Vestibular Oriented Research Meeting, February 16 – 17, 2021
Podium Abstracts
Abstract 1An Experimental and Computational Study of Recovery Nystagmus
Jacob M Pogson, Dale C Roberts, Jorge Otero-Millan, David S Zee, Bryan K Ward
Neurology and Otolaryngology, School of Medicine, The Johns Hopkins University
Reversal of spontaneous nystagmus direction—recovery nystagmus (RN)—occurs after vertigo attacks in Meniere’s Disease (MD) (McClure, 1978; 1981; Young, et al. 2019), which suggests that it may serve as a diagnostic marker for MD (Young, et al. 2019). RN is thought to reflect static vestibular adaptation as reported before e.g., to sustained caloric and rotatory chair stimuli and is distinct from post-rotatory nystagmus (Young & Oman, 1970; Malcom & Jones, 1970; Jareonsettasin, et al. 2016). Two related and unresolved questions are the locations of vestibular adaptation in MD attacks and how these cause RN. Resolving these questions may be useful to understand the causes of vertigo attacks in MD.
Long-duration magnetic vestibular stimulation (MVS) allows the time-course of vestibular adaptation to be studied by artificially inducing a sustained vestibular asymmetry that mimics transient changes in peripheral vestibular activity. MVS is thought to generate a constant fluid force on both lateral canal cupula, equivalent to a constant acceleration that activates the vestibulo-ocular reflex (VOR) pathway (Roberts, et al. 2011). Vestibular adaptation can be measured through changes in the velocity of the primary ‘per’ VOR nystagmus and the presence of a secondary ‘post’ response (Jareonsettasin, et al. 2016). We studied a mimic of RN to MVS in normal subjects and built a linear control system model.
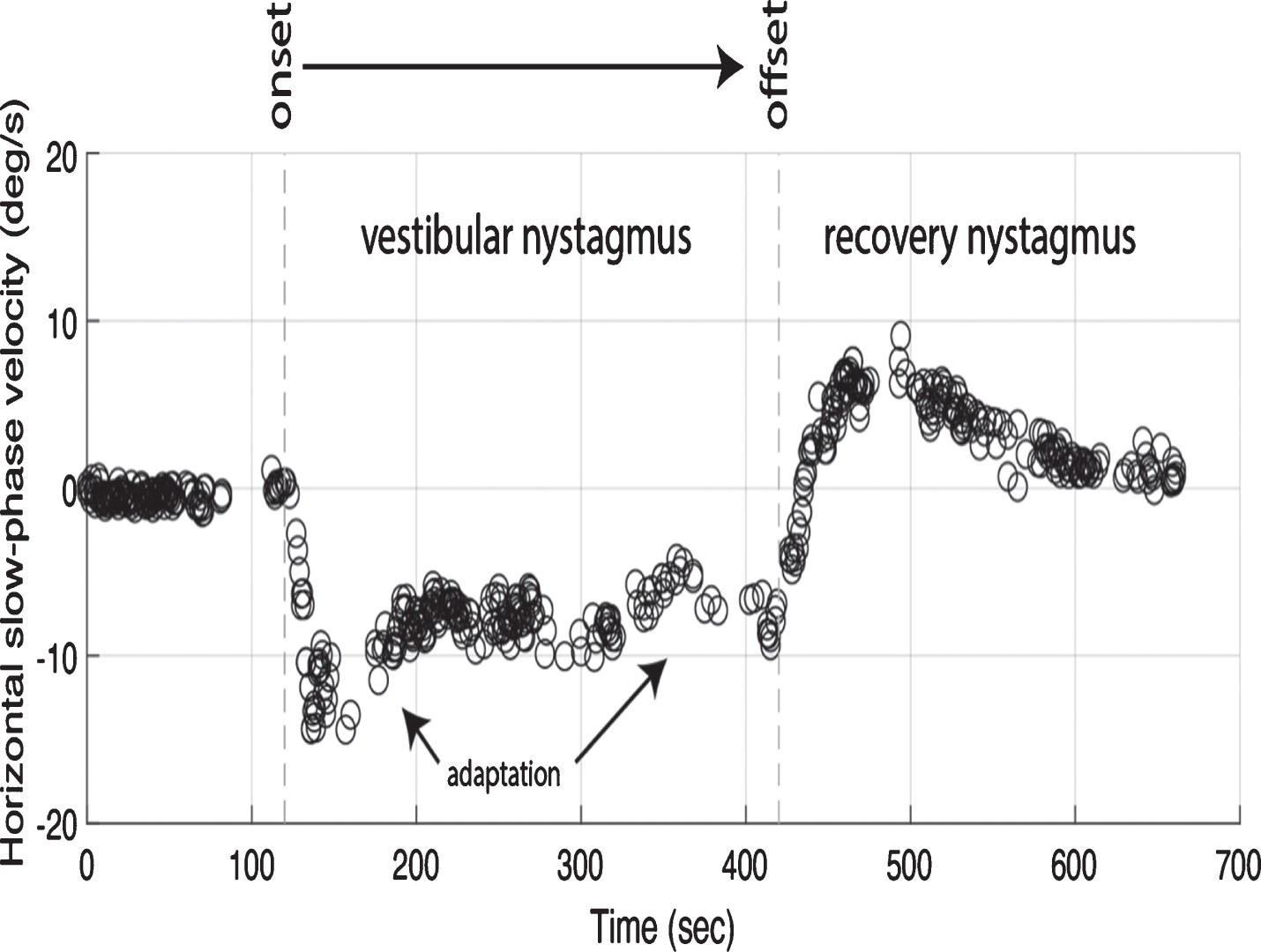
Five healthy participants underwent binocular three-dimensional 100 Hz video-oculography during five minutes of 7 Tesla MVS while lying in the supine position. Pre-exposure horizontal nystagmus slow-phase velocity (SPV) was mean(SE) 1.0(0.3) deg/sec (Figure 1). Per-exposure SPV showed a stimulus onset to peak latency of 69(28.2) seconds and amplitude of 15(5.2) deg/s, followed by a decay to a more steady-state of 9(2.0) deg/s over 105(37.4) seconds. Leaving the magnet, post-exposure nystagmus showed a rapid direction reversal to a peak SPV amplitude of -7(2.6) deg/s at a latency of 42(2.0) seconds, also followed by a decay to baseline (<2 deg/s) over (182(35.6) seconds). Non-parametric tests found that nystagmus time-course dynamics (latency and amplitude) were similar (p > 0.1) between per- and post-exposure, and that the per-exposure SPV was larger (p < 0.01) than the pre- and post- baseline nystagmus.
A model with parameters from primates (Goldberg & Fernandez, 1971; Merfeld, 1995), using canal, afferent, as well as central velocity storage (Laurens & Angelaki, 2011) with adaptation time-constants (Jareonsettasin, et al. 2016), showed a qualitatively similar time-course for both per- and post- stimulus. Because subjects in MVS are required to lay in the supine position, we also found that the addition of a cerebellar rotational feedback component (which combines canal and otolith signals, Laurens & Angelaki, 2011) in the model suppressed SPV amplitudes in the supine position compared to the upright position.
In conclusion, we found experimentally that MVS can mimic RN and that mathematical models of MVS RN are consistent with longer centrally mediated adaptation that dominates the constant MVS response to trigger RN.
Abstract 2The Gait Disorientation Test: A Novel Screening Test for Vestibular Dysfunction
Colin R. Grove, PT, DPT, MS, NCS1, Bryan C. Heiderscheit, PT, PhD, FAPTA2, G Mark Pyle, MD2, Susan L. Whitney, DPT, PhD, NCS, FAPTA3
1University of Wisconsin Hospital and Clinics Verona, WI
2University of Wisconsin-Madison Madison, WI
3University of Pittsburgh Pittsburgh, PA
Vestibular hypofunction may affect tens of millions of adults in Europe and the United States. Screening persons suspected to have vestibular loss is an important step in determining whether a diagnostic workup, referral to specialists, and/or vestibular rehabilitation is/are needed. Barriers to effective screening include the need for specialized training to administer and interpret tests; the cost, size, and complexity of related equipment; and/or suboptimal discriminative validity of available tests. Therefore, the purpose of this study was to develop a novel screening method for vestibular dysfunction. We hypothesized that performance of walking under challenging sensory conditions would discriminate vestibular-impaired from healthy adults. Seventeen healthy adults (39.3 ± 11.2 years old, 13 female) and 17 adults with laboratory-confirmed peripheral vestibular hypofunction (61.1 ± 10.2 years, 9 female) participated. In this prospective study, participants underwent the horizontal and vertical non-instrumented dynamic visual acuity test (NI-DVAT); the Five-times Sit-to-stand Test (FTSTST); next-generation Sensory Organization Test (NG-SOT); and the Functional Gait Assessment (FGA). The Gait Disorientation Test (GDT) was developed using FGA1 (walking, eyes open) and FGA8 (walking, eyes closed). We calculated the GDT result by subtracting the time needed to complete FGA1 from the time needed to complete FGA8. We assessed the criterion validity of these tests using the area under the curve (AUC) from receiver operator characteristic analyses and computed the sensitivity and specificity (95% confidence interval [CI]). The AUCs (95% CI; threshold) were as follows: horizontal NI-DVAT = 0.83 (0.69, 0.97; 3 line change), vertical NI-DVAT = 0.86 (0.73, 0.99; 3 line change), FTSTST = 0.85 (0.72, 0.97; 8.7 s), NG-SOT composite score = 0.93 (0.86, 1; 75.7), FGA total score = 0.95 (0.87, 1; 28.5), and GDT = 0.91 (0.82, 0.99; 4.5 s). The sensitivity and specificity (95% CI) of the GDT were 82% (57%, 96%). Analyzing the GDT result in parallel with using a threshold of 8 seconds for its walking eyes closed component provided 98% sensitivity and 81% specificity. The GDT is a straightforward, low-tech, low-cost tool that providers may use to identify vestibular-impaired adults. The discriminative ability of the GDT exceeds or is comparable to tests that require specialized training, complex and expensive equipment, and/or more time to perform.
Acknowledgement: The project described was supported by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grants UL1TR000427 and TL1TR002375. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abstract 3Are differing symptoms sets site specific in the vestibular patient
A.I. Mallinson, N.S. Longridge
Division of Otolaryngology, Faculty of Medicine, University of British Columbia; Neuro-otology unit, Vancouver General Hospital, Vancouver, BC, Canada
Patients with so-called “traditional” complaints of dizziness (i.e. true vertigo) often have accompanying nausea and imbalance. Complaints of nausea, as we know can at times be debilitating (i.e. symptoms of a vestibular deficit predominating over signs).
Many patients have non traditional complaints which are still of vestibular origin. It is understood that the silent vestibular system can also produce an autonomic symptom set which can override clinical signs. The motion sick individual can attest to the reality of this symptom set. In some patients symptoms of nausea are the sole complaint. In brief, vestibular disfunction sometimes generates symptoms predominantly or even solely. The de novo development or worsening of motion sickness, either as carsickness or as the development of the symptom set which is now referred to as PPPD, is strongly suggestive of the development of vestibular pathology. Sometimes these patients with “symptoms only” have concerns that they will be dismissed as psychiatric, as they appear healthy.
When the “inner reference” task of the vestibular system, (i.e. constantly sensing earth vertical) is not performed normally some of these patients’ symptoms are constant and although they often improve to some level, they often subsequently “plateau” and are left with subtle complaints of either imbalance or nausea.
We wondered why we see two different groups of patients in the clinical setting The crucial otolithic role in the production of motion sickness on land was originally outlined by Preber. Complaints of movement and nausea (often lumped under the category of “space motion sickness”) are also suffered by returning astronauts. It was emphasized by Parker (1998) that the otoliths also play a major role in the development of this. It has also been suggested to be partly attributable to otolith asymmetry or to a canal/otolith conflict. However it has also been shown that this conflict was responsible for postural instability and disorientation in astronauts after landing.
These patients, often referred to in the literature as otolithic patients, often have abnormal otolithic tests, and in one study we carried out, 100% of patients with nausea predominating had an otolithic abnormality, and in all but one patient, it was the only diagnostic abnormality found. This confirmed to us that complaints of nausea, in the absence of true spinning vertigo are of otolithic origin.
What are the differences between these two groups of patients. We report on 50 patients seen sequentially complaining of either nausea only or imbalance only. We assessed all patients with a detailed history, along with a full set of diagnostic tests. We analyzed all data and compared results between two groups we delineated; “nausea only” and “imbalance only”, in an attempt to try and elucidate any difference between these two groups of patients; both of whom by history have complaints suggesting otolithic pathology.
Abstract 4Visual and vestibular deficits are associated with lower functional mobility and higher symptom burden in adults with persistent symptoms after a mild traumatic brain injury
Linda D’Silva PT, PhD1, Prabhakar Chalise, PhD2, Michael Rippee, MD3, Hannes Devos, PT1
1Department of Physical Therapy and Rehabilitation Science
2Department of Biostatistics and Data Science
3Department of Neurology
1,2,3 University of Kansas Medical Center, Kansas City, KS.
Background: Oculomotor and vestibular impairments are common after a mild traumatic brain injury (mTBI);1-3 however, the impact of these deficits on mobility and symptom severity in older adults with persistent symptoms is unclear.
Purpose: To examine the impact of visual-vestibular abnormalities on functional mobility, post-concussion symptom severity, perception of handicap due to dizziness and complaints of mental fatigue in adults between 40 to 75 years of age with persistent symptoms (3 months to 2 years post-injury) after mTBI.
Outcomes: Oculomotor deficits examined included smooth pursuit and saccade abnormalities (categorical variables), depth perception, near-point convergence, static visual acuity (SVA) and processing time; vestibular deficits were assessed by the head thrust test (categorical variable), and dynamic visual acuity loss in the pitch and yaw planes using the Bertec Vision Advantage.™ Functional mobility was assessed by the Functional Gait Assessment (FGA),4 and symptoms measured by the Post-Concussion Symptom Scale (PCSS), Dizziness Handicap Inventory (DHI), and Mental Fatigue Scale (MFS). Correlations between variables were examined by Spearman’s correlations. The FGA was log transformed before performing stepwise multivariable linear regression because it did not follow the normal distribution.
Results: Twenty- three individuals (mean age 55.7 ± 1.9 years; 19 females and 4 males), mean duration since injury 33.23 ± 5.1 weeks (range: 12-92 weeks) completed the study. FGA performance [mean 21.04 ± 1.3 (range: 7-29)], and PCSS scores [(mean score 61.59 ± 6.4; range (9-110)] indicated large variation in sample characteristics. DHI scores (mean 50.43 ± 5.3) and MFS scores (mean 20.3 ± 1.7) reflected substantial symptoms of dizziness and fatigue. FGA performance was correlated with PCSS (r=-0.50, p=0.03), specifically the somatic (r=-0.53, p=0.01) and cognitive (r=-0.46, p=0.03) symptoms on the PCSS. Smooth pursuit (β=-0.45, p=0.02) and SVA (β= -0.4, p=0.03) explained 40% of the variance in FGA performance. Smooth pursuit abnormalities (β=0.50, p=0.01) and positive head thrust (β=0.40, p=0.05) explained 38% of the variance in the PCSS scores. FGA performance was correlated with the DHI score (r=-0.60, p=0.004). Impaired saccades explained 21% of the variance in DHI scores. Although, mental fatigue scale score were not correlated with the FGA (r=-0.33, p=0.12), MFS score were correlated with the PCSS (r=0.70, p<0.001) and DHI (r=0.80, p<0.001), indicating higher symptom burden is correlated with higher mental fatigue.
Conclusions: Oculomotor and vestibular deficits were associated with poorer performance on mobility tasks, higher severity of post-concussion symptoms, and higher perception of handicap due to dizziness, in the adult population, months to years after mTBI. Higher symptom burden was associated with higher mental fatigue. A combination of these factors has the potential to reduce activity and participation levels. Future studies examining the effect of targeted visual and vestibular exercise programs on improving mobility and reducing symptom burden are necessary.
Acknowledgements: Funded by the Physical Therapy and Rehabilitation Science Department at the University of Kansas Medical Center.
1. Wright WG, Tierney RT, McDevitt J. Visual-vestibular processing deficits in mild traumatic brain injury. Journal of vestibular research : equilibrium & orientation. 2017;27(1):27-37.
2. Skóra W, Stanczyk R, Pajor A, Jozefowicz-Korczynska M. Vestibular system dysfunction in patients after mild traumatic brain injury. Ann Agric Environ Med. 2018;25(4):665-668.
3. King JE, Pape MM, Kodosky PN. Vestibular Test Patterns in the NICoE Intensive Outpatient Program Patient Population. Military medicine. 2018;183(suppl_1):237-244.
4. Wrisley DM, Marchetti GF, Kuharsky DK, Whitney SL. Reliability, internal consistency, and validity of data obtained with the functional gait assessment. Physical therapy. 2004;84(10):906-918.
Keynote Address 1Quick clinical update on the treatment of vestibular disorders and ataxia
Michael Strupp
Department of Neurology, LMU, Munich, Germany
We provide a “flash light update” on the treatment of peripheral and central vestibular disorders, including cerebellar ataxia.
Peripheral vestibular disorders
Benign paroxysmal positional vertigo (BPPV)
Liberatory maneuvers. Based on our biophysical model of BPPV1, we demonstrated in a prospective randomized three-nation trial that the new “SemontPLUS maneuver” (SM+) is more effective than the “SM”. (https://n.neurology.org/content/94/15_Supplement/2338). Vitamin D. In our recently completed prospective study in 680 patients with BPPV, other vestibular and other neurological disorders, we could not support the hypothesis of a specific vitamin D deficit in patients with BPPV.
Pharmacotherapy of acute unilateral vestibulopathy (AUV)
Corticosteroids. A trial shows that the earlier the treatment the better the outcome2.
Betahistine. In an animal model of AUV, it was demonstrated that the activation of the H1 receptor is relevant for betahistine’s benefits in central vestibular compensation3.
Menière’s disease (MD)
Betahistine. There is some evidence that higher dosages of betahistine are effective for prophylactic treatment of MD. The combination of betahistine with the MAO inhibitor selegiline evidently increases its efficacy. The most likely mode of action is the activation of the H1 receptor, which is also found in the human inner ear, and which leads to increased membrane permeability.
Vestibular paroxysmia (VP)
In an RCT, it was demonstrated that oxcarbazepine significantly reduces the number of attacks in VP4; however the drop-out rate was 60%. Lacosamide is also effective and well tolerated5.
Central vestibular disorders and cerebellar ataxia Vestibular migraine (VM)
In an RCT, we found that metoprolol (95 mg/d) was not superior to placebo for the prophylactic treatment of VM6.
Episodic ataxia type 2
In an RCT, we demonstrated that fampridine and acetazolamide are both effective and that fampridine has fewer side effects7.
Cerebellar ataxia (CA)
A new promising drug for the treatment of certain types of CA, namely lysosomal storage diseases, is the modified amino acid acetyl-L-leucine, as we demonstrated in animal models and clinical studies8-10.
References
1. Obrist D, Nienhaus A, Zamaro E, Kalla R, Mantokoudis G, Strupp M. Determinants for a Successful Semont Maneuver: An In vitro Study with a Semicircular Canal Model. Front Neurol 2016;7:150.
2. Sjogren J, Magnusson M, Tjernstrom F, Karlberg M. Steroids for Acute Vestibular Neuronitis-the Earlier the Treatment, the Better the Outcome? Otol Neurotol 2019;40:372-374.
3. Chen ZP, Zhang XY, Peng SY et al. Histamine H1 Receptor Contributes to Vestibular Compensation. J Neurosci 2019;39:420-433.
4. Bayer O, Bremova T, Strupp M, Hufner K. A randomized double-blind, placebo-controlled, cross-over trial (Vestparoxy) of the treatment of vestibular paroxysmia with oxcarbazepine. J Neurol 2018;265:291-298.
5. Strupp M, Elger C, Goldschagg N. Treatment of vestibular paroxysmia with lacosamide. Neurol Clin Pract 2019;9:539-541.
6. Strupp M, Bayer O, Feil K, Straube A. Prophylactic treatment of migraine with and without aura with acetyl-DL-leucine: a case series. J Neurol 2019;266:525-529.
7. Muth C, Teufel J, Schoels L et al. Fampridine and acetazolamide in EA2 and related familial EA: a prospective randomized placebo-controlled cross-over trial. Neurology Clinical Practise. In press.
8. Kaya E, Smith DA, Smith C, Boland B, Strupp M, Platt FM. Beneficial Effects of Acetyl-DL-Leucine (ADLL) in a Mouse Model of Sandhoff Disease. J Clin Med 2020;9(4).
9. Bremova-Ertl T, Platt F, Strupp M. Sandhoff Disease: Improvement of Gait by Acetyl-DL-Leucine: A Case Report. Neuropediatrics 2020.
10. Kaya E, Smith DA, Smith C et al. Acetyl-Leucine slows disease progression in lysososmal storage diseases. Brain Communications. In press.
Abstract 5Restoring vestibular afferent dynamics improves accuracy of prosthesis-evoked vestibulo-ocular reflex (VOR) responses
Kantapon Pum Wiboonsaksakul1, Dale C Roberts2, Charles C Della Santina2, Kathleen E Cullen1
1Department of Biomedical Engineering, Johns Hopkins University
2Department of Otolaryngology-Head and Neck Surgery, Johns Hopkins University School of Medicine
An exciting and emerging approach to treat patients with impaired vestibular function is a prosthesis that senses head rotation and transforms this movement into vestibular afferent stimulation, substituting for the damaged periphery. Early results from clinical trials, while encouraging, have shown only partial functional improvement. Bridging a gap between basic science knowledge and clinical applications, we implemented biomimetic dynamics in vestibular prostheses for the first time. We asked whether representing the natural dynamics of vestibular afferents in the mapping between head motion and afferent stimulation would result in better performance. To test this proposal, we compared vestibulo-ocular reflex (VOR) responses evoked by the static mapping used by all current devices (no dynamics) to those evoked by 4 newly implemented mappings representing and exceeding the characteristic high-pass dynamics of vestibular afferent processing. Testing was done in two monkeys with profound bilateral vestibular loss that had been implanted with a prosthesis. VOR eye movements were first quantified in response to sinusoidal stimulation that spanned the natural frequency range (0.2 – 20 Hz). We found that afferent-like high-pass mappings evoked more robust VORs with more precise timing. In contrast, the standard static mapping showed a gain decline and increasingly sluggish timing with increasing frequency. Furthermore, mappings with high-pass dynamics exceeding natural range, produced an undesirable phase advance. VOR eye movements were also quantified in response to transient stimulation and similar trends were observed. Overall, using a mapping that mimicked the afferent subclass known to provide primary contribution to the VOR yielded optimal performance. This suggests that endogenous afferent dynamics are well matched to produce accurate VOR response and advocates for a more biomimetic prosthesis design. Together, these results confirm that the implementation of biomimetic mappings in vestibular prostheses can optimize functional outcomes for patients.
