Nystagmus characteristics of healthy controls
Abstract
BACKGROUND:
Healthy controls exhibit spontaneous and positional nystagmus which needs to be distinguished from pathological nystagmus.
OBJECTIVE:
Define nystagmus characteristics of healthy controls using portable video-oculography.
METHODS:
One-hundred and one asymptomatic community-dwelling adults were prospectively recruited. Participants answered questions regarding their audio-vestibular and headache history and were sub-categorized into migraine/non-migraine groups. Portable video-oculography was conducted in the upright, supine, left- and right-lateral positions, using miniature take-home video glasses.
RESULTS:
Upright position spontaneous nystagmus was found in 30.7% of subjects (slow-phase velocity (SPV)), mean 1.1±2.2 degrees per second (°/s) (range 0.0 – 9.3). Upright position spontaneous nystagmus was horizontal, up-beating or down-beating in 16.7, 7.9 and 5.9% of subjects. Nystagmus in at least one lying position was found in 70.3% of subjects with 56.4% showing nystagmus while supine, and 63.4% in at least one lateral position. While supine, 20.8% of subjects showed up-beating nystagmus, 8.9% showed down-beating, and 26.7% had horizontal nystagmus. In the lateral positions combined, 37.1% displayed horizontal nystagmus on at least one side, while 6.4% showed up-beating, 6.4% showed down-beating. Mean nystagmus SPVs in the supine, right and left lateral positions were 2.2±2.8, 2.7±3.4, and 2.1±3.2°/s. No significant difference was found between migraine and non-migraine groups for nystagmus SPVs, prevalence, vertical vs horizontal fast-phase, or low- vs high-velocity nystagmus (<5 vs > 5°/s).
CONCLUSIONS:
Healthy controls without a history of spontaneous vertigo show low velocity spontaneous and positional nystagmus, highlighting the importance of interictal nystagmus measures when assessing the acutely symptomatic patient.
1Introduction
In the pursuit of studying, quantifying and classifying the nystagmus characteristics of common causes of vertigo, it is imperative to first distinguish what constitutes the typical spectrum of eye-movements in healthy controls without a history of vertigo. Important findings regarding the nystagmus characteristics of known pathologies including vestibular migraine (VM), episodic ataxia, benign paroxysmal positional vertigo (BPPV) and Menière’s Disease (MD) shed light on the range of possible eye movements in central and peripheral vestibular disorders [14, 29, 33, 37].
Previous studies have shown that nystagmus can occur in normal controls, observable without visual fixation [2, 20, 21, 24, 32]. Further in-depth study on the nystagmus patterns and slow-phase velocities (SPV) will help to arrive at reasonable normative ranges that may be used to gauge whether a pattern of nystagmus fall within (or outside) the normal limits. In this study we present the nystagmus findings of 101 healthy adults without a history of recurrent vertigo lasting more than five minutes.
Novel aspects of this study include collection of video data using stand-alone portable video- goggles which could in the future be useful as a diagnostic technique for episodic vertigo. We also undertook statistical comparison of nystagmus slow-phase velocities between those with and without a migraine history and comparison of normal controls against vestibular migraine sufferers experiencing ictal episodes of vertigo, who also demonstrate low velocity nystagmus.
Community-dwelling adults ≥18 years old were prospectively recruited between June 2017 and February 2020, with informed consent (Sydney, Australia). Healthy controls were given a questionnaire regarding their vertigo and headache history. Subjects answering ‘yes’ to having had an episode of spontaneous vertigo lasting >5 minutes in the past were excluded from the study. Participants with a previous diagnosis of BPPV (now asymptomatic) were included in the study. Based on their questionnaire answers, subjects were further sub-categorized into a migraine (M) or non-migraine (nM) group.
We used the Headache Classification Committee of the International Headache Society (IHS) [13] classification for Migraine without Aura, to assist classification. Participants in the migraine group were those who answered ‘yes’ to having had a headache history including the following: B) lasting >4 hours, and answered ‘yes’ to two of the following: C1) unilateral location, C2) pulsating or throbbing quality, C3) moderate or severe pain level, C4) aggravation by normal movement (e.g. walking or climbing stairs), and answered ‘yes’ to at least one of the following: D1) headache caused nausea, D2) headache caused photophobia or phonophobia. IHS Criterion A (at least five attacks of headache) and D (not better accounted for by another International Classification of Headache Disorders criteria) were not included in the questionnaire.
