Sustained acoustic medicine treatment of discogenic chronic low back pain: A randomized, multisite, double-blind, placebo-controlled trial
Abstract
BACKGROUND:
Sustained acoustic medicine (SAM) is a noninvasive long-term treatment that provides essential mechanical and thermal stimulus to accelerate soft tissue healing, alleviate pain, and improve physical activity. SAM increases localized deep tissue temperature, blood flow, cellular proliferation, migration, and nutrition exchange, resulting in reduced inflammation and an increased rate of tissue regeneration.
OBJECTIVE:
To assess the efficacy of SAM treatment of discogenic back pain in the lower spinal column to reduce pain, improve quality of life, and lower pharmacotherapy use.
METHODS:
Sixty-five subjects with chronic low back pain were randomly assigned to SAM (
RESULTS:
SAM treatment significantly reduced chronic lower back pain from baseline relative to placebo treatment (
CONCLUSION:
Daily, home-use SAM treatment significantly improves the clinical symptoms of chronic lower back pain, improves physical mobility, and reduces daily medication use. SAM treatment is well-tolerated by patients and may be considered a safe, non-invasive treatment option for chronic discogenic, lower back pain.
1.Introduction
Lower back pain is a prevalent health problem and affects people of all ages, from children to the elderly. Sixty to 85% of the population experiences lower back pain at least once in their lifetime, with the highest prevalence in people between 40 and 69 years old. Chronic lower back pain (e.g., back pain greater than 3 months) impacts 10% to 23.3% of the adult population in the United States [1, 2]. The highest prevalence of lower back pain is in women between 40 to 80 years old [3]. The annual cost of lower back pain management in the United States exceeds $100 billion [4, 5]. While only 1.2% of patients receive surgery within the first year of diagnosis, they account for approximately $784 million in annual healthcare cost [6]. The largest portion of the cost is associated with indirect economic costs such as lost workdays and reduced overall productivity. Besides economic effects, lower back pain significantly affects the quality of life and daily activities, leading to depression and anxiety for many patients [7, 8, 9].
Back pain is a complex pathology. It can be due to trauma or degeneration involving spine structure, including muscles, fascia, ligaments, tendons, facet joints, neurovascular elements, vertebrae, and intervertebral discs. In trauma or degeneration, physical damage and improper healing can lead to chronic localized inflammation and pain [10, 11]. The intervertebral disc degradation (herniated disks) reduces the intervertebral space, thus changing the local biochemical and biomechanical function, leading to localized chronic inflammation, degeneration of nucleus pulposus cells, and pain [11, 12]. Accelerated spinal degeneration has been shown to reduce the space between two vertebrae as the intervertebral disc and associated elements break down, resulting in lower back pain spreading out to the lower limbs. Studies have reported that the level of disc herniation does not correlate with the severity of pain and physical mobility. The physical damage is typically confirmed using MRI and CT imaging [13]. Clinically, 54% of back pain patients have recurrent pain at 6 months, and 47% of patients reported recurrent pain at 24 months with physical damage to spinal structures [14].
Considering the complication of lower back pain, multiple modes of treatment are used concurrently, including behavioral management and nonpharmacological, pharmacological, and surgical treatments. The first line of treatment for lower back pain includes strength and stabilization exercises, physical therapy, cognitive therapy, nonpharmacological therapies, and pharmacological approaches [15, 16, 17, 18]. Physical therapy, cognitive therapy, and other nonpharmacological therapies may be effective but take a long time and persistence. Pharmacological therapies are effective but have multi-organ adverse effects and are not recommended for long-term use [17, 19]. Finally, surgical treatment is considered in trauma or after the failure of other therapies, which may include implantable devices [20, 21].
The intervertebral structure is highly mechanosensitive and requires mechanical stimulus to recover and regenerate [22]. Ultrasound is an acoustic wave providing alternating mechanical force [23, 24, 25]. Studies have shown that ultrasound increases cellular migration, proliferation, and localized vascularization, reducing inflammation and accelerating soft tissue healing [23, 24, 26, 27, 28, 29, 30]. The Food and Drug Administration (FDA) has approved ultrasound treatment systems for non-union fracture healing, musculoskeletal pain, and soft tissue injuries as a standalone or combination therapy [31, 32, 33, 34, 35].
Sustained acoustic medicine (SAM) is an FDA-approved, non-invasive, long-term source of high-dose, high-frequency, continuous ultrasound that provides 18,720 joules of energy over 4 hours of treatment [36, 37]. Clinical studies have shown that SAM application has limited adverse effects, reduces chronic musculoskeletal pain (e.g., soft-tissue injuries including tendinopathy, osteoarthritis, and myofascial pain), accelerates soft tissue healing, improves patients’ quality of life [37, 38, 39, 40, 41, 42]. SAM increases localized temperature deep into skeletal muscle (greater than 5 cm deep and 8∘C), blood flow, cellular proliferation, migration, and nutrition exchange, resulting in reduced inflammation and an increased rate of tissue regeneration, providing significant pain reduction and functional gains [35, 38, 40, 43]. Clinical studies on SAM have established the clinical effectiveness of treatment in upper and lower limbs and joints, but there is limited data specifically evaluating the efficacy of SAM on chronic discogenic lower back pain. Chronic lower back pain significantly affects mobility and quality of life. We aim to evaluate SAM as an alternative deep-penetrating treatment option for chronic lower back pain.
This study aims to determine the efficacy and safety of SAM treatment in alleviating chronic lower back pain over an 8-week treatment. We hypothesized that 8 weeks of SAM would result in more significant pain reduction, improved quality of life, reduced medication use, and improved physical activity limitation compared to placebo treatment.
