Bone mineral density and serum 25-hydroxyvitamin D in patients with idiopathic benign paroxysmal positional vertigo
Abstract
The aim of this study was to evaluate the relationship between bone mineral density (BMD) and 25-hydroxyvitamin D with the occurrence and recurrence of BPPV. The records of 130 idiopathic BPPV patients (55±12 years old, 30 men and 100 women) and 130 age- and sex-matched controls who underwent bone mineral densitometry between April 2012 and September 2015 were reviewed retrospectively. We compared the BMD and serum 25-hydroxyvitamin D between the patients and controls, and also compared the BMD between recurrent and non-recurrent BPPV groups. Among the female subjects, the BPPV group showed a significantly decreased BMD compared to the controls (p < 0.05). The men in the control group had significantly higher 25-hydroxyvitamin D levels than the men with BPPV (p < 0.05). Sixty-three patients (48%) reported recurrent attacks of BPPV. The women with recurrent BPPV were significantly older and showed a significantly lower BMD than non-recurrent women (p < 0.001). However, multiple regression analysis revealed that age alone was significantly associated with the recurrence of BPPV in women. BMD in women and serum 25-hydroxyvitamin D levels in men are associated with the occurrence of BPPV. Only age is an independent predictor of recurrence, though a low BMD and age correlate with the recurrence of BPPV.
1Introduction
Benign paroxysmal positional vertigo (BPPV) is one of the most common peripheral vestibular disorders encountered in neurotology clinics and accounts for nearly 20% of vertigo [9, 16]. The canalith repositioning procedure (CRP) provides successful treatment for most BPPV patients, but some patients require repetitive CRPs. In the literature, BPPV recurrence generally varies from 20% to 30%, although rates as high as 50% have been reported [3, 22, 28]. It is therefore important to figure out predisposing factors to help prevent the occurrence and recurrence of BPPV. Although BPPV management is well established, the underlying causes of otoconial degeneration and dislodgement from the utricle are not fully known. Several predisposing factors for BPPV have been reported and linked to recurrence, including advanced age, female sex, osteoporosis, trauma, and other inner ear disorders [1, 7, 19]. Several studies suggested a correlation between BPPV with osteoporosis and vitamin D deficiency, implying that abnormal calcium metabolism may underlie BPPV [4, 12, 21, 27]. These studies used bone mineral density (BMD), a measurement of calcium levels in particular bone areas, as an indirect clinical indicator of osteoporosis and demonstrated a clear correlation between BPPV and BMD score. Some of these studies suggested that the occurrence of BPPV might be associated with osteoporosis or osteopenia [11, 13, 21, 33], BPPV recurrence might be clinically predicted by a decreased BMD score [11, 32], and medications used to treat osteoporosis or osteopenia might be helpful for preventing the occurrence and recurrence of BPPV [18].
The aim of this study was to evaluate the relationship between BMD, 25-hydroxyvitamin D, and the occurrence and recurrence of idiopathic BPPV. We compared BMD and serum 25-hydroxyvitamin D in BPPV patients with age- and sex-matched controls and compared BMD between recurrent and non-recurrent BPPV groups.
2Patients and methods
2.1Study population
A retrospective chart review of 161 consecutive BPPV patients who underwent bone mineral densitometry of the anterior-posterior lumbar spine and femur between April 2012 and September 2015 was conducted. Diagnosis of BPPV was based on history of vertigo or physical examination. Brief episodes of vertigo induced by changes in head position were considered as highly suggestive of BPPV. Routine physical examination included cranial nerve examination and positioning testing, such as Dix-Hallpike test or roll test, in which BPPV patients exhibit positional nystagmus. Of the 161 patients, 31 patients with a history of inner ear diseases, including vestibular neuritis, labyrinthitis, Meniere’s disease, ear surgery, and otitis media, a history of head trauma during the months preceding BPPV attack, or a history of lumbar spine or femur fracture, any of which can influence on BMD, were excluded. The remaining 130 patients with idiopathic BPPV (41 patients with physical examination-diagnosed BPPV and 89 patients with history-diagnosed BPPV) who underwent bone mineral densitometry were selected for analysis. Another 130 age- and sex-matched subjects with BMD and serum 25-hydroxyvitamin D measurements from the institute’s Health Promotion Center were included in the control group after excluding subjects with a history of dizziness or fracture.
BPPV patients were further classified into recurrent and non-recurrent groups. BPPV recurrence was defined as a relapse of vertigo that occurred 1 or more months after successful CRP at our clinic or two or more treatments for BPPV in other clinics during the preceding year.
This study was conducted under the review and approval of the Institutional Review Board of our institution.
2.2Determinations of BMD and biochemical tests
BMD (g/cm2) was measured in all subjects at the total femur, femoral neck, trochanter, and anterior-posterior lumbarspine (L1–L4) using dual-energy X-ray absorptiometry (DEXA Lunar, Prodigy Advance instrument; General Electric Madison, WI; software version 9.30.044). The in vivo precision of the machine was 1.25% for the femoral neck and 0.67% for the lumbar spine. A T-score, derived from the DEXA measurement, expressed an individual’s BMD (g/cm2) in standard deviations relative to the mean BMD (g/cm2) of a young adult population of the same sex. BMD was defined according to World Health Organization criteria, with normal as T-score ≥ –1.0, osteopenia as –2.5 < T-score < –1.0, and osteoporosis as a T-score ≤ –2.5 on the basis of the lowest T-score available.
