Efficacy of platelet-rich plasma injection in the treatment of frozen shoulder: A systematic review and meta-analysis
Abstract
BACKGROUND:
Frozen shoulder (FS) is characterized by progressive shoulder pain and a limited range of motion. Recently, platelet-rich plasma (PRP) injection is a newly developed treatment option for patients with FS and its efficacy needs to be examined.
OBJECTIVE:
By conducting a systematic review and meta-analysis, this study attempted to evaluate the efficacy of PRP injection in the treatment of patients with FS.
METHODS:
PubMed, EMBASE, Web of Science, Elsevier, The Cochrane Library, WanFang Data and CNKI databases were searched up to May 31, 2020. This study included randomized controlled trials as well as prospective cohort studies. Two reviewers independently screened the title, abstract and full text in order to extract data from qualified studies. The main outcome was pain visual analogue score (VAS) while the secondary outcome was range of motion (ROM) of the shoulder joint that consists of four parts: internal rotation, flexion, external rotation and abduction.
RESULTES:
Three randomized controlled trials and one prospective cohort study met the inclusion criteria. Accordingly, a total of 359 cases were analyzed and followed up to 3 months. The control group included corticosteroids (CS), ultrasound therapy, and stellate ganglion block. Compared to other groups, VAS was statistically significant after 1 month and 3 months of treatment (SMD:
CONCLUSIONS:
The corresponding findings illustrate that compared to other non-operative treatments, local injection of PRP can effectively improve pain and shoulder motion in patients with FS. However, due to the short follow-up time and limitations regarding the quantity and quality of studies, the above conclusions require further elucidation by performing additional high-quality studies.
1.Introduction
Frozen shoulder (FS), which is also referred to as adhesive capsulitis, is characterized by progressive shoulder pain and a limited range of motion [1]. The incidence of FS in the general population is about 2%–5%, mainly in the middle-aged and elderly between 40–70 years of age. The incidence of FS in diabetic patients reached as high as 20%. Its etiology is complicated as joint degeneration and chronic strain related [2, 3]. The basic pathology involves soft tissue fibrosis of the articular cavity, capsule, ligament and aseptic inflammation [4]. The long-term delay of the disease negatively impacts patients’ psychology and aggravates their pain. Currently, non-operative treatment includes e.g. non-steroidal anti-inflammatory drugs, intra-articular injection of CS, suprascapular nerve block, and physical therapy [5, 6, 7, 8, 9]. NSAIDs may relieve pain and reduce sleep disturbance, but they do not have a substantial effect on recovery.
Intra-articular injection of CS is still one of the most commonly used methods for the treatment of FS. However, CS injection has been found to be associated with hyperglycemia, which is harmful to articular cartilage, increasing the risk of tendon rupture, local skin depigmentation and subcutaneous tissue atrophy [10]. Although many treatment methods exist, their overall effect is not ideal, and they contain side effects. Therefore, how to improve the curative rate of FS and reduce the side effects of treatment has become a focus of current research.
Platelet-rich plasma (PRP) injection is a new form of treatment, which is a concentrate of PRP obtained by repeated centrifugation of the patients’ peripheral blood [11]. PRP contains many components, such as platelet-derived growth factor (PDGF), platelet-derived epidermal growth factor (PDEGF), transforming growth factor
Because of its advantages, PRP injection has been gradually used in the clinical treatment of osteoarthritis, lateral epicondylitis, rotator cuff injury, tendon disease, metatarsal fasciitis and other diseases demonstrating good effects in tissue repair. Systematic reviews have also shown that PRP can effectively treat tendon, ligament, cartilage and muscle injuries [15]. However, some studies confirmed that there was no superior clinical benefit of PRP compared with the control for knee osteoarthritis [39] or achilles tendon rupture [40]. Hall et al. [16] reported the outcomes of PRP in the treatment of rotator cuff repair through shoulder arthroscopy. After two years of follow-up, no related complications occurred, and the pain visual analogue score (VAS) and range of motion (ROM) scores of all patients increased compared with those before the operation. Nevertheless, a systematic review suggested that more studies should be conducted in the future to confirm reliable results due to the low quality of the methodology. Besides, in-depth studies are required to confirm reliable results for efficacy of PRP for rotator cuff repair [38]. Over the past few years, increasing studies have been conducted on the application of injection of PRP for the treatment of FS. An existing study suggested that in the rat FS model, PRP injection into the glenohumeral joint inhibited strong structural changes in the posterior synovial membrane of rats in an in vivo shoulder contracture model, which did not cause any side effects and was considered to be safe [37]. Havva et al. [26] found that PRP injection could effectively alleviate pain, improve range of motion of shoulder joint and enhance functionality in patients suffering from FS and chronic shoulder pain. In 2020, Hüma et all. injected PRP into the shoulder joint of a patient with chronic kidney disease and it was found that the symptoms of FS could be mitigated [33]. Some studies have qualitatively evaluated the safety and efficacy of PRP injection in FS. Although numerous studies have reported that PRP injection is a promising alternative treatment option for FS, there have been rare clinical trials for quantitative analysis to prove this theory. In this study, the clinical literature related to the use of topical PRP injections for the treatment of FS was comprehensively searched. Relevant literature was combined through systematic reviews and meta-analyses. The aim of this study was to understand the efficacy of local injection of PRP in the treatment of FS and to compare the efficacy of PRP with conservative treatment.
2.Methods
The meta-analysis was registered in INPLASY under registration number INPLASY202060097.
2.1Literature search
Seven electronic databases including PubMed, EMBASE, Web of Science, Elsevier, The Cochrane Library, WanFang Data and CNKI, as well as the references of related experiments, were searched using from the time of establishment of the database to May 31, 2020. The search criteria adopted a combination of subject words and free words: “Frozen Shoulder”, “Bursitides” [Mesh], “Pes Anserine Bursiti*”, “Adhesive Capsuliti*” or “Adhesive Capsulitis of the Shoulder”, and “platelet-rich plasma” [Mesh], “platelet-rich fibrin matrix”, “platelet gel” or “PRP”, using the Boolean operator “and or”. This study included all published randomized controlled trials as well as prospective cohort studies. Pretrials, observational studies, case reports, reviews, and basic or animal studies were excluded.
2.2Inclusion criteria
Patients older than 18 years old with a clinical diagnosis of FS (pain caused by active extension of shoulder pronation and elbow extension according to symptoms).
2.3Exclusion criteria
Disorder was secondary to inflammatory joint disease; Presence of any kind of shoulder musculoskeletal pathological or neurological disorder; Anemia (hemoglobin level
2.4Quality assessment of the studies
Two evaluators evaluated the bias risk included in the study according to the bias risk assessment tool for RCT in the Cochrane manual, and the prospective study was evaluated in the form of non-random study indicators. Differences were resolved by consensus or through consultation with senior inspectors.
2.5Data extraction
Two researchers independently screened the literature, extracted the data and cross-checked them. If any objections were present, they were discussed and resolved amongst themselves or were referred to a third researcher for assistance. If there was a lack of information, the original author was attempted to be contacted for supplementation. The extracted data included: The basic information contained in the study included the authors’ names, year of publication and country basic characteristics of the subjects including sample size and age details of the intervention and treatment process key elements of bias risk assessment, and key data on outcome indicators.
2.6Statistical analysis
Since different follow-up times were used in the included articles, we collected and calculated data over approximately the same time range. The data for the first month and the third week were combined, and the follow-up data for the twelfth week and the third month were combined. The average difference (MD) and 95% CI were calculated using continuity variables for VAS score and ROM improvement. Here,
Figure 1.