2.Methods
A prospective, randomized, double-blinded, multi-site, placebo-controlled study in the outpatient community hospital pain management clinics of Ithaca, NY, and Chapel Hill, NC, United States, was conducted from November 2015 to April 2016. This study was approved by the Medical Ethical Committee at the institutional review board of Schuman (#2015/20140901), and the trial was registered with the United States National Institutes of Health Clinical Trails registry (NCT02609854). Written informed consent was obtained from all subjects prior to participation. The study was conducted in accordance with relevant guidelines, regulations, and the World Medical Association Declaration of Helsinki. Funding for the study was provided by the National Space Biomedical Research Institute, a subsidiary of The National Aeronautics and Space Administration of the United States of America, to evaluate emerging medical technologies for space-relevant human health concerns.
Recruitment strategies involved posters, flyers, and clinic/hospital pull-up displays to inform potential subjects of the chronic lower back pain research study. The recruiters had a bachelor’s degree or higher with a minimum experience of 10 years in health care sciences. Potential subjects were initially screened over the phone for general eligibility by the study site research assistants. Phone screening covered symptomology, study intervention ability to apply treatment to the lower lumbar region, and length of study involvement. Any subject passing the initial screening was advised to consult with their primary healthcare or pain management provider to confirm clearance prior to study participation.
2.1Inclusion criteria
All potential subjects were evaluated by physical examination conducted by board-certified physicians, blood tests, and radiographs to identify any exclusion factors. Board-certificated radiologists interpreted the radiographs. Ambulatory male and female patients 20 to 60 years of age with lower back pain for more than 3 months presenting with or without associated leg pain, MRI confirmation of lower lumbar spine herniated disc (L1 – L5), mean Numeric Rating Scale (NRS) pain of four or more out of ten the week preceding enrollment and 2-weeks of baseline pain measures, and capable of self-applying SAM treatment to the lower lumbar region (L1 – L5) were included in this study.
2.2Exclusion criteria
The subjects were excluded if they had arthritis, bone spur, stenosis, fusion, or implants near the herniated disc. Patients with active infections, open sores or wounds, undergoing chemotherapy or having known neuropathy, hereditary disposition to excessive bleeding, and peripheral artery disease were also excluded. Patients with malignancy or metastasis on the vertebra, acute compression fracture, and collagen disease, such as ankylosing spondylitis, were excluded. Evidence of nerve root, spinal cord, or cauda equina compression; severe spinal stenosis indicated by signs of neurogenic claudication; grade 3 to 4 spondylolisthesis; fibromyalgia or systemic/inflammatory disorder; as well as any other current lower extremity musculoskeletal injuries were excluded. The latter included any medical condition limiting mobility or pregnancy. In addition, patients who had a prior diagnosis of dementia were excluded. All potential subjects underwent the Mini Mental State Examination, and those with a score of less than 24 were excluded. Finally, subjects who did not show the ability to use the SAM device properly failed to follow the instructions, were unable to walk, or participated in other clinical trials within the last 30 days were excluded from the study.
2.3Study procedures
Eligible and willing subjects provided written informed consent, underwent basic demographic and vital measures, and completed a 2-week (minimum of 14 days) daily pain diary prior to randomization in the placebo-controlled study. Study arms were randomized with a Microsoft® Excel RAND function computer-generated random number allocation list of active and non-active ultrasound transducer emitters provided by the manufacturer. Subjects were sequentially enrolled into either the active group (active SAM device) or the placebo group (SAM device with deactivated ultrasound emitting transducers). Treatment allocation was blinded from the clinical sites and research staff enrolling patients and performing data entry, and all study devices and materials appeared and operated equivalently. Study participants were also blinded to treatment group allocation and were informed that they may or may not receive active intervention. The study biostatistician held the device status key for analysis and unblinding.
Figure 1.
Sustained Acoustic Medicine (SAM) application to lower back. The ultrasound delivery system spreads ultrasound diathermy to the size of the star-shaped ultrasound coupling patch.
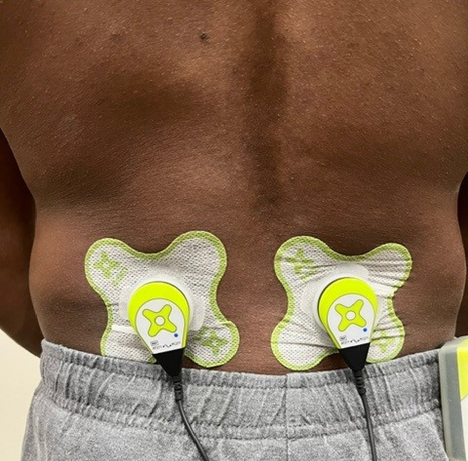
All subjects were provided with a power controller, 2 applicators, ultrasound coupling bandages, an ultrasound gel bottle, a Y-adapter, a charger, and the user manual. All patients were trained on how to use the SAM device properly to ensure it would not interfere with their daily life routine and provide the essential treatment to the spinal column. The ultrasound applicator(s) were placed bilaterally on either side of the herniation approximately 3 to 5 cm from the centerline, ensuring ultrasonic coverage of the injury site, as shown in Fig. 1. The ultrasound gel coupling patch secured each applicator in place on the back and filled the space between the ultrasound transducer and the skin to provide little or no loss of acoustic intensity propagation into deep tissue. The SAM treatment was to be administered during normal daily activities, including deskwork, light chores, and exercise. The device was to be removed prior to any bathing or aquatic activity.
The active SAM device was programmed to deliver continuous high-frequency 3MHz, an intensity of 132 mW/cm2, and a total power of 1.3W, providing deep (5 cm) ultrasound stimulation for a total of 18,720 joules of energy over 4 hours of treatment. The non-active device functioned identically to the active device with a timer, power, and all user indications. However, power to the ultrasound transducer crystal was internally disconnected to prevent ultrasound energy delivery by the manufacturer. The active and placebo treatment was administered for 4 hours daily during day-to-day activities at the site of pain (L1 – L5), excluding water-related activities (potentially immersing in the device). In addition, the subjects were provided with a daily diary to record changes in the time of treatment, effects on pain, and day-to-day activities. Weekly patient video phone calls and bi-weekly in-person reviews were conducted to ensure subjects were completing reporting and addressing any study-related questions with the research staff.
