The effect of migraine and motion sickness on symptoms evoked by the caloric vestibular test
Abstract
BACKGROUND:
The caloric vestibular test (CVT) may evoke headache and vestibular symptoms in susceptible people. Patients with migraines have higher susceptibility to motion sickness. In migraines, impaired habituation to repetitive stimuli is a well-known interictal abnormality.
OBJECTIVE:
This study is aimed at evaluating CVT-evoked headache, nausea, vomiting, and imbalance in patients with and without migraine and/or motion sickness.
METHODS:
A retrospective data analysis was performed on 554 patients with a complaint of dizziness who underwent bithermal CVT at a tertiary referral center. The occurrences of CVT-evoked headache, nausea, vomiting, and imbalance were observed in four groups: patients with only migraine (MG; n = 94), those with only motion sickness (MSG; n = 89), those with migraine and motion sickness (MMSG; n = 122), and those without migraine and motion sickness (non-MMSG; n = 146). The differences between the groups were assessed.
RESULTS:
The mean ages of groups were similar (p = 0.534). The proportions of females were higher in the MG, MSG, and MMSG (p = 0.001). The severity of nausea and headache for each gender was higher in the MG, MSG, and MMSG (p < 0.001). Vomiting was more common in MMSG among males (p = 0.003), while there was no difference between groups among females (p = 0.099). Imbalance was more common in MMSG among females (p < 0.001). A relationship was detected between age and imbalance (p < 0.001), where an increased risk for imbalance was evident with greater age. Three patients in the MMSG needed hospitalization after CVT.
CONCLUSIONS:
Special caution is needed when performing caloric testing for patients with migraines or MS since CVT-evoked symptoms may occur with higher incidence and intensity, which might be related to a lack of habituation in neuronal information processing after robust sensory stimuli like CVT.
Abbreviations
CVT | caloric vestibular test |
DHI | Dizziness Handicap Inventory |
HPA | hypothalamic-pituitary-adrenal |
ICHD | International Classification of Headache Disorders |
VN | vestibular nuclei |
MS | motion sickness |
MMSG | with migraine and motion sickness group |
non-MMSG | without migraine and motion sickness group |
MG | with only migraine group |
MSG | with only motion sickness group |
SPEV | slow-phase velocity |
1Introduction
Migraine is an extremely prevalent and complex neurological disorder that affects multiple cortical, subcortical, and brainstem areas that regulate autonomic, affective, cognitive, and sensory functions [11]. As such, it is evident that the brains of those with migraines differ from those without them [7]. Furthermore, different neural networks interact with each other to allow people with migraines to cope with stressors [11].
In the pathophysiology of migraines, clinical neurophysiology and functional neuroimaging studies show that the brain is “hyperexcitable” or “hypersensitive” [2, 14]. Electrophysiological studies provide a noninvasive and easy way to access the nervous system. In contrast with healthy controls, people with migraines show an average habituation deficit to repetitive stimuli in stimulus-related evoked potentials [10, 30]. One explanation for this lack of habituation in those with migraines could be reduced intracortical inhibition or increased cortical excitability. In general, when the brain encounters a stressful trigger or condition, it responds adaptively by means of behavioral or physiological mechanisms. However, maladaptive brains with migraines might not be able to suppress stressful triggers to give an immediate adaptive response and to protect the body from stressors [8, 31].
Motion sickness (MS) is generally accepted to be based on some form of sensory conflict or sen-sory mismatch between actual patterns versus expected invariant patterns of vestibular, visual, and kinaesthetic inputs [25]. Identification of sensory conflict neurons in the vestibular nuclei (VN) and cerebellum has provided new knowledge regarding sensory conflict theory [16]. The contribution of sensory conflict neurons to VN-autonomic regulation is still ambiguous, but downstream pathophysiological mechanisms of MS have been updated in recent studies, which emphasize visceral vestibular convergence, vestibulo-thermal regulation, and MS-related endocrine [40]. Nausea and vomiting may be accompanied by a host of significant symptoms, including headache, drowsiness, sweating, facial pallor, cold sweating, increased salivation, sensations of bodily warmth, dizziness, loss of appetite, and increased sensitivity to odors [25]. Many of these features of motion sickness resemble symptoms of acute vestibular disease [26].
The caloric vestibular test (CVT) is a part of videonystagmography and is used for comparison of the relative sensitivity of right versus left peripheral vestibular function [37]. It is well known that vestibular stimulation or imbalance is a stressful condition in itself [36]. As an outcome of the stimulation of the vestibular system, CVT may evoke nausea, vomiting, or imbalance in susceptible people, eliciting an acute and reversible alert-like reaction that involves the sympathetic adrenal-medullar system and hypothalamic-pituitary-adrenal (HPA) axis responses [15]. It has been shown that the ability to habituate to vestibular stimulation relates to one’s susceptibility to motion sickness [19].
Since those who suffer from migraines have a higher incidence of MS [9, 17, 21, 22], hypersensitivity of vestibular sensory processing is more likely to occur. In light of the pathophysiology described above, we hypothesize that CVT-related side effects might be more robust and disturbing in patients with migraine and/or motion sickness because of their maladaptive or hypersensitive brains. Therefore, this study is aimed at evaluating CVT-evoked headache, nausea, vomiting, and imbalance after the procedure in patients with only migraine, with only MS, with migraine and MS, and without migraine and MS.