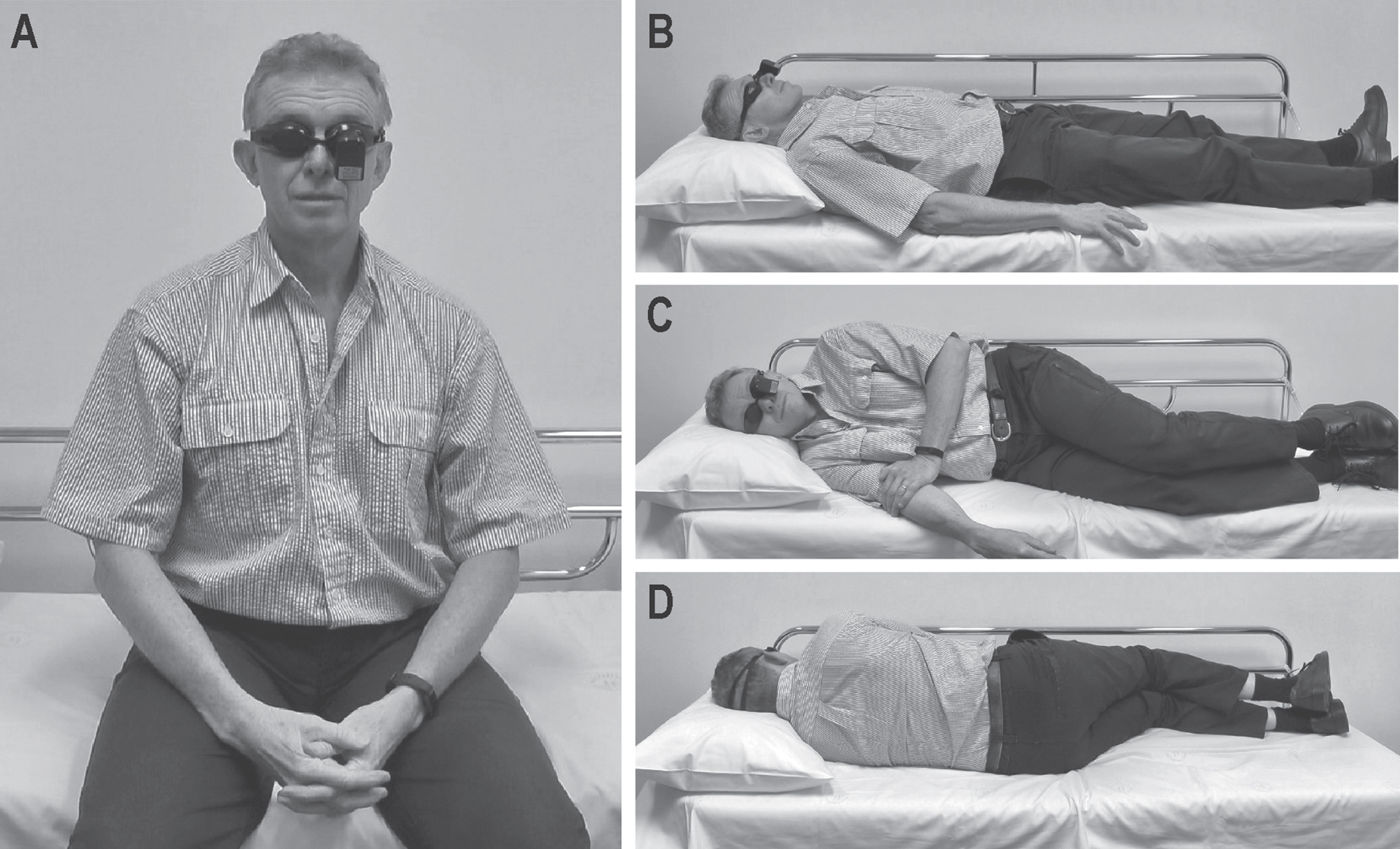
Video-oculography without visual fixation was conducted with all participants and included the following positions; 1) sitting upright, looking straight ahead for 15 seconds(s), then for 30s each; 2) lying supine, 3) lying on their right side, and 4) lying on their left side (Fig. 1). In all testing positions, participants were asked to maintain their eyes in a centre gaze position. All participants were asymptomatic from vertigo and headache at the time of testing and no visible spontaneous nystagmus could be seen with the naked eye. No adverse effects were reported.
Fig.1
Positions conducted during video-oculography; A) sitting upright, B) supine, C) right-lateral, and D) left-lateral.