Abstract 6New findings on vestibular impairment in healthy community volunteers and virologically-controlled HIV+ female subjects
Cohen, Helen S1, Plankey, Michael W2, Sangi-Haghpeykar, Haleh3
1Dept of Otolaryngology – Head and Neck Surgery, Baylor College of Medicine, Houston, TX, USA
2Department of Medicine, Georgetown University Medical Center, Washington, DC, USA
3Dept of Obstetrics and Gynecology, Baylor College of Medicine
The common wisdom is that young adults rarely develop vestibular disorders and HIV causes vestibular disorders. The evidence to support those assertions, however, is weak. We tested 284 healthy male and female control subjects who were volunteers from the general community and we tested a sample of 63 female HIV+ female subjects and 22 female HIV- subjects who were being followed in a longitudinal study of HIV disease. Subjects were tested on the standard clinical battery of objective diagnostic tests. Among healthy controls, we found evidence of vestibular impairments in all age decades. Thus, independent of age, all adults who complain of dizziness and imbalance should be assessed for vestibular disorders. HIV+ subjects, who were taking antiretroviral medication, did not differ significantly from HIV- controls. These data suggest that HIV, itself, is not associated with vestibular impairment, in people with good virologic control.
Supported by: NIH grant R01- DC009031.
Abstract 7Identification of a genetic variant underlying familial cases of recurrent benign paroxysmal positional vertigo
Yinfang Xu1*, Yan Zhang1*, Ivan A. Lopez2, Jacey Hilbers1, Anthony J. Griswold4, Akira Ishiyama2, Susan Blanton3,4, Xue Zhong Liu3,4, and Yunxia Wang Lundberg1
1Vestibular Genetics Laboratory, Boys Town National Research Hospital, Omaha, NE 68131, USA
2Department of Head and Neck Surgery, “David Geffen” School of Medicine at the University of California at Los Angeles, CA 90095, USA
3Department of Otolaryngology, the University of Miami Miller School of Medicine, Miami, FL 33136, USA
4Dr. John T. Macdonald Foundation, Department of Human Genetics and John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, Miami, FL
*These authors contributed equally to the work
Benign paroxysmal positional vertigo (BPPV) is the most common cause of vertigo in humans, yet the molecular etiology is completely unknown. Evidence suggests that genetic factors may play an important role in some cases of idiopathic BPPV, particularly in familial cases, but the responsible genetic variants have not been identified. In this study, we performed whole exome sequencing [including untranslated regions (UTRs)] of 12 families and Sanger sequencing of additional 30 families with recurrent BPPV in Caucasians from the United States (US) Midwest region, to identify the genetic variants responsible for heightened susceptibility to BPPV. Fifty non-BPPV families were included as controls. In silico and experimental analyses of candidate variants show that an insertion variant rs113784532 (frameshift causing truncation) in the neural cadherin gene PCDHGA10 (protocadherin-gamma 10) is an exceedingly strong candidate (p=1.2x10-4 vs. sample controls; p=1.5x10-100 vs. ExAC data; p= 4.9x10-4 vs. NHLBI exome data). The mutant protein forms large aggregates in BPPV samples even at young ages, and affected subjects carrying this variant have an earlier onset of the condition than those without (average 44.0 versus 54.4 years old, p=0.054). In both human and mouse inner ear tissues, PCDHGA10 is expressed in the ganglia, hair cells and the vestibular transitional epithelia. Fluorescent RNA in situ hybridization using mouse inner ear tissues shows that expression increases with age. In summary, our data show that a variant in the PCDHGA10 gene may be involved in causing or aggravating some familial cases of recurrent BPPV.
Acknowledgements: Part of the work was supported by grants from the National Institute on Deafness and Other Communication Disorders (NIDCD): K18DC014748 to YWL, and U24DC015910 to IAL and AI.
Abstract 8Deficits in Standing Balance Control in mTBI Subjects with Chronic Balance Complaints
Robert J. Peterka, Ph.D.1, Kody R. Campbell, Ph.D.2, Lucy Parrington, Ph.D.2, Laurie A. King, Ph.D.2
1Oregon Health & Science University and VA Portland Health Care System, Portland OR
2Oregon Health & Science University, Portland OR
Dizziness and balance complaints are common following a mild Traumatic Brain Injury (mTBI). While most recover over time, dizziness symptoms persist in about 28% of people with mTBI (Theadom et al. 2016). We used a Central SensoriMotor Integration (CSMI) test paradigm (Peterka et al. 2018) to evaluate sensory integration and motor activation characteristics of the balance control system in 51 mTBI subjects with unresolved, self-reported balance complaints at least 3 months post-injury in comparison to results from 58 control subjects. The CSMI test derives parameters of a closed-loop feedback control model of the balance control system that account for experimentally measured anterior-posterior center-of-mass body sway, which is evoked by low-amplitude (2° peak-to-peak) pseudorandom rotations of the stance surface and/or visual surround. The parameters include ‘sensory weights’ that represent the relative contributions of proprioceptive, visual, and vestibular cues to balance control and ‘motor activation’ factors that quantify the generation of corrective ankle torque. During eyes-closed surface-tilt CSMI tests, balance relies on orientation information derived from vestibular inputs and proprioception. Experimental results showed essentially identical reliance on vestibular cues among mTBI and control subjects (mean vestibular weight 0.48 ± 0.07SD for mTBI, N=48 with 3 unable to complete eyes-closed testing, compared to 0.49 ± 0.08SD for controls, N=58). Motor activation is represented by two parameters: a ‘stiffness factor’ and ‘damping factor’ that determine how much ankle torque is generated per unit of body sway angle and angular velocity, respectively, derived from a weighted combination of orientation information from contributing sensory systems. In contrast to the indistinguishable sensory contributions to balance, the motor activation components were reduced in mTBI compared to control subjects (6% reduction in the stiffness factor and 7% reduction in the damping factor). Contributing to these average reductions, there appeared to be a notable subset of 12 of 48 (25%) mTBI subjects with lower stiffness factor values and 4 of 48 (8%) with lower damping factor values than all but one of the control subjects. Functionally, low motor activation, and particularly low stiffness, results in a sloppy, under-controlled balance system where large body sways can be evoked by relatively small perturbations. In contrast, preliminary results from an ongoing study applying CSMI methods to subjects with more acute mTBI identified a lower percentage of subjects with low stiffness 5 of 68 (7%) or damping 1 of 68 (1%) suggesting that low motor activation may be a maladaptive compensation that develops over a period of months.
References
[1] Peterka et al. (2018). Frontiers in Neurology 9:1045.
[2] Theadom et al. (2016). Br J Gen Pract 66(642).
Acknowledgements: Supported by the Assistant Secretary of Defense for Health Affairs under Awards W81XWH-15-1-0620 and W81XWH-17-1-0424.
Keynote Address 2Visual control of Postural Balance
Prof Adolfo M. Bronstein MD PhD
Imperial College London
In order to isolate the visual contribution to the control of postural balance, experiments in which subjects are exposed to large-field visual motion (optokinetic) stimuli will be reviewed. In these situations, at motion onset, the visual stimulus signals subject self-motion but inertial (vestibulo-proprioceptive) cues do not. Visually evoked postural responses (VEPR) thus induced can be quickly suppressed by cognitive status or simple repetition of the stimulus, provided alternative (non visual) and reliable self-motion cues are available. I will present a conceptual model of the visual control of balance in which the process of assessing the reliability and visuo-vestibular matching is carried out by a General comparator able to control the gain of the visuo-postural system. Complexity and congruency in the visual stimulus itself are assessed by a Visual comparator, e.g. the presence of motion parallax in the visual stimulus can reverse the sway response direction. VEPR can also be re-oriented according to the position of the eyes in the head and the head on the trunk. This indicates that ocular and cervical proprioceptors must also access the Gain control mechanism so that visual stimuli can recruit and silence different postural muscles appropriately. The overall gain of the visuo-postural system is also influenced by less easily defined idiosyncratic factors, such as visual dependence and psychological traits; interestingly both these factors have been found to be associated with poor long term outcome in vestibular disorders. The experimental results and model will hopefully convince you that the visuo-postural system is a wonderful example of interaction between physics (e.g. stimuli geometry, body dynamics) and the border zone between neurology and psycho-somatic medicine. (Reference: Prog Brain Res. 2019;248:285-302. doi: 10.1016/bs.pbr.2019.04.023.)
Abstract 9Noisy Galvanic Vestibular Stimulation Improves Visual Perceptual Thresholds
Jamie Voros, Rachel Rise, Sage Sherman, Allison P. Anderson, PhD, and Torin K. Clark, PhD
Bioastronautics Laboratory, University of Colorado, Boulder, CO
Sensory noise is often considered to be a limiting factor for perceptual precision. While counterintuitive, stochastic resonance (SR) is a mechanism by which adding noise to a sensory channel can improve perception and information throughput (1). SR and the resulting benefits have been shown to exist within the vestibular perceptual channels. For example, applying low levels of noisy galvanic vestibular stimulation (nGVS) at each subject’s ‘optimal’ level reduced postural sway compared to a preceding sham presentation (2). Other studies have found vestibular perceptual thresholds (i.e., how small of a motion can be reliably perceived) were improved with an individualized level of nGVS (3). In fact, by measuring vestibular perceptual thresholds with a range of varying nGVS levels, Galvan-Garza and colleagues were able to show a characteristic SR curve’s improvement as nGVS level was increased, followed by a return to the sham threshold performance level when too much nGVS was applied (4). Each of these studies suggests “in channel” SR exists for the vestibular sensory channel. Other studies, have suggested the presence of “cross modal” SR, in which for example, auditory white noise is applied to improve tactile perceptual thresholds (5-6). Here we aim to investigate whether cross modal SR exists within the vestibular channel. We applied bilateral nGVS to electrodes on the mastoids at current levels of 0.1, 0.2, ..., 1 mA and, in each case, measured auditory and visual thresholds. Auditory stimuli were 1kHz pure tones 0.25 seconds in duration (or no tone), while visual stimuli were Gabor patches with vertical gratings (or no gratings and just visual static noise), and thresholds were assessed using common two interval, two altered forced choice tasks (e.g., was the auditory beep in the first interval or the second?). After identifying the nGVS level for each subject that produced their best thresholds, we retested sham (i.e., with GVS electrodes applying no stimulation) and the best nGVS level for an independent assessment. We found evidence for vestibular cross-modal SR in that visual perceptual thresholds were improved by 18% with the best nGVS level, compared to sham (p = 0.026). In the 7 of 9 subjects that had improvements, the benefit averaged 26%. Further, subjects with higher (worse) thresholds in sham had greater improvements with the best nGVS (p = 0.005). Auditory thresholds were not significantly different with the best nGVS level compared to sham. These results show the first evidence for cross-modal SR improvements from the application of white noise to the vestibular system. The mechanism of cross-modal SR remains unclear, but has been suggested to occur in multimodal neurons (5). The importance of integrating visual verticality cues with vestibular information to sense spatial orientation may encourage GVS-visual cross-modal SR.
References
(1) Moss et al (2004). Clinical Neurophysiology 115(2).
(2) Mulavara et al (2011). Experimental Brain Research 210(2).
(3) Keywan et al (2018). Frontiers in Neurology 9(83).
(4) Galvan-Garza et al (2018). Brain Stimulation 11(4).
(5) Lugo et al (2008). PLoS ONE 3(8).
(6) Manjarrez et al (2007) Neuroscience Letters 3(415).
Abstract 10The Effects of Training on Visual-Vestibular Heading Perception and Balance in Older and Younger Adults
Grace A. Gabriel, MA1, Laurence R. Harris, PhD2
Denise Y. P. Henriques, PhD3 Maryam Pandi, MSc4 Jennifer L. Campos1
1University of Toronto, Department of Psychology; The KITE Research Institute, UHN
2York University, Department of Psychology
3York University, Department of Kinesiology
4The KITE Research Institute, UHN
When walking, driving, or simply standing still, our brains process and integrate several sensory inputs (e.g., visual and vestibular) to ensure safe balance and mobility. Importantly, age-related changes to such multisensory processes may have detrimental effects on self-motion perception. While there is some evidence suggesting that multisensory processes may be improved through training, it is currently unknown whether the accuracy and precision of visual-vestibular self-motion estimates can also be improved through training. It is also unknown whether potential training effects might be modulated by age. In this study, we investigated whether the precision and accuracy of visual-only, vestibular-only, and combined visual-vestibular heading estimates could be improved by training, and whether any such training effects might be different in older versus younger adults. We also investigated whether visual-vestibular training effects might transfer to benefits in standing balance performance.
Older (n = 11) and younger (n = 11) adults were seated in a 6-degrees-of-freedom motion simulator and asked to judge and report verbally in which direction they had been moved (“forward-left” or “forward-right”). Each participant completed three baseline motion conditions: 1) vestibular-only (passive physical motions in the dark), 2) visual-only (cloud of dots via head-mounted display), and 3) bimodally (vestibular and visual combined). An adaptive staircase procedure was used to determine heading judgement precision (just-noticeable difference; JND) and accuracy (point of subjective equality; PSE). We also measured participants’ standing balance using a forceplate to record centre-of-pressure path length. Baseline measures of sensory, motor, and cognitive abilities were also collected. Participants then completed a 3-day customized training paradigm involving 900 bimodal heading trials during which they were provided with feedback (“correct” / “incorrect”) following each of their heading judgements. The heading angles of the training trials were centred around true straight ahead with a range of ±67% of each participant’s PSE for their baseline bimodal condition. For example, if the PSE was -10°, training trials would be chosen in the range +6.7° and – 6.7°. After training, we reassessed participants’ PSEs and JNDs in all three psychophysical heading estimation conditions (vestibular, visual, and bimodal), as well as their standing balance performance.
Participants were more accurate in the vestibular-only condition relative to the visual or bimodal conditions (p = .016), and less precise in the visual-only condition compared to the bimodal condition (p = .008) across all pre- and post-training sessions. Participants’ overall heading precision increased following training (p = .010), independent of age. Older adults demonstrated some evidence of improved postural stability following training (p = .056).
Overall, these preliminary results suggest that heading estimation can benefit from customized training, and that older adults in particular may experience a transfer of visual-vestibular training effects that results in more stable standing balance.
Acknowledgements: Funded by VISTA Grant awarded to L. R. Harris, D. Y. P. Henriques, and J. L. Campos and an NSERC Doctoral Award to G. Gabriel.
Abstract 11The vestibular system in everyday life: lessons from physiology, modelling and clinic.
Jean Laurens, PhD.
Ernst Strüngmann Institute (ESI) for Neuroscience in Cooperation with Max Planck Society
In this talk, I will review recent developments in the system neuroscience study of vestibular function and propose future directions.
Since the 1960’s, investigators in the vestibular field have combined VOR recordings, self-motion experiments, neurophysiology and modelling to understand how the brain processes vestibular signals experienced when the body is being moved ‘passively’, for instance when sitting in an aircraft or a laboratory stimulator. The theoretical concept of internal model, proposed in the 1980’s [1] and supported by behavioural experiments [2], has since been confirmed by neurophysiological investigations.
However, more recent studies [3] have shown that many central vestibular neurons respond much less to ‘active’, self-generated movements compared to passive motion, indicating that the brain uses signals derived from motor efferences to cancel self-generated vestibular signals. These findings have cast doubts on whether internal models computations revealed by passive motion experiments are relevant during natural, active self-motion.
Recently [4], we resolved this issue by demonstrating that self-motion perception during active and passive motion is explained by early formulations of the internal model framework [1], where the brain uses motor efference copies to anticipate body motion and to predict how the vestibular sensors will be activated. Unpredicted vestibular signal resulting from motor errors or external perturbations are used to update internal models of motion and initiate corrective movements. Thus, self-motion perception in everyday life is primarily driven by motor actions, and the vestibular system acts a corrective feedback mechanism.
Yet, fundamental questions remain. If self-motion perception is governed by efference copies, so long as we don’t make errors, then is the vestibular system truly necessary? And are passive motion experiments still relevant for studying vestibular function?
To answer the first question, I will propose that the internal model framework implies that vestibular feedback is particularly important when the consequences of motor actions are less predictable, for instance when walking on unstable surfaces, which corresponds to the patterns of deficits observed in patients suffering from bilateral vestibular hypofunction. This, in turn, indicates that the internal model framework is a promising way to studying vestibular deficits, as well as sensory substitution and vestibular prosthetics, as has been done in many recent studies.
To answer the second question, I will point out that if the role of the vestibular system is to provide corrective feedback in response to motor errors or external perturbations, then inducing motor errors or applying external motion is the logical way to study it. Thus, traditional motion platforms should remain an efficient way to deliver well-controlled stimuli to study central vestibular pathways, so long as the role of the vestibular system during natural motion remains appreciated.
These results reconciliation once diverging research directions based on passive and active motion, and suggest how to integrate them for future fundamental and clinical vestibular research.
References
[1] C. M. Oman, ‘A heuristic mathematical model for the dynamics of sensory conflict and motion sickness’, Acta Oto-Laryngologica, vol. 94, no. sup392, pp. 4–44, 1982
[2] D. M. Merfeld, ‘Modeling the vestibulo-ocular reflex of the squirrel monkey during eccentric rotation and roll tilt’, Exp Brain Res, vol. 106, no. 1, 1995
[3] K. E. Cullen, ‘The vestibular system: multimodal integration and encoding of self-motion for motor control’, Trends in Neurosciences, vol. 35, no. 3, pp. 185–196, 2012
[4] J. Laurens and D. E. Angelaki, ‘A unified internal model theory to resolve the paradox of active versus passive self-motion sensation’, Elife, vol. 6, p. e28074, 2017.
Abstract 12Auditory contributions to spatial encoding while walking in a virtual environment
Corey S. Shayman1, Erica M. Barhorst-Cates, PhD2, Timothy E. Hullar, MD3, Jeanine K. Stefanucci, PhD2, Sarah H. Creem-Regehr, PhD2
1MD-PhD Program, University of Utah, Salt Lake City, UT
2Department of Psychology, University of Utah, Salt Lake City, UT
3NCRAR, Department of Veterans Affairs, Portland, OR, Department of Neurology, OHSU, Portland, OR
Vestibular, visual, and proprioceptive information are well known to participate in maintaining balance and spatial encoding. Recent experiments have demonstrated external auditory stimuli to be useful as environmental landmarks in order to improve stability in people with baseline instability. This has been shown both during quiet stance and while walking (as measured by gait speed) in conditions without visual cues. Individuals with imbalance or vestibular impairment also suffer from navigational inaccuracies while ambulating. Here, we studied the ability of auditory landmarks to improve performance during a navigational and spatial updating task.
Seventeen healthy adults completed a “triangle completion task” in a virtual environment (HTC Vive). This task requires spatially updating the relative location of a target during translations and rotations. Participants walked to a target starting location, specified visually as a black pole. Upon arrival at the starting location, participants then walked towards a second pole (orange), then a third pole (orange). In the control condition, subjects walked the two segments of a triangle with visual cues present before turning toward their starting point in the dark. On reaching the third marker, visual cues were removed, and participants were tasked with orienting back towards their starting location. In the spatial-audition condition, the starting point was marked with a virtual 60 dB point-source of white noise that was inactivated at the same time visual cues were removed. This noise source was perceived as remaining constant in position by manipulating the relative level between ears during head movement.
Mean angular error was 30.1 deg in the absence of the spatial auditory cue, improving to 22.8 deg with spatial sound (paired t-test, p=0.049, Cohen’s d = 0.58). Those with the greatest baseline challenge navigating improved the most. In the extreme case, spatial auditory cues improved mean angular error from 67.1 to 27.2 deg.
Environmental auditory landmarks may serve to improve navigational and spatial encoding accuracy during ambulation, a problematic task for many people with vestibular loss. The design demonstrates the utility of virtual reality for studying sensory integration during ambulation by allowing rigorous control of sensory inputs. Spatial auditory cues can be considered a useful modality contributing to successful balance and ambulation.
Abstract 13Causal roles for human dorsal parietal and medial prefrontal cortex in perception of the subjective visual vertical
Paul CJ Taylor1, 2, 3, 4, Lina Willacker1, 2
1Department of Neurology, University Hospital, LMU Munich, Germany
2German Center for Vertigo and Balance Disorders, University Hospital, LMU Munich, Germany,
3Faculty of Philosophy and Philosophy of Science, LMU Munich, Germany
4Munich Center for Neurosciences – Brain and Mind, LMU Munich, Munich, Germany
Numerous cortical areas are active while participants relate together visual and egocentric vestibular information. Although dorsal parietal and medial prefrontal cortical activity correlate with visual-vestibular task performance, it is not clear what causal role these dorsal cortical areas may play n the human. We used transcranial magnetic stimulation (TMS) to interfere with activity in parietal or medial prefrontal cortices in groups of between 16-20 healthy people, while they performed the subjective visual vertical (SVV) task. Participants reported with a button press whether a flashed line was tilted counterclockwise or clockwise of true vertical. By fitting psychometric functions, we measured perceptual performance in terms of bias (also referred to as accuracy) versus precision (or sensitivity, threshold, reliability, sigma).
In the first study (Willacker et al. 2019), participants were sorted into two groups of 16 according to their baseline bias at SVV i.e. those with either a slight counterclockwise versus clockwise bias when judging a line to be truly vertical. Right parietal TMS facilitated verticality perception, reducing the difference between groups - affecting bias, with no effect on precision. ERPs suggested that the behavioural TMS effect occurred through normalizing individual SVV biases. No such effects occurred with control stimulation and tasks.
In the second study (Willacker et al. 2020), to ensure a high perceptual demand (putatively necessary to demonstrate a dorsal medial involvement) SVV lines were presented inside pop-out targets within a visual search array. Perceptual performance was analysed before and after theta-burst TMS stimulation of the medial frontal cortex, a control site, or no stimulation, in three groups of 20 people. Medial frontal stimulation improved the precision of verticality judgments with no effects on bias.
Taken together, we suggest that human dorsal cortical regions play roles in SVV perception which are causal, dissociable, and attentional.
References
Willacker, L., Roccato, M., Can, B., Dieterich, M., Taylor, P.C. (2020). Reducing variability of perceptual decision making with offline theta-burst TMS of dorsal medial frontal cortex. Brain Stimulation 13(6):1689-1696.
Willacker, L., Dowsett, J., Dieterich, M., Taylor, P.C. (2019). Egocentric processing in the roll plane and dorsal parietal cortex: A TMS-ERP study of the subjective visual vertical. Neuropsychologia 127:113-122.
Acknowledgements: Funded by German Federal Ministry of Education and Research (BMBF, German Center for Vertigo and Balance Disorders, Grant code 801210010-20) and DFG (TA 857/3-1, TA 857/3-2).
