Non-motor impairments affect walking kinematics in Parkinson disease patients: A cross-sectional study
Abstract
BACKGROUND:
In patients with Parkinson disease (PD), severe postural and gait impairments are rarely observed in early stage of disease and non-motor symptoms (NMS) are often overlooked.
OBJECTIVE:
This observational study aimed to characterize the impact of non-motor impairments on walking kinematics in early stages PD patients, and to assess the differences of gait parameters and NMS between PD patients with and without mild cognitive impairment (MCI).
METHODS:
Twenty-six patients with Modified Hoehn and Yahr Scale score≤2 were evaluated for NMS using Kings Parkinson’s Pain Scale, Parkinson Fatigue Severity scale, Parkinson Anxiety Scale, Beck Depression Inventory and Epworth Sleepiness Scale, kinematic parameters through an inertial sensor and cognitive performance by a comprehensive neuropsychological battery.
RESULTS:
Fatigue had a moderate negative correlation with step cadence, and a moderate to strong positive correlation with gait duration, Timed Up and Go (TUG) and TUG Dual Task (p < 0.01). Pain showed positive moderate correlation with gait duration (p < 0.01). Twelve patients resulted affected by MCI and reported significantly worse scores in gait duration, pain and fatigue (p < 0.05). According to cognitive z scores, PD-MCI group showed a moderate negative correlation between visuospatial abilities and fatigue (p < 0.05).
CONCLUSIONS:
NMS significantly affect walking kinematics whereas a limited role of cognitive status on motor performance occur in the early PD stages.
1Introduction
Parkinson disease (PD) is a progressive neurodegenerative condition with a prevalence of 1-2 out of 1000 people (GBD 2016 Parkinson’s Disease Collaborators) and an incidence of 10 to 20 per 100000 people/year, with predictable increase in the next decade (Tysnes & Storstein, 2017).
In PD patients, abnormal gait patterns occur, characterized by reduced speed, arm swing and step length, and difficulty in dissociating arm and trunk movements (Morris, Iansek, Matyas, & Summers, 1994), while, in the advanced stages of the disease, shuffling, festination, and freezing episodes are frequently observed (Mirelman et al., 2019). Several non-motor symptoms (NMS) are commonly observed in PD (Lee & Koh, 2015).
Up to 40–50%of patients with PD are affected by mood and cognitive disorders (Reijnders, Ehrt, Weber, Aarsland, & Leentjens, 2008), occurring even 4–6 years before motor symptoms. Among these, PD patients might experience Mild Cognitive Impairment (MCI) and Dementia. MCI occurs in 25–50%of PD patients (Weil, Costantini, & Schrag, 2018) and might involve a single or multiple domains (as attention, memory, executive functions, language, and visuospatial abilities) without interference with social or occupational functioning (Yarnall et al., 2014).
Several studies reported MCI-related gait changes in the general population, particularly in terms of reduced speed, stride length and time, and anterior-posterior and mediolateral sway position (Bahureksa et al., 2017). On the contrary, decline in spatiotemporal gait parameters may be predictive of cognitive impairment across domains such as memory, executive function, language, visuospatial (Savica et al., 2017).
In PD patients, cognitive impairment seems to be typically associated with gait alterations, such as slower speed and shorter steps as well as poor postural control (Kim et al., 2018).
In the early stage of PD, patients are unlikely to be affected by severe postural and gait impairments (Palakurthi et al., 2019; Kwon et al., 2017) and NMS are often underestimated.
The aim of this paper is to characterize the influence of non-motor impairments on walking kinematics in early stages PD patients. Moreover, we also assess the gait parameters according to the cognitive status and the correlations among non-motor and walking parameters.
2Materials and methods
2.1Participants
We recruited consecutive patients diagnosed with PD according to the United Kingdom Parkinson’s disease Society Brain Bank criteria (Gibb, & Lees, 1988). Inclusion criteria were: a) age ≥ 45 years; b) modified Hoehn and Yahr Scale (mH&Y) score≤2 in “ON” stage; c) disease duration lower than 5 years; d) optimized and stable PD drug therapy for at least four weeks before the enrollment. Exclusion criteria were: a) dementia associated with PD according to consensus criteria (Emre et al., 2007); b) diagnosis of atypical or secondary parkinsonism; c) clinically significant comorbidities (i.e., cardiovascular or cerebrovascular disease, renal or hepatic insufficiency). All participants signed informed consent. The study was conducted in accordance to the Declaration of Helsinki.
2.2Outcomes
All patients were assessed by dedicated evaluation protocol, including collection of anamnestic and anthropometric data, and the administration of the following outcome measures:
– Unified Parkinson’s Disease Rating Scale (UPDRS) III for PD motor symptoms (G Fahn S, Elton R and Members of the UPDRS Development Committee, 1987);
– Kings Parkinson’s Pain Scale (KPPS) for pain symptoms (Chaudhuri et al., 2015);
– Parkinson Fatigue Severity scale (PFSS) for fatigue symptoms (Brown, Dittner, Findley, & Wessely, 2005);
– Parkinson Anxiety Scale (PAS) to evaluate anxiety symptoms (Leentjens et al., 2014);
– Beck Depression Inventory (BDI) for depressive symptoms (Visser, Leentjens, Marinus, Stiggelbout, & van Hilten, 2006);
– Epworth Sleepiness Scale (ESS) for daily sleepiness (Kumar, Bhatia, & Behari, 2003).
Functional evaluation consisted of measurement of the kinematic parameters during 10 meter-walking test [cadence (steps/min), speed (m/s), stride length (m), duration (s)], and duration of both Time Up and Go (TUG), and TUG Dual Task (TUGdt, consisting of TUG combined with a verbal task), through an inertial sensor (G-WALK®, BTS) to the skin of lumbar region, at level of L5 vertebra.