2Materials and methods
2.1Patients (Figure 1)
We retrospectively analyzed our database for this study. Among 3099 patients, we identified the medical records of 676 adult patients and who underwent bithermal CVT at a tertiary referral center in three years who had a complaint of dizziness without a previous diagnosis of any vestibular or hearing disorder, a headache of secondary nature, any unstable medical or psychiatric condition, pregnancy, or breastfeeding. Patients were excluded if their ages were not between 18 and 60 years (n = 40) or if their Dizziness Handicap Inventory (DHI) [27] scores were not between 36 and 52 points (n = 82). The 25-item DHI evaluates the self-perceived handicapping effects imposed by dizziness [27]. A retrospective data analysis was performed on 554 patients whose ages were between 18 and 60 years and DHI scores between 36 and 52 points (moderate handicap).
Fig. 1
Patient Inclusion Flowchart. CVT = caloric vestibular test, DHI = Dizziness Handicap Inventory, MG = with migraine group, MSG = with motion sickness, MMSG = with migraine and motion sickness group, non-MMSG = without migraine and motion sickness group.
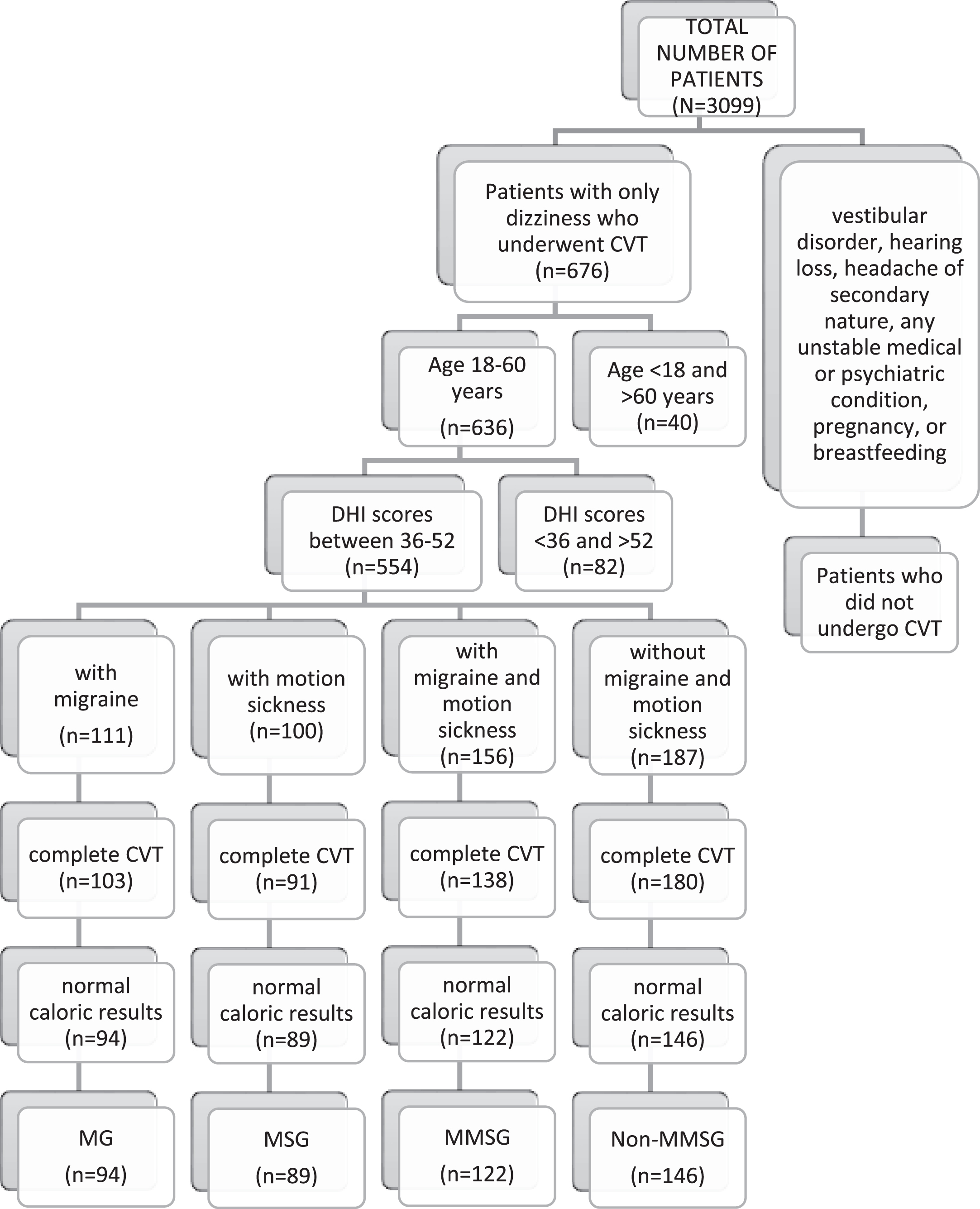
We divided the patients into four groups: those with only migraines (MG), those with only MS (MSG), those with migraine and MS (MMSG), and those without migraine and MS (non-MMSG). Premature termination of the test can have causes other than migraine and MS, such as anxiety [32], so the patients who did not complete all four irrigations of CVT were excluded from the study. Patients with normal (symmetrical) caloric responses were included in the study due to decreased susceptibility to MS in unilateral and bilateral vestibular loss [26]. The menstrual cycling of females in the groups was not differentiated in the analysis.
