2010): restoration, recruitment, and retraining neural of the regions. This experience-dependent neuroplasticity is comprised of biochemical, cellular, physiological, and structural level changes. With accommodative training, repeated stimulation using a combination of positive and negative lenses with increasing task difficulty (i.e., task loading) resulted in the overall improvement of accommodative function. Given the extensive neuronal areas involved in the control of accommodation, it is difficult to speculate on precisely which specific areas of the brain have regained normal activity, since the brain may use different strategies (restoration/recruitment/retraining) to recover from the functional loss. Functional neuroimaging studies are therefore necessary to correlate these relearned accommodative behavioral changes. A proposed mechanism is presented in Fig. 11.
Fig. 11
Proposed neural mechanisms of TBI causing accommodative dysfunction. WM –white matter, NPA –near point of accommodation.
5Conclusions
Two critical oculomotor components of the near triad, namely accommodation and pupil, manifest many abnormalities and dysfunctions following mTBI: responses are slowed, sometimes delayed, and frequently more variable. Fortunately, a range of proven rehabilitative approaches have evolved to reduce the common visual symptoms of blurred vision and light sensitivity, to improve the patient’s quality of life, both vocationally and avocationally. To further validate oculomotor therapy effects, functional and structural brain imaging studies are warranted to correlate with the behavioral changes.
Conflict of interest
None to report.
References
1 | Adoni, A. & McNeet, M. ((2007) ). The pupillary response in traumatic brain injury: a guide for trauma nurses. Journal of Trauma Nursing, 14: (4), 191–196. |
2 | Al-Qurainy, I. A. ((1995) ). Convergence insufficiency and failure of accommodation following midfacial trauma. British Journal of Oral and Maxillofacial Surgery, 33: , 71–75. |
3 | Bazarian, J. J. , Zhong, J. , Blyth, B. , Zhu, T. , Kavcic, V. & Peterson, D. ((2007) ). Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. Journal of Neurotrauma, 24: , 1447–1459. |
4 | Bigler, E. D. ((2007) ). Anterior and middle cranial fossa in traumatic brain injury: relevant neuroanatomy and neuropathology in the study of neuropsychological outcome. Neuropsychology, 21: , 515–531. |
5 | Bohlmann, B. J. & France, T. D. ((1987) ). Persistent accommodative spasm nine years after head trauma. Journal of Clinical Neuroophthalmology, 7: , 129–134. |
6 | Brahm, K. D. , Wilgenburg, H. M. , Kirby, J. , Ingalla, S. , Chang, C. & Goodrich, G. L. ((2009) ). Visual impairment and dysfunction in combat-injured servicemembers with traumatic brain injury. Optometry and Vision Science, 86: , 817–825. |
7 | Brown, S. ((2003) ). Effect of whiplash injury on accommodation. Clinical and Experimental Ophthalmology, 31: , 424–429. |
8 | Burke, J. P. , Orton, H. P. , West, J. , Strachan, I. , Hockey, M. S. & Ferguson, D. G. ((1992) ). Whiplash and its effect on the visual system. Graefes Archives of Clinical and Experimental Ophthalmology, 230: , 335–339. |
9 | Capo-Aponte, J. E. , Beltran, T. A. , Walsh, D. V. , Cole, W. R. & Dumayas, J. Y. ((2018) ). Validation of visual objective biomarkers for acute concussion. Military Medicine, 183: (suppl. 1), 9–17. |