Global cognitive functioning was assessed by means of the Montreal Cognitive Assessment (MoCA) (Nasreddine et al., 2005).
Moreover, PD patients performed a comprehensive neuropsychological battery including the following tests:
– Trial Making Test A (TMT-A) and digit span backward for attention and working memory (Giovagnoli, 1996);
– Modified Card Sorting Test, number of achieved categories and letter fluency task for executive functions (Caffarra, Vezzadini, Dieci, Zonato, & Venneri, 2004);
– Prose recall test and Rey’s Auditory Verbal Learning Test - delayed recall for immediate and delayed memory (Novelli et al., 1986; Carlesimo, Caltagirone, & Gainotti, 1996);
– Copying drawings and Judgment of Line Orientation test for visuospatial abilities (Siciliano et al., 2016).
Cognitive impairment was defined when patients obtained neuropsychological tests scores at least 1.5 standard deviation below the normative data for the Italian population (Siciliano et al., 2017).
Performance on the individual neuropsychological test was transformed into z-scores. Subsequently, a composite summary index for each cognitive domain was derived from the corresponding averages of the respective neuropsychological tests (i.e. attention and working memory z-score, memory z-score, executive functions z-score, visuospatial abilities z-score, and language z-score).
Identification of MCI was based on the Movement Disorders Society (MDS) Task Force level II criteria (Litvan I et al., 2012). Moreover, we distinguished patients with PD-MCI single domain (SD) (i.e., impairments in two tests within a single cognitive domain) and multiple domains (MD) (i.e., impairments in at least one test in two or more cognitive domains).
A physiatrist performed the motor evaluation, whereas an experienced neuropsychologist carried out the assessment of cognitive functions. All patients were evaluated in “OFF” therapeutic state.
2.3Statistical analysis
Statistical analysis was carried out using Statistical Package for the Social Sciences 25 (SPSS 25; IBM Corp., Armonk, NY, USA) software to perform correlation analysis among kinematic parameters, NMS and corrected MoCA score.
Student’s t-test was used to compare gait parameters and NMS in PD patients with and without MCI, after applying Levene’s test for variance. In the PD-MCI subgroup, kinematic parameters and NMS scores were correlated with cognitive z-scores. Normality was checked through Shapiro-Wilk test. Correlation analysis was performed using Pearson’s correlation coefficient or Spearman’s rank correlation, in case of non-parametric variables. We considered a significance threshold of p < 0.05. We considered following cut-offs and strength for correlation coefficient: 0–0.19 considered as very weak, 0.2–0.39 as weak, 0.40–0.59 as moderate, 0.6–0.79 as strong and 0.8–1 as very strong correlation.
3Results
Twenty-six PD patients (18 males; 8 females) were recruited. Demographic and anthropometric characteristics are shown in Table 1. Two patients were naive to drug therapy for PD (7.7%), 10 patients were taking levodopa alone (38.5%), 3 were taking dopaminergic agonists (11.5%), and 11 were treated with a combination of levodopa and dopaminergic agonists (42.3%).
Table 1
Clinical characteristics of our PD population (N = 26)
| Age (years) | 65.38±8.69 |
| BMI (kg/m2) | 28.71±4.55 |
| Gender | |
| Male (%) | 18 (69.23 %) |
| Female (%) | 8 (30.77 %) |
| Disease duration (years) | 2.84±1.25 |
| Phenotype | |
| Akinetic | 13 (50%) |
| Tremor | 13 (50%) |
| mH&Y score | |
| 1 | 10 (38.46%) |
| 1.5 | 1 (3.84%) |
| 2 | 15 (57.7%) |
| UPDRS III score | 23.61±8.39 |
Continuous variables are expressed as mean±standard deviation; discrete ones are expressed as total number (%). PD: Parkinson disease; BMI: Body Mass Index; mH&Y: Modified Hoehn and Yahr Scale; UPDRS: Unified Parkinson’s Disease Rating Scale.
The scores of NMS scales are shown in Table 2. Patients reported mild musculoskeletal pain, slight daily sleepiness, moderate fatigue symptoms, and no depression or anxiety.
Table 2
Scores of non-motor symptom scales in our PD population
| Kings Parkinson’s Pain Scale (KPPS) | 8.96±10.70 |
| Epworth Sleepiness Scale (ESS) | 6±13.58 |
| Parkinson Fatigue Severity (PFS) Scale | 2.77±1.28 |
| Parkinson Anxiety Scale (PAS) total score | 10.38±8.33 |
| Beck Depression Inventory (BDI II) | 9±7.27 |
Continuous variables are expressed as mean±standard deviation. PD: Parkinson disease.
Spatiotemporal gait parameters, TUG and TUGdt duration are shown in Table 3. These data suggest an acceptable postural control with slight alterations in walking parameters (particularly speed, cadence, and stride length), TUG and TUGdt duration (TUGdt value > 10%ΔTUG was considered as pathological) (Lopes et al., 2020).
Table 3
Kinematic parameters during walking, TUG and TUGdt duration
| Parameter | Mean value (±SD) |
| Gait speed (m/s) | 1.48±0.88 |
| Gait duration (s) | 15.46±2.57 |
| Cadence (steps/min) | 112.42±8.65 |
| Stride length (m) | 1.60±1.01 |
| TUG (s) | 13.27±3.13 |
| TUGdt (s) | 15.60±4.57 |
Continuous variables are expressed as mean±standard deviation. SD: standard deviation; TUG: Timed up and go; TUGdt: Timed up and go DualTask.
