Association between dopamine receptor D2 Taq IA gene polymorphism and persistent postural-perceptual dizziness
Abstract
BACKGROUND:
Persistent postural-perceptual dizziness (PPPD) is a chronic dizziness, its pathogenesis is unknown by now.
OBJECTIVE:
To study the relationship between the DRD2 gene TaqIA polymorphisms and PPPD, and further to explore the molecular mechanism underlying this disease.
METHODS:
43 patients diagnosed with PPPD and 45 randomly selected cases (matched by age and sex) were included in the study and control group, respectively. DRD2 gene TaqIA polymorphisms were detected in all participants by polymerase chain reaction (PCR)combined with the restriction fragment length polymorphism (RFLP) method.
RESULTS:
In the study group, frequencies of the A1 and A2 TaqIA alleles (65.1% and 34.9%, respectively) were significantly different to those in the control group (46.7% and 53.3%, respectively; P < 0.05). The allele frequency in the study group for the A1/A1 genotype was 34.9%, for A1/A2 was 60.5%, and for A2/A2 was 4.6%, all of which were significantly higher than the control group (24.4%, 44.5%. and 31.1%, respectively; P < 0.01).
CONCLUSIONS:
Our findings indicate that the DRD2 TaqIA A1 allele is possibly the susceptibility polymorphism for PPPD, and that the A2/A2 genotype has a potentially protective role for PPPD. However, larger independent studies are required for further validation.
1Introduction
Persistent postural-perceptual dizziness (PPPD) is a common clinical condition characterized by chronic dizziness, as defined by the subcommittee of the Bárány Society in 2017. PPPD includes core clinical features described over the last 30 years in syndromes like phobic postural vertigo (PPV) and chronic subjective dizziness (CSD) and the diagnostic criteria for PPPD were established at the committee for the Classification of Vestibular Disorders of the Bárány Society [4, 12, 14]. The diagnostic criteria of PPPD include [12]: A. One or more symptoms of dizziness, unsteadiness, or nonspinning vertigo are present on most days for 3 months and more; B. Symptoms are present without specific provocation but are exacerbated by 1. Upright posture; 2. Active or passive motion without regard to direction or position; 3. Exposure to moving visual stimuli or complex visual patterns; C. The disorder usually begins shortly after an event that causes acute vestibular symptoms or problems with balance, though less commonly; D. Symptoms cause significant distress or functional impairment; E. Symptoms are not better attributed to another disease or disorder. In terms of pathogenesis, although most PPPD patients suffer from acute vestibular function disorder, such as benign positional paroxysmal vertigo (BPPV), not all the patients with acute vestibular peripheral vertigo develop PPPD. A personality analysis study has found that neuroticism is one of the main risk factors of PPPD [13, 15]. Although research has shown that the dopamine receptor D2 (DRD2/ANKK1) TaqIA single nucleotide polymorphism which was cataloged as rs1800497 is associated with neurotic personality [6–8], no correlation between DRD2 gene TaqIA polymorphisms and PPPD has been reported so far. Therefore, this study aims to analyze the relation between DRD2 gene TaqIA polymorphisms and PPPD, and to further discuss the molecular mechanism underlying PPPD.
2Methods
2.1Patients selection
43 patients diagnosed with PPPD were selected from the outpatient and inpatient clinics of the Department of Neurology at Yantaishan Hospital, between January 2017 and June 2017, and assigned to the study group. The control group included 45 cases whose gender and age characteristics were matched with those of the study group during the same time period of hospitalization. The control subjects were randomly selected from 368 patients, which initially presented with acute vertigo (including BPPV, vestibular neuronitis, Meniere’s disease and others), but were fully recovered within three months (up to 6 months follow-up), without receiving treatment with selective serotonin reuptake inhibitors (SSRIs) or serotonin and norepinephrine reuptake inhibitors (SNRIs). The study was approved by the Ethics Committee of Yantaishan Hospital of Shandong Province and written informed consent was obtained from all the participants after they were acquainted with all the procedures. (Reference number: 20161107).