Abstract 14Spatial updating of stimulus location of high uncertainty during dynamic orienting behavior
Jesse J. Heckman, MSc, Wouter B.C., Mattheussens, MSc, Marc M. Van Wanrooij, PhD, A. John Van Opstal, PhD, Annemiek D. Barsingerhorn, PhD
Department of Biophysics, Donders Centre for Neuroscience, Donders Institute for Brain, Cognition, and Behaviour, Radboud University, Nijmegen.
During everyday orienting behavior our brain continuously processes and integrates a wide range of information from various modalities in order to obtain an accurate percept of targets in the real world. Here, the visuomotor system must incorporate visual information with extraretinal sources, such as vestibular signals, muscle proprioception, and efference copies, to maintain a stable visual representation and distinguish target motion from self-motion. This process of accounting for intervening eye and head movements during orientation is called spatial updating.
Visual flashes during self motion of the eyes and head will produce brief visual streaks on the retina that may provide information about target motion relative to the eye. Previous work by Van Barneveld et al (2011) found that when the brain is unsure about visual stimulus motion there is no spatial updating. In their study participants were rotated sinusoidally around the earth bound vertical axis with a single low frequency and stimulus uncertainty was controlled by 3 different stimulus durations effectively manipulating the size of the visual streak on the retina. Only for the shortest stimulus duration (0.5 ms) did spatial updating cease. In that case, the retinal error fully accounted for the target as if the stimulus was physically attached to the eye.
Presently, we aim to follow up on this study by extending the paradigm and by incorporating a multidimensional concept of stimulus uncertainty, i.e. we will vary vestibular stimulation frequency, visual flash duration, and visual intensity of the stimulus. We measure human orienting behavior in a custom-build 2-axis vestibular stimulator by combining 3D eye- and head tracking data. Preliminary results indicate that spatial updating is used to locate head-fixed targets in space in both head-fixed and head-free conditions. In contrast to Van Barneveld et al (2011), our data suggests that even for stimuli shorter than 1ms spatial updating still occurs. This discrepancy may be explained by differences in vestibular stimulation frequency, the subjects’ prior knowledge about the setup and stimulation, and/or the experimental paradigm. However, our data does indicate that reaction times are significantly shorter for longer stimulus durations (100ms) compared to shorter stimulus durations (<4ms). The goal of this study is to research gaze control during visual-vestibular integration, and results may have implications for eye-head orienting models.
References:
1. Van Barneveld et al. (2011). The Journal of Neuroscience, July 20, 2011 • 31(29):10558 –10568
Acknowledgements: Funded by EU Horizon 2020 ERC advanced grant “ORIENT” 693400.
Abstract 15Individual Differences in Spatial Acuity and the Ability to Balance without Gravitational Cues
Vivekanand Pandey Vimal Ph.D, Paul DiZio Ph.D and James R. Lackner Ph.D
Ashton Graybiel Spatial Orientation Lab, Brandeis University, Waltham, MA
In our prior studies we secured blindfolded subjects into a device that was programmed to behave like an inverted pendulum1-5. Subjects used a joystick to orient themselves to the direction of balance in the Horizontal Roll Plane2-5, where there are no position relevant gravitational cues. Collectively, subjects showed minimal learning and a characteristic pattern of positional drifting. However, individual subjects showed wide differences with some showing robust learning and others becoming worse on most performance measures over time5. Our goal was to determine whether spatial acuity could explain the individual differences in active balancing. We exposed blindfolded subjects to passive movement profiles with different frequency components and had them press a joystick trigger to indicate every time they passed the start point. Spatial acuity was better for Horizontal Roll Plane motion profiles with 0.15 Hz than 0.03 Hz components, extending the idea that canal thresholds are lower for higher frequency motions. On comparing subjects’ passive spatial acuity to their active balancing performance, we found no relation between passive spatial accuracy and active balance control ability. Thresholds alone did not predict learning and performance when balancing without gravity dependent position signals. We did find significant correlations between passive spatial acuity in the Vertical Roll Plane, where subjects have task relevant gravitational cues, and active balancing in the Horizontal Roll Plane during early learning. These correlations appeared only after a resource demanding task had been administered. Individual differences in balance control without gravity cues thus relate to a variety of factors that become relevant at different stages of learning.
References
1. Vimal. et al. Experimental Brain Research (2016)
2. Vimal. et al. Experimental Brain Research (2017)
3. Vimal. et al. Experimental Brain Research (2018)
4. Vimal. et al. Experimental Brain Research (2019)
5. Vimal. et al. Aerospace Medicine and Human Performance (2020)
Acknowledgements: VPV was supported by the Translational Research Institute for Space Health through NASA NNX16AO69A. The MARS device was provided by Air Force Office of Scientific Research AFOSR FA9550-12-1-0395.
Abstract 16Predictive processing by Purkinje cells in the anterior vermis during active versus passive self-motion
Omid Zobeiri1, Kathleen Cullen2
1Department of Biomedical Engineering, McGill University
2Department of Biomedical Engineering, Johns Hopkins University
The ability to distinguish between self-generated (reafference) vs. externally-applied (exafference) sensory signals is fundamental for ensuring accurate motor control as well as perceptual stability. This is particularly evident in the context of the vestibular system, in which the same central neurons that receive direct afferent input also project to motor neurons that control vestibulo-spinal reflexes (VSR). Notably, while VSRs are essential for providing a postural response to unexpected perturbation, they are impeding during self-generated head motion. Previous studies by our group have shown that central VSR neurons selectively code passive head movements. Accordingly, here we recorded from Purkinje cells in the vestibular cerebellum (anterior vermis) in rhesus monkeys during comparable active & passive rotational and translational head movements. We first recorded neuronal responses to vestibular passive stimulation alone and neck proprioceptive passive stimulation alone. We found that the Simple spike activity encoded both stimuli in a direction-dependent manner. Accordingly, for each Purkinje cell, we first developed a model of the dynamics of simple spike response based on passive head and body movements kinematics in each direction. We then passively applied both vestibular and proprioceptive stimuli simultaneously (i.e., passive head-on-body rotations) and found that Purkinje cells linearly integrated these two inputs. Then to compare each neuron’s responses to active versus passive movements, we fit comparable models to neuronal responses during preferred and non-preferred active head movements. We found that neuronal sensitivities were markedly attenuated in the active condition (~60%, p<0.01). Finally, we tested whether the attenuated responses during the active movement is a result of neck motor inputs to the Purkinje cells. We found that while in the majority of the Purkinje cells, the neck motor signals affect the simple spike firing, a simple linear model that integrate motor signal with the sensory feedback cannot explain the suppressed simple spike response during active movements. Taken together, these results provide new insights into the computations performed by Purkinje cells in anterior vermis that underlie the suppression of vestibular reafference, suggesting that the cerebellar Purkinje cells implement nonlinear sensorimotor integration to differentially encode externally-applied vs. self-generated head movements and suppress vestibular reafference signal.
Keynote Address 4Zonal patterning of the vestibular organs and their functions
Doris K. Wu, Kazuya Ono, Youngrae Ji and Yosuke Tona
National Institute on Deafness and Other Communication Disorders, National Institutes of Health, Bethesda, Maryland 20892
The vestibular system of the mammalian inner ear consists of five sensory organs: two maculae for detecting linear acceleration and three cristae for detecting angular acceleration. Each sensory organ contains a specialized region in the center known as the central zone in the cristae and the striola in the maculae of the utricle and saccule. In addition, each macula can be divided into two regions by a line of polarity reversal (LPR), across which hair bundles on top of the sensory hair cells are arranged in opposite orientation. Although the striola/central zone and LPR are highly specialized features that undoubtedly contribute differently to the vestibular function, the precise role of each of these features is unclear and nor is it clear how these specialized regions and morphological features come about during development. Our recent results indicate that the striola/central zone is established during development by a low level of retinoic acid (RA), generated by a RA degradation enzyme, Cyp26b1, whereas the extrastriola/peripheral zone, the sensory tissue surrounding the striola/central zone, requires a higher level of RA generated by a RA synthesizing enzyme, Raldh3 (1). As a result, the knockout of Cyp26b1 in mice resulted in a reduction of the striola/central zone whereas the knockout of Raldh3 resulted in striola/central zone expansion.
In contrast to the striola/central zone, the LPR in the two maculae is established by the restricted expression of a transcription factor Emx2 to only one of the two regions in each macula, thus rendering all the hair cells within its domain to establish the hair bundle on the opposite side of a hair cell (2). Thus, all the hair bundles in the maculae are unidirectional in an Emx2 knockout whereas ectopic Emx2 also causes similar phenotype of unidirectional hair cells but they are in the opposite direction from the Emx2 knockout.
Although the knockout of Cyp26b1 or Emx2 are lethal, the conditional knockout (cKO) of these genes in the inner ear are viable. We are currently assessing the behavioral consequences of Cyp26b1 cKO (absence of the striola and central zones) and Emx2 cKO (absence of the LPR) mice. The Cyp26b1 cKO mice can swim but lack detectable vestibular evoked potential (VsEP). In contrast, Emx2 cKO mice show differential directional sensitivity to VsEP, consistent with the unidirectional hair bundle orientation pattern in the maculae. These mice also exhibit subtle but significant deficits in swimming, compared to controls. The implication of these deficits in vestibular functions will be discussed.
References
1) Ono et al., (2020) Nature Commun 11(1):63.
2) Jiang et al., (2017) eLife 6: e23661.
Abstract 17Preferential Representation of Otolithic Versus Semi-Circular Canal Input in the Rat Hippocampus
Martin Hitier,1, Yan-Feng Zhang1, Go Sato1, Stephane Besnard2, Yiwen Zheng1, Paul F. Smith1
1Dept. of Pharmacology and Toxicology, School of Biomedical Sciences and Brain Health Research Centre, University of Otago, Dunedin, New Zealand
2INSERM, U1075, COMETE, 1400, Caen, France
A number of studies in humans have reported that spatial memory impairment with aging is significantly predicted by deficits in cervical vestibular-evoked myogenic potentials (cVEMPs), indicative of saccular function (see Agrawal et al., 2020 for a review). Likewise, cVEMP deficits have been linked statistically to an increased risk of Alzheimer’s Disease (see Agrawal et al., 2020 for a review). These results are consistent with animal studies which have demonstrated that mice without otoconia, but with normal semi-circular canal function, exhibit spatial memory deficits as well as dysfunction of thalamic head direction cells and hippocampal place cells (see Smith, 2019 for a review). In this study we selectively electrically stimulated the utricle and saccule of the rat and recorded local field potentials (LFPs) in the hippocampus. We found that the amplitudes of LFPs evoked in the CA1 and CA3 regions of the hippocampus, by stimulation of the utricle or saccule, were significantly greater than those evoked by stimulation of any of the 3 semi-circular canals (P = 0.0001). This was particularly notable in the contralateral hippocampus for saccular stimulation, for which LFP amplitude was greater than utricular stimulation. These results suggest that otolithic information, especially from the saccule, is preferentially represented in the rat hippocampus, which might explain the statistical relationship between age-related spatial memory deficits and cVEMP deficits in humans.
References
Agrawal Y., Smith, P.F., Rosenberg, P.B. (2020) Vestibular impairment, cognitive decline and Alzheimer’s Disease: Balancing the evidence. Aging and Mental Health. 24(5): 705-708.
Smith, P.F. (2019) The growing evidence for the importance of the otoliths for spatial memory. Frontiers in Neural Circuits. 3(66): 1-14.
Abstract 18Computational Model of Potassium Transport in the Vestibular System
Robert M. Raphael
Department of Bioengineering, Rice University
Computational modeling of biological processes is a powerful tool for obtaining an integrated understanding of how complex systems function and dysfunction. In the inner ear, maintenance of a high potassium concentration (~150 mm) in the endolymphatic fluid is essential for hearing and balance. During sensory transduction, hair cell mechanotransduction channels are continually draining potassium ions from the endolymph. The resupply of potassium is an energy intensive process carried out by specialized epithelial cells - marginal cells in the cochlea and vestibular dark cells in the vestibule. These cells have extensive basolateral infoldings rich in mitochondria and a high density of the Na+-K+-ATPase pump. The biophysics of vestibular dark cell ion transport is not fully understood. To advance this research, we extended a previously developed integrated mathematical model of ion transport across the marginal/dark cells (Qurashi, et al. Am. J. Phys. 2007) by implementing a 15-state Post-Albers model of the Na+-K+-ATPase that includes explicit affinities for Na+ and K+ on both sides of the membrane and voltage dependent dissociation constants. The model contains mathematical expressions for known ion transporters at the basal and apical faces of dark cell. This extended model allows us to simulate the effects of energetic depletion by studying how potassium transport across the epithelium depends on ATP concentration. The results indicate that the current carried by the Na+-K+-ATPase, the K+ carried by the Na+-K+-Cl– cotransporter (NKCC1) and the net K+ current across the epithelium (iKte) all begin to decline when the ATP concentration on the basolateral side falls. Of particular physiological significance is that the model predicts that iKte reverses direction meaning that potassium will be transported out of the endolymph. The influence of extracellular K+ and Cl– on the transepithelial K+ current can also be simulated, advancing our understanding of the function and dysfunction of ion transport in the inner ear. The transepithelial model can be linked to existing models of hair cell mechanotransduction, providing a multiscale model of ion homeostasis in the vestibular endolymph. Using finite element software, the pathway of potassium flow from the vestibular dark cells to the hair cells can be simulated. The overall model is able to make quantitative predictions on how alterations in the conductance of specific channels and transporters that can result from genetic mutations or drug exposure, affect ion transport and sensory transduction. These predictions may provide important clues to mechanisms of hidden vestibular loss and suggest strategies for pharmacological intervention in vestibular disorders.
Abstract 19Computational Model of Ephaptic and Potassium Mechanisms of Non-Quantal Transmission at the Vestibular Hair Cell-Calyx Synapse
Aravind Chenrayan Govindaraju1,2, Anna Lysakowski3, Ruth Anne Eatock4, Robert Raphael1
1Department of Bioengineering, Rice University, Houston, TX
2 Applied Physics Graduate Program, Rice University, Houston, TX
3 Department of Neurobiology, University of Chicago, Chicago, IL
4 Department of Anatomy and Cell Biology, University of Illinois at Chicago, IL
Sensory hair cells of the vestibular inner ear detect and relay information on head motion to afferent neurons, which in turn guide motor reflexes that maintain gaze, balance, and our sense of orientation. Afferent neurons form large cup-shaped terminals (calyces) on type I hair cells that transmit to calyces by both quantal (Q) release of glutamate from vesicles and non-quantal (NQ) flow of ions through the basolateral (pre-synaptic) membrane of the hair cell into the synaptic cleft and through the inner (post-synaptic) calyx membrane. Reference electrodes cannot access the enclosed vestibular hair cell-calyx (VHCC) synapse without disrupting its structure and function. As a result, ion concentrations [Ion] and electric potential (φ) within the synaptic cleft (SC) cannot be measured and the voltage of the post-synaptic membrane cannot be directly obtained. This has posed a barrier to understanding NQ transmission. We have developed a computational biophysical model of the synapse to overcome this limitation. To simulate the dynamic behavior of the system, the VHCC model uses expressions for K+ and Na+ electrodiffusion in the cleft, simplified Hodgkin-Huxley-style ion currents based on whole-cell recordings, and the cable equation. The input to the model is a step or sinusoidal deflection of the hair bundle, and the outputs include the spatio-temporal profile of K+ and Na+ within the synaptic cleft and the change in electrical potential within the synaptic cleft and the afferent neuron. Intracellular potentials within the hair cell and the afferent neuron are calculated as a function of varying [K^+ ]_SC (K+ modulation), and φ_SC (Ephaptic coupling) which alter driving forces of currents across the presynaptic and postsynaptic membranes. By allowing [K^+ ]_SC or φ_SC to be held constant as required, the model allows the separation of these two processes which cannot be achieved experimentally. Simulations capture frequency independence of ephaptic coupling and low-pass behavior of K+ modulation at the synaptic cleft and show that together these process can account for the recorded NQ behavior at the synapse. Currents through the low-voltage-activated potassium conductance (gK,L) on the hair cell and Kv7.4 and HCN channels on the post-synaptic membrane were compared. At rest and during stimulation of the hair bundle, the currents through gK,L and Kv7.4 were an order of magnitude greater than current through HCN. The VHCC model is capable of capturing the putative roles of channels and transporters and exploring their interactions in an in-silico physiological representation of the vestibular hair cell-calyx synapse. gK,L and Kv7.4 appear to be the foremost mediators of transmission during physiological operation. Model simulations suggest that both frequency independent ephaptic coupling and low-pass K+ modulation both accelerate non-quantal transmission between the hair cell and afferent neuron.
Acknowledgements: Supported by NIH-NIDCD R01 DC012347
Abstract 20RotaRod testing on FAM136a and DTNA transgenic mice in light and dark conditions shows implications for contributing to loss of vestibular function in Meniere’s Disease patients
Anna Lysakowski1, Nora Laban1, Rose Bahari2, Jacob Kulaga1, Joseph Lesus1, Heba Sattar3, Zahid Abdul4, Abd-Al-Rahman Al-Rifai2, Vidya Babu2, Suhitha Irukulla2, David Marbina2, Ishita Bhuptani2, Basil Zakkar5, Teresa Requena6,
1Dept. of Anatomy and Cell Biology
2Dept. of Biological Sciences
3Dept. of Bioengineering, 4Dept. of Chemistry
5Dept. of Economics, Univ. of Illinois at Chicago, Chicago, IL, USA, 6Centre for Discovery Brain Sciences, Univ. of Edinburgh, Scotland, UK
Meniere’s Disease (MD) is an inner ear disorder that causes vertigo and hearing loss (Lopez-Escamez et al., 2015). While there are many possible causes for MD, there is strong evidence for some genetic causes. In recent studies, a Whole Exome Sequencing (WES) study was done on a multi-generational Spanish family with MD (Requena et al., 2015) and in cases of sporadic MD in a South Korean population (Oh et al., 2019) to discover rare mutations in FAM136a and DTNA genes in both studies. FAM136a is a mitochondrial gene of unknown function and DTNA is a gene coding for α-dystrobrevin, a cytoskeletal protein that assists in stabilizing synapses (Grady et al., 2006).
Transgenic mice (on a C57BL/6 background strain) for these two genes were obtained from Sanger (FAM136a) and Jackson (DTNA) labs. The effects of these genes on vestibular function, was checked using a behavioral assay in a RotaRod apparatus. Both light and dark conditions were tested. The RotaRod was programmed to produce 3 training runs and 3 acceleration runs, all in light conditions. Training and acceleration runs were similar: 10 secs at 5 rpm and acceleration from 5 to 44 rpm over 40 secs. Mice were tested for additional acceleration runs in the dark, to remove visual input and provide a vestibular challenge. Three-way ANOVAs were used to analyze the results.
Wild type (WT), heterozygote (HET), and knockout (KO) mice of each gene and sex, were tested on a monthly basis from 1 to 24 months old. Mouse ages were analyzed both unbinned and binned by age in 6-month increments (1-6, 7-12, 13-18, 19-24 months). We found significant differences across age for both FAM136a and DTNA mice, with older mice performing worse than younger mice. Both males and females performed worse in dark compared to light conditions. In addition, there was a tendency for KOs to perform worse in the dark compared to WTs.
These data support a correlation between the MD-related genes, FAM136a and DTNA, and their effect on vestibular function. An expected age-related effect was found, along with a tendency for genotype differences. These results have implications for future diagnostic approaches and treatments of MD.
Support: American Hearing Research Foundation grant
References
Grady et al. (2006) J Neurosci 26:2841-2851.
Lopez-Escamez et al. (2015) J Vestibular Res 25:1-7.
Requena et al. (2015) Human Molec Genet 24:1119-1126.
Oh et al. (2019) Front Neurol 10:1424.
Keynote Address 5“When I’m 64”: a glimpse into the ageing vestibular system
Alan M. Brichta1, Lauren A. Poppi1, Mark J. Bigland1, Rebecca Lim1, Doug W. Smith1
1School of Biomedical Sciences and Pharmacy, Faculty of Health and Medicine, The University of Newcastle, Callaghan, New South Wales, Australia; Hunter Medical Research Institute, New Lambton, Australia
Like all peripheral sensory systems, the vestibular system is vulnerable to deterioration with age.1,2 Our current understanding of the ageing vestibular system comes from histological and functional studies in human and animal models. However, this understanding remains incomplete. Here we describe two important components of the vestibular periphery that undergo significant age-related changes: vestibular hair cells; and the Efferent Vestibular System (EVS).
Loss of vestibular hair cells has long been considered a major culprit in age-related decline of vestibular function.3 However, despite the obvious appeal of this simple (and convenient) explanation, more recent quantitative stereological evidence suggests vestibular hair cells are remarkably resilient to the effects of ageing over the average lifespan of humans and gerbils.4,5 In human studies, significant declines in hair cell numbers only become evident in the 8th and 9th decades of life,{Lopez, 2005 #5893} while deterioration of vestibular function has been detected two or three decades earlier 6,7. Therefore, this discrepancy is likely due to hair cell functional deficits preceding hair cell loss.8 In age-related studies, functional deficits in cells are often due to compromised mitochondrial activity.9 This would negatively impact hair cells, which are critically dependent on mitochondria10 for energy production and calcium buffering.10,11 In contrast to many ageing studies, we saw no evidence of age-related mitochondrial DNA (mtDNA) mutations, but we did find a marked age-related reduction in mtDNA copy number in vestibular hair cells. Given the importance of mtDNA in mitochondrial oxidative phosphorylation and hair cell function, our findings support the notion that in older animals, vestibular hair cells are compromised and on the brink of an energy crisis.
Another general consequence of ageing is the decline of central cholinergic networks.12 Since vestibular organs receive cholinergic EVS projections from the brainstem and efferent action modulates the activity of hair cells and afferent fibers, this raises the intriguing possibility that inner ear cholinergic synaptic transmission might be affected in the aged. Interestingly, although our immunofluorescent studies showed no evidence for gross anatomical differences in cholinergic efferent terminals, a preliminary genome-wide gene expression study indicated cholinergic signaling genes were perturbed in the vestibular organs of aged mice. qPCR revealed expression of the nicotinic receptor subunit genes Chrna1, Chrna9, and Chrna10 and the Ca2+-activated K+ channel (BK) gene, Kcnma1, were significantly reduced in aged compared to young adult mice. The functional consequence of reduced expression was confirmed using patch clamp recordings and showed ACh-evoked current amplitudes in type II hair cells were significantly attenuated in aged animals compared to young adults.
Together, our findings support the notion that ageing impairs multiple sites in the vestibular system, including hair cell function and cholinergic signaling in the vestibular periphery. These two changes alone could lead to altered vestibular function and potentially affect important vestibular-mediated reflexes such as the VOR.