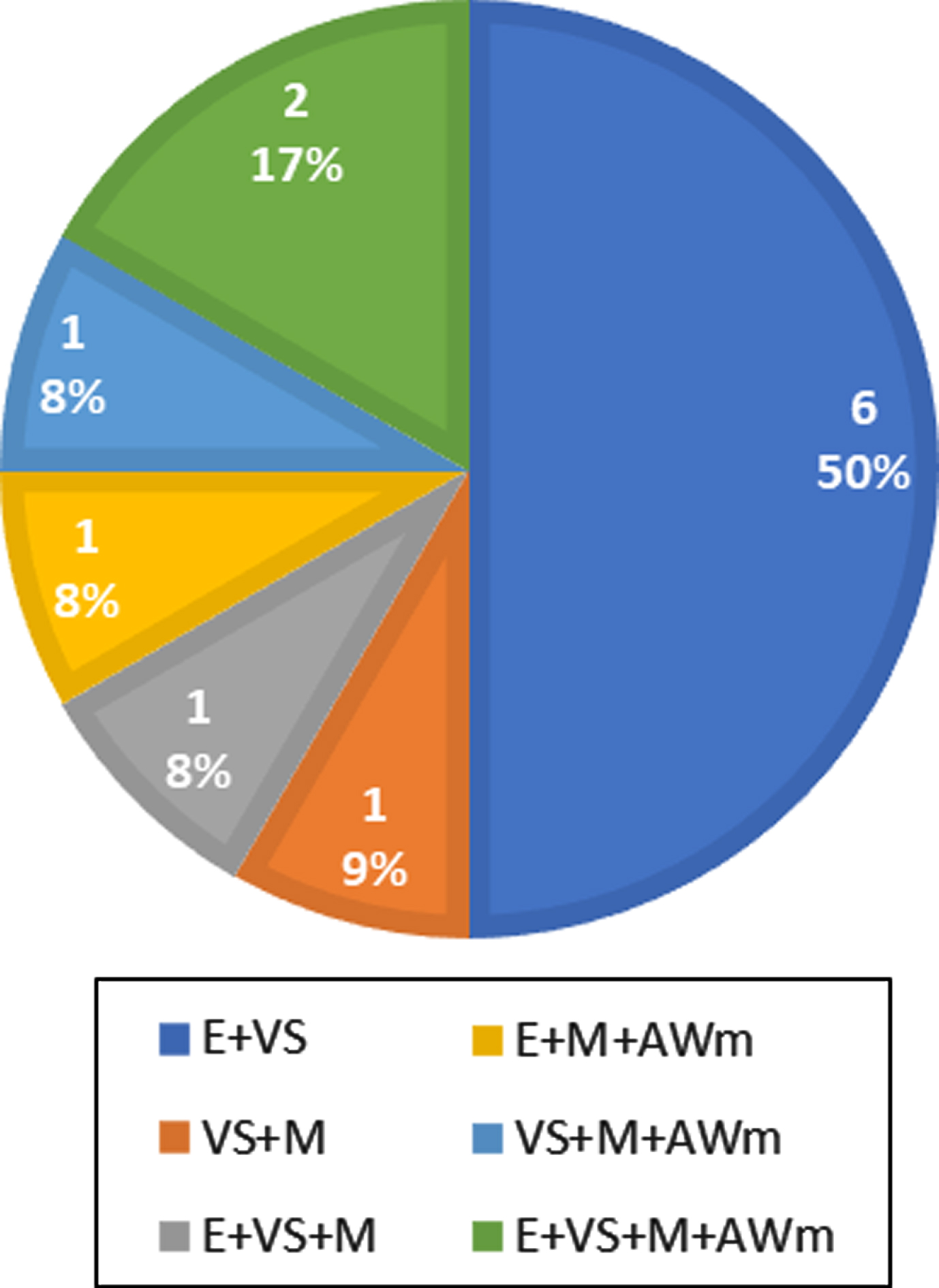
Neuropsychological evaluation revealed a corrected MoCA score of 24.78±2.09 (mean±standard deviation) and 12 patients were classified as MCI-MD (6 females; 6 males). Data about cognitive functions in our PD-MCI population are shown in Fig. 1.
Fig. 1
Impaired cognitive functions in 12 patients with diagnosis of MCI-MD. (MCI-MD: Mild Cognitive Impairment Multi Domain; E: executive functions; VS: visuospatial abilities; M: memory; AWm: attention/working memory)
According to the Shapiro-Wilk test, cadence, TUG duration, ESS, PAS, BDI-II and the cognitive z-scores for memory, executive and visuospatial functions were normally distributed.
The correlation analysis for fatigue showed a moderate negative correlation with step cadence (p < 0.01), and a moderate to strong positive correlation with gait duration (p < 0.01), TUG (p < 0.01) and TUGdt (p < 0.01). Pain showed positive moderate correlation with gait duration (p < 0.01) and TUGdt (p < 0.05), while anxiety showed weak negative correlation with cadence (p < 0.05) and positive correlation with gait duration (p < 0.05). Finally, also BDI-II showed a positive weak correlation with gait duration (p < 0.05).
MoCA scores showed a weak negative correlation with TUGdt (p < 0.05) (Table 4).
