Rehabilitation effect of core muscle training combined with functional electrical stimulation on lower limb motor and balance functions in stroke patients
Abstract
BACKGROUND:
Studies have shown that core muscle training can accelerate the recovery of motor function in stroke patients. However, there are no relevant reports to show the effect of core muscle training combined with functional electrical stimulation (FES) on the rehabilitation of stroke patients.
OBJECTIVE:
This study aimed to observe the efficacy of core muscle training combined with FES on motor and balance functions of lower limbs in stroke patients.
METHODS:
This study selected and divided 120 stroke patients with hemiplegia admitted to our hospital into the control and observation groups. Patients in the control group just received core muscle training; while patients in the observation group were treated by core muscle training combined with FES. Both groups were treated for 8 weeks. Subsequently, the clinical data and information of all patients were collected and counted. Muscle strength changes were observed by detecting paralytic dorsiflexor (pDF), plantar flexor (pPF), knee extensor (pKE), and knee flexor (pKF) before and after treatment. Motor and balance abilities of both groups were scored through the 10-meter walking test (10 MWT), Berg balance scale (BBS), functional ambulation category (FAC) scale, timed up and go (TUG) test, and lower extremity motricity index (MI-Lower).
RESULTS:
No significant difference was found in clinical data between the two groups. The intensity of pDF, pPF, pKE, and pKF significantly increased in both groups after treatment, and the intensity of these parameters was higher in the observation group relative to the control group. Additionally, 10 MWT and TUG test scores of patients in the observation group were notably decreased while the BBS and MI-Lower scores were significantly increased after treatment compared with those in the control group.
CONCLUSION:
Core muscle training combined with FES can significantly improve the rehabilitation effect of lower limb motor and balance functions in stroke patients.
1.Introduction
Stroke has become a serious problem globally. Notably, approximately 15 million new stroke cases have been reported each year globally. The United States reported over 795,000 patients with stroke, including 610,000 new and 185,000 recurrent patients. Additionally, stroke is responsible for approximately 140,000 deaths annually in the United States, accounting for approximately 1 in 20 deaths [1, 2]. Patients with stroke have a 1%–4% probability of developing post-stroke movement disorder (PSMD). PSMD is not only a leading cause of mobility impairments and disability but also directly affects the function and quality of life of stroke survivors [3]. Movement disorders secondary to stroke are varied, with different natural courses, prognoses, and treatment and idiopathic. Additionally, movement disorders may simultaneously occur with stroke or present as delayed stroke sequelae [4]. Briefly speaking, movement disorders can be divided into hyperkinesia and abnormal involuntary movements. Generally, patients with movement disorders are mainly manifested as athetosis, dystonia, tremor, myoclonus, asterixis, stereotypes, akathisia, convulsions, vascular parkinsonism, progressive supranuclear palsy, and other hypokinetic diseases of insufficient movement or slowness (bradykinesia) [5]. Studies have claimed that approximately 30% of patients with chronic stroke suffer from persistent independent walking impairment [6]. Subsequent studies have paid attention to the management of patients’ lower limb function recovery and achieved good effects [7, 8]; however, further improvement remains needed.
Afferent stimulation was applied to stimulate the nervous system to improve movement disorders during the rehabilitation management of patients with stroke. Afferent stimulation shows more pronounced clinical benefits compared with other interventions in several articles. Successful recovery of upper limb motor function in patients with stroke with simple rehabilitation techniques, such as massage and vibration, was reported as early as 1915 [9]. Subsequent studies have further demonstrated that cutaneous and proprioceptive afferent motor stimulus information contributes to improving motor performance and promoting effective motor learning through increasing cortical excitability in the stimulated body parts [10]. Interestingly, studies in recent years have pointed out the superior effects of functional electrical stimulation (FES) on motor function recovery in patients with stroke, as electrosensory input alters the sensory and motor cortex [11]. Currently, transcutaneous electrical nerve stimulation (TENS), peroneal nerve FES, and tibialis anterior muscle FES are considered effective electrical stimulation modalities. Some studies have stated that FES is more effective than TENS [12] because it can promote voluntary muscle activity, reduce foot drop and spasm, and maintain long-term sensorimotor cortical reorganization [13]. Moreover, FES can promote lower limb motor function rehabilitation and improve the surface electromyography signal of lower limbs in patients with stroke [14]. FES is an effective treatment for patients with stroke. Many other rehabilitation methods are available. For instance, core muscle training presents beneficial effects on trunk function, standing balance, and mobility in patients with stroke and can accelerate motor function recovery in patients with stroke, as proved by many clinical studies [15]. FES of the gastrocnemius and biceps femoris in a linear or interval pattern combined with cycling has been reported to promote rehabilitation in patients with stroke and improve functional activity and speed in stroke survivors [16, 17]. However, their research only focuses on a small range of core muscle training. Concurrently, little is known about the effect of core muscle training combined with FES on the rehabilitation of patients with stroke because previous studies have demonstrated that core muscle group training and FES can improve lower extremity motor function and balance function, respectively. Therefore, this study hypothesized that combining the two approaches might produce better rehabilitation outcomes and that using core muscle group training as a control group would better compare the efficacy of the two interventions in combination.