Portable video-oculography goggles were used to record a single video for each subject, recorded at 30 Hz. The pupil was then tracked and analysed with a program using threshold-based ellipse fitting (LabVIEW, National Instruments; Austin, Texas). The pupil-analysis system was calibrated using head-fixed targets validated against manual tracing and virtual simulations. Eye-movements were analysed in both the horizontal and vertical planes, and the nystagmus slow-phase velocity (SPV) was obtained, measured in degrees per second (°/s). There was no lower limit placed on the nystagmus SPV reported, however a minimum of three beats of nystagmus within 15 seconds of recording was required for analysis. The nystagmus plane (vertical or horizontal) which was found to have the more significant SPV was deemed the dominant plane, and only the SPV for that plane was used for statistical analysis.
Statistical analysis was performed using IBM SPSS Statistics for Windows, Version 26 (Armonk, NY). Linear regression equations were used to assess the difference in nystagmus velocities between the non-migraine and the migraine groups, and the relationship between nystagmus and age. Binary regression equations were used to determine the likelihood of nystagmus presence in both groups. The SPV data is positively skewed, but the means and standard deviations are included in Tables 1 and 2 alongside non-parametric measures to facilitate comparison with previous papers.
Table 1
Nystagmus Slow-Phase Velocity (°/s), by Fast-Phase
| Sitting Upright | Supine | Right-Lateral | Left-Lateral | |||||||||||||
| Horiz. | DBN | UBN | Nil. | Horiz. | DBN | UBN | Nil. | Horiz. | DBN | UBN | Nil. | Horiz. | DBN | UBN | Nil. | |
| % | 16.8 | 6.0 | 6.9 | 70.3 | 26.7 | 89 | 19.8 | 44.6 | 39.6 | 6.0 | 8.9 | 45.5 | 34.7 | 6.8 | 4.0 | 54.5 |
| N | 17 | 6 | 7 | 71 | 27 | 9 | 20 | 45 | 40 | 6 | 9 | 46 | 35 | 7 | 4 | 55 |
| Mean | 3.4 | 4.8 | 3.7 | – | 3.9 | 3.9 | 4.1 | – | 4.7 | 6.2 | 4.6 | – | 4.2 | 6.2 | 3.1 | – |
| SD | 2.0 | 3.3 | 2.3 | – | 2.5 | 2.5 | 3.3 | – | 2.9 | 5.9 | 3.2 | – | 3.0 | 5.4 | 1.7 | – |
| Min. | 0.4 | 0.5 | 0.8 | – | 1.0 | 1.4 | 0.7 | – | 1.4 | 1.5 | 1.5 | – | 0.8 | 1.3 | 1.2 | – |
| Q1 | 1.8 | 1.4 | 1.5 | – | 2.0 | 2.1 | 2.0 | – | 2.3 | 2.1 | 2.9 | – | 2.1 | 2.0 | 1.4 | – |
| Med. | 3.5 | 5.1 | 4.3 | – | 3.0 | 3.3 | 3.4 | – | 3.7 | 2.8 | 3.4 | – | 3.1 | 4.1 | 3.1 | – |
| Q3 | 4.8 | 7.6 | 5.4 | – | 5.3 | 5.4 | 6.4 | – | 6.2 | 13.6 | 5.5 | – | 5.1 | 11.0 | 4.7 | – |
| Max. | 7.1 | 9.3 | 7.2 | – | 10.8 | 8.9 | 14.1 | – | 11.4 | 14.0 | 12.5 | – | 12.7 | 16.0 | 4.9 | – |
Nystagmus characteristics of 101 normal controls by nystagmus fast-phase, in four positions. [RBN; Right-beating nystagmus, LBN; Left-beating nystagmus, UBN; Up-beating nystagmus, DBN; Down-beating nystagmus; Max.; Maximum, Med.; Median, Min.; Minimum, Nil.; No nystagmus, Q1; First quartile, Q3; Third quartile, SD; Standard deviation, °/s; Degrees per second, %; Percentage of total subjects].
Table 2
Nystagmus Slow-Phase Velocity (°/s) by Position
| Sitting Upright | Supine | Right-Lateral | Left-Lateral | |
| Mean | 1.1 | 2.2 | 2.6 | 2.0 |
| SD | 2.1 | 2.9 | 3.4 | 3.2 |
| Min. | 0.0 | 0.0 | 0.0 | 0.0 |
| Q1 | 0.0 | 0.0 | 0.0 | 0.0 |
| Med. | 0.0 | 1.4 | 1.7 | 0.0 |
| Q3 | 1.5 | 3.4 | 3.9 | 3.0 |
| Max. | 9.3 | 14.1 | 14.0 | 16.0 |
Nystagmus slow-phase velocity characteristics of 101 healthy normal controls in four positions, all fast-phase directions combined. [Max; Maximum, Med.; Median, Min; Minimum, Q1; First quartile, Q3; Third quartile, SD; Standard Deviation, °/s; Degrees per second].