In the MG, the CVT-evoked symptoms of 94 patients were analyzed after excluding 17 patients due to premature termination of CVT (n = 8: nausea = 2, vomiting = 1, anxiety = 5) or abnormal caloric results (n = 9). In the MSG, the CVT-evoked symptoms of 89 patients were analyzed after excluding 11 patients due to premature termination of CVT (n = 9: nausea = 3, vomiting = 2, anxiety = 4) or abnormal caloric results (n = 2). In the MMSG, the CVT-evoked symptoms of 122 patients were analyzed after excluding 34 patients due to premature termination of CVT (n = 18: nausea = 6, vomiting = 3, anxiety = 9) or abnormal caloric results (n = 16). In the non-MMSG, the CVT-evoked symptoms of 146 patients were analyzed after excluding 41 patients due to premature termination of CVT (n = 7: nausea = 2, anxiety = 5) or abnormal caloric results (n = 34). All patients included in MG and MMSG were diagnosed by a neurologist according to the International Classification of Headache Disorders –third beta edition (ICHD-3 beta) (Third Edition) [34]. All migraine subtypes were included in the study and were not differentiated in the analysis.
2.2CVT procedure
All patients included in the study completed a bithermal water caloric test (Difra Aquastar, Eupen, Belgium). An otoscopic examination was conducted to ensure that the ear canal was free of obstructions that could affect the quality of the irrigation and to ensure that there was no perforation of the tympanic membrane. All patients were informed in detail about what to expect before the irrigation began. Infrared goggles were placed over the eyes for video-oculographic recording in a “vision-denied” condition (Micromedical by Interacoustics, Assens, Denmark). The patient’s head was elevated to a 30° angle to place the horizontal canal into a vertical plane so that the horizontal canal was positioned optimally for stimulation.
The caloric testing began with 30 seconds of cold-water irrigations, followed by 30 seconds of warm-water irrigations for each ear with a post-irrigation interval of 5 minutes, as recommended by the British Society of Audiology (1999) [29]. The patient was asked to suppress the caloric-induced nystagmus shortly after the peak of the caloric response by staring at a fixed target, which was an illuminated LED on the inside surface of the video goggles. Alertness was maintained by counting numbers or engaging in conversation with the patient. Only patients with normal caloric responses were included in the study to make sure that strong nystagmus was evoked, but the relation between slow-phase velocity (SPEV) and CVT-evoked symptoms was not assessed since no consistent relationship has been shown between SPEV and the likelihood of a patient feeling nauseous in previous studies [32, 39].
2.3Evaluation of CVT-evoked symptoms
The occurrences of CVT-evoked headache, nausea, vomiting, and imbalance were observed for 30 minutes after all four irrigations were completed. Headache intensity was measured on a 4-point scale where 0 = no headache; 1 = mild headache; 2 = moderate headache; and 3 = severe headache, as recommended by the International Headache Society for controlled trials of acute treatment of migraine attacks in adults (Fourth edition) [20]. Nausea intensity was measured on a similar 4-point scale where 0 = no nausea; 1 = mild nausea; 2 = moderate nausea; and 3 = severe nausea. Vomiting and imbalance following the CVT were observed and noted as absent or present by a clinician.
Tandem walking was explained and demonstrated to all patients. They were instructed to walk 5 steps with the eyes open before and after CVT while placing one foot in front of the other and touching toe to heel with each step. All patients included in the study were able to perform the tandem walk before CVT. Inability to perform or complete the 5 steps tandem walk after CVT was evaluated as imbalance.
It is our routine clinical procedure to obtain written informed consent from each subject following a detailed explanation of the CVT. The study was conducted under the ethical principles stated in the Declaration of Helsinki, and the objectives and protocol were approved by the Medical Research Ethics Committee of Acibadem University and Acibadem Healthcare Group with an approval number 2021-06/21.
2.4Statistical methods
The data were summarized as means, standard deviations, numbers, and percentages. The age distribution between groups was compared using one-way ANOVA and the post-hoc Bonferroni method. The relationship between age and symptoms was analyzed using an independent t-test and one-way ANOVA. The comparison of DHI median scores between the groups was done using p-values, the Kruskal-Wallis test, and degree-of-freedom analysis. The association between groups and other variables was evaluated using Pearson’s chi-squared test. Statistical analysis was performed using STATISTICA 13.0 software (TIBCO Software Inc. Statistica, version 13, 2018). The statistical significance level was taken as p < 0.05, and Bonferroni-adjusted p values are given.
