Visual stress responses to static images are associated with symptoms of Persistent Postural Perceptual Dizziness (PPPD)
Abstract
BACKGROUND:
Images that deviate from natural scene statistics in terms of spatial frequency and orientation content can produce visual stress (also known as visual discomfort), especially for migraine sufferers. These images appear to over-activate the visual cortex.
OBJECTIVE:
To connect the literature on visual discomfort with a common chronic condition presenting in neuro-otology clinics known as persistent postural perceptual dizziness (PPPD). Patients experience dizziness when walking through highly cluttered environments or when watching moving stimuli. This is thought to arise from maladaptive interaction between vestibular and visual signals for balance.
METHODS:
We measured visual discomfort to stationary images in patients with PPPD (N = 30) and symptoms of PPPD in a large general population cohort (N = 1858) using the Visual Vertigo Analogue Scale (VVAS) and the Situational Characteristics Questionnaire (SCQ).
RESULTS:
We found that patients with PPPD, and individuals in the general population with more PPPD symptoms, report heightened visual discomfort to stationary images that deviate from natural spectra (patient comparison, F (1, 1865) = 29, p < 0.001; general population correlations, VVAS, rs (1387) = 0.46, p < 0.001; SCQ, rs (1387) = 0.39, p < 0.001). These findings were not explained by co-morbid migraine. Indeed, PPPD symptoms showed a significantly stronger relationship with visual discomfort than did migraine (VVAS, zH = 8.81, p < 0.001; SCQ, zH = 6.29, p < 0.001).
CONCLUSIONS:
We speculate that atypical visual processing –perhaps due to a visual cortex more prone to over-activation –may predispose individuals to PPPD, possibly helping to explain why some patients with vestibular conditions develop PPPD and some do not.
1Introduction
Persistent postural perceptual dizziness (PPPD) is a condition characterised by postural instability and dizziness when exposed to self-movement and challenging visual environments [15, 63]. Common triggers include supermarket aisles, action movies, and crowded streets. The condition is common, chronic and debilitating [14, 56, 61, 67]. It often develops following a vestibular insult [1, 6, 51, 63], but it is also associated with other central disorders such as anxiety [5, 31, 53, 62, 64, 65] and migraine [51–53, 63, 66].
A leading theory suggests that PPPD is caused by a persistent over-dependence on visual information for postural control relative to vestibular cues, even after the original vestibular insult recovers [6, 10, 11, 23, 55]. However, we have recently reported that symptoms of PPPD are also common in the general population, with 9%of individuals scoring above the patient 25th percentile score on questionnaire measures of PPPD [52]. Furthermore, the distribution of PPPD symptoms lies on a continuous spectrum in the general population rather than being bimodal (i.e., with or without symptoms). Therefore, we now posit that some visual/vestibular systems have a predisposition to eliciting such symptoms, even before any vestibular damage.
Most research on PPPD has focused on the interactions between visual, vestibular and proprioceptive cues for postural control and locomotion, alongside associated psychogenic factors such as anxiety, and differences in brain structure and connectivity between vestibular, visual and spatial processing areas [27, 39, 40, 43, 44, 48, 50, 70, 74, 75]. A consensus picture is emerging of reduced activity and structural differences in areas associated with vestibular processing and spatial cognition, and altered connectivity within and between these regions [27, 30, 40, 44, 70, 75], consistent with down-weighting of vestibular information. In turn, there appears to be greater connectivity for visual areas, suggesting up-weighting of visual information [39, 48, 70], as well as altered connectivity with areas associated with attention and emotion [39, 48].
However, little research has explored whether individuals with PPPD, beyond relying more on visual information, might also process visual information in an atypical way. One fMRI study has reported that the strength of visual cortical activity correlates positively with dizziness symptoms in PPPD patients when visual motion stimuli are presented [54], which raises the question of whether the visual cortex is hyper-responsive in individuals with visually-induced dizziness.
If visual processing is in some way atypical in PPPD, this could exacerbate difficulties when vision becomes the primary sense for maintaining postural control. This paper explores whether individuals with PPPD, or those with increased symptoms of PPPD, report differences in their visual experience when viewing complex and challenging –but static –visual images.
1.1Natural and unnatural visual scenes
Human visual systems are thought to be optimised to process scenes with the characteristics of the natural environment, which tends to contain a broad range of orientations and a predominance of low spatial frequencies [19, 49]. For example, a natural Welsh landscape, with gentle hills, clouds and foliage has the consistent structure of natural scenes, with big objects providing most of the contrast variation in the scene and small objects or details providing less contrast variation. This property is reflected in the amplitude spectrum of the spatial frequencies that compose the scene: as spatial frequency increases, amplitude tends to reduce in a characteristic way [19, 21].
Efficient information processing requires sparse coding [60]. Having more neural activity in the visual cortex is not necessarily a good thing for processing visual information. Efficient processing would be expected to entail relatively sparse activity. Natural scenes and images that share these properties require less neural energy to process –perhaps because they are the environments in which we evolved and developed to perceive [19, 20, 46, 60]. Images that deviate from these statistical properties appear to be processed less efficiently: they are discriminated less well [22, 35, 47], they are subordinate in binocular rivalry [3], and they tend to produce a larger neural response [28, 38].
Many of the environments that elicit PPPD symptoms appear to deviate dramatically from natural scene statistics. A supermarket, for example, is highly cluttered with small objects and contains many more cardinal (horizontal/vertical) than oblique orientations. We informally observed that these types of environment show similarities to images that are known to produce visual discomfort (also known as visual stress) in individuals without dizziness. These uncomfortable images contain a limited range of orientations and a predominance of mid-high spatial frequencies [18, 33, 45, 49]. Particularly uncomfortable are images with an excess of aligned orientations with contrast energy at spatial frequencies around 3 cycles/degree, which corresponds to the peak sensitivity of the visual system [4, 7].
In the present study, we asked patients with PPPD and a large general population sample to rate a set of images for the amount of visual discomfort they experienced when viewing them. It should be emphasised that all the images were static, so discomfort did not depend on image motion or simulated self-motion that would be expected to be problematic in PPPD through interaction with balance processing. Instead, discomfort to static images could indicate that individuals with PPPD respond to visual information differently –compared to individuals without PPPD. This could potentially exacerbate dizziness symptoms if vision is relied upon for postural control.