This study received local ethics committee approval for the use of human participants (Protocol No X13-0425 & HREC/13/RPAH/591) and written informed consent was obtained from all participants in accordance with the 1964 Declaration of Helsinki and its later amendments.
2Results
One hundred and one participants were included in the study with a mean age of 44.4 years (±18.7, range 18–86 years) including 55 women and 46 men. No participants reported headache or became vertiginous during the test. In all cases, the nystagmus we observed (without fixation) was persistent and lasted for the duration of the manoeuvre (sitting up, supine, lateral positions).
In total, 29.7% (n = 30) of subjects had nystagmus in the upright position, and in at least one of the three lying positions, while 40.6% (n = 41) had positional nystagmus only. Only 0.99% (n = 1) of subjects had upright position nystagmus without positional nystagmus, and 28.7% (n = 29) showed no nystagmus in any position. Detailed nystagmus results can be found in Tables 1 and 2.
When separated into three age groups (A:<40 years old, B: 40–60 years old, and C:>60 years old), there was no significant difference in overall nystagmus means between age groups A (1.7°/s) and B (2.6°/s, p = 0.062), and between A and C (1.3°/s, p = 0.266). However, there was a significant difference between age groups B and C (p = 0.008, CI: 0.338 – 0.250).
2.1Spontaneous nystagmus: Sitting position
Spontaneous nystagmus observed while sitting was found in 30.7% of subjects with a mean SPV of 1.1 °/s (±2.1, range 0 – 9.3°/s, median 0.0°/s). Nystagmus was horizontal in 16.7% and vertical in 13.7% (up-beating in 7.8%, down-beating in 5.9%) (Fig. 2, Videos 1–5). Two subjects (ages 25 and 56 years) displayed square-wave jerk eye movements in all positions. Another displayed a persistent, mild torsional nystagmus component in all positions. Comparison of M and nM groups showed no significant difference in spontaneous nystagmus SPV (p = 0.870), prevalence of nystagmus (p = 0.335) or the likelihood of demonstrating high (>5 °/s) vs low (<5 °/s) velocity nystagmus (p = 0.960).
Fig.2
Nystagmus traces in the horizontal and vertical planes in a 29-year-old normal control. In the upright and supine position there is spontaneous up-beating nystagmus, with right-beating nystagmus visible in either lateral position. [H; Horizontal (plane), RBN; Right-beating nystagmus, UBN; Up-beating nystagmus, V; Vertical (plane), °; Degrees, °/s; Degrees per second. Grey bars indicate blinking artefact.].
![Nystagmus traces in the horizontal and vertical planes in a 29-year-old normal control. In the upright and supine position there is spontaneous up-beating nystagmus, with right-beating nystagmus visible in either lateral position. [H; Horizontal (plane), RBN; Right-beating nystagmus, UBN; Up-beating nystagmus, V; Vertical (plane), °; Degrees, °/s; Degrees per second. Grey bars indicate blinking artefact.].](https://content.iospress.com:443/media/ves/2020/30-6/ves-30-6-ves200022/ves-30-ves200022-g002.jpg)
2.2Positional nystagmus
Nystagmus was observed in at least one of the three lying positions in 70.3% of subjects.
2.2.1Supine position
Nystagmus in the supine position was observed in 56.4% of subjects. While supine, nystagmus was horizontal (26.7%), up-beating (20.8%), or down-beating (8.9%). Mean nystagmus SPV while supine was 2.2°/s (±2.9, range 0 – 14.1, median 1.4°/s). Comparison of the M and nM groups showed no significant difference in supine nystagmus SPV (p = 0.883). Eleven subjects displayed persistent positional nystagmus with velocities > 10°/s, however, no vertigo was reported during assessment.
2.3Positional nystagmus: Lateral positions
In at least one lateral position, 63.4% of subjects showed nystagmus, which was horizontal (37.1%), up-beating (6.4%), or down-beating (6.4%). The difference in nystagmus SPV between the right- and left-lateral positions was not significant (p = 0.111). Ten subjects showed persistent apogeotropic nystagmus in either lateral position (one from the M group) while four showed geotropic nystagmus (two from the M group).
In comparison with nystagmus observed in the sitting position, there was a significant enhancement in nystagmus SPV in all three lying positions (supine p < 0.001, CI: 0.543 – 1.655; right lateral p < 0.001, CI: 0.841 – 2.127, left lateral p = 0.002, CI: 0.328 – 1.459).
