Treatment of vestibular disorders with weak asymmetric base-in prisms: An hypothesis with a focus on Ménière’s disease
Abstract
BACKGROUND:
Regular treatments of Ménière’s disease (MD) vary largely, and no single satisfactory treatment exists. A complementary treatment popular among Dutch and Belgian patients involves eyeglasses with weak asymmetric base-in prisms, with a perceived high success rate. An explanatory mechanism is, however, lacking.
OBJECTIVE:
To speculate on a working mechanism explaining an effectiveness of weak asymmetric base-in prims in MD, based on available knowledge.
METHODS:
After describing the way these prisms are prescribed using a walking test and its effect reported on, we give an explanation of its underlying mechanism, based on the literature.
RESULTS:
The presumed effect can be explained by considering the typical star-like walking pattern in MD, induced by a drifting after-image comparable to the oculogyral illusion. Weak asymmetric base-in prisms can furthermore eliminate the conflict between a net vestibular angular velocity bias in the efferent signal controlling the VOR, and a net re-afferent ocular signal.
CONCLUSIONS:
The positive findings with these glasses reported on, the fact that the treatment itself is simple, low-cost, and socially acceptable, and the fact that an explanation is at hand, speak in favour of elaborating further on this treatment.
1Introduction
To treat vestibular diseases, numerous methods do exist today, mainly aimed at reducing the severity of the associated symptoms. Usual treatments include oral pharmacotherapy, physical or behavioural therapy, intratympanic pharmacotherapy, or surgery aiming at a complete functional ablation of the organs of balance [80]. Of all vestibular diseases, Ménière’s disease (MD) is probably the most publicly known. MD is generally considered an idiopathic syndrome of vestibular endolymphatic hydrops, characterized by a triad of symptoms consisting of episodes of vertigo, sensorineural hearing loss, and fluctuating aural symptoms, most often tinnitus [6, 40, 54, 81]. Of this triad of symptoms, it is the unpredictable and incapacitating vertigo and nausea during acute attacks that affect quality of life most [19, 84]. During latent phases of MD hearing loss and tinnitus may persist, as may mild vertigo giving rise to postural instability and oscillopsia in many patients. Although a definite curative treatment is still lacking also in MD, apart from the numerous treatment methods mentioned, many MD patients in particular rely on complementary therapies [58, 86].
One of the complementary treatments to which a relatively large number of Ménière patients in the Netherlands and Belgium have turned for more than 70 years, involves optical treatment using weak asymmetric base-in prisms [27, 58, 94, 96, 97], here referred to as WABIPs. As yet, no prospective randomized controlled trial (RCT) has experimentally confirmed that these WABIPs are effective, nor have possible mechanisms been described supported by the literature. Given the positive results reported so far [22, 58, 101, 103], and to initiate and explicate a discussion about the use of these prisms based on scientific arguments, rather than on opinions, also facilitating an RCT, in this paper we elaborate on a possible mechanism for the effect of these WABIPs. Here, we take their prescription as explained below as the basic assumption, elaborating on existing data and theories taken from the literature, and do not add new experimental data. Yet, as a result of this thought experiment we arrive at a new hypothesis.
To present a comprehensive report, we first give a short review of the current knowledge on the diagnosis, epidemiology, pathology and current treatments of MD (Section 2). A description of the WABIPs and how they are prescribed and applied is given next (Section 3), followed by a possible mechanism thereof (Section 4). A discussion finally concludes the paper (Section 5). The reader familiar with the literature on MD may proceed with Section 3.
2Ménière’s disease
2.1Diagnosis
Based on the AAO HNS criteria [21], the most recently published diagnostic criteria for MD as jointly formulated by several societies1 [54] include two categories: definite and probable MD. Definite MD is described as an idiopathic syndrome of endolymphatic hydrops and is diagnosed when all of the following four criteria are met:
1. two or more spontaneous attacks of vertigo lasting between 20 minutes and 12 hours each,
2. audiometric test results showing a low- to medium-frequency hearing loss in the affected ear on at least one occasion before, during, or after one of the vertigo attacks,
3. fluctuating aural symptoms in the affected ear, including a loss of hearing, tinnitus, or fullness of that ear,
4. the symptom set which cannot better be accounted for by another vestibular diagnosis.
In probable MD, the patient meets criteria 1, 3, and 4, but does not have audiometrically determined hearing loss. A case of probable MD could therefore also be, for example, a case of vestibular migraine [52].
Of all the symptoms associated with MD, vertigo and nausea are reported to have the largest negative impact on the quality of life [47, 48, 64, 108].
2.2Epidemiology
Epidemiological studies on MD are scarce and findings show large variations. A Swedish study reported an overall incidence of 46 new cases per 100 000 per year [87], while a Finnish study reported a yearly incidence of 4.3 per 100 000 and a prevalence of 43 per 100 000 [49]. In the US a prevalence of 190 total cases per 100 000 was found [39]. In the Netherlands and Belgium, the incidence is estimated to be between 40–50 new cases per 100 000 per year and the prevalence estimated at a total of 300 per 100 000 [50]. This wide range in incidences and prevalences can be assumed to be due to methodological differences, changes in the diagnostic criteria, and differences among the included populations.
2.3Pathology
A histological hallmark of MD was discovered early in the 20th century in post-mortem temporal bones of Ménière patients [36, 107]. In all cases studied, an endolymphatic hydrops was observed, i.e., an enlargement of the endolymphatic space within the inner ear [31, 70]. Yet, an extensive review of the literature on endolymphatic hydrops did not provide unequivocal support for the hypothesis that an endolymphatic hydrops alone causes MD [29]. Both MD and the endolymphatic hydrops are therefore still referred to as idiopathic.
2.4Current treatment options
Today, Ménière patients are offered an abundance of medical and complementary healthcare treatments. This abundance implies that none of the current treatment options satisfactorily alleviates all associated symptoms in all patients. Moreover, most of these treatments have not been tested through randomized controlled trials [81], several have been debunked (see below), and patients often report to benefit from a combination of treatments [78].
A primary treatment widely recommended is the reduction of salt intake and the use of diuretics to lower the volume and pressure of endolymph [78, 81]. Second, one of the most prescribed regular treatments in Europe is the use of betahistine [58, 81]. Next to betahistine, cinnarizine is also prescribed, particularly in the Netherlands [58]. A more invasive treatment shown to be effective in a large number of Ménière’s patients, involves intratympanic steroids [53] and gentamicin injections, the latter functionally destroying the vestibular end organ [69, 78, 81]. Another invasive treatment involves surgery of the labyrinth, such as endolymphatic sac surgery, vestibular nerve section and labyrinthectomy [78, 81]. In addition to these treatments, many patients use hearing aids to compensate for their hearing loss and/or tinnitus [58, 81].
2.5Placebo effect
A non-negligible effect of many therapies concerns the placebo effect [68]. Because of the erratic exacerbating and remitting nature of MD, the placebo effect has been assumed to be of special interest here as well [38]. The functional effect of betahistine, for example, has seriously been doubted, and its placebo effect, though not proven, made plausible [1]. Moreover, the same has been reported to hold for endolymphatic sac (or shunt) surgery [4, 12, 13, 92, 93, 105]. Endolymphatic sac surgery, for example, has been shown to be as effective as a simple mastoidectomy, with 70% of the patients reporting alleviation of the Ménière symptoms in both groups [12, 13].