2.4Primary outcome measure
The NRS pain score was the primary outcome measure. NRS pain is an 11-point scale, with 0 being no pain and 10 being the most pain. NRS score was assessed at baseline and reassessed every two weeks for 8-weeks (bi-weekly) in-person follow-up. A minimally clinically important reduction in pain was defined as 2 points on the NRS scale.
Patients were also instructed to record the incremental change in back pain in a daily diary during treatment over the 8-week period. Pain scores were recorded immediately before treatment, 30 minutes into the treatment, 2 hours into treatment, and immediately after treatment.
2.5Secondary outcome measures
The Global Rating of Change GROC score was measured at the end of week 8. The GROC assesses a patient’s level of back pain well-being on a 15-point scale ranging from (
The use of prescription opioid pain medication, morphine milligram equivalent (MME) dosage, and over-the-counter NSAIDS were tracked on study enrollment and study completion. This included physician medication reports and patient diary documentation of medication usage. Patients were required not to increase pain medication usage during the study period. Patients were allowed to reduce their medication usage if it did not increase their pain, which was evaluated by daily diary tracking (both by the patient and staff during on-site meetings).
A functional back pain treatment survey regarding walking, gardening, and lifestyle activities based on the modified Oswestry disability index (ODI) questionnaire was also completed at the end of the intervention. Subject satisfaction with treatment was evaluated with a yes/no questionnaire at the completion of the study in regard to ease of use, continued use, and effectiveness of treatment.
2.6Statistics
All data were analyzed using The R Project for Statistical Computing using an intention-to-treat analysis. The Kolmogorov-Smirnov test was used to determine if data were normally distributed. No evidence of non-normality was found to merit the use of non-parametric tests on the primary or secondary outcome measures. A repeated measure ANOVA and
A sample calculation for the primary outcome measure of NRS pain was conducted based on previous therapeutic ultrasound clinical trials on chronic low back pain with an average pain reduction of 2 points after intervention. To detect this difference with 90% power and
Table 1
Patient demographic information for enrolled subjects
| Patient demographic data | |||
|---|---|---|---|
| Variable | Active ultrasound | Placebo ultrasound | |
|
| 33 | 32 | NA |
| Sex (M/F) | 14/19 | 12/20 | NA |
| Age, years | 50.2 | 47.0 | 0.2931 |
| BMI | 29.8 | 29.6 | 0.8911 |
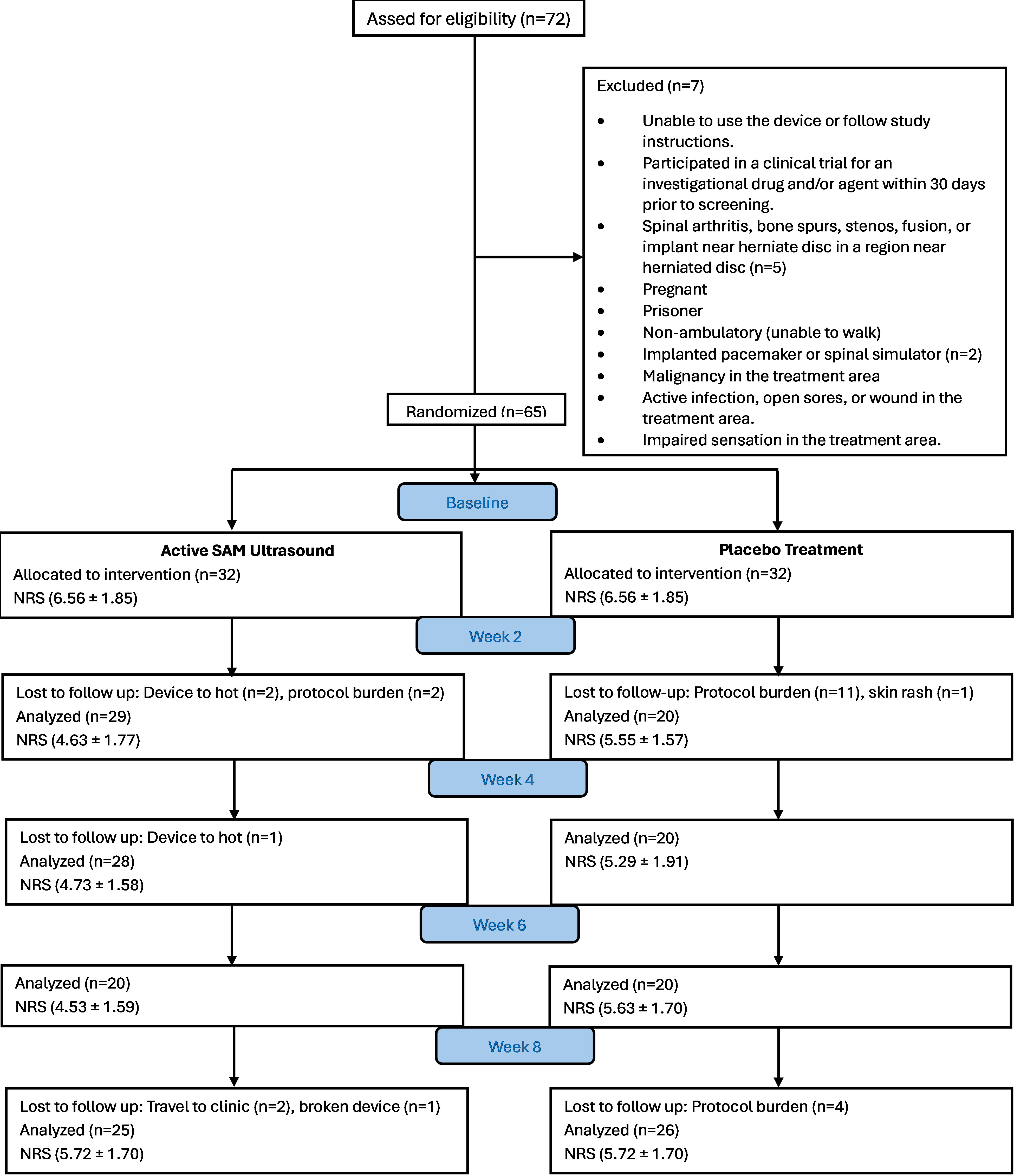
Figure 2.
