Assessment of short- and long-term functionality and quality of life in patients with post-acute COVID-19 syndrome
Abstract
BACKGROUND:
Although the number of new cases of coronavirus 2019 (COVID-19) has been drastically reduced worldwide, patients who demonstrate long-term symptoms need more attention from health systems, as these symptoms can negatively affect functionality and quality of life.
OBJECTIVE:
To evaluate muscle function and quality of life at 3, 6, 9 and 12 months in patients with post-acute COVID-19 syndrome and to assess their associations with general fatigue and lung function.
METHODS:
This observational and longitudinal study evaluated patients with post-acute COVID-19 syndrome. Participants were subjected to the following evaluations: Short Form-36; handgrip strength; Functional Assessment of Chronic Illness Therapy-Fatigue scale; and spirometry.
RESULTS:
Among the 350 participants who were evaluated in the third month, 74.6%, 61.4% and 45.4% reported general fatigue, dyspnoea and cough, respectively. In the comparisons between the third month and the sixth month, there were significant increases in Functional Assessment of Chronic Illness Therapy-Fatigue scale, pulmonary function and several Short Form-36 domains. In the comparisons between the sixth month and the ninth month, there was a significant increase only in the social functioning domain of the Short Form-36. In the comparisons between the ninth month and the twelfth month, there was an increase only in some Short Form-36 domains. Significant correlations were observed between the Short Form-36 domains with Functional Assessment of Chronic Illness Therapy-Fatigue scale, handgrip strength and pulmonary function.
CONCLUSION:
In patients with post-acute COVID-19 syndrome, there was a progressive improvement in quality of life, general fatigue and pulmonary function during the 12 months of follow-up, with this improvement being more pronounced in the first 6 months. There was a relationship between functionality and quality of life in these patients.
1.Introduction
According to the World Health Organization (WHO), viral diseases continue to emerge and represent a serious public health problem. More recently, we experienced the coronavirus 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. Among the most severe clinical manifestations of acute disease are severe pneumonia, sepsis and septic shock. However, COVID-19 has much greater consequences than generating an acute disease and may cause long-term symptoms or even permanent sequelae that can significantly impact functionality and quality of life (QoL) [2, 3, 4].
Maximum forces exerted by humans are important in many aspects of life, and its measurements are often used not only in ergonomics but also in rehabilitation [5]. Applying force manually is the most common method of operating hand tools and moving objects [6]. Handgrip strength (HGS) is not only an indicator of the individual’s performance, but it can also be used to reduce persistent diseases, as muscle difficulty is strongly linked to practical restrictions and physical disability [7]. In patients with post-acute COVID-19 syndrome (PACS), the worse their HGS and general fatigue is, the more impaired their physical function [8]. In addition, a history of previous hospitalization for COVID-19 can cause subsequent worsening of physical function. In this sense, the evaluation of functional limitations including muscular dysfunctions of the hands is necessary in COVID-19 survivors to estimate the long-term burden of the disease [4].
Although it is believed that the vast majority of patients recover from their functional limitations, muscle involvement in COVID-19 can be problematic, producing myalgia and muscle weakness with the potential to further deteriorate QoL [9]. Assessing non-hospitalized adults with PACS, a Brazilian study showed worse functionality and postural balance than controls, which was associated with lower HGS, general fatigue and poor QoL [10]. Still in the Brazilian population, another study showed that the reduced functional exercise capacity in women with PACS was strongly explained by the deterioration of muscle strength and pulmonary function [11]. In a large cohort of hospitalized patients in Brazil, the authors showed that general fatigue and arthralgia were among the most prevalent symptoms in PACS [12]. However, these studies did not assess the longitudinal changes in functionality and QoL of these patients, which are important to allow the design of new rehabilitation strategies.
There is evidence that SARS-CoV-2 can impair physical function, deteriorate lung function and significantly reduce QoL [13, 14]. Several studies have evaluated the QoL of patients with PACS in the short- and medium-term, although they have not focused on the long-term changes and the impact of abnormalities on functionality and QoL [15, 16, 17, 18]. Since muscles and lungs are often affected in the acute phase of COVID-19 [4], understanding how PACS-affected individuals react to physical, muscular and respiratory dysfunction over time will allow tracing the development profile of PACS for these abnormalities. With this, it will be possible to identify how such limitations will affect the functionality and QoL of these subjects and to promote monitoring to prevent the development of significant changes in physical, muscle and respiratory functions. The objectives of the present study were to evaluate muscle function and QoL at 3, 6, 9 and 12 months in patients with PACS and to assess their associations with general fatigue and pulmonary function.
2.Materials and methods
2.1Participants
From October 2020 to July 2022, an observational and longitudinal study was conducted with 350 survivors of COVID-19 (of 315 eligible) aged
In addition to the assessment in the third month (T1), these participants were subsequently evaluated in case of persistent symptoms (diagnosis of PACS) in the sixth month (T2), ninth month (T3) and twelfth month (T4). Patient appointments were confirmed the day before with a phone call.