3Results
The mean ages of the groups were similar (p = 0.534) (Table 1), but the gender distributions were significantly different between groups (p = 0.001). The proportions of females were higher in the MG, MSG, and MMSG than the non-MMSG (Table 1). Hence, we compared the symptoms of the groups separately for each gender (Table 2).
Table 1
Mean age and gender distributions
| Non-MMSG | MG | MSG | MMSG | Statistics, d.f. | p value | ||
| Age | Mean±SD | 36.79±8.98 | 38.23±8.82 | 37.86±10.65 | 38.30±9.29 | F = 0.73, df = 3.446 | 0.534 |
| Gender | Female (n, %) | 82 (56.16%) | 69 (74.19%) | 65 (73.03%) | 93 (76.23%) | χ2 = 15.61, df = 3 | 0.001 |
| Male (n, %) | 64 (43.84%) | 24 (25.81%) | 24 (26.97%) | 29 (23.77%) |
MG = with only migraine group; MSG = with only motion sickness group; MMSG = with migraine and motion sickness group; non-MMSG = without migraine and motion sickness group.
Table 2
Comparison of symptoms between groups among females and males
| Female | Male | ||||||||||||
| Non-MMSG (n = 82) | MG (n = 69) | MSG (n = 65) | MMSG (n = 93) | χ2, d.f. | Bonf. adj. p value | Non-MMSG (n = 64) | MG (n = 24) | MSG (n = 24) | MMSG (n = 29) | χ2, d.f. | Bonf. adj. p value | ||
| Nausea | 0 (n, %) | 50 (60.98%) | 15 (21.74%) | 1 (1.54%) | 15 (16.13%) | 107.82, df = 9 | < 0.001 | 44 (68.75%) | 9 (37.50%) | 0 (0.00%) | 4 (13.79%) | 64.78, df = 9 | < 0.001 |
| 1 (n, %) | 10 (12.20%) | 20 (28.99%) | 7 (10.77%) | 30 (32.26%) | 8 (12.50%) | 7 (29.17%) | 1 (4.17%) | 9 (31.03%) | |||||
| 2 (n, %) | 11 (13.41%) | 28 (40.58%) | 31 (47.69%) | 22 (23.66%) | 7 (10.94%) | 5 (20.83%) | 13 (54.17%) | 6 (20.69%) | |||||
| 3 (n, %) | 11 (13.41%) | 6 (8.70%) | 26 (40.00%) | 26 (27.96%) | 5 (7.81%) | 3 (12.50%) | 10 (41.67%) | 10 (34.48%) | |||||
| Vomiting | Absent (n, %) | 78 (95.12%) | 62 (89.86%) | 51 (78.46%) | 81 (87.10%) | 9.85, df = 3 | 0.099 | 63 (98.44%) | 22 (91.67%) | 22(91.67%) | 20 (68.97%) | 17.47, df = 3 | 0.003 |
| Present (n, %) | 4 (4.88%) | 7 (10.14%) | 14 (21.54%) | 12 (12.90%) | 1 (1.56%) | 2 (8.33%) | 2 (8.33%) | 9 (31.03%) | |||||
| Headache | 0 (n, %) | 73 (89.02%) | 5 (7.25%) | 29 (44.62%) | 40 (43.01%) | 131.00, df = 9 | < 0.001 | 60 (93.75%) | 2 (8.33%) | 11 (45.83%) | 11 (37.93%) | 87.13, df = 9 | < 0.001 |
| 1 (n, %) | 8 (9.76%) | 27 (39.13%) | 24 (36.92%) | 19 (20.43%) | 4 (6.25%) | 6 (25.00%) | 7 (29.17%) | 6 (20.69%) | |||||
| 2 (n, %) | 1 (1.22%) | 24 (34.78%) | 11 (16.92%) | 11 (11.83%) | 0 (0.00%) | 8 (33.33%) | 4 (16.67%) | 4 (13.79%) | |||||
| 3 (n, %) | 0 (0.00%) | 13 (18.84%) | 1 (1.54%) | 23 (24.73%) | 0 (0.00%) | 8 (33.33%) | 2 (8.33%) | 8 (27.59%) | |||||
| Imbalance | Absent (n, %) | 82 (100%) | 66 (95.65%) | 64 (98.46%) | 75 (80.65%) | 31.20, df = 3 | < 0.001 | 64 (100%) | 22 (91.67%) | 24 (100%) | 27 (93.10%) | 8.06, p = 3 | 0.224 |
| Present (n, %) | 0 (0.00%) | 3 (4.35%) | 1 (1.54%) | 18 (19.35%) | 0 (0.00%) | 2 (8.33%) | 0 (0.00%) | 2 (6.90%) | |||||
| Hospitalization | Absent (n, %) | 82 (100%) | 69 (100%) | 65 (100%) | 91 (97.85%) | 4.67, df = 3 | 0.922 | 64 (100%) | 24 (100%) | 24 (100%) | 28 (96.55%) | 3.19, df = 3 | 1.000 |
| Present (n, %) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 2 (2.15%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 1 (3.45%) | |||||
Bonf. adj. = Bonferroni adjusted; MG = with only migraine group; MSG = with only motion sickness group; MMSG = with migraine and motion sickness group; non-MMSG = without migraine and motion sickness group. 0 = non; 1 = mild; 2 = moderate; and 3 = severe.