Routine laboratory examinations of intact parathyroid hormone (PTH) and 25-hydroxyvitamin D were conducted in patients with BPPV between April 2012 and September 2015 similar to the time of measuring the bone mineral densitometry. Serum 25-hydroxyvitamin D concentrations (reference value at our institution, 8.0–51.9 ng/mL) were measured by radioimmunoassay (Cobra II Auto-Gamma Counting System; Packard, Minnesota, USA) in patients and controls. In BPPV patients, intact PTH concentrations (reference value at our institution, 10–65 pg/mL) were also analyzed by radioimmunoassay (Cobra II Auto-Gamma Counting System; Packard), while serum-ionized calcium concentrations (reference value at our institution, 3.9–4.5 mg/dL) were measured using a Stat Profile Critical Care Xpress (Nova Biomedical, Waltham, MA).
We compared patient BMD and serum 25-hydroxyvitamin D levels with controls. To determine the effects of osteoporosis on BPPV recurrence, we compared BMD, PTH-intact, and 25-hydroxyvitamin D between recurrent and non-recurrent BPPV groups.
2.3Statistical analyses
Statistical analyses included a Chi-square test for dichotomous variables and a Student’s t test for continuous variables to compare control and BPPV groups as well as women and men. Univariate logistic regression analysis was performed to detect variables associated with BPPV recurrence, and multivariate logistic regression analyses were adjusted for age to evaluate the independent prognostic value of BMD. All p values < 0.05 were considered significant. The SPSS statistical software package (version 21.0; IBM Corporation, Armonk, NY) was used for all statistical analyses.
3Results
The 130 eligible patients included 100 women and 30 men with a mean age of 54.9±12.2 years (range, 20–87 years). The mean age was not significantly different between women and men (54.9±12.7 years vs. 54.8±10.6 years, p > 0.05). Idiopathic BPPV occurred most commonly during ages 50–59 in both women and men (37 women (37%) and 13 men (43%)). There were no significant differences in age or sex between patients and controls.
3.1Comparison of BMD and serum 25-hydroxyvitamin D between BPPV patients and controls