The static images had previously been rated as either low or high discomfort by general population participants, and this was supported by an analysis of their spectral content that measured the degree to which they deviated from natural scenes in terms of orientation distribution and spatial frequency content [49] (cf. below). We investigated whether patients with PPPD, and individuals in the general population with more PPPD symptoms, rated the ‘high-discomfort’ images as more uncomfortable than did those with fewer PPPD symptoms.
1.2Association with migraine
It has been known for some time that individuals with migraine report a general increase in visual discomfort to images that deviate from the statistical properties of natural scenes [25, 41, 59, 72, 73]. They also show heightened neural responses in the visual cortex when viewing these images, suggesting that visual processing areas may be hyper-responsive in migraine [9, 28].
Migraine is associated with PPPD [52, 63], so we would expect that individuals with both migraine and visually-induced dizziness symptoms might also report increased visual discomfort. Of greater interest is whether the relationship between PPPD symptoms and visual discomfort only exists when there is co-occurring migraine, or if it exists irrespective of migraine. Therefore, when assessing the association between PPPD symptoms and visual discomfort, we control for the presence of co-morbid migraine.
2Method
2.1Participants
Patient cohort: Thirty patients were recruited from the vestibular clinic at University Hospital Wales (UHW). All patients had received a diagnosis of PPPD from a clinical scientist in Audiology or a Consultant Audiovestibular Physician, following the ICVD criteria [63] and common tests to examine vestibular functioning, including Halmagyi bedside head thrust testing, Video Head Impulse testing (vHIT using Synapsys system, Synapsys Solutions Ltd, West Sussex, UK), Videonystagmography (typically saccades, pursuit, gaze using GN system) and (sometimes) caloric testing if deemed necessary. Some patients had additional vestibular conditions (see Table 1). The average age of participants was 44 (sd = 14.3, range 11–67), 60%were female.
Table 1
Number and percent of patients who reported a secondary vestibular condition in addition to PPPD
| Number (percent) | |
| Vestibular Migraine | 4 (13%) |
| Labyrinthitis | 5 (17%) |
| Ménière’s disease | 2 (7%) |
| BPPV | 4 (13%) |
| Vestibular Neuritis | 3 (10%) |
General population cohort: Surveys were sent to 18,683 members of a community public health participant list in Wales, and we received approximately 2000 responses. Participants who reported a current diagnosis of any common vestibular-related conditions were excluded from all analyses (N = 193). Following this exclusion, we obtained 1858 responses for discomfort image ratings and migraine screening questionnaire. Of these, 1845 provided age and 1853 provided gender information. Of these, 1797 completed the visual vertigo analogue scale (VVAS) and 1435 completed the situational characteristics questionnaire (SCQ). A total of 1392 participants had a full set of responses on all measures. The average age of participants was 55 (range 18–88), 74%were female. No payment or compensation was offered to participants. All procedures were approved by the School of Psychology, Cardiff University, ethics committee.
2.2Materials
All aspects of the survey were delivered via Qualtrics (Provo, UT), an online survey tool.
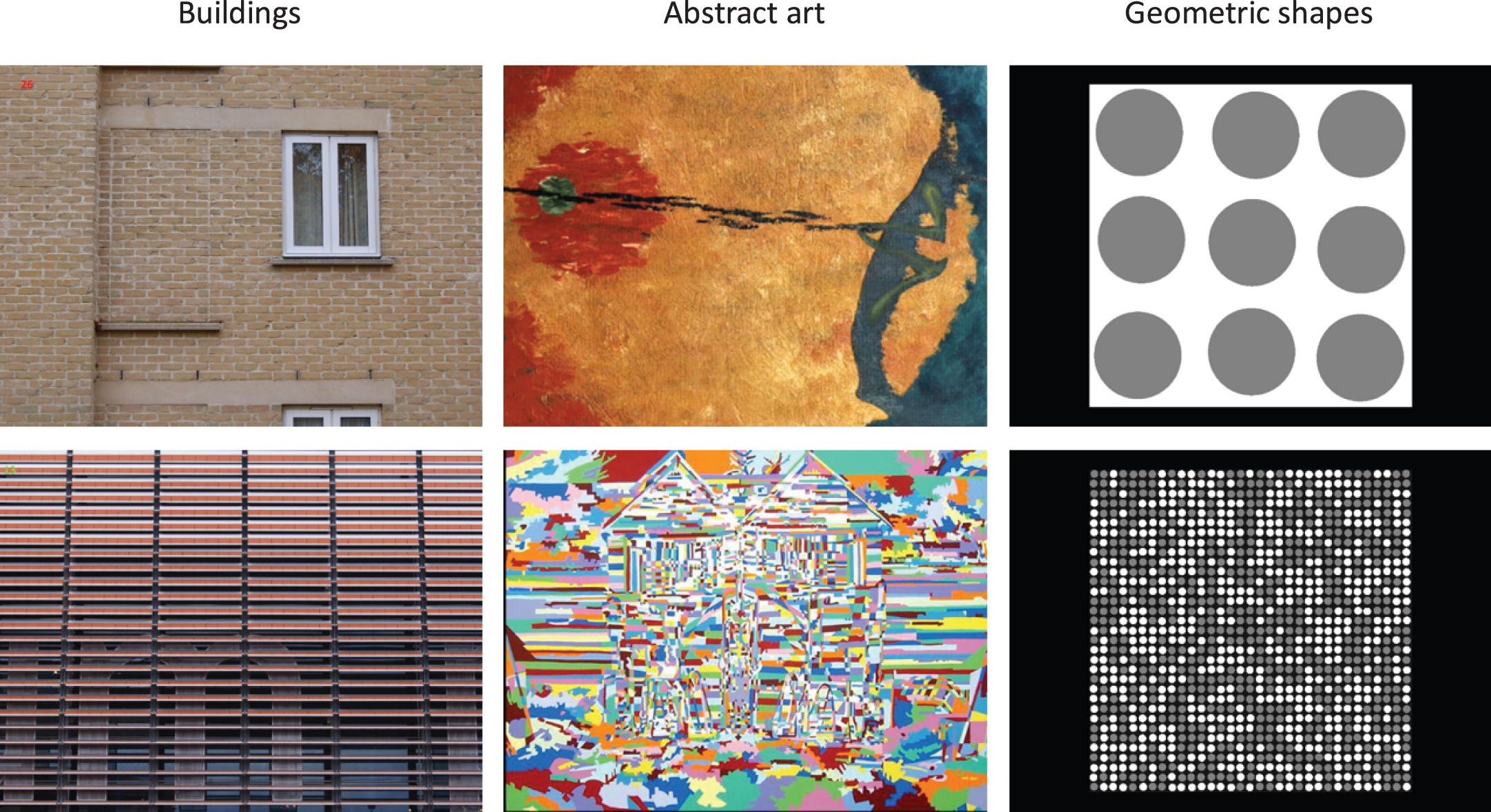
Visual discomfort images: We selected a random subset of 20 images from Penacchio and Wilkins (2015), which had been rated as high discomfort (n = 10) or low discomfort (n = 10). Images were taken from three categories: photographs of buildings, abstract art, and geometric shapes. Examples are shown in Fig. 1. Spectral analysis from Penacchio and Wilkins (2015) showed that the high-discomfort images contain a predominance of medium-high spatial frequencies and a narrower range of orientations, while the low-discomfort images conformed more to the statistical properties of natural scenes. This was reflected by a greater deviation of the amplitude spectrum of high-discomfort images, than low-discomfort images, from the average amplitude spectrum of 2000 natural images (the mean ratio of deviation of the high-discomfort images in the Van Hateren and van der Schaaf database [69] is 32.2, sd = 28.5, compared a mean deviation ratio of 3.9, sd = 2.4, for low-discomfort images).