CONSORT flow diagram of study inclusion, randomization and follow-up.
3.Results
3.1Patient distribution
Seventy-two subjects were screened for eligibility and 65 subjects were eligible for randomization (Fig. 2). The active group had 33 subjects (14 males, 19 females, average age 50.2
Table 2
Back pain reduction from baseline (NRS) and mean change from baseline (NRS) for all study participants
| Week | Active | Placebo | Mean difference 95% CI | |
|---|---|---|---|---|
| Primary outcome NRS data | ||||
| Baseline | 7.04 | 6.56 | 0.48 ( | 0.4767 |
| 2 weeks | 4.63 | 5.55 | 0.0635 | |
| 4 weeks | 4.73 | 5.29 | 0.2655 | |
| 6 weeks | 4.53 | 5.63 | 0.0184 | |
| 8 weeks | 4.24 | 5.72 | 0.0079 | |
| NRS mean change from baseline 95% CI | ||||
| 2 weeks | 0.0001 | |||
| 4 weeks | 0.0001 | |||
| 6 weeks | 0.0001 | |||
| 8 weeks | 0.0001 | |||
3.2Primary outcomes
Table 2 shows a gradual decrease in the active group’s pain score relative to the placebo group, with a statically significant change in pain recorded after 6-weeks (mean NRS difference
Figure 3.
Back pain percent reduction from baseline.
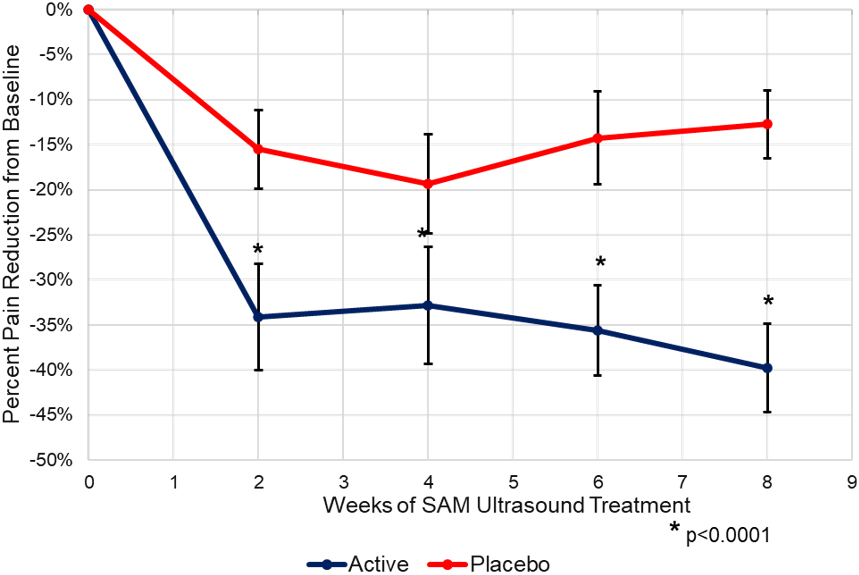
The longitudinal analysis shows a significant and clinically relevant decrease in pain from the baseline after 2-weeks of treatment (Fig. 3). A 35% decrease in pain (2.40-point NRS pain reduction) was seen during the first 2-weeks of treatment from the baseline in the active SAM group and up to a 45% decrease (3.15-point NRS pain reduction) in pain at the week 8 study completion (
Table 3
Back pain reduction from baseline (NRS) and mean change from baseline (NRS) for the participants who completed the full 8-week intervention
| Week | Active ( | Placebo ( | Mean | |
|---|---|---|---|---|
| Primary outcome NRS data (Completers) | ||||
| Baseline | 7.07 | 6.31 | 0.76 ( | 0.1138 |
| 2 Weeks | 4.59 | 5.41 | 0.1613 | |
| 4 Weeks | 4.60 | 5.39 | 0.1410 | |
| 6 Weeks | 4.51 | 5.63 | 0.0293 | |
| 8 Weeks | 4.24 | 5.72 | 0.0079 | |
| NRS mean change from baseline 95% CI | ||||
| 2 Weeks | 0.0007 | |||
| 4 Weeks | 0.0001 | |||
| 6 Weeks | 0.0001 | |||
| 8 Weeks | 0.0001 | |||
A subgroup pain reduction analysis was completed on participants who completed the full 8 weeks of the study in both the active (
A secondary subgroup analysis of non-completers (
Figure 4.
Back pain reduction (NRS) by week during treatment administration.