Flow diagram of study inclusion.
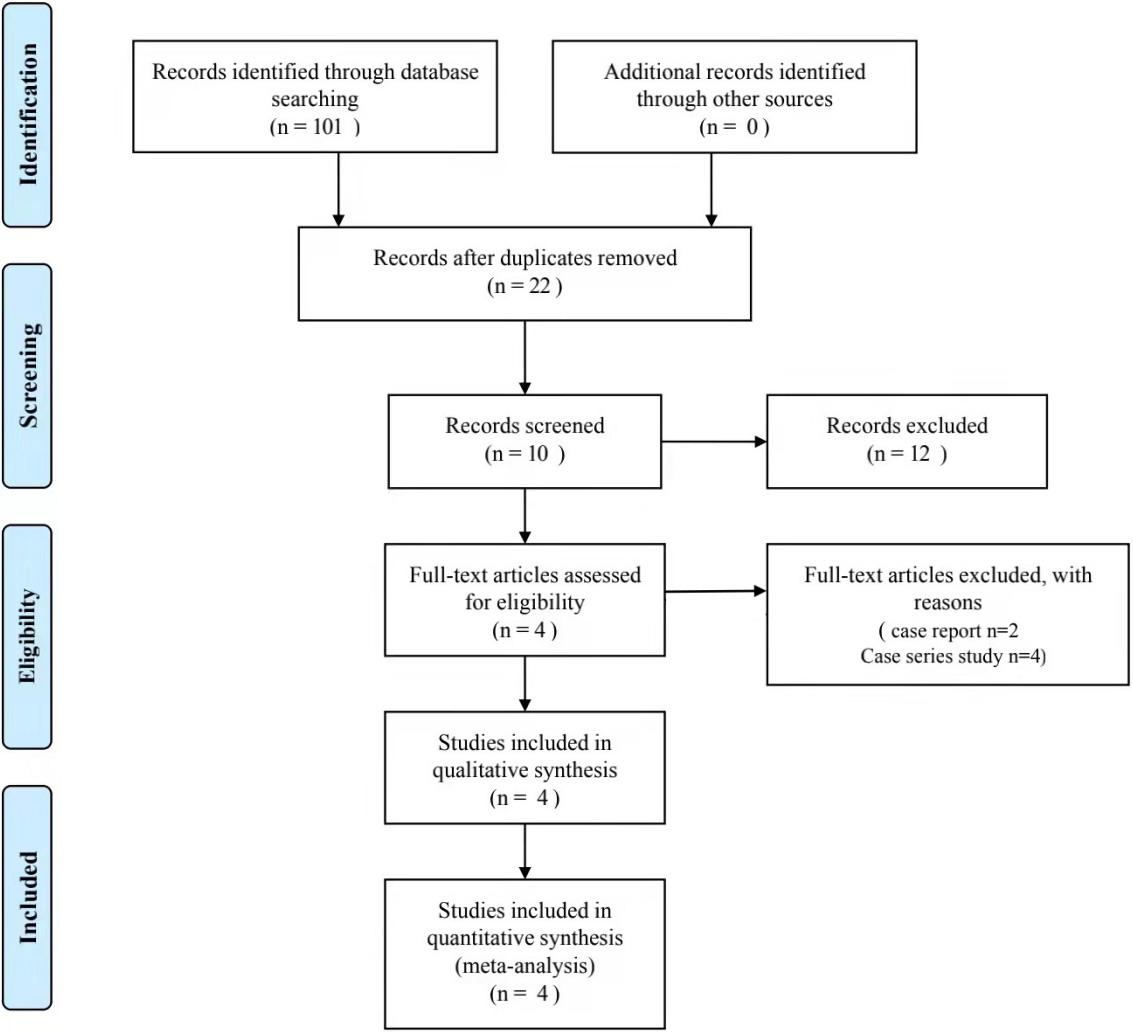
3.Results
3.1Study identification and selection
Figure 1 depicts the study selection and inclusion. Accordingly, potential studies were initially detected, and following a layer-by-layer screening, one prospective study and three randomized trials were included in the final quality assessment and data extraction. Of the four studies [17, 18, 19, 20], three reported a VAS (
3.2Risk of bias assessment
All trials were assessed by two reviewers in accordance with the Cochrane Manual Risk of Bias assessment tool to assess the bias in the included studies (Figs 2 and 3). One of the included studies was a prospective cohort study using the Newcastle-Ottawa Scale (NOS) for assessment [20] (Fig. 3). Sequence generation and allocation were adequately reported by two studies [18, 19], except for one study [17] where the concealment of allocation from the investigators and sequence generation was unclear (unclear risk of bias). Two studies [18, 19] were considered at a low risk for detection bias because of the blinding of the outcome assessor, except for one study [17] in which the outcome assessor was not reported (unclear risk of bias). Patients in two studies [18, 19] were blinded to their intervention group (low risk of bias), except for one study [17] in which the patients were not blinded to their intervention group (unclear risk of bias). None of the studies reported significant loss of follow-up (low risk of bias).
Figure 2.
Quality assessment of RCT studies.
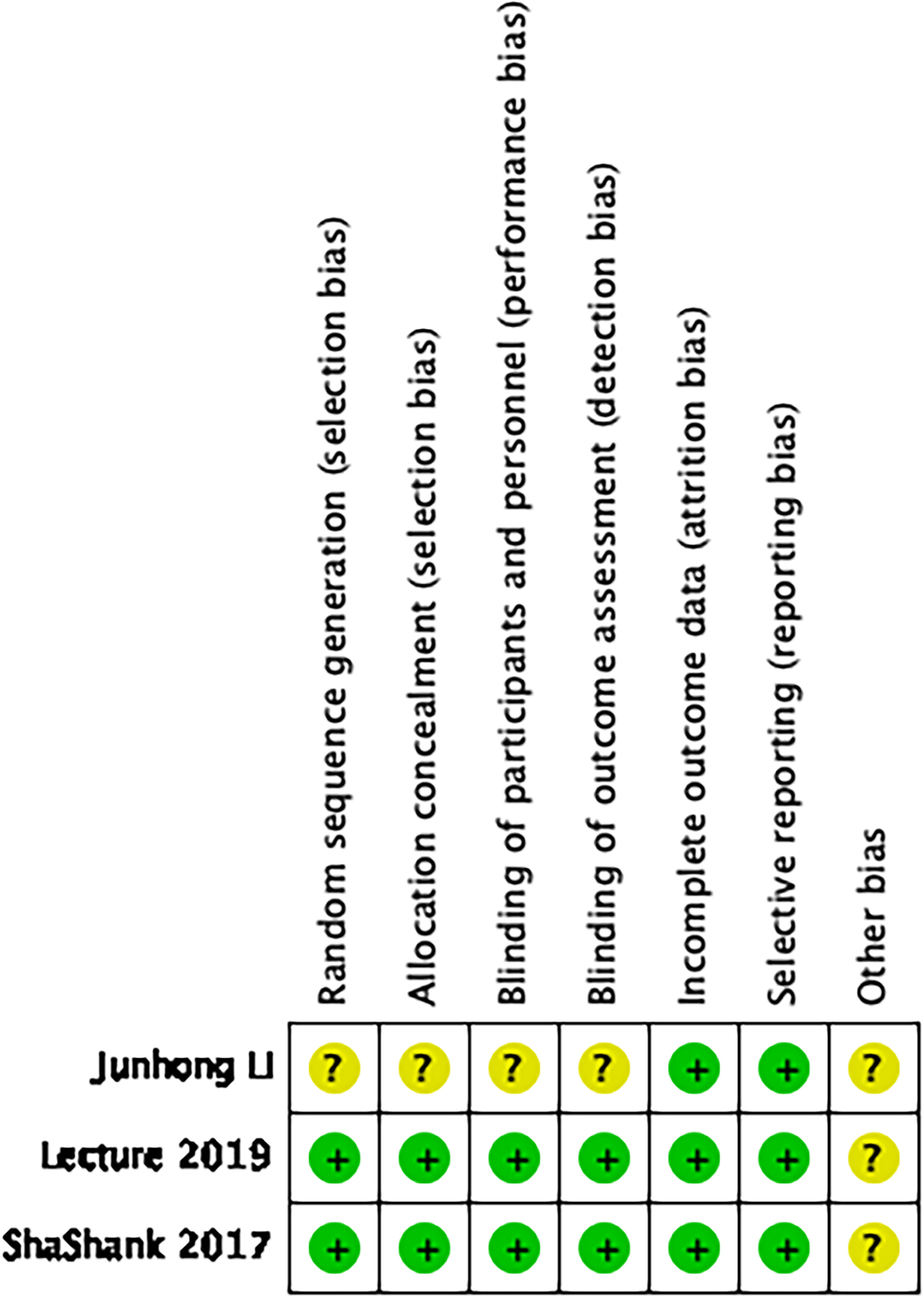
Figure 3.