Fig. 1
Example low discomfort images (top row) and high discomfort images (bottom row) used in the study.
On a standard 22 inch monitor with a viewing distance of 60 cm, the images subtended 25°×15° visual angle. The images were imbedded in a Qualtrics questionnaire and were viewed on participants’ personal devices, so they were rendered at different sizes and resolutions across participants; we asked participants at the beginning of the questionnaire to use the device with the biggest screen available (e.g., tablet preferable over a phone). The majority of general population participants used a computer monitor or a tablet to view the images (computer = 53%, tablet = 24%, phone = 23%). The visual angle of the images may not have differed much between computers and tablets, since the latter are generally held closer. But the visual angle is likely to have been smaller for phones. Since this was a large population survey, we had to accept this source of variability.
Visual Vertigo Analogue Scale (VVAS): The VVAS [13] is a short questionnaire that asked respondents to indicate on a rating scale from 0–10 the amount of dizziness they experience in 9 different situations. These situations are known triggers for patients with PPPD and include walking down a supermarket aisle, walking across a patterned floor, and going to the cinema. The items are then averaged, and this average score is multiplied by 10. The total score an individual could achieve by rating all situations a 10 (maximum dizziness) is 100. Note that the original version of the VVAS asks patients to place a mark on a continuous line on a piece of paper. We had to adapt this for delivery online. In our version, participants were asked to place a virtual line between 0 and 10, delimitated by increments of 1. However, since we do not use the score of the VVAS in an absolute sense, but merely to correlate with visual discomfort, this difference in method is not important here. Furthermore, the scale worked well insofar as patients scored higher than members of the general population and internal consistency was excellent (Cronbach’s alpha above 0.9, [52]).
Situational Characteristics Questionnaire (SCQ): The SCQ [32] was originally developed as a measure of space and motion discomfort, however, this condition is now considered to fall under the umbrella term of PPPD [63]. The SCQ is a 20 item questionnaire that, like the VVAS, also asks about discomfort in situations that trigger visually-induced dizziness and discomfort. Situations are rated between 0 and 3 and scores are normalised by subtracting responses to paired situations that are not commonly associated with visually-induced dizziness. The final score is obtained by dividing the summed ratings across all items by the total number of items and then multiplying by 10, therefore, the maximum score that can be given is 30. The SCQ was an optional questionnaire in the survey and consequently we are missing data for 425 participants (total N = 1465). The SCQ in our sample had acceptable internal consistency with a Cronbach’s alpha of 0.79.
Migraine Screen Questionnaire (MS-Q): This is a five item screening tool developed to identity migraine based on the criteria of the International Headache Society [37]. Participants answer yes/no questions about headache episodes they experience, such as ‘Do you usually suffer from nausea when you have a headache?’ and ‘Does light or noise bother you when you have a headache?’. Participants must response ‘yes’ to four or more of the five questions to have a result of probable migraine. The MS-Q is found to have good internal consistency (Cronbach’s alpha = 0.82) and high sensitivity (0.82–0.93) and specificity (0.81–0.97) in both neurological clinics and primary care [36, 37].
2.3Analysis
Our measure of visual discomfort was the ‘discomfort index’, defined as the difference in ratings between the low and high discomfort images. A greater score indicates an aversion to images that deviate from natural scene statistics rather than a bias to use higher ratings for all images. To provide reassurance that participants were appropriately using the scale, we checked that the high discomfort images were consistently rated as producing more visual discomfort than the low discomfort images: patients, t (29) = 11, p < 0.001, d = 1.96; general population, t (1857) = 42, p < 0.001, d = 0.97). To compare patient scores to controls whilst controlling for age, gender and migraine, we used ANCOVA. To assess the correlation between discomfort scores and PPPD symptoms in the general population we used Spearman correlations. For each analysis we used the maximum number of participants with complete responses for the information needed for that analysis.