2.Materials and methods
2.1Study object
This study selected 120 patients with stroke with hemiplegia receiving rehabilitation treatment at China-Japan Friendship Hospital from December 2021 to May 2022. They were randomly divided into the control and observation groups following the FES application. The control group just performed core muscle training, and the observation group received core muscle training
The inclusion criteria were: (1) the stroke was determined by magnetic resonance imaging and computed tomography (CT); (2) patients suffered from the stroke for the first time and the course of the disease lasted for
The exclusion criteria were: patients who (1) suffered from cognitive dysfunction; (2) severe cardiopulmonary, hepatic and renal dysfunctions, and other serious diseases; (3) lower limb dysfunction caused by other nervous and osteoarticular system diseases; (4) have an abdominal hernia, abdominal pathology or surgery [18].
The study conformed to the Declaration of Helsinki. The study procedures were approved by the ethics committee of the China-Japan Friendship Hospital (2021-124-K82).
2.2Treatment methods
The control group conducted core muscle training only, while the observation group performed core muscle training
FES, which is a low-frequency pulse electrotherapy, was conducted using a TENS temeco electrical stimulation therapeutic apparatus developed in the United States (Wuxi, China). Specifically, all electrodes were arranged vertically along the midline. The first electrode was placed over the hyoid bone, the second over the superior notch of the thyroid cartilage immediately below the first electrode, and the third and fourth at equal distances between the first and second electrodes. Electrical stimulation intensity was set as 0–30 mA (stimulation intensity was limited by patient tolerance), frequency as 80 Hz, and resistance as 1000 Q. Further, biphasic square waves were applied with a wave width of 700 ms, 20 beats/min, 17 beats/day, and 5 beats/week. The treatment of both groups lasted 8 weeks [19].
Core muscle training. 1. Double bridge exercise: a supine position was required first, followed by hip and knee flexion; the buttock and waist were lifted in a “half-bridge” shape through the force from the lower back; the buttock and the waist were slowly put down after 15 s, then repeated for a total of 10 sets. 2. Single-leg bridge exercise: patients were asked to a supine position and their arms were interlaced in front of their chests; one leg was straight placed on the yoga ball and the other leg was bent; the low back and hip were slowly lifted under the force of the low back and buttock, making the shoulder, abdomen, and the leg that placed on the yoga ball straight; the same action was performed on the other leg 15 s later; the above action performed on both legs was set as one group, repeated for a total of 10 sets. 3. Push-ups: a prone position was asked, then the upper body was raised by supporting the bed with both hands; next, the abdominal muscles were stretched as much as possible until the contraction of low back muscles was felt; the head was lifted as far back as possible; the pelvis pressed close to the bed, and the body was slowly back to the starting position after 20 s, repeated for a total of 10 sets. 4. Lateral leg raises: the patient lay laterally on the ground, with his right hand supporting his head and left hand placed in front of the navel; the body was adjusted in a straight line through inspiratory, and the left leg was slowly lifted; the leg was slowly put down and close together to the other leg on the time of expiration after 5 s; the hip was kept perpendicular to the ground during this period, and the exercise was performed on the other side of the leg again upon rest supine to adjust breathing, repeated for a total of 10 sets. 5. Kneeling diagonal support: the patient was asked for a crawling kneeling posture; hands and knees were close to the ground; the hands were as wide as the shoulders; the knees were located below the hip; and the back was kept a straight line; later, one upper limb was extended, and the diagonal lower limb was slid backward for a straightening, i.e., arms and contralateral leg were extended while the back remained horizontal; the body returned to the starting position after 10 s, and the other side of the upper limb and contralateral lower limb were asked to repeat the above action; pelvic stability was maintained and both shoulders were flat during the whole exercise, repeated for a total of 10 sets. More attention should be paid to the left abdominal muscle in core muscle group training for patients with right hemiplegia to help support and control the left side of the trunk. More attention should be paid to the training of the right abdominal and dorsal muscles for patients with left hemiplegia. Additionally, the location and intensity of stimulation are selected following the specific situation in training assisted by FES. All the above exercises were asked for 10 repetitions each time, 5 days per week, for 8 consecutive weeks. Family members were required to assist and monitor the training of patients after discharge [20].