2.1.1Case inclusion criteria [12, 15]
Case inclusion criteria were as follows: (1) Persistent non-vertiginous dizziness or subjective imbalance lasting 3 months or more; (2) hypersensitivity to motion stimuli; (3) visual vertigo; (4) absence of active of neuro-otology and other nervous system diseases; (5) no clear cause of dizziness by drug treatment; (6) neural imaging examination normal; and (7) balance function is normal or mildly abnormal, but not enough to make a diagnosis (mildly abnormal means current or previous vestibular problem does not fully explain the patient’s symptoms).
2.1.2Case exclusion criteria [15]
Case exclusion criteria were as follows: (1) Patients diagnosed with anxiety and depression in addition to having dizziness symptoms (which means they have anxiety or depression core symptoms such as tension, irritability or low spirits et al.); (2) patients who refuse to enroll in the follow-up investigation; (3) patients with other types of dizziness such as Chronic cerebral circulation insufficiency (CCCI), Multiple sclerosis and Dandy syndrome et al.; (4) patients with other serious chronic diseases requiring long-term use of medications; and (5) patients whose medical records are incomplete.
2.2Gene polymorphism detection method
Genomic DNA was extracted from peripheral blood lymphocytes. DRD2 gene TaqIA polymorphisms were detected by the polymerase chain reaction (PCR)-restriction fragment length polymorphism (RFLP) method. PCR primers were designed using the Premier 5.0 software and manufactured by the Invitrogen company; the primer sequences are as follows:
Forward primer: 5’-CCGTCGACGGCTGGCC AAGTTGTCTA-3’, Reverse primer: 5’-CCGTCG ACCCTTCCTGAGTGTCATCA-3’, the PCR product was 310 bp.
PCR reactions contained 10 mM Tris-HCl (pH 9.0), 50 mM KCl, 1.5 mM MgCl2, 200 uM each dNTP, 0.2 uM of each primer and 1.25 units Taq DNA polymerase in a 25 ul volume containing 50 ng sample DNA [5]. The reaction conditions were set as follows: pre-denaturation at 95°C for 4 min; 35 cycles of denaturation at 94°C for 45 s, annealing at 56°C for 30 s, and extension at 72°C for 1 min; final extension at 72°C for 10 min. PCR products were digested with 5μl of Taq - restriction enzymes, in a 65°C water bath for 4 h, and then the digested products were separated by 2.5% agarose gel electrophoresis for genotyping. The TaqIA polymorphism A1 allele, which does not contain a restriction site, was detected by the presence of a 310 bp fragment. The A2 allele, which has one restriction site, was detected by the presence of a 180 bp and a 130 bp fragment. A1/A2 heterozygous genotypes were defined when all the three products (310 bp, 180 bp and 130 bp) were detected after enzyme digestion.
2.3Statistical analysis
A database was established using Excel 2010, and SPSS 19.0 was used as a platform to perform statistical analysis. The sample group representative was determined by the Hardy - Weinberg equilibrium method. Data was compared by the chi-square test, with P < 0.05 used to define statistical significance.
3Results
3.1Patient characteristics
The male: female ratio in PPPD was approximately 1:2 (14/29). In the other acute vertigo patients the ratio was close to 1:1 (196/172), and the difference between the two groups was statistically significant (P < 0.05).
In order to avoid the bias introduced by gender and age differences, we randomly selected 45 patients from other acute vertigo cases as the control group, which we paired with the study group by gender and age. The study group included 43 cases, with age ranging from 27 to 76 years, (mean: 52.45±13.36 years). The control group was composed of 45 cases with ages from 22 to 74 years (mean: 53.28±13.22 years). The two groups showed normal distribution with respect to patient age, and the χ2-test showed no statistical difference between the groups (χ2 = 2.885, P = 0.718). Other patient characteristics including education, occupation, marital status, place of residence, family income, smoking history, drinking history, history of hypertension, migraine, hyperlipidemia, diabetes and coronary atherosclerotic heart disease, showed no statistical difference between the two groups (P > 0.05) (Table 1).