The severity of nausea and headache for each gender was significantly higher in the MG, MSG, and MMSG than the non-MMSG (p < 0.001) (Table 2). A significant difference was found between the groups for vomiting among males (p = 0.003), where the MMSG had a significantly higher occurrence of vomiting than the other groups (Table 2). There was no significant difference between the groups for vomiting among females (p = 0.099). A significant difference was found between the groups for imbalance among females (p < 0.001), where the MMSG had a significantly higher occurrence of imbalance than the other groups (Table 2). There was no significant difference between the groups for imbalance among males (p = 0.224).
Age and imbalance were statistically relevant, but age was not related to other symptoms. Since there were very few patients with imbalance, we were able to compare the mean ages of patients according to imbalance occurrence (absent/present) in only the MMSG. A significant association was present between age and imbalance (p < 0.001), where an increased risk for imbalance was evident with greater age (Table 3).
Table 3
Relationship between age and imbalance
| Imbalance | t, d.f. | Bonferroni adjusted | ||
| Age | Absent | Present | p value | |
| All groups | 36.95±8.91 | 50.15±8.13 | 7.37 df = 448 | < 0.001 |
| MMSG | 36.30±8.08 | 48.50±8.48 | 6.12 df = 120 | < 0.001 |
MMSG = with migraine and motion sickness group.
We also analyzed the association between nausea/vomiting and imbalance in the MMSG but did not detect a significant association between nausea and imbalance (p = 0.225) or vomiting and imbalance (p = 0.313) (Table 4). CVT-evoked symptoms disappeared within a couple of hours in the majority of patients. However, three patients (two females and one male) in the MMSG required hospital admission to achieve optimal management due to the severity of headache or nausea/vomiting. None of the patients in the MG, MSG, or non-MMSG required hospitalization.
Table 4
The association between nausea/vomiting and imbalance
| MMSG | Statistics, d.f. | Bonferroni adjusted | |||
| Imbalance present | Imbalance absent | p value | |||
| Nausea | 0 (n) | 13 | 6 | χ2 = 4,361, df = 3 | 0.225 |
| 1 (n) | 35 | 4 | |||
| 2 (n) | 24 | 4 | |||
| 3 (n) | 30 | 6 | |||
| Vomiting | Absent (n) | 86 | 15 | χ2 = 1,018, df = 1 | 0.313 |
| Present (n) | 16 | 5 | |||
MMSG = with migraine and motion sickness group.
We analyzed the median DHI scores (Table 5) of the groups and detected a statistically significant difference between the non-MMSG and the other groups (non-MMSG and MG (p < 0.0001), non-MMSG and MSG (p < 0.0001), non-MMSG and MMSG (p < 0.0001)). The median DHIs did not differ significantly between the other groups (MSG and MG (p = 1.00), MG and MMSG (p = 0.377), MSG-MMSG (p = 0.310)).
Table 5
DHI Score Analysis of the Groups
| DHI | ||||||
| Group | n | Min-Max | Median [Q1 - Q3] | p | Kruskal-Wallis Test Statistics | Degree of Freedom |
| MMSG | 122 | 36–52 | 44 [42–46] | < 0,0001 | 106,841 | 3 |
| MG | 94 | 36–52 | 42 [40–44] | |||
| MSG | 89 | 36–52 | 42 [40–44] | |||
| NON-MMSG | 146 | 36–46 | 40 [38–40] | |||
DHI = Dizziness Handicap Inventory, MG = with migraine group, MSG = with motion sickness, MMSG = with migraine and motion sickness group, non-MMSG = without migraine and motion sickness group.
4Discussion
The results of the present study showed that the MG, MSG, and MMSG had a higher intensity of headaches and higher frequency of vestibular symptoms compared to the non-MMSG after CVT. Our results agree with those of previous studies indicating that vertigo induced by vestibular stimulation (rotation/caloric testing) can act as a specific migraine trigger and cause nausea and vomiting [15, 32, 33, 39]. The present study evaluated the CVT-evoked symptoms in patients with moderate dizziness who were referred for vestibular function testing. Although the results were similar in terms of vestibular symptoms, the present study is differentiated in the following ways in comparison to previous studies on CVT-evoked symptoms evaluated in volunteers [15, 32, 33, 39]. The first difference is the reflection of real-life clinical practice by retrospective analysis of patients who underwent caloric testing at a tertiary referral center. Second, a detailed analysis was conducted on the CVT-evoked symptoms of headache, imbalance, nausea, and vomiting. Providing real-world evidence might be crucial since it has been shown that women report a greater history of MS than men when asked to answer questionnaires on their MS history. Furthermore, those reporting that they are prone to MS are less likely to volunteer for MS-provoking experiments [23].
Nausea was the most common CVT-evoked symptom in all groups, including the non-MMSG in this study. These findings are similar to those of Vitkovic et al., who reported that patients with migraine and MS were four times more likely to feel nausea with increased emetic response to vestibular stimulation during CVT [39]. The present results might indicate that severe nausea and vomiting can result from migraine alone and MS alone, as well as both migraine and MS in females. The reason is that no significant difference was found between groups for vomiting among females (p = 0.099). However, although MS alone can cause severe nausea in males, vomiting is not likely to be triggered among them unless they have both migraine and MS since vomiting was significantly more common in males in the MMSG (p = 0.003) in our study.