Quality assessment of nonrandomized trials.

3.3Research characteristics
Of the included studies, one study compared PRP with procaine
Table 1
Characteristics of the included studies
| Reference | Group | Age (years) | Numbers | Intervention | Outcome measure | Follow-up |
|---|---|---|---|---|---|---|
| Lin et al. [17] | PRP, Procaine | 59.8 | N | 3 times, 2 ml, every 2 weeks | VAS, UCLA | 1w, 1m, 3m, 6m |
| Shashank et al. [18] | PRP, CS, Ultrasonic therapy | 51.9 | N | Single injection 2 ml, single injection 2 ml (80 mg), 7 minutes 1.5 W/cm | VAS, QuickDASH, ROM | 3w, 6w, 12w |
| Lecturer et al. [19] | SGB | 55.2 | N | Single, SGB | NRS, QuickDASH, ROM | 1m, 3m, 6m |
| Barman et al. [20] | PRP, CS | 50.0 | n | Single injection 4 ml, single injection (CS 2 ml | VAS, ROM | 3w, 6w, 12w |
Abbreviations: PRP: platelet-rich plasma; CS: Corticosteroid; DASH: Disabilities of the Arm, Shoulder and Hand Score; ROM: range of motion, shoulder joint; VAS: visual analog scale; UCLA: University of California at Los Angeles Shoulder Scale; SGB: stellate ganglion block; NRS: Numerical Rating Scale.
3.4Meta-analysis results
Among the included studies, VAS and ROM were the most commonly used tools in evaluating the efficacy of PRP injection or other treatments. The data of the first and third months after the intervention were calculated. According to the data provided when the study summarized the data, there was no significant difference in the standardized mean of VAS score at enrollment (SMD:
Figure 4.
Meta-analysis results of VAS scores between PRP group and control group before intervention.
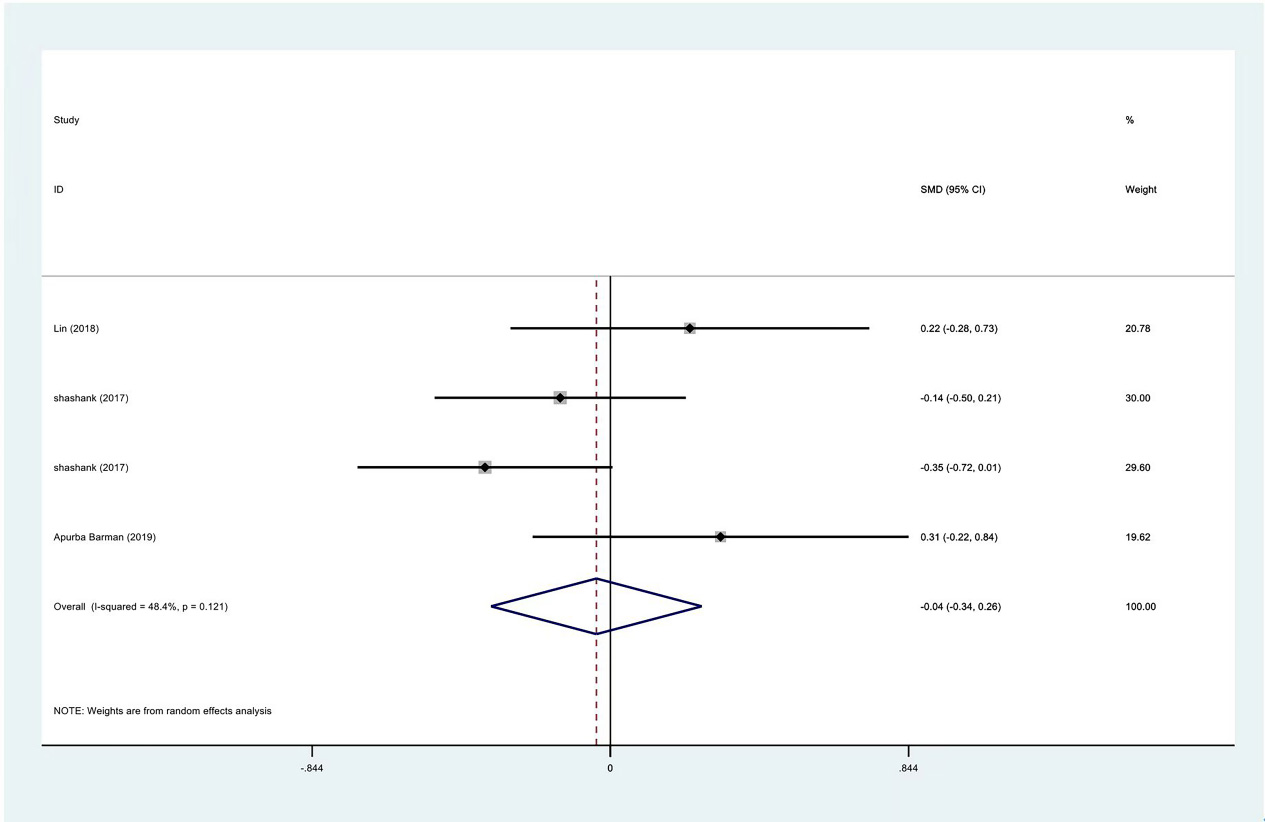
Figure 5.
Meta-analysis results of VAS scores between PRP group and control group at 1 month.