3Results
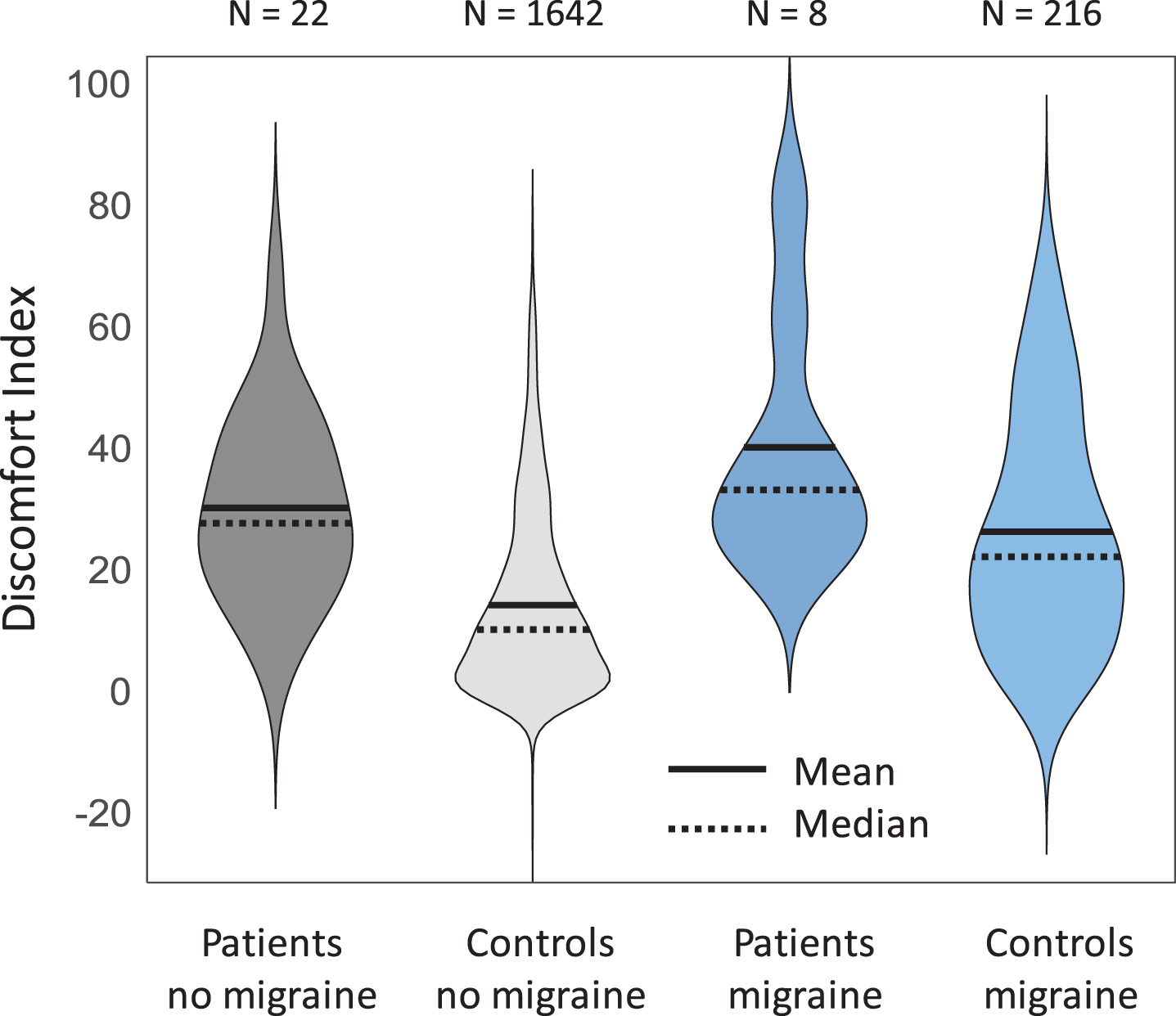
As hypothesised, patients with PPPD had a significantly higher discomfort index than participants from the general population cohort, after controlling for age, gender and migraine (see Fig. 2; F (1, 1865) = 29, p < 0.001; this analysis used all general population participants who returned discomfort scores, age, gender and migraine information, and did not report vestibular deficits; note also that patients also scored the low-discomfort images more highly than controls, ruling out an explanation that the difference in index reflects lower scores for low-discomfort images rather than higher scores for high-discomfort images).
Fig. 2
Violin plots showing higher discomfort index (difference in discomfort ratings between high and low discomfort images) in patients than in the general population controls, separated by migraine. In the statistical analysis (see text) we also controlled for age and gender. Black solid lines indicate mean, and dotted lines median.
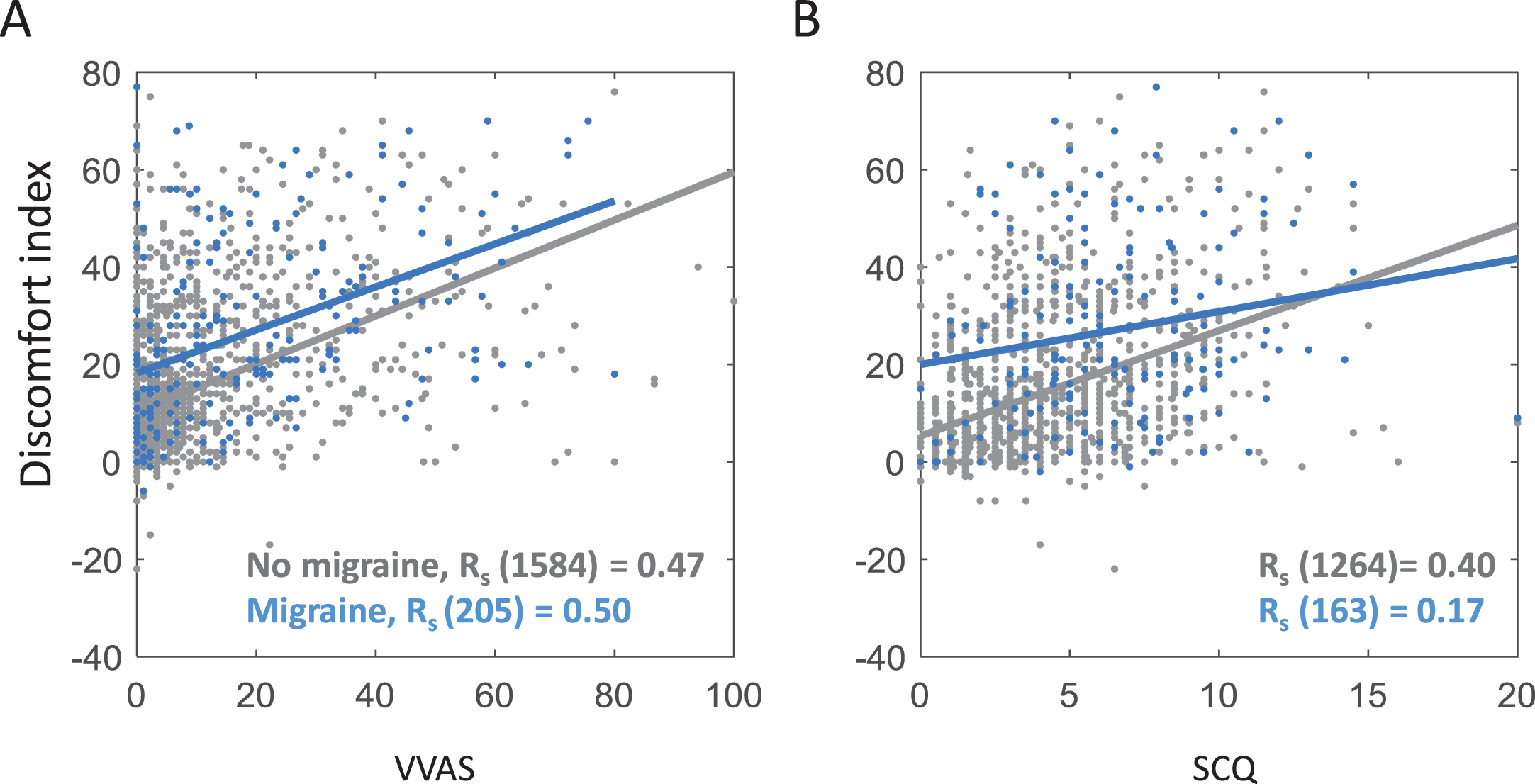
Secondly, as hypothesised, the visual discomfort index correlated positively with both measures of PPPD symptoms in general population cohorts, while controlling for age, gender and migraine (VVAS, rs (1387) = 0.46, p < 0.001; SCQ, rs (1387) = 0.39, p < 0.001); these analyses used all general population participants who returned discomfort scores, VVAS or SCQ, and age, gender and migraine information, and did not report vestibular deficits). Importantly this correlation was strongly present both in participants with migraine and those without migraine; see Fig. 3.