Table 1
Comparison of general information between the two group
| Observation group ( | Control group ( |
|
| |
|---|---|---|---|---|
| Age (year) | 41.77 | 42.68 | 0.185 | |
| Gender (%) | 1.319 | 0.251 | ||
| Male | 36 (60.0) | 42 (70.0) | ||
| Female | 24 (40.0) | 18 (30.0) | ||
| Bleeding type (%) | 1.656 | 0.198 | ||
| Cerebral hemorrhage | 30 (50.0) | 23 (38.3) | ||
| Cerebral infarction | 30 (50.0) | 37 (61.7) | ||
| Hemiplegia (%) | 1.645 | 0.200 | ||
| Left | 24 (40.0) | 31 (51.7) | ||
| Right | 36 (60.0) | 29 (48.3) | ||
| Time from disease occurrence to treatment | 15.33 | 15.57 | 0.407 |
2.3Clinical data collection
The gender, age, disease course, primary disease, affected side, and other clinical information of each patient with stroke with hemiplegia participating in this study were recorded in detail and are statistically analyzed.
2.4Outcome measures
The muscle strength changes of paralytic dorsiflexor (pDF), plantar flexor (pPF), knee extensor (pKE), and knee flexor (pKF) of each patient were observed before treatment or 8 weeks after treatment to clear the lower limb muscle strength, balance, and motor ability of patients. Patients were given a 10-meter walking test (10 MWT) and a timed up and go (TUG) test following the scoring requirements. Additionally, patients’ balance, walking, and lower limb movement abilities were scored through the Berg balance scale (BBS), FAC, and lower extremity motricity index (MI-Lower). Two doctors with
2.5Statistics and analysis
All study data were analyzed by Statistical Package for the Social Sciences version 26.0 software. The enumeration data were expressed as
3.Results
3.1Baseline characteristics of two patient groups
The observation group was composed of 36 males and 24 females, aged 32–50 years, with an average age of 41.77