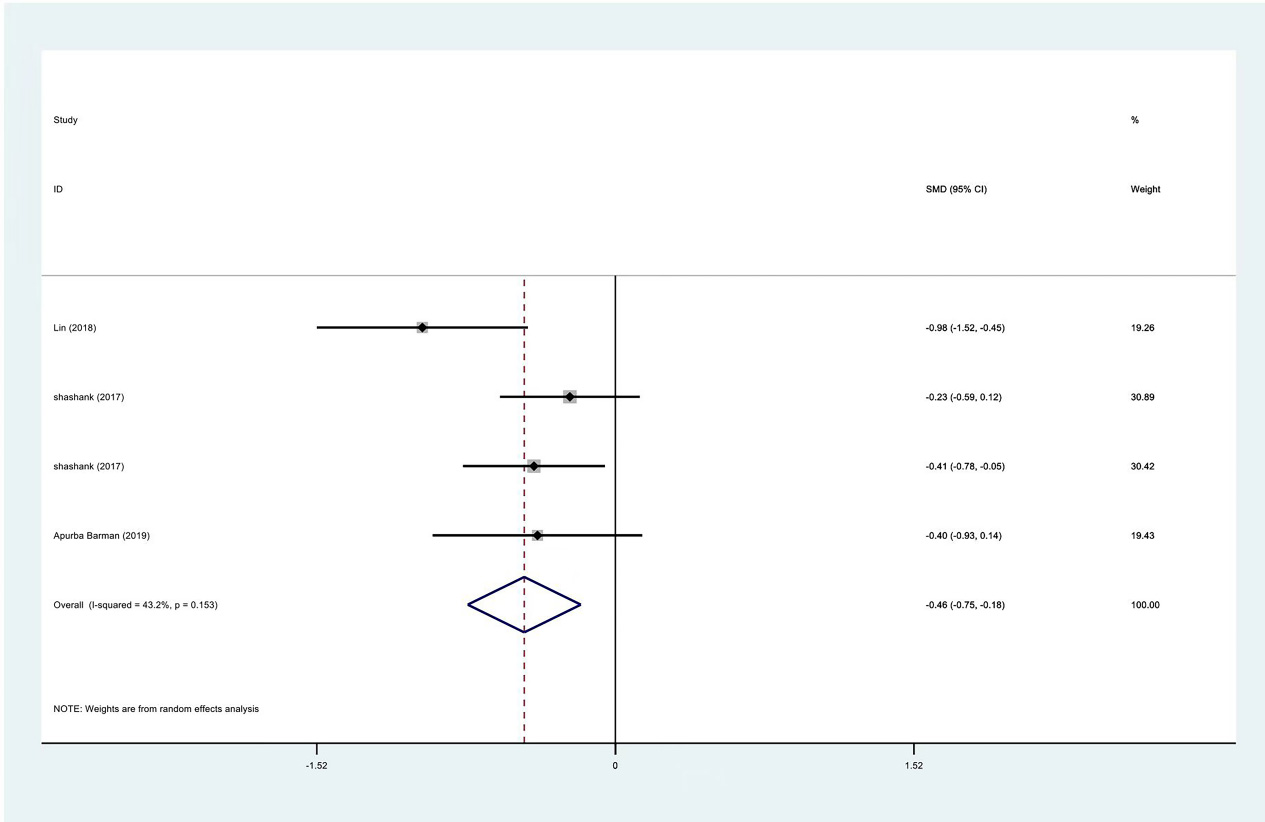
Figure 6.
Meta-analysis results of VAS scores between PRP group and control group at 3 months.
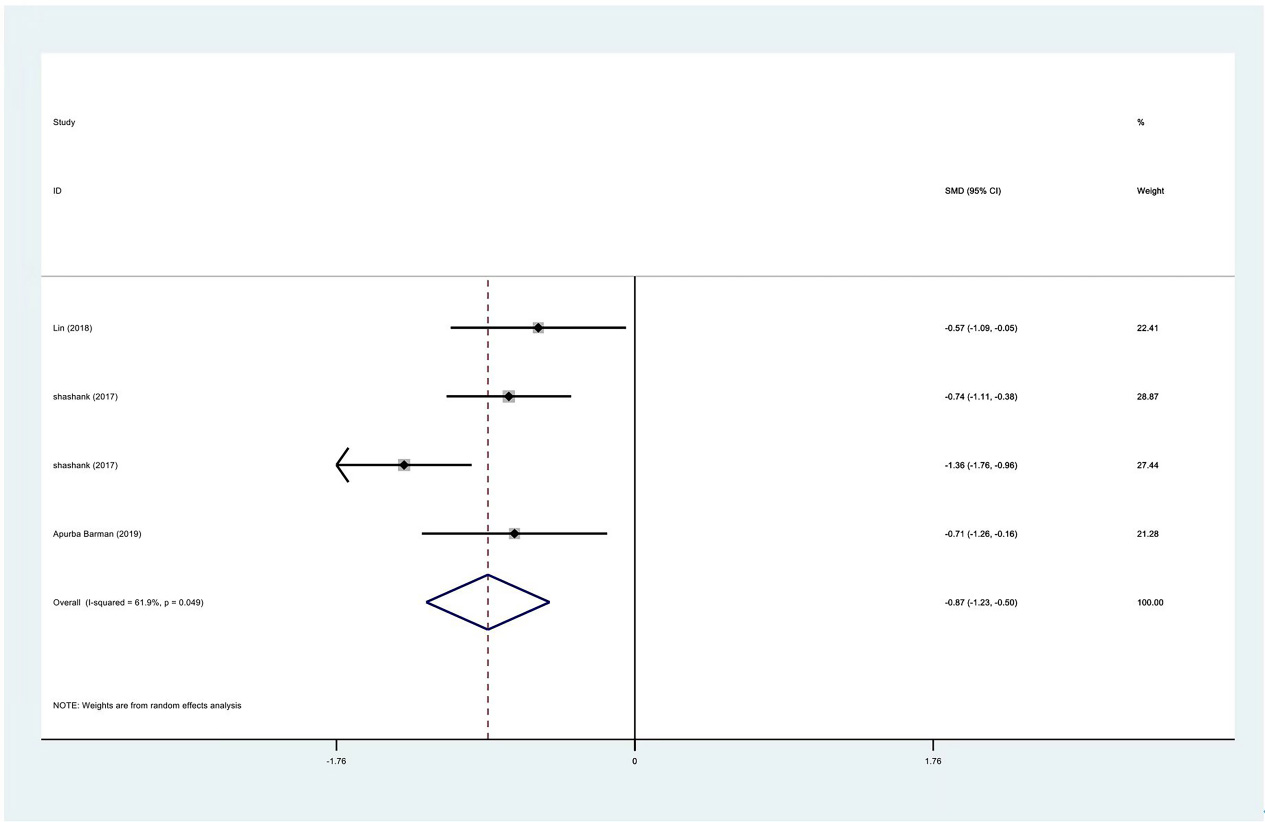
Figure 7.
Egger test of VAS scores between PRP group and control group at 1 month.

Figure 8.
Egger test of VAS scores between PRP group and control group at 3 months.

Figure 9.
Meta-analysis results of internal rotation between PRP group and control group at 1 month.