The project was approved by the Brazilian National Research Ethics Committee (number CAAE-30135320.0.0000.5259) and was conducted in accordance with the principles of the Declaration of Helsinki. All participants signed an informed consent form.
2.2Instruments and measurements
QoL was assessed using the Short Form-36 (SF-36), which is a multidimensional instrument with questions grouped into two major components (physical and mental) that allow measurement of the health state transition. Scores can vary between 0 and 100, and higher values indicate better QoL. The 36 questions that make up the SF-36 are grouped into 8 domains, as follows: physical functioning, physical role limitations, bodily pain, general health perceptions, vitality, social functioning, emotional role limitations and mental health [20]. The Portuguese translation of the SF-36 is adequate, given the completeness of responses and its internal consistency [21]. In Portuguese version, the Cronbach’s alpha was 0.82 for the physical and 0.87 for the mental dimension [22].
Handgrip strength (HGS) was assessed using a digital handheld dynamometer (SH5001, Saehan Corporation, Yangsan, Korea). Participants were instructed to sit with neutral shoulders, elbows bent at 90
General fatigue was assessed using the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) scale. It is a specific fatigue assessment scale that has good representativeness of the individual’s condition and is considered an easy-to-apply instrument. The FACIT-F scale is a 13-item scale that aims to evaluate the overall fatigue of the individual correlated with his or her daily activities. These items provide a score ranging from 0 to 52. The lower the score is, the lower the fatigue index [24, 25]. The FACIT-F scale has been used in clinical populations and is valid for use in the Portuguese language. Internal consistency reliability is good, with Cronbach’s alpha of the 0.93 [26].
Pulmonary function was assessed using a volume spirometer (Vitatrace VT 130 SL, Codax Ltda, Rio de Janeiro, Brazil) following the recommendation of the American Thoracic Society/European Respiratory Society [27]. The following variables were evaluated: forced vital capacity (FVC), forced expiratory volume in one second (FEV
2.3Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics version 26.0 software (IBM Corp., Armonk, NY, USA). To verify the homogeneity of the sample, the Shapiro-Wilk test was used. The results are expressed as the median (interquartile range) for numerical data and as the frequency and percentage for categorical data. In the longitudinal analysis, the comparison of physical function, pulmonary function and QoL parameters over time (T1, T2, T3 and T4) was performed by the Wilcoxon signed-rank test. The correlation between the absolute deltas of the SF-36 domains and the parameters of physical, muscular and respiratory function was analysed using the Spearman correlation coefficient. The significance level adopted was 5%.