Table 1
Patients’ demographic and sociological characteristics
| Indicators | Study group(n = 43) | control group(n = 45) | χ2 | P | |
| Gender | Male | 14 (32.6%) | 15 (33.3%) | 0.006 | 0.938 |
| Female | 29 (67.4%) | 30 (66.7%) | |||
| Education level | Senior high school or lower | 12 (27.9%) | 16 (35.6%) | 0.593 | 0.441 |
| Senior high school or higher | 31 (72.1%) | 29 (64.4%) | |||
| Occupation | Unemployed | 5 (11.6%) | 7 (15.6%) | 1.533 | 0.675 |
| Farmer | 6 (14.0%) | 9 (20%) | |||
| Worker | 17 (39.5%) | 18 (40%) | |||
| Cadre or other | 15 (34.9%) | 11 (24.4%) | |||
| Marital status | Unmarried | 3 (7.0%) | 4 (8.9%) | 0.225 | 0.894 |
| Married | 32 (74.4%) | 34 (75.5%) | |||
| Divorced or widowed | 8 (18.6%) | 7 (15.6%) | |||
| Residence | Rural areas and suburbs | 15 (34.9%) | 14 (31.1%) | 0.142 | 0.707 |
| City | 28 (65.1%) | 31 (68.9%) | |||
| Income(RMB/year) | Less than 60,000 | 11 (25.6%) | 14 (31.1%) | 0.331 | 0.565 |
| More than 60,000 | 32 (74.4%) | 31 (68.9%) | |||
| Smoking history | No | 29 (67.4%) | 27 (60%) | 1.225 | 0.542 |
| Less than 20 / day | 5 (11.6%) | 4 (8.9%) | |||
| More than20 / day | 9 (21%) | 14 (31.1%) | |||
| Drinking history | No | 29 (67.4%) | 32 (71.1%) | 0.139 | 0.709 |
| Yes | 14 (32.6%) | 13 (28.9%) | |||
| Hypertension | No | 30 (69.8%) | 31 (68.9%) | 0.008 | 0.929 |
| Yes | 13 (30.2%) | 14 (31.1%) | |||
| Headache history | No | 26 (60.5%) | 33 (73.3%) | 1.648 | 0.199 |
| Yes | 17 (39.5%) | 12 (26.7%) | |||
| Hyperlipidemia | No | 28 (65.1%) | 27 (60%) | 0.246 | 0.620 |
| Yes | 15 (34.9%) | 18 (40%) | |||
| Diabetes | No | 35 (81.4%) | 39 (86.7%) | 0.457 | 0.499 |
| Yes | 8 (18.6%) | 6 (13.3%) | |||
| Coronary atherosclerotic heart disease | No | 34 (79.1%) | 38 (84.4%) | 0.427 | 0.513 |
| Yes | 9 (20.9%) | 7 (15.6%) |
The two groups of patients were also evaluated with the adult Eysenck Personality Questionnaire Inventory (EPQI), with special emphasis on extraversion (E) and neuroticism (N). The results showed that introverted PPPD patients accounted for 44.2% of the group, which was higher than the control group (26.7%), but there was not statistically significant difference between the groups (P > 0.05). In contrast, analysis of the emotional aspects of the EPQI, revealed that the number of neurotic patients in the PPPD group (67.4%) was significantly higher than those in the control group (37.8%; P < 0.01) (Table 2).
Table 2
Adult EPQI scores of patients included in the study
| Group Introversion extroversion | χ2 | P | Neuroticism stability | χ2 | P | |
| SG | 19 (44.2%) 24 (55.8%) | 2.95 | 0.08 | 29 (67.4%) 14 (32.6%) | 7.75 | 0.00 |
| CG | 12 (26.7%) 33 (73.3%) | 8 | 5 | 17 (37.8%) 28 (62.2%) | 6 | 5 |
SG = study group, CG = control group.
The digested PCR products of the amplified dopamine D2 receptor gene TaqIA polymorphism are shown in Fig. 1.
Fig.1
Sampling electrophoresis figure. M is the Marker, 1, 4and 5 are respectively A1/A1, A1/A2 and A2/A2 gene type in patients group; 2, 3 and 6 are respectively A1/A1, A1/A2, A2/A2 gene type in the control group.