3Utermöhlen’s weak asymmetric base-in prisms
3.1Prisms
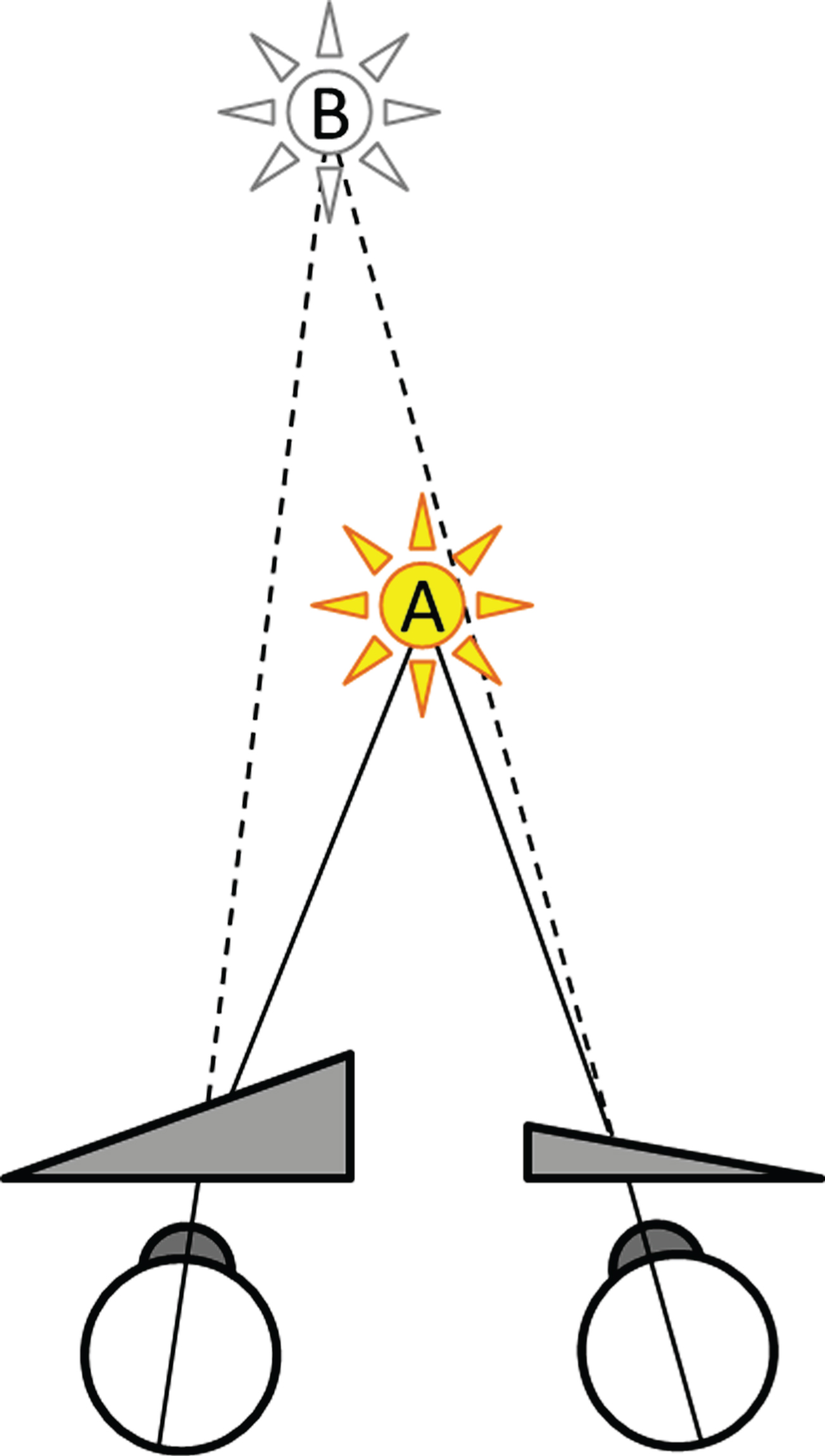
Prismatic glasses are classically known from treatments for ophthalmologic visuo-spatial deficits like strabismus [67], and more recently also for neurological visuo-spatial disorders like unilateral spatial neglect [62, 77]. MD patients have been assumed to benefit from prism glasses as well. This effect was first proposed by Utermöhlen, a Dutch ear, nose and throat (ENT) and eye specialist [94–98, 102]. The glasses prescribed by Utermöhlen have three distinct characteristics. First, the total (added absolute) power of both prisms does not exceed 3 prism dioptres (Δ). Second, the amount of prismatic power is asymmetrically divided over the two eyes. Due to this asymmetry, a real object at point A is projected onto an imaginary point B that is shifted laterally with respect to its original location A (Fig. 1). This shift also holds for the visual straight-ahead in the direction of the strongest prism, and requires a (version induced) gaze shift. Third, the bases of the prisms are always positioned inward, or nasally. This positioning results in the projection of A onto B, located further away than A, which may compensate for a convergence insufficiency. Based on these characteristics, Vente & Meulenbroeks [103] referred to these prisms as weak asymmetric base-in prisms (WABIPs), to set them apart from other kinds of prism prescriptions.
Fig.1
Optical effect of WABIPs. A real object at point A, located here on the visual straight-ahead, is project by WABIPs onto an imaginary point B, further away, and shifted laterally in the direction of the strongest prism.
3.2The extended Utermöhlen test
Since Utermöhlen first described his method in 1941, several Dutch medical doctors, affiliated with an informal ad hoc Utermöhlen Working Group, have applied this method. Together they determined the method of WABIPs prescription as reported on here.
First, a careful medical anamnesis is taken to exclude comorbidities that may hamper the application of the prism glasses and excluding those for whom no benefit is anticipated. Explicit exclusion criteria are amblyopia, absence of stereopsis, and previous vestibular or strabismus surgery. Although WABIPs are prescribed by optometrists and orthoptists, the importance of the medical history is taken as the main reason to advocate the application of these glasses by medical doctors. The patient is then optometrically tested and given his or her optimal refraction, stereopsis being tested last. The Maddox test is applied next [55, 61], both at a far and at a near distance to determine especially a larger than normal exophoria for near vision [29, 79]. These results determine the total added (absolute) prism power of the WABIPs [91]. To determine the asymmetry of the prism powers, De Wit and Visser [27] adapted Utermöhlen’s original walking test [94, 97]. In their extended Utermöhlen test (EUT), the patient walks ahead with eyes open in a straight path towards an additional bright vertical luminous line, for a distance of approximately 5 meters in a normally lit room (initial phases in Fig. 2). At 0.5 m in front of the line the patient stops and is asked to focus on the luminous line for approximately 10 s, creating a clear retinal after-image. After the luminous line is turned off and the room lights dimmed, the patient is instructed to close the eyes and to walk 5 m backwards along the same straight path. Different from Utermöhlen, who then stopped, the EUT requires the patient then to walk forward again towards the extinguished luminous line and back again for about 5 times while keeping the eyes closed. During this procedure, no mention is made of the after-image, nor its apparent motion (see below).
Fig.2
Sketch of the Extended Utermöhlen Test as seen from above, including the assumed explanatory variables elaborated on in section 4. Left panel: A healthy subject walks in the initial phase toward a fixation light, imprinting an after-image. After closing the eyes and in a dimly lit room, the subject is instructed to walk back and forth five times in total, and succeeds in doing so without significant deviations. Note that the after-image moves with the subject. Centre panel: A unilateral MD patient with a left-side affected labyrinth (darkened), when instructed to do the same, typically deviates in the direction of the affected side when walking backward, and continues rotating during the remainder of the EUT. Right panel: The same patient shows a straightened walking pattern when wearing WABIPs, particularly effective while imprinting an afterimage during the initial phase of the EUT, with the glasses preventing a drift of the after-image during its remainder. The arrows represent the vestibular efferent orientation bias (e) and ocular re-afferent orientation (a) as explained further in the text.