Figure 1.
Lower extremity muscle strength changes in two groups A/B/C/D, The detection for lower extremity muscle strength-related indicators, including paralytic dorsiflexor (pDF) (A), plantar flexor (pPF) (B), knee extensor (pKE) (C), and knee flexor (pKF) (D), ** vs Control,
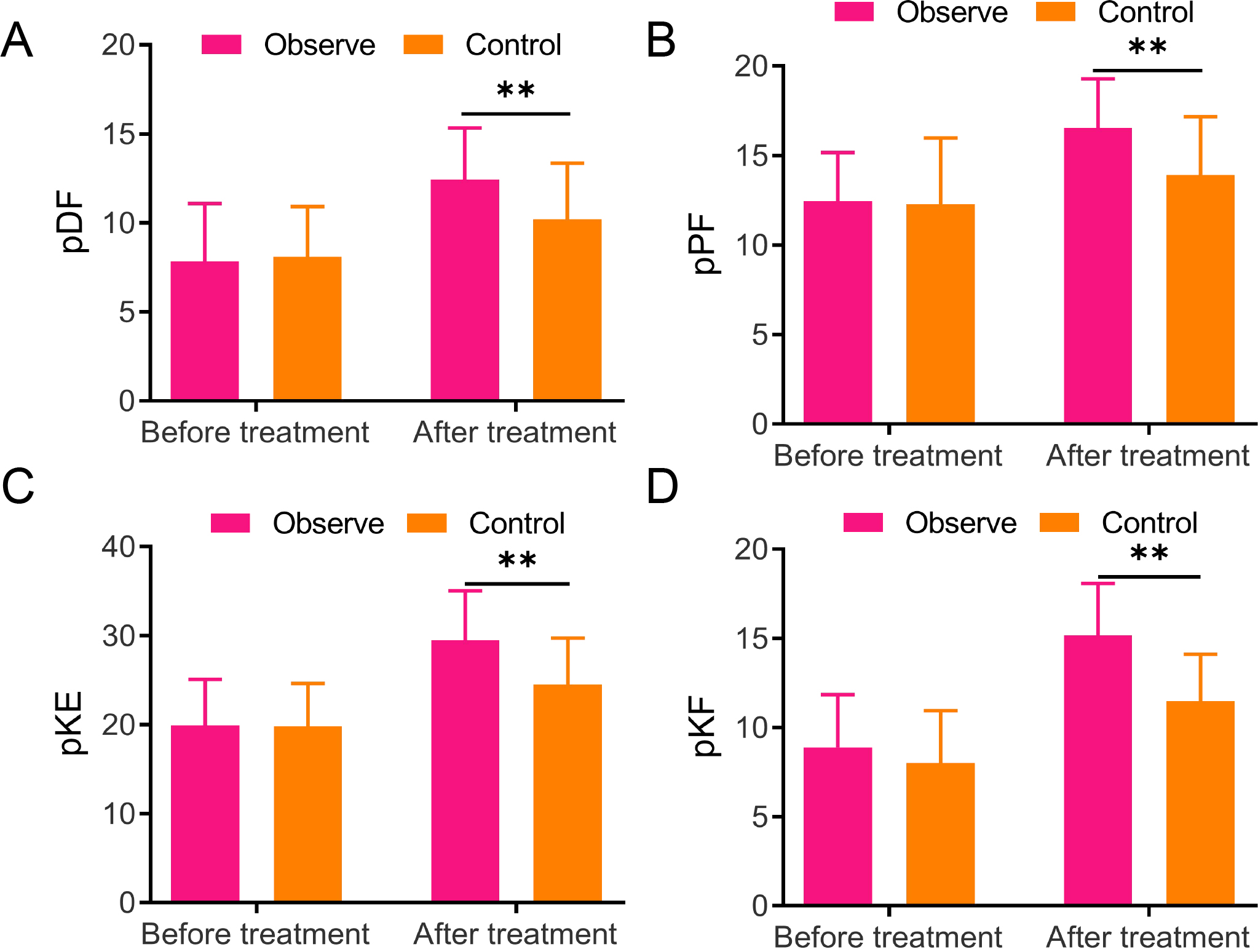
3.2Changes in lower extremity muscle strength in two groups
Lower extremity muscle strength changes before and 8 weeks after treatment were detected to observe the rehabilitation effects of the two groups, respectively. No significant difference was found in the muscle strength of the pDF, pPF, pKE, and pKF between the two groups, but they increased in both groups after treatment. Notably, the observation group exhibited much higher muscle strength of pDF (12.43
Figure 2.
Comparisons between the two groups in the 10-meter walking test, Berg balance scale, functional ambulation category, timed up and go, and lower extremity motricity index scores A, 10-meter walking test (10 MWT); B, Berg balance scale (BBS); C, Functional ambulation category (FAC); D, Timed up and go (TUG); E, Lower extremity motricity index (MI-Lower), ** vs Control,
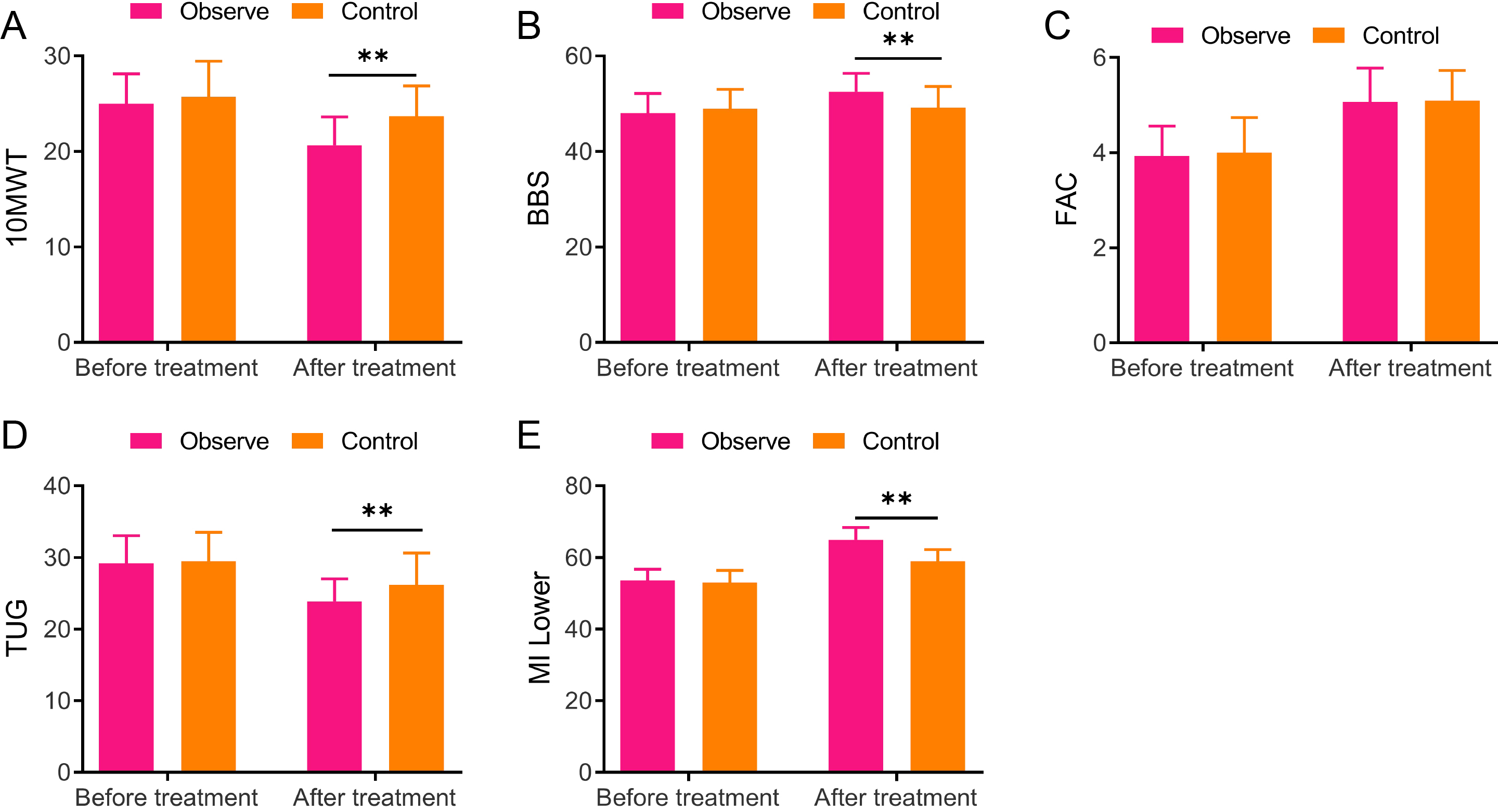
3.3Comparison of the 10-meter walking test, Berg balance scale, functional ambulation category, timed up and go, and lower extremity motricity index scores between the two groups
The 10 MWT, BBS, FAC, TUG, and MI-Lower of both groups were scored before and 8 weeks after treatment in light of the scale requirement. Briefly, no obvious difference was found in 10 MWT, BBS, FAC, TUG, and MI-Lower scores between the two groups before treatment. Both groups demonstrated decreased 10 MWT and TUG scores while increased BBS, FAC, and MI-Lower scores after treatment, relative to those before treatment. Moreover, the observation group displayed greatly higher scores of BBS (52.5