10 | Capó-Aponte, J. E. , Urosevich, T. G. , Temme, L. A. , Tarbett, A. K. & Sanghera, N. K. ((2012) ). Visual dysfunctions and symptoms during the subacute stage of blast-induced mild traumatic brain injury. Military Medicine, 177: , 804–813. |
11 | Capo-Aponte, J. E. , Urosevich, T. G. , Walsh, D. V. , Temme, L. A. & Tarbett, A. K. ((2013) ). Pupillary light reflex as an objective biomarker for early identification of blast-induced mTBI. Journal of Spine, S4: , 004. |
12 | Chan, R. V. & Trobe, J. D. ((2002) ). Spasm of accommodation associated with closed head trauma. Journal of Neuroophthalmology, 22: , 15–17. |
13 | Chang, T. T. , Ciuffreda, K. J. & Kapoor, N. ((2007) ). Critical flicker frequency and related symptoms in mild traumatic brain injury. Brain Injury, 21: , 1055–1062. |
14 | Citri, A. & Malenka, R. C. ((2008) ). Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology, 33: , 18–41. |
15 | Ciuffreda, K. J. ((2002) ). The scientific basis for and efficacy of optometric vision therapy in nonstrabismic accommodative and vergence disorders. Optometry, 73: , 735–762. |
16 | Ciuffreda, K. J. ((2006) ). Accommodation, the pupil, and presbyopia. In: Benjamin, W.J. (ed.), Borish’s Clinical Refraction, pp. 77–120, W.B. Saunders. |
17 | Ciuffreda, K. J. , Ciuffreda, Y. H. , Kapoor, N. & Suchoff, I. B. ((2001) ). Oculomotor consequences of acquired brain injury. In: Suchoff IB, Kapoor N, Ciuffreda KJ, editors. Visual and Vestibular Consequences of Acquired Brain Injuries, Santa Ana, CA: Optometric Extension Program Foundation. |
18 | Ciuffreda, K. J. , Joshi, N. R. & Truong, J. Q. (2017). Understanding the effects of mild traumatic brain injury on the pupillary light reflex. Concussion, CNC36. |
19 | Ciuffreda, K. J. , Kapoor, N. , Rutner, D. , Suchoff, I. B. , Han, M. E. & Craig, S. ((2007) ). Occurrence of oculomotor dysfunctions in acquired brain injury: a retrospective analysis. Optometry, 78: , 55–61. |
20 | Ciuffreda, K. J. & Kruger, P. B. ((1988) ). Dynamics of human voluntary accommodation. American Journal of Optometry & Physiological Optics, 65: , 365–370. |
21 | Ciuffreda, K. J. , Ludlam, D. P. , Yadav, N. K. & Thiagarajan, P. ((2016) ). Traumatic brain injury: visual consequences, diagnosis, and treatment. In: Yanoff, M. (ed.), Advances in Ophthalmology and Optometry, pp. 307–333, Elsevier. |
22 | Ciuffreda, K. J. , Rutner, D. , Kapoor, N. , Suchoff, I. B. , Craig, S. & Han, M. E. ((2008) ). Vision therapy for oculomotor dysfunctions in acquired brain injury: A retrospective analysis. Optometry, 79: , 18–22. |
23 | Ciuffreda, K. J. , Tannen, B. , Singman, E. L. & Han, E. ((2021) , in press). Evaluation and treatment of visual dysfunction. In: Zasler, N.D., Katz, D.I. & Zafonte, R.D. (eds.), Brain Injury Medicine, Demos Medical. |
24 | Digre, K. B. & Brennan, K. C. ((2012) ). Shedding light on photophobia. Journal of Neuro-Ophthalmology, 32: , 68–81. |
25 | Duane, A. J. ((1922) ). Studies in monocular and binocular accommodation, with their clinical application. Transactions of American Ophthalmological Society, 20: , 132–157. |
26 | Gianutsos, R. , Ramsey, G. & Perlin, R. R. ((1988) ). Rehabilitative optometric services for survivors of acquired brain injury. Archives of Physical Medicine and Rehabilitation, 69: , 573–578. |
27 | Gilmartin, B. ((1986) ). A review of the role of sympathetic innervation of the ciliary muscle in ocular accommodation. Ophthalmic & Physiological Optics, 6: , 23–37. |