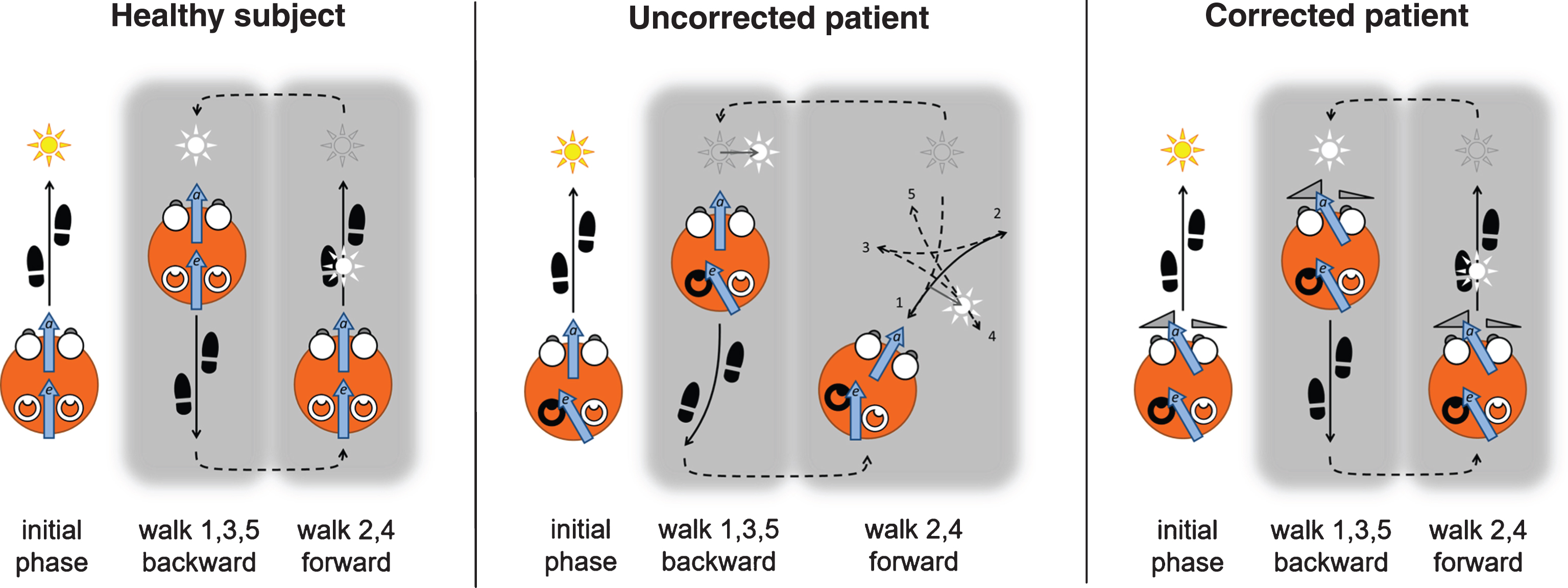
As reported by De Wit & Visser [27], the walking pattern of Ménière patients in the EUT typically deviates from the straight path, resembling a star-like figure, or “marche-en-étoile” as described by [5] and shown in the center panel of Fig. 2. Ménière patients most often deviate towards the affected side [27]. The patient is then given WABIPs adjusted to the patient’s optimal refraction, and the EUT is repeated. The asymmetry in prism power is determined by the deviation on the EUT, prism with the highest power typically placed on the presumed affected side. With the correct WABIPs, the patient then walks along straight lines throughout the EUT as shown in the right panel of Fig. 2. If there is a deviation remaining, the asymmetry of the prisms is adjusted accordingly, and the EUT repeated.
3.3WABIPs therapy
Finally, the patient is given the prescription of the optimal refraction with the correct prisms added, to be worn in daily life, both during latent phases and during attacks. Multifocal glasses are typically advised against for their known vertiginous effects. During attacks, the patient is advised to fixate visually on a point straight ahead whilst wearing the WABIPs. Patients are lastly advised to return for a repeat consultation after one year. During this visit, the EUT is repeated, possibly resulting in an adapted prescription.
3.4Alleviation of vertigo in Ménière’s disease by WABIPs
Due to the lack of RCT data, the assumed effectiveness of WABIPs is largely based on anecdotal evidence [27, 94–98], references of particular interest, but typically not complying with current standards for scientific research. In addition, two retrospective surveys have been published more recently [58, 101], one case report [22], and one prospective double blind experimental study [103].
In the retrospective survey by Vente et al. [101], the effect of WABIPs has been reported in a group of 580 patients diagnosed with unilateral MD by an ENT specialist. The effect was self-rated on a four-point scale: 1 = no or only minor improvement experienced; 2 = undoubted improvement experienced but incidental vomiting still occurring; 3 = rare attacks of short duration (less than one minute) without vomiting, and antivertigo medication discontinued; 4 = complete cessation of attacks and discontinuation of antivertigo medication. Overall, 93% of their cohort reported an improvement (scores ≥2), 73% reported only rare short or no attacks without medication (scores 3 and 4). They furthermore observed that 76% showed an EUT deviation to the same side as diagnosed by the ENT specialist, of which group 95% reported an improvement (scores ≥2). Moreover they observed that 36% of their cohort showed a significant convergence insufficiency (see above), 96% of these patients reporting an improvement (scores ≥2). 29% fell in both categories (ipsilateral EUT and a significant convergence insufficiency), of whom 98% reported an improvement (scores ≥2). 93% of the latter group even reported to be free of attacks and medication (score 4).
A case study was presented by Crowley [22], applying the method to an MD patient, who remained without symptoms for at least 20 months after prescription.
Marsman [58] published data on the effectiveness of WABIPs, based on a survey among 305 Dutch and Belgian Ménière patients, 69% of whom had experienced symptoms for more than 5 years. The aim of this survey was to discover which therapies were used by these patients to alleviate their symptoms. This study showed that medication (typically betahistine) was used by most patients (90%), followed by WABIPs (34%). Moreover, 62% of the betahistine users considered the medication effective, whereas the users of the WABIPs reported 78% effectiveness.
The only notice of a prospective WABIPs study comes from Vente & Meulenbroeks [103]. They applied the EUT to 21 unilateral Ménière patients. Fourteen of these patients were prescribed WABIPs as described above, while the other 7 were given their individually determined optimal WABIPs, however, base-out (i.e., placed temporally) as a placebo control group. Effects were rated by means of a diary. Four patents were excluded due to incomplete data. Ten out of the 11 remaining patients (91%) with base-in prisms experienced a favourable result, in particular fewer vertigo attacks, as compared to 2 out of the remaining 6 (33%) of the control group. In those showing a clear convergence insufficiency, 5/5 (100%) had favourable results with base-in prisms and 1/5 (20%) with base-out prisms. The latter may also find support by further anecdotal evidence that, in many patients returning with complaints, their prisms were shown to be finally applied in the wrong orientation. These prospective study results are in support of those from the retrospective study by Vente et al. [101].
The high success rate of WABIPs observed suggests that there is more to it than merely a placebo effect, although caution is always warranted. In the sacculotomy, for example, the reported success rates were also high, around 80%, yet proven not more effective than a placebo procedure. In favour of an actual effect are: 1) the relatively high success rates in patient improvement reported (up to 98%); 2) the fact that by considering ipsilateral EUT and ENT based diagnoses and a convergence insufficiency both had a positive effect on this rate; and 3) the difference in results between base-in and base-out prisms. All of these observations speak in favour of WABIPs, or at least of further studies on the effectiveness and mechanism of WABIPs in MD.
Although these observations may not be taken as solid proofs of effectiveness each individually, and do not render an RCT superfluous, they do speak in favour of WABIPs when considered together in a maximum likelihood or Bayesian way. Stated more carefully, they at least speak in favour of further studies on the effectiveness and mechanism of these WABIPs.
4Proposed mechanism of action of WABIPs
4.1The star-walk induced by a drifting after-image
To explain the star-walk or “marche-en-étoile” in Ménière patients (see section 3.2), we here refer exclusively to unilateral defects, or at least a clear asymmetry in vestibular function. Moreover, despite the fact that MD has been judged idiopathic, and the hydrops has not been confirmed to be a necessary and sufficient condition for the disease [30], there is little doubt as to its vestibular cause [6, 40, 54, 81]. In particular, it has been shown that MD is characterised by vestibular hypofunction [56, 57, 65, 71, 80]. Also it can be assumed that in unilateral MD, the functioning of the semi-circular canals (SCC) is at issue, as revealed by the VOR [3, 23, 32, 80], in particular induced by caloric irrigation. The left- and rightward nystagmus then shows a different amplitude when the left and right ear of Ménière’s patients are irrigated separately with warm and cold water. The asymmetry is referred to as directional preponderance [36, 43, 56, 57, 65, 71]. Caloric stimulation has been explained by endolymphatic convection within the SCC, in particular the horizontal canal that comes most close to the auditory duct [42]. Of these eye movements, typically the low-frequency hypofunction of the affected ear is associated with this directional preponderance [37, 59]. Interestingly, the head impulse test (HIT), typically investigating the effect of high-frequency head motion, did not reveal this preponderance in MD [51, 56, 57, 65, 66, 71, 82]. A tentative conclusion is that unilateral MD can (partially) be characterised by a low-frequency hypofunction of the affected canals.