Fig. 3
Scatterplots showing Spearman correlations between the two measures of PPPD A = Visual Vertigo Analogue Scale (VVAS), B = Situational Characteristics Questionairre (SCQ) and visual discomfort index (high discomfort image rating –low discomfort image rating) for both participants with migraine (blue) and those without (grey), controlling for age and gender.
Migraine prevalence was 12%, and as expected this correlated both with visual discomfort (rs (1388) = 0.21, p < 0.001) and with PPPD symptoms (VVAS, rs (1388) = 0.2, p < 0.001; SCQ, rs (1388) = 0.18, p < 0.001), while controlling for age and gender. However, as already shown in Fig. 3, migraine does not account for the association between visual discomfort index and PPPD symptoms. Indeed, the correlation between PPPD symptoms and visual discomfort was significantly stronger than the correlation between migraine and visual discomfort (VVAS, zH = 8.81, p < 0.001; SCQ, zH = 6.29, p < 0.001; only participants who had complete data on all questionnaires were included in these partial correlation analyses; N = 1392).
4Discussion
Images that deviate from natural scene statistics in terms of the distribution of spatial frequencies and edge-orientations tend to produce some visual discomfort or visual stress [18, 33, 45, 49]. These same images seem to over-activate visual cortex and are processed less well [3, 22, 24, 26, 28, 35, 47]. We found that patients with PPPD report greater visual discomfort to a random selection of these images than do individuals from a large general population cohort. Likewise, within the general population, individuals with more symptoms of PPPD tended to report greater visual image discomfort.
4.1Visual processing of static images
All the images were static, so discomfort did not depend on the kind of motion in a video or simulated self-motion that would be expected to interact with balance processing and would be expected to be problematic in PPPD. These findings were not explained by co-morbid migraine, which has a known association with visual discomfort [25, 41, 59, 72, 73] and PPPD [51, 63, 66]. Indeed, PPPD symptoms showed a significantly stronger relationship with visual discomfort than did migraine.
We believe that these findings constitute preliminary evidence that visual processing itself (not just reliance on, and integration of, visual signals for balance) might be atypical in individuals with PPPD. A possible hypothesis is that some visual cortices are prone to a type of over-activation that renders them inefficient at processing certain kinds of scenes –ones that deviate markedly from natural scene statistics. These scenes are already known to be processed less well on average than images with natural statistics [22, 35, 47]. There is some initial evidence that dizziness symptoms in PPPD might correlate with increased neural activity in the visual cortex [54] –although that study explored motion stimuli and not static images. What such over-activation means remains unknown, and the reason why some visual systems may be more prone to it would only be speculation at this time.
In migraine and in Meares-Irlen Syndrome (visual stress) it has been suggested that increased visual discomfort might be due to hyper-responsivity in the visual cortex, though again, what this means mechanistically remains unknown [9, 12, 17, 28, 71–73]. There is some indication of greater or wider response in visual cortex when viewing pattern-glare-inducing stimuli or simply reading words [8, 28, 29]. If the visual cortex is hyper-responsive, this could be associated with higher levels of neural noise and lower levels of inhibition, which results in poorer visual discrimination abilities [16, 42, 45]. In psychophysical experiments, individuals with migraine tend to show higher contrast sensitivity thresholds and motion discrimination thresholds [12, 57, 68], which could be driven by a lack of inhibition relative to excitation in the visual cortex [2, 34, 45]. Poorer contrast sensitivity in migraine is also related to poorer performance on motion processing tasks [58]. It is not known if individuals with PPPD also show higher contrast sensitivity thresholds or general difficulties with spatial and motion discrimination, and this would seem to be an important avenue for future research. Poorer visual discrimination, or more aversive responses to visual scenes, might render the visual signal a less reliable cue for postural control, which could especially destabilise PPPD patients.
4.2Relationship to moving images or self-movement
While our study explored discomfort in response to static, not moving, images, it remains the case that PPPD is normally associated with moving around environments or watching moving images on a screen. But symptoms tend not to occur for movement through all environments; some are worse than others. It seems that a combination of movement and cluttered scenes –such as supermarkets –are especially problematic. It seems plausible –though it is currently speculation - that hyper-activity to scenes with structured repetition deviating from natural scenes could make it especially difficult for a visual system to extract efficiently accurate self-movement signals. In this view, static deviation from natural scenes is merely uncomfortable; adding movement to such scenes introduces a further problem of integration across senses, and becomes potentially nauseating. If vestibular signals have also been compromised for some reason, or if vestibular processing networks are atypical [27, 30, 39, 40, 44, 48, 70, 75], this cumulation of factors may be what leads to debilitating PPPD.
In the future, it would be interesting to extend the approach of studying visual discomfort when viewing static images to the types of visual motion stimuli that individuals with PPPD are particularly sensitive to. It may be possible to build a computational model that captures the spatial frequency and orientation content of moving images and combines this with a measurement of the visual motion within the stimuli. This tool could be used to predict the types of stimuli that are most likely to trigger symptoms in individuals with PPPD or are more effective for visual desensitization.
5Limitations
One limitation of the current research is that the high discomfort images could have produced feelings of dizziness or nausea in individuals with high PPPD symptoms, and participants might have relied on these feelings to rate the images, rather than visual discomfort per se. However, anecdotal reports from patients suggest that stationary images are much less likely to trigger dizziness symptoms, especially if they are fairly small in size, as ours were. Furthermore, the instructions were clear that participants should rate the images based on visual discomfort. In future studies, it might be best practice to also ask participants to report any dizziness they experience when viewing the images, so that this can be controlled for in the analysis.