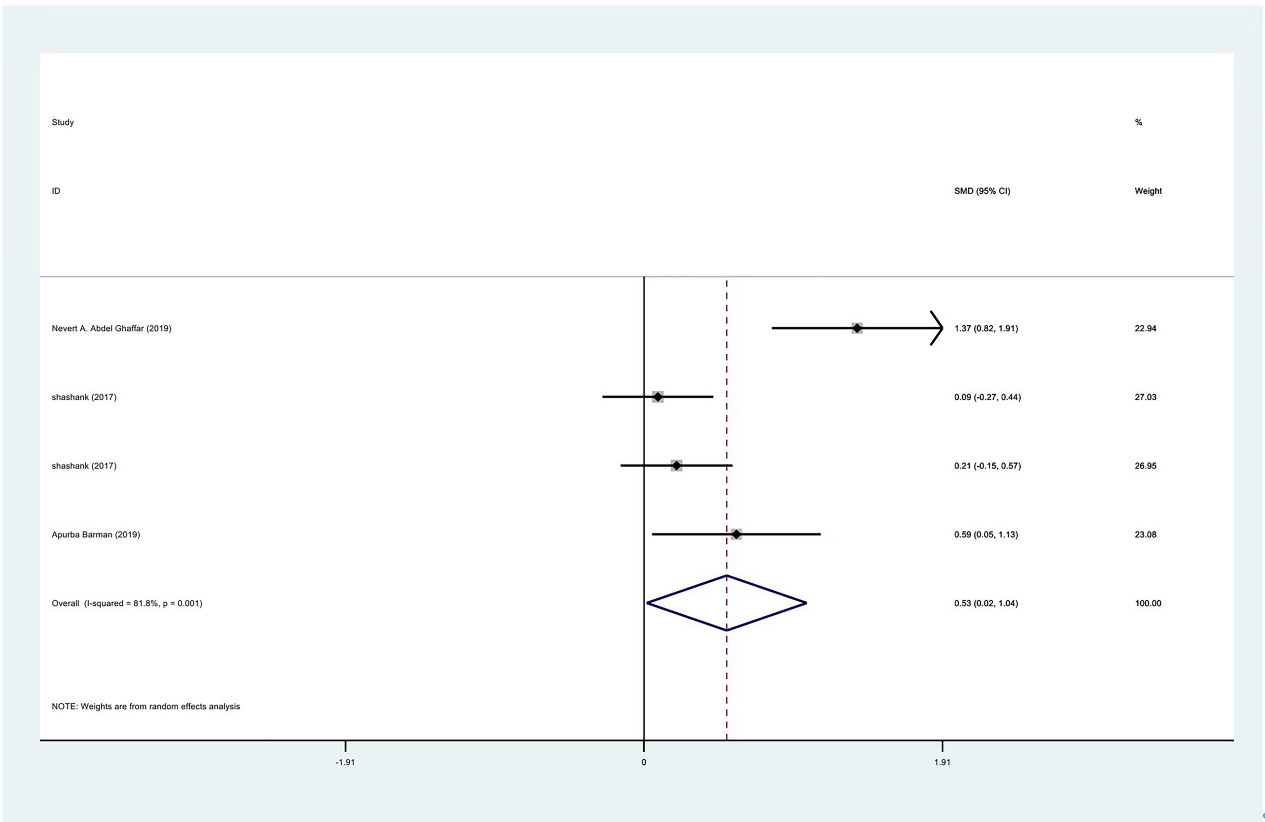
Figure 10.
Meta-analysis results of external rotation between PRP group and control group at 1 month.
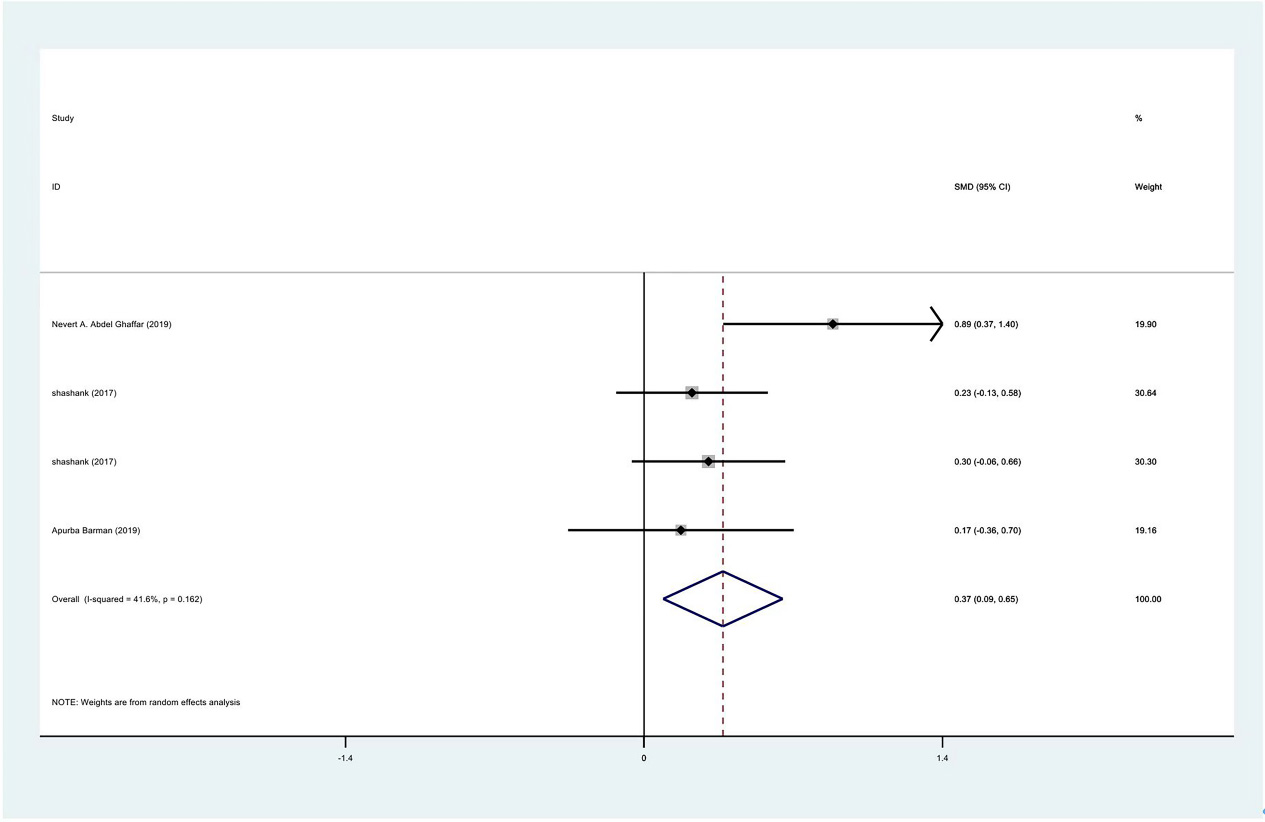
Figure 11.
Meta-analysis results of abduction between PRP group and control group at 1 month.
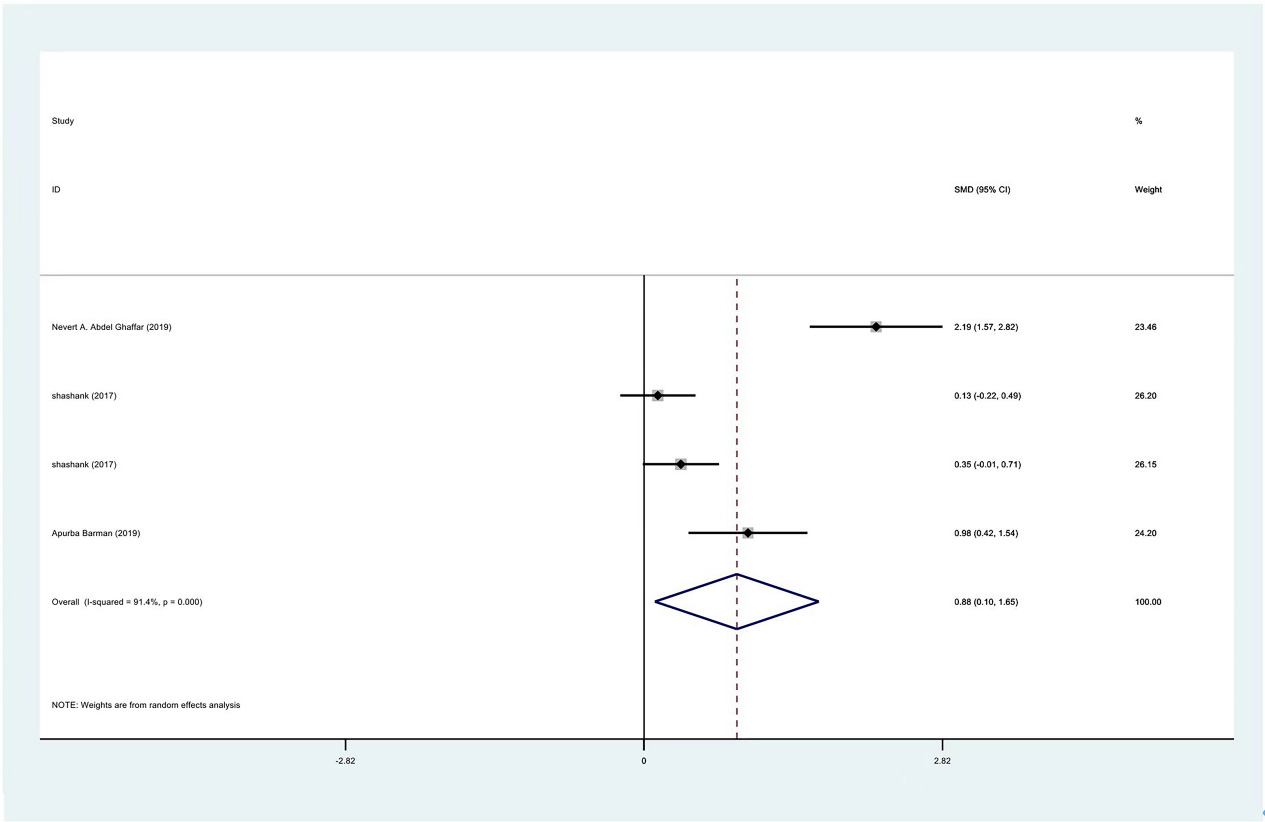
Figure 12.