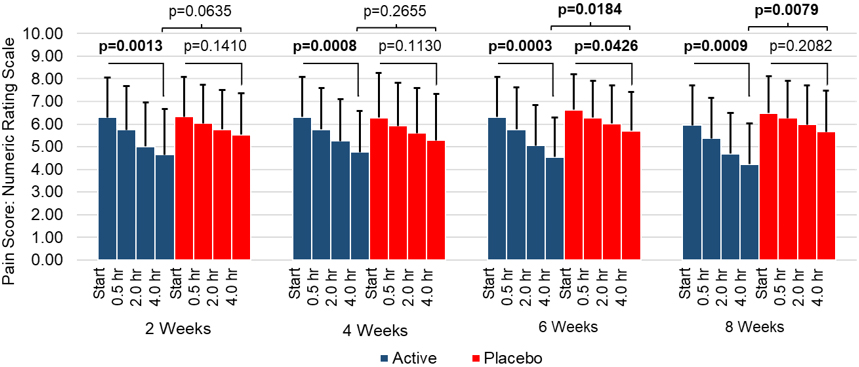
The within-day-treatment change in the pain during intervention was recorded in daily diaries and analyzed biweekly (Fig. 4). A significant decrease in pain scores was recorded during treatment in the active SAM group across all eight weeks. A significant difference in pain scores between active and placebo SAM groups was also observed at 6 weeks (
3.3Secondary outcome measures
The active SAM group reported significant improvement in lower back well-being as measured by the GROC compared to the placebo group at the end of the intervention. Mean GROC for the active SAM group was 3.67
The active SAM group reported a 22.5% reduction in pain medication with no change reported in the placebo group (mean difference 22.52%, 95% CI: 10.94% to 34.11%,
At the 8-week exit survey, the majority of active SAM treatment group subjects reported a reduction in back pain (100%,
Table 4
Secondary outcome measures including GROC, medication, function, and patient receptivity
| Secondary outcomes GROC, medication, life activity, compliance | ||||
|---|---|---|---|---|
| Outcome | Active | Placebo | Mean difference 95% CI | |
| Health improvement score (GROC) | 3.67 ( | 0.19 ( | 3.48 (2.71 to 4.24) | 0.0001 |
| Reduction in medication use | 22.5% ( | 00.0% ( | 22.5% (10.9% to 34.1%) | 0.0004 |
| Did treatment reduce your back pain? | 100% ( | 25% ( | 75% (55% to 95%) | 0.0001 |
| Did treatment improve your quality of life? | 100% ( | 31% ( | 69% (48% to 90%) | 0.0001 |
| Did your functional activity, such as walking, playing, gardening, etc., improve? | 95% ( | 19% ( | 76% (56% to 97%) | 0.0001 |
| Was treatment an effective solution for your back pain? | 100% ( | 13% ( | 88% (72% to 103%) | 0.0001 |
| Would you like to continue to use treatment after the study? | 100% ( | 19% ( | 81% (63% to 99%) | 0.0001 |
| Do you have a daily use requirement for treatment? | 100% ( | 31% ( | 69% (48% to 90%) | 0.0001 |
| Was treatment easy to use as recommended? | 100% ( | 80% ( | 20% (2% to 38%) | 0.0327 |
4.Discussion
Lower back pain is a clinical challenge with complex epidemiology and pathogenesis [10, 11]. Acute pain can be treated with physical exercise and therapy, but chronic low back pain causes significant socio-economic effects and requires lifelong treatment, including analgesics and NSAIDs [10, 15, 19, 44]. The long-term use of these drugs leads to an opioid epidemic and adverse effects on multiple organs [17, 44, 45, 46, 47, 48, 49, 50, 51, 52]. The lower back plays an essential role in day-to-day activity, and chronic pain significantly limits day-to-day activities and impairs mobility to the extent of causing physical disability and depression [53, 54]. Recent advancements have explored nonpharmacological therapies [18, 55]. This study shows the effectiveness of the non-invasive, self-administered, in-home use of SAM for the treatment of chronic, discogenic low back pain. SAM delivers continuous ultrasound at the high-frequency 3 MHz, an intensity of 132 mW/cm2, and a total power of 1.3 W, providing deep (
Multiple studies have shown the effectiveness of SAM in increasing musculoskeletal tissue regeneration, pain management, and mobility. This includes a recent systematic review and meta-analysis on SAM treatment for musculoskeletal pain and soft tissue healing by Winkler et al. 2021 and a 135 subject clinical study by Jarit et al. 2023 demonstrating both pain and health improvements for soft-tissue injuries, including the back. To our knowledge, this is the first RCT to evaluate SAM’s clinical effectiveness and safety on discogenic, chronic, and low back pain. The encouraging data from this study confirms previous findings and shows that SAM can be used to treat lower back pain as a standalone therapy. In addition, the cross-sectional analysis of data with active and placebo groups shows that the active group significantly improves pain after 8 weeks of treatment (8 weeks
Interestingly, a comparison from baseline shows the highest, approximately 35%, decrease in pain occurs during the first 2-weeks of treatment in the active group (14 treatment sessions). However, only a 5% decrease in pain was recorded in the following 6 weeks, and another 5% decrease occurred after 8 weeks of treatment (56 treatment sessions). Over the course of 8 weeks, the difference between active and placebo pain reduction also increased. These findings suggest that patients and prescribing physicians may be able to modulate treatment use to reduce daily application burden while still achieving clinically meaningful pain reduction and quality life improvement.
The use of therapeutic ultrasound administered in the clinical setting for the management of chronic low back pain has been investigated in prior RCTs. Haile et al. 2021 recently conducted a systematic review of ultrasound therapy RCTs and found that five studies demonstrated ultrasound therapy significantly reduced lower back pain scores when sequentially administered over a regular treatment period (typically 10 to 12 treatment sessions over 3 to 6 weeks) [56]. The authors concluded that based on the literature, ultrasound therapy may be considered a non-drug and non-invasive alternative treatment for lower back pain. The SAM long-duration ultrasound device used in this study enabled patients to receive multi-hour ultrasound treatment daily in the home setting for 8 weeks (56 treatment sessions). The data from the study shows that regular home treatment with SAM has a significant clinical benefit for patients, including greater pain reduction, health improvement, and reduction of lost time from clinic visits and associated costs. The minimal clinically important difference (MCID) for chronic low back pain treatment ranges in the literature from 11% to greater than 50% change on the Oswestry disability index depending on intervention type [57]. After 8 weeks of daily sustained acoustic medicine treatment, the active SAM group significantly exceed MCID change, with subjects reporting a mean improvement ranging from 69% to 88% over placebo intervention (
Ebadi et al. have previously shown continuous ultrasound efficacy (1 MHz and 1.5 W/cm2) for 4 weeks, with 10 treatments showing significant lumbar improvement in mobility and global visual analog scale (VAS) pain [58]. Durmus et al. treated lower back pain in the lumbar spine with 10 treatments of continuous ultrasound at 1 MHz and 1 W/cm2 over 3 weeks, demonstrating significant improvement relative to placebo treatments [59]. Tantawy et al. treated 15 chronic lumbar pain patients with 1 MHz continuous ultrasound at 1 W/cm2 intensity for 10 mins for 2 days /week over 8 weeks and reported a significant reduction in VAS pain scores and an increase in ROM in a comparative study [60]. These studies use short-duration continuous ultrasound in conjunction with exercise compared to standalone SAM therapy, which uses long-duration continuous ultrasound in the comfort of home during daily activities. In addition, the SAM allows daily treatment over 8 weeks compared to limited weekly sessions delivered by a healthcare provider. This study also reports on daily changes in pain and quality of life and conducts longitudinal bi-weekly analysis over 8 weeks, further confirming the cumulative effects of SAM therapy on chronic discogenic back pain.