28 | Goodrich, G. L. , Kirby, J. , Cockerham, G. , Ingalla, S. P. & Lew, H. L. ((2007) ). Visual function in patients of a polytrauma rehabilitation center: A descriptive study. Journal of Rehabilitation Research and Development, 44: , 929–936. |
29 | Green, W. ((2009) ). Dynamic and static aspects of accommodation in mild traumatic brain injury (Master’s thesis). SUNY College of Optometry. https://dspace.sunyconnect.suny.edu/handle/1951/44795 |
30 | Green, W. , Ciuffreda, K. J. , Thiagarajan, P. , Szymanowicz, D. , Ludlam, D. P. & Kapoor, N. ((2010) ). Accommodation in mild traumatic brain injury. Journal of Rehabilitation Research and Development, 47: , 183–199. |
31 | Greenwald, B. D. , Kapoor, N. & Singh, A. D. ((2012) ). Visual impairments in the first year after traumatic brain injury. Brain Injury, 26: , 1338–1359. |
32 | Harrison, R. J. ((1987) ). Loss of fusional vergence with partial loss of accommodative convergence and accommodation following head injury. Binocular Vision, 2: , 93–100. |
33 | Heath, G. G. ((1956) ). Components of accommodation. American Journal of Optometry and Archives of the American Academy of Optometry, 33: , 569–579. |
34 | Hebb, D. O. ((1949) ). The organization of Behavior. NewYork: John Wiley and Sons Inc. |
35 | Hsu, J. , Stec, M. , Ranaivo, H. R. , Srdanovic, N. & Kurup, S. P. ((2021) ). Concussion alters dynamic pupillary light responses in children. Journal of Child Neurology, 36: , 195–202. |
36 | Hung, G. K. & Ciuffreda, K. J. ((1988) ). Dual-mode behaviour in the human accommodation system. Ophthalmic and Physiological Optics, 8: , 327–332. |
37 | Johnston, M. V. ((2009) ). Plasticity in the developing brain: Implications for rehabilitation. Developmental Disabilities Research Reviews, 15: , 94–101. |
38 | Kapoor, N. & Ciuffreda, K. J. ((2002) ). Vision disturbances following traumatic brain injury. Current Treatment Options in Neurology, 4: , 271–280. |
39 | Kardon, R. ((2003) ). Regulation of light through the pupil. In: Kaufman, P. & Alm, A. (eds.), Adler’s Physiology of the Eye, pp. 502–525, Mosby. |
40 | Kowal, L. ((1992) ). Ophthalmic manifestations of head injury. Australian New Zealand Journal of Ophthalmology, 20: , 35–40. |
41 | Leslie, S. ((2001) ). Accommodation in acquired brain injury. In: Suchoff IB, Kapoor N, Ciuffreda KJ, editors. Visual and Vestibular Consequences of Acquired Brain Injuries, Santa Ana, CA: Optometric Extension Program Foundation. |
42 | Loewenfeld, I. E. & Lowenstein, O. ((1993) ). The Pupil: Anatomy, Physiology, and Clinical Applications. Iowa State University Press, Ames and Wayne State University Press, Detroit. |
43 | Lv, X. , Chen, Y. , Tan, W. , Yu, Y. , Zou, H. , Shao, Y. , Zan, S. , Tao, J. & Miao, W. ((2020) ). Functional neuroanatomy of the human accommodation response to an “E” target varying from -3 to -6 diopters. Frontiers in Integrative Neuroscience, 14: , 1–11. |
44 | Master, C. L. , Podolak, O. E. , Ciuffreda, K. J. , Metzger, K. B. , Joshi, N. R. et al. ((2020) ). Utility of the pupillary light reflex as a physiologic biomarker for adolescent sport-related concussion. JAMA Ophthalmology, 138: , 1135–1141. |
45 | May, P. J. , Warren, S. , Bohlen, M. O. , Barnerssoi, M. & Horn, A. K. ((2016) ). A central mesencephalic reticular formation projection to the Edinger-Westphal nuclei. Brain Structure & Function, 221: , 4073–4089. |
46 | May, P. J. , Billig, I. , Gamlin, P. D. & Quinet, J. ((2019) ). Central mesencephalic reticular formation control of the near response: lens accommodation circuits. Journal of Neurophysiology, 121: , 1692–1703. |