Meta-analysis results of flexion between PRP group and control group at 1 month.
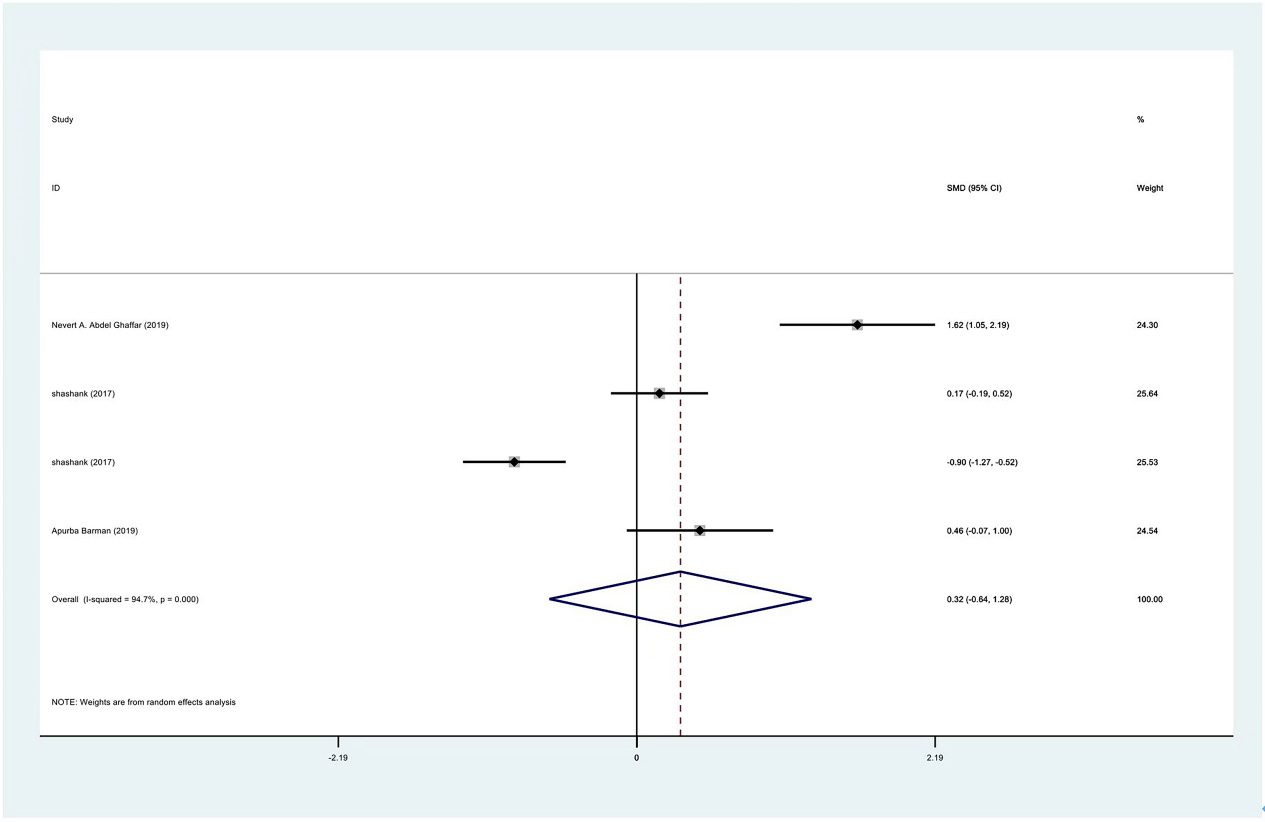
Figure 13.
Meta-analysis result of internal rotation between PRP group and control group at 3 months.
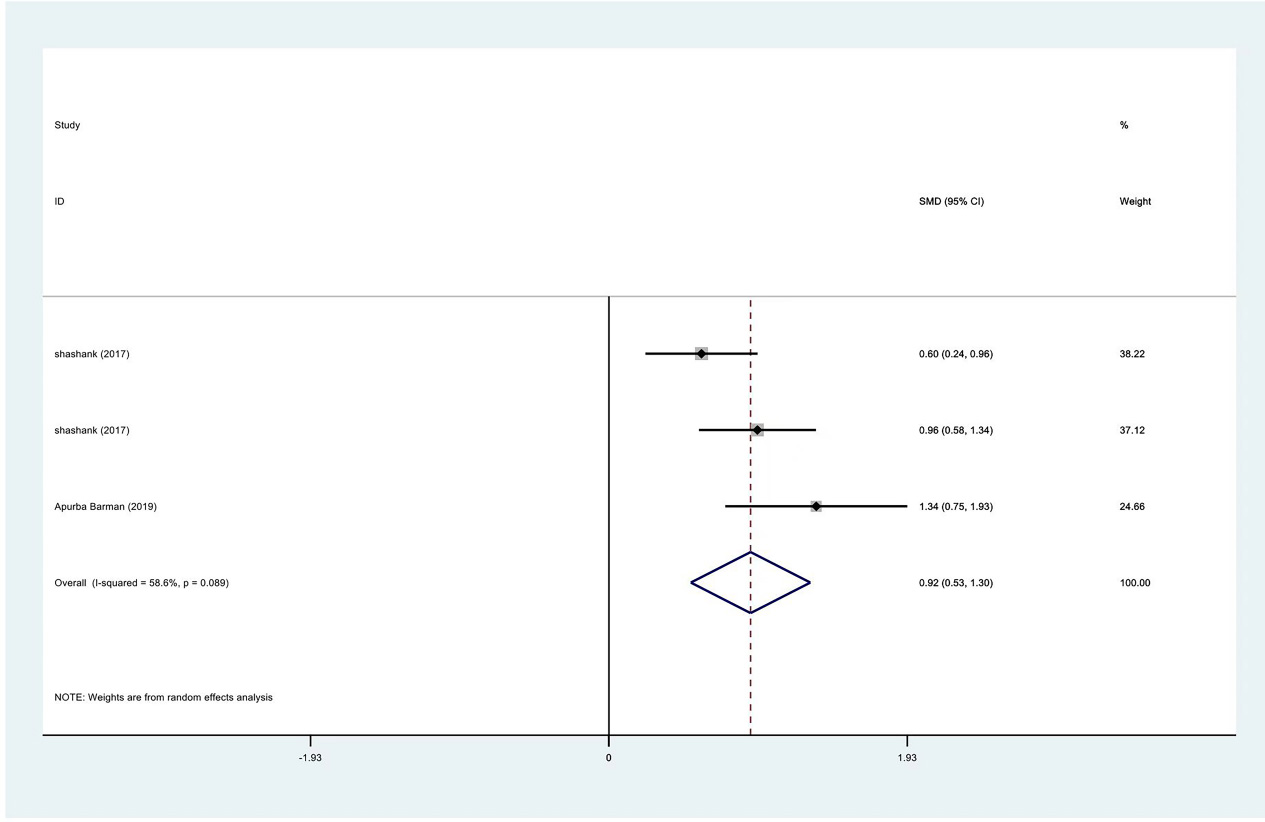
Figure 14.