The study is not without some limitations. A significantly larger number of dropouts occurred in the placebo arm due to the protocol burden on the subjects, which could potentially affect the study results. Since the clinical benefit of ultrasound therapy and home-use SAM intervention has been evaluated with placebo control, future studies should consider utilizing intervention arms with alternative treatments, such as corticosteroids or oral/topical medication, and recruit a higher sample size to reduce patient attrition. Expanded and comparative study arms will be helpful for clinical decision-making in the use of SAM treatment in the care continuum. Additionally, a longer-term intervention and follow-up period could help determine the lasting clinical benefit for patients.
5.Conclusion
This double-blind, placebo-controlled, randomized clinical trial in patients with discogenic chronic low back pain demonstrated that 18,720 joules of daily 3 MHz SAM treatment had a significant beneficial effect on pain, health, function, and reduction of medication use, including NSAIDs and opioids compared to the control group. SAM treatment has a role in managing chronic low back pain symptoms with limited side effects so that patients can improve their quality of life.
Author contributions
Study conception and design: RO and TM. Data collection and analysis: all authors. Drafting of the manuscript: all authors. Critical revision of the manu- script: all authors. All authors read and approved the final manuscript.
Datat availibilty
Data is available from the corresponding author upon reasonable request.
Ethical approval
The study has been performed in accordance with the Declaration of Helsinki and later amendments and was approved by the Medical Ethical Committee at the institutional review board of Schuman (# 2015/20140901).
Funding
This work is supported by the National Space Biomedical Research Institute (Grant no. SMST03901).
Informed consent
All participants provided written informed consent.
Acknowledgments
None to report.
Conflict of interest
The authors declare that they have no conflict of interest.
References
[1] | de Souza IMB, Sakaguchi TF, Yuan SLK, Matsutani LA, do Espirito-Santo AS, Pereira CAB, et al. Prevalence of low back pain in the elderly population: a systematic review. Clinics (Sao Paulo). (2019) ; 74: : e789. |
[2] | Deyo RA, Mirza SK, Martin BI. Back pain prevalence and visit rates: estimates from US. national Surveys. 2002. Spine (Phila Pa 1976). (2006) ; 31: (23): 2724-7. |
[3] | Jacqueline W. Lucas MPH, Eric M. Connor BS, Jonaki Bose, M.Sc. Back, Lower Limb, and Upper Limb Pain Among U.S. Adults, (2019) ; [cited 2023 9/28/203]. Available from: https//www.cdc.gov/nchs/products/databriefs/db415.htm. |
[4] | Freburger JK, Holmes GM, Agans RP, Jackman AM, Darter JD, Wallace AS, et al. The rising prevalence of chronic low back pain. Arch Intern Med. (2009) ; 169: (3): 251-8. |
[5] | Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. (2006) ; 88: (Suppl 2): 21-4. |
[6] | Kim LH, Vail D, Azad TD, Bentley JP, Zhang Y, Ho AL, et al. Expenditures and Health Care Utilization Among Adults With Newly Diagnosed Low Back and Lower Extremity Pain. JAMA Netw Open. (2019) ; 2: (5): e193676. |
[7] | Zemedikun DT, Kigozi J, Wynne-Jones G, Guariglia A, Roberts T. Methodological considerations in the assessment of direct and indirect costs of back pain: A systematic scoping review. PLoS One. (2021) ; 16: (5): e0251406. |
[8] | Baumeister H, Knecht A, Hutter N. Direct and indirect costs in persons with chronic back pain and comorbid mental disorders – a systematic review. J Psychosom Res. (2012) ; 73: (2): 79-85. |
[9] | Wami SD, Abere G, Dessie A, Getachew D. Work-related risk factors and the prevalence of low back pain among low wage workers: results from a cross-sectional study. BMC Public Health. (2019) ; 19: (1): 1072. |
[10] | Biyani A, Andersson GB. Low back pain: pathophysiology and management. J Am Acad Orthop Surg. (2004) ; 12: (2): 106-15. |
[11] | Li W, Gong Y, Liu J, Guo Y, Tang H, Qin S, et al. Peripheral and Central Pathological Mechanisms of Chronic Low Back Pain: A Narrative Review. J Pain Res. (2021) ; 14: : 1483-94. |
[12] | Adams MA. Biomechanics of back pain. Acupunct Med. (2004) ; 22: (4): 178-88. |
[13] | Qurain T, Alshammari LRAS, Sreeja Mannickal Thankappan, Meshari T Alshammari. Correlation between Pain, Disability and Levels of Disc Herniation in Michigan State University Grade-3 Disc Prolapsed Patients using Magnetic Resonance Imaging: A Cross-sectional Study. Journal of Clinical and Diagnostic Research. (2022) ; 16: (1): 4. |
[14] | Mehling WE, Gopisetty V, Bartmess E, Acree M, Pressman A, Goldberg H, et al. The prognosis of acute low back pain in primary care in the United States: a 2-year prospective cohort study. Spine (Phila Pa 1976). (2012) ; 37: (8): 678-84. |