To provide insight into the clinical significance of the results, we calculated the effect size in Jeffreys’s Amazing Statistics Program version 0.10.2 (https://jasp-stats.org). To provide context for interpreting the null findings, a post hoc power analysis was performed using GPower 3.1.1 software based on the differences for the three evaluation moments (T1-T2, T2-T3 and T3-T4).
3.Results
Among the 365 patients who were evaluated for inclusion in the study, 15 were excluded for the following reasons: diagnosis without confirmation by RT-PCR (
In the sixth month after COVID-19, 111 participants returned due to the permanence of symptoms, 29 (26.1%) with general fatigue, 26 (23.4%) with dyspnoea and 21 (18.9%) with cough. Thus, 31.7% of the initial sample still met the criteria for the diagnosis of PACS. In the comparisons between T1 and T2 (Table 1), there were significant increases in FACIT-F (
Table 1
Longitudinal changes in physical function, lung function and quality of life over 12 months after COVID-19
| Variable | Comparison between the 3rd and 6th month ( | Comparison between the 6th and 9th month ( | Comparison between the 9th and 12th month ( | ||||||
|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T2 | T3 | T3 | T4 | ||||
| Physical function | |||||||||
| HGS (kgf) | 25.9 (18.1–33) | 25 (19–35.4) | 0.081 | 28 (19–35.3) | 26 (20–34) | 0.33 | 27 (20–35.8) | 27.5 (18.5–38.8) | 0.99 |
| FACIT-F (points) | 28 (20–36) | 32 (24–41.2) | 0.0001 | 33 (25–42) | 35.5 (21.8–43.3) | 0.56 | 33.8 (21.3–43.8) | 39 (24.8–42) | 0.36 |
| Spirometry | |||||||||
| FVC (% predicted) | 80.2 (69.6–92.6) | 84.2 (75.9–91.7) | 0.001 | 83 (74.5–90.1) | 85.3 (74.9–92.9) | 0.17 | 86.8 (76.8–94.3) | 84.4 (74.1–94.8) | 0.55 |
| FEV | 82.8 (70.7–93.8) | 84.9 (75.7–93.1) | 0.006 | 83.2 (72.3–91.1) | 83.2 (76.7–94.7) | 0.30 | 82.2 (77.2–95) | 83.2 (72.2–92.4) | 0.13 |
| FEV | 80.8 (75.1–85.3) | 81.5 (74.6–85.3) | 0.40 | 81.4 (74.9–85.9) | 80.3 (76.7–85.3) | 0.51 | 78.8 (76.1–84.1) | 79.1 (74.2–84.3) | 0.077 |
| SF-36 (points) | |||||||||
| Physical functioning | 35 (17.5–50) | 40 (25–60) | 0.003 | 40 (23.8–56.3) | 45 (28.8–66.3) | 0.14 | 42.5 (25–63.8) | 45 (25–65) | 0.66 |
| Physical role limitations | 0 (0–25) | 25 (0–50) | 0.001 | 25 (0–25) | 25 (0–50) | 0.31 | 30 (0–68.8) | 35 (0–75) | 0.42 |
| Bodily pain | 40 (20–62) | 41 (31–63) | 0.015 | 41 (31–72.5) | 41 (31–54.5) | 0.47 | 41 (31–59.3) | 52 (31–54) | 0.060 |
| General health perceptions | 40 (30–57) | 47 (30–62) | 0.29 | 50 (32–67) | 45 (32–58.3) | 0.21 | 40 (30.5–60.8) | 52 (40–69.5) | 0.003 |