Subjects with non-stereotyped positional nystagmus patterns in either lateral position (such as nystagmus whilst only one side, or vertical nystagmus while one side and horizontal while on the other) totalled 34.7%. The difference in nystagmus SPV in all lateral positions between the M and nM sub-groups was not significant (p = 0.644 and p = 0.806 for right- and left-lateral positions, respectively). There was no significantly increased likelihood that the M group would display nystagmus of any velocity while in a lateral position (p = 0.213) as compared with the nM group.
2.4Nystagmus comparison between normal controls and vestibular migraine
Using previously published data [37], spontaneous (6.6°/s) and positional (10.3°/s) nystagmus velocities in patients during episodes of vestibular migraine were statistically compared with healthy controls from the current study which showed a significant difference (p < 0.001, CI: 3.828 – 12.503, p < 0.001, CI: 3.943 – 12.656, respectively), with VM patients showing higher nystagmus velocities [37]. There was also a significantly higher likelihood of any visible spontaneous upright (65.7%) or positional (89.6%) nystagmus in the VM group during an episode of vertigo, as compared with normal controls (p < 0.001, CI: 0.120 – 0.447, p = 0.003, CI: 0.006 – 0.338, respectively).
3Discussion
There has been a limited number of publications on nystagmus in normal controls with over 100 subjects, measuring both nystagmus incidence and SPVs, highlighting the importance of renewed in-depth investigation into this phenomenon. From the work of previous investigators, it is understood that healthy normal controls without vestibular or autonomic symptoms may exhibit low velocity nystagmus when examined under fixation-denied conditions, however the reported SPV ranges and spontaneous vs positional occurrence varies widely, possibly due to small study subject numbers.
Spontaneous nystagmus while sitting upright without visual fixation is reported in healthy controls with authors citing occurrences between 20 – 40% [2, 20, 21, 23, 24], while other studies cite fewer occurrences of 5 – 6.6% [11, 12, 32]. The spontaneous nystagmus was reported as horizontal in 0 – 10%, and vertical in 3.4 – 30% [2, 11, 20]. In the studies which measured nystagmus SPVs, the reported means were generally low, with ranges of 0.7 – 5.0°/s [2, 20, 32].
Positional nystagmus in healthy controls is described with occurrence between 22.5 – 88% [2, 11, 12, 20, 21, 23, 24, 30]. Some studies found that horizontal positional nystagmus was the most common pattern [21, 30], while other studies found vertical nystagmus was observed most frequently [2]. Previous studies agree that torsional nystagmus was either exceedingly rare or absent [20, 21]. Geotropic and apogeotropic positional nystagmus was a common finding with reported occurrence between 8 – 32% [21, 23, 24].
Spontaneous vertical nystagmus is described in central disorders including vestibular migraine, demyelination, cerebellar disease and posterior circulation ischaemia [6, 26, 34, 35]. Down-beating nystagmus has been attributed to damaged vertical smooth-pursuit and has been reported in lesions affecting cerebellar pathways including infarction, demyelination, paraneoplastic syndrome, spinocerebellar ataxias, or Chiari Malformation [3, 15, 22, 27, 28]. Episodic Ataxia (Type II) may be associated with interictal spontaneous down-beating nystagmus [15]. Spontaneous up-beating nystagmus is rare and has been described in Wernicke’s encephalopathy [16] and lesions within the paramedian medulla [8]. Lying in the supine position may also provoke or enhance nystagmus generated by Chiari malformation or posterior fossa space occupying lesions [4, 7, 17, 19, 31, 36]. In a study of patients with a diagnosis of cerebellar disease, it was found that 24% showed nystagmus while upright, 64% of which were down-beating and 15% horizontal [9]. Persistent apogeotropic positional nystagmus is reported in lateral canal cupulolithiasis which demonstrates asymmetric slow-phase velocities in either lateral position, unlike what we observed [1]. Medulloblastoma or other lesions within the vestibulocerebellum including the uvula, ventral uvula, nodulus, or tonsil regions are rare central causes of apogeotropic nystagmus [5, 15]. Although vertical positional nystagmus of peripheral origin is most commonly due to posterior canal BPPV, it is easily identified by its pattern of paroxysmal torsional/up-beating nystagmus upon provocative positional testing [33]. Studies have speculated that vertical positional nystagmus in normal controls may be at least partially due to the effect of gravity on the otolith organs [18].
Recent studies indicate that acutely symptomatic patients with vestibular migraine demonstrate low amplitude spontaneous and positional nystagmus, the slow-phase velocities of which overlap with those recorded in normal controls [34, 37]. However, our VM cohort demonstrated ictal spontaneous nystagmus in 65.7% of patients, whereas fewer than half as many (30.4%) of the normal controls in this study demonstrated this. The higher prevalence of nystagmus and the higher SPV in these acutely symptomatic patients indicates that the eye movements recorded in VM did indeed represent the ictus [37].