Table 4
Correlation coefficients and p-values between walking kinematics parameters, non-motor symptom scores and cognitive scores
| KPSS | ESS | PFS | PAS TS | BDI-II | Corrected MoCA | |
| Gait duration (s) | 0.593** | 0.309 | 0.633** | 0.390* | 0.393* | 0.245 |
| 0.004 | 0.125 | 0.001 | 0.049 | 0.047 | 0.227 | |
| Gait speed (m/s) | –0.248 | –0.183 | –0.280 | 0.009 | –0.167 | 0.059 |
| 0.222 | 0.370 | 0.166 | 0.966 | 0.414 | 0.775 | |
| Cadence (steps/min) | –0.189 | –0.177 | –0.501** | –0.393* | –0.200 | 0.277 |
| 0.354 | 0.386 | 0.009 | 0.047 | 0.328 | 0.170 | |
| Stride length (m) | –0.280 | –0.207 | –0.223 | 0.070 | –0.119 | 0.042 |
| 0.167 | 0.311 | 0.274 | 0.734 | 0.564 | 0.839 | |
| TUG (s) | 0.356 | 0.236 | 0.569** | 0.151 | 0.161 | –0.377 |
| 0.075 | 0.246 | 0.002 | 0.463 | 0.432 | 0.058 | |
| TUGdt (s) | 0.452* | 0.080 | 0.654** | 0.273 | 0.314 | –0.393* |
| 0.020 | 0.699 | 0.000 | 0.178 | 0.118 | 0.047 |
*p < 0.05, **p < 0.01. KPSS: Kings Parkinson’s Pain Scale; ESS: Epworth Sleepiness Scale; PFS: Parkinson Fatigue Severity Scale; PAS TS: Parkinson Anxiety Scale total score; BDI-II: Beck Depression Inventory; TUG: Timed Up and Go; TUGdt: Timed Up and Go Dual Task; MoCA: Montreal Cognitive Assessment.
Based on the neuropsychological evaluation, gait duration was significantly different between PD-MCI and PD patients without cognitive impairment (Parkinson’s disease normal cognition, PD-NC) (Table 5). As shown in Table 6, PD-MCI patients reported significantly worse scores in outcome measures for pain and fatigue (p < 0.05).
Table 5
Differences of walking kinematics parameters between the two groups (with and without MCI)
| Parameter | PD-MCI | PDNC | p-value |
| Gait speed (m/s) | 1.35±0.71 | 1.63±1.08 | 0.445 |
| Gait duration (s) | 16.53±2.31 | 14.55 ±2.51 | 0.049* |
| Cadence (steps/min) | 110.52±9.27 | 114.05±8.07 | 0.310 |
| Stride length (m) | 1.78±1.20 | 1.45±0.85 | 0.430 |
| TUG (s) | 14.22±3.08 | 12.47±3.06 | 0.161 |
| TUGdt (s) | 16.72±5.37 | 14.64±3.71 | 0.257 |
Continuous variables are expressed as mean±standard deviation. PD-MCI: Parkinson’s disease with Mild Cognitive Impairment; PDNC: normal cognition Parkinson’s disease; TUG: Timed up and go; TUGdt: Timed up and go DualTask; *p < 0.05.
Table 6
Differences of non-motor symptom scores between the two groups (with and without MCI)
| Parameter | PD-MCI | PDNC | p-value |
| Kings Parkinson’s Pain Scale (KPPS) | 13.75±11.95 | 4.86±7.76 | 0.032* |
| Epworth Sleepiness Scale (ESS) | 5.08±3.17 | 6.78 ±3.85 | 0.235 |
| Parkinson Fatigue Severity (PFS) Scale | 3.32±1.24 | 2.31±1.78 | 0.045* |
| Parkinson Anxiety Scale (PAS) total score | 13.08±8.88 | 8.07±7.38 | 0.129 |
| Beck Depression Inventory (BDI II) | 11.25±7.87 | 7.07±6.37 | 0.148 |
Continuous variables are expressed as mean±standard deviation. PD-MCI: Parkinson’s disease with Mild Cognitive Impairment; PDNC: Parkinson’s disease normal cognition; *p < 0.05.
The correlation analysis for cognitive z-scores in the PD-MCI group showed a moderate negative correlation between visuospatial abilities and fatigue (Table 7).
Table 7
Correlation coefficients and p-values among cognitive subitems, walking kinematics parameters and non-motor symptom scores in PD-MCI group
| Cognitive z-score | Gait duration (s) | Gait speed (m/s) | Cadence (steps/min) | Stride length (m) | TUG (s) | TUGdt (s) | KPSS | ESS | PFS | PAS TS | BDI-II |
| Executive functions z-score | –0.022 | –0.047 | 0.064 | –0.030 | –0.151 | –0.094 | –0.110 | 0.332 | 0.068 | 0.041 | –0.088 |
| 0.916 | 0.820 | 0.755 | 0.885 | 0.462 | 0.649 | 0.591 | 0.097 | 0.742 | 0.844 | 0.670 | |
| Visuospatial abilities z-score | –0.364 | 0.212 | 0.227 | 0.281 | –0.318 | –0.289 | –298 | 0.109 | –0.410* | –0.292 | –0.217 |
| 0.068 | 0.297 | 0.265 | 0.164 | 0.113 | 0.153 | 0.140 | 0.595 | 0.037 | 0.148 | 0.286 | |
| Attention/ working memory z-score | –0.105 | 0.013 | 0.037 | 0.048 | –0.228 | –0.264 | –0.248 | 0.070 | –0.109 | –0.015 | 0.148 |
| 0.610 | 0.948 | 0.859 | 0.815 | 0.263 | 0.192 | 0.222 | 0.734 | 0.597 | 0.942 | 0.469 | |
| Memory z-score | –0.013 | –0.089 | 0.156 | –0.046 | –0.086 | 0.068 | –0.170 | –0.119 | 0.207 | –0.157 | 0.084 |
| 0.950 | 0.066 | 0.448 | 0.823 | 0.363 | 0.741 | 0.407 | 0.563 | 0.310 | 0.444 | 0.682 |
*p < 0.05, **p < 0.01. KPSS: Kings Parkinson’s Pain Scale; ESS: Epworth Sleepiness Scale; PFS: Parkinson Fatigue Severity Scale; PAS TS: Parkinson Anxiety Scale total score; BDI-II: Beck Depression Inventory; TUG: Timed Up and Go; TUGdt: Timed Up and Go Dual Task.