47 | Monteira, M. L. , Curi, A. L. , Pereira, A. , Chamon, W. & Leite, C. C. ((2003) ). Persistent accommodative spasm after severe head trauma. British Journal of Ophthalmology, 87: , 243–244. |
48 | McKay, R. E. , Kolm, M. A. , Schwartz, E. S. & Larson, M. D. ((2020) ). Evaluation of two portable pupillometers to assess clinical utility. Concussion, 5: , CNC82. |
49 | Myers, G. A. & Stark, L. ((1990) ). Topology of the near response triad. Ophthalmic and Physiological Optics, 10: , 175–181. |
50 | Nichols, A. & Schulman, R. ((2020) ). Objective measurement of sustained pupillary constriction: a pilot study using an app-based pupillometer. Vision Development & Rehabilitation, 6: (1), 57–63. |
51 | Ohtsuka, K. , Maeda, S. & Oguri, N. ((2002) ). Accommodation and convergence palsy caused by lesions in the bilateral rostral superior colliculus. American Journal of Ophthalmology, 133: , 425–427. |
52 | Ohtsuka, K. & Sawa, M. ((1997) ). Frequency characteristics of accommodation in a patient with agenesis of the posterior vermis and normal subjects. British Journal of Ophthalmology, 81: , 476–480. |
53 | Richter, H. O. , Lee, J. T. & Pardo, J. V. ((2000) ). Neuroanatomical correlates of the near response: Voluntary modulation of accommodation/vergence in the human visual system. European Journal of Neuroscience, 12: , 311–321. |
54 | Richter, H. O. , Costello, P. , Sponheim, S. R. , Tee, J. L. & Pardo, J. V. ((2004) ), European Journal of Neuroscience, 20: , 2722–2732. |
55 | Roca, P. D. ((1972) ). Ocular manifestations of whiplash injuries. Annals of Ophthalmology, 4: , 63–73. |
56 | Rouse, M. W. , Borsting, E. J. , Mitchell, G. L. , Scheiman, M. , Cotter, S. A. , Cooper, J. , Kulp, M. T. , London, R. & Wensveen, J. Convergence Insufficiency Treatment Trial Group. ((2004) ). Validity and reliability of the revised convergence insufficiency symptom survey in adults. Ophthalmic and Physiological Optics, 24: , 384–390. |
57 | Rucker, J. C. , Buettner-Ennever, J. , Straumann, D. & Cohen, B. ((2019) ). Case studies in neuroscience: instability of the visual near triad in traumatic brain injury—evidence for a putative convergence integrator. Journal of Neurophysiology, 122: , 1254–1263. |
58 | Rutner, D. , Kapoor, N. , Ciuffreda, K. J. , Craig, S. , Han, E. & Suchoff, I. B. ((2006) ). Occurrence of ocular disease in traumatic brain injury in a selected sample: a retrospective analysis. Brain Injury, 20: (10), 1079–1086. |
59 | Scheiman, M. & Gallaway, M. ((2001) ). Vision therapy to treat binocular vision disorders after acquired brain injury: factors affecting prognosis. In: Suchoff IB, Kapoor N, Ciuffreda KJ, editors.Visual andVestibular Consequences of Acquired Brain Injuries, Santa Ana, CA: Optometric Extension Program Foundation. |
60 | Schnider, C. M. , Ciuffreda, K. J. , Copper, J. & Kruger, P. B. ((1984) ). Accommodation dynamics in divergence excess exotropia. Investigative Ophthalmology and Vision Science, 25: , 414–418. |
61 | Semmlow, J. L. , Hung, G. K. , Horng, J. L. & Ciuffreda, K. J. ((1993) ). Initial control component in disparity vergence eye movements. Ophthalmic and Physiological Optics, 13: , 48–55. |
62 | Snell, R. S. & Lemp, M. A. ((1998) ). Clinical Anatomy of the Eye. 2nd ed. Malden, MA: Blackwell Science. |
63 | Suchoff, I. B. , Ciuffreda, K. J. & Kapoor, N. (2001). Visual and Vestibular Consequences of Acquired Brain Injury. Optometric Extension Program Foundation. |