Our study limitations include subject convenience sampling, which may produce bias and thus the results may not be generalisable to a larger population. No formal recording was made of the subjects’ eye movements with fixation. As no formal gaze-holding, saccades, pursuit, or head impulse testing was conducted on the normal controls, and without the benefit of a full medical history, neurological examination and imaging, there is a chance of the inclusion of normal subjects with sub-clinical central or peripheral vestibular disorders. These limitations should be considered when comparing oculomotor findings in neuro-otology patients to normal subjects, as these study results may overestimate the ‘normal’ range of nystagmus eye movement. Furthermore, substances such as alcohol and nicotine may increase nystagmus, while some medications such as benzodiazepines are known to reduce nystagmus, and were not controlled for [10, 18, 25, 27].
Thus, one third of healthy controls and two thirds of vestibular migraine sufferers show low velocity nystagmus. In the context of the interictal patient, it is important that low velocity nystagmus is interpreted with caution, since it may be unrelated to the underlying pathology. If encountered in an acutely symptomatic patient, it is best to seek a comparison with the interictal state. Rather than seeking a “cut-off” SPV value for pathological nystagmus, it is best that spontaneous nystagmus is interpreted in the context of the clinical syndrome and ictal status.
4Conclusion
Healthy normal controls without a self-reported history of spontaneous vertigo show spontaneous and positional nystagmus when observed without visual fixation. A small number of outliers demonstrate high velocity nystagmus > 10 deg/s. This study highlights the importance of recording inter-ictal nystagmus when interpreting the patterns of ictal nystagmus in patients with episodic vertigo.
Study funding
This research was funded by the Garnett Passe and Rodney Williams Memorial Foundation, and the National Health and Medical Research Council of Australia. These funding sources have no role in any aspect of the study or the decision to submit for publication.
Declaration of conflicts of interest
A Young receives scholarship funding from the University of Sydney, and reports no conflict of interest. M Welgampola receives funding from the Garnett Passe and Rodney Williams Memorial Foundation, and the National Health and Medical Research Council of Australia, and reports no conflict of interest. M D’Souza, S Rosengren and A Bradshaw report no conflict of interest.
Ethical standard
This study received local ethics committee approval (Protocol No X18-0087) and written informed consent was obtained in accordance with the 1964 Declaration of Helsinki and its later amendments.
Author contributions
A Young designed the study, collected and analysed the data, created the original figures, conducted statistical analysis, drafted and approved the final manuscript. S Rosengren edited the manuscript content, assisted with the statistical analysis and approved the final manuscript. A Bradshaw created the original figures, and approved the final manuscript. M D’Souza conducted statistical analysis, edited the manuscript content and approved the final manuscript. M Welgampola edited the manuscript content and approved the final manuscript.
Video 1. Fixation-denied video of a 29-year-old normal control showing mild spontaneous up-beating nystagmus in the upright position and when lying supine, and right-beating nystagmus in either lateral position.
Video 2. Fixation-denied video of a 61-year-old normal control showing no nystagmus in either the upright or supine position but showing apogeotropic positional nystagmus in either lateral position; left-beating nystagmus in the right lateral position, right-beating nystagmus in the left-lateral position.
Video 3. Fixation-denied video of a 55-year-old normal control showing spontaneous down-beating nystagmus in all positions.
Video 4. Fixation-denied video of a 45-year-old normal control showing no spontaneous nystagmus in the upright position, with right-beating nystagmus while supine, and geotropic nystagmus in either lateral position; right-beating nystagmus in the right-lateral position, left-beating nystagmus in the left-lateral position.
Video 5. Fixation-denied video of a 40-year-old normal control showing spontaneous right-beating nystagmus in the upright position, no nystagmus while lying supine, and geotropic nystagmus in either lateral position; right-beating nystagmus in the right-lateral position, left-beating nystagmus in the left-lateral position.
Supplementary material
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/VES-200022.