4Discussion
Motor and non-motor symptoms, especially cognitive dysfunctions, worsen over the PD course, ultimately resulting in functional independence loss (Gaßner et al., 2017) through a dangerous duet between impairment of walking kinematics and NMS. This concept undoubtedly characterizes advanced stages of PD, but the role of the interplay between cognitive status and motor performance in early stage PD patients is still debated.
Our data suggest significant correlations between walking kinematics and non-motor impairments in early stage PD patients.
In our PD population, the analysis of spatio-temporal gait parameters showed an increased stride length, with a reduction in number of steps/minute (De Ridder et al., 2019; Pinto et al., 2019); moreover, we observed a longer duration in both TUG and TUGdt. These findings are consistent with the characteristics of early PD gait reported by other Authors (Pistacchi et al., 2017).
For what concerns NMS, fatigue showed moderate to strong correlations with lower step cadence, longer gait duration in 10-m gait, TUG and TUGdt, suggesting a negative impact of this distressing symptom on both motor function and complex attentional skills. Our findings support the conclusions of some studies that consider fatigue as one of the strongest contributing factors to perceived walking difficulties in people with PD (Kader, Ullén, Iwarsson, Odin, & Nilsson, 2017; Christiansen, Schenkman, McFann, Wolfe, & Kohrt, 2009). Moreover, our results about the negative impact of fatigue on TUGdt duration support the hypothesis that PD patients experience a “central” fatigue that affects both motor (e.g., objective motor fatigability) and mental dimensions (Herlofson, Ongre, Enger, Tysnes, & Larsen, 2012).
Pain is another disabling NMS that is underestimated in PD patients, as well as its impact on motor performance in this population. Our results showed moderate correlations of pain with both longer 10-meter walking and TUGdt duration. Interestingly, it has been demonstrated the role of chronic pain as distractor that contributes to slower gait speed also in healthy older adults, thus implicating an attentional involvement (Leveille et al., 2017). These findings are consistent with those reported in our population of PD patients. More recently, huge interest for both diagnosis and management of pain in this population fosters the development of a new classification of PD-related pain, aiming to provide a mechanism-based treatment (Mylius et al., 2021).
For what concern our data about gait parameters according to the cognitive status, no differences were reported in speed and cadence between PD-MCI and PD-NC patients, suggesting a limited role of cognitive status on motor performance in the early PD stages.
Otherwise, it should be considered that walking is a complex task (Gaßner et al., 2017) characterized by some locomotor parameters that are dependent upon the integrity of specific brain control networks, involved in cognition. For example, gait speed and variability that are typically impaired in PD patients, are associated with frontoparietal and dorsal attention control network, respectively (Lo et al., 2017; Klobušiaková, Mareček, Fousek, Výtvarová, & Rektorová, 2019).
Moreover, PD-MCI patients showed worse scores in all outcomes except for ESS, compared to those without cognitive impairment. In particular, pain and fatigue resulted more disabling in the PD-MCI group. More in details, a correlation between visuospatial deficit and fatigue has been found in the PD-MCI group, in accordance with a previous work (Kluger et al., 2017).
In our opinion, assessment of cognitive status in early PD patients should be properly addressed to comprehensively counteract the progression of motor impairment, considering that cognitive issues can even anticipate motor symptoms (Aarsland D, 2016).
Our findings support prompt identification and management of cognitive impairment in PD patients, thus minimizing its consequences on motor performance at the beginning of the disease. Recently published clinical guidelines, recommend a combined pharmacological and non-pharmacological (i.e., therapeutic exercise) approach to better manage both motor and non-motor symptoms in PD patients (Martignon et al., 2021), but a consensus about the effectiveness of this strategy in early PD patients has not been reached so far.
As implications for clinical practice, our results suggest a routine assessment of motor and non-motor symptoms since the beginning of PD. A multidisciplinary approach to counteract these symptoms, through pharmacological and non-pharmacological treatment options, might reduce the functional burden in these patients. Moreover, an early individualized rehabilitation plan could enhance the effectiveness of drug therapy and patient’ compliance, along with benefits in terms of quality of life (McGinley, et al., 2012).
The main strength of our study consists in accurate selection of study population according to the gold standards for the identification of motor-related disability and cognitive status in early PD patients. Furthermore, we carried out an instrumental assessment of gait parameters by using a wearable inertial sensor that has been validated and considered as a reliable and feasible method to assess walking kinematics in several diseases (Celik, Stuart, Woo, & Godfrey, 2021). Recent findings support the use of these devices in PD considering their high sensitivity and representativeness of real-world scenarios (Petraglia et al., 2019). Therefore, the use of wearable devices might be helpful in the identification and monitoring of gait changes in PD population.
However, our study has some limitations. The rigorous inclusion criteria (i.e., mH&Y score≤2, patient evaluated in “OFF” stage) result in poor external validity, considering huge variability of PD clinical status (i.e., motor evaluation in “ON” status). The gender distribution underrepresents female participants. This topic should be considered for future research, due to the high prevalence of depression observed in PD females (Perrin et al., 2017) and its potential impact on gait performance. Moreover, the heterogeneity of the effectiveness of drug therapy for PD on investigated outcome measures might affect our findings. It would be interesting to investigate other NMSs (e.g. visceral impairments as cardiovascular, gastrointestinal and urinary issues) on motor performance in PD patients.
5Conclusions
This observational study showed that NMS may be associated with walking performance, since in the early stage of PD. The main findings of this study are the significant correlations among fatigue, attentional skills, and gait pattern alterations. On the other side, cognitive status seems to have a limited role on motor performance in early PD patients.