Acknowledgements: Funded by National Health and Medical Research Council of Australia; Garnett Passe and Rodney Williams Memorial Foundation.
1. Zalewski CK. Aging of the Human Vestibular System. Semin Hear. 2015;36(3):175-96.
2. Brosel S, Laub C, Averdam A, Bender A, Elstner M. Molecular aging of the mammalian vestibular system. Ageing Res Rev. 2016;26:72-80.
3. Rosenhall U. Degenerative patterns in the aging human vestibular neuro-epithelia. Acta oto-laryngologica. 1973;76(2):208-20.
4. Kevetter GA, Zimmerman CL, Leonard RB. Hair cell numbers do not decrease in the crista ampullaris of geriatric gerbils. Journal of Neuroscience Research. 2005;80(2):279-85.
5. Lopez I, Ishiyama G, Tang Y, Frank M, Baloh RW, Ishiyama A. Estimation of the number of nerve fibers in the human vestibular endorgans using unbiased stereology and immunohistochemistry. Journal of neuroscience methods. 2005;145(1-2):37-46.
6. Agrawal Y, Zuniga MG, Davalos-Bichara M, Schubert MC, Walston JD, Hughes J, et al. Decline in semicircular canal and otolith function with age. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2012;33(5):832-9.
7. Bermúdez Rey MC, Clark TK, Wang W, Leeder T, Bian Y, Merfeld DM. Vestibular Perceptual Thresholds Increase above the Age of 40. Front Neurol. 2016;7:162.
8. Bigland MJ, Brichta AM, Smith DW. Effects of Ageing on the Mitochondrial Genome in Rat Vestibular Organs. Curr Aging Sci. 2018;11(2):108-17.
9. Martin LJ. Biology of mitochondria in neurodegenerative diseases. Prog Mol Biol Transl Sci. 2012;107:355-415.
10. Spinelli KJ, Klimek JE, Wilmarth PA, Shin JB, Choi D, David LL, et al. Distinct energy metabolism of auditory and vestibular sensory epithelia revealed by quantitative mass spectrometry using MS2 intensity. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(5):E268-77.
11. Lenzi D, Roberts WM. Calcium signalling in hair cells: multiple roles in a compact cell. Current opinion in neurobiology. 1994;4(4):496-502.
12. Schliebs R, Arendt T. The cholinergic system in aging and neuronal degeneration. Behav Brain Res. 2011;221(2):555-63.
Poster Abstracts
Examining the impact of balance-related tasks, with and without a head-referencing device, on working memory task performance in typically-developing children.
Rebecca S. Benjamin1,2, Sharon L. Cushing1,2,3,4, Alan W. Blakeman2, Jennifer L. Campos5,6, Blake C. Papsin1,2,3,4, Karen A. Gordon1,2,3,4,7
1Institute of Medical Sciences, University of Toronto, Toronto, ON, Canada
2Archie’s Cochlear Implant Laboratory, Hospital for Sick Children, Toronto, ON, Canada
3Department of Otolaryngology, Head and Neck Surgery, Hospital for Sick Children, Toronto, ON, Canada4Department of Otolaryngology, Head and Neck Surgery, University of Toronto, Toronto, ON, Canada5Department of Psychology, University of Toronto, Toronto, ON, Canada
6KITE, Toronto Rehabilitation Institute, University Health Network, Toronto, ON, Canada
7Department of Communication Disorders, Hospital for Sick Children, Toronto, ON, Canada
Objectives: In this study, we aim to: 1) determine the effects of balance-related tasks on cognitive load in typically-developing children and 2) assess potential decrements in cognitive task performance when children use a new vestibular prosthesis, the BalanCI.
Background: Balance problems are common in children with profound deafness (Kaga et al., 2008). The BalanCI is an investigative prosthesis that aims to support balance in children with vestibulo-cochlear loss who use cochlear implants to hear (Wolter et al., 2020). The BalanCI integrates with cochlear implants and provides head-referencing information using auditory input so children can make postural adjustments and remain upright. It is possible, however, that interpreting and reacting to input delivered by the BalanCI may introduce additional cognitive loads, potentially impacting balance and concurrent tasks, thereby reducing its effectiveness. As a first step, in the present study we tested typically-developing children to evaluate the hypotheses that: 1) children perform more poorly on working memory tasks while engaged in a balance stance compared to when they are seated due to a prioritization of balance over working memory tasks (Mersmann et al., 2013); and 2) BalanCI use may result in reduced performance on working memory tasks compared to when no device is used, especially for verbal working memory tasks, due to competing cognitive resources and a potential overload in auditory working memory (Mayer & Moreno, 1998).
Methods: 14 typically-developing children (5 females) aged 7-18 (mean age ± SD = 13.57 ± 2.85 years) completed verbal and visuospatial working memory tasks. The verbal working memory task consisted of an auditory backwards digit span task, and the visuospatial working memory task consisted of a visual backwards dot matrix task. Each task was completed in three different balance conditions: while the child was seated (little balance required), while the child stood in tandem stance with the BalanCI off, and while the child stood in tandem stance with the BalanCI on. Auditory feedback from the BalanCI was played through insert earphones. Working memory task performance was measured through span length and number of correct trials for each balance condition. Repeated-measures ANOVAs were conducted on working memory span and number of trials correct across balance conditions for each working memory task.
Results: No main effects of balance condition were observed for either working memory task, such that no significant differences were found in performance between sitting vs. standing or when the BalanCI was on vs. off (p’s > 0.1).
Conclusions: These results suggest that BalanCI use does not negatively affect verbal or visuospatial task performance in typically-developing children. This work provides the basis for assessing balance-related effects on cognitive load with and without the BalanCI in children who use bilateral cochlear implants to hear and also have vestibular loss.
References
(1) Kaga, K., Shinjo, Y., Jin, Y., & Takegoshi, H. (2008). Vestibular failure in children with congenital deafness. International journal of audiology, 47(9), 590-599.
(2) Wolter, N. E., Gordon, K. A., Campos, J. L., Madrigal, L. D. V., Pothier, D. D., Hughes, C. O., ... & Cushing, S. L. (2020). BalanCI: Head-Referenced Cochlear Implant Stimulation Improves Balance in Children with Bilateral Cochleovestibular Loss. Audiology and Neurotology, 25(1-2), 60-71.
(3) Mersmann, F., Bohm, S., Bierbaum, S., Dietrich, R., & Arampatzis, A. (2013). Young and old adults prioritize dynamic stability control following gait perturbations when performing a concurrent cognitive task. Gait & posture, 37(3), 373-377.
(4) Mayer, R. E., & Moreno, R. (1998). A split-attention effect in multimedia learning: Evidence for dual processing systems in working memory. Journal of educational psychology, 90(2), 312.
Characterizing responses of distinct body segments used to maintain balance after floor perturbations in typically-developing children
Rebecca S. Benjamin1,2, Sharon L. Cushing1,2,3,4,*, Alan W. Blakeman2, Jennifer L. Campos5,6, Blake C. Papsin1,2,3,4, Karen A. Gordon1,2,3,4,7
1Institute of Medical Sciences, University of Toronto, Toronto, ON, Canada
2Archie’s Cochlear Implant Laboratory, Hospital for Sick Children, Toronto, ON, Canada
3Department of Otolaryngology, Head and Neck Surgery, Hospital for Sick Children, Toronto, ON, Canada4Department of Otolaryngology, Head and Neck Surgery, University of Toronto, Toronto, ON, CanadaON, Canada5Department of Psychology, University of Toronto, Toronto, ON, Canada
6KITE, Toronto Rehabilitation Institute, University Health Network, Toronto, ON, Canada
7Department of Communication Disorders, Hospital for Sick Children, Toronto, ON, Canada
Objective: The aim of this study was to characterize responses at specific body segments to perturbations of the floor in typically-developing children.
Background: Compensatory movements to balance perturbations have previously been quantified using center of pressure (De Sousa et al., 2012), center of mass in relation to the base of support (Patel & Bhatt, 2015) and stepping responses (Connor et al., 2019). These measures characterize the ability to maintain balance but do not provide insight into how distinct segments of the body coordinate to remain upright in response to balance perturbations. Healthy adults typically use an ankle strategy (rotation of body through the ankles) or a hip strategy (rotation of body through the hips) to maintain balance when perturbed (Rogers & Mille, 2018). The hypothesis in the present study was that typically-developing children use the ankle strategy in response to forwards and backwards perturbations and the hip strategy in response to left and right perturbations, with greater movement magnitudes with greater perturbations.
Methods: 14 typically-developing children (5 females) ages 7-18 (mean age ± SD = 13.57 ± 2.85 years) participated in the study. They were asked to stand on a treadmill and stay upright in response to 3 magnitudes of perturbation in forward (peak velocities: small = 0.1 m/s, medium = 0.3 m/s, large = 0.6 m/s), backward (small = 0.1 m/s, medium = 0.4 m/s, large = 0.9 m/s), left and right (small = 0.2 m/s, medium = 0.4 m/s, large = 0.6 m/s) directions (acceleration = 3 m/s2, duration = 700 ms). Magnitude and direction of perturbations were presented in a random order and with a jittered temporal onset. Children wore a 5-point harness to ensure their safety. Motion capture markers were placed on the head (4 markers), upper body (3 markers), pelvis (3 markers) and one on each foot to measure responses to perturbations. Changes in position of motion capture markers over time were quantified by path length, root-mean-square change, and area under the curve for displacement in the x, y and z axes, and for rotation in the pitch, roll and yaw planes.
Results: Participants were able to maintain their balance during all perturbations without falling. Preliminary analyses of forwards and backwards perturbations suggest movement magnitude decreasing from the head to the feet, indicating the potential use of an ankle strategy. This pattern was less clear in response to left and right perturbations and requires further analyses. Preliminary analyses also reveal larger movement magnitudes with larger perturbations across directions. Further analyses will characterize the time course and assess variability in responses to determine whether individual children use unique postural strategies to maintain balance.
Conclusions: This work will define normative measures and outcomes to be used in our parallel studies that aim to improve balance and reduce risk of falling in children with auditory and vestibular dysfunction.
References
1) De Souza, F. M. B., Pereira, R. P., Minuque, N. P., Do Carmo, C. M., De Mello, M. H. M., Villaça, P., & Tanaka, C. (2012). Postural adjustment after an unexpected perturbation in children with haemophilia. Haemophilia, 18(3), e311-e315.
2) Patel, P., & Bhatt, T. (2015). Adaptation to large-magnitude treadmill-based perturbations: improvements in reactive balance response. Physiological reports, 3(2), e12247.
3) Conner, B. C., Petersen, D. A., Pigman, J., Tracy, J. B., Johnson, C. L., Manal, K., ... & Crenshaw, J. R. (2019). The cross-sectional relationships between age, standing static balance, and standing dynamic balance reactions in typically developing children. Gait & posture, 73, 20-25.
4) Rogers, M. W., & Mille, M. L. (2018). Balance perturbations. In Handbook of Clinical Neurology (Vol. 159, pp. 85-105). Elsevier.
Impaired vestibular reflexes response and vestibulo ocular reflex adaptation in a mouse model of spinocerebellar ataxia type 6
Hui Ho Vanessa Chang1, Alanna J. Watt2 and Kathleen E. Cullen1
1Dept. of Physiology, McGill University, Montreal, QC, H3G 1Y6, Canada.
2Dept. of Biology, McGill University, Montreal, QC, H3A 1B1, Canada.
Spinocerebellar Ataxia Type 6 (SCA6) is a mid-life onset neurodegenerative disease that affects motor coordination. This autosomal dominant disease is caused by the expansion of a CAG repeat tract in a CACNA1A gene that encodes the a1A subunit of the P/Q type voltage-gated Ca2+ channel. A hyper-expanded polyglutamine (84Q) mouse model of SCA6 (SCA684Q/84Q), is characterized by impaired locomotive function. Using both in vitro and in vivo recordings, we have previously shown that, in this same mouse model, the firing precision of cerebellar Purkinje cells in lobule 3, areas of the cerebellum generally associated with locomotion, is significantly reduced (Jayabal et al., 2016). In addition, SCA684Q/84Q mice showed reduction in complex spike firing rate and increase in the duration of pause after the appearance of complex spikes.
Previous studies have demonstrated cell death across the entire cerebellar cortex in SCA6 patients. Given the involvement of cerebellum in generation of and motor learning in the vestibulo ocular reflex (VOR) and optokinetic reflex (OKR), we hypothesized that SCA684Q/84Q mice would likely show deficits during these oculomotor behaviors. To test this hypothesis and to understand the pathophysiology of SCA6 mice in more detail, we quantified their eye movements to characterize their vestibular-ocular reflex (VOR), optokinetic reflex (OKR), and VOR adaptation. Vestibular rotational stimuli were delivered to a head-restrained mouse at frequencies and velocities spanning the range of those comprising natural behaviors (0.2-2 Hz and 16 deg/s). VOR eye movement responses were measured in both dark and light conditions. OKR were evoked by rotation of visual stimulus at the same frequencies and velocities. For VOR adaptation, gain decrease was induced by presenting visual and vestibular stimuli (2Hz and peak velocity of 16 deg/s) that were at the same speed and exactly in phase. The gain-down training lasted for 30 minutes. Our preliminary data show that SCA684Q/84Q mice had a significant reduction of VOR gain at 0.8, 1 and 2 Hz and a ~35% reduction of OKR gain without a change in phase compared to litter-matched control WT mice. Additionally, during VOR motor learning, SCA684Q/84Q mice only showed 16% decrease in VOR gain whereases WT mice showed over 40% VOR gain decrease. Finally, we found that SCA64Q/84Q mice also generated slower saccades than the control mice. Taken together, our results confirm our original hypothesis that neuronal responses are altered in the floccular lobe of SCA684Q/84Q mice, a region of the cerebellum known to play a vital role in the calibration of the VOR pathway as well as generation of the optokinetic responses.
A large body pitch combined with translational motion induces a deviation of subjective haptic vertical in a natural environment
Hiu Mei Chow1, Lothar Spillmann2, Matt Oxner3, Kenzo Sakurai4, Chia-huei Tseng5
1Department of Ophthalmology and Visual Sciences, University of British Columbia, Vancouver, Canada
2Neurology Clinic, University of Freiburg, Freiburg, Germany
3School of Psychology, Victoria University of Wellington, Wellington, New Zealand
4Department of Human Sciences, Tohoku Gakuin University, Japan
5Research Institute of Electrical Communication, Tohoku University, Japan
Accurate representation of verticality is important to daily activities such as posture maintenance and navigation. Laboratory studies often investigate the role of body orientation and translational motion on verticality perception independently. In contrast, those two factors can co-occur and interact in natural environments, for example, on a mountain tram. Here we capitalize on the Hong Kong (HK) Peak Tram, a mode of public transportation in HK, to investigate these factors. Passengers of the Peak Tram experience both body pitch changes and translational motion as the tram ascends or descends the mountain along a dynamically changing mountain slope. We hypothesized that body pitch and translational motion interacted to cause misperception of verticality on the HK Peak Tram. If this was the case, we expected to find a systematic deviation of subjective haptic vertical (SHV) on a tilted moving tram, but not a tilted stopped tram or on a horizontally moving tram.
Six to eight healthy adults were asked to adjust a rod’s orientation to match with their perceived direction of gravity in various set-ups with their eyes closed. The difference between this rod and the true vertical was termed SHV error. Our results showed that SHV error increased linearly as a function of body pitch on a moving Peak Tram. This SHV error was as large as 10 degrees when participants were tilted at 26 degrees on the Peak Tram, larger than that reported in laboratory studies with a similar body pitch without translational motion (Schoene, 1964; Bortolami et al., 2006). By contrast, SHV error was close to zero when the same group of participants provided SHV settings on the Peak Tram stopped at a terminus at a slope of 26 degrees. Congruently, their SHV error was close to zero when participants provided SHV settings on a horizontal moving tramcar.
Our results suggest that sensory inputs about body orientation are transformed quickly for the setting of SHV in a natural environment. The large and persistent SHV error observed on the HK Peak Tram was due to a combination of large body pitch and translational motion. Visual cues can be ruled out as the cause of SHV error, as these results were found when observers had their eyes closed. Instead, we speculate that vestibular inputs encoding translational motion might have been misattributed to tilt when the body pitch was large.
Central Vestibular Integration Functions are Associated with Walking Capacity, Balance, Fatigue, and Disease Severity in People with Multiple Sclerosis
Graham Cochrane, B.A.1,2, Jennifer Christy, PT, Ph.D.1, Robert Motl, Ph.D.1
1Department of Physical Therapy, School of Health Professions, University of Alabama at Birmingham, Birmingham AL
2Medical Scientist Training Program, School of Medicine, University of Alabama at Birmingham, Birmingham AL
Balance and fatigue, two of the most debilitating symptoms of multiple sclerosis (MS), have been associated with each other in people with multiple sclerosis (PwMS). This relationship has been hypothesized to be due to shared central sensory processing pathways. However, few studies have investigated the associations between other vestibular functions and fatigue in PwMS. In addition, while vestibular functions have previously been explored in PwMS, no study has comprehensively examined them in the same cohort of PwMS and examined how those functions change over the course of disease. Our goals were to complete an extensive battery of vestibular function tests in PwMS and people without MS as well as assess fatigue severity and walking capacity to explore relationships between vestibular functions and fatigue. We completed a three-hour battery (1.5 hours over two days) in 40 PwMS (EDSS of 1.0-6.5, median: 2.5) ages 21-55 and in a semi-age- and sex-matched sample of 20 people without MS. This battery of tests included several measures of VOR (rotary chair VOR, computerized dynamic visual acuity (cDVA), and video head-impulse), subjective visual vertical (SVV) (rotary chair and bucket test), cervical and ocular VEMPS, balance and gait function (Sensory Organization Test (SOT), 6-minute walk (6MW), and Functional Gait Assessment (FGA)), and fatigue surveys (Modified Fatigue Impact Scale (MFIS), Fatigue Severity Scale (FSS)). Bivariate statistical tests were completed between all measures between those with and without MS, as well as between PwMS with an EDSS less than 3.0 and an EDSS >=3.0. Spearman correlations were calculated between vestibular functions and fatigue survey scores, SOT, 6MW distance, and FGA. Our results indicate that reflexive vestibular functions (simple VOR, VEMPs) and SVV deviations are not impaired in PwMS nor change over the course of MS disease severity. However, vestibular functions requiring central integration (VOR suppression, cDVA, SVV variance) were impaired in PwMS and degraded further over the course of disease. SVV variance and SOT were the only vestibular measure statistically correlated, albeit weakly, with MFIS and FSS. SVV variance showed strong relationships with SOT, 6MW, and FGA performance. We hypothesize that SVV variance may be a measure of central sensory processing of vestibular information in PwMS related to physical outcome measures and may serve as a central vestibular outcome variable for future studies of vestibular function.
Vestibular-auditory crossmodal aftereffects: the effect of vestibular adaptation on auditory motion perception
Luigi F. Cuturi, Ph.D.1, Silvia Zanchi, Ph.D. student1,2,3 , Monica Gori, Ph.D.1
1Unit for Visually Impaired People (U-VIP), Italian Institute of Technology, Genoa, Italy
2Robotics Brain and Cognitive Sciences (RBCS), Italian Institute of Technology, Genoa, Italy;
3University of Genoa, Italy
Aftereffect paradigms have been considered as the psychologist’s electrode, as these paradigms allow researchers to observe behavioral proof of neural response to environmental changes. In the context of vestibular research, within vestibular domain aftereffects [1] as well as visuo-vestibular aftereffects [2] have been observed. In the present research, we focus on the interaction between vestibular and auditory processing of motion. To this aim, we employ a motion-nulling procedure, which finds the auditory motion stimulus that cancels vestibular induced aftereffects. On each trial, participants are presented with an adapting stimulus consisting in a long lasting yaw rotation (5 s duration, 80° rotation , peak velocity 30°/s), delivered via a Rotational-Translational chair [3]; and a subsequent test stimulus, a pink noise sound moving eccentrically from the 0° azimuth position (i.e. aligned with participant’s nose) virtually rendered on headphones. Participants’ task is to discriminate the direction of the auditory motion stimulus, either to the right or left. In separate blocks of trials, we test a baseline condition with no vestibular adaptation but auditory-only stimulation, and two adaptation conditions depending on the direction of the adapting stimulus, either clockwise or counterclockwise. For each condition, we calculate the point of subjective equality (PSE) which corresponds to the auditory motion stimulus perceived to be equally moving to the right or left; we take the shift of the PSE as a measurement of aftereffect. Our results show a shift of the PSE consequent to adaptation in the counterclockwise condition and mild aftereffects in the clockwise condition. To our knowledge, these findings are the first report of crossmodal aftereffects induced by vestibular stimulation in the auditory domain. The presence of such aftereffects suggests that acoustic and vestibular domains may share common calibration processes of motion information. Considering the influence of visual experience on multisensory calibration processes [4,5], in future research we will investigate how visual impairment such as blindness influences the emergence of self-motion aftereffects across sensory modalities.
References
1. Crane, B.T. Fore-aft translation aftereffects. Exp. brain Res. 2012, 219, 477–87.
2. Cuturi, L.F.; MacNeilage, P.R. Optic Flow Induces Nonvisual Self-Motion Aftereffects. Curr. Biol. 2014, 24, 2817–2821.
3. Cuturi, L.F.; Torazza, D.; Campus, C.; Merello, A.; Lorini, C.; Crepaldi, M.; Sandini, G.; Gori, M. The RT-Chair : a Novel Motion Simulator to Measure Vestibular Perception. 2020, 3318–3322.
4. Cuturi, L.F.; Gori, M. The Effect of Visual Experience on Perceived Haptic Verticality When Tilted in the Roll Plane. Front. Neurosci. 2017, 11.
5. Gori, M.; Amadeo, M.B.; Campus, C. Spatial metric in blindness: behavioural and cortical processing. Neurosci. Biobehav. Rev. 2020.
Visually induced self-motion (vection) can be altered by cognitive factors and personality traits.
Sarah D’Amour1,3, Laurence R. Harris1, Stefan Berti2, Behrang Keshavarz3,4
1Centre for Vision Research, York University, Toronto, Canada
2Department of Clinical and Neuropsychology, Johannes Gutenberg University Mainz, Germany
3KITE, Toronto Rehabilitation Institute–University Health Network, Toronto, Canada
4Department of Psychology, Ryerson University, Toronto, Canada
During natural self-motion, both the vestibular and visual systems are stimulated and their signals informing the brain about the movement are in agreement. However, the sense of self-motion can be evoked in a stationary observer by visual motion alone: a phenomenon known as vection. When visual motion consistent with rotation is provided to a stationary observer, the visual and vestibular signals are initially in conflict but, if the motion is maintained at a constant angular velocity, the conflict subsides over several seconds and a strong sense of self-motion can be evoked. How much can high-level cognitive factors such as expectation or the tendency to depersonalize, influence this interpretation of the visual motion?