| Vitality | 35 (25–50) | 45 (30–60) | 0.0001 | 45 (25–70) | 50 (30–65) | 0.68 | 55 (22.5–65) | 62.5 (35–70) | 0.037 |
| Social functioning | 50 (25–62.5) | 50 (25–75) | 0.001 | 50 (25–65.6) | 56.3 (37.5–75) | 0.019 | 56.3 (37.5–75) | 75 (50–100) | 0.022 |
| Emotional role limitations | 0 (0–66.7) | 33.3 (0–83.4) | 0.028 | 33.3 (0–66.7) | 33.3 (0–66.7) | 0.58 | 33.3 (0–33.3) | 33.3 (0–91.7) | 0.10 |
| Mental health | 56 (40–76) | 60 (40–80) | 0.020 | 62 (40–81) | 64 (43–77) | 0.72 | 66 (41–79) | 74 (49–80) | 0.048 |
The values shown are the median (interquartile range). List of abbreviations: HGS – handgrip strength, FACIT-F – Functional Assessment of Chronic Illness Therapy-Fatigue, FVC – forced vital capacity; FEV
Figure 1.
Relationships of the Functional Assessment of Chronic Fatigue Illness Therapy (FACIT-F) with the domains of the Short Form-36 (SF-36) for 3rd to 6th month absolute deltas: physical functioning (
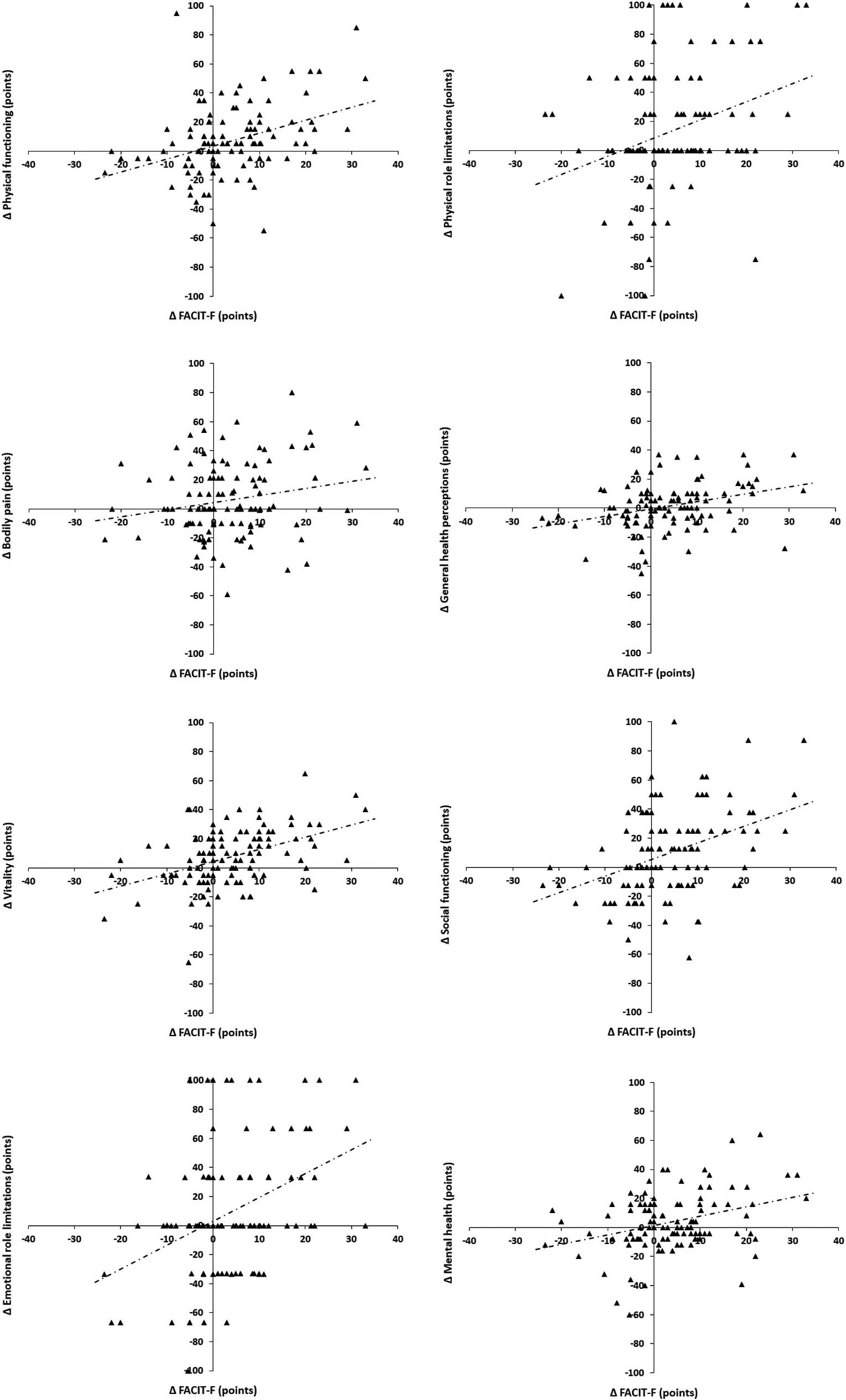
Table 2
Spearman’s correlation coefficients between quality of life, physical function and lung function
| SF-36 | ||||||||||||||||
| Physical functioning | Physical role limitations | Bodily pain | General health perceptions | Vitality | Social functioning | Emotional role limitations | Mental health | |||||||||
| 3rd to 6th month | ||||||||||||||||
| HGS | 0. | 069 | | 019 | 0. | 029 | 0. | 030 | 0. | 030 | 0. | 111 | 0. | 047 | 0. | 031 |
| FACIT-F | 0. |
388 | 0. |
282 | 0. | 168 | 0. |
351 | 0. |
427 | 0. |
379 | 0. |
307 | 0. |
271 |
| FVC | 0. | 127 | 0. | 125 | 129 | | 031 | 0. | 073 | 0. | 032 | 0. | 090 | 064 | ||
| FEV | 0. | 134 | 0. | 172 | 059 | 0. | 023 | 0. | 066 | 0. | 042 | 0. | 043 | 123 | ||
| FEV | 0. | 098 | 0. | 078 | 0. | 039 | 0. | 101 | 0. | 120 | 0. | 147 | 0. | 016 | 021 | |
| 3rd to 9th month | ||||||||||||||||
| HGS | 0. |
394 | 0. | 090 | 0. | 181 | 003 | 0. |
368 | 0. |
298 | 0. | 129 | 0. | 077 | |
| FACIT-F | 0. |
440 | 0. | 110 | 0. | 112 | 0. |
375 | 0. |
600 | 0. | 280 | 0. | 132 | 0. |
471 |
| FVC | | 056 | 0. | 192 | 0. | 088 | 0. | 195 | 0. | 055 | 0. |
318 | 0. | 197 | 0. | 096 |
| FEV | 0. | 022 | 0. | 274 | 0. | 082 | 0. | 205 | 0. | 147 | 0. | 211 | 0. | 216 | 0. | 018 |
| FEV | 0. | 082 | 0. | 034 | 0. | 047 | 011 | 018 | | 150 | | 065 | 206 | |||
| 3rd to 12th month | ||||||||||||||||
| HGS | 0. |
459 | 0. |
386 | 0. | 214 | 0. |
366 | 0. |
403 | 0. |
491 | 0. | 139 | 0. | 214 |
| FACIT-F | 0. |
615 | 0. |
361 | 0. |
453 | 0. | 224 | 0. |
568 | 0. | 128 | 0. | 247 | 0. |
455 |
| FVC | 0. |
462 | 0. |
420 | 0. | 165 | 0. | 063 | 0. | 169 | 0. | 326 | 0. | 035 | 014 | |
| FEV | 0. |
579 | 0. | 348 | 0. | 167 | 0. | 177 | 0. | 198 | 0. | 253 | 0. | 121 | 0. | 000 |
| FEV | 0. | 233 | 065 | 0. | 016 | 0. | 091 | 0. | 096 | 135 | 0. | 027 | 0. | 002 | ||
The values in bold refer to significant differences.