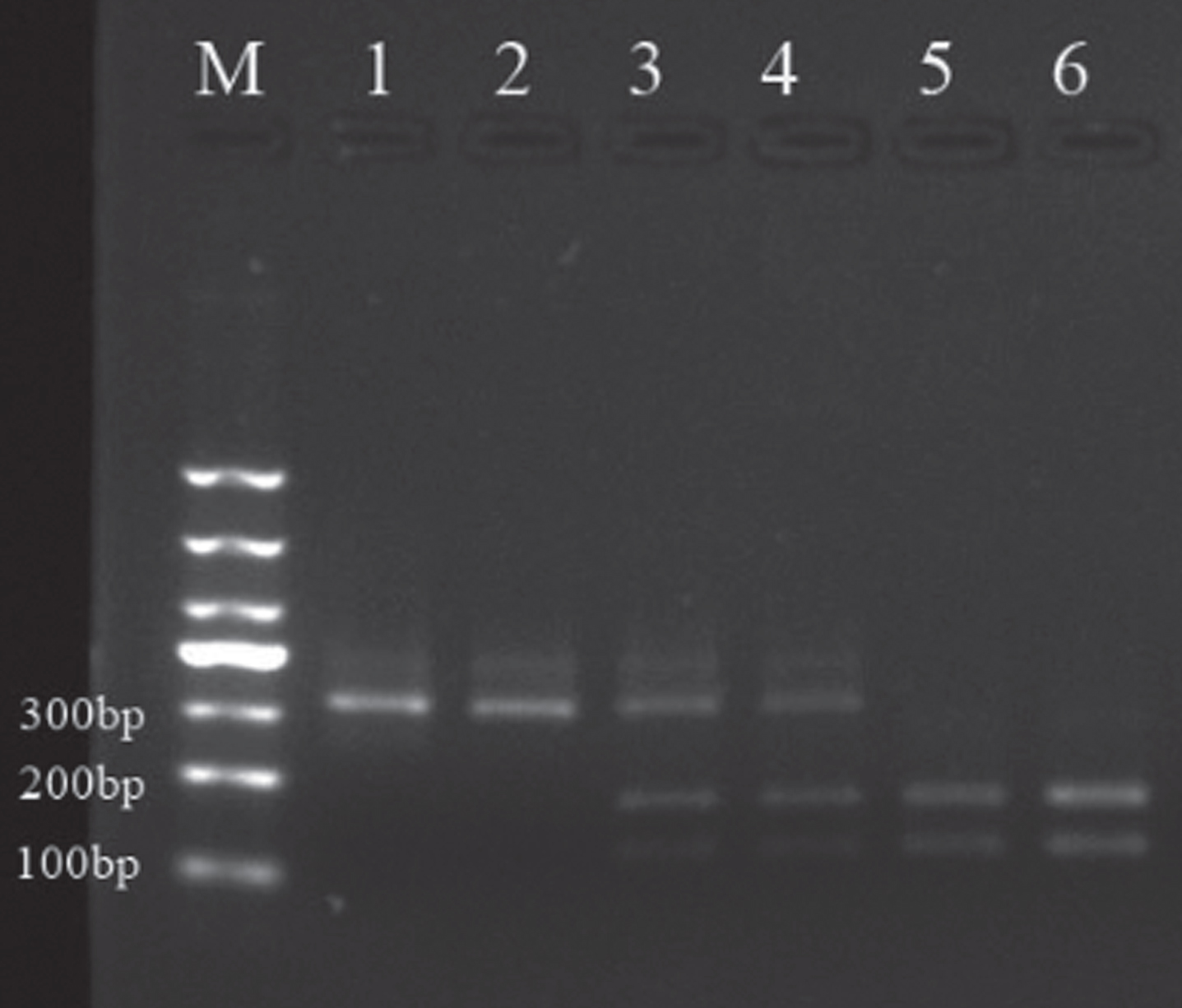
3.2Genotyping and allele frequency distribution
In the experimental group the frequency of the A1/A1 genotype was 34.9%, the A1/A2 frequency was 60.5%, and the A2/A2 ratio was 4.6%. In contrast, the genotype frequency of the control group was 24.4% for A1/A1, 44.6% for A1/A2, and 31.1% for A2/A2, and the genotype distribution was consistent with the Hardy Weinberg equilibrium law (χ2 = 4.80, P > 0.05; χ2 = 0.211, P > 0.05; respectively). Overall, the genotype frequency differences between the two groups were statistically significant (χ2 = 10.358, P = 0.006), (Table 3).