We quantified how cognitive manipulations such as contextual information (i.e., expectation) and plausibility (i.e., chair configuration) might alter the vection experience. We also explored how individual traits such as field dependence, depersonalization, anxiety, and social desirability might be related to vection. Fifty-one healthy adults were exposed to an optic flow stimulus that consisted of horizontally moving black-and-white bars presented on three adjacent monitors to evoke circular vection. Participants were divided into three groups and given experimental instructions carefully worded to induce either strong, weak, or no expectation with regard to the intensity of vection. In addition, the configuration of the chair was modified during the experiment to give the participants the impression that it could or could not rotate. Vection onset time, duration, and intensity were recorded.
Results showed that expectation did not affect duration or onset of vection but did alter perceived vection intensity - but only when the chair was in the configuration in which participants believed it could actually rotate. Positive correlations for vection measures with field dependency and depersonalization but no sex-related effects were also found.
Our results show that the interpretation of visual motion as self-motion during a visual-vestibular conflict can be influenced by cognitive factors and individual traits. Interestingly, cognitive factors did not affect vection onset time indicating that the interaction between the vestibular “stationary” signal and the visual motion cues is robust to cognitive manipulations. Instead, the interpretation of the “steady state” visually induced sensation of self-movement (i.e., vection intensity) can be altered by cognition. We conclude that the sensation of vection is not a purely perceptual phenomenon but can be affected by top-down mechanisms.
Acknowledgements: LRH is a recipient of NSERC Discovery grants.
Fear Avoidance Beliefs Are Associated with Disability in Persons with Vestibular Disorders
Pamela M. Dunlap, DPT, PhD1, Patrick J. Sparto, PT, PhD2, Gregory F. Marchetti, PT, PhD3, Joseph M. Furman, MD, PhD4, Jeffrey P. Staab, MD, MS5, Anthony Delitto, PT, PhD2, Brooke N. Klatt, DPT, PhD2, Susan L. Whitney, DPT, PhD2
1Department of Epidemiology, University of Pittsburgh, Pittsburgh, PA
2Department of Physical Therapy, University of Pittsburgh, Pittsburgh, PA
3Department of Physical Therapy, Duquesne University, Pittsburgh, PA
4Department of Otolaryngology, University of Pittsburgh, Pittsburgh, PA
5Departments of Psychiatry and Psychology and Otolaryngology Head and Neck Surgery, Mayo Clinic, Rochester, MN
Background/Objective: Fear and avoidance of movements and activities can lead to abnormal sensory-motor processing, space and motion discomfort, and can inhibit vestibular compensation mechanisms in persons with vestibular disorders. The purpose of this study was to determine the association between fear avoidance beliefs and disability at three-month follow-up in persons with vestibular disorders while accounting for demographic and clinical characteristics.
Methods: This prospective cohort study included participants over 18 years of age who reported dizziness. Participants were recruited from a balance disorders clinic and outpatient physical therapy clinics. All participants completed the Vestibular Activities Avoidance Instrument (VAAI) and the Hospital Anxiety and Depression Scale (HADS) at baseline and the Vestibular Activities and Participation measure (VAP), dizziness Visual Analogue Scale (VAS), and 12-item Short Form Health Questionnaire (SF-12) at baseline and three-month follow-up. A modified version of the VAAI including nine items abstracted from the 81-item VAAI was utilized in this study. The relationships between 9-item VAAI scores (0-54) and follow-up measures of disability were assessed using Spearman’s correlation coefficients. A multivariate linear regression model was analyzed to determine the effect of fear avoidance beliefs on follow-up VAP score while accounting for baseline outcome measures.
Results: Participants (n=404) with a mean age of 54±17 years completed the baseline assessment and 71% (n=286) completed the three-month assessment. The mean 9-item VAAI score was 25 (SD=14) at baseline and was significantly associated with VAP (ρ=0.54), SF-12 component scores (ρ=-0.53; -0.44), and dizziness VAS at follow-up (ρ=0.37), (p<0.001). Approximately 37% of the variation in VAP score at follow-up was predicted by 9-item VAAI score, dizziness VAS, and HADS-D score when considered together (R2=0.37, p<0.001).
Conclusions: Fear avoidance beliefs are associated with measures of disability at three-month follow-up and are predictive of activity limitations and participation restrictions at three months when controlling for baseline dizziness and depression symptom severity. Measurement of fear avoidance beliefs may provide important prognostic information in persons with vestibular disorders, suggesting that an assessment of fear avoidance beliefs could be used by clinicians to identify individuals at greater risk of disability after a vestibular disorder.
References
Furman & Jacob. (2001) J Anxiety Disord. 15(1-2): 9-26.
Yardley & Redfern. (2001) J Anxiety Disord. 15(1-2): 107-119.
Yardley et al. (1992) Psychol Health. 6(1-2):85-96.
Pothier et al. (2018) JAMA Otolaryngol Head Neck Surg. 144(10):906-912.
Herdman et al. (2020) J Psychosom Res. 132:109969.
Optical Coherence Tomography Imaging of the Murine Vestibular System
Govindaraju AC, Majid M, Raphael RM
Department of Bioengineering, Rice University, Houston, TX
The vestibular system is responsible for our sense of orientation and balance. Disorders of the vestibular system can cause diseases such as Meniere’s disease for which there are no effective clinical treatments. The vestibular semicircular canals and end-organs are contained within the temporal bone, and a major barrier to vestibular research is the inability to noninvasively image internal vestibular structures within the bony and membranous labyrinths and to study how these structures are perturbed in disease states. We have utilized optical coherence tomography (OCT) to image the vestibular system in isolated and intact murine temporal bones. OCT is a form of optical interferometry which enables imaging biological tissue at greater depths than optical or fluorescence microscopy. After sacrifice, murine temporal bones were rapidly removed and placed on the stage of a Thorlabs Ganymede Spectral Domain 620C OCT Imaging system. Images of vestibular compartments were acquired in 2D mode for cross-sectional imaging and 3D mode for volume imaging. The data were analyzed in ThorImage OCT software to obtain dimensions of internal structures and reconstruct vestibular anatomy. OCT imaging through the temporal bone readily permitted imaging of the semicircular canals located just beneath the bone. Imaging of a transverse cross section of the semicircular canal reveals distinct compartments delineating the boundaries between the perilymph and the endolymph. 3D imaging of the semicircular canals along the axis of curvature also revealed the presence of membranous structures and enabled imaging of connective tissue between the outer boundary of the duct and the bony labyrinth – structures previously only seen in electron microscopy. We have also demonstrated that OCT can image internal structures in the various vestibular end-organs: the ampulla, the saccule and the utricle. The results establish that OCT can non-invasively image through the murine temporal bone and visualize the internal structures of the vestibular system. The implementation of OCT introduces a powerful method for studying normal vestibular anatomy and physiology as well as testing hypotheses related to the causes and treatments of vestibular disorders.
Acknowledgements: We are grateful to Anna Lysakowski for discussions on vestibular anatomy and financial support from the NSF IGERT Program.
Visual-vestibular conflict detection is modulated by motor signals
Savannah Halow, BS1, James Liu, MS2, Eelke Folmer, Ph.D.2, Paul MacNeilage, Ph.D.1
1Department of Psychology, University of Nevada, Reno NV
2Department of Computer Science and Engineering, University of Nevada, Reno NV
Head movement relative to the stationary environment gives rise to congruent vestibular and visual optic flow signals. The resulting percept of a stationary visual environment depends on mechanisms that compare visual and vestibular signals to evaluate their congruence. Here we investigate the efficiency of these mechanisms and how it depends on fixation behavior as well as on the active versus passive nature of the head movement. Sensitivity to conflict was measured by modifying the gain on visual motion relative to head movement on individual trials and asking subjects to report whether the gain was too low or too high. Low and high gains result in percepts of the environment moving with or against head movement, respectively. Fitting a psychometric function to the resulting data yields two key parameters to characterize performance; the standard deviation (SD) and mean of the cumulative Gaussian fit. The mean indicates the single visual gain value that is perceived to match head movement. The SD indicates the range of gains that are compatible with perception of a stationary visual environment, referred to by Wallach as the Range of Immobility (Wallach, 1985). Experiments were conducted using a head-mounted display capable of rendering visual scene motion contingent on head motion, with fixation behavior monitored by an embedded eye tracker. The experimental design included combinations of active or passive head movement together with head-fixed or scene-fixed fixation. During active conditions, subjects rotated their heads in yaw ~15 deg/s over ~1 sec. Each subject’s movements were recorded and played back via rotating chair during the passive condition. During head-fixed and scene-fixed fixation the target moved with the head or scene, respectively. Sensitivity (quantified by SD) was better during active than passive head movement, likely due to increased precision on the head movement estimate arising from motor prediction and neck proprioception. Sensitivity was also better during scene-fixed than head-fixed fixation, perhaps due to decreased velocity of retinal image motion and increased precision on the estimate of retinal image motion under these conditions. The gain perceived as matching (quantified by the mean) also depended on motor signals. Gains were closer to unity during scene-fixed fixation and during active head movement, and decreased in the other conditions. These findings quantify how visual-vestibular conflict detection is modulated by eye and neck motor signals.
References
(1) Wallach (1985). Perceiving a stable environment. Scientific American 252 (118-125).
Acknowledgments: Research was supported by NIGMS of NIH under grant number P20 GM103650.
Whole-Motion 3-D Modeling of Spatial Orientation
Jan E. Holly, Ph.D., Seungjae Lee, B.A., and Chido Mpofu, B.A.
Colby College, Waterville, ME
For spatial orientation models, a goal that remains elusive is to predict the perceived 3-D motion as a whole. This is true regardless of the implementation, whether by Kalman filters, differential equations, internal models, Bayesian models, or variations of these. Examples of such motions include off-vertical axis rotation (OVAR) [6], horizontal linear oscillation [1], and forward motion through a curve [5]. In these motions without vision, models typically fail to predict translation and tilt in phase and the resulting perceived cone-shaped motion during OVAR as well as the hilltop illusion during horizontal oscillation, and fail to predict a face-forward subjective heading during motion through a curve. This is because models have primarily focused on predicting individual components of perception. That endeavor now forms a springboard for fully 3-D whole-motion model development. The goal of the present study was to develop a model that predicts perception of 3-D motion through a curve in a way that is consistent with subject reports [4,5], with the aim of identifying principles of perception that have been missed by earlier models. This research is along the lines of previous modeling that correctly predicts a cone-shaped perception during OVAR [3], and that focuses on 3-D motion as a whole, including heading, in a centrifuge [2]. As background: The core of existing models is the concept of perceptual experience and familiarity. Besides the 3-D laws of physics, there are three main principles that are implemented in all 3-D models: Perceived rotation tends toward zero, perceived linear velocity tends toward zero, and the resultant force tends to be aligned with vertical. The Whole-Motion Model of [2] implemented a further refinement that forward velocity is more familiar than backward velocity. The present study used the Standard Model (which captures the core 3-D dynamics of existing spatial disorientation models) and the Whole-Motion Model of [2] as a starting point, and then tested additional hypotheses about perception by modeling their consequences in comparison with known subject reports [4,5]. Three main results: (1) The beginning of the motion is the main determinant of the ensuing perception. (2) Perceptual GIF-resolution (GIF = gravito-inertial force) can be modified or superseded by familiarity of motion as whole. (3) The acceleration-velocity-position relationship can be superseded by familiarity of motion as whole. Conclusion: Models that aim to fully predict spatial disorientation in 3-D must incorporate not only the 3-D physics and dynamics of components, but also the familiarity of patterns of whole 3-D motions.
References
[1] Glasauer (1995). Acta Otolaryngol Suppl 520:37–40.
[2] Holly, Vrublevskis, Carlson (2008). J Vestib Res 18:171–186.
[3] Holly, Wood, McCollum (2010). Biol Cybern 102:9–29.
[4] Ivanenko, Grasso, Israël, Berthoz (1997). J Physiol 502:223–233.
[5] Nooij, Nesti, Bülthoff, Pretto (2016). Exp Brain Res 234:2323–2337.
[6] Wood, Reschke, Sarmiento, Clément (2006). Exp Brain Res 182:365–377.
Acknowledgements: Funded in part by Colby College Research Grants.
Does visually induced motion sickness affect driving performance in virtual reality?
Elizaveta Igoshina, MA1,4, Frank Russo, PhD1,3,5, Robert Shewaga, MSc5, Bruce Haycock, PhD2,5, Behrang Keshavarz, PhD3,5
1Department of Psychology, The University of Toronto, Canada
2Institute for Aerospace Studies, The University of Toronto, Canada
3Department of Psychology, Ryerson University, Canada
4Department of Neurosciences and Mental Health, The Hospital for Sick Children, Canada
5KITE - Toronto Rehabilitation Institute, Canada
Virtual reality (VR) driving simulation technologies have a myriad of applications from entertainment, to scientific and medical research. However, they also are known to cause visually induced motion sickness (VIMS), a special form of traditional motion sickness1,2. Common side effects of VIMS include nausea and disorientation2,3, suggesting VIMS can bias driving performance. Thus, the goals of this study were to (1) investigate how VIMS affects performance in a simulated driving task and (2) examine a potential treatment to reduce VIMS through in-vehicle ventilation. Twenty-four participants were engaged in a driving task where they reacted to hazards, obeyed speed limits, and completed common driving maneuvers. Driving performance (Objective 1) was evaluated based on various common and novel driving criteria, and compared between high- and low VIMS groups. To study the effect of airflow on VIMS (Objective 2), for half of the participants the car vents were directed to the driver’s head and torso having airflow directly contact the driver’s skin, creating the experimental group: airflow (direct, indirect). The level of VIMS was measured before and after the simulated drive and participants were retroactively divided into VIMS groups (high, low). We found (1) no differences in driving performance between participants who experienced high- and low VIMS, indicating driving performance was not influenced by VIMS. Also, we found (2) no differences in VIMS severity between airflow groups, indicating that both direct and indirect airflow are equally effective at keeping VIMS experienced in a driving simulator relatively low. In conclusion, (1) as driving-performance was not affected by VIMS, results obtained during a driving simulation study from participants who experience high levels of VIMS can be treated equal to participants who experience low levels of VIMS. Furthermore, (2) as our findings suggest that the direction of airflow provided during a simulated drive does not influence VIMS, both direct and indirect airflow can be used as a low cost, effective means of reducing VIMS in VR driving simulators.
References
1. Classen, S., Bewernitz, M., & Shechtman, O. (2011). Driving simulator sickness: an evidence-based review of the literature. American journal of occupational therapy, 65(2), 179-188.
2. Keshavarz, K., Hetch, H., Lawson, B. D. (2014). Visually Induced Motion Sickness: Causes, Characteristics, and Countermeasures. In E. E. Editor & F.F. Editor (Eds.), Handbook of Virtual Environments: Design, Implementation, and Applications, Second Edition (647-698). Taylor & Francis Group.
3. Kennedy, R. S., Drexler, J., & Kennedy, R. C. (2010). Research in visually induced motionsickness. Applied Ergonomics, 41, 494-503. doi:10.1016/j.apergo.2009.11.006
The perception of object velocity is biased and less precise during visually induced self-motion
Björn Jörges, PhD, Laurence R. Harris, PhD
Center for Vision Research, York University, Toronto, Canada
When a moving observer views a moving object, the object’s physical speed cannot be extracted from its retinal speed alone. When observer and object move in the same direction, the retinal speed of the object is partially cancelled out, and when they move in opposite directions, the object’s retinal speed is increased. Observers must thus obtain an accurate estimate of their own velocity and compensate for that to recover an object’s velocity accurately. Estimating self-motion speed should be facilitated when visual and vestibular cues are congruent. When a static observer experiences optic flow compatible with self-motion, compensation may be incomplete, leading to biases in judgments of object speed (Hypothesis 1). Furthermore, self-motion information is noisier than retinal information concerning object motion [1], especially when only visual cues are available [2]. Subtracting noisy self-motion information from retinal motion to estimate target velocity should thus decrease precision (Hypothesis 2). We presented two displays: a test display and a probe display, in a 3D virtual environment. In the test display, a target object moved linearly to the left or right in the fronto-parallel plane. In the probe display, a cloud of smaller objects travelled in the same direction. Participants judged whether the cloud or the object moved faster. In the test display the target object moved at constant speeds in front of a textured backdrop while floating above a textured ground plane. The speed of the cloud in the probe display was determined by a PEST staircase. While observing the target object, participants were moved visually either in the same direction (reduced retinal speed), in the opposite direction (increased retinal speed), or remained static. In two control conditions, we tested for possible effects of induced motion. In control 1, the object moved against a blank backdrop (self-motion, but no induced motion), and in control 2, the backdrop moved but the ground plane remained stationary (induced motion, but no self-motion). Regarding Hypothesis 1: participants judged object motion in the opposite direction condition as faster than in the static condition, but still compensated for about 85% of self-motion due to self-motion. There was no difference in accuracy between the same direction and static conditions, indicating full compensation. Regarding Hypothesis 2: precision was decreased in the opposite direction condition, while we found no difference in precision between the static and same direction conditions.. This asymmetry might be due to the fact that mean retinal speeds were lower when observer and target moved in the same direction, which should increase discriminability. A decrease in precision elicited by self-motion may thus be offset by an increase in precision due to lower retinal speed. In the blank wall condition (control 1), we found results similar to the main experimental conditions, while the moving wall condition (control 2) elicited no differences. Induced motion is thus unlikely to have played a role in our results. Further research is necessary to determine how the availability of vestibular cues can remedy accuracy or precision losses during self-motion.
Acknowledgements: LRH is supported by an NSERC discovery grant. BJ is supported by the Canadian Space Agency.
References
[1] Dokka et al. (2015). Cerebral Cortex, 25(3).
[2] Fetsch et al. (2010). European Journal of Neuroscience, 31(10).
Does Migraine Affect BPPV? A Retrospective Analysis of Severity, Recurrence, and Falls
Eric K. Kim, BA1, Lauren Pasquesi, AuD2, Jeffrey D. Sharon, MD3
1University of California San Francisco, School of Medicine, San Francisco, CA
2University of California San Francisco, Balance and Falls Center, San Francisco, CA
3University of California San Francisco, Department of Otolaryngology-Head and Neck Surgery, San Francisco, CA, San Francisco, CA
Benign paroxysmal positional vertigo (BPPV) is the most common type of vertigo and can have a significant impact on one’s quality of life.1 A previous study found that migraine has been associated with a higher risk for BPPV.2 Given this epidemiological risk factor, we wanted to examine whether migraine is associated with a more severe presentation of BPPV. We conducted a retrospective chart review of 227 patients (102 with migraine and 125 without migraine) diagnosed with posterior canal BPPV based on characteristic nystagmus from 01/2015 to 06/2020 at a tertiary referral center. One hundred and forty-two patients had complete DHI data (52 with migraine and 90 without migraine). Patients with migraine were diagnosed with posterior canal BPPV at an earlier age than patients without migraine (60.3 vs. 65.5, p=0.004), which validates the finding of another retrospective study.3 However, there was no significant difference in their intake Dizziness Handicap Index (DHI) between the migraine group and non-migraine group (46.5 vs. 43.3, p>0.05). While one study has shown that residual post-BPPV dizziness is more severe in patients with migraine, our finding demonstrates that DHI at the time of evaluation was comparable between the two groups.4 Furthermore, there were no significant differences in the rates of self-reported falls (43.7% vs. 55.3%, p>0.05) or BPPV recurrence (69.0% vs. 74.2%, p>0.05). These findings suggest that migraine alone does not reliably predict the severity of BPPV but that the presence of migraine is associated with earlier onset posterior canal BPPV.
References
1. Kim et al. (2014) New England Journal of Medicine 20(370).
2. Chu et al. (2015) Journal of Headache and Pain 16(62).
3. Faralli et al. (2014) B-ENT 10(2).
4. Whitney et al. (2005) Otology & Neurotology 26(5).
Perception of translation and the impact of orientation and gravity
Megan Kobel, Au.D., Daniel Merfeld, Ph.D.
The Ohio State University, Columbus OH
The otolith organs detect both gravity and linear acceleration and these must be disambiguated for accurate perception of both. While subjects without vestibular dysfunction have been reported to show similar sensitivity to earth-vertical (i.e. parallel to gravity) and earth-horizontal (i.e. perpendicular to gravity) motions (MacNeilage et al., 2010), complete bilateral vestibular loss patients display a larger impact of vestibular loss on earth-vertical superior-inferior (z-axis) translations than earth-horizontal inter-aural (y-axis) translations (Valko et al., 2012). Given these differing conclusions, we set out to comprehensively assess the impact of (1) direction of motion relative to the head (i.e. otoliths), (2) direction of motion relative to gravity, and (3) head orientation relative to gravity (i.e. body position) on translation perception. Vestibular thresholds for 1 Hz translations were quantified for 3 directions in head coordinates (inter-aural (y-axis; y-translation), naso-occipital (x-axis; x-translation), and superior-inferior (z-axis; z-translation) translations using standard methods in a Moog 6DOF motion platform in young healthy subjects (n=12, ages 21-36). Thresholds for all three of these head-axes were assessed in three body orientations (upright, supine, and ear-down), which, unlike the earlier studies, provided a comprehensive design that included nine conditions. We identified a significant main effect for motion direction relative to gravity on translation perception (F=96.358, p<0.0001) as thresholds for earth-vertical translations were significantly higher than earth-horizontal translations. The impact of motion relative to gravity was only seen for y-translation and x-translation thresholds, while z-translation thresholds were not significantly impacted; this may have occurred because any such effect during z-axis translations was offset by the other effects (e.g. motion relative to head and head orientation). Motion relative to the head (F=21.634, p<0.0001) was also identified as a significant main effect as z-translation thresholds were significantly higher than both y-translation and x-translation thresholds while y-translations and x-translations were statistically indistinguishable. A statistically significant main effect of head orientation was also found (F=12.002, p<0.0001) suggesting upright thresholds are lower than those measured when tilted from upright (i.e. supine and ear-down); however, this main effect may be an artifact of our design, which was unbalanced due to physical constraints (e.g. earth-vertical z-translation must be performed while upright), and could capture the large impact of orientation relative to gravity on both y-translations and x-translations. Overall, this study gives fundamental insights into vestibular processing and suggests that translation perception is impacted by motion relative to the head, motion relative to gravity, and head orientation. These results also provide essential normative data needed for future implementation of perceptual thresholds in vestibular diagnosis.
References
1) MacNeilage et al, J. Neurosci., 2010.
2) Valko et al, J. Neurosci., 2012.