FVC (
In the ninth month after COVID-19, 46 participants returned due to continuing symptoms, with 9 (19.6%) experiencing general fatigue, 8 (17.4%) experiencing dyspnoea and 4 (8.7%) experiencing cough. In the comparisons between T2 and T3 (Table 1), no significant increase was observed for HGS, FACIT-F or pulmonary function. Regarding the QoL, there was a significant increase only in the social functioning domain of the SF-36 (
In the twelfth month after COVID-19, 32 participants returned due to the continuing symptoms, 6 (18.8%) with general fatigue, 5 (15.6%) with dyspnoea and 2 (6.2%) with cough. In the comparisons between T2 and T3 (Table 1), no significant increase was observed for HGS, FACIT-F or pulmonary function. Regarding the QoL, there was an increase in the following SF-36 domains: general health perceptions (
Additionally, we evaluated the associations between changes in T1, T2, T3 and T4 (Table 2). When the absolute deltas between T1 and T2 were evaluated, we observed significant correlations between the FACIT-F and all domains of the SF-36 (except bodily pain) (Fig. 1). When the absolute deltas between T1 and T3 were evaluated, we observed significant correlations between FACIT-F and physical functioning, general health perceptions, vitality and mental health domains of SF-36. Significant correlations were observed between HGS and physical functioning, vitality and social functioning domains of SF-36. Additionally, significant correlations were observed between the FVC and the SF-36 social functioning domain. Finally, when the absolute deltas between T1 and T4 were evaluated, we observed significant correlations between the FACIT-F and the SF-36 physical functioning, physical role limitations, bodily pain, vitality and mental health domains. Significant correlations were observed between the HGS and the physical functioning, physical role limitations, general health perceptions, vitality and social functioning domains of the SF-36. Additionally, significant correlations were observed between the FVC and the SF-36 physical functioning and physical role limitations domains.
Based on an a priori type I error
4.Discussion
There is growing interest in long-term sequelae after recovery from the acute phase of COVID-19. In the 1-year follow-up of a sample of patients with PACS, the main finding of the present study was the improvement of QoL, general fatigue and pulmonary function in the first 6 months of follow-up without improvement in peripheral muscle strength (HGS). In these patients, the improvement after 6 months is slower, occurring especially in the components that involve QoL (both physical and mental). In addition, there was an association between general fatigue and the domains of the physical and mental components of QoL in the first 6 months after acute infection. After this period, there was an association of QoL with muscle function and lung function in the subsequent months until the end of follow-up at 1 year. To our knowledge, this is the first study that evaluated the associations of QoL with functionality in the follow-up of patients with PACS in the course of 1 year.
In patients with PACS, the persistence of symptoms negatively affects QoL, making it necessary to monitor symptoms in the medium and long term. In our sample, all SF-36 domains were below the expected values for the Brazilian population from the third to the twelfth month after acute infection of COVID-19 [29]. Moreover, there was an increase in almost all dimensions of the SF-36 over the 12 months, with values higher than the minimal clinically important difference (MCID) of 5 points described in the SF-36 [30]. Contrary to our results, O’Brien et al. [31] did not notice significant changes in the mean scores of the eight SF-36 domains during the 1-year period, although these authors noted values below the normative data of the population and evaluated only patients with previous hospitalizations. Although our sample was not included in any physical reconditioning program, the improvement in functionality and QoL, especially in the first 6 months, suggests that therapeutic strategies may be more beneficial in this initial period of PACS, especially in those who have a significant deterioration of physical capacity and QoL [32].
General fatigue has been described as one of the main symptoms of PACS, occurring in 34.8% to 90% of cases depending on the evaluation tool and the time in which it is evaluated after the acute phase of the disease [14, 33]. In fact, approximately three-quarters of our sample reported general fatigue in the third month, and the presence of this symptom progressively decreased during follow-up. Considering the median value of the FACIT-F scale, there was a variation from 28 to 39 points between the third and twelfth months; this variation is greater than the MCID described for the FACIT-F of 4 points [34], although still below the normative value of 40.1 points [35]. Throughout the 12-month follow-up, we observed significant correlations between changes in the FACIT-F scale and the SF-36 domains, especially with the vitality domain, which best reflects the general fatigue in patients with PACS [36]. In agreement with our findings, Rass et al. [37] observed that impairments in the physical health component of the SF-36 were strongly related to general fatigue in a sample of patients with PACS. In fact, the significant load of general fatigue in patients with PACS is related to a high degree of difficulty in performing activities of daily living, deterioration of physical function and, therefore, worsening of QoL [38].