4.Discussion
This study aimed to investigate the effects of core muscle group training and FES on the lower limbs of patients with stroke. Our study revealed that the combined rehabilitation program of core muscle group training combined with FES achieved better rehabilitation results through dual training for lower limb muscle strength and balance ability of patients with stroke. This rehabilitation program is a comprehensive and personalized rehabilitation program that can better meet the rehabilitation needs of different patients.
PSMD, accounting for 22% of all secondary dyskinesias, is one of the main causes impairing the mobility of patients [4]. Therefore, rehabilitation treatment is particularly important for patients with stroke. Strokes mainly fall into hemorrhagic stroke and ischemic stroke. Despite diverse lesions of stroke, stroke-induced movement disorders have no obvious specificity, and the influence of age, stroke types, and onset latency on abnormal movement outcomes remains uncertain [5]. The causes for the above differences remain unclear. The incidence of movement disorders caused by strokes has different clinical investigation results. Some studies proposed a higher prevalence of PSMD in patients with hemorrhagic stroke [21]. However, the systematic evaluation by Suri et al. revealed that two-thirds of the 284 cases of PSMD were secondary to ischemic stroke rather than hemorrhagic stroke [22]. All in all, many factors affected the movement disorders of patients with stroke. This study revealed that the general information, including gender, age, time from disease occurrence to treatment, and condition, was not significantly different, thus the experiment for efficacy analysis could be continued.