Acknowledgements: Funded by NIH/NIDCD R01-DC014924
Feasibility of VestAid: A Tablet-based Technology for Objective Exercise Monitoring in Vestibular Rehabilitation
Klatt1, BN; Hovareshti2, P; Holt2, LS; Zalkin2, C; Tolani2, D; Whitney1, SL
1School of Health and Rehabilitation Sciences, University of Pittsburgh
2Intelligent Automation, Inc.
The feasibility and acceptability of VestAid, a low-cost home-exercise system that helps patients perform prescribed vestibulo-ocular reflex (VORx1) exercises was tested. The system is implemented as a tablet-based app for the patient and a web-based portal for the physical therapist with the physical therapist inputing the parameters of the exercises. Video instructions on the tablet help patients recall how to perform the VOR exercises, and a metronome guides head speed during the exercises. VestAid uses the tablet camera to automatically assess compliance and performance, collects symptom data before and after each exercise, and provides physical therapists with near real-time objective (head speed, gaze compliance) and subjective (pre- and post- exercise symptoms, perceived difficulty) metrics of exercise performance.
The feasibility and acceptability of VestAid in a study of ten participants with various vestibular diagnoses was assessed. Twelve VOR x1 with a stable target and horizontal head movement exercises were selected with different parameters (optotype: size and color, background: color, patterns, movement of the pattern). The exercises were categorized as easy, moderate, and difficult by study team physical therapists. The easy exercises had stationary backgrounds and lower contrast between the optotype and the background, whereas the more difficult exercises had higher contrast, complex patterns, and moving backgrounds. The order of the exercises was randomized.
Participants (mean age = 45, SD = 19; 6 females) completed each of the twelve 30-second VOR x1 exercises once. Participants self-determined the rest break between exercises. All 10 participants successfully completed each of the 12 exercises. Participants were asked about their experience and completed usability surveys using 8 qualitative open-ended questions and 10 statements to which they indicated their agreement (strongly disagree, disagree, neutral, agree, or strongly agree). For the statement “it was easy to rate the difficulty of the exercises,” responses included “strongly agree” (n=4), “agree” (n=5), and “neutral” (n=1). The study team categorized the twelve VOR x1 exercises as either Easy or Hard (six of each). After completing an exercise, each patient rated the perceived difficulty using a 0 to 10 scale (where 0 is extremely easy and 10 is extremely hard). The mean rating of the Easy exercises across participants was 2.7/10, SD = 1.9. The mean rating for the hard exercises across participants was 4.8/10, SD = 2.1. Patients with vestibular disorders were able to complete the trials without adverse events and their ratings were consistent with the difficulty ratings of physical therapists, suggesting that the VestAid device has clinical utility.
Supported by: the US Army Medical Research and Materiel Command under Contract Number W81XWH-18-C-0030. The views, opinions and/or findings contained in this report are those of the author(s) and should not be construed as an official Department of the Army position, policy, or decision unless so designated by other documentation.
Investigating neural encoding in the vestibular nuclei using neuropixel probes
Aamna Lawrence1, Hui Ho Vanessa Chang2, Kathleen E Cullen1
1Department of Biomedical Engineering, Johns Hopkins University, Baltimore, MD, USA
2Department of Physiology, McGill University, Montreal, QC, Canada
Understanding neural circuits and how they generate behavior and perception critically depends on the accurate measurements of neuronal activity. Rapid progress in semiconductor technology has led to the development of high-density silicon probes with many densely packed sites that provide high spatial resolution for electrical measurements. Furthermore, such probes have successfully captured the coordinated activity of neurons, thereby providing insights about population encoding. While deep subcortical brain regions, like the brainstem, have been relatively less explored using the neuropixel probes, in our present study, we were able to successfully isolate single units in the vestibular nuclei using chronically-implanted Neuropixel IMEC 3B probes with 960 recording sites in head-restrained mice. The probe was positioned in the brain, such that there were approximately 112 recording sites in the medial vestibular nuclei. Spike sorting algorithms, namely, Kilosort2 and JRClust, were used for isolating the units. In total, we were able to isolate up to ten head movement-sensitive neurons by passively moving the head using the vestibular ocular reflex paradigm. The units showed robust head velocity sensitivity and no sensitivity for eye position and eye velocity during the static eye position and optokinetic reflex evoking paradigms, as inferred from their firing rates. The head velocity sensitivity (0.25 ± 0.12 (sp/s) / (deg/s)) and the baseline firing rate (18.56 ± 9.03 sp/s) of the neurons agreed with those reported by Beraneck and Cullen (2007) using tungsten microelectrodes. Moreover, our analysis of isolated units provided consistent results across both the above-mentioned spike sorting software. Additionally, we computed the pairwise cross-correlation between the single-units of the neuropixel probes and found synchrony between them, shown previously by Dale and Cullen (2015) in the nucleus prepositus, establishing the role of the connexin-36 gap junctions in the electrical coupling of the vestibular nuclei neurons. Overall, our results highlight the neuropixel probe’s success to investigate deep brain regions and simultaneously record many single units, thereby extending our ability to probe these circuits beyond the capabilities of the conventional tungsten and glass microelectrodes, and find relationships between the isolated neurons. Future experiments will address population encoding during more naturalistic head movements in mice using neuropixel probes.
References
Beraneck, M., & Cullen, K. E. (2007). Journal of Neurophysiology, 98(3), 1549-1565.
Dale, A., & Cullen, K. E. (2015). Journal of Neuroscience, 35 (10), 4287-4295.
Nonhuman primates provide a platform for postural testing in health, vestibular loss, and with vestibular implants
Olivia M.E. Leavitt1, Charles C. Della Santina2, Kathleen E. Cullen1
1Biomedical Engineering
2Otolaryngology, Johns Hopkins School of Medicine, Baltimore, MD
Vestibular loss results in impairments in balance and gait. An important development for restoring sensation following profound bilateral vestibular loss (BVL) is a vestibular implant which stimulates vestibular afferents in response to head rotations. Vestibular implants are in clinical trials and improve patient outcomes, but further research can better optimize their performance[1]. In response to sinusoidal tilt perturbations, normal human subjects undergo a transition from a strategy maintaining body position relative to the surface (platform-fixed) to maintaining the head position in space (head-fixed) at 0.5 Hz[2]. Prediction is involved; during unpredictable pseudorandom tilts subjects are less stable with either strategy[2]. Further, regardless of predictability, subjects with bilateral vestibular loss demonstrate greater postural instability at low frequencies during platform tilts[2]. The extent to which vestibular implants can restore balance in these conditions, particularly in animals, is not known. Experimentation in animal models is essential to further prosthesis development by probing how vestibular circuits involved in balance respond to prosthetic stimulation.
Here, we tested whether balance strategies persist in a monkey model and whether a vestibular implant restores balance, using sinusoidal and pseudorandom tilt stimuli. We trained two adult rhesus macaques, one normal and one with BVL and a multichannel vestibular implant, to stand in an enclosure on a motion platform. The animal’s motion was tracked using markerless pose estimation (DeepLabCut), a head-mounted IMU, and a force plate. Sinusoid stimuli included 6 frequencies of sine wave roll tilts of constant peak velocity, and pseudorandom stimuli were constructed from a pseudorandom ternary sequence. Following habituation to a baseline stimulation rate, the vestibular implant was used to apply stimulation to either the horizontal or posterior semicircular canal. As observed in humans, during sinusoidal roll tilts the normal monkey demonstrated a transition from a platform-fixed to a head-fixed strategy. At low frequencies the body moved little relative to the platform, and at high frequencies the head remained stationary in space. The monkey’s transition frequency was 1 Hz, higher than humans’ due to biomechanical differences. The transition frequency persisted during pseudorandom stimuli, but with greater instability than sinusoidal, as with normal humans. The BVL animal’s postural responses mirrored those of human subjects with BVL: greater instability during low-frequency sinusoidal stimuli, and less stable response to pseudorandom than sinusoidal stimuli. Prosthetic stimulation of the horizontal canal did not reduce the postural instability observed in the BVL monkey. Prosthetic stimulation of the posterior canal did reduce postural instability from BVL at low frequencies. Together, these results indicate that rhesus provide a useful model of posture in normal and BVL conditions for probing how vestibular circuits involved in balance respond to prosthetic stimulation, and that prosthetic stimulation of vestibular afferents improves posture.
[1] P. J. Boutros et al., “Continuous vestibular implant stimulation partially restores eye-stabilizing reflexes,” JCI Insight, vol. 4, no. 22, p. e128397, 2019.
[2] L. Assländer and R. J. Peterka, “Sensory reweighting dynamics in human postural control,” J Neurophysiol, vol. 111, pp. 1852–1864, 2014.
Computerized Rotational Head Impulse Test: Test-Retest Reliability using a head-fixed target
Roxanne Loock1, Barbara Heinze (PhD)1, Alex Kiderman (PhD)2 Jorge E. González (PhD)3
1Department of Speech-Language Pathology and Audiology, University of Pretoria, South Africa
2Neurolign, USA, LLC
3Department of Communication Sciences and Disorders, East Carolina University, Greenville, NC, USA
Introduction: The computerized rotational head impulse test (crHIT) was recently developed to overcome limitations of the video head impulse test (vHIT) and assess the functioning of the lateral semicircular canals (SCC) in a more objective manner. Instead of an examiner applying rapid, brief and unpredictable head rotations, as during the vHIT, the crHIT utilizes computer-controlled whole-body rotations. The crHIT has displayed good test-retest reliability using a stationary target, however this system can also be adapted for use with head-fixed targets such as those utilized during suppression head impulse (SHIMP) testing. Since the crHIT is newly developed, its test-retest reliability for clinical use with a moving target needed to be determined.
Methods: Thirty-one healthy adult participants, between the ages of 18 and 40, with normal lateral SCC functioning and no symptoms or history of vestibular dysfunction were assessed with the crHIT using head-fixed targets. These participants were assessed on three separate occasions; the second evaluation took place one to six hours after the initial evaluation and the third evaluation 24 hours to two weeks after the second evaluation.
Results: A one-way repeated measures ANOVA with a Greenhouse-Geisser correction demonstrated significant differences in the aVOR suppression latencies, as well as the aVOR gain values, across the three testing sessions, with these latencies decreasing both across the three testing intervals as well as within the individual testing sessions.
Conclusion: Further investigation is required to determine the physiological mechanisms underlying the decreased aVOR suppression latencies across multiple testing sessions. However, it is suspected that aVOR and saccadic adaptation mechanisms have potentially contributed to the decrease in aVOR suppression latencies and aVOR gain values across multiple testing sessions.
The impact of persistent and resurgent voltage-gated sodium currents on excitability in vestibular ganglion neurons
Selina Baeza Loya, Ruth Anne Eatock, Ph.D.
Department of Neurobiology, University of Chicago, Chicago IL
Background: The vestibular inner ear transmits head-motion information to the brain via two populations of primary vestibular ganglion neurons (VGNs), which differ in the regularity of action potential (AP) timing. The two kinds of AP timing (regular and irregular) represent rate and temporal encoding, respectively (Jamali et al., Nat Comm 7:13229, 2016). Understanding the impact of diverse ionic currents on spike timing regularity and waveform is therefore crucial to understanding how different sensory coding strategies arise. Although voltage-gated sodium (NaV) currents are responsible for the rising phase of APs, their contributions to differences in AP regularity are not fully understood. Rodent vestibular ganglia express all NaV channel α and β subunits, including NaV1.6 which mediates NaV currents in vestibular calyceal terminals (Rennie and Meredith, J Neurophysiol 124:510, 2020). NaV currents through a given α subunit can be transient (inactivating current), persistent (non-inactivating current), and/or resurgent (current flow after relief from inactivation block). We are interested in how these modes of NaV current influence AP firing in VGNs.
Methods: We use biophysical and computational approaches to investigate. Whole-cell patch-clamp electrophysiological recordings were taken from mouse VGNs (postnatal days, P, 3-25) that were isolated and cultured overnight. However, it is difficult to experimentally distinguish the effects of transient, resurgent, and persistent currents on spiking because we lack methods to isolate components. We therefore modified the Kalluri group’s computational conductance-based VGN model (Hight and Kalluri, J Neurophysiol 116:503, 2016; Ventura and Kalluri, J Neurosci 39:2860, 2019), which included generic transient NaV current, by adjusting and adding expressions for transient, persistent, and resurgent NaV components with properties based on our data.
Results: In a sample of 68 VGNs, all had large transient NaV currents that were blocked by 1 μM TTX; 37 had persistent NaV current (P3-25), 5 had resurgent NaV current (P17-20), and 3 (P17-20) had both persistent and resurgent currents. Application of the NaV1.6 blocker 4,9-anhydro-tetrodotoxin (4,9-ah-TTX) indicated that both transient and persistent currents had a substantial NaV1.6 component. In current clamp, 4,9-ah-TTX decreased neuronal excitability: increasing current threshold for spiking in all VGNs, and decreasing AP rate in sustained (regular) VGNs. In the computational model, adding persistent and resurgent current components had a negligible effect on firing by the model transient (irregular) VGN. For the model sustained (regular) VGN, adding persistent and resurgent currents decreased spike latency (delay relative to current step onset) and increased spike rate.
Conclusions: Increasing NaV channel availability in the after-spike interval with persistent and resurgent currents may enhance the excitability of regular VGNs and so help to shape sensory encoding by the vestibular inner ear.
Supported by: NIH DC012347 and HHMI Gilliam Fellowship for Advanced Study
The reduction in the velocity storage time constant due to unilateral hypofunction is a response to changing peripheral signal-to-noise characteristics
Amsal Madhani, Research Associate, Faisal Karmali, Ph.D, Richard F. Lewis, MD
Jenks Vestibular Physiology Lab, MEEI; Harvard Medical School - Boston, MA.
The velocity storage mechanism in a central mechanism that processes peripheral vestibular cues. This includes the elongation of the semicircular time constant of approximately 5 s to the central velocity storage time constant, which is roughly 15-25 s in individuals with no known vestibular pathology. The velocity storage time constant is an important clinical parameter which varies with age, pathology and stimulus amplitude. In fact, the velocity storage time constant is a key diagnostic parameter for peripheral loss, with a time constant of 6-12 s suggesting unilateral hypofunction. Despite its clinical utility, there is little mechanistic understanding of why unilateral hypofunction should result in a lower velocity storage time constant. It has been hypothesized that Bayesian optimal processing determines velocity storage dynamics based on the statistics of vestibular noise and experienced motion. Specifically, while a longer time constant would be advantageous because this would make the vestibulo-ocular reflex accurate over a longer period of time, it has been argued that this would amplify neural noise and thus, make the VOR less precise. In particular, it has been hypothesized that the brain determines the optimal velocity storage time constant based on vestibular noise to determine the optimal tradeoff between being accurate and being precise. In this study we applied a Bayesian optimal Kalman filter model to determine the ideal velocity storage time constant for unilateral hypofunction. Since the exact effect of unilateral hypofunction on vestibular noise is unknown, we developed four scenarios based on findings in the literature. In all scenarios, the models predicted a velocity storage time constant that was substantially lower than for normal subjects. In particular, one plausible model predicted velocity storage time constants between 7 and 10 s, which is consistent with clinical findings. This suggests that this clinically-relevant change results from the brain optimizing velocity storage in response to a change in the peripheral signal-to-noise ratio. These results complement our existing work showing that age-related time constant variations are explained by an optimal adjustment in response to hair cell death.
Acknowledgements: Funded by NIDCD R01-DC018287-01
KV1.8 potassium channels in mouse vestibular hair cells
Martin HR1, Lumbreras V1, Pregernig G2, Negi S2, So KS2, Kowalczyk M2, McCarthy R2, Palermo AT2, Price SD3, Lysakowski A3, Burns JC2, Eatock RA1
1University of Chicago
2Decibel Therapeutics, Inc
3University of Illinois at Chicago
Potassium (K+) currents in vestibular hair cells play dynamic roles in shaping receptor potentials. Voltage-gated K+ (KV) channels are strikingly different in the type I and type II hair cells (HCI and HCII) of amniote vestibular organs. HCI have a non-inactivating low-voltage-activated K+ conductance (gK,L) that strongly affects the gain and time course of receptor potentials and participates in non-quantal transmission with the post-synaptic calyx. HCII lack gK,L; as in most hair cells, their KV channels activate positive to resting potential, including an inactivating A-type K+ conductance (gA). Here we report evidence that KV1.8 channel expression plays a role in the expression of both gK,L and gA. KV1.8 (gene name: Kcna10) is highly expressed in mouse vestibular hair cells (Scheffer et al., 2015) and, in a constitutive knockout of KV1.8 (B6-Kcna10TM45 mice; Lee et al., 2013), the vestibular evoked potential, VsEP, in response to head accelerations was strongly reduced.
To investigate possible contributions of KV1.8 channels to hair cell voltage-gated currents, we recorded from hair cells in semi-intact preparations of the utricles of B6-Kcna10TM45 mice (postnatal days 10-300). Sectioned utricular epithelia were stained with anti-KV1.8 (Alomone). The level of Kcna10 transcripts in subtypes of mouse utricular hair cells was assessed using an existing single-cell RNA-Seq database that spans embryonic (E14.5) to adult ages.
HCI in null mutants lacked gK,L: relative to littermate controls, steady-state maximal conductance density was reduced 95% and voltage of half-maximal activation (Vhalf) was shifted +40 mV. HCII in null mutants lacked gA: relative to littermate controls, peak maximal conductance density was reduced 66-84% and inactivation time course was greatly slowed. The HCI and HCII conductances that depend on KV1.8 expression (gK,L and gA) have very different voltage dependence: gK,L Vhalf –82 ± 1 mV (mean ± SE, n=45); gA Vhalf –22 ± 1 mV (29). Antibody labeling of null and wildtype utricles shows specific expression in HCI and HCII basolateral membranes, and single-cell RNAseq shows Kcna10 expression in both HCI and HCII, with higher levels in HCI.
If KV1.8 is a pore-forming subunit in both gK,L and gA, then the voltage dependence and inactivation rate of these conductances depend on additional factors that are differentially expressed in HCI and HCII. Single-cell RNA data suggest candidates: ß subunits KV1ß1 and KV1ß2 are enriched in HCI and HCII, respectively, and the A-type channel subunit KV1.4 is enriched in HCII. Pharmacological experiments on HCII suggest that gA may be a heteromer of KV1.4 and another KV1 a subunit, for which KV1.8 is a possible candidate.
References
Lee et al (2013). Hearing Research 300.
(2) Scheffer et al (2015). Journal of Neuroscience 35(16).
Acknowledgements: Supported by NSF Graduate Research Fellowship to HRM and NIH grant R01 DC012347. We thank T.B. Friedman and S.M. Jones for the generous gift of B6-Kcna10TM45 mice.
How differences in perceived orientation affect visual self-motion and the perceptual upright
Meaghan McManus, Dr. Laurence R. Harris
Centre for Vision Research, York University
How we interpret our visual environment affects how we interpret ourselves within that environment. If a person and their environment are tilted together some people experience a visual reorientation illusion (VRI) where they feel upright (Howard & Hu, 2001). In situations where VRI are likely to occur visually-induced self-motion (vection) is enhanced (McManus & Harris, 2019 VSS). This suggests that participants who report VRIs might have a higher visual weighting due to ignoring the gravity vector (Howard & Hu, 2001).
Here we investigated the connection between VRIs and sensory weighting using virtual reality. Perceived self-motion was measured by having participants complete a visual self-motion task where they visually moved to previously seen target locations while standing, supine, and prone. Shorter travel distances (higher gain) indicated a stronger vection experience. Several different measures were used to estimate participant’s sensitivity to VRIs, including a questionnaire and self-report, and they were divided into VRI and non-VRI groups. The perceptual upright (PU; Dyde et al. 2006) was then measured while sitting or lying on their side to estimate the weightings of vision, body, and gravity. Participants reported whether an ambiguous symbol in various orientations appeared as a “p” or “d” as the visual background orientation was varied. The PU was defined as midway between the orientations of maximum ambiguity and the weighting of each cue determined geometrically.
Using a linear mixed model, we assessed whether VRI grouping affected the gain of perceived self-motion. There was a main effect of VRI on gain (F(1, 304.543)= 4.888, p< 0.028) in which the VRI group had a higher gain compared to the no-VRI group (mean difference= 7.50%, SE= 3.40%). For the PU task, t-tests were used to compare between VRI groups for each of the sensory weights. The vision and body weightings did not differ between the VRI and non-VRI groups, however the VRI group had a significantly higher weighting of gravity (mean difference= 6.70%, SE= 3.06%, p=0.035).
Despite having a perceived orientation seemingly dominated by vision and enhanced perceived self-motion, VRI-vulnerable people’s PU is actually more influenced by gravity. Instead of placing more weight on vision, VRI-vulnerable individuals might be more sensitive to visual-vestibular conflict, where placing more weight on gravity allows them greater sensitivity in detecting a conflict between the gravity and visually indicated directions of up. Experiencing a VRI may represent a resolution of such a conflict.
References
(1) Dyde, R. T., Jenkin, M. R., & Harris, L. R. (2006). Experimental Brain Research 173(4), 612-622.
(2) Howard, I. P., & Hu, G. (2001). Perception 30(5), 583 600.
(3) McManus, M., & Harris, L. R. (2019). Journal of Vision 19(10), 237.
Acknowledgments: LRH is supported by a Discovery Grant from the Natural Sciences and Engineering Research Council (NSERC) of Canada and the Canadian Space Agency. MM holds a research studentship from the NSERC CREATE program.
Dopamine decreases voltage-gated sodium currents in vestibular calyx terminals
Frances L. Meredith Ph.D. and Katherine J. Rennie Ph.D.
Department of Otolaryngology, University of Colorado School of Medicine, Aurora CO 80045
Type I vestibular hair cells are innervated by large afferent calyx terminals and efferent fibers make synapses with the outer face of calyces. Although acetylcholine is believed to modulate afferent transmission, other efferent neurotransmitters may also be involved. We have recently reported that calyx terminals in thin slices of gerbil cristae express tetrodotoxin-sensitive Na+ currents with transient, persistent and resurgent components (Meredith and Rennie 2020). Dopamine is a candidate efferent neurotransmitter in the vestibular system and we investigated the effect of dopamine on Na+ currents in calyces. Calyx terminals were isolated along with their accompanying type I hair cells from the cristae of male and female gerbils aged 21-27 days. Whole cell voltage clamp recordings were obtained from isolated calyces as described previously (Rennie and Streeter 2006). Large transient Na+ currents were present in all isolated calyces, but resurgent Na+ currents were only detected in 1/15 cells studied. Perfusion of dopamine (100 μM) in the extracellular solution significantly reduced peak transient Na+ currents in 5/7 cells tested. Dopamine application reduced peak transient Na+ currents to 82.8 ± 17.3 % (mean ± SD) of control and partial or complete recovery was observed on return to control extracellular solution.