Osteoporosis was more frequent in women with BPPV than in controls, and osteopenia or osteoporosis were more frequent in female and male BPPV patients than in controls (Fig. 1). In total of female and male, L2, L3, L4, L1-2, L1-3, L2-3, L2-4, and L3-4BMD (g/cm2) were significantly lower in BPPV patients compared with controls (p < 0.05), and T-scores of L1, L2, L3, L4, L1-2, L1-3, L1-4, L2-3, L2-4, L3-4, and the lowest T-score were also significantly lower in BPPV patients compared with controls (p < 0.05).
Fig.1
Proportions of osteopenia and osteoporosis in BPPV patients and control groups according to sex. The proportion of osteoporosis (T-score ≤ –2.5) was higher in BPPV patients than controls.
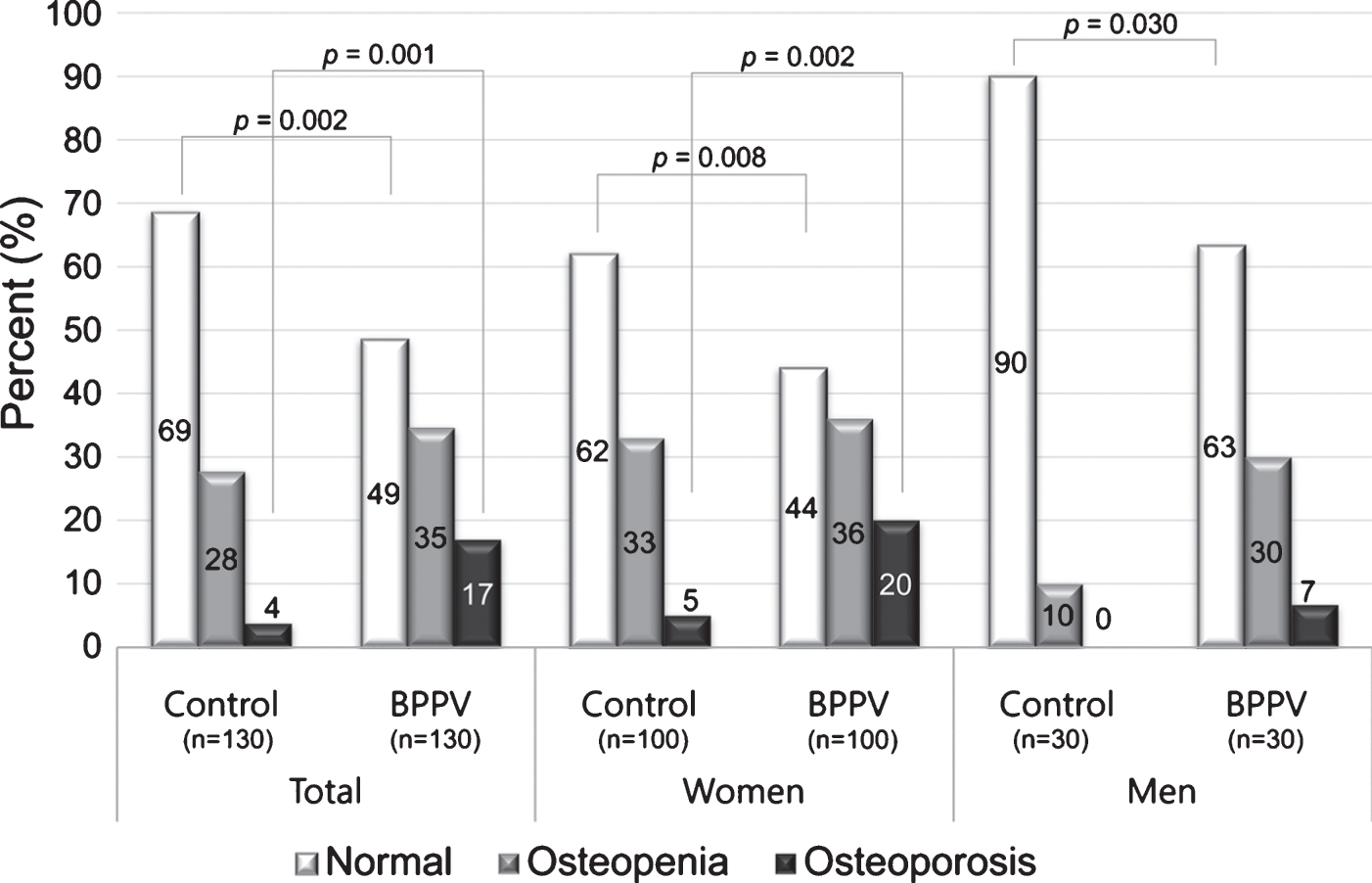
In women the differences in BMD (g/cm2) and T-scores between the BPPV and control groups were significant in all measured areas (lumbar spine and femur). However, serum 25-hydroxyvitamin D concentrations were not significantly different between the two groups in women (p > 0.05). In contrast, in men, serum 25-hydroxyvitamin D concentrations were significantly decreased in BPPV patients compared with controls (p = 0.001) whereas BMD parameters showed no significant differences between the two groups (Supplementary Table S1 and Fig. 2).
Fig.2
Differences in the lowest T-score and serum 25-hydroxyvitamin D concentrations between women and men in BPPV and control groups. The T-scores of women were significantly lower than men in both the BPPV and control groups (A). In the control group, the mean serum 25-hydroxyvitamin D concentration was significantly higher in men compared with women (B). The error bar indicates one standard deviation from the mean.
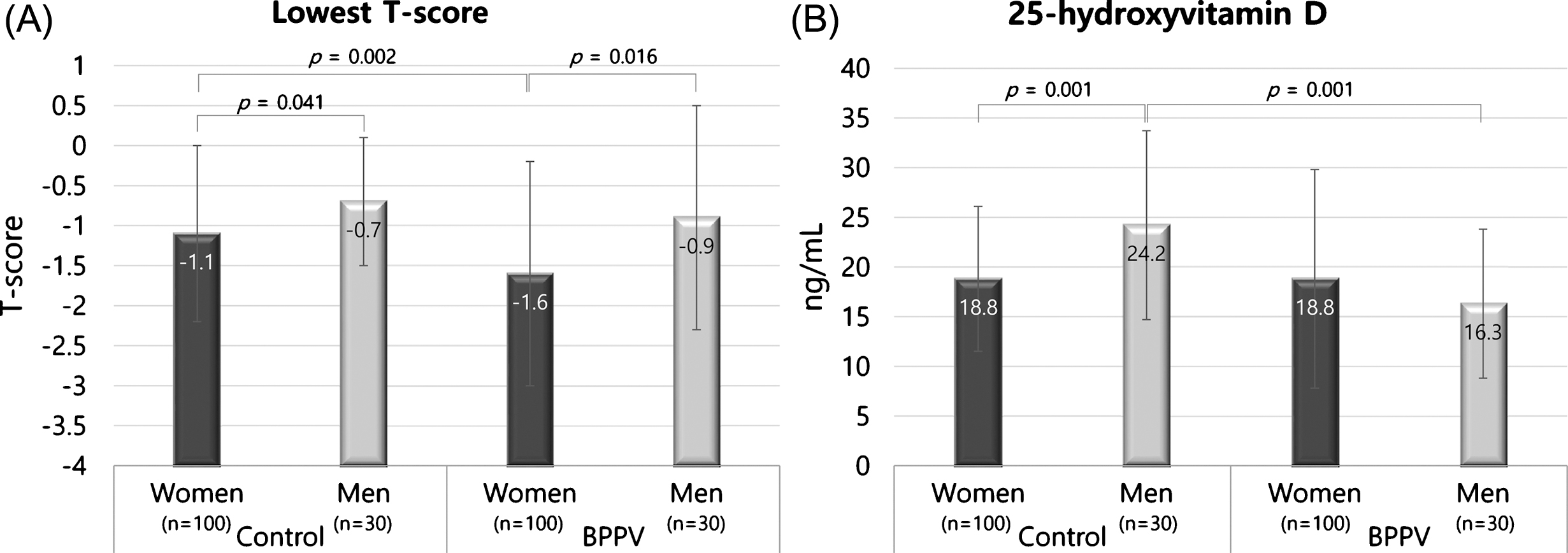
The T-scores of women were significantly lower compared with men in both the BPPV and control groups (L1, L3, L1-2, L1-3, L2-3, L2-4, L3-4, femur neck, femur trochanter, total femur and lowest T-score in the BPPV group and L4, L3-4, femur neck, total femur and lowest T-score in the control group, p < 0.05) (Fig. 2A). The mean serum 25-hydroxyvitamin D concentrations were not significantly different between female and male BPPV patients (p > 0.05), while the mean serum 25-hydroxyvitamin D concentrations were significantly higher in men in the control group (p = 0.01) (Fig. 2B).