Another limitation is that image size could not be controlled across participants because different viewing devices were used. However, we still observed a very strong difference in ratings between high and low discomfort images, and replicated previous findings of a relationship between discomfort ratings and migraines.
PPPD is associated with anxiety [5, 31, 53, 62, 64, 65] and therefore it is always important to consider whether anxiety mediates the relationship between PPPD and other factors. In a parallel paper exploring the role of anxiety in PPPD, we found that anxiety is also related to visual discomfort, but it does not entirely explain the relationship between visual discomfort and PPPD [53]. There is shared variance between all three factors, and it is plausible that experiencing discomfort in some visual environments could elicit anxiety and this could mediate some of the relationship with dizziness.
6Conclusion
In summary, we found that patients with PPPD and individuals with more PPPD symptoms report higher visual discomfort to images that deviate from natural spectra than individuals with few PPPD symptoms. Images that produce visual discomfort tend to share similarities with the types of challenging, highly cluttered environments that trigger PPPD symptoms. Although PPPD is often described as a condition driven by vestibular deficiencies and later visual dependence, our results suggest that visual processing in PPPD may also be atypical. Future research should explore whether known associations in migraine between visual discomfort, cortical hyper-excitability and visual discrimination deficits are also observed in PPPD.
Acknowledgments
This study funded by Wellcome [104943/Z/14/Z], Wellcome and Cardiff University ISSF [097824/Z/11/Z], and Health and Care Research Wales [SCF–18–1504]. The project was facilitated by HealthWise Wales, the Health and Care Research Wales initiative, which is led by Cardiff University in collaboration with SAIL, Swansea University. We would also like to thank the Clinical Audiology team at University Hospital Wales for their assistance in recruiting the patients.
References
[1] | Adamec I. , Meaški S.J. , Skorić M.K. , Jažić K. , Crnošija L. and Habek M. , O-01 Persistent postural-perceptual dizziness: clinical and neurophysiological study, Clinical Neurophysiology 130: ((2019) ), e21. |
[2] | Aurora S. and Wilkinson F. , The brain is hyperexcitable in migraine, Cephalalgia 27: ((2007) ), 1442–1453. |
[3] | Baker D.H. and Graf E.W. , Natural images dominate in binocular rivalry, Proceedings of the National Academy of Sciences 106: ((2009) ), 5436–5441. |
[4] | Barten P.G. , Contrast sensitivity of the human eye and its effects on image quality, Spie optical engineering press Bellingham, WA, (1999) . |
[5] | Brandt T. and Dieterich M. , Phobischer attacken-schwankschwindel, ein neues syndrom, Münch Med Wochenschr 128: ((1986) ), 247–250. |
[6] | Bronstein A.M. , Visual vertigo syndrome: clinical and posturography findings, Journal of Neurology, Neurosurgery & Psychiatry 59: ((1995) ), 472–476. |
[7] | Campbell F.W. and Robson J.G. , Application of Fourier analysis to the visibility of gratings, The Journal of Physiology 197: ((1968) ), 551. |
[8] | Chouinard B.D. , Zhou C.I. , Hrybouski S. , Kim E.S. and Cummine J. , A functional neuroimaging case study of Meares–Irlen syndrome/visual stress (MISViS), Brain Topography 25: ((2012) ), 293–307. |
[9] | Coppola G. , Pierelli F. and Schoenen J. , Is the cerebral cortex hyperexcitable or hyperresponsive in migraine?, Cephalalgia 27: ((2007) ), 1427–1439. |
[10] | Cousins S. , Cutfield N.J. , Kaski D. , Palla A. , Seemungal B.M. , Golding J.F. , Staab J.P. and Bronstein A.M. , Visual dependency and dizziness after vestibular neuritis, PloS one 9: ((2014) ), e105426. |
[11] | Cousins S. , Kaski D. , Cutfield N. , Arshad Q. , Ahmad H. , Gresty M.A. , Seemungal B.M. , Golding J. and Bronstein A.M. , Predictors of clinical recovery from vestibular neuritis: a prospective study, Annals of Clinical and Translational Neurology 4: ((2017) ), 340–346. |
[12] | Cucchiara B. , Datta R. , Aguirre G.K. , Idoko K.E. and Detre J. , Measurement of visual sensitivity in migraine: validation of two scales and correlation with visual cortex activation, Cephalalgia 35: ((2015) ), 585–592. |
[13] | Dannenbaum E. , Chilingaryan G. and Fung J. , Visual vertigo analogue scale: an assessment questionnaire for visual vertigo, Journal of Vestibular Research 21: ((2011) ), 153–159. |
[14] | Dieterich M. , Staab J. and Brandt T. , Brandt, Functional (psychogenic) dizziness, in: Handbook of clinical neurology, Elsevier, (2016) , pp. 447–468. |
[15] | Dieterich M. and Staab J.P. , Functional dizziness: from phobic postural vertigo and chronic subjective dizziness to persistent postural-perceptual dizziness, Current Opinion in Neurology 30: ((2017) ), 107–113. |
[16] | Edden R.A. , Muthukumaraswamy S.D. , Freeman T.C. and Singh K.D. , Orientation discrimination performance is predicted by GABA concentration and gamma oscillation frequency in human primary visual cortex, Journal of Neuroscience 29: ((2009) ), 15721–15726. |
[17] | Evans B. , Wilkins A. , Brown J. , Busby A. , Wingfield A. , Jeanes R. and Bald J. , A preliminary investigation into the aetiology of Meares—Irlen syndrome, Ophthalmic and Physiological Optics 16: ((1996) ), 286–296. |
[18] | Fernandez D. and Wilkins A.J. , Uncomfortable images in art and nature, Perception 37: ((2008) ), 1098–1113. |
[19] | Field D.J. , Relations between the statistics of natural images and the response properties of cortical cells, Journal of the Optical Society of America 4: ((1987) ), 2379–2394. |