References
[1] | Argaet E.C. , Bradshaw A.P. and Welgampola M.S. , Benign positional vertigo, its diagnosis, treatment and mimics, Clinical Neurophysiology Practice 4: ((2019) ), 97–111. |
[2] | Bisdorff A. , Sancovic S. , Debatisse D. , Bentley C. , Gresty M. and Bronstein A. , Positional nystagmus in the dark in normal subjects, Neuro-Ophthalmology 24: (1) ((2000) ), 283–290. |
[3] | Bussiere M. , Al-Khotani A. , Steckley J.L. , Nicolle M. and Nicolle D. , Paraneoplastic downbeat nystagmus, Can J Ophthalmol 43: (2) ((2008) ), 243–245. |
[4] | Buttner U. , Helmchen C. and Brandt T. , Diagnostic criteria for central versus peripheral positioning nystagmus and vertigo: A review, Acta Otolaryngol 119: (1) ((1999) ), 1–5. |
[5] | Choi J.-Y. , Glasauer S. , Kim J.H. , Zee D.S. and Kim J.-S. , Characteristics and mechanism of apogeotropic central positional nystagmus, Brain 141: (3) ((2018) ), 762–775. |
[6] | Choi J.H. , Oh E.H. , Park M.G. , Baik S.K. , Cho H.J. , Choi S.Y. , Lee T.H. , Kim J.S. and Choi K.D. , Early mri-negative posterior circulation stroke presenting as acute dizziness, J Neurol 265: (12) ((2018) ), 2993–3000. |
[7] | Choi J.Y. and Kim J.S. , Nystagmus and central vestibular disorders, Curr Opin Neurol 30: (1) ((2017) ), 98–106. |
[8] | Eggers S.D.Z. , Bisdorff A. , Von Brevern M. , Zee D.S. , Kim J.S. , Perez-Fernandez N. , Welgampola M.S. , Della Santina C.C. and Newman-Toker D.E. , Classification of vestibular signs and examination techniques: Nystagmus and nystagmus-like movements: Consensus document of the committee for the international classification of vestibular disorders of the bárány society, Journal of Vestibular Research: Equilibrium and Orientation 29: (2-3) ((2019) ), 57–87 Newman. |
[9] | Feil K. , Strobl R. , Schindler A. , Krafczyk S. , Goldschagg N. , Frenzel C. , Glaser M. , Schoberl F. , Zwergal A. and Strupp M. , What is behind cerebellar vertigo and dizziness?, Cerebellum ((2018) ). |
[10] | Fetter M. , Haslwanter T. , Bork M. and Dichgans J. , New insights into positional alcohol nystagmus using three-dimensional eye-movement analysis, Ann Neurol 45: (2) ((1999) ), 216–223. |
[11] | Geisler C. , Bergenius J. and Brantberg K. , Nystagmus findings in healthy subjects examined with infrared videonystagmoscopy, ORL J Otorhinolaryngol Relat Spec 62: (5) ((2000) ), 266–269. |
[12] | Hajioff D. , Barr-Hamilton R. , Colledge N. , Lewis S. and Wilson J. , Re-evaluation of normative electronystagmography data in healthy ageing, Clinical Otolaryngology & Allied Sciences 25: (4) ((2000) ), 249–252. |
[13] | Headache classification committee of the international headache society (ihs) the international classification of headache disorders, 3rd edition, Cephalalgia 38: (1) ((2018) ), 1–211. |
[14] | Hirai C. , Yamamoto Y. , Takeda T. , Tasaki A. , Inaba Y. , Kiyokawa Y. , Suzuki Y. and Tsutsumi T. , Nystagmus at the onset of vertiginous attack in meniere’s disease, Otol Neurotol 38: (1) ((2017) ), 110–113. |
[15] | Ilg W. , Branscheidt M. , Butala A. , Celnik P. , De Paola L. , Horak F.B. , Schols L. , Teive H.a.G. , Vogel A.P. , Zee D.S. and Timmann D. , Consensus paper: Neurophysiological assessments of ataxias in daily practice, Cerebellum ((2018) ). |
[16] | Kattah J.C. , Tehrani A.S. , Du Lac S. , Newman-Toker D.E. and Zee D.S. , Conversion of upbeat to downbeat nystagmus in wernicke encephalopathy, Neurology 91: (17) ((2018) ), 790–796 Newman. |
[17] | Kim J.-S. and Zee D.S. , Benign paroxysmal positional vertigo, New England Journal of Medicine 370: (12) ((2014) ), 1138–1147. |
[18] | Kim J. , Somers J. , Stahl J. , Bhidayasiri R. and Leigh R. , Vertical nystagmus in normal subjects: Effects of head position, nicotine and scopolamine, Journal of Vestibular Research 10: (6) ((2000) ), 291–300. |