3.2Comparison of BMD, PTH-intact, and 25-hydroxyvitamin D between recurrent and non-recurrent BPPV groups
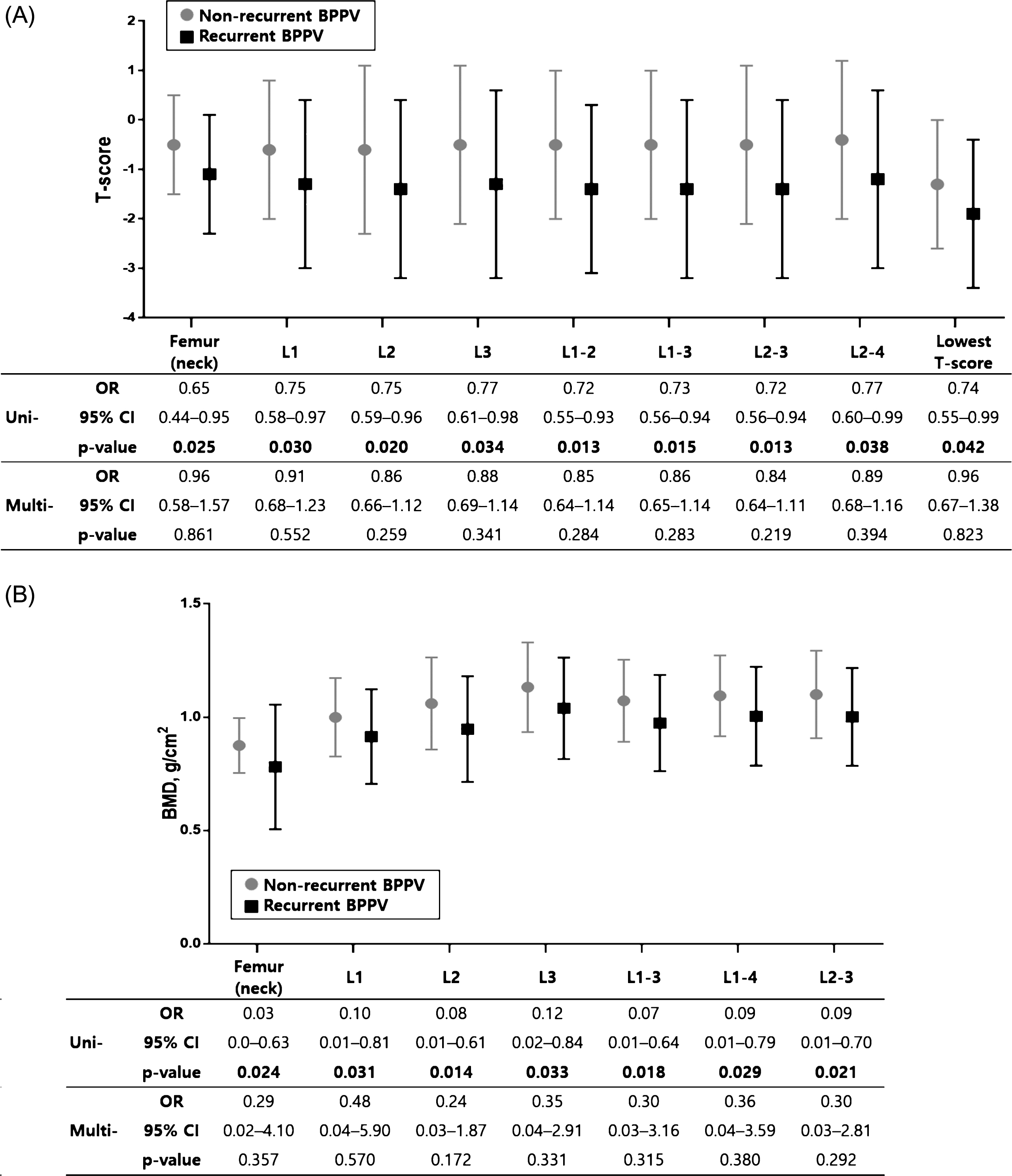
Sixty-three patients (48%) reported recurrent attacks of BPPV, 50 (50%) in women and 13 (43%) in men, with no significant association between sex and recurrence (p = 0.52). In 18 cases, BPPV recurred 1 or more months after successful CRP in our clinic, and 50 patients reported two or more BPPV treatments in other clinics during the preceding year. When comparing women with and without recurrence, the prevalence of osteoporosis was higher in women with recurrent BPPV (Fig. 3). In women there were significant differences in BMD between recurrent and non-recurrent patients in univariate logistic regression analyses. BMD (g/cm2) of the femur (neck), L1, L2, L3, L1-3, L1-4 and L2-3 were significantly lower in women with recurrence compared with women without recurrence, and T-scores of the femur (neck), L1, L2, L3, L1-2, L1-3, L2-3 and L2-4, and the lowest T-score were also significantly decreased in women with recurrence compared with women without recurrence (p < 0.05, Supplementary Table S2 and Fig. 4). However, multivariate logistic regression analyses adjusted for age revealed that T-score and BMD (g/cm2) in women were not significantly different between recurrent and non-recurrent BPPV subjects (p > 0.05, Fig. 4). Age was significantly higher in women with recurrent BPPV than in women without recurrence (59.1±11.9 years in recurrent BPPV vs. 50.8±12.2 years in non-recurrent BPPV, p = 0.002). PTH-intact and 25-hydroxyvitamin D were not associated with recurrence of BPPV (p > 0.05, Supplementary Table S2).