[20] | Field D.J. , What is the goal of sensory coding? Neural Computation 6: ((1994) ), 559–601. |
[21] | Geisler W.S. , Visual perception and the statistical properties of natural scenes, Annu Rev Psychol 59: ((2008) ), 167–192. |
[22] | Geisler W.S. , Perry J.S. , Super B. and Gallogly D. , Edge co-occurrence in natural images predicts contour grouping performance, Vision Research 41: ((2001) ), 711–724. |
[23] | Guerraz M. , Yardley L. , Bertholon P. , Pollak L. , Rudge P. , Gresty M. and Bronstein A. , Visual vertigo: symptom assessment, spatial orientation and postural control, Brain 124: ((2001) ), 1646–1656. |
[24] | Haigh S.M. , Barningham L. , Berntsen M. , Coutts L.V. , Hobbs E.S. , Irabor J. , Lever E.M. , Tang P. and Wilkins A.J. , Discomfort and the cortical haemodynamic response to coloured gratings, Vision Research 89: ((2013) ), 47–53. |
[25] | Harle D.E. , Shepherd A.J. and Evans B.J. , Visual stimuli are common triggers of migraine and are associated with pattern glare, Headache: The Journal of Head and Face Pain 46: ((2006) ), 1431–1440. |
[26] | Hibbard P.B. and O’Hare L. , Uncomfortable images produce non-sparse responses in a model of primary visual cortex, Royal Society open science 2: ((2015) ), 140535. |
[27] | Hoppes C.W. , Sparto P.J. , Whitney S.L. , Furman J.M. and Huppert T.J. , Changes in cerebral activation in individuals with and without visual vertigo during optic flow: A functional near-infrared spectroscopy study, NeuroImage: Clinical 20: ((2018) ), 655–663. |
[28] | Huang J. , Cooper T.G. , Satana B. , Kaufman D.I. and Cao Y. , Visual distortion provoked by a stimulus in migraine associated with hyperneuronal activity, Headache: The Journal of Head and Face Pain 43: ((2003) ), 664–671. |
[29] | Huang J. , Zong X. , Wilkins A. , Jenkins B. , Bozoki A. and Cao Y. , fMRI evidence that precision ophthalmic tints reduce cortical hyperactivation in migraine, Cephalalgia 31: ((2011) ), 925–936. |
[30] | Indovina I. , Riccelli R. , Chiarella G. , Petrolo C. , Augimeri A. , Giofrè L. , Lacquaniti F. , Staab J.P. and Passamonti L. , Role of the insula and vestibular system in patients with chronic subjective dizziness: an fMRI study using sound-evoked vestibular stimulation, Frontiers in Behavioral Neuroscience 9: ((2015) ), 334. |
[31] | Jacob R.G. , Redfern M.S. and Furman J.M. , Space and motion discomfort and abnormal balance control in patients with anxiety disorders, Journal of Neurology, Neurosurgery & Psychiatry 80: ((2009) ), 74–78. |
[32] | Jacob R.G. , Woody S.R. , Clark D.B. , Lilienfeld S.O. , Hirsch B.E. , Kucera G.D. , Furman J.M. and Durrant J.D. , Discomfort with space and motion: a possible marker of vestibular dysfunction assessed by the situational characteristics questionnaire, Journal of Psychopathology and Behavioral Assessment 15: ((1993) ), 299–324. |
[33] | Juricevic I. , Land L. , Wilkins A.J. and Webster M.A. , Visual discomfort and natural image statistics, Perception 39: ((2010) ), 884–899. |
[34] | Khalil N. and Legg N. , Pathophysiology of migraine: a study using VEP and contrast sensitivity, New advances in headache research. 3rd edn. London: Smith-Gordon ((1989) ), 57–61. |
[35] | Knill D.C. , Field D.J. and Kerstent D. , Human discrimination of fractal images, JOSA A 7: ((1990) ), 1113–1123. |
[36] | Láinez M.J. , Castillo J. , Domínguez M. , Palacios G. , Díaz S. and Rejas J. , New uses of the Migraine Screen Questionnaire (MS-Q): validation in the Primary Care setting and ability to detect hidden migraine. MS-Q in Primary Care, BMC Neurology 10: ((2010) ), 39. |
[37] | Láinez M.J. , Domínguez M. , Rejas J. , Palacios G. , Arriaza E. , Garcia-Garcia M. and Madrigal M. , Development and validation of the Migraine Screen Questionnaire (MS-Q), Headache: The Journal of Head and Face Pain 45: ((2005) ), 1328–1338. |
[38] | Le A.T. , Payne J. , Clarke C. , Kelly M.A. , Prudenziati F. , Armsby E. , Penacchio O. and Wilkins A.J. , Discomfort from urban scenes: Metabolic consequences, Landscape and Urban Planning 160: ((2017) ), 61–68. |
[39] | Lee J.O. , Lee E.S. , Kim J.S. , Lee Y.B. , Jeong Y. , Choi B.S. , Kim J.H. and Staab J.P. , Altered brain function in persistent postural perceptual dizziness: a study on resting state functional connectivity, Human Brain Mapping 39: ((2018) ), 3340–3353. |
[40] | Li K. , Si L. , Cui B. , Ling X. , Shen B. and Yang X. , Altered spontaneous functional activity of the right precuneus and cuneus in patients with persistent postural-perceptual dizziness, Brain Imaging and Behavior (2019), 1–11. |
[41] | Marcus D.A. and Soso M.J. , Migraine and stripe-induced visual discomfort, Archives of Neurology 46: ((1989) ), 1129–1132. |
[42] | McKendrick A. and Badcock D. , Motion processing deficits in migraine, Cephalalgia 24: ((2004) ), 363–372. |
[43] | Na S. , Im J.J. , Jeong H. , Lee E.-S. , Lee T.-K. , Chung Y.-A. and Song I.-U. , Cerebral perfusion abnormalities in patients with persistent postural-perceptual dizziness (PPPD): a SPECT study, Journal of Neural Transmission 126: ((2019) ), 123–129. |
[44] | Nigro S. , Indovina I. , Riccelli R. , Chiarella G. , Petrolo C. , Lacquaniti F. , Staab J.P. and Passamonti L. , Reduced cortical folding in multi-modal vestibular regions in persistent postural perceptual dizziness, Brain Imaging and Behavior 13: ((2019) ), 798–809. |
[45] | O’Hare L. and Hibbard P.B. , Visual processing in migraine, Cephalalgia 36: ((2016) ), 1057–1076. |
[46] | Olshausen B.A. and Field D.J. , Sparse coding with an overcomplete basis set: A strategy employed by V1?, Vision Research 37: ((1997) ), 3311–3325. |
[47] | Parraga C.A. , Troscianko T. and Tolhurst D.J. , The human visual system is optimised for processing the spatial information in natural visual images, Current Biology 10: ((2000) ), 35–38. |