An issue of particular interest concerns the output of the coding for angular velocity over a wide range of typically natural head movements [33, 88]. Neural integration of the (most) affected canal afferents, characterised by hypofunction, and the normal (or less affected) afferents of the contralateral canals, then likely results in a net angular velocity offset in the efferent (motor) signals controlling the VOR. This offset we will further refer to as the vestibular bias (see also Fig. 2). When relatively large, this bias may lead to spontaneous overt nystagmus in the acute phase of MD [80, 83], and to a covert, i.e., not manifest, nystagmus [106] when relatively small in the latent phase of MD. The latter may be explained by cancellation of the vestibular component by visual cues resulting in fixation suppression [20], or by the optokinetic reflex [23, 32]. Alternatively, or in addition to that, the vestibular bias may be insufficient to overcome a possible muscle resistance to movement [23, 90]. A refinement of the tentative conclusion given above therefore is that unilateral MD can (partly) be characterised by a vestibular bias, resulting in normal eye movements in its latent phase, and possibly leading to spontaneous nystagmus in its acute phase.
The EUT is typically applied in a latent phase of MD. Despite the lack of evidence for the involvement of overt eye movements, Kapteyn & De Wit [44] reported an apparent drift of the after-image in the EUT. The importance of this after-image is given by the observation that MD patients do not show the typical EUT star-walk when they only walk towards an extinguished fixation object in the initial phase with eyes open [27]. De Wit c.s. [26, 27] related the drift of the after-image to the opto- or oculogyral illusion (OGI), however without further explaining it. The OGI refers to a drift of either a true subject-fixed target, or an inherently subject-fixed after-image, in the same direction as an actual self-rotation in healthy subjects [14, 16, 17, 18, 28, 34, 60, 99]. Interestingly, the OGI is present even at otherwise perceptually subthreshold physical angular motion. Like MD, the OGI has been shown to be of vestibular origin, e.g., it is absent in labyrinthine defective subjects [60]. Moreover, eye movements have been excluded as a causal factor for the OGI [16, 18, 28, 41, 106]. At low accelerations in particular, the OGI has been explained by a discrepancy between efferent (motor) signals and re-afferent2 (sensory) ocular signals [16, 28, 106]. The results of Carriot et al. [16], for example, showed “that at very low acceleration rates, the OGI is closely related to the suppression of nystagmus, and to incomplete suppression coupled with retinal slip at higher acceleration rates (> = approx 5/s2)”. Interestingly, Sugie [89] already gave an explicit account of the perceived or subjective target position in the OGI, long before Carriot’s account of it, based on an efference copy [104] or corollary discharge [85]. Essentially, Sugie [89] explicitly reckoned actual target position (T), the net vestibular efferent motor signal driving the eye muscles (e, also shown in Fig. 2), and a net re-afferent (sensory) signal accounting for eye position (a, also shown in Fig. 2), predicting that in general perceived target position S is given by S = T − (e−a), but see note3. If, for example, the eye is actively moving (as indicated by both e and a that will normally be equal then) while looking at an Earth-stationary target (e.g., T = 0), the perceived target motion indeed equals the true target motion, i.e., S = T = 0. It also nicely explains the simple observation that pushing the eye-ball with a finger from the side makes the world seem to move. In this case there is no true target motion and no efferent signal (T = e = 0), but there is an afferent signal, resulting in S = a ≠ 0. Lastly, and most importantly here, if there were to be a vestibular efference copy due to a small angular motion (e ≠ 0), that for the reasons given above does not result in an actual eye movement (a = 0), a target moving with the subject (T = 0) would be perceived to be moving, i.e., S =−e. Because the efference copy is opposite the self-motion, the latter thus explains the oculogyral illusion: a perceived target motion in the direction of the self-motion. In case of an erroneous vestibular bias in MD, this likewise explains the star-walk in the EUT as shown in Fig. 2. This conclusion, however, requires the additional assumption that the drifting after-image in the EUT is associated with the visual straight ahead. This assumption seems to be justified by De Wit & Visser [27], who mentioned that “during the first phase of our [extended Utermöhlen] test, the walk with open eyes to an orientation point, probably a ‘memory’ is built up in the locomotor system, as well as in the vestibular system. This memory then, would maintain the right direction during the walk with eyes closed. We have experienced that when the subject performs the marche-en-étoile with no previous ‘orientation walk’ his sense of direction is noticeably more disturbed - as when the subject moves his head during the test”.
A detail here concerns the star-walk itself, in which the patient walks backward in one direction, and forward in the other (Fig. 2). This change of direction, however, is consistent with the observation that the patient shows a continuous unidirectional yaw rotation throughout the EUT with eyes closed. Moreover, the latter observation is also consistent with the assumption that this rotation is brought about by the vestibular bias, coding for velocity. Here, it might be questioned whether patients are aware of their deviate walking pattern, which awareness might undermine the test. Anecdotal evidence, however, showed that patients are typically in disbelief about their deviate walking pattern when informed about that afterwards. Moreover, in the latent phase, the small vestibular bias only results in a small deviation of the subjective straight-ahead, a subtlety corrected for by weak prisms, typically not considered for ophthalmologic deficits [15].
Although we here focussed on the case of a relatively small vestibular bias with a possible covert effect on eye movements, a large bias with an overt nystagmus in MD does not contradict the reported observations [27]. After all, the drift of the after-image in the oculogyral illusion is in the same direction as the implied self-motion, which motion may lead to a slow phase VOR in the opposite direction, then causing a real permanent fixation spot to move in the same direction as the after-image. The fact that according Sugie [89] this would not give rise to a perceived target motion (e−a = 0 from which it follows that S = T = 0), may be explained by considering Alexander’s law [35]. This law states that nystagmus slows down in the direction of the slow phase, possibly causing a discrepancy between the efferent and re-afferent signals e and a. This discrepancy may as yet give rise to a perceived target motion, as with the OGI. We are aware of the fact that this explanation is hypothetical, and needs further consideration and validation.
Another detail concerns Carriot at al. their observation [16] that “After constant velocity is achieved, the OGI remains at considerable amplitude [...]. This pattern suggests that velocity storage signals also contribute and have different time constants for affecting visual perception of target location and for driving eye movements”. Here, velocity storage refers to the neural mechanism required to explain the phenomenon that the duration of nystagmus induced by a sudden change in angular self-motion is typically longer than can be explained by canal dynamics only [72, 76]. Although a continuous drifting of the after-image in MD patients and the resulting persistent walking deviation in the EUT would not require velocity storage, this mechanism is likely at issue, probably even more so because velocity storage and its involvement with motion sickness has been explained using efference copies [9, 10, 25], and the observations that velocity storage is affected in patients with a unilateral hypofunction, like that in MD [45, 56, 57, 65, 71].
The tentative conclusion so far can thus be extended further by stating that the typical EUT star-walk in unilateral MD can be explained by the vestibular bias, making an after-image associated with the straight-ahead to drift comparable to the OGI.
4.2The star-walk corrected by WABIPs
The correct WABIPs in front of the eyes during the first walk of the EUT with eyes open causes a lateral shift of the visual straight-ahead in the direction of the strongest prism (see Figs. 1 and 2). This implies that the eyes are shifted statically towards the affected side. While walking towards the luminous line with these prisms in a lit room with eyes open, the visual information allows the patient to walk in a straight line, while building up a “memory” of straight-ahead in the vestibulo-spinal system. An analogue may be that correct driving in a car is independent of the orientation of our hands on the wheel. It is important to note here that the retinal images with and without prisms are equal. In that sense, we deliberately refer to the conflict at issue here as a vestibulo-ocular, rather than a visual-vestibular conflict.