Studies show that migraine and MS patients may have an abnormal autonomic function compared to healthy subjects [3]. Therefore, the autonomic nervous system might be responsible for the higher severity of CVT-evoked symptoms in cases of mig-raine and MS. The basis of nausea in migraine was once thought to be gastric stasis [28]. However, today, it is known that when improving gastric stasis in migraineurs with a migraine attack, nausea still remains until the migraine attack is successfully treated [28]. Therefore, it is assumed that gastric stasis is an epiphenomenon but not the cause of nausea and vomiting, which is a central process [28].
It is well known that there is a convergence of vestibular, visual, proprioceptive, and visceral system information in the brainstem and cerebellum. These extensive interactions modulate somatic and autonomic responses to self-movement and moving environments [5]. Hypersensitivity or intensified functional interactions between the vestibular nuclei, solitary nucleus, and trigeminal nucleus caudalis may cause an increased prevalence of MS in migraineurs [24].
Severe headache consistent with migraine criteria were evoked in the majority of both female and male patients in the MG and MMSG, while none of the patients in the non-MMSG had severe headache after CVT (Table 2). Moderate headache was mostly evoked in the MSG among both genders in our study (Table 2). Structural and functional changes across cortical and brainstem networks in migraineurs are likely to contribute to the initiation of headache attacks [38]. Patients with moderate or severe headache may have a lower threshold for a trigger like CVT because of a reduced intracortical inhibition or an increased cortical excitability. During a migraine attack, the primary symptom is typically headache, but attacks are associated with other accompanying symptoms like nausea and vomiting [34]. Likewise, nausea and vomiting may be accompanied by headache in MS [25].
Imbalance might be the most severe response to CVT. None of patients in the non-MMSG showed imbalance. Imbalance was mostly evoked in females in the MMSG and in males in the MG and MMSG (Table 2). MS alone was not likely to cause CVT-evoked imbalance, and there was no significant relation between nausea/vomiting and imbalance in our groups of patients (Table 4). Our results indicated that being female (p < 0.001) and greater age (p < 0.001) were additional significant factors for CVT-evoked imbalance. Migraine has been known to predominantly occur among females, but the mechanism for the gender difference in migraine is not clear, even if endogenous sex steroids are considered to play a relevant role [35].
The patients with imbalance after CVT might be those who have the highest dysfunction in vestibular sensory processing. Migraine has been shown to be associated with a risk of falling, and patients exhibit a higher prevalence of impairment in balance, falls, and fear of falling [12]. Vestibular habituation deficit mainly due to migraine [10, 30] and age-dependent white-matter changes [4], and the age-dependent changes in the vestibulo-ocular reflex [1] might be related to changes in balance control after CVT in our group of patients.
One male and two female patients needed to be hospitalized to control their headaches and/or vestibular symptoms after the procedure. The majority of migraine patients have normal caloric responses, and the rate of abnormal caloric test results in those with migraine was ranged between 8 and 25%in previous studies [9, 17, 21, 22]. A hyper-responsive caloric pattern can also be seen in those with migraine, which has reported rates of 15.8–16.6%in previous studies [9, 17, 21, 22]. This may cause significant discomfort leading to premature termination of the test. Therefore, monothermal air calorics, which provide less powerful exposure than bithermal water calorics, might be the protocol of choice in patients with migraine and/or MS when CVT is needed. Although water irrigations were performed in our study, air CVT was also shown to be an activator of the HPA axis [15], and a large increase in cortisol levels after air CVT was demonstrated [18].
The comparison of DHI scores between the groups showed that patients with migraine, motion sickness, or both had a decrease in their self-perceived handicap from dizziness according to their DHI score. However, the occurrence of CVT symptoms might not be directly related to the DHI score since no significant differences were detected between the groups except with the non-MMSG and other groups. In recent years, CVT has not been part of the care routine for every patient with dizziness, imbalance, or vertigo at our clinic. While trying to convince most of our patients to undergo CVT in the past years, we have already started to obtain patient consent and decrease the number of CVTs at the time of the study based on our clinical experience with the occurrence of CVT-evoked symptoms. The present study was aimed to document our clinical experience.
In view of the results of the present study, we have started to perform it even less since every year, approximately one-third of the patients (28.5%, un-published data) who are referred for vestibular evaluation to our clinic have migraine or motion sickness. In addition, anxiety was the most common reason leading to premature termination of the test in all of our groups of patients, including the ones in the non-MMSG. Therefore, we also modified our CVT protocol to monothermal (warm) air calorics, and we no longer use water calorics. We only perform bithermal CVT in selected cases when monothermal results are not enough for decision-making. Therefore, we currently only perform CVT if we need additional confirmation of the side or site of lesions. The results of the present study indicate that clinicians need to reconsider the necessity of CVT and should be more cautious when doing caloric testing when needed if a patient has migraines or MS.