Paralysis, which is one of the most common manifestations of stroke, is a phenomenon caused by a patient’s inability or reduced ability to activate motor units of the will [23]. Clinically, paralysis is manifested as muscle weakness, reduced muscle activation speed, and the inability of the affected limbs to produce useful functional movements [24]. The combination of hypertonic flexion and somatosensory abnormalities in the lower limbs is often manifested as knee extension and functional leg extension difficulties, severely limiting the functional workspace of patients [25]. FES, which is a method of inducing muscle contraction to assist or restore motor function, has been applied in gait rehabilitation in patients with gait defects after neurological disorders [26]. Recently, a study reported the role of FES in the common peroneal nerve through the long-term use of the foot drop stimulator significantly. Specifically, the stimulator increases the motor-evoked potential by transcranial magnetic stimulation, maximal voluntary contraction, and maximal motor waves from common peroneal nerve stimulation in patients with non-progressive and progressive diseases (such as multiple sclerosis) [27]. Overall, periodic FES application in the common peroneal nerve enhances motor cortex region activation and its residual descending connections. Additionally, TENS significantly increases ankle plantar flexion in patients with chronic stroke [28]. Hence, electrical stimulation is a means of rehabilitating the muscle condition of stroke. This study revealed that the patient’s pDF, pPF, pKE, and pKF were improved after 8 weeks of core muscle training combined with FES. In particular, FES effectively improves muscle weakness and muscle activation reduction in patients with stroke.
Additionally, patients with stroke also present symptoms, such as ungraded muscle contraction, poor motor coordination, poor endurance, spasm, and impaired balance in their lower limbs, which seriously affect their normal walking ability [29]. Approximately 30% of stroke survivors are unable to walk independently in the 6 months following a stroke, and the leading cause of walking dysfunction is the inability to ankle dorsiflexion during the gait swing phase. Reduced ankle dorsiflexion, knee flexion, or hip flexion can make the foot fail to completely disengage from the floor during the gait swing phase, resulting in difficult and unsafe walking [30]. Fortunately, electrical stimulation can improve the walking ability of patients [31]. The rehabilitation effect of FES and neuromuscular electrical stimulation on lower extremity function in patients with subacute stroke has been compared in a study. Shortly speaking, both stimulations can effectively improve the motor function of lower limbs in patients, and FES shows a better improvement relative to neuromuscular electrical stimulation [32]. This study scored patients using different scales. The observation group presented lower TUG scores while higher BBS scores than the control group according to the outcomes. Previous studies used TAG as a common physical performance test that focuses on observing a subject’s ability to perform sequential motor tasks related to walking and turning. Generally, TAG is commonly employed to assess mobility, balance, and motor ability in elderly or patients with balance disorders [33]. Similarly, BBS is a commonly applied scale for evaluating patients’ balance ability [34]. Our experiment revealed a shorter time spending on the 10 MWT and notably increased MI-Lower score in the observation group than both in the control group. MI-Lower, which is one of the indicators for effective outcomes of stroke clinically, is usually utilized to evaluate the initial functions of lower limb muscles or bones in patients [35]. The present study revealed significantly lower 10MWT and TUG scores and significantly higher BBS, FAC and MI-Lower scores in both groups after treatment relative to the pre-treatment period. In particular, FES combined with core muscle group training treatment significantly improved balance, walking ability, and lower limb motor ability in patients with stroke after treatment. Thus, all of these results indicate that patients treated with FES combined with core muscle group training have better recovery of lower extremity motor ability.
Notably, this study demonstrated many unresolved issues. First, the sample size was too small, and our conclusions need to be validated by testing them on a larger number of patients. Second, we need to further clarify the specific mechanism of action of functional electrical stimulation combined with core muscle group training for patients with stroke.
5.Conclusion
The results of our clinical trial demonstrated that the combination of FES and core muscle training contributed to muscle balance and motor ability recovery in the lower extremities of patients with stroke. However, patients in this study are regional, thus the source of patients should be further expanded to fully observe the curative effect of FES.
Funding
This work was supported by the Central Health Research Project (No. 2020YB25).
Ethics statement
The study conformed to the Declaration of Helsinki. The study procedures were approved by the ethics committee of the China-Japan Friendship Hospital (2021-124-K82).
Informed consent
All subjects signed the consent form before participating in the study.
Author contributions
Study concept and design: ZL, JWG; Analysis and interpretation of data: RG, CL; Drafting of the man- uscript: ZL, JWG; Critical revision of the manuscript for important intellectual content: ZL, JWG; Statistical analysis: RG, CL; Study supervision: ZL, JG, RG, CL. All authors read and approved the final manuscript.
Acknowledgments
The authors are grateful to Beijing Health Vocational College and China-Japan Friendship Hospital for their support of this research.
Conflict of interest
The authors report no conflict of interest.