The most crucial assumption pointed to by Kapteyn & De Wit [44] and De Wit & Visser [27] then is that the eyes are now allowed to be oriented in the direction preferred by the vestibular bias. The conflict between the vestibular efferent bias (e) and the ocular re-afferent signal (a) is then eliminated, thus also eliminating a drift of the after-image during the remainder of the EUT as explained by the reasoning given above, based on Sugie [89]. Moreover, this reasoning also effectively explains why, during the remainder of the EUT, the patient walks in a straight-ahead fashion again, because the subjective straight-ahead associated with the true straight-ahead in the initial walk with eyes open does not drift away anymore. To close the loop: the right WABIPs are typically chosen such that the after-image does not drift anymore as implied by the patient walking straight ahead.
It can therefore be concluded even more specifically that the typical EUT star-walk in MD may be explained by a conflict between the vestibular efferent bias and a re-afferent ocular signal. This conflict 1) causes an after-image associated with the straight-ahead to drift comparable to the OGI; and 2) can be eliminated by giving the eyes the same bias as preferred by the vestibular system using WABIPs.
4.3The therapeutic effect of WABIPs
In the latent phase of unilateral MD, patients can be assumed to be continuously stressed by the conflict between the vestibular efferent velocity bias (e) and the re-afferent ocular signal (a). Even though this stress may be otherwise unnoticed, it makes sense to assume that it can accumulate over time, giving rise to symptoms of dizziness, vertigo and even nausea. A comparable phenomenon concerns motion sickness. Motion sickness due to mild motion may also go unnoticed initially, but can accumulate giving rise ocular, disorienting, and vertiginous and/or nauseating effects [46, 73], showing a certain overlap with the disorienting symptoms associated with MD. Moreover, the most popular theory on motion sickness by Reason & Brand [73] has been further elaborated to include equal assumptions about efference copies and afferent signals as applied by Sugie [7, 8, 9, 11, 63, 89]. Note that the discomforting conflict in Ménière does not require actual, or more specifically exogenous body motion. The crucial point is that the assumed motion in MD is endogenous, i.e., caused by the malfunctioning organs of balance, or more specifically by the net vestibular angular velocity bias referred to above. Interestingly, Vente et al. [100] also reported on a positive effect of WABIPs on motion sickness. It therefore makes sense to assume that by eliminating the vestibulo-ocular conflict e−a in the EUT, also the vestibulo-ocular stress and accumulated symptoms typical for MD will be eliminated, or more likely reduced, by continuously wearing WABIPs.
As mentioned in section 2, vertigo and nausea are reported to have the largest negative impact on the quality of life in MD [47, 48, 64, 108]. This includes negative beliefs about the consequences of vertigo, typically increasing the level of anxiety experienced [48]. Because here it is assumed that WABIPs are especially effective with respect to vertigo and nausea, WABIPs can also be assumed to reduce the level of anxiety. This therefore concerns a psychological effect, intensifying the positive effect on the quality of life as a whole.
WABIPs have also been reported to be of benefit during attacks in the acute phase of MD. Although we are not aware of, and cannot think of any reason why WABIPs might have a direct effect on the functioning of the end-organ and cochlea, in particular a hydrops, we may yet speculate on a couple of reasons. The first reason concerns the effect of prisms on the VOR, which, although likely small, may not be negligible. This comes about by easing visual fixation, allowing the visual system to regain dominance over the vestibular system, thus reducing vertigo. An additional effect that may be at issue, relates to Alexander’s law again [35]. Because the strongest prism is typically placed at the side of affected labyrinth, this will force the eyes to look in that direction, thus effectively attenuating the nystagmus, which attenuation may have an additional positive effect. Furthermore, because the vestibulo-ocular stress and resulting anxiety have been reduced during the latent phase, this reduction may ease the patient from the start of the acute phase onwards. Because at the end, like with pain in general, it is the perception of the attack that counts, it may also make sense to assume that the reduction of the vestibulo-ocular stress keeps minor attacks below the threshold for perception, which may even explain a reduction in frequency thereof.
With respect to the accumulation of stress yielding the negative effects associated with MD, a difference has been reported between short- and long-term effects [2, 74, 75]. Although referring to prism effects in healthy subjects, these references suggest that the long term tonic imbalance between the healthy and affected vestibular organ may lead to habituation specifically by means of velocity storage. Moreover, this seems to be related to an enhancement of the impaired low-frequency vestibular responses [32]. Interestingly, velocity storage has been manipulated by means of VOR adaptation, particularly in the mal-de-débarquement syndrome [24]. The latter syndrome refers to symptoms shared with motion sickness (as discussed above) and MD, caused by a traumatic event typically involving motion, lasting for months to years, and in which no clinical deficit of vestibular function can be proven. The latter points to a central effect, in which velocity storage may be at issue. This observation may also have consequences for the time required to wear WABIPs, i.e., the possibility that after a certain time, the CNS has eliminated the vestibulo-ocular stress internally, after which the prisms are not required anymore. The importance with respect to WABIPs of velocity storage and habituation is yet unclear and remains to be explored.
5Discussion
The final conclusion reached, based on the literature above, is that the typical star-walk observed in the extended Utermöhlen test (EUT) during a latent phase of unilateral Ménière’s disease (MD) can be explained by a conflict between the vestibular efferent bias (e) and a re-afferent ocular signal indicative for eye position (a), which 1) makes an after-image of a target (T) associated with the straight-ahead to drift subjectively (S), comparable to the OGI according S = T − (e−a); 2) which conflict e−a can be eliminated by giving the eyes a bias as preferred by the efference copy using weak asymmetric base-in prisms (WABIPs) effectively making a = e; and 3) wearing these WABIPs on a daily basis prevents this conflict accumulating into vertigo and nausea.
The focus of the reasoning given above concerned the asymmetry of the prisms resulting in a lateral displacement of the visual field and straight-ahead, and version eye movements. The reasoning about the base-in position of the prisms was assumed to compensate for a convergence insufficiency. Yet, the latter may also have an effect on the alleviation of vertigo and nausea, because a convergence insufficiency may lead to an erroneous estimate of depth, based on the difference between the re-afferent signals of both eyes. This can therefore be assumed to also impede the perception of self-motion, which, in turn may add to the vestibulo-ocular stress.
A complicating factor in the application of WABIPs in MD results from the simple observation that ENT doctors generally do not prescribe eyeglasses, whereas eye doctors generally do not treat Ménière patients. Moreover, from an ophthalmologic point of view, the weakness of the prisms can cause doubt, as can the phenomenon of adaptation to, or “eating up” of prisms [15]. Yet, it should be reckoned that these objections relate to ophthalmologic disorders, while the WABIPs as explained here typically concern a vestibular disorder. If the effectiveness of WABIPs could be definitively proven and its underlying mechanism described here validated, this would offer the patient suffering from MD a cheap and easy treatment for improving their quality of life, and possibly preventing the use of medication and/or surgery, including the complete ablation of the organs of balance.
In this paper we deliberately focussed on MD, mainly because the scarce data that has been presented on WABIPs in the literature did focus on MD. Furthermore there is some understanding about its origin, and the disease can be characterized by explicit criteria. The reasoning underlying the hypothesis described here, and the ensuing benefit of WABIPs and other (vertical) prisms alike, may, however, equally hold for unilateral vestibular diseases different from but related to MD.
We therefore finally conclude that: 1) the positive results of WABIPs reported on so far, 2) the fact that the treatment itself is relatively simple, non-invasive, low-cost and has a high degree of patient acceptance; and 3) there is support from the literature for a mechanism as reported on here, all speak in favour of further studies on the effectiveness and mechanism of WABIPs in Ménière’s and related diseases.
Notes
1 The Classification Committee of the Bárány Society, the Japan Society for Equilibrium Research, the European Academy of Otology and Neurotology, the Equilibrium Committee of the American Academy of Otolaryngology-Head and Neck Surgery and the Korean Balance Society.