Finally, instrumental assessment of walking kinematics should be considered as mandatory to better characterize motor impairment in PD patients. This strategy will improve management planning, particularly about rehabilitation interventions, by encouraging exercise prescription already in early PD patients.
Conflict of interest
None to report.
References
1 | Aarsland, D. ((2016) ). Cognitive impairment in Parkinson’s disease and dementia with Lewy bodies, Parkinsonism & Related Disorders, 22: (Suppl 1), 148. |
2 | Bahureksa, L. , Najafi, B. , Saleh, A. , Sabbagh, M. , Coon, D. , Mohler, M. J. , & Schwenk, M. ((2017) ). The Impact of Mild Cognitive Impairment on Gait and Balance: A Systematic Review and Meta-Analysis of Studies Using Instrumented Assessment, Gerontology, 63: (1), 67–83. |
3 | Brown, R. G. , Dittner, A. , Findley, L. , & Wessely, S. C. ((2005) ). The Parkinson fatigue scale, Parkinsonism & Related Disorders, 11: (1), 49–55. |
4 | Caffarra, P. , Vezzadini, G. , Dieci, F. , Zonato, F. , & Venneri, A. ((2004) ). Modified Card Sorting Test: normative data, Journal of Clinical and Experimental Neuropsychology, 26: (2), 246–250. |
5 | Carlesimo, G. A. , Caltagirone, C. , & Gainotti, G. ((1996) ). The Mental Deterioration Battery: normative data, diagnostic reliability and qualitative analyses of cognitive impairment. The Group for the Standardization of the Mental Deterioration Battery, European Neurology, 36: (6), 378–384. |
6 | Celik, Y. , Stuart, S. , Woo, W. L. , & Godfrey, A. ((2021) ). Gait analysis in neurological populations: Progression in the use of wearables, Medical Engineering & Physics, 87: , 9–29. |
7 | Chaudhuri, K. R. , Rizos, A. , Trenkwalder, C. , Rascol, O. , Pal, S. , Martino, D. , Carroll, C , Paviour, D. , Falup-Pecurariu, C. , Kessel, B. , Silverdale, M. , Todorova, A. , Sauerbier, A. , Odin, P. , Antonini, A. , Martinez-Martin, P. , & EUROPAR and the IPMDS Non Motor PD Study Group ((2015) ). King’s Parkinson’s disease pain scale, the first scale for pain in PD: An international validation, Movement disorders: official journal of the Movement Disorder Society, 30: (12), 1623–1631. |
8 | Christiansen, C. L. , Schenkman, M. L. , McFann, K. , Wolfe, P. , & Kohrt, W. M. ((2009) ). Walking economy in people with Parkinson’s disease, Movement disorders: official journal of the Movement Disorder Society, 24: (10), 1481–1487. |
9 | De Ridder, R. , Lebleu, J. , Willems, T. , De Blaiser, C. , Detrembleur, C. , & Roosen, P. ((2019) ). Concurrent Validity of a Commercial Wireless Trunk Triaxial Accelerometer System for Gait Analysis. jsr, Journal of Sport Rehabilitation, 28: (6), 2018–0295. |
10 | Emre, M. , Aarsland, D. , Brown, R. , Burn, D. J. , Duyckaerts, C. , Mizuno, Y. , Broe, G. A. , Cummings, J. , Dickson, D. W. , Gauthier, S. , Goldman, J. , Goetz, C. , Korczyn, A. , Lees, A. , Levy, R. , Litvan, I. , McKeith, I. , Olanow, W. , Poewe, W. , Quinn, N. ,. . . Dubois, B. ((2007) ). Clinical diagnostic criteria for dementia associated with Parkinson’s disease, Movement disorders: official journal of the Movement Disorder Society, 22: (12), 1689–1837. |
11 | Fahn, S , Elton, R. and and Members of the UPDRS Development Committee (1987) The Unified Parkinson–s Disease Rating Scale. In: Fahn, S., Marsden, C.D., Calne, D.B. and Goldstein, M., Eds., Recent Developments in Parkinson–s Disease, Vol. 2, |
12 | Federico, A. , Tinazzi, M. , & Tamburin, S. ((2018) ). MoCA for cognitive screening in Parkinson–s disease: Beware of floor effect. Movement disorders: official journal of the Movement Disorder Society, 33: (3), 499. https://doi.org/10.1002/mds.27329 |
13 | Gaßner, H. , Marxreiter, F. , Steib, S. , Kohl, Z. , Schlachetzki, J. , Adler, W. , Eskofier, B. M. , Pfeifer, K. , Winkler, J. , & Klucken, J. ((2017) ). Gait and Cognition in Parkinson’s Disease: Cognitive Impairment Is Inadequately Reflected by Gait Performance during Dual Task, Frontiers in Neurology, 8: , 550. |
14 | GBD 2016 Neurology Collaborators ((2019) ). Global, regional, and national burden of neurological disorders,: a systematic analysis for the Global Burden of Disease Study, The Lancet Neurology, 18: (5), 459–480. |
15 | Gibb, W. R. , & Lees, A. J. ((1988) ). A comparison of clinical and pathological features of young- and old-onset Parkinson’s disease, Neurology, 38: (9), 1402–1406. |
16 | Giovagnoli, A. R. , Del Pesce, M. , Mascheroni, S. , Simoncelli, M. , Laiacona, M. , & Capitani, E. ((1996) ). Trail making test: normative values from normal adult controls, Italian Journal of Neurological Sciences, 17: (4), 305–309. |