Meta-analysis results of external rotation between PRP group and control group at 3 months.
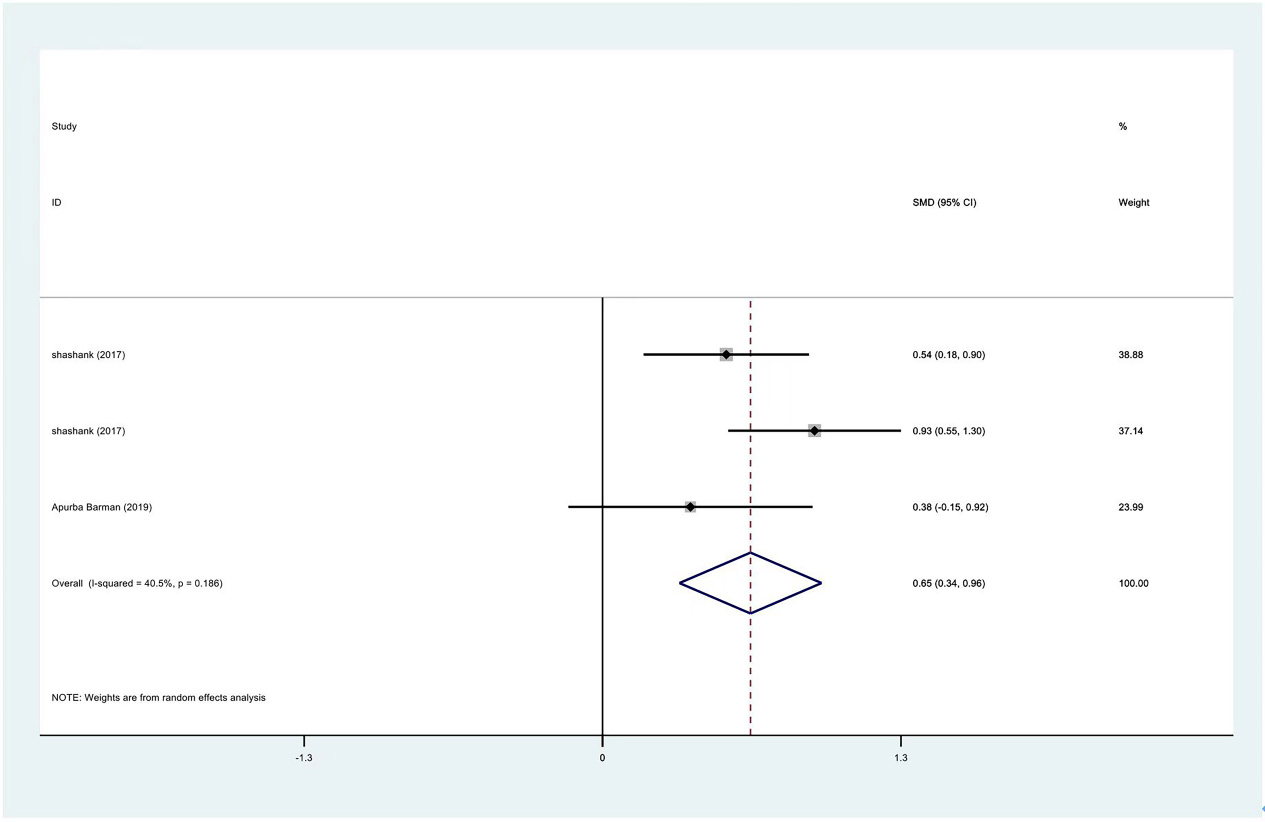
Figure 15.
Meta-analysis results of flexion between PRP group and control group at 3 months.
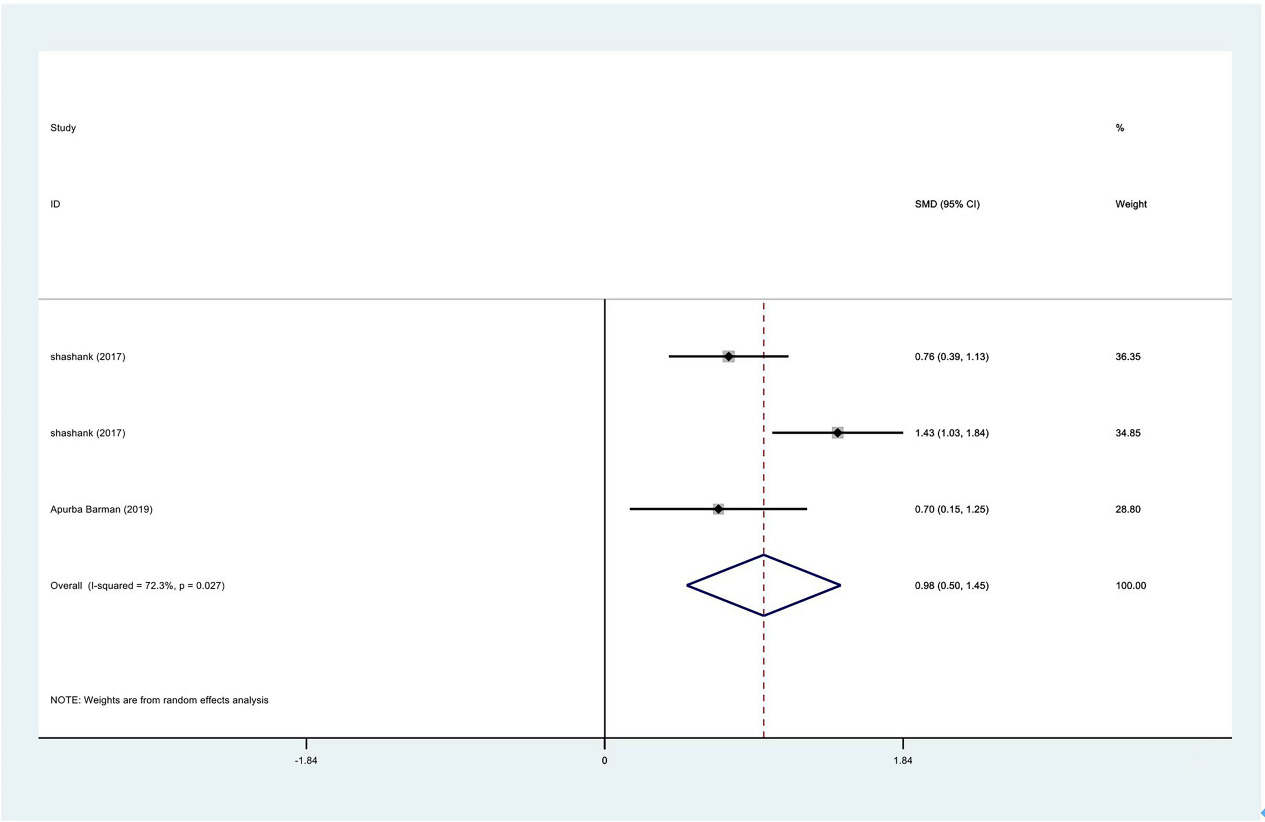
Figure 16.