References
[1] | Benesch C, Glance LG, Derdeyn CP, et al. Perioperative Neurological Evaluation and Management to Lower the Risk of Acute Stroke in Patients Undergoing Noncardiac, Nonneurological Surgery: A Scientific Statement From the American Heart Association/American Stroke Association. Circulation. (2021) ; 143: (19): e923-e946. |
[2] | Hosp JA, Dressing A, Engesser A, et al. The Role of Ascending Ventral-Tegmental Fibers for Recovery after Stroke. Ann Neurol. (2022) . |
[3] | Jochems ACC, Munoz Maniega S, Clancy U, et al. Associations of Peak-Width Skeletonized Mean Diffusivity and Post-Stroke Cognition. Life (Basel). (2022) ; 12: (9): 1362. |
[4] | Zanon Zotin MC, Schoemaker D, Raposo N, et al. Peak width of skeletonized mean diffusivity in cerebral amyloid angiopathy: Spatial signature, cognitive, and neuroimaging associations. Front Neurosci. (2022) ; 16: : 1051038. |
[5] | Xie H, Xiong D, Zhu P, et al. Effectiveness and safety of repetitive transcranial magnetic stimulation on memory disorder in stroke: A protocol for systematic review and meta-analysis. Medicine (Baltimore). (2022) ; 101: (40): e30933. |
[6] | Anwer S, Waris A, Gilani SO, et al. Rehabilitation of Upper Limb Motor Impairment in Stroke: A Narrative Review on the Prevalence, Risk Factors, and Economic Statistics of Stroke and State of the Art Therapies. Healthcare (Basel). (2022) ; 10: (2): 190. |
[7] | Ghayour Najafabadi M, Shariat A, Dommerholt J, et al. Aquatic Therapy for improving Lower Limbs Function in Post-stroke Survivors: A Systematic Review with Meta-Analysis. Top Stroke Rehabil. (2022) ; 29: (7): 473-489. |
[8] | Cui W, Huang L, Tian Y, et al. Effect and mechanism of mirror therapy on lower limb rehabilitation after ischemic stroke: A fMRI study. NeuroRehabilitation. (2022) ; 51: (1): 65-77. |
[9] | Butera C, Guerriero R, Amadio S, et al. Functional end-plate recovery in long-term botulinum toxin therapy of hemifacial spasm: a nerve conduction study. Neurol Sci. (2013) ; 34: (2): 209-215. |
[10] | Gandhoke GS, Belykh E, Zhao X, Leblanc R, Preul MC. Edwin Boldrey and Wilder Penfield’s Homunculus: A Life Given by Mrs. Cantlie (In and Out of Realism). World Neurosurg. (2019) ; 132: : 377-388. |
[11] | Tendler A, Roth Y, Barnea-Ygael N, Zangen A. How to Use the H1 Deep Transcranial Magnetic Stimulation Coil for Conditions Other than Depression. J Vis Exp. (2017) ; (119): 55100. |
[12] | Robbins SM, Houghton PE, Woodbury MG, Brown JL. The therapeutic effect of functional and transcutaneous electric stimulation on improving gait speed in stroke patients: a meta-analysis. Arch Phys Med Rehabil. (2006) ; 87: (6): 853-859. |
[13] | Thompson AK, Lapallo B, Duffield M, Abel BM, Pomerantz F. Repetitive common peroneal nerve stimulation increases ankle dorsiflexor motor evoked potentials in incomplete spinal cord lesions. Exp Brain Res. (2011) ; 210: (1): 143-152. |
[14] | Zhang XH, Liu JY, Han P, Wang YL, Xiao P. Clinical Efficacy of Functional Electrical Stimulation-assisted Rehabilitation Cycling on the Function of Lower Limbs in Patients with Stroke. Curr Neurovasc Res. (2021) ; 18: (3): 318-323. |
[15] | Haruyama K, Kawakami M, Otsuka T. Effect of Core Stability Training on Trunk Function, Standing Balance, and Mobility in Stroke Patients. Neurorehabil Neural Repair. (2017) ; 31: (3): 240-249. |
[16] | Huang JL, Fu YP, Gan W, et al. Hepatic stellate cells promote the progression of hepatocellular carcinoma through microRNA-1246-RORα-Wnt/β-Catenin axis. Cancer Lett. (2020) ; 476: : 140-151. |
[17] | Shariat A, Najafabadi MG, Ansari NN, et al. The effects of cycling with and without functional electrical stimulation on lower limb dysfunction in patients post-stroke: A systematic review with meta-analysis. Neuro Rehabilitation. (2019) ; 44: (3): 389-412. |
[18] | Garrido MM, Alvarez EE, Acevedo PF, et al. Early transcranial direct current stimulation with modified constraint-induced movement therapy for motor and functional upper limb recovery in hospitalized patients with stroke: A randomized, multicentre, double-blind, clinical trial. Brain Stimul. (2022) ; 16: (1): 40-47. |