Fig.3
Proportions of osteopenia and osteoporosis in BPPV patients with and without recurrence according to sex. The proportion of osteoporosis (T-score ≤–2.5) was higher in women with recurrence than in women without recurrence.
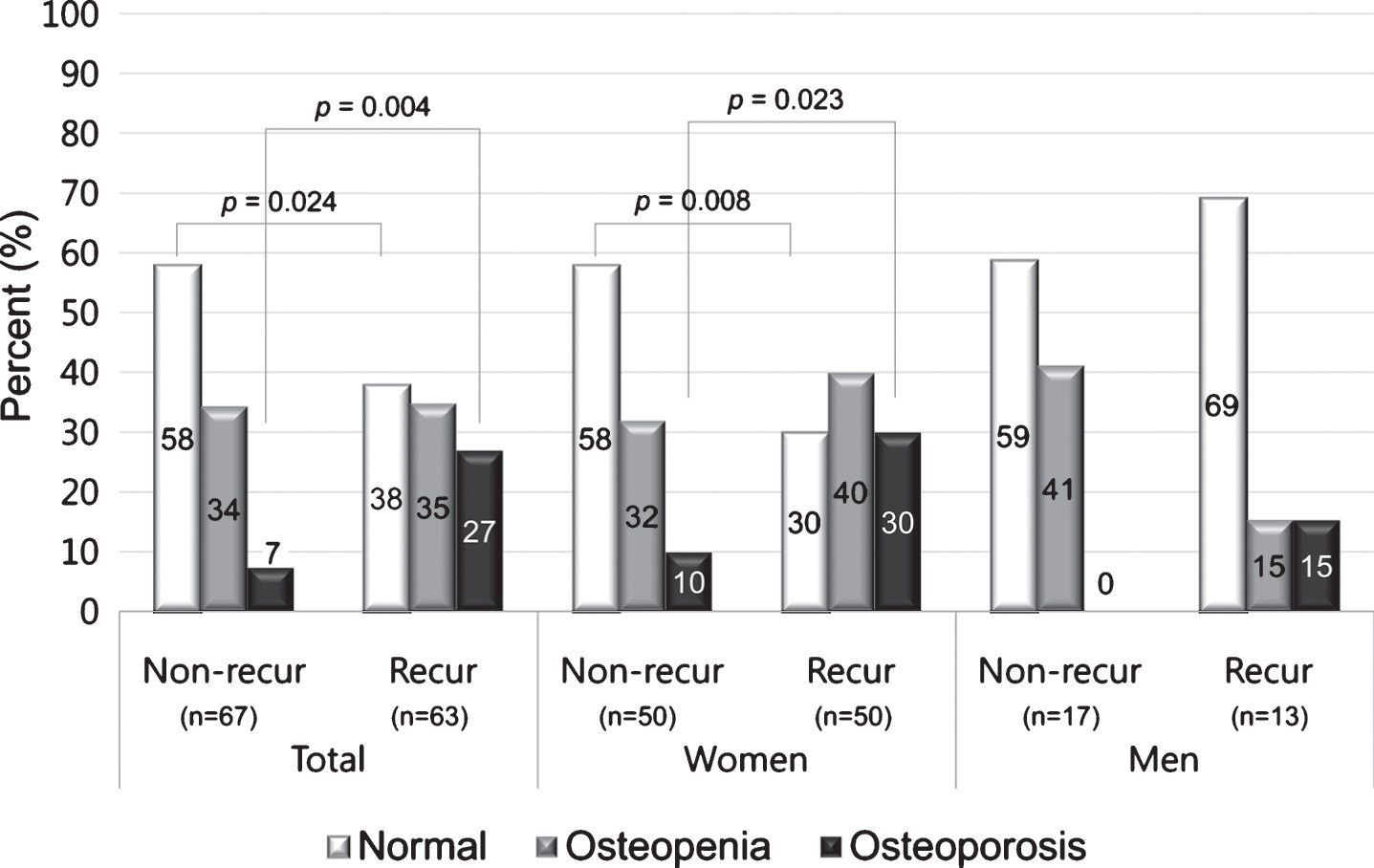
Fig.4
Comparison of T-score and BMD (g/cm2)in women with and without BPPV recurrence. In univariate logistic regression analyses, T-score (A) and BMD (g/cm2) (B) were significantly lower in women with recurrence compared to women without recurrence. However, these differences were not significant in multivariate logistic regression analyses adjusted for age. The error bar indicates one standard deviation from the mean. The mean±standard deviation values are presented in Table 4. Bold face indicates P < 0.05. Uni-, univariate logistic regression analyses; Multi-, multivariate logistic regression analyses; OR, odds ratio; CI, confidence interval.