4.1Limitations of the study
The retrospective data analysis in our study provided real-world evidence about CVT-evoked symptoms at a tertiary referral center. However, there were some limitations. It has been reported that nausea and vomiting during CVT are more frequent in patients with vestibular migraine [39]. A major drawback of this study was that the subgroups of patients with vestibular migraine were not identified in the MG and MMSG since insufficient data were available to retrospectively diagnose definite/probable VM in every patient. Therefore, it is not possible to say that vestibular migraine might have been present in patients who had moderate or severe headache, needed to be hospitalized, or experienced imbalance after CVT.
Second, using a validated questionnaire for MS is not a routine procedure at our clinic. Most of the patients in this study were asked about MS. If they declared MS, their statements were accepted as correct. Therefore, enough data to determine the severity of MS in each patient were not present. Third, the link between anxiety and migraine is well known [6], but we were not be able to assess anxiety, psychiatric comorbidities, or their relationship with the patients’ symptom severity due to a limitation of the methodology in the study. Nevertheless, despite the stated limitations, our study achieves success in showing differences in response to CVT-evoked symptoms between patients with and without migraine and/or MS.
5Conclusion
CVT in patients with migraine and/or MS may evoke headache, nausea, vomiting, and imbalance, and prolonged symptoms may rarely require hospitalization. Special caution is needed for patients with migraine and/or MS since CVT-evoked symptoms may occur with higher incidence and intensity. Over the years, since the caloric test was first described in the early 1900 s by Robert Bárány, a presumption that the doctor knows best has been exchanged for one where the patient knows best if properly informed [13]. Therefore, before performing CVT, each patient should receive a list of probable CVT-evoked symptoms with additional information regarding the higher incidence and intensity of migraines and MS.
References
[1] | Alberts B.B. , Selen L.P. and Medendorp W.P. , Age-related reweighting of visual and vestibular cues for vertical perception, Journal of neurophysiology 121: ((2019) ), 1279–1288. |
[2] | Aurora S. and Wilkinson F. , The brain is hyperexcitable in migraine, Cephalalgia 27: ((2007) ), 1442–1453. |
[3] | Aurora S.K. , Kori S.H. , Barrodale P. , McDonald S.A. and Haseley D. , Gastric Stasis in Migraine: More Than Just a Paroxysmal Abnormality During a Migraine Attack: CME, Headache: The Journal of Head and Face Pain 46: ((2006) ), 57–63. |
[4] | Baezner H. , Blahak C. , Poggesi A. , Pantoni L. , Inzitari D. , Chabriat H. , Erkinjuntti T. , Fazekas F. , Ferro J. and Langhorne P. , Association of gait and balance disorders with age-related white matter changes: the LADIS study, Neurology 70: ((2008) ), 935–942. |
[5] | Balaban C.D. , Projections from the parabrachial nucleus to the vestibular nuclei: potential substrates for autonomic and limbic influences on vestibular responses, Brain research 996: ((2004) ), 126–137. |
[6] | Best C. , Eckhardt-Henn A. , Tschan R. and Dieterich M. , Psychiatric morbidity and comorbidity in different vestibular vertigo syndromes, Journal of Neurology 256: ((2009) ), 58–65. |
[7] | Borsook D. , Goadsby P.J. and Hargreaves R. , The migraine brain: imaging structure and function, Oxford University Press, (2012) . |
[8] | Borsook D. , Maleki N. , Becerra L. and McEwen B. , Understanding migraine through the lens of maladaptive stress responses: a model disease of allostatic load, Neuron 73: ((2012) ), 219–234. |
[9] | Brey R.L. , Both migraine and motion sickness may be due to low brain levels of serotonin, Neurology 65: ((2005) ), E9–E10. |
[10] | Brighina F. , Palermo A. and Fierro B. , Cortical inhibition and habituation to evoked potentials: relevance for pathophysiology of migraine, The journal of headache and pain 10: ((2009) ), 77–84. |
[11] | Burstein R. , Noseda R. and Borsook D. , Migraine: multiple processes, complex pathophysiology, Journal of Neuroscience 35: ((2015) ), 6619–6629. |
[12] | Carvalho G. , Almeida C. , Florencio L. , Pinheiro C. , Dach F. , Bigal M. and Bevilaqua-Grossi D. , Do patients with migraine experience an increased prevalence of falls and fear of falling? A cross-sectional study, Physiotherapy 104: ((2018) ), 424–429. |
[13] | Coggon J. and Miola J. , Autonomy, liberty, and medical decision-making, The Cambridge law journal 70: ((2011) ), 523. |
[14] | Coppola G. , Pierelli F. and Schoenen J. , Is the cerebral cortex hyperexcitable or hyperresponsive in migraine? Cephalalgia 27: ((2007) ), 1427–1439. |