[19] | Man B, Li WW, Xu JF, Wang Q. Clinical study on tri-tongue acupuncture combined with low-frequency electrical stimulation for treating post-stroke dysarthria. World J Clin Cases. (2022) ; 10: (34): 12587-12593. |
[20] | Li R, Li L, Chen Q. Effect of Respiratory Training Combined with Core Muscle Training on the Overall Motor Function and Activities of Daily Living of Patients with Early and Midterm Stroke. J Healthc Eng. (2022) ; 2022: : 2830711. |
[21] | Alarcon F, Zijlmans JC, Duenas G, Cevallos N. Post-stroke movement disorders: report of 56 patients. J Neurol Neurosurg Psychiatry. (2004) ; 75: (11): 1568-1574. |
[22] | Suri R, Rodriguez-Porcel F, Donohue K, et al. Post-stroke Movement Disorders: The Clinical, Neuroanatomic, and Demographic Portrait of 284 Published Cases. J Stroke Cerebrovasc Dis. (2018) ; 27: (9): 2388-2397. |
[23] | Liu X, Han K, Lu X. Stroke-mimicking unilateral hypokalemic paralysis and literature review. Am J Emerg Med. (2022) ; 58: : 349 e341-349 e343. |
[24] | Mizuta N, Hasui N, Nakatani T, et al. Walking characteristics including mild motor paralysis and slow walking speed in post-stroke patients. Sci Rep. (2020) ; 10: (1): 11819. |
[25] | Zhan J, Pan R, Zhou M, et al. Electroacupuncture as an adjunctive therapy for motor dysfunction in acute stroke survivors: a systematic review and meta-analyses. BMJ Open. (2018) ; 8: (1): e017153. |
[26] | Chaikho L, Clark E, Raison M. Transcutaneous Functional Electrical Stimulation Controlled by a System of Sensors for the Lower Limbs: A Systematic Review. Sensors (Basel). (2022) ; 22: (24): 9812. |
[27] | Everaert DG, Thompson AK, Chong SL, Stein RB. Does functional electrical stimulation for foot drop strengthen corticospinal connections? Neurorehabil Neural Repair. (2010) ; 24: (2): 168-177. |
[28] | Kwong PWH, Chan KL, Choi HY, et al. Immediate effects of transcutaneous electrical nerve stimulation on gait patterns in chronic stroke survivors: A single group, pretest-posttest clinical trial. Hum Mov Sci. (2022) ; 83: : 102948. |
[29] | Zhao R, Lu J, Xiao Y, et al. Effects of Gaze Stabilization Exercises on Gait, Plantar Pressure, and Balance Function in Post-Stroke Patients: A Randomized Controlled Trial. Brain Sci. (2022) ; 12: (12): 1694. |
[30] | Rossler R, Rommers N, Kim EK, et al. Timed up-and-go performance is associated with objectively measured life space in patients 3 months after ischemic stroke: a cross-sectional observational study. J Neurol. (2022) ; 1-11. |
[31] | Kapadia N, Masani K, Catharine Craven B, et al. A randomized trial of functional electrical stimulation for walking in incomplete spinal cord injury: Effects on walking competency. J Spinal Cord Med. (2014) ; 37: (5): 511-524. |
[32] | Huang S, Zhang Y, Liu P, et al. Effectiveness of contralaterally controlled functional electrical stimulation vs. neuromuscular electrical stimulation for recovery of lower extremity function in patients with subacute stroke: A randomized controlled trial. Front Neurol. (2022) ; 13: : 1010975. |
[33] | Khan F, Abusharha S, Alfuraidy A, et al. Prediction of Factors Affecting Mobility in Patients with Stroke and Finding the Mediation Effect of Balance on Mobility: A Cross-Sectional Study. Int J Environ Res Public Health. (2022) ; 19: (24): 16612. |
[34] | Blaszcz M, Prucnal N, Wrzesniewski K, et al. Physical Activity, Psychological and Functional Outcomes in Non-Ambulatory Stroke Patients during Rehabilitation-A Pilot Study. J Clin Med. (2022) ; 11: (24): 7260. |
[35] | Zhou ZQ, Hua XY, Wu JJ, et al. Combined robot motor assistance with neural circuit-based virtual reality (NeuCir-VR) lower extremity rehabilitation training in patients after stroke: a study protocol for a single-centre randomised controlled trial. BMJ Open. (2022) ; 12: (12): e064926. |