17 | Herlofson, K. , Ongre, S. O. , Enger, L. K. , Tysnes, O. B. , & Larsen, J. P. ((2012) ). Fatigue in early Parkinson’s disease. Minor inconvenience or major distress? European Journal of Neurology, 19: (7), 963–968. |
18 | Kader, M. , Ullén, S. , Iwarsson, S. , Odin, P. , & Nilsson, M. H. ((2017) ). Factors Contributing to Perceived Walking Difficulties in People with Parkinson’s Disease, Journal of Parkinson’s Disease, 7: (2), 397–407. |
19 | Kim, S. M. , Kim, D. H. , Yang, Y. , Ha, S. W. , & Han, J. H. ((2018) ). Gait Patterns in Parkinson’s Disease with or without Cognitive Impairment, Dementia and Neurocognitive Disorders, 17: (2), 57–65. |
20 | Klobušiaková, P. , Mareček, R. , Fousek, J. , Výtvarová, E. , & Rektorová, I. ((2019) ). Connectivity Between Brain Networks Dynamically Reflects Cognitive Status of Parkinson’s Disease: A Longitudinal Study, Journal of Alzheimer’s disease: JAD, 67: (3), 971–984. |
21 | Kluger, B. M. , Pedersen, K. F. , Tysnes, O. B. , Ongre, S. O. , Øygarden, B , & Herlofson, K. ((2017) ). Is fatigue associated with cognitive dysfunction in early Parkinson’s disease? Parkinsonism & Related Disorders, 37: , 87–91. |
22 | Kumar, S. , Bhatia, M. , & Behari, M. ((2003) ). Excessive daytime sleepiness in Parkinson’s disease as assessed by Epworth Sleepiness Scale (ESS). Sleep Medicine, 4: (4), 339–342. https://doi.org/10.1016/s1389-9457(03)00105-9 |
23 | Kwon, K. Y. , Lee, H. M. , Kang, S. H. , Pyo, S. J. , Kim, H. J , & Koh, S. B. ((2017) ). Recuperation of slow walking in de novo Parkinson’s disease is more closely associated with increased cadence, rather than with expanded stride length, Gait & posture, 58: , 1–6. |
24 | Lee, H. M. , & Koh, S. B. ((2015) ). Many Faces of Parkinson’s Disease: Non-Motor Symptoms of Parkinson’s Disease, Journal of Movement Disorders, 8: (2), 92–97. |
25 | Leentjens, A. F. , Dujardin, K. , Pontone, G. M. , Starkstein, S. E. , Weintraub, D. , & Martinez-Martin, P. ((2014) ). The Parkinson Anxiety Scale (PAS): development and validation of a new anxiety scale, Movement disorders: official journal of the Movement Disorder Society, 29: (8), 1035–1043. |
26 | Leveille, S. G. , Hausdorff, J. M. , McLean, R. , Shi, L. , Dong, Z. , Manor, B. , & Bean, J. ((2017) ). Attention mediates the relation between pain and gait in older adults, Innovation in Aging, 1: (Suppl 1), 736. |
27 | Litvan, I. , Goldman, J. G. , Tröster, A. I. , Schmand, B. A. , Weintraub, D. , Petersen, R. C. , Mollenhauer, B. , Adler, C. H. , Marder, K. , Williams-Gray, C. H. , Aarsland, D. , Kulisevsky, J. , Rodriguez-Oroz, M. C. , Burn, D. J. , Barker, R. A. , & Emre, M. ((2012) ). Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines, Movement disorders: official journal of the Movement Disorder Society, 27: (3), 349–356. |
28 | Lo, O. Y. , Halko, M. A. , Zhou, J. , Harrison, R. , Lipsitz, L. A. , & Manor, B. ((2017) ). Gait Speed and Gait Variability Are Associated with Different Functional Brain Networks, Frontiers in aging neuroscience, 9: , 390. |
29 | Lopes, L. , Scianni, A. A. , Lima, L. O. , de Carvalho Lana, R. , & Rodrigues-De-Paula, F. ((2020) ). The Mini-BESTest is an independent predictor of falls in Parkinson Disease, Brazilian Journal of Physical Therapy, 24: (5), 433–440. |
30 | Martignon, C. , Pedrinolla, A. , Ruzzante, F. , Giuriato, G. , Laginestra, F. G. , Bouça-Machado, R. , Ferreira, J. J. , Tinazzi, M. , Schena, F. , & Venturelli, M. ((2021) ). Guidelines on exercise testing and prescription for patients at different stages of Parkinson’s disease, Aging Clinical and Experimental Research, 33: (2), 221–246. |
31 | McGinley, J. L. , Martin, C. , Huxham, F. E. , Menz, H. B. , Danoudis, M. , Murphy, A. T. , Watts, J. J , Iansek, R. , & Morris, M. E. ((2012) ). Feasibility, safety, and compliance in a randomized controlled trial of physical therapy for Parkinson’s disease, Parkinson’s Disease, 2012: , 795294. |
32 | Mirelman, A. , Bonato, P. , Camicioli, R. , Ellis, T. D. , Giladi, N. , Hamilton, J. L. , Hass, C. J. , Hausdorff, J. M. , Pelosin, E. , & Almeida, Q. J. ((2019) ). Gait impairments in Parkinson’s disease, The Lancet. Neurology, 18: (7), 697–708. |
33 | Morris, M. E. , Iansek, R. , Matyas, T. A. , & Summers, J. J. ((1994) ). The pathogenesis of gait hypokinesia in Parkinson’s disease, Brain: a journal of neurology, 117: (Pt 5), 1169–1181. |