4Discussion
Otoconia are a result of inorganic calcium carbonate accumulation onto an organic matrix core composed of glycoproteins [14, 25, 26]. Otoconia are in a dynamic state, and calcium is required for their mineralization and turnover [23, 24]. Calcium and carbonate levels in the endolymph should be kept at critical level to initiate and maintain the mineralization of the otoconia protein matrix and avoid unnecessary mineralization [10, 34, 36]. It has been suggested that disturbance of calcium metabolism induced by osteoporosis/osteopenia can lead to BPPV. The epithelial Ca2 + channel transport system, Na+/Ca2 + exchangers, and plasma membrane Ca2 + pumps expressed in the inner ear contribute to this critical balance of calcium levels by transepithelial absorption of Ca2 + from the endolymph of the inner ear [36]. Vitamin D regulates the expression of some Ca2 + binding proteins via vitamin D receptors in the epithelial cells of the inner ear [36]. Therefore, it has been speculated that vitamin D deficiency also contributes to the development of BPPV by abnormal calcium metabolism in the inner ear. Thus, in patients with BPPV and osteoporosis, disturbance of calcium metabolism might represent a common pathogenesis that results in calcium deficiency-related degradation and fragmentation of otoconia, as has been described for bones [20].
Several studies have demonstrated an apparent association between BPPV and BMD score. Vibert et al. reported that 75% (24/32)of women with BPPV (aged 50–85 years) had osteopenia/osteoporosis, suggesting a correlation [33]. Jang et al. showed that women with idiopathic BPPV had significantly lower BMD values compared to age-matched women without idiopathic BPPV, and patients with low BMD had an increased recurrence rate [11]. Jeong et al. found that prevalence of osteopenia and osteoporosis was higher in idiopathic BPPV patients (n = 209) than in controls in both women and men [13]. In a retrospective chart review, Mikulec et al. found a significant negative association between BPPV and treated osteoporosis in women aged 51 to 60 years (n = 260) [18]. Buki et al. showed that vitamin D levels were lower in patients with BPPV recurrence, and that the recurrence rate decreased with vitamin D supplementation [4].
Most of these studies included women, especially older women, as subjects. This is probably because BPPV is more common in women and typically occurs between the ages of 41 and 60 years. However, BPPV can occur at any age and in men. The association between osteoporosis and BPPV in young and male patients remains unclear. Thus, we evaluated the correlation of BMD scores and serum levels of 25-hydroxyvitamin D with BPPV in both women and men. We found a significant correlation between BMD scores and idiopathic BPPV in women and a significant correlation between serum levels of 25-hydroxyvitamin D and idiopathic BPPV in men. These differences between women and men might be due to gender differences in the prevalence and stage of osteoporosis. The prevalence of osteoporosis, as well as awareness and treatment, are higher in women [5, 15]. In our study, the proportion of patients with normal BMD (T-score ≥–1.0) was lower in the BPPV group than in the control group, regardless of gender. However, the level of vitamin D in men with BPPV was lower than in the control group. Women in both the BPPV and control groups showed low levels of vitamin D. A previous study defined serum 25-hydroxyvitamin D levels of ≤20 ng/mL as a vitamin D deficiency [31]. This suggests that women and men in the BPPV group are in different stages of abnormal calcium metabolism – vitamin D deficiency with a higher percentage ofosteopenia/osteoporosis in women and vitamin D deficiency with a much lower percentage of osteopenia/osteoporosisin men (Figs. 1 and 2). In the literature, many authors have suggested that low serum 25-hydroxyvitamin D represents a risk factor for osteoporosis [2, 6, 17]. Thus, vitamin D deficiency in men with BPPV might predict osteoporosis in the future.
We found that 63 patients (48%) experienced at least one episode of BPPV recurrence. In addition to recurrent forms of BPPV induced by surgery, head trauma, or inner ear disease, many factors appear to be related to recurrence, such as advanced age, female gender, osteoporosis, and other systemic disease such as hypertension, diabetes, and depression [7, 8]. Our study also revealed that age was a significant factor for BPPV recurrence. It was recently demonstrated that the number of otoconia in the utricle and saccule, as well as type I and II sensory hair cells, declines with age [35]. It is well known that degradation and fragmentation of otoconiais an age-related process of demineralization, resulting in balance disorder [34, 35]. The presence of osteoporosis or another systemic disease during aging may facilitate otoconial demineralization and detachment from the otoconial bed [34].
In contrast to our study, there are several studies that found an association of reduced BMD and low levels of vitamin D with development/recurrence of BPPV in both women and men [29, 30]. But the importance of our study is that we identify gender differences in predisposing factors for the development of idiopathic BPPV.
The present study had several limitations, including those inherent to a retrospective analysis; as our study was cross-sectional, we are unable to establish a causal relationship between osteoporosis and the development of BPPV and we are unable to obtain the blood samples at constant time. There is the seasonality of vitamin D and PTH and vitamin D levels change over weeks, therefore, the timing of blood draws to measure vitamin D level relative to BPPV episode is crucial. We need further prospective study with the constant time of blood sampling to emphasize the relationship between vitamin D level with BPPV. Because our hospital is tertiary hospital, our patients included those previously diagnosed with BPPV in primary clinic, thus there is a limitation that patients with acute vertigo who exhibit positional nystagmus are rare. So we included and analyzed patients with highly suggestive of BPPV (history-diagnosed BPPV) together in this study. When we repeated same analysis with 41 patients with physical examination-diagnosed-BPPV and control group, the result that the women in the BPPV group showed a significantly decreased BMD compared to the controls and the men in the control group had significantly higher 25-hydroxyvitamin D levels than the men with BPPV was not changed. The other limitation of our study is that we evaluated only idiopathic BPPV. We were unable to identify the association between comorbidities and BPPV, both of which are affected by aging.