[19] | Lea J. , Lechner C. , Halmagyi G.M. and Welgampola M.S. , Not so benign positional vertigo: Paroxysmal downbeat nystagmus from a superior cerebellar peduncle neoplasm, Otology & neurotology 35: (6) ((2014) ), e204–e205. |
[20] | Levo H. , Aalto H. and Petteri T. , Hirvonen, Nystagmus measured with video-oculography: Methodological aspects and normative data, ORL J Otorhinolaryngol Relat Spec 66: (3) ((2004) ), 101–104. |
[21] | Martens C. , Goplen F.K. , Nordfalk K.F. , Aasen T. and Nordahl S.H.G. , Prevalence and characteristics of positional nystagmus in normal subjects, Otolaryngology–Head and Neck Surgery ((2016) ), 0194599816629640. |
[22] | Marti S. , Bockisch C.J. and Straumann D. , Prolonged asymmetric smooth-pursuit stimulation leads to downbeat nystagmus in healthy human subjects, Invest Ophthalmol Vis Sci 46: (1) ((2005) ), 143–149. |
[23] | Mcauley J.R. , Dickman J.D. , Mustain W. and Anand V.K. , Positional nystagmus in asymptomatic human subjects, Otolaryngology–Head and Neck Surgery 114: (4) ((1996) ), 545–553. |
[24] | Mulch G. and Lewitzki W. , Spontaneous and positional nystagmus in healthy persons demonstrated only by electronystagmography: Physiological spontaneous nystagmus or “functional scar”?, Arch Otorhinolaryngol 215: (2) ((1977) ), 135–145. |
[25] | Romano F. , Tarnutzer A.A. , Straumann D. , Ramat S. and Bertolini G. , Gaze-evoked nystagmus induced by alcohol intoxication, J Physiol 595: (6) ((2017) ), 2161–2173. |
[26] | Serra A. , Chisari C.G. , Matta M. , Eye movement abnormalities in multiple sclerosis: Pathogenesis, modeling, and treatment, Frontiers in neurology 9: (2018), 31. |
[27] | Strupp M. , Kremmyda O. , Adamczyk C. , Bottcher N. , Muth C. , Yip C.W. and Bremova T. , Central ocular motor disorders, including gaze palsy and nystagmus,S, J Neurol 261: (Suppl 2) ((2014) ), 542–558. |
[28] | Strupp M. , Schüler O. , Krafczyk S. , Jahn K. , Schautzer F. , Büttner U. and Brandt T. , Treatment of downbeat nystagmus with 3,4-diaminopyridine: A placebo-controlled study, Neurology 61: (2) ((2003) ), 165–170. |
[29] | Strupp M. , Zwergal A. and Brandt T. , Episodic ataxia type 2, Neurotherapeutics 4: (2) ((2007) ), 267–273. |
[30] | Sunami K. , Tochino R. , Zushi T. , Yamamoto H. , Tokuhara Y. , Iguchi H. , Takayama M. , Konishi K. and Yamane H. , Positional and positioning nystagmus in healthy subjects under videonystagmoscopy, Acta Otolaryngol Suppl(554) ((2004) ), 35–37. |
[31] | Taylor R.L. , Chen L. , Lechner C. , Aw S.T. and Welgampola M.S. , Vestibular schwannoma mimicking horizontal cupulolithiasis, Journal of Clinical Neuroscience 20: (8) ((2013) ), 1170–1173. |
[32] | Van Der Stappen A. , Computerized electronystagmography: Normative data revisited, Acta Otolaryngol 120: (6) ((2000) ), 724–730. |
[33] | Von Brevern M. , Bertholon P. , Brandt T. , Fife T. , Imai T. , Nuti D. and Newman-Toker D. , Benign paroxysmal positional vertigo: Diagnostic criteria, J Vestib Res 25: (3-4) ((2015) ), 105–117 Newman. |
[34] | Von Brevern M. , Zeise D. , Neuhauser H. , Clarke A.H. and Lempert T. , Acute migrainous vertigo: Clinical and oculographic findings, Brain 128: (2) ((2005) ), 365–374. |
[35] | Wagner J.N. , Glaser M. , Brandt T. and Strupp M. , Downbeat nystagmus: Aetiology and comorbidity in 117 patients, Journal of Neurology, Neurosurgery & Psychiatry 79: (6) ((2008) ), 672–677. |
[36] | Williams L.G. , Brimage P. , Lechner C. , Taylor R.L. , Masters L. and Welgampola M.S. , Lhermitte–duclos disease presenting with atypical positional nystagmus, Journal of Clinical Neuroscience 21: (9) ((2014) ), 1647–1649. |
[37] | Young A.S. , Lechner C. , Bradshaw A.P. , Macdougall H.G. , Black D.A. , Halmagyi G.M. and Welgampola M.S. , Capturing acute vertigo: A vestibular event monitor, Neurology 92: (24) ((2019) ), e2743–e2753. |