HGS evaluates the static forces compressing around the dynamometer and is a practical, fast and viable tool for the study of vitality and physical fitness [7]. HGS can be used as a reference to establish reaction time, and problems with HGS are indicators of difficulties in performing everyday tasks and worse performance at work [6]. In our sample, the median HGS value was 27.2 kgf in the third month, which is well below the value described in healthy Brazilian adults (38.0
Individuals with PACS should be carefully monitored for respiratory dysfunction as a sequel of SARS-CoV-2 acute lung injury [41]. In the present study, we observed a gradual improvement in pulmonary function throughout follow-up, mainly between 3 and 6 months. Following a cohort of patients with PACS for 1 year, Zhou et al. [42] observed mean values of pulmonary function higher than those of our sample, which can be explained at least in part by the predominance of nonsevere cases in their study. During the pandemic, it is important to evaluate the relationship between lung function and QoL in COVID-19 survivors. We observed a correlation between the physical functioning domain of SF-36 and pulmonary function, which is in agreement with the study by Bardakci et al. [16]. Notably, we evaluated the correlations between the absolute deltas of the QoL time intervals and the absolute deltas of the time intervals of the functionality variables. This provided a more reliable idea of the improvements over time, rather than the strictly cross-sectional analysis performed by most studies [16, 41, 42].
Our study has several limitations. First, we did not use a control group that could assist in the interpretation of our results. Also, the initial sample was not followed up during the 12 months, although we defined the persistence of symptoms in the subsequent months (diagnosis of PACS) as a follow-up criterion, and this definition is more compatible with the “real world”. Third, because the results of the baseline data are unknown, our findings cannot be attributed exclusively to COVID-19. This is because previous musculoskeletal and cardiopulmonary diseases may have affected our results. Despite these limitations, our results may contribute to the design of studies focused on muscle and cardiopulmonary reconditioning, especially those focused on the first 6 months of PACS. This could contribute to a faster recovery of patients’ physical capacity and consequently QoL, in addition to reducing public spending on social programs aimed at this population that may affect work activities.
5.Conclusions
Our results show that in patients with PACS, there is progressive improvement in QoL, general fatigue and pulmonary function during the 12 months of follow-up, and this improvement is more pronounced in the first 6 months after the acute phase of COVID-19. There is a relationship between general fatigue and QoL in the first 6 months and between functionality and QoL in the subsequent period until completing 1 year. Thus, we believe that assessment of muscle function and QoL can improve the understanding of the medium- and long-term consequences of COVID-19 and thus provide opportunities to apply tailored interventions in patients with PACS.
Ethical approval
The project was approved by the Brazilian National Research Ethics Committee (number CAAE-30135320.0.0000.5259).
Funding
The study was funded by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; Grant number #302215/2019-0), Brazil; the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ; Grant numbers #E-26/211.024/2019, #E-26/211.187/2021, #E-26/211.104/2021, and #E-26/200.929/2022), Brazil, and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Finance Code 001, 88881.708719/2022-01, and 88887.708718/2022-00), Brazil.
Informed consent
All participants signed an informed consent form.
Author contributions
Conceptualization and design: J.E.A.V., T.T.M., A.J.L.; Data collection: T.T.M., L.B.M., M.S.C., A.T.A.G., A.J.L.; Interpretation: J.E.A.V., A.J.L.; Writing – original draft: J.E.A.V., T.T.M., L.B.M., M.S.C., A.T.A.G., A.J.L.; All authors read and approved the final manuscript.
Acknowledgments
The authors would like to thank the entire team of professionals at the Post-COVID Outpatient Clinic at Piquet Carneiro Policlinic, State University of Rio de Janeiro, Rio de Janeiro, Brazil.
Conflict of interest
None of the authors have any conflict of interest to declare.