[48] | Passamonti L. , Riccelli R. , Lacquaniti F. , Staab J.P. and Indovina I. , Brain responses to virtual reality visual motion stimulation are affected by neurotic personality traits in patients with persistent postural-perceptual dizziness, Journal of Vestibular Research (2019), 1–10. |
[49] | Penacchio O. and Wilkins A.J. , Visual discomfort and the spatial distribution of Fourier energy, Vision Research 108: ((2015) ), 1–7. |
[50] | Pollak L. , Osherov M. , Berkovitz N. , Beckerman I. , Stryjer R. and Tal S. , Magnetic resonance brain imaging in patients with visual vertigo, Brain and behavior 5: ((2015) ), e00402. |
[51] | Popkirov S. , Staab J.P. and Stone J. , Persistent postural-perceptual dizziness (PPPD): a common, characteristic and treatable cause of chronic dizziness, Practical Neurology 18: ((2018) ), 5–13. |
[52] | Powell G. , Derry-Sumner H. , Rajenderkumar D. , Rushton S.K. and Sumner P. , Persistent postural perceptual dizziness is on a spectrum in the general population, Neurology (2020). |
[53] | Powell G. , Derry-Sumner H. , Shelton K. , Rushton S. , Hedge C. , Rajenderkumar D. and Sumner P. , Visually-induced dizziness is associated with sensitivity and avoidance across all senses, Journal of Neurology (2020). |
[54] | Riccelli R. , Passamonti L. , Toschi N. , Nigro S. , Chiarella G. , Petrolo C. , Lacquaniti F. , Staab J.P. and Indovina I. , Altered Insular and Occipital Responses to Simulated Vertical Self-Motion in Patients with Persistent Postural-Perceptual Dizziness, Frontiers in Neurology 8: ((2017) ). |
[55] | Schniepp R. , Wuehr M. , Huth S. , Pradhan C. , Brandt T. and Jahn K. , Gait characteristics of patients with phobic postural vertigo: effects of fear of falling, attention, and visual input, Journal of Neurology 261: ((2014) ), 738–746. |
[56] | Sezier A.E.I. , Saywell N. , Terry G. , Taylor D. and Kayes N. , Working-age adults’ perspectives on living with persistent postural-perceptual dizziness: a qualitative exploratory study, BMJ Open 9: ((2019) ), e024326. |
[57] | Shepherd A.J. , Visual contrast processing in migraine, Cephalalgia 20: ((2000) ), 865–880. |
[58] | Shepherd A.J. , Beaumont H.M. and Hine T.J. , Motion processing deficits in migraine are related to contrast sensitivity, Cephalalgia 32: ((2012) ), 554–570. |
[59] | Shepherd A.J. , Hine T.J. and Beaumont H.M. , Color and spatial frequency are related to visual pattern sensitivity in migraine, Headache: The Journal of Head and Face Pain 53: ((2013) ), 1087–1103. |
[60] | Simoncelli E.P. and Olshausen B.A. , Natural image statistics and neural representation, Annual Review of Neuroscience 24: ((2001) ), 1193–1216. |
[61] | Staab J.P. , Chronic subjective dizziness, CONTINUUM: Lifelong Learning in Neurology 18: ((2012) ), 1118–1141. |
[62] | Staab J.P. , Psychiatric Considerations in the Management of Dizzy Patients, in: Vestibular Disorders, Karger Publishers, (2019) , pp. 170–179. |
[63] | Staab J.P. , Eckhardt-Henn A. , Horii A. , Jacob R. , Strupp M. , Brandt T. and Bronstein A. , Diagnostic criteria for persistent postural-perceptual dizziness (PPPD): consensus document of the committee for the classification of vestibular disorders of the bárány society, Journal of Vestibular Research 27: ((2017) ), 191–208. |
[64] | Staab J.P. , Rohe D.E. , Eggers S.D. and Shepard N.T. , Anxious, introverted personality traits in patients with chronic subjective dizziness, Journal of Psychosomatic Research 76: ((2014) ), 80–83. |
[65] | Staab J.P. and Ruckenstein M.J. , Which comes first? Psychogenic dizziness versus otogenic anxiety, The Laryngoscope 113: ((2003) ), 1714–1718. |
[66] | Staab J.P. and Ruckenstein M.J. , Expanding the differential diagnosis of chronic dizziness, Archives of Otolaryngology–Head & Neck Surgery 133: ((2007) ), 170–176. |
[67] | Strupp M. , Glaser M. , Karch C. , Rettinger N. , Dieterich M. and Brandt T. , The most common form of dizziness in middle age: phobic postural vertigo, Der Nervenarzt 74: ((2003) ), 911–914. |
[68] | Tibber M.S. , Kelly M.G. , Jansari A. , Dakin S.C. and Shepherd A.J. , An inability to exclude visual noise in migraine, Investigative ophthalmology & visual science 55: ((2014) ), 2539–2546. |
[69] | Van J.H. , Hateren and A. van der Schaaf, Independent component filters of natural images compared with simple cells in primary visual cortex, Proceedings of the Royal Society of London. Series B: Biological Sciences 265: ((1998) ), 359–366. |
[70] | Van Ombergen A. , Heine L. , Jillings S. , Roberts R.E. , Jeurissen B. , Van Rompaey V. , Mucci V. , Vanhecke S. , Sijbers J. and Vanhevel F. , Altered functional brain connectivity in patients with visually induced dizziness, NeuroImage: Clinical 14: ((2017) ), 538–545. |
[71] | Wilkins A. , Huang J. and Cao Y. , Prevention of visual stress and migraine with precision spectral filters, Drug Development Research 68: ((2007) ), 469–475. |
[72] | Wilkins A.J. , Visual stress, Oxford University Press, (1995) . |
[73] | Wilkins A.J. , Nimmo-Smith I. , Tait A. , McMANUS C. , SALA S.D. , Tilley A. , Arnold K. , Barrie M. and Scott S. , A neurological basis for visual discomfort, Brain 107: ((1984) ), 989–1017. |
[74] | Woll J. , Sprenger A. and Helmchen C. , Postural control during galvanic vestibular stimulation in patients with persistent perceptual–postural dizziness, Journal of Neurology 266: ((2019) ), 1236–1249. |
[75] | Wurthmann S. , Naegel S. , Steinberg B.S. , Theysohn N. , Diener H.-C. , Kleinschnitz C. , Obermann M. and Holle D. , Cerebral gray matter changes in persistent postural perceptual dizziness, Journal of Psychosomatic Research 103: ((2017) ), 95–101. |