Table 3
Dopamine receptor D2 gene Taq IA allele frequency distribution in both groups
| Group | Genotype frequency | ||||
| AIA1 | AIA2 | A2A2 | χ2 | P | |
| SG | 15 (34.9%) | 26 (60.5%) | 2 (4.6%) | 10.358 | 0.006 |
| CG | 11 (24.4%) | 20 (44.5%) | 14 (31.1%) | ||
Furthermore, the A1 and A2 allele frequencies between the two groups were significantly different (χ2 = 6.066, P = 0.014); The odds ratio (OR) was 2.13, which indicated that the A1 allele is likely the susceptibility polymorphism of PPPD.
4Discussion
PPPD is a disorder characterized by chronic dizziness and accompanied by core symptoms like sensitivity to motion stimulation, discomfort at open spaces, and varying degrees of anxiety and phobic behaviors. This condition has been previously described as“phobic postural vertigo (PPV)”, space motion discomfort (SMD), visual vertigo (VV) and chronic subjective dizziness (CSD) in the last 150 years [4].The pathogenesis of PPPD is unknown by now. In recent years it has been shown that neuroticism is one of the major risk factors for developing PPPD, and that the dopamine receptor D2 gene TaqIA polymorphism is associated with neuroticism [6–8]. Therefore, it can be speculated that the DRD2 gene TaqIA polymorphism may be involved in the molecular mechanism of PPPD pathogenesis.
To further test this hypothesis, we gathered data from 43 patients diagnosed with PPPD and from 45 cases diagnosed with acute vestibular vertigo but fully recovered within 6 months in the same time period of hospitalization. The results showed that the A1 allele was obviously more abundant in the PPPD group than in the control group, and the difference was statistically significant (P < 0.05); the odds ratio (OR) was 2.13 which implied that the A1 allele may be a susceptibility gene for PPPD. In the control group, the frequency distribution of the A2/A2 genotype was 31.1%, which is significantly higher than in the experimental group (4.6%; P < 0.01). This finding has not been reported previously, and suggests the protective role of the A2/A2 genotype against developing PPPD.
Some scholars have demonstrated that the DRD2 receptors regulate dopamine concentration in the synapses through a negative feedback regulation mechanism [3]. Another study has shown that the D2 receptors density in the brain caudate nucleus declined by about 30% in people carrying the A1 allele, relative to A1 allele non-carriers [10]. Therefore, it can be speculated that patients with the A1 allele polymorphism may have reduced DRD2 density in related areas of the brain, such as in the striate body, which makes them more susceptible to psychological problems. Under an external environmental stimuli, such as tension, it can affect the development and migration of dopaminergic neurons, and the establishment of synaptic connections [1, 11] but at the same time it can increase the reactivity of the hypothalamus - pituitary - adrenal axis [9]. Through the feedback regulation and the excitation of the presynaptic membrane dopaminergic activity, the body can regulate the release of norepinephrine, and further enhance the sympathetic nerve excitability, which make the body to become more sensitive and react excessively, leading to neurotic behavior [2]. Erik G Jonsson has reported that the DRD2 gene TaqIA polymorphism is associated with the dopamine D2 receptor concentration [7]. The significantly more frequent A1 allele in PPPD patients in our study may reflect reduction in the number of DRD2 molecules in brain areas, such as in the striate body in the PPPD brain. Eysenck personality rating testing revealed that under some environmental conditions or when suffering from acute vestibular vertigo, PPPD patients were more prone to developing neurotic personality than control patients. This could be explained by the fact that PPPD patients more frequently carried the A1 allele of the DRD2 gene TaqIA polymorphism, indicating that the A1 allele may be implicated in the molecular mechanisms underlying the onset of PPPD. Therefore, the A1 allele may be one of the susceptibility gene polymorphisms for developing PPPD.
5Conclusion
DRD2 gene TaqIA polymorphisms might be involved in the pathogenesis of PPPD, and the A1 allele may be one of the susceptibility genes for PPPD, while the A2 allele may have a protective role against PPPD, Large clinical trials are necessary to further validate our results.
Acknowledgments
We gratefully acknowledge the support, advice, and recommendations offered by numerous colleagues throughout this endeavor. In particular, we appreciate to Dr. Jiang weihui of the rehabilitation department for her selfless help in completing all the scale statistical analysis of the enrolled patients. We also appreciate the theoretical and technical guidance given by Dr Yan mengjun and Dr Wang lei from Shandong Qunying Medical Research Co, LTD.