34 | Mylius, V. , Perez Lloret, S. , Cury, R. G. , Teixeira, M. J. , Barbosa, V. R. , Barbosa, E. R. , Moreira, L. I. , Listik, C. , Fernandes, A. M. , de Lacerda Veiga, D. , Barbour, J. , Hollenstein, N. , Oechsner, M. , Walch, J. , Brugger, F. , Hägele-Link, S. , Beer, S. , Rizos, A. , Chaudhuri, K. R. , Bouhassira, D. , & Ciampi de Andrade, D. ((2021) ). The Parkinson disease pain classification system: results from an international mechanism-based classification approach, Pain, 162: (4), 1201–1210. |
35 | Nasreddine, Z. S. , Phillips, N. A. , Bédirian, V. , Charbonneau, S. , Whitehead, V. , Collin, I. , Cummings, J. L. , & Chertkow, H. ((2005) ). The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment, Journal of the American Geriatrics Society, 53: (4), 695–699. |
36 | Novelli, G. , Papagno, C. , Capitani, E. , Laiacona, M. , Cappa, S.F. , & Vallar, G. ((1986) ). Three clinical tests for the assessment of verbal long term memory... Three clinical tests for the assessment of verbal long term memory function norms from normal subjects, Archivio di Psicologia Neurologia e Psichiatria, 47: (2), 278–296. |
37 | Palakurthi, B. , & Burugupally, S. P. ((2019) ). Postural Instability in Parkinson’s Disease: A Review, Brain Sciences, 9: (9), 239. |
38 | Perrin, A. J. , Nosova, E. , Co, K. , Book, A. , Iu, O. , Silva, V. , Thompson, C. , McKeown, M. J. , Stoessl, A. J. , Farrer, M. J. , & Appel-Cresswell, S. ((2017) ). Gender differences in Parkinson’s disease depression, Parkinsonism & Related Disorders, 36: , 93–97. |
39 | Petraglia, F. , Scarcella, L. , Pedrazzi, G. , Brancato, L. , Puers, R. , & Costantino, C. ((2019) ). Inertial sensors versus standard systems in gait analysis: a systematic review and meta-analysis, European Journal of Physical and Rehabilitation Medicine, 55: (2), 265–280. |
40 | Pinto, C. , Schuch, C. P. , Balbinot, G. , Salazar, A. P. , Hennig, E. M. , Kleiner, A. , & Pagnussat, A. S. ((2019) ). Movement smoothness during a functional mobility task in subjects with Parkinson’s disease and freezing of gait - an analysis using inertial measurement units, Journal of Neuroengineering and Rehabilitation, 16: (1), 110. |
41 | Pistacchi, M. , Gioulis, M. , Sanson, F. , De Giovannini, E. , Filippi, G. , Rossetto, F. , & Zambito Marsala, S. ((2017) ). Gait analysis and clinical correlations in early Parkinson’s disease, Functional Neurology, 32: (1), 28–34. |
42 | Reijnders, J. S. , Ehrt, U. , Weber, W. E. , Aarsland, D. , & Leentjens, A. F. ((2008) ). A systematic review of prevalence studies of depression in Parkinson’s disease, Movement disorders: official journal of the Movement Disorder Society, 23: (2), 183–313. |
43 | Savica, R. , Wennberg, A. M. , Hagen, C. , Edwards, K. , Roberts, R. O. , Hollman, J. H. , Knopman, D. S. , Boeve, B. F. , Machulda, M. M. , Petersen, R. C. , & Mielke, M. M. ((2017) ). Comparison of Gait Parameters for Predicting Cognitive Decline: The Mayo Clinic Study of Aging, Journal of Alzheimer’s disease: JAD, 55: (2), 559–567. |
44 | Siciliano, M. , Santangelo, G. , D’Iorio, A. , Basile, G. , Piscopo, F. , Grossi, D. , & Trojano, L. ((2016) ). Rouleau version of the Clock Drawing Test: age- and education-adjusted normative data from a wide Italian sample, The Clinical Neuropsychologist, 30: (sup1), 1501–1516. |
45 | Siciliano, M. , Trojano, L. , De Micco, R. , De Mase, A. , Garramone, F. , Russo, A. , Tedeschi, G. , & Tessitore, A. ((2017) ). Motor, behavioural, and cognitive correlates of fatigue in early, de novo Parkinson disease patients, Parkinsonism & related disorders, 45: , 63–68. |
46 | Tysnes, O. B. , & Storstein, A. ((2017) ). Epidemiology of Parkinson’s disease, Journal of Neural Transmission (Vienna, Austria:), 124: (8), 901–905. |
47 | Visser, M. , Leentjens, A. F. , Marinus, J. , Stiggelbout, A. M. , & van Hilten, J. J. ((2006) ). Reliability and validity of the Beck depression inventory in patients with Parkinson’s disease. Movement disorders: official journal of the Movement Disorder Society, 21: (5), 668–672. https://doi.org/10.1002/mds.20792 |
48 | Weil, R. S. , Costantini, A. A. , & Schrag, A. E. ((2018) ). Mild Cognitive Impairment in Parkinson’s Disease-What Is It? Current Neurology and Neuroscience Reports, 18: (4), 17. |
49 | Yarnall, A. J. , Breen, D. P. , Duncan, G. W. , Khoo, T. K. , Coleman, S. Y. , Firbank, M. J. , Nombela, C. , Winder-Rhodes, S. , Evans, J. R. , Rowe, J. B. , Mollenhauer, B. , Kruse, N. , Hudson, G. , Chinnery, P. F. , O’Brien, J. T. , Robbins, T. W. , Wesnes, K. , Brooks, D. J. , Barker, R. A. , Burn, D. J. , . . . ICICLE-PD Study Group ((2014) ). Characterizing mild cognitive impairment in incident Parkinson disease: the ICICLE-PD study, Neurology, 82: (4), 308–316. |