[15] | Cozma S. , Ghiciuc C.M. , Damian L. , Pasquali V. , Saponaro A. , Lupusoru E.C. , Patacchioli F.R. and Dima-Cozma L.C. , Distinct activation of the sympathetic adreno-medullar system and hypothalamus pituitary adrenal axis following the caloric vestibular test in healthy subjects, PloS one 13: ((2018) ), e0193963. |
[16] | Cullen K.E. , The vestibular system: multimodal integration and encoding of self-motion for motor control, Trends in neurosciences 35: ((2012) ), 185–196. |
[17] | Cuomo-Granston A. and Drummond P.D. , Migraine and motion sickness: what is the link? Progress in neurobiology 91: ((2010) ), 300–312. |
[18] | Dagilas A. , Kimiskidis V. , Aggelopoulou M. , Kapaki E. , Fitili C. , Libitaki G. , Papagiannopoulos S. , Kazis D. , Kazis A. and Aidonis A. , Changes in blood neurotransmitter and steroid levels during evoked vertigo, Otology & Neurotology 26: ((2005) ), 476–480. |
[19] | Dai M. , Kunin M. , Raphan T. and Cohen B. , The relation of motion sickness to the spatial–temporal properties of velocity storage, Experimental brain research 151: ((2003) ), 173–189. |
[20] | Diener H.-C. , Tassorelli C. , Dodick D.W. , Silberstein S.D. , Lipton R.B. , Ashina M. , Becker W.J. , Ferrari M.D. , Goadsby P.J. and Pozo-Rosich P. , Guidelines of the International Headache Society for controlled trials of acute treatment of migraine attacks in adults, Cephalalgia 39: ((2019) ), 687–710. |
[21] | Drummond P.D. , Triggers of motion sickness in migraine sufferers, Headache: The Journal of Head and Face Pain 45: ((2005) ), 653–656. |
[22] | Eckhardt-Henn A. , Best C. , Bense S. , Breuer P. , Diener G. , Tschan R. and Dieterich M. , Psychiatric comorbidity in different organic vertigo syndromes, Journal of neurology 255: ((2008) ), 420–428. |
[23] | Flanagan M.B. , May J.G. and Dobie T.G. , Sex differences in tolerance to visually-induced motion sickness, Aviation, space, and environmental medicine 76: ((2005) ), 642–646. |
[24] | Furman J.M. and Marcus D. , Migraine and motion sensitivity, CONTINUUM: Lifelong Learning in Neurology 12: ((2006) ), 116–134. |
[25] | Golding J.F. and Gresty M.A. , Motion sickness and disorientation in vehicles, Oxford textbook of vertigo and imbalance. Oxford University Press, Oxford ((2013) ), 293–306. |
[26] | Golding J.F. and Patel M. , Meniere’s, migraine, and motion sickness, Acta oto-laryngologica 137: ((2017) ), 495–502. |
[27] | Jacobson G.P. and Newman C.W. , The development of the dizziness handicap inventory , Archives of Otolaryngology–Head & Neck Surgery 116: ((1990) ), 424–427. |
[28] | Láinez M.J. , García-Casado A. and Gascón F. , Optimal management of severe nausea and vomiting in migraine: improving patient outcomes, Patient related outcome measures 4: ((2013) ), 61. |
[29] | Lightfoot G. , BSA recommended procedure, British journal of audiology 33: ((1999) ), 349–351. |
[30] | Magis D. , Lisicki M. and Coppola G. , Highlights in migraine electrophysiology: Are controversies just reflecting disease heterogeneity? Current opinion in neurology 29: ((2016) ), 320–330. |
[31] | Montagna P. , Pierangeli G. and Cortelli P. , The primary headaches as a reflection of genetic darwinian adaptive behavioral responses, Headache: The Journal of Head and Face Pain 50: ((2010) ), 273–289. |
[32] | Moran M. and Vitkovic J. , Perceptual differences in vestibular migraine populations in response to vestibular function testing, Australian and New Zealand Journal of Audiology, The 33: ((2013) ), 1. |
[33] | Murdin L. , Davies R.A. and Bronstein A.M. , Vertigo as a migraine trigger, Neurology 73: ((2009) ), 638–642. |
[34] | Olesen J. , International classification of headache disorders, The Lancet Neurology 17: ((2018) ), 396–397. |
[35] | Sacco S. , Ricci S. , Degan D. and Carolei A. , Migraine in women: the role of hormones and their impact on vascular diseases, The journal of headache and pain 13: ((2012) ), 177–189. |
[36] | Saman Y. , Bamiou D. , Gleeson M. and Dutia M.B. , Interactions between stress and vestibular compensation–a review, Frontiers in neurology 3: ((2012) ), 116. |
[37] | Shepard N. and Jacobson G. , The caloric irrigation test, in: Handbook of clinical neurology, Elsevier, (2016) , pp. 119–131. |
[38] | Tolner E.A. , Chen S.-P. and Eikermann-Haerter K. , Current understanding of cortical structure and function in migraine, Cephalalgia 39: ((2019) ), 1683–1699. |
[39] | Vitkovic J. , Paine M. and Rance G. , Neuro-otological findings in patients with migraine-and nonmigraine-related dizziness, Audiology and Neurotology 13: ((2008) ), 113–122. |
[40] | Zhang L.L. , Wang J.Q. , Qi R.R. , Pan L.L. , Li M. and Cai Y.L. , Motion sickness: current knowledge and recent advance, CNS neuroscience & therapeutics 22: ((2016) ), 15–24. |