[15] | Amir Qaseem TJW, Robert M. McLean, and Mary Ann Forciea. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline From the American College of Physicians. Annals of Internal Medicine. (2017) . |
[16] | Varrassi G, Moretti B, Pace MC, Evangelista P, Iolascon G. Common Clinical Practice for Low Back Pain Treatment: A Modified Delphi Study. Pain Ther. (2021) ; 10: (1): 589-604. |
[17] | Enthoven WT, Roelofs PD, Deyo RA, van Tulder MW, Koes BW. Non-steroidal anti-inflammatory drugs for chronic low back pain. Cochrane Database Syst Rev. (2016) ; 2: (2): CD012087. |
[18] | Ketenci A, Zure M. Pharmacological and non-pharmacological treatment approaches to chronic lumbar back pain. Turk J Phys Med Rehabil. (2021) ; 67: (1): 1-10. |
[19] | Roelofs PD, Deyo RA, Koes BW, Scholten RJ, van Tulder MW. Nonsteroidal anti-inflammatory drugs for low back pain: an updated Cochrane review. Spine (Phila Pa 1976). (2008) ; 33: (16): 1766-74. |
[20] | Baliga S, Treon K, Craig NJ. Low Back Pain: Current Surgical Approaches. Asian Spine J. (2015) ; 9: (4): 645-57. |
[21] | Liu C, Ferreira GE, Abdel Shaheed C, Chen Q, Harris IA, Bailey CS, et al. Surgical versus non-surgical treatment for sciatica: systematic review and meta-analysis of randomised controlled trials. BMJ. (2023) ; 381: : e070730. |
[22] | Searle A, Spink M, Ho A, Chuter V. Exercise interventions for the treatment of chronic low back pain: a systematic review and meta-analysis of randomised controlled trials. Clin Rehabil. (2015) ; 29: (12): 1155-67. |
[23] | Izadifar Z, Babyn P, Chapman D. Mechanical and Biological Effects of Ultrasound: A Review of Present Knowledge. Ultrasound Med Biol. (2017) ; 43: (6): 1085-104. |
[24] | Przystupski D, Ussowicz M. Landscape of Cellular Bioeffects Triggered by Ultrasound-Induced Sonoporation. Int J Mol Sci. (2022) ; 23: (19). |
[25] | Whitney NP, Lamb AC, Louw TM, Subramanian A. Integrin-mediated mechanotransduction pathway of low-intensity continuous ultrasound in human chondrocytes. Ultrasound Med Biol. (2012) ; 38: (10): 1734-43. |
[26] | Chung JI, Min BH, Baik EJ. Effect of Continuous-Wave Low-Intensity Ultrasound in Inflammatory Resolution of Arthritis-Associated Synovitis. Phys Ther. (2016) ; 96: (6): 808-17. |
[27] | Papadopoulos ES, Mani R. The Role of Ultrasound Therapy in the Management of Musculoskeletal Soft Tissue Pain. Int J Low Extrem Wounds. (2020) ; 19: (4): 350-8. |
[28] | Best TM, Wilk KE, Moorman CT, Draper DO. Low Intensity Ultrasound for Promoting Soft Tissue Healing: A Systematic Review of the Literature and Medical Technology. Intern Med Rev (Wash D C). (2016) ; 2: (11). |
[29] | de Lucas B, Perez LM, Bernal A, Galvez BG. Ultrasound Therapy: Experiences and Perspectives for Regenerative Medicine. Genes (Basel). (2020) ; 11: (9). |
[30] | Uddin SM, Hadjiargyrou M, Cheng J, Zhang S, Hu M, Qin YX. Reversal of the detrimental effects of simulated microgravity on human osteoblasts by modified low intensity pulsed ultrasound. Ultrasound Med Biol. (2013) ; 39: (5): 804-12. |
[31] | Dijkman BG, Sprague S, Bhandari M. Low-intensity pulsed ultrasound: Nonunions. Indian J Orthop. (2009) ; 43: (2): 141-8. |
[32] | Gebauer D, Mayr E, Orthner E, Ryaby JP. Low-intensity pulsed ultrasound: effects on nonunions. Ultrasound Med Biol. (2005) ; 31: (10): 1391-402. |
[33] | Moghaddam A, Yildirim TM, Westhauser F, Danner W, Swing T, Bruckner T, et al. Low intensity pulsed ultrasound in the treatment of long bone nonunions: Evaluation of cytokine expression as a tool for objectifying nonunion therapy. J Orthop. (2016) ; 13: (4): 306-12. |
[34] | Nolte PA, van der Krans A, Patka P, Janssen IM, Ryaby JP, Albers GH. Low-intensity pulsed ultrasound in the treatment of nonunions. J Trauma. (2001) ; 51: (4): 693-702; discussion -3. |
[35] | Uddin SMZ, Komatsu DE, Motyka T, Petterson S. Low-Intensity Continuous Ultrasound Therapies-A Systematic Review of Current State-of-the-Art and Future Perspectives. J Clin Med. (2021) ; 10: (12). |
[36] | Best TM, Moore B, Jarit P, Moorman CT, Lewis GK. Sustained acoustic medicine: wearable, long duration ultrasonic therapy for the treatment of tendinopathy. Phys Sportsmed. (2015) ; 43: (4): 366-74. |
[37] | Langer MD, Lewis GK, Jr. Sustained Acoustic Medicine: A Novel Long Duration Approach to Biomodulation Utilizing Low Intensity Therapeutic Ultrasound. Proc SPIE Int Soc Opt Eng. (2015) ; 9467. |
[38] | Draper DO. The Benefits of Long Duration Ultrasound. Biomedical Journal of Scientific & Technical Research. (2019) ; 18: (4). |
[39] | Draper DO, Klyve D, Ortiz R, Best TM. Effect of low-intensity long-duration ultrasound on the symptomatic relief of knee osteoarthritis: a randomized, placebo-controlled double-blind study. J Orthop Surg Res. (2018) ; 13: (1): 257. |