2 The addition of “re-” to “afferent”, refers to the fact that specifically afferents are meant as a result of a an action realised by a specific efferent signal.
3 In fact, Sugie used E’v instead of e, i.e., a signal predicted based on the efference copy and knowledge of the muscle-eye dynamics (which he called a “simulator”), and the actual eye position E instead of a. The former takes into account the fact that eye position can be driven by a signal coding for velocity. The latter can be inferred by the brain from, for example, a re-afferent signal. The given equation follows in a straightforward fashion from the flow-diagram presented in Fig. 1 from Sugie’s paper.
Acknowledgments
This study has been funded by the Stichting Hoormij, the Dutch association of patients with a hearing disorder, based on a legacy. The Utermöhlen Working Group is acknowledged for their cooperation and willingness to provide the methodological information.
References
[1] | Adrion A. , Fischer C.S. , Wagner J. , Gurkov R. , Mansmann U. and Strupp M. , Efficacy and safety of betahistine treatment in patients with Meniere’s disease: Primary results of a long term, multicentre, double blind, randomised, placebo controlled, doe defining trial (BEMED trial), British Medical Journal 352: ((2016) ), h6816. |
[2] | Alexander M.S. , Flodin B.W.G. and Marigold D.S. , Prism adaptation and generalization during visually guided locomotor tasks, Journal of Neurophysiology 106: ((2011) ), 860–871. |
[3] | Angelaki D.E. and Cullen K.E. , Vestibular system: The many facets of a multimodal sense, Annual Reviews on Neuroscience 31: ((2008) ), 125–150. |
[4] | Arenberg I.K. , Placebo effect for Ménière’s disease sac shunt surgery disputed, Archives of Otolaryngology 107: ((1981) ), 773–774. |
[5] | Babinski J. and Weill G.A. , Désorientation et déséquilibration spontanée et provoquée. La deviation angulaire, Comptes-rendus de la Société de Biologie 74: ((1913) ), 852–855. |
[6] | Belinchon A. , Perez-Garrigues H. and Tenias J.M. , Evolution of symptoms in Ménière’s disease, Audiology & Neuro-otology 17: ((2012) ), 126–132. |
[7] | Bles W. , Bos J.E. , de Graaf B. , Groen E. and Wertheim A.H., Motion sickness: Only one provocative conflict? Brain Research Bulletin 47: ((1998) ), 481–487. |
[8] | Bos J.E. and Bles W. , Modelling motion sickness and subjective vertical mismatch detailed for vertical motions, Brain Research Bulletin 47: ((1998) ), 537–542. |
[9] | Bos J.E. and Bles W. , Theoretical considerations on canal-otolith interaction and an observer model, Biological Cybernetics 86: ((2002) ), 91–207. |
[10] | Bos J.E. , Bles W. and de Graaf B. , Eye movements to yaw, pitch, and roll about vertical and horizontal axes: Adaptation and motion sickness, Aviation Space and Environmental Medicine 73: ((2002) ), 436–444. |
[11] | Bos J.E. , Bles W. and Groen E.L., A theory on visually induced motion sickness, Displays 29: ((2008) ), 47–57. |
[12] | Bretlau P. , Placebo effect in surgery for Ménière’s disease: A three-year follow-up study of patients in a double-blind placebo controlled study on endolymphatic sac shunt surgery, American Journal of Otology 5: ((1984) ), 558–561. |
[13] | Bretlau P. , Thomsen J. , Tos M. and Johnsen N.J. , Placebo effect in surgery for Ménière's disease: Nine-year follow-up, American Journal of Otology 10: ((1989) ), 259–261. |
[14] | Byford G.B. , Eye movements and the optogyral illusion, Aerospace Medicine 34: ((1963) ), 119–123. |
[15] | Campos E.C. and Catellani T. , Further evidence for the fusional nature of the compensation (or ‘eating up’) of prisms in concomitant strabismus, International Ophthalmology 1: ((1978) ), 57–62. |
[16] | Carriot J. , Bryan A. , DiZio P. and Lackner J.R. , The oculogyral illusion: Retinal and oculomotor factors, Experimental Brain Research 209: ((2011) ), 415–423. |
[17] | Clark B. and Stewart J.D. , Comparison of sensitivity of the perception of bodily rotation and the oculogyral illusion,&, Psychophysics 3: ((1968) ), 253–256. |
[18] | Clark B. and Stewart J.D. , Effects of angular acceleration on man: Thresholds for the perception of rotation and the oculogyral illusion, Aerospace Medicine 40: ((1969) ), 952–956. |
[19] | Cohen H. , Ewell L.R. and Jenkins H.A. , Disability in Meniere’s Disease, Archives of Otolaryngology, Head and Neck Surgery 121: ((1995) ), 9–33. |
[20] | Collins W.E. , Special effects of brief periods of visual fixation on nystagmus and sensations of turning, Aerospace Medicine 39: ((1968) ), 257–266. |
[21] | Committee on Hearing and Equilibrium, Guidelines for the diagnosis and evaluation of therapy in Ménière’s disease, Otolaryngology Head and Neck Surgery 113: ((1995) ), 181–185. |
[22] | Crowley T. , A case report of the use of prism glasses to alleviate the effects of Meniere’s disease, British Orthoptic Journal 60: ((2003) ), 52–54. |
[23] | Cullen K.E. , The vestibular system: Multimodal integration and encoding of self-motion for motor control, Trends in Neurosciences 35: ((2012) ), 185–196. |
[24] | Dai M. , Cohen B. , Smouha E. and Cho C. , Readaptation of the vestibulo-ocular reflex relieves the mal de debarquement syndrome, Frontiers in Neurology 5: ((2014) ), 1–6. |
[25] | Dai M.J. , Kunin M. , Raphan T. and Cohen B. , The relation of motion sickness to the spatial-temporal properties of velocity storage, Experimental Brain Research 15: ((2003) ), 73–189. |
[26] | de Wit G. , Vente P.E.M., Bos J.E. and Bles W., Het snelle labyrint ten dienste van het trage oog [The fast labyrinth for the purpose of the slow eye], Nederlands Tijdschrift voor KNO-Heelkunde 5: ((1999) ), 7–10. |
[27] | de Wit G. and Visser B.P., The ‘Marche-En-Étoile’ revised: The influence of after-images and prism-glasses on starwalks, Acta Otolaryngologica 105: ((1988) ), 553–557. |
[28] | Evanoff J.N. and Lackner J.R. , Some proprioceptive influences on the spatial displacement component of the oculogyral illusion, Perception & Psychophysics 43: ((1988) ), 526–530. |
[29] | Evans B.J.W. , An open trial of the Institute Free-space Stereogram (IFS) excercises, British Journal of Optometry & Dispensing 8: ((2000) ), 5–14. |
[30] | Foster C.A. and Breeze R.E. , Endolymphatic hydrops in Ménières disease: Cause, consequence, or epiphenomenon? Otology and Neurotology 34: ((2013) ), 1210–1214. |
[31] | Gibson R.W.P. , Hypothetical mechanism for vertigo in Meniere’s disease, Otolaryngologic Clinics of North America 43: ((2010) ), 1019–1027. |
[32] | Goldberg J. , Wilson V. , Cullen K. , Angelaki D. , Broussard D. , Buttner-Ennever J. , Fukushima K. , Minor L. , The Vestibular System: A Sixth Sense, Oxford University Press, ((2012) ). |
[33] | Goldberg J.A. and Fernandez C. , Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey; II: Response to sinusoidal stimulation and dynamics of peripheral vestibular system, Journal of Neurophysiology 34: ((1971) ), 661–675. |