References
[1] | Akers K.G. , Nakazawa M. , Romeo R.D. , Connor J.A. , McEwen B.S. and Tang A.C. , Early life modulators and predictors of adult synaptic plasticity, Eur j Neurosci 24: ((2006) ), 547–554. |
[2] | Barbato G. and Della C. , Monica, A. Costanzo and V. De Padova, Dopamine activation in Neuroticism as measured by spontaneous eye blink rate, Physiology & Behavior 105: ((2012) ), 332–336. |
[3] | Brake W.G. , Zhang T.Y. , Diorio J. , Meaney M.J. and Gratton A. , Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats, Eur J Neurosic 19: ((2004) ), 1863–1874. |
[4] | Dieterich M. and Staab J.P. , Functional dizziness: From phobic postural vertigo and chronic subjective dizziness to persistent postural-perceptual dizziness, Curr Opin Neurol 30: ((2017) ), 107–113. |
[5] | Grandy D.K. , Zhang Y. and Civelli O. , PCR detection of the Taq A RFLP at the DRD2 locus, Hum Mol Genet 2: ((1993) ), 2197. |
[6] | Hui I.H. , Cheng C.C. , Yang Y.K. , Yeh T.L. , Chen P.S. and Chiu N.T. , Correlation between striatal dopamine D2 receptor density and neuroticism in community volunteers, Psychiatry Research: Neuroimaging 138: ((2005) ), 259–264. |
[7] | Jönsson E.G. , Cichon S. , Gustavsson J.P. , Grünhage F. , Forslund K. , Mattila-Evenden M. , Rylander G. , Asberg M. , Farde L. , Propping P. and Nöthen, M.M. Association between a promoter dopamine D2 receptor gene variant and the personality trait detachment, Biol Psychiatry 53: ((2003) ), 577–584. |
[8] | Kazantseva A. , Gaysina D. , Malykh S. and Khusnutdinova E. , The role of dopamine transporter (SLC6A3) and dopamine D2 receptor/ankyrin repeat and kinase domain containing 1 (DRD2/ANKK1) gene polymorphisms in personality traits, Prog Neuropsychopharmacol Biol Psychiatry 35: ((2011) ), 1033–1040. |
[9] | Liu D. , Diorio J. , Tannenbaum B. , Caldji C. , Francis D. , Freedman A. , Sharma S. , Pearson D. and Meaney M.J. , Maternal care, hippocampal glucocorticoid receptors and pypothalamic-pituitary-adrenal responses to stress, Science 277: ((1997) ), 1659–1662. |
[10] | Noble E.P. , Blum K. , Ritchie T. , Montgomery A. and Sheridan P.J. , Allelic association of the D2 dopamine receptor gene with receptor-blinding characteristics in alcoholism, Arch Gen Psychiatry 48: ((1991) ), 648–654. |
[11] | Roceri M. , Hendriks W. , Racagni G. , Ellenbroek B.A. and Riva M.A. , Early maternal deprivation reduces the expression of BDNF and NMDA receptor subunits in rat hippocampus, Mol Paychiatry 7: ((2002) ), 609–616. |
[12] | Staab J.P. , Eckhardt-Henn A. , Horii A. , Jacob R. , Strupp M. , Brandt T. and Bronstein A. , Diagnostic criteria for persistent postural-perceptual dizziness (PPPD): Consensus document of the committee for the Classification of Vestibular Disorders of the Bárány Society, J Vestib Res 27: ((2017) ), 191–208. |
[13] | Staab J.P. , Rohe D.E. , Eggers S.D. and Shepard N.T. , Anxious, introverted personality traits in patients with chronic subjective dizziness, J Psychosom Res 76: ((2014) ), 80–83. |
[14] | Yan Z.H. and Chen C.C. , Research progress for persistent postural-perceptual dizziness, Natl Med J China 97: ((2017) ), 1118–1120. |
[15] | Yan Z.H. , Cui L.P. and Yu T.X. , H Liang, Y. Wang and C.C. Chen, Analysis on the characteristics of the Persistent Postural-Perceptual Dizziness—a clinical study in China, International Journal of Audiology 56: ((2017) ), 33–37. |