[40] | Draper DO, Wells A, Wilk K. Efficacy of Sustained Acoustic Medicine as an Add-on to Traditional Therapy in Treating Sport-related Injuries: Case Reports. Glob J Orthop Res. (2020) ; 2: (4). |
[41] | Lewis GK, Jr., Langer MD, Henderson CR, Jr., Ortiz R. Design and evaluation of a wearable self-applied therapeutic ultrasound device for chronic myofascial pain. Ultrasound Med Biol. (2013) ; 39: (8): 1429-39. |
[42] | Walters R, Kasik J, Ettel C, Ortiz R. Evaluation of Sustained Acoustic Medicine for Treating Musculoskeletal Injuries in Military and Sports Medicine. Open Orthop J. (2022) ; 16. |
[43] | Rigby JH, Taggart RM, Stratton KL, Lewis GK, Jr., Draper DO. Intramuscular Heating Characteristics of Multihour Low-Intensity Therapeutic Ultrasound. J Athl Train. (2015) ; 50: (11): 1158-64. |
[44] | Atchison JW, Herndon CM, Rusie E. NSAIDs for musculoskeletal pain management:current perspectives and novel strategies to improve safety. J Manag Care Pharm. (2013) ; 19: (9 Suppl A): S3-19. |
[45] | Gudin J, Kaufman AG, Datta S. Are Opioids Needed to Treat Chronic Low Back Pain? A Review of Treatment Options and Analgesics in Development. J Pain Res. (2020) ; 13: : 1007-22. |
[46] | Licciardone JC, Gatchel RJ, Aryal S. Effects of Opioids and Nonsteroidal Anti-Inflammatory Drugs on Chronic Low Back Pain and Related Measures: Results from the PRECISION Pain Research Registry. Tex Med. (2018) ; 114: (10): e1. |
[47] | Migliorini F, Maffulli N, Baroncini A, Eschweiler J, Tingart M, Quack V. Opioids for chronic low back pain management: a Bayesian network meta-analysis. Expert Rev Clin Pharmacol. (2021) ; 14: (5): 635-41. |
[48] | Nury E, Schmucker C, Nagavci B, Motschall E, Nitschke K, Schulte E, et al. Efficacy and safety of strong opioids for chronic noncancer pain and chronic low back pain: a systematic review and meta-analyses. Pain. (2022) ; 163: (4): 610-36. |
[49] | O’Leary K. Opioids unhelpful for acute low-back and neck pain. Nat Med. (2023) . |
[50] | Petzke F, Klose P, Welsch P, Sommer C, Hauser W. Opioids for chronic low back pain: An updated systematic review and meta-analysis of efficacy, tolerability and safety in randomized placebo-controlled studies of at least 4 weeks of double-blind duration. Eur J Pain. (2020) ; 24: (3): 497-517. |
[51] | Vanneman ME, Larson MJ, Chen C, Adams RS, Williams TV, Meerwijk E, et al. Treatment of Low Back Pain With Opioids and Nonpharmacologic Treatment Modalities for Army Veterans. Med Care. (2018) ; 56: (10): 855-61. |
[52] | Vraa ML, Myers CA, Young JL, Rhon DI. More Than 1 in 3 Patients With Chronic Low Back Pain Continue to Use Opioids Long-term After Spinal Fusion: A Systematic Review. Clin J Pain. (2021) ; 38: (3): 222-30. |
[53] | Mirzamohammadi E, Ghandhari H, Pirbornatan M, Mohammadi S, Hosseininejad M. Assessment of disability levels in patients with low back pain based on the type of lumbar spinal disorder. J Back Musculoskelet Rehabil. (2021) ; 34: (1): 131-7. |
[54] | Ren XS, Selim AJ, Fincke G, Deyo RA, Linzer M, Lee A, et al. Assessment of functional status, low back disability, and use of diagnostic imaging in patients with low back pain and radiating leg pain. J Clin Epidemiol. (1999) ; 52: (11): 1063-71. |
[55] | Cashin AG RR, Wand BM, O’Connell NE, Lee H, Bagg MK, O’Hagan E, Maher CG, Furlan AD, Tulder MW, McAuley JH. Non-pharmacological and non-surgical treatments for low back pain in adults: an overview of Cochrane Reviews. The Cochrane Database of Systematic Reviews. (2021) ; 8. |
[56] | Haile G, Hailemariam TT, Haile TG. Effectiveness of Ultrasound Therapy on the Management of Chronic Non-Specific Low Back Pain: A Systematic Review. J Pain Res. (2021) ; 14: : 1251-7. |
[57] | Schwind J, Learman K, O’Halloran B, Showalter C, Cook C. Different minimally important clinical difference (MCID) scores lead to different clinical prediction rules for the Oswestry disability index for the same sample of patients. J Man Manip Ther. (2013) ; 21: (2): 71-8. |
[58] | Ebadi S, Ansari NN, Naghdi S, Jalaei S, Sadat M, Bagheri H, et al. The effect of continuous ultrasound on chronic non-specific low back pain: a single blind placebo-controlled randomized trial. BMC Musculoskelet Disord. (2012) ; 13: : 192. |
[59] | Durmus D, Durmaz Y, Canturk F. Effects of therapeutic ultrasound and electrical stimulation program on pain, trunk muscle strength, disability, walking performance, quality of life, and depression in patients with low back pain: a randomized-controlled trial. Rheumatol Int. (2010) ; 30: (7): 901-10. |
[60] | Tantawy SA, Abdelbasset WK, Kamel DM, Alrawaili SM, Alsubaie SF. Laser photobiomodulation is more effective than ultrasound therapy in patients with chronic nonspecific low back pain: a comparative study. Lasers Med Sci. (2019) ; 34: (4): 793-800. |