[34] | Graybiel A. and Hupp D.I. , The oculo-gyral illusion. A form of apparent motion which may be observed following stimulation of the semicircular canals, Journal of Aviation Medicine 17: ((1946) ), 3–27. |
[35] | Hallpike C.S. , The caloric test: A review of its principles and practice with especial reference to the phenomenon of directional preponderance, In: The vestibular system and its diseases. Wolfson RJ (ed) Univ. Pennsylvania Press, Philadelphia, Pa ((1966) ), 207–217. |
[36] | Hallpike C.S. and Cairns H. , Observations on the pathology of Ménière’s syndrome, Journal of Laryngololy and Otology 53: ((1938) ), 625–655. |
[37] | Halmagyi G.M. , Cremer P.D. , Anderson J. , Murofushi T. and Curthoys I.S. , Isolated directional preponderance of caloric nystagmus: II. A neural network model, American Journal of Otology 21: ((2000) ), 568–572. |
[38] | Hamill T.A. , Evaluation of Treatment Outcomes, Journal of the American Academy of Audiology 17: ((2006) ), 27–37. |
[39] | Harris J.P. and Alexander T.H. , Current-day prevalence of Ménière’s syndrome, Audiology and Neurotology 15: ((2010) ), 318–322. |
[40] | Havia M. and Kentala E. , Progression of symptoms of dizziness in Menière’s disease, Head Neck 130: ((2004) ), 431–435. |
[41] | Howard I.P. and Templeton W.B. , Human spatial orientation. Wiley, London, ((1966) ). |
[42] | Jongkees L.B.W. , Value of the caloric test of the labyrinth, Archives of Otolaryngology 48: ((1948) ), 402–417. |
[43] | Jongkees L.B.W. and Philipszoon A.J. , The caloric test in Ménière’s disease, Acta Otolaryngologica 58: ((1964) ), 168–170. |
[44] | Kapteyn T.S. , de Wit G. , After-image walking test to detect the influence of the vestibular system on the external eye muscle balance during the latent phase of Meniere’s disease. In: lgarashi & Black (eds.) Vestibular and Visual Control on Posture and Locomotor Equilibrium (7th lnt. Symof the lnt. Soc. Posturography, Houston, Texas, 1983), Karger, Basel ((1985) ), 110–113. |
[45] | Katsarkas A. , Smith H. and Galiana H. , Head-shaking nystagmus (HSN): The theoretical explanation and the experimental proof, Acta Otolaryngologica 120: ((2000) ), 177–181. |
[46] | Kennedy R.S. , Lane N.E. , Berbaum K.S. and Lilienthal M.G. , Simulator sickness questionnaire: An enhanced method for quantifying simulator sickness, International Journal of Aviation Psychology 3: ((1993) ), 203–220. |
[47] | Kirby S.E. and Yardley L. , Understanding psychological distress in Ménière’s disease: A systematic review, Psychology and Health Medicine 13: ((2008) ), 257–273. |
[48] | Kirby S.E. and Yardley L. , Cognitions associated with anxiety in Ménière’s disease, Journal of Psychosomatic Research 66: ((2009) ), 111–118. |
[49] | Kotimäki J. , Sorri M. , Aantaa E. and Nuutinen J. , Prevalence of Meniere disease in Finland, Laryngoscope 109: ((1999) ), 748–753. |
[50] | Kuks J.B.M. and Snoek J.W. , Klinische neurologie, Bohn Stafleu van Loghum, ((2007) ). |
[51] | Lee S. , Kee H. , Sheen S.S. , Choi BY. , Koo J. and Kim K. , Head-shaking and vibration-induced nystagmus during and between the attacks of unilateral Ménière’s disease, Otology & Neurotology 36: ((2015) ), 865–872. |
[52] | Lempert T. , Olesen J. , Furman J. , Waterston J. , Seemungal B. , Carey J. , Bisdorff A. , Versino M. , Evers S. and Newman-Toker D. , Vestibular migraine: Diagnostic criteria, Journal of Vestibular Research 22: ((2012) ), 167–172. |
[53] | Liu B. , Leng Y. , Zhou R. , Liu J. , Liu D. , Zhang S.L. and Kong W.J. , Intratympanic steroids injection is effective for the treatment of drop attacks with Meniere’s disease and delayed endolymphatic hydrops - A prospective study, Medicine 95: ((2016) ), e5767. |
[54] | Lopez-Escamez J.A. , Carey J. , Chung W.H. , Goebel J.A. , Magnusson M. , Mandalà M. , Newman-Toker D.E. , Strupp M. , Suzuki M. , Trabalzini F. and Bisdorff A. , Diagnostic criteria for Ménière’s disease, Journal of Vestibular Research 25: ((2015) ), 1–7. |
[55] | Maddox E.E. , The clinical use of prisms and the decentering of lenses, 2nd ed. John Wright and Co, Bristol, UK, ((1893) ). |
[56] | Maire R. and van Melle G. , Vestibulo-ocular reflex characteristics in patients with unilateral Ménière’s disease, Otology & Neurotology 29: ((2008) ), 693–698. |
[57] | Manzari L. , Burgess A.M. , MacDougall H.G. , Bradshaw A.P. and Curthoys I.S. , Rapid fluctuations in dynamic semicircular canal function in early Ménière’s disease, European Archives of Oto-Rhino-Laryngology 268: ((2011) ), 637–639. |
[58] | Marsman M. , De ziekte van Ménière [Ménière’s disease]. Wetenschapswinkel University Groningen, Netherlands, in commission of the Nederlandsee Vereniging Voor Slechthorenden, ((2011) ). |
[59] | Mateijsen D.J. , Hengel P.W. , Kingma H. , Oreel M.A. , Wit H.P. and Albers F.W. , Vertigo and electronystagmography in uni- and bilateral Ménière’s disease, Otorhinolaryngology 63: ((2001) ), 341–348. |
[60] | Miller E.F. and Graybiel A. , Thresholds for the perception of angular acceleration as indicated by the oculogyral illusion, Perception & Psychophysics 17: ((1975) ), 329–332. |
[61] | Morgan M.W. , The Maddox classification of vergence eye movements, American Journal of Optometry & Physiological Optics 57: ((1980) ), 537–539. |
[62] | Nijboer T. , Nys G. , van der Smagt M. , van der Stigchel S. and DijkermanH., Repetitive long-term prism adaptation permanently improves the detection of contralesional visual stimuli in a patient with chronic neglect, Cortex 47: ((2011) ), 734–740. |
[63] | Oman C.M. , A heuristic mathematical model for the dynamics of sensory conflict and motion sickness, Acta Otolaryngologica Suppl 392: ((1982) ), 1–44. |
[64] | Orji F.T. , The Influence of psychological factors in Ménière’s disease, Annals of Medical Health Science Research 4: ((2014) ), 3–7. |
[65] | Park H.J. , Migliaccio A. , Della Santina C.C. , Minor L.B. and Carey J.P., Search-coil head-thrust and caloric tests in Ménière’s disease, Acta Otolaryngologica 125: ((2005) ), 852–857. |
[66] | Pérez P. , Florente J.L. , Gomez J.R. , del Campo A. , Lopez A. and Suarez C., Functional significance of peripheral head-shaking nystagmus, Laryngoscope 114: ((2004) ), 1078–1084. |
[67] | Pigassou-Albouy R. , A discussion of prism therapy for strabismus, Journal of Ophthalmic Nursing & Technology 7: ((1988) ), 18–25. |
[68] | Price D.D. , Finniss D.G. and Benedetti F. , A comprehensive review of the placebo effect: Recent advances and current thought, Annual Review of Psychology 59: ((2008) ), 565–590. |
[69] | Pullens B. and van Benthem P.P. , Intratympanic gentamicin for Ménière’s disease or syndrome, Cochrane Database Syst Rev ((2011) ), CD008234. |
[70] | Pyykkö I. , Nakashima T. , Yoshida T. , Zou J. and Naganawa S. , Meniere’s disease: A reappraisal supported by a variable latency of symptoms and the MRI visualisation of endolymphatic hydrops, British Medical Journal Open 3: ((2013) ), 1–10. |