To our knowledge, this is the first study to identify gender differences in predisposing factors for the development of idiopathic BPPV. As the link between bone turnover and idiopathic BPPV appears to be strong, with a common pathogenesis of abnormal calcium metabolism, our findings suggest that otologists should manage bone turnover-associated disorders when treating patients with idiopathic BPPV. Furthermore, we should be conscious of gender differences in factors associated with idiopathic BPPV. The measurement of BMD and serum levels of vitamin D might be helpful in diagnosing and managing BPPV patients.
5Conclusion
A large percentage of idiopathic BPPV patients have osteopenia/osteoporosis. The T-score and BMD (g/cm2) are associated with the occurrence of idiopathic BPPV, especially in women, while the serum levels of vitamin D are significantly decreased in men with idiopathic BPPV. These findings suggest that defective calcium metabolism underlies idiopathic BPPV in both women and men. Although the prevalence of osteoporosis is higher in women with recurrent BPPV, multiple regression analysis indicate that advanced age is the only independent predictor of BPPV recurrence. Further studies with a larger population are needed to define these relationships.
Conflict of interest statements
There are no conflicts of interest.
Financial disclosure
The authors have no other funding or financial relationships.
Supplementary material
[1] The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/VES-170625.
References
[1] | Baloh R.W. , Honrubia V. and Jacobson K. , Benign positional vertigo: Clinical and oculographic features in 240 cases, Neurology 37: ((1987) ), 371–378. |
[2] | Bischoff-Ferrari H.A. , Dietrich T. , Orav E.J. and Dawson-Hughes B. , Positive association between 25-hydroxy vitamin D levels and bone mineral density: A population-based study of younger and older adults, Am J Med 116: ((2004) ), 634–639. |
[3] | Brandt T. , Huppert D. , Hecht J. , Karch C. and Strupp M. , Benign paroxysmal positioning vertigo: A long-term follow-up (6-17 years) of 125 patients, Acta Otolaryngol 126: ((2006) ), 160–163. |
[4] | Buki B. , Ecker M. , Junger H. and Lundberg Y.W. , Vitamin D deficiency and benign paroxysmal positioning vertigo, Med Hypotheses 80: ((2013) ), 201–204. |
[5] | Cawthon P.M. , Gender differences in osteoporosis and fractures, Clin Orthop Relat Res 469: ((2011) ), 1900–1905. |
[6] | Collins D. , Jasani C. , Fogelman I. and Swaminathan R. , Vitamin D and bone mineral density, Osteoporos Int 8: ((1998) ), 110–114. |
[7] | De Stefano A. , Dispenza F., Suarez H., Perez-Fernandez N., Manrique-Huarte R., Ban J.H., Kim M.B., Strupp M., Feil K., Oliveira C.A., Sampaio A.L., Araujo M.F., Bahmad F. Jr, Gananca M.M., Gananca F.F., Dorigueto R., Lee H., Kulamarva G., Mathur N., Di Giovanni P., Petrucci A.G., Staniscia T., Citraro L. and Croce A., A multicenter observational study on the role of comorbidities in the recurrent episodes of benign paroxysmal positional vertigo, Auris Nasus Larynx 41: ((2014) ), 31–36. |
[8] | De Stefano A. , Kulamarva G. and Dispenza F., Malignant paroxysmal positional vertigo, Auris Nasus Larynx 39: ((2012) ), 378–382. |
[9] | Hotson J.R. and Baloh R.W. , Acute vestibular syndrome, N Engl J Med 339: ((1998) ), 680–685. |
[10] | Hughes I. , Thalmann I. , Thalmann R. and Ornitz D.M. , Mixing model systems: Using zebrafish and mouse inner ear mutants and other organ systems to unravel the mystery of otoconial development, Brain Res 1091: ((2006) ), 58–74. |
[11] | Jang Y.S. and Kang M.K. , Relationship between bone mineral density and clinical features in women with idiopathic benign paroxysmal positional vertigo, Otology & Neurotology 30: ((2009) ), 95. |
[12] | Jeong S.H. , Kim J.S. , Shin J.W. , Kim S. , Lee H. , Lee A.Y. , Kim J.M. , Jo H. , Song J. and Ghim Y. , Decreased serum vitamin D in idiopathic benign paroxysmal positional vertigo, J Neurol 260: ((2013) ), 832–838. |
[13] | Jeong S.H.H. , Choi S.H. , Kim J.Y.Y. , Koo J.W.W. , Kim H.J. and Kim J.S. , Osteopenia and osteoporosis in idiopathic benign positional vertigo, Neurology 72: ((2009) ), 1069–1076. |
[14] | Johnsson L.G. , Rouse R.C. , Wright C.G. , Henry P.J. and Hawkins J.E. Jr , Pathology of neuroepithelial suprastructures of the human inner ear, Am J Otolaryngol 3: ((1982) ), 77–90. |