References
[1] | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. (2020) ; 382: (18): 1708-20. |
[2] | Leung T, Chan A, Chan EW, Chan V, Chui C, Cowling, BJ, et al. Short- and potential long-term adverse health outcomes of COVID-19: A rapid review. Emerg Microbes Infect. (2020) ; 9: (1): 2190-9. |
[3] | Wu Z, Mcgoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in china: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. (2020) ; 323: (13): 1239-42. |
[4] | Achkar M, Jamal O, Chaaban T. Post-COVID lung disease(s). Ann Thorac Med. (2022) ; 17: (3): 137-44. |
[5] | Roman-Liu D, Tokarski T. Upper limb strength in relation to upper limb posture. Int J Ind Ergon. (2005) ; 35: : 19-31. |
[6] | Liao K-H. Experimental study on gender differences in hands and sequence of force application on grip and hand-grip controlInt. J Occup Saf Ergon. (2014) ; 20: (1): 77-90. |
[7] | Jain R, Meena ML, Sain MK, Dangayach GS. Impact of posture and upper-limb muscle activity on grip strength. Int J Occup Saf Ergon. (2019) ; 25: (4): 614-20. |
[8] | Mittal J, Ghosh A, Bhatt Sp, Anoop S, Ansari Ia, Misra A. High prevalence of post COVID-19 fatigue in patients with type 2 diabetes: A case-control study. Diabetes Metab Syndr. (2021) ; 15: (6): 102302. |
[9] | Tuzun S, Keles A, Okutan D, Yildiran T, Palamar D. Assessment of musculoskeletal pain, fatigue and grip strength in hospitalized patients with COVID-19. Eur J Phys Rehabil Med. (2021) ; 57: (4): 653-62. |
[10] | de Sousa KCA, Gardel DG, Lopes AJ. Postural balance and its association with functionality and quality of life in non-hospitalized patients with post-acute COVID-19 syndrome. Physiother Res Int. (2022) ; 27: (4): e1967. |
[11] | de Oliveira TCP, Gardel DG, Ghetti ATA, Lopes AJL. The Glittre-ADL test in non-hospitalized patients with post-COVID-19 syndrome and its relationship with muscle strength and lung function. Clin Biomech. (2022) ; 100: : 105797. |
[12] | de Oliveira JF, de Ávila RE, de Oliveira NR, Sampaio NCS, Botelho M, Gonçalves FA, et al. Persistent symptoms, quality of life, and risk factors in long COVID: A cross-sectional study of hospitalized patients in Brazil. Int J Infect Dis. (2022) ; 122: : 1044-51. |
[13] | Cao J, Zheng X, Wei W, Chu X, Chen X, Wang Y, et al. Three-month outcomes of recovered COVID-19 patients: Prospective observational study. Ther Adv Respir Dis. (2021) ; 15: : 17534666211009410. |
[14] | Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med. (2021) ; 27: (4): 601-15. |
[15] | González J, Zuil M, Benítez ID, de Gonzalo-Calvo D, Aguilar M, Santisteve S, et al. One year overview and follow-up in a post-COVID consultation of critically ill patients. Front Med. (2022) ; 9: : 897990. |
[16] | Bardakci MI, Ozturk EN, Ozkarafakili MA, Ozkurt H, Yanc U, Sevgi DY. Evaluation of long-term radiological findings, pulmonary functions, and health-related quality of life in survivors of severe COVID-19. J Med Virol. (2021) ; 93: (9): 5574-81. |
[17] | McFann K, Baxter BA, LaVergne SM, Stromberg S, Berry K, Tipton M, et al. Quality of life (QoL) is reduced in those with severe COVID-19 disease, post-acute sequelae of COVID-19, and hospitalization in United States adults from northern Colorado. Int J Environ Res Public Health. (2021) ; 18: (21): 11048. |
[18] | Anastasio F, Barbuto S, Scarnecchia E, Cosma P, Fugagnoli A, Rossi G, et al. Medium-term impact of COVID-19 on pulmonary function, functional capacity and quality of life. Eur Respir J. (2021) ; 58: (3): 2004015. |
[19] | National Institute for Health and Care Excellence, (2020) . COVID-19 rapid guideline: managing the long-term effects of COVID-19. Available in: https://www.nice.org.uk/guidance/ng188. |
[20] | Brazier JE, Harper R, Jones NM, O’Cathain A, Thomas KJ, Usherwood T, et al. Validating the SF-36 health survey questionnaire: New outcome measure for primary care. BMJ. (1992) ; 305: (6846): 160-4. |
[21] | Laguardia J, Campos MR, Travassos CM, Najar AL, Anjos LA, Vasconcellos MM. Psychometric evaluation of the SF-36 (v.2) questionnaire in a probability sample of Brazilian households: Results of the survey Pesquisa Dimensões Sociais das Desigualdades (PDSD), Brazil, 2008. Health Qual Life Outcomes. (2011) ; 9: : 61. |
[22] | Severo M, Santos AC, Lopes C, Barros H. Reliability and validity in measuring physical and mental health construct of the Portuguese version of MOS SF-36. Acta Med Port. (2006) ; 19: (4): 281-7. |
[23] | Nonato CP, Azevedo BLPA, Oliveira JGM, Gardel DG, de Souza DCN, Lopes AJ. The Glittre Activities of Daily Living Test in women with scleroderma and its relation to hand function and physical capacity. Clin Biomech. (2020) ; 73: : 71-7. |