[71] | Rambold H. , Economic management of vertigo/dizziness disease in a county hospital: Video-head-impulse test vs. caloric irrigation, European Archives of Otorhinolaryngology 272: ((2014) ), 2621–2628. |
[72] | Raphan T. , Matsuo V. and Cohen B. , Velocity storage in the vestibulo-ocular reflex arc (VOR), Experimental Brain Research 35: ((1979) ), 229–248. |
[73] | Reason J.T. , Brand J.J. , Motion sickness, Academic Press, London, ((1975) ). |
[74] | Redding G.M. , Rossetti Y. and Wallace B. , Applications of prism adaptation: A tutorial in theory and method, Neuroscience and Biobehavioral Reviews 29: ((2005) ), 431–444. |
[75] | Redding G.M. and Wallace B. , Strategic calibration and spatial alignment: A model from prism adaptation, Journal of Motor Behavior 34: ((2002) ), 126–138. |
[76] | Robinson D.A. , Linear addition of optokinetic and vestibular signals in the vestibular nucleus, Experimental Brain Research 30: ((1977) ), 447–450. |
[77] | Rossetti Y. , Rode G. , Pisella L. , Farné A. , Li L. , Boisson D. and Perenin M.T. , Prism adaptation to a rightward optical deviation rehabilitates left hemispatial neglect, Nature 395: ((1998) ), 166–169. |
[78] | Sajjadi H. and Paparella M.M. , Ménière’s disease, Lancet 372: ((2008) ), 406–414. |
[79] | Scheiman M. , Gwiazda J. and Li T. , Non-surgical interventions for convergence insufficiency, Cochrane Database Systematic Reviews 16: ((2011) ), CD006768. |
[80] | Schubert M.C. and Minor L.B. , Vestibulo-ocular physiology, Physical Therapy 84: ((2004) ), 373–385. |
[81] | Sharon J.D. , Trevino C. , Schubert M.C. and Carey J.P. , Treatment of Menière’s Disease, Current Treatment Options in Neurology 17: ((2015) ), 341. |
[82] | Shin J.E. , Kim C.H. and Park H.J. , Vestibular abnormality in patients with Ménière’s disease and migrainous vertigo, Acta Otolaryngologica 133: ((2013) ), 154–158. |
[83] | Smith P.F. and Curthoys I.S. , Mechanisms of recovery following unilateral labyrinthectomy: A review, Brain Research Reviews 14: ((1989) ), 155–180. |
[84] | Söderman A.C.H. , Bagger-Sjöbäck D. , Bergenius J. and Langius A. , Factors influencing quality of life in patients with Ménière’s disease, identified by a multidimensional approach, Otology & Neurotology 23: ((2002) ), 941–948. |
[85] | Sperry R.W. , Neural basis of the spontaneous optokinetic response produced by visual inversion, Journal of Comparative and Physiological Psychology 43: ((1950) ), 482–489. |
[86] | Stichting Hoormij, Alternatieve behandelingen [Complementary treatments], Stichting Hoormij at www.stichtinghoormij.nl/pages/nl-nl/meniere/alternatieve-behandelingen-1, ((2016) ). |
[87] | Stahle J. , Stahle C. and Kaufman Arenberg I. , Incidence of Ménière’s disease, Archives of Otolaryngology 104: ((1978) ), 99–102. |
[88] | Steinhausen W. , Ueber den Nachweis der Bewegung der Cupula in der intakten Bogengangsampulle des Labyrinthes bei der naturlichen rotatorischen und calorischen Reizung, Pflü gers Archiv für die gesamte Physiologie 228: ((1931) ), 322–328. |
[89] | Sugie N. , Investigation of visual perception of position based on the reafferent theory, Biological Cybernetics 21: ((1976) ), 17–22. |
[90] | Sylvestre P. and Cullen K.E. , Quantitative analysis of abducens neuron discharge dynamics during saccadic and slow eye movements, Journal of Neurophysiology 82: ((1999) ), 2612–2632. |
[91] | Teitelbaum B. , Pang Y. and Krall J. , Effectiveness of base in prism for presbyopes with convergence insufficiency, Optometry and Vision Science 86: ((2009) ), 153–156. |
[92] | Thomsen J. , Bonding P. , Becker B. , Stage J. and Tos M. , The non-specific effect of endolymphatic sac surgery in treatment of meniere’s disease - a prospective, randomized controlled study comparing ”classic” endolymphatic sac surgery with the insertion of a ventilating tube in the tympanic membrane, Acta Otolaryngologica 118: ((1998) ), 769–773. |
[93] | Thomsen J. , Bretlau P. , Tos M. and Johnsen N.J. , Placebo effect in surgery for Meniere’s disease, Archives of Otolaryngology 107: ((1981) ), 271–277. |
[94] | Utermohlen G.P. , Het prisma-effect bij de ziekte van Ménière [The prism effect in Ménière’s disease], Nederlands Tijdschrift voor Geneeskunde 85: ((1941) ), 1183–1192. |
[95] | Utermohlen G.P. , Nadere gegevens omtrent de toepassing van het prisma-effect bij de ziekte van Meniere [Additional data on the application of the prism effect in Ménière’s disease], Nederlands Tijdschrift voor Geneeskunde 88: ((1944) ), 836–837. |
[96] | Utermohlen G.P. , De prisma-therapie getoetst aan 160 lijders aan het syndroom van Meniere [The prism therapy examined in 160 patients with Ménière’s disease], Nederlands Tijdschrift voor Geneeskunde 91: ((1947) ), 124–126. |
[97] | Utermohlen G.P. , Prisma-therapie van de ziekte van Meniere [Prism therapy in Ménière’s disease], Nederlands Tijdschrift voor Geneeskunde 91: ((1947) ), 1080–1088. |
[98] | Utermohlen G.P. , De nawerking van het prisma-effect bij M. Meniere [The aftereffect of the prism effect in Ménière’s disease], Nederlands Tijdschrift voor Geneeskunde 92: ((1948) ), 3592–3594. |
[99] | van Dishoeck H.A.E. , Spoor A. and Nijhoff P., The opto-gyral illusion and its relation to the nystagmus of the eyes, Acta Otolaryngologica 44: ((1954) ), 597–607. |
[100] | Vente P.E.M. , Bos J.E. and de Wit G. , Motion sickness amelioration induced by prism spectacles, Brain Research Bulletin 47: ((1998) ), 503–505. |
[101] | Vente P.E.M. , Bos J.E. , Wertheim A.H. and de Wit G. , Prism spectacles as a brace for vestibular asymmetry - Study of it’s effect on Meniere patients, Abstract Barany Society Meeting, Seatle, WA, 26-29 September, Journal of Vestibular Research 11: ((2002) ), 268. |
[102] | Vente P.E.M. , Mallows K. , Utermohlen V. , Historical research into a treatment for Meniere’s. A pioneer makes the connection (Utermohlen 1873-1962). In: Proc. 4th Int. Symp. on Meniere’s Disease, Paris, France, April 11-14, 1999. Sterkers O, Ferrary E, Dauman R, Sauvage JP, Tran Ba Huy P (eds) Kugler Publications, The Hague ((2000) ), 739–740. |
[103] | Vente P.E.M. and Meulenbroeks A.A.W.M. , Prism glasses as a therapy for Ménière’s vertigo evaluated. Pilot study, XXVIIth Barany Society Meeting ((2012) ). |
[104] | von Holst E. and Mittelstaedt H., Das Reafferenzprinzip, Naturwissenschaften 37: ((1950) ), 464–476. |
[105] | Welling D.B. and Nagaraja N.H. , Endolymphatic mastoid shunt: A reevaluation of efficacy, Otolaryngolgy, Head and Neck Surgery 122: ((2000) ), 340–345. |
[106] | Whiteside T.C.D. , Graybiel A. and Niven J.I. , Visual illusions of movement, Brain 88: ((1965) ), 193–210. |
[107] | Yamakawa K. , Über die pathologische Veränderung bei einem Meniere-Kranken, Proc. 42nd Annual Meeting, Journal of the Otolaryngological Society of Japan ((1938) ), 2310–2312. |
[108] | Yardley L. , Dibb B. and Osborne G. , Factors associated with quality of life in Ménière’s disease, Clinical Otolaryngology and Allied Sciences 28: ((2003) ), 436–441. |