[15] | Juby A.G. and Davis P. , A prospective evaluation of the awareness, knowledge, risk factors and current treatment of osteoporosis in a cohort of elderly subjects, Osteoporos Int 12: ((2001) ), 617–622. |
[16] | Labuguen R.H. , Initial evaluation of vertigo, Am Fam Physician 73: ((2006) ), 244–251. |
[17] | Malavolta N. , Pratelli L. , Frigato M. , Mule R. , Mascia M.L. and Gnudi S. , The relationship of vitamin D status to bone mineral density in an Italian population of postmenopausal women, Osteoporos Int 16: ((2005) ), 1691–1697. |
[18] | Mikulec A.A. , Kowalczyk K.A. , Pfitzinger M.E. , Harris D.A. and Jackson L.E. , Negative association between treated osteoporosis and benign paroxysmal positional vertigo in women, J Laryngol Otol 124: ((2010) ), 374–376. |
[19] | Oghalai J.S. , Manolidis S. , Barth J.L. , Stewart M.G. and Jenkins H.A. , Unrecognized benign paroxysmal positional vertigo in elderly patients, Otolaryngol Head Neck Surg 122: ((2000) ), 630–634. |
[20] | Parham K. and Kuchel G.A. , A geriatric perspective on benign paroxysmal positional vertigo, J Am Geriatr Soc 64: ((2016) ), 378–385. |
[21] | Parham K. , Leonard G. , Feinn R.S. , Lafreniere D. and Kenny A.M. , Prospective clinical investigation of the relationship between idiopathic benign paroxysmal positional vertigo and bone turnover: A pilot study, Laryngoscope 123: ((2013) ), 2834–2839. |
[22] | Perez P. , Franco V. , Cuesta P. , Aldama P. , Alvarez M.J. and Mendez J.C. , Recurrence of benign paroxysmal positional vertigo, Otol Neurotol 33: ((2012) ), 437–443. |
[23] | Preston R.E. , Johnsson L.G. , Hill J.H. and Schacht J. , Incorporation of radioactive calcium into otolithic membranes and middle ear ossicles of the gerbil, Acta Otolaryngol 80: ((1975) ), 269–275. |
[24] | Ross M.D. , Calcium ion uptake and exchange in otoconia, Adv Otorhinolaryngol 25: ((1979) ), 26–33. |
[25] | Ross M.D. , Pote K.G. , Rarey K.E. and Verma L.M. , Microdisc gel electrophoresis in sodium dodecyl sulfate of organic material from rat otoconial complexes, Ann N Y Acad Sci 374: ((1981) ), 808–819. |
[26] | Sans A. , Dechesne C.J. and Dememes D. , The mammalian otolithic receptors: A complex morphological and biochemical organization, Adv Otorhinolaryngol 58: ((2001) ), 1–14. |
[27] | Shudong Y. , Fenye L. , Zhixin C. and Qirong W. , Association between osteoporosis and benign paroxysmal positional vertigo: A systematic review, BMC Neurology 14: ((2014) ), 110. |
[28] | Steenerson R.L. , Cronin G.W. and Marbach P.M. , Effectiveness of treatment techniques in 923 cases of benign paroxysmal positional vertigo, Laryngoscope 115: ((2005) ), 226–231. |
[29] | Talaat H.S. , Abuhadied G. , Talaat A.S. and Abdelaal M.S. , Low bone mineral density and vitamin D deficiency in patients with benign positional paroxysmal vertigo, Eur Arch Otorhinolaryngol 272: ((2015) ), 2249–2253. |
[30] | Talaat H.S. , Kabel A.M. , Khaliel L.H. , Abuhadied G. , El-Naga H.A. and Talaat A.S. , Reduction of recurrence rate of benign paroxysmal positional vertigo by treatment of severe vitamin D deficiency, Auris Nasus Larynx 43: ((2016) ), 237–241. |
[31] | Thacher T.D. and Clarke B.L. , Vitamin D insufficiency, Mayo Clin Proc 86: ((2011) ), 50–60. |
[32] | Toshiaki Y. , Shiho S. , Yachiyo S. , Takayuki M. , Nobuya F. and Hiroshi H. , Osteoporosis as a risk factor for the recurrence of benign paroxysmal positional vertigo, The Laryngoscope 123: ((2013) ), 2813–2816. |
[33] | Vibert D. , Kompis M. and Hausler R. , Benign paroxysmal positional vertigo in older women may be related to osteoporosis and osteopenia, Ann Otol Rhinol Laryngol 112: ((2003) ), 885–889. |
[34] | Vibert D. , Sans A. , Kompis M. , Travo C. , Muhlbauer R.C. , Tschudi I. , Boukhaddaoui H. and Hausler R. , Ultrastructural changes in otoconia of osteoporotic rats, Audiol Neurootol 13: ((2008) ), 293–301. |
[35] | Walther L.E. and Westhofen M. , Presbyvertigo-aging of otoconia and vestibular sensory cells, J Vestib Res 17: ((2007) ), 89–92. |
[36] | Yamauchi D. , Raveendran N.N. , Pondugula S.R. , Kampalli S.B. , Sanneman J.D. , Harbidge D.G. and Marcus D.C. , Vitamin D upregulates expression of ECaC1 mRNA in semicircular canal, Biochem Biophys Res Commun 331: ((2005) ), 1353–1357. |