Dopamine and agonists of dopamine receptors were reported to decrease action potential firing rate in frog semicircular canal afferents (Andrianov et al. 2009), but the effect of dopamine on firing in mammalian vestibular afferents remains unknown. Our preliminary results show a reduction in transient Na+ current in response to dopamine suggesting that dopamine may decrease firing in calyx afferents. Future studies will examine the possible roles of D1 and D2 receptor subtypes in dopaminergic signaling in vestibular afferents.
1. Andrianov GN, Ryzhova IV, Tobias TV. Neurosignals 17:222-228, 2009.
2. Meredith FM, Rennie KJ. J. Neurophysiol. 124: 510-524, 2020.
3. Rennie KJ, Streeter MA. J. Neurophysiol. 95: 26-32, 2006.
Acknowledgement: Supported by NIH/NIDCD DC016860.
Multisensory effects on illusory self-motion (vection): The role of visual, auditory, and tactile cues
Brandy Murovec, BSc1, Julia Spaniol, PhD1, Jennifer Campos, PhD2, Behrang Keshavarz, PhD2
1Ryerson University, Toronto, ON
2Toronto Rehabilitation Institute, Toronto, ON
Virtual reality (VR) is being increasingly used in a variety of domains such as training, research, and entertainment. One of the most critical components to an immersive experience in VR is vection, defined as the illusion of self-motion. Traditionally, vection has been described as a visual phenomenon, but more recent research has shown that vection is also influenced by a variety of other senses. The goal of the present study was to further investigate the role of multisensory cues on vection using novel visual, auditory, and tactile stimuli. To achieve this, 24 younger adults participated in a study at KITE-Toronto Rehabilitation Institute’s dome-shaped, immersive VR laboratory equipped with floor to ceiling curved projection screens (240° horizontal and 110° vertical field-of-view) and a 7-speaker surround sound system. Participants were seated in a rotatable chair in the center of the lab and were exposed to a revolving stimulus aimed to induce the illusion of rotational self-motion along the vertical body axis (i.e., circular vection). The rotating stimulus contained visual (photorealistic virtual office scene), auditory (three stationary sound sources placed within the same virtual office scene), and/or tactile (a circular handrail within reach that rotates around the participants) cues. All participants were exposed to trials that either included a single sensory input (visual, auditory, or tactile cues alone), a combination of two cues, or a combination of all three sensory cues presented together. The size of the visual field of view (FOV) was manipulated at 4 levels (small, medium, full, and no visuals). Following each trial, data on vection intensity and duration was collected verbally using scales ranging from 0-10 and 0-100%, respectively. Results showed that all three sensory cues successfully induced vection when presented in isolation, with visual cues eliciting the most compelling sensation as expected. Additionally, main effects of auditory and tactile cues suggested overall more intense and prolonged vection when these cues were provided. In the restricted FOV conditions, a multisensory effect was found, indicating stronger vection intensity and longer vection duration in conditions which contained multiple sensory cues compared to a single sensory cue alone. Our findings support the idea that vection is not purely a visually driven phenomenon, but rather a multisensory experience which can be bolstered with the addition of auditory and tactile cues. The present study delivered valuable insights towards understanding how multisensory stimulation influences vection and may inform strategies to improve the level of realism and immersion for an optimal VR experience.
Keywords: Vection, Self-motion, Multisensory
Age, motion cues, and baseline vestibular functioning predict driving performance in a simulated driving task
Robert J. Nowosielski, MSc1, Bruce Haycock, PhD2, Behrang Keshavarz, PhD3, Jennifer Campos, PhD3
1The KITE Research Institute, University Health Network (UHN), Department of Psychology, University of Toronto, Toronto, Canada
2The KITE Research Institute, UHN; Institute for Aerospace Studies, University of Toronto, Toronto, Canada
3The KITE Research Institute, UHN; Department of Psychology, Ryerson University, Toronto, Canada
Vestibular function is known to change with age, but the effects of these changes on many functional behaviours requiring self-motion perception is largely unknown. Driving is a complex task that involves different types of motion cues that are sensed via the vestibular system and are used to guide behaviour. However, it is not clear whether there are age-related differences in how different types of motion cues affect driving performance. Driving simulators are valuable tools for examining driving performance in a safe and controlled way, yet they differ widely in fidelity, including their motion capabilities. Using KITE’s high-fidelity, 6 degree-of-freedom driving simulator, we measured the driving performance of older and younger drivers across three different physical motion conditions: no motion (fixed base), rotational motion (yaw), and full motion (yaw, pitch, roll, and translational motion) using a between-subjects design (2 age (older vs. younger) x 3 motion conditions (fixed, yaw, full)). We tested 34 younger adults (18 – 35 years) and 32 older adults (65+ years) using two, 15-minute driving scenarios for each motion condition. All participants first completed a 15-minute fixed-based driving scenario at baseline. Driving performance was measured using several driving parameters, such as speed variability and lane deviations. Baseline measures of dynamic visual acuity and posturography (center of pressure length), both indirect measures of vestibular function, were also evaluated. The main objectives of this study were: 1) To evaluate how different types of motion affect simulated driving performance; 2) To examine the relationship between baseline measures of vestibular functioning and driving performance; 3) To determine whether there are age-related differences observed for 1 and 2. We hypothesized an additive and beneficial effect of motion (no motion to yaw, yaw to full motion) on driving performance (e.g. reduced speed variability, reduced lane deviations), with older adults benefiting more from additional motion components than younger adults. Our preliminary results demonstrate that, compared to younger adults, older adults demonstrated a greater improvements in lane keeping and steering reversals with the addition of yaw motion, and a significant decrease in steering reversals and lane departures with the addition of full motion, despite driving more poorly overall. Additionally, both younger and older adults demonstrated a reduction in speed variability with the addition of yaw and full motion. Furthermore, across groups, poorer dynamic visual acuity at baseline was associated with more speed variability and more steering reversals. Less stable standing balance (i.e. greater center of pressure path length) at baseline was associated with a more lane departures, more variable lane positioning and more steering reversals. Overall, these findings suggest that older adults may benefit more from the addition of motion than do younger adults. Exploring the relationship between the baseline measures of vestibular functioning and driving performance may also provide additional insights into the importance of motion cues during simulated driving and how they may be influenced by vestibular abilities.
Defining the vestibulopathy of vestibular migraine: ictal and interictal changes on neurovestibular testing
John G. Oas, MD
The Ohio State University, Columbus OH
The mechanisms that define the transient vestibulopathy of vestibular migraine remain elusive. Previous reports of the vestibular test findings in neurovestibular test cases suggest both ictal (during an event) and interictal (in between events) abnormalities. The ictal findings previously reported mostly include spontaneous or positional nystagmus, with one case (Na et al, 2019) presenting with a reversible unilateral peripheral vestibular loss (caloric paresis, changes in the gain and phase lead of the vestibuloöcular reflex (VOR) as well as video head impulse changes). Interictal findings have demonstrated a return to normal, a caloric paresis without changes in the VOR (Dimitri et al, 2001), and a sensitization of self-motion perception (King et al, 2019). We report here in detail a case of definite vestibular migraine (ICHD-3 A1.6.5 criteria) with both ictal and post-ictal (20 days later) neurovestibular test findings showing changes in VOR gain and phase consistent with peripheral vestibular hypofunction, intact utricular otolith function well as dysfunction affecting gaze-holding and visual tracking as well and the fixation suppression of the VOR without caloric paresis that disappeared 20 days later. We compare this case along with the neurovestibular test finding in a case of autopsy proven VV2 variant Creutzfeldt-Jakob Disease and a case of Gaucher’s Disease. In these latter two cases, the pathology suggests a brainstem localization for the VOR changes. These ictal findings in vestibular migraine suggest transient (reversible) vestibulopathy localized to the brainstem in the vestibular nuclei and affecting central oculomotor pathways during the vestibular migraine event.
References: 1. Na et al; (2019) J Neurol 266:242. 2. Dimitri et al; (2001) J Vest Res 11(1):53. 3. King et al; (2019) Sci Rep 9(1):14323.
Bouton afferent terminals activity from mouse utricle
Omar Lopez-Ramirez1+, Antonia Gonzalez-Garrido1+, Ruth Anne Eatock1
1Department of Neurobiology, University of Chicago, Chicago IL 60637
+Equal contribution.
The activity of vestibular afferent neurons drives reflexes that stabilize gaze and head position. In mammals, vestibular afferent neurons terminate on hair cells with either bouton endings on type II hair cells or calyceal endings on type I hair cells. These terminals distribute differently in the functional zones of the sensory epithelium. The utricular epithelium, has striolar (central) and extrastriolar (peripheral) regions with striking anatomical and functional differences, including a difference in spike timing regularity. Most afferents make both endings, but the striola has calyx-only terminals and the extrastriola has a small number of bouton-only terminals. While whole-cell recordings from vestibular afferent calyces are available in some rodent species, there have been no reported recordings from vestibular bouton terminals. Here, we report preliminary observations on the membrane conductances and action potential properties of mammalian vestibular bouton terminals.
We used whole-cell patch clamp to record from boutons in the in vitro semi-intact preparation of CD1 mouse utricular epithelia (P10-21), with physiological solutions at room temperature. This preparation conserves the hair cells, primary afferent innervation and mechanosensory pathway, allowing the study of primary afferent synapse mechanisms. Bouton morphology was revealed by fluorescent dye from the recording pipette. Bouton endings were tested for voltage-dependent conductances, voltage responses to current injection, and synaptic transmission evoked by deflecting the bundles of type I and type II hair cells. We recorded from striolar (S, 4) and extrastriolar (ES, 10) boutons.
Boutons had HCN and voltage-gated K (KV) channels (IKDR, KLV and A-type). Striolar boutons were larger (3.4 ± 0.7 pF) than extrastriolar (2.1 ± 0.1 pF; p = 0.03) and, similar to calyces, had more negative resting potentials (-74.3 ± 1.4 mV vs. -67.7 ± 1.7 mV; p = 0.04), input resistances did not differ significantly (S: 181.3 ± 16.5 M?; ES: 255.7 ± 51.7 M?; p = 0.4). As with calyces, positive current steps evoked sustained firing in some boutons and transient firing in others. In dimorphic afferents, we demonstrate electrotonic propagation of non-quantal calyceal responses to bouton terminals in the same afferent. We recorded both regular and irregular spontaneous activity of bouton endings, without clear correlation with zone. In one case, we recorded a bouton on a bouton-only extrastriolar afferent; it had relatively small capacitance (1.8 pF), high input resistance (691.2 M? vs 255.7 ± 51.7 M? for 8 dimorphic ES boutons), small voltage-gated currents, and a transient spiking response to injected currents with a low current threshold (40 pA).
These preliminary bouton data show a range of voltage-gated conductances and firing properties that have also been described in calyceal recordings. These data will help to build a model of how information from boutons and calyces is electrically integrated in vestibular afferent firing.
Supported by: NIDCD (R01 DC012347).
Objective detection of vestibular evoked myogenic potentials
Daniel J. Romero, Au.D.1 , Erin G. Piker, Au.D., Ph.D.1, Christopher Zalewski, Ph.D.2, Christopher Clinard, Ph.D.3
1Department of Communication Sciences and Disorders, James Madison University, Harrisonburg, VA
2National Institutes on Deafness and Other Communication Disorders (NIDCD), Bethesda, MD
3Department of Communication Sciences and Disorders, James Madison University, Harrisonburg, VA
The cervical and ocular vestibular evoked myogenic potential (cVEMP and oVEMP) are objective measures of otolith function; yet, the presence or absence of the response depends on visual detection by an examiner. Visual detection becomes difficult and interrater reliability decreases when there is reduced vestibular function or when the level of muscle contraction (electromyography or EMG) is low (Arnold, 1985; Obeidat & Lewis Bell, 2019). High stimulus levels and maximal EMG result in more robust responses, and are now considered a requirement during routine VEMP testing (Rosengren et al., 2019). More recent investigations examining the high levels of stimulation used during VEMP testing have raised concerns regarding noise exposure in children (Rodriguez et al., 2018). In addition, the effect of age on EMG results in more variable responses along with a difficulty reaching higher levels of muscle contraction (Akin et al., 2011). A detection method that allows for lower stimulus levels and less reliance on maximum EMG activation during the test is needed.
Objective detection algorithms, such as fixed single point (Fsp), were introduced in auditory evoked potentials to alleviate the burden of visual detection. When applied to the waveform plotted across time, Fsp estimates the signal and noise components by calculating the variance within a specified time window (i.e. signal) and compares it to the variance of a noise estimate represented at single point in time (e.g. -2 msec). The result is a ratio between the signal and noise and the response is determined to be present or absent using an F-test (Elberling & Don, 1984). Fsp has a wide range of clinical applications in auditory evoked potentials and there is emerging evidence that Fsp can be applied to VEMPs.
The purpose of this investigation was to characterize the behavior of Fsp in cVEMPs and oVEMPs and compare Fsp to visual detection in a group of young healthy participants. Air-conducted cVEMPs and oVEMPs were measured in response to a 500 Hz toneburst. For cVEMPs, the stimulus level was systematically varied between 90 - 123 dB peak SPL and EMG target levels ranged between 10 – 150 μV. For oVEMPs, only the stimulus level was manipulated. Fsp was applied to every recording offline using a custom algorithm written in MATLAB.
Several preliminary results are reported. When applied to VEMP detection, Fsp values increased as stimulus level increased in cVEMPs and oVEMPs, however Fsp values remained significant at lower stimulus levels. In cVEMPs, Fsp values were comparable across different levels of EMG activation and maximum EMG activation did not yield larger Fsp values. Finally, when Fsp was used to detect VEMPs as opposed to visual detection, reliable and robust Fsp values were obtained even in cases where the level of the stimulus and muscle contraction were low. The results from this investigation show the feasibility of utilizing an objective detection algorithm in vestibular evoked potentials and have the potential to shift conventional thinking about the role of stimulus level and muscle contraction during routine VEMP testing.
References:
Akin, F. W., Murnane, O. D., Tampas, J. W., & Clinard, C. G. (2011). The effect of age on the vestibular evoked myogenic potential and sternocleidomastoid muscle tonic electromyogram level. Ear and Hearing, 32(5), 617–622. https://doi.org/10.1097/AUD.0b013e318213488e
Arnold, S. A. (1985). Objective versus visual detection of the auditory brain stem response. Ear and Hearing, 6(3), 144–150. https://doi.org/10.1097/00003446-198505000-00004
Elberling, C., & Don, M. (1984). Quality Estimation of Averaged Auditory Brainstem Responses. Scandinavian Audiology, 13(3), 187–197. https://doi.org/10.3109/01050398409043059
Obeidat, F. S., & Lewis Bell, S. (2019). Objective methods to measure vestibular evoked myogenic potential response saccular tuning curves. International Journal of Audiology, 58(11), 724–732. https://doi.org/10.1080/14992027.2019.1613574
Rodriguez, A. I., Thomas, M. L. A., Fitzpatrick, D., & Janky, K. L. (2018). Effects of high sound exposure during air-conducted vestibular evoked myogenic potential testing in children and young adults. Ear and Hearing, 39(2), 269–277. https://doi.org/10.1097/AUD.0000000000000484
Rosengren, S. M., Colebatch, J. G., Young, A. S., Govender, S., & Welgampola, M. S. (2019). Vestibular evoked myogenic potentials in practice: Methods, pitfalls and clinical applications. Clinical Neurophysiology Practice, 4, 47–68. https://doi.org/10.1016/j.cnp.2019.01.005
Acknowledgments: Funded by the American Academy of Audiology Foundation (Dept ID# 550967).
Modeling the Interaction among Three Cerebellar Disorders of Eye Movements: Periodic Alternating, Gaze-evoked and Rebound Nystagmus
Ari A Shemesh1, Koray Kocoglu2, Gülden Akdal2,3, Rahmi Tümay Ala3, G. Michael Halmagyi4, David S Zee1,5 and Jorge Otero-Millan1,6
1Department of Neurology, The Johns Hopkins University School of Medicine, Baltimore, Maryland, USA
2Department of Neurosciences, Institute of Health Sciences, Dokuz Eylül University, İzmir, Turkey
3Department of Neurology, Faculty of Medicine, Dokuz Eylül University, İzmir, Turkey
4Department of Neurology, Royal Prince Alfred Hospital and University of Sydney, Sydney, Australia
5Departments of Ophthalmology, Otolaryngology-Head and Neck Surgery and Neuroscience, The Johns Hopkins University School of Medicine, Baltimore, Maryland, USA
6School of Optometry, University of California Berkeley, Berkeley, California, USA
A woman, age 44, with a positive anti-YO paraneoplastic cerebellar degeneration syndrome and normal imaging developed an ocular motor disorder including periodic alternating nystagmus (PAN), gaze-evoked nystagmus (GEN) and rebound nystagmus (RN). During fixation there was typical PAN but changes in gaze position evoked complex, time-varying oscillations of GEN and RN.
Methods: The patient’s eye movements were recorded with an infrared video goggles system. Experiment 1 demonstrated the oscillatory behavior of the vestibular system in isolation from the gaze-holding system. The patient was asked to fixate a central target for 5 min. Experiments 2 and 3 were designed to elicit GEN and RN. For experiment 2 the patient was asked to alternate her gaze every 10 seconds between center fixation and a left target, between center and a right target, or between the right and left target, for a total of three minutes. Left and right target positions were at 30 degrees. For experiment 3, the patient fixated a central target for three minutes after a sustained attempt to look eccentrically to right or left 40 degrees, one minute for each eccentric gaze position.
Modelling: To unravel the pathophysiology of this unusual pattern of nystagmus, we developed a mathematical model of normal function of the circuits mediating the vestibular-ocular reflex1 and gaze-holding including their adaptive mechanisms.
Results: Simulations showed that all the finding in our patient of apparently complex time varying oscillations of GEN and RN, could be explained by two, small, isolated changes in cerebellar circuits: reducing the time constant of the gaze-holding integrator to ~ 2s, producing GEN and RN, and increasing the gain of the vestibular velocity-storage positive feedback loop by about 1.5, producing PAN.
Conclusion:
(1) The gaze- and time-varying pattern of nystagmus in our patient can be accounted for by superposition of one model of the vestibular system that produces typical PAN and another model of the gaze-holding network that produces typical GEN and RN, without requiring a new oscillator in the gaze-holding system or a more complex, nonlinear interaction between the two models. (2) This analysis also suggests a bedside strategy for uncovering gaze-evoked and rebound nystagmus in the setting of a time-varying nystagmus such as PAN. (3) Our results support current ideas of compartmentalization of cerebellar functions for the control of the vestibular velocity-storage mechanism (nodulus and ventral uvula) and for holding horizontal gaze steady (the flocculus and tonsil).
References
(1) Leigh RJ, Robinson DA, Zee DS (1981) A hypothetical explanation for periodic alternating nystagmus: instability in the optokinetic-vestibular system. Ann N Y Acad Sci 374:619–635.
Modelling the effect of gravity on Periodic Alternating Nystagmus
Ari A Shemesh1, Koray Kocoglu2, Gülden Akdal2,3, Rahmi Tümay Ala3, G. Michael Halmagyi4, David S Zee1,5 and Jorge Otero-Millan1,6
1Department of Neurology, The Johns Hopkins University School of Medicine, Baltimore, Maryland, USA
2Department of Neurosciences, Institute of Health Sciences, Dokuz Eylül University, İzmir, Turkey
3Department of Neurology, Faculty of Medicine, Dokuz Eylül University, İzmir, Turkey
4Department of Neurology, Royal Prince Alfred Hospital and University of Sydney, Sydney, Australia
5Departments of Ophthalmology, Otolaryngology-Head and Neck Surgery and Neuroscience, The Johns Hopkins University School of Medicine, Baltimore, Maryland, USA
6School of Optometry, University of California Berkeley, Berkeley, California, USA
Introduction: Periodic alternating nystagmus (PAN) is a rare oscillatory ocular motor disorder. The effects of gravity on the dynamic behavior of PAN can be studied by monitoring the nystagmus in head positions where “virtual” head rotation signals of PAN (virtual because the head is not rotating) are not about an axis parallel to the pull of gravity. Previous studies of patients with PAN reached different conclusions about gravity and PAN. Furman et al., in four patients, reported PAN was unaffected by gravity in supine and ear-down positions1. In contrast, Chung et al., in a single patient, showed suppression of PAN over a timescale of a few minutes during ear-down positions2.
What neuronal circuits account for the difference in the effects of gravity among PAN patients? We considered the effects of ablation of the nodulus and ventral uvula in monkeys since these lesions cause PAN, impaired tilt suppression of post rotatory nystagmus3, and eliminate the steady-state response during off-vertical axis (OVAR) rotation4. The steady-state response of OVAR and tilt suppression of post rotatory nystagmus are normally generated through the vestibular velocity-storage integrator via a “rotation feedback” loop that realigns a central estimate of gravity with gravitoinertial acceleration (GIA). The rotation feedback functions mathematically as a cross-product. Its inputs are the GIA as sensed by the otolith, and the central estimated gravity. Its output is a velocity signal that is injected into the velocity storage integrator. Together with the results of the ablations studies, we hypothesize that variations in the gain of rotation feedback account for the difference in the effects of gravity among PAN patients.
Methods: In a patient with PAN eye movements were recorded in upright, ear down and supine positions.
Modelling: We used a model of how the brain resolves the tilt-translation ambiguity5,6, but also incorporated an unstable, oscillatory vestibular system generating PAN7.
Results: PAN was suppressed in our patient in ear-down positions, in a similar pattern to that of Chung et al.2 This effect was simulated by reducing the gain of the projection of the rotation feedback loop to the velocity-storage integrator to approximately 15% of its normal value. Moreover, by disconnecting the rotation feedback (gain = zero) the model simulated PAN that is unaffected by gravity as reported by Furman et al1.
Conclusions:
(1) The slowly oscillating “virtual” head rotation signals of PAN are mathematically integrated in supine and ear-down positions leading to an erroneous estimate of the orientation of the head to gravity since the head is stationary.
(2) In PAN, the rotation feedback loop, normally used to resolve the tilt translation ambiguity, may malfunction.
(3) A low gain of rotation feedback can lead to suppression of PAN in head down positions, but the effect takes a few minutes.
(4) With zero gain rotation feedback PAN is unaffected by head down positions.
(5) In patients with PAN, understanding how brain resolves the tilt-translation ambiguity can enhance both the clinician’s ability to localize neurological disorders and our knowledge of how the normal brain functions.
References
(1) Furman JM, Wall C, Pang DL (1990) Vestibular function in periodic alternating nystagmus. Brain J Neurol 113 (Pt 5):1425–1439.