[24] | Mosher CE, Duhamel KN. An examination of distress, sleep, and fatigue in metastatic breast cancer patients. Psychooncology. (2012) ; 21: (1): 100-7. |
[25] | Lima TRL, Kasuki L, Gadelha M, Lopes AJ. Physical exercise improves functional capacity and quality of life in patients with acromegaly: A 12-week follow-up study. Endocrine. (2019) ; 66: (2): 301-9. |
[26] | Acaster S, Dickerhoof R, DeBusk K, Bernard K, Strauss W, Allen LF. Qualitative and quantitative validation of the FACIT-fatigue scale in iron deficiency anemia. Health Qual Life Outcomes. (2015) ; 13: : 60. |
[27] | Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardization of spirometry. Eur Respir J. (2005) ; 26: (2): 319-38. |
[28] | Pereira CAC, Sato T, Rodrigues SC. New reference values for forced spirometry in white adults in Brazil. J Bras Pneumol. (2007) ; 33: (4): 397-406. |
[29] | Laguardia J, Campos MR, Travassos C, Najar AL, Anjos LA, Vasconcellos MM. Brazilian normative data for the Short Form 36 questionnaire, version 2. Rev Bras Epidemiol. (2013) ; 16: (4): 889-97. |
[30] | Kosinski M, Zhao SZ, Dedhiya S, Osterhaus JT, Ware JE Jr. Determining minimally important changes in generic and disease-specific health-related quality of life questionnaires in clinical trials of rheumatoid arthritis. Arthritis Rheum. (2000) ; 43: (7): 1478-87. |
[31] | O’Brien K, Townsend L, Dowds J, Bannan C, Nadarajan P, Kent B, et al. 1-year quality of life and health-outcomes in patients hospitalised with COVID-19: A longitudinal cohort study. Respir Res. (2022) ; 23: (1): 115. |
[32] | Dalbosco-Salas M, Torres-Castro R, Leyton AR, Zapata FM, Salazar EH, Bastías GE, et al. Effectiveness of a primary care telerehabilitation program for post-COVID-19 patients: A feasibility study. J Clin Med. (2021) ; 10: (19): 4428. |
[33] | Lopes AJ, Litrento PF, Provenzano BC, Carneiro AS, Monnerat LB, da Cal MS, et al. Small airway dysfunction on impulse oscillometry and pathological signs on lung ultrasound are frequent in post-COVID-19 patients with persistent respiratory symptoms. PLoS One. (2021) ; 16: (11): e0260679. |
[34] | Hewlett S, Dures E, Almeida C. Measures of fatigue: Bristol Rheumatoid Arthritis Fatigue Multi-Dimensional Questionnaire (BRAF MDQ), Bristol Rheumatoid Arthritis Fatigue Numerical Rating Scales (BRAF NRS) for severity, effect, and coping, Chalder Fatigue Questionnaire (CFQ), Checklist Individual Strength (CIS20R and CIS8R), Fatigue Severity Scale (FSS), Functional Assessment Chronic Illness Therapy (Fatigue) (FACIT-F), Multi-Dimensional Assessment of Fatigue (MAF), Multi-Dimensional Fatigue Inventory (MFI), Pediatric Quality Of Life (PedsQL) Multi-Dimensional Fatigue Scale, Profile of Fatigue (ProF), Short Form 36 Vitality Subscale (SF-36 VT), and Visual Analog Scales (VAS). Arthritis Care Res. (2011) ; 63: (Suppl 11): S263-S86. |
[35] | Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: Properties, applications, and interpretation. Health Qual Life Outcomes. (2003) ; 1: : 79. |
[36] | Ouanes S, Al-Amin H, Hussein NB, Khan F, Shahrani A, David P, et al. Physical and psychosocial well-being of hospitalized and non-hospitalized patients with COVID-19 compared to the general population in Qatar. Front Psychiatry. (2021) ; 12: : 792058. |
[37] | Rass V, Ianosi BA, Zamarian L, Beer R, Sahanic S, Lindner A, et al. Factors associated with impaired quality of life three months after being diagnosed with COVID-19. Qual Life Res. (2022) ; 31: (5): 1401-14. |
[38] | Townsend L, Dowds J, O’Brien K, Sheill G, Dyer AH, O’Kelly B, et al. Persistent poor health after COVID-19 is not associated with respiratory complications or initial disease severity. Ann Am Thorac Soc. (2021) ; 18: (6): 997-1003. |
[39] | Neves RS, Lopes AJ, Menezes SLS, Lima TRL, Ferreira AS, Guimarães FS. Hand grip strength in healthy young and older Brazilian adults: development of a linear prediction model using simple anthropometric variables. Kinesiology, (2017) ; 49: (2): 208-16. |
[40] | Bobos P, Nazari G, Lu Z, MacDermid JC. Measurement properties of the hand grip strength assessment: A systematic review with meta-analysis. Arch Phys Med Rehabil. (2020) ; 101: (3): 553-65. |
[41] | Okan S, Okan F, Yücesoy FD. Evaluation of pulmonary function and exercise capacity after COVID-19 pneumonia. Heart Lung. (2022) ; 54: : 1-6. |
[42] | Zhou F, Tao M, Shang L, Liu Y, Pan G, Jin T, et al. Assessment of sequelae of COVID-19 nearly 1 year after diagnosis. Front Med. (2021) ; 8: : 717194. |