64 | Suchoff, I. B. , Kapoor, N. , Waxman, R. & Ference, W. ((1999) ). The occurrence of ocular and visual conditions in a non-selected acquired brain-injured patient sample. Journal of American Optometric Association, 70: , 301–308. |
65 | Stelmack, J. A. , Frith, T. , Koevering, D. V. , Rinne, S. & Stelmack, T. R. ((2009) ). Visual function in patients followed at a Veterans Affairs polytrauma network site: An electronic medical record review. Optometry, 80: , 419–424. |
66 | Stern, C. D. ((2011) ). Photophobia, light, and color in acquired brain injury. In: Suter, P.S. & Harvey, L.H. (eds.), Visual Rehabilitation, pp. 283–300, CRC press. |
67 | Suter, P. S. & Harvey, L. H. (eds.) ((2011) ). Vision Rehabilitation, CRC press. |
68 | Thiagarajan, P. (2012). Oculomotor rehabilitation for reading dysfunction in mild traumatic brain injury (Doctoral thesis).SUNYCollege of Optometry. https://dspace.sunyconnect.suny.edu/handle/1951/60654 |
69 | Thiagarajan, P. & Ciuffreda, K. J. ((2014) ). Effect of oculomotor rehabilitation on accommodative responsivity in mild traumatic brain injury. Journal of Rehabilitation Research & Development, 51: , 175–192. |
70 | Thiagarajan, P. & Ciuffreda, K. J. ((2015) ). Pupillary responses to light in chronic, non-blast mTBI. Brain Injury, 29: (12), 1420–1425. |
71 | Thiagarajan, P. & Ciuffreda, K. J. ((2015) ). Short-term persistence of oculomotor rehabilitative changes in mild traumatic brain injury (mTBI): A pilot study of clinical effects. Brain Injury, 29: , 1475–1479. |
72 | Trenerry, M. R. , Crosson, B. , DeBoe, J. & Leber, W. R. ((1989) ). Professional manual: visual search and attention test. Psychological Assessment Resources, Lutz. |
73 | Truong, J. Q. (2016). Mild traumatic brain injury and photosensitivity: Objective pupillometric findings (Doctoral thesis). SUNY College of Optometry. https://dspace.sunyconnect.suny.edu/handle/1951/67743 |
74 | Truong, J. Q. & Ciuffreda, K. J. ((2016) a). Comparison of pupillary dynamics to light in the mild traumatic brain injury (mTBI) and normal populations. Brain Injury, 30: (11), 1378–1389. |
75 | Truong, J. Q. & Ciuffreda, K. J. ((2016) b). Quantifying pupillary asymmetry through objective, binocular pupillometry in the normal and mild traumatic brain injury (mTBI) populations. Brain Injury, 30: (11), 1372–1377. |
76 | Truong, J. Q. & Ciuffreda, K. J. ((2016) c). Objective pupillary correlates of photosensitivity in the normal and mild traumatic brain injury populations. Military Medicine, 181: (10), 1382–1390. |
77 | Truong, J. Q. , Joshi, N. R. & Ciuffreda, K. J. ((2018) ). Influence of refractive error on pupillary dynamics in the normal and mild traumatic brain injury (mTBI) populations. Journal of Optometry, 11: (2), 93–102. |
78 | Truong, J. Q. , Ciuffreda, K. J. , Han, E. & Suchoff, I. B. ((2014) ). Photosensitivity in mild traumatic brain injury (mTBI): a retrospective analysis. Brain Injury, 28: (10), 1283–1287. |
79 | Warriach, Z. & Kleim, J. A. ((2010) ). Neural plasticity: the biological substrate for neurorehabilitation. American Academy of Physical Medicine and Rehabilitation, Suppl. 2: , S208–S219. |
80 | Zasler, N. D. , Katz, D. I. & Zafonte, R. D. (eds.) ((2021) ). Brain Injury Medicine, Demos Medical. |
81 | Zost, M. G. ((2001) ). Diagnosis and management of visual dysfunction in cerebral injury. In: Maino DM, editor. Diagnosis and management of special populations. Santa Ana, CA: Optometric Extension Program Foundation, 75–134. |