(2) Chung SJ, Park SH, Kim JS, Oh SW, Kim HJ, Park JH, Huh YE (2014) Positional suppression of periodic alternating nystagmus. J Neuroophthalmol 34(2):162-4.
(3) Waespe W, Cohen B, Raphan T (1985) Dynamic modification of the vestibulo-ocular reflex by the nodulus and uvula. Science 228:199–202.
(4) Angelaki DE, Hess BJM (1995) Lesion of the nodulus and ventral uvula abolish steady-state off-vertical axis otolith response. J Neurophysiol 73(4):1716-20.
(5) Glasauer S, Merfeld DM (1997) Modelling three-dimensional vestibular responses during complex motion simulation. In: Fetter M, Haslwanter T, Misslisch H, Tweed D (eds) Three-dimensional kinematics of eye head and limb movements. CRC Press, pp 387-398.
(6) Laurens J, Angelaki DE (2011) The functional significance of velocity storage and its dependence on gravity. Exp Brain Res 210(0):407-422.
(7) Leigh RJ, Robinson DA, Zee DS (1981) A hypothetical explanation for periodic alternating nystagmus: instability in the optokinetic-vestibular system. Ann N Y Acad Sci 374:619–635.
Characterization of human head orientation during natural behavior
Christian B. Sinnott, MA1, Peter A. Hausamann1,2, Paul R. MacNeilage, Ph.D.1
1University of Nevada, Reno; Reno NV
2Technische Universität München; Munich, Germany
Estimating head orientation relative to gravity is important for a range of perceptual and motor behaviors including oculomotor reflexes, postural reflexes, and perception of upright. To optimize precision, accuracy and efficiency of estimation, the nervous systems should incorporate prior knowledge about the typical distribution of head orientations that are encountered on a day-to-day basis. However, previous work characterizing the statistics of human head orientation during natural behaviors outside the laboratory has been limited. Mobile methods of head tracking based on inertial measurement units (IMUs) have been used to measure linear acceleration and angular velocity of the head, but head orientation is generally not reported. It is difficult to reconstruct orientation of the head relative to gravity without additional magnetometer measurements and sensor fusion via Kalman filtering. Even after filtering, these estimation methods can be noisy and subject to drift. Here, we present head orientation data collected using a mobile head-tracking system that implements visual-inertial simultaneous localization and mapping (VI-SLAM), a method that is robust to the error and drift that affects pure IMU-based systems. In this study, participants wore an Intel RealSense T265 tracking camera on the head during 5 hours of normal, everyday activity. The camera was connected to a mini-computer (UpBoard Core) worn on a belt. The distribution of pitch and roll angles measured across all participants and conditions was centered close to upright, and pitch was more variable than roll. We discuss the implications of these statistical measures for probabilistic models of both encoding and decoding at various stages of the vestibular processing pathways.
Response of the Vestibular System to Microgravity Environments
John Sommerfeldt, MD
Walter Reed National Military Medical Center
Up to 80% of astronauts and 90-100% of pilots experience vestibular disequilibrium during their career, termed space adaptation syndrome or spatial disorientation respectively. While symptoms are often transient and lack long-term effects, the consequences can be devastating, accounting for over one-third of all fatal mishaps in the U.S. Air Force with a direct cost of over $2.3 billion from 1993-2013. The primary objective of this study was to review vestibular system function and alterations when exposed to microgravity. Management methods were reviewed in the literature as well as through discussion with NASA flight surgeons. A literature search was performed, and abstracts were read for potentially relevant articles. Relevant articles were read in their entirety. The vestibular system utilizes semicircular canals and otolith organs in a normal (terrestrial) environment. Three paired semicircular canals provide the vestibular component of orientation in a terrestrial environment. The utricle and saccule primarily detect vertical and horizontal acceleration and provide primary vestibular orientation during space flight. Two primary theories explain the etiology of space adaptation syndrome: the otolith theory, with symptoms secondary to decreased vestibular input following unloading of otoconia in microgravity, and the fluid shift theory, with symptoms secondary to development of endolymphatic hydrops following body fluid shift toward the head. Although many treatment options have been tried, no prophylaxis has proved universally effective. Antihistamines and restriction of head motion for the first 48 hour of space flight are the primary management options, with emerging evidence for sensory adaptability training and biofeedback devices.
References
1) Previc FH. Intravestibular Balance and Motion Sickness. Aerosp Med Hum Perform 2018;89:130-40.
2) Alford BR, Atkins JH Jr. Historical ties between otolaryngology--head and neck surgery and space medicine. Otolaryngol Head Neck Surg. 1991 Feb;104(2):153-6. doi: 10.1177/019459989110400201. PMID: 1901142.
3) Drake RL, Vogl AW, Mitchell AWM. Gray’s Atlas of Anatomy. 3 ed, 2021.
4) Arriaga MA, Brackmann DE. Cummings Otolaryngology: Head and Neck Surgery. 7 ed, 2021.
5) Newman, David G. “An Overview of Spatial Disorientation as a Factor in Aviation Accidents and Incidents.” ATSB TRANSPORT SAFETY INVESTIGATION REPORT, 2007.
6) Poisson RJ, Miller ME. Spatial disorientation mishap trends in the U.S. Air force 1993-2013. Aviat Space Environ Med. 2014 Sep;85(9):919-24. doi: 10.3357/ASEM.3971.2014. PMID: 25197890.
7) Boril, J., Smrž, V., Petru, A., Blasch, E., Leuchter, J., Frantis, P., & Jalovecky, R. (2018). Survey of Spatial Disorientation and Sensory Illusion among Air Force Pilots. 2018 IEEE/AIAA 37th Digital Avionics Systems Conference (DASC), 1-7.
8) Lawson BD, Rupert AH, McGrath BJ. The Neurovestibular Challenges of Astronauts and Balance Patients: Some Past Countermeasures and Two Alternative Approaches to Elicitation, Assessment and Mitigation. Front Syst Neurosci. 2016 Nov 22;10:96. doi: 10.3389/fnsys.2016.00096. PMID: 27920669; PMCID: PMC5118654.
The author reports no funding or other conflicts of interest to report
The improvement potential of the vestibular patient according to the saccade classification
Liraz Tencer-Lagziel MScPT, PhD Candidate1, Eli Carmeli PhD 1, Hadas Ben Rubi Shimron BPT2, Oz Zur PhD, PT2,3
1Department of Physical Therapy, University of Haifa, Haifa, Israel
Hadas Ben Rubi Shimron,
2The Israeli Center for Treating Dizziness and Balance Disorders, Raanana, Israel
3Department of Physical Therapy, Ben Gurion University of the Negev, Beer Sheva, Israel
Individuals with acute unilateral vestibulopathy (AUV) have slower eye velocity in response to head movements and consequently, retinal slip during head motion. This is one of the more effective error signals that drive Vestibulo ocular reflex (VOR) adaptations. Adaptations can be accomplished using corrective, compensatory saccades to augment the reduced slow phase component of the VOR. Vestibular rehabilitation (VR) for patients with AUV aims to stimulate the vestibular compensation processes. The intervention includes eye and head movements to improve visual-vestibular interactions and tolerance for movements, as well as exercises while standing and walking to improve static and dynamic postural control. Forty-six patients who were diagnosed with AUV underwent an examination including video head impulse test (vHIT), vestibular and balance assessment and behavioral questionnaires. Based on the initial vHIT results, patients were classified into 3 subgroups: normal pattern (n=13), scattered pattern (n=27) and gathered pattern (n=6). The examination was conducted within 2 weeks of the onset of symptoms and 2 months after VR. We evaluated whether the saccade classification affects a patient’s ability to improve balance. We found (a) significant differences between the 3 groups in the following measures: VOR gain (p=0.0032), covert average count (p=0.011), overt average count (p<.0001), short anxiety screening test (p=0.018), and Modified Clinical Test of Sensory Interaction on Balance path length (p=0.0325). (b) Patients with gathered saccades demonstrated better improvement compared to the normal and scattered groups in terms of vestibular measures, anxiety levels and balance. Our study may reveal unique head movement strategies that optimize performance and promote recovery of the vestibular system based on the saccade classification. Our study may lead to more effective VR strategies and could provide new information about the changing relationships over time of the vestibular system, postural control and balance. Randomized controlled trial ASMC 0067-17; NCT0327177.
Changes in behavioral features after vestibular rehabilitation
Liraz Tencer-Lagziel MScPT, PhD Candidate1, Oz Zur PhD, PT 2,3, Eli Carmeli PhD1
1Department of Physical Therapy, University of Haifa, Haifa, Israel
2The Israeli Center for Dizziness and Balance Disorders, Raanana, Israel
3Department of Physical Therapy, Ben Gurion University of the Negev, Beer Sheva, Israel
1Department of Physical Therapy, University of Haifa, Haifa, Israel
Individuals with acute unilateral vestibulopathy (AUV) report a variety of symptoms, including vertigo, nausea, postural instability and anxiety. These symptoms can cause emotional distress, hinder the ability to perform activities of daily living or work and appear to affect health-related quality of life. Vestibular rehabilitation (VR) for patients with AUV aims to stimulate the vestibular compensation processes and thus, reduce anxiety and improve quality of life. The intervention includes eye and head movements to improve visual-vestibular interactions and tolerance for movements. Exercises in standing and walking positions are also included to improve static and dynamic postural control. Our goals were to determine how changes in the vestibular system due to VR translated to behavioral changes and to assess whether a 2-month VR program (Intervention group, n=32) would have a stronger effect on behavioral changes compared with participants who received only exercise guidance (Control group, n=14). After 2 months of intervention, we found significant differences between groups, with an advantage for the intervention group compared to the control group in the following measures: Activities specific Balance Confidence scale (p=0.044), Short Anxiety Screening Test (p<0.0001), Dizziness Handicap Inventory (p=0.002), UCLA Dizziness Questionnaire (p=0.043), and the components of role emotional (p=0.0313) of the SF-36 questionnaire. Our results indicate the importance of vestibular rehabilitation on behavioral elements, in addition to the clinical improvements. This type of rehabilitation enable to maintain holistic and broad treatment to the vestibular patient. Randomized controlled trial ASMC 0067-17; NCT03271775.
In patients diagnosed with posterior BPPV, how effective are different BPPV-repositional maneuvers when complete resolution of symptoms is required?
Sebastian H. Valsted, Medical Student, Andreas T. Larsen, Medical Student
Aalborg University Hospital, Aalborg, Denmark
Background: Benign Paroxysmal Positional Vertigo (BPPV) is the most frequent cause of vertigo in adults. There are three main types of BPPV: posterior, horizontal and anterior, with posterior BPPV (P-BPPV) accounting for approximately 80% of BPPV cases. BPPV is characterized by short episodes of vertigo after positional changes such as turning the head, lying down and getting up. The diagnostics of P-BPPV is typically performed using a Dix-Hallpike (DH)-test. Today the most frequently used treatment is the Epley maneuver, but other opportunities are present. It is still not known which maneuver is the most efficient treating BPPV when complete resolution of vertigo symptoms is required.
Methods:
Study design: PubMed, Embase and Cochrane library were searched to identify randomized controlled trials and systematic reviews.
Objective: to assess the effectiveness of different P-BPPV-RMs.
Data collection and synthesis: study selection was carried out by two reviewers. Cochrane risk of bias tool was used for assessment of methodological quality and results were evaluated according to the GRADE method. Outcomes were analysed in RevMan (5.4) and reported in relative risk (RR). Statistically heterogeneity was quantified using I2 statistics.
Outcomes: the main outcome is complete resolution of vertiginous symptoms. Secondary outcomes include cervical- and back pain, post treatment dizziness and nausea, conversion of a positive DH-test to a negative DH-test.
Results: Eight studies were selected including 595 patients. Based on GRADE assessment the quality of the results was graded low or very low.
Primary outcomes: six studies reported dichotomous outcome. No difference was found between the RMs looking at total remission of vertigo. The Epley- compared to the Semont maneuver: RR 1.18 [95% CI 0.85, 1.65, p=0.33]. The Epley- compared to the Li maneuver: RR 0.95 [95% CI 0.71, 1.28, p=0,76]. The Epley- compared to the Gans maneuver: RR 1.50 [95% CI 0.96, 2.35, p=0.08]. Two studies reported continuous outcome. No difference between the RMs was found.
Secondary outcomes: two studies reported a difference in conversion of a positive DH-test to a negative DH-test between the Epley- and the Gans maneuver RR 1,53 [95% CI 1.09, 2.16, p=0.02] and between the Epley- and the Semont maneuver RR 2.95 [95% CI 1.10, 7.91, p=0.03]. One study reported no difference in back pain RR 13.0 [95% CI 0.76, 220.96, p=0.08] and cervical pain RR of 7.0 [95% CI 0.38, 129.93, p=0.19] between the Epley- and the Gans maneuver.
Conclusion: The evidence shows that the Epley maneuver is equally as good as the Semont-, Li- and Gans maneuver regarding total remission of vertiginous symptoms. A statistically significant difference in favour of the Epley maneuver compared to the Semont- and Gans maneuver was found regarding conversion of a positive to a negative DH-test. No differences regarding back- and cervical pain were reported. The quality of the results was graded low and very low, highlighting the need of further high-quality standardized studies.
Vestibular Noise as a Predictor of Postural Sway
Andrew R. Wagner, PT, DPT, NCS, Megan J. Kobel, AuD, and Daniel M. Merfeld, PhD
The Ohio State University, Columbus, OH
Recently, it was shown that an increase in 0.2 Hz roll-tilt perceptual thresholds was associated with a significant increase in the likelihood of failing to complete a “foam, eyes-closed” standing balance task [1–3]. This finding suggested that increased vestibular noise, as quantified by vestibular thresholds, was associated with increased fall risk, but the categorical (pass/fail) nature of the balance test prevents further inference into the relationship between vestibular noise and postural sway. To address this limitation, we measured 0.2, 0.5, and 1.0 Hz head centered roll-tilt vestibular thresholds alongside static postural sway in a group of 20 healthy young adult volunteers (26±3.9; 15 female). A tri-axial force plate was used to quantify movement of the center of pressure (CoP) while subjects stood with the feet together on a medium density foam pad (3 trials of 60 seconds). As a primary measure of postural sway, we quantified the root mean square (RMS) displacement separately in both the anteroposterior and mediolateral direction. In addition, we analyzed the CoP time series using multi-scale entropy and detrended fluctuation analysis to determine if vestibular noise was associated with the complexity of postural sway. We hypothesized that increases in vestibular noise (i.e., increased thresholds) would be associated with greater postural sway in corresponding planes of motion (e.g., that higher roll-tilt thresholds would be associated with increased mediolateral postural sway).
We found that 0.5 Hz, but not 0.2 or 1.0 Hz, roll tilt thresholds showed a significant positive correlation with RMS displacement of the CoP in the mediolateral (β = 3.07, p = 0.011, 95% CI: 0.80-5.34), and to a lesser extent in the anteroposterior (β=3.46, p=0.037, 95% CI: 0.24-6.70)) direction, consistent with our hypothesis. No significant association was seen between the DFA scaling exponent or multi-scale entropy complexity index and any frequency of threshold (p > 0.05). These data provide further support to the previously demonstrated association between tilt thresholds and balance by using a continuous (rather than pass/fail) measure of postural control. In addition, these results suggest that vestibular noise associated with 0.5 Hz roll tilt may be most predictive of postural sway.
Acknowledgements: Funded by NIH/NIDCD R01-DC014924, DoD Congressionally Directed Medical Research Program (CDMRP) grant W81XWH1920003, and the Foundation for Physical Therapy Research.
1. Bermúdez Rey, M.C., Clark, T.K., Wang, W., Leeder, T., Bian, Y., Merfeld, D.M.: Vestibular Perceptual Thresholds Increase above the Age of 40. Front. Neurol. 7, (2016). https://doi.org/10.3389/fneur.2016.00162
2. Karmali, F., Bermúdez Rey, M.C., Clark, T.K., Wang, W., Merfeld, D.M.: Multivariate Analyses of Balance Test Performance, Vestibular Thresholds, and Age. Front. Neurol. 8, 578 (2017). https://doi.org/10.3389/fneur.2017.00578
3. Beylergil, S.B., Karmali, F., Wang, W., Bermúdez Rey, M.C., Merfeld, D.M.: Vestibular roll tilt thresholds partially mediate age-related effects on balance. In: Progress in Brain Research. pp. 249–267. Elsevier (2019)
A Pragmatic Approach to Multisensory Fusion for Body Spatial Awareness
Bridgett Wallace, PT, DPT1 and Fernando Santos, PT, PhD2
1360 balance & dizziness
2Bertec Corporation
Studies have highlighted the role of multisensory fusion as a critical component of body spatial awareness to optimize postural control. Such research focuses on the integration of somatosensory, visual, and vestibular inputs through a complex reweighting of all sensory cues to produce successful motor responses for maintaining balance. Thus, if a sensory pathway is impaired, it is expected the central nervous system (CNS) would rely more heavily on the remaining intact systems. For example, if an individual has a vestibular impairment, it is suspected he or she would rely more heavily on visual or somatosensory inputs. More recently, studies have investigated the effects multisensory integration as related to self-identification versus self-location, which are identified as primary components of body self-consciousness. Findings are suggesting multisensory inputs are more successfully integrated when rehabilitative interventions focus on the self-perception of balance strategies for navigating body, eye, and head movements as part of a body self-consciousness paradigm for optimizing postural control. The two primary aims of our discussion are to (1) explore the variability of the center of mass (COM) in stable versus unstable surfaces when combined with stable versus unstable visual environments and (2) review the impact of clear instructions about attending to self-motion on therapy outcomes as related to sensory reweighting for improving body spatial awareness under sensory conflict.
References
(1) Britton and Arshad (2019). Frontiers in Neurology 10(63).
(2) Haran and Keshner; (2008). Journal of Neurological Physical Therapy 32(4).
Modulation of deep mesencephalic nucleus to locomotion in rhesus monkeys
Rui-Han Wei, Ph.D., Erez Gugig, Ph.D., Oliver Stanley, B.D. and Kathleen Cullen Ph.D.
Department of Biomedical Engineering, School of Medicine, Johns Hopkins University, Baltimore, MD
The deep mesencephalic nucleus (DpMe) is a large the midbrain reticular area which plays a key role in integrating and processing sensory, attention, and limbic inputs. Its diverse connections include spinal, cortical, basal ganglia, and limbic inputs as well as outputs to thalamic nuclei and reticulospinal areas (Rodríguez M et al., 2001). DpMe cells have been shown to respond to passive vestibular stimuli, which suggests that this region plays an important role in vestibulo-motor control (Aravamuthan BR and Angelaki D 2012). However, DpMe responses to locomotion have not yet been characterized. Here we analyzed single-unit extracellular activities from the DpMe in rhesus monkeys during passive vestibular stimulation (translations and rotation), head-fixed treadmill walking, head-fixed and head-free ground walking. 3Dgyroscopes/3Daccelerometers were used to record the head motion. 4 High-speed cameras were used for motion recording synchronously. Deep-learning computer-vision toolbox (DeepLabCut) was used to extract the animals’ 3D posture for gait analysis. We identified that DpMe cells demonstrated a significant increase in activity during head-fixed treadmill walking, head-fixed and head-free ground walking when compared to resting activities. Notably, DpMe cells demonstrated phase-dependent modulation during walking, even in the absence of vestibular stimulation. Modulation patterns varied between DpMe cells- some cells demonstrated peak activity during swing phase, and others during stance. Taken together these results suggest that DpMe neurons play a critical role in processing multi-sensory input during the complex sensorimotor activities generated in everyday life, such as walking. In addition, our findings also advance our knowledge of the neural circuits that coordinate gait and balance and thus have important implications for the development of novel treatments and interventions.
References
(1) Rodríguez M. et al., (2001) The Journal of Comparative Neurology 438.
(2) Aravamuthan BR and Angelaki D (2012) Neuroscience 223(25)
Robust biases in the estimation of yaw rotation and the potential influence of auditory landmarks.
Silvia Zanchi, Ph.D. student1,2,3, Luigi F. Cuturi, Ph.D.1, Giulio Sandini, Professor2, Monica Gori, Ph.D.
1Unit for Visually Impaired People (U-VIP), Italian Institute of Technology, Genoa, Italy;
2Robotics Brain and Cognitive Sciences (RBCS), Italian Institute of Technology, Genoa, Italy;
3University of Genoa, Italy
Navigation and orientation strategies involve relying on internal cues such as vestibular signals, which provide information about acceleration and direction of movements regardless of external cues in the environment. However, perceptual readout of vestibular information could be biased in certain circumstances even in a healthy population [1; 2]. Environmental signals provide useful information to overcome the ambiguity of internal cues. Our goal was to investigate whether external auditory cues, namely landmarks, could aid self-motion perception. We hypothesized that, in the presence of potential perceptual biases, auditory landmarks would improve accuracy of vestibular perception of self-motion. We asked healthy participants to perform a passive self-motion discrimination task. Healthy participants were seated on a Rotational Translational Chair (RT-Chair; [3]) and they were rotated along the earth-vertical axis. They were asked to perform a passive self-motion discrimination task by indicating whether they felt to be rotated more or less than 45° azimuth, which was the middle amplitude between the two considered points of reference at azimuth 0° (i.e. aligned with participant’s nose) and 90°, with no visual information available. The following four conditions were tested: in two conditions vestibular-only self-motion stimuli were presented, with rotations toward the right (i) and left (ii); in other two conditions, each auditory landmark was presented before and after the experienced movement, with rotations toward the right (iii) and left (iv). We used virtually spatialized sounds as external auditory landmarks, in correspondence of the two points of reference (azimuth = 0° and 90°), presented via headphones. We calculated the point of subjective equality (PSE) for each participant and condition, a measure of accuracy that indicated the physical movement perceived as a 45° yaw rotation. In each condition, all participants showed a strong overestimation of the perceived 45° yaw rotation. Against our initial hypothesis, when auditory landmarks were present, perceptual biases were of comparable magnitude to those in the vestibular-only conditions. These results showed a robust overestimation bias and showed that auditory landmarks did not influence estimations. Since landmarks were presented only before and after self-motion stimulation, the absence of acoustic information during motion might have reduced the potential effect of external cues to overcome the vestibular bias. Further research will determine whether coherent moving acoustic signals can enhance accuracy in self-motion perception.
References
1. Israël, I., Sievering, D., & Koenig, E. (1995). Self-rotation estimate about the vertical axis. Acta oto-laryngologica, 115(1), 3-8.
2. Cuturi, L. F., & MacNeilage, P. R. (2013). Systematic biases in human heading estimation. PloS one, 8(2), e56862.
3. Cuturi, L. F., Torazza, D., Campus, C., Merello, A., Lorini, C., Crepaldi, M., ... & Gori, M. (2020, July). The RT-Chair: a Novel Motion Simulator to Measure Vestibular Perception. In 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC).