Meta-analysis results of abduction between PRP group and control group at 3 months.
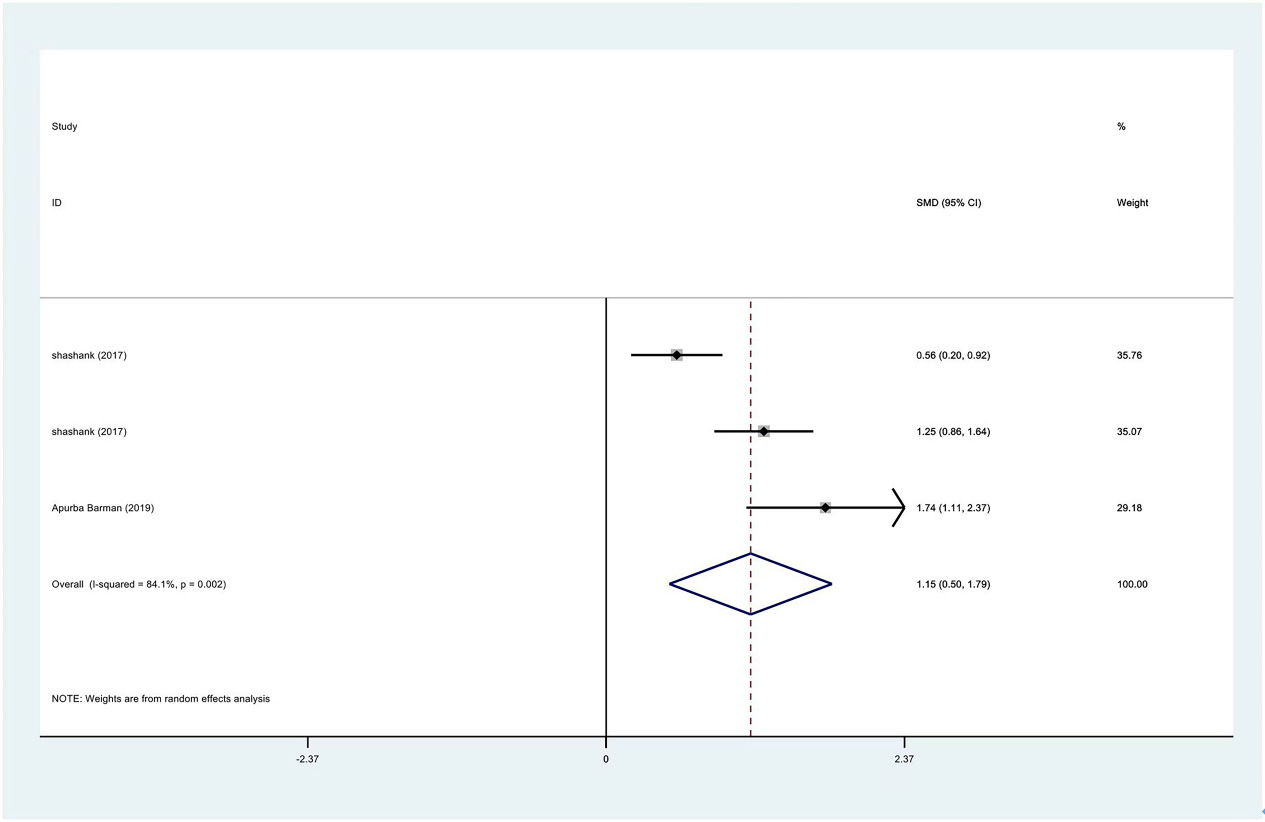
The range of motion of the shoulder joint consists of four parts: internal rotation, flexion, external rotation and abduction. The results showed that the internal rotation, external rotation and abduction were all improved in the PRP treatment group, compared to the control group at 1 month (SMD: 0.53, 95% Cl: 0.02 to 1.04,
4.Discussion
This is the first systematic review with meta-analysis performed to evaluate the efficacy of nonoperative PRP injections for FS. The systematic review suggested that patients who received the treatment for FS with PRP injections could be expected to have improved clinical outcomes at short-term follow-up as compared with patients who received other treatments. Of all clinical outcomes assessed in this systematic review, PRP injection was more effective than other treatments with regards to pain relief and functional improvement in FS patients and had fewer side effects. However, in this systematic review, limited high-quality studies regarding the use of non-operative PRP in FS were identified. There were only three randomized controlled trials and one cohort study. All studies had relatively limited sample sizes between 60 to 120 participants. All qualifying trials had different PRP injection protocols including four trials that utilized multiple serial PRP injections with varying intervals. Accordingly, although all included studies have reported that PRP has a significant therapeutic effect on patients with FS compared with other treatments, considerable high-quality clinical studies are still needed in the future.
Table 2
Results of the meta-analysis
| Follow-up | Evaluation tools | Studies | Patients (PRP/other treatments) | SMD | 95% CI | I2 | |
|---|---|---|---|---|---|---|---|
| 1 month | VAS | 3 | 120/175 | Yes | 43.20% | ||
| Internal rotation | 3 | 122/177 | 0.53 | 0.02 to 1.04 | Yes | 81.80% | |
| Flexion | 3 | 122/177 | 0.32 | No | 94.70% | ||
| External rotation | 3 | 122/177 | 0.37 | 0.009 to 0.65 | Yes | 41.60% | |
| Abduction | 3 | 122/177 | 0.88 | 0.10 to 1.65 | yes | 91.40% | |
| 3 months | VAS | 3 | 120/175 | Yes | 61.90% | ||
| Internal rotation | 3 | 122/177 | 0.92 | 0.53 to 1.30 | Yes | 58.60% | |
| Flexion | 3 | 122/177 | 0.98 | Yes | 72.30% | ||
| External rotation | 3 | 122/177 | 0.65 | 0.34 to 0.96 | Yes | 40.50% | |
| Abduction | 3 | 122/177 | 1.15 | 0.50 to 1.79 | Yes | 84.10% |
The better efficacy of PRP in the treatment of FS may be related to the pathogenesis of FS. Although the pathogenesis of FS has not been fully elucidated, it is recognized that it is chronic aseptic inflammation. Various studies have shown that inflammatory cytokines such as tumor necrosis factor-
Recently, PRP has been widely studied and developed in the field of basic disciplines and has been proven to repair chronic muscle injury by basic experiments and clinical studies around the world. Hammond et al. [27] showed that in repeated small stress stretch, muscle activity was significantly improved in a muscle injury model, and the recovery time was shortened after treatment with PRP. Bubnov et al. [28] found that during the one-month follow-up, compared to conventional conservative treatment, ultrasound-guided PRP injection plus conservative treatment was found to be more effective in relieving pain in athletes with acute muscle injury, and the scores of muscle strength and subjective function were observed to be significantly improved. Christos et al. [34] found that during the 6-month follow-up, compared to whole blood injection, PRP injection significantly relieved pain in tennis elbow. Another 2-year follow-up study demonstrated that PRP injection improved upper limb function and relieved pain in patients with chronic lateral epicondylitis compared to corticosteroid injection. These results were consistent with the effect of PRP injection in the treatment of patients with FS, indicating that PRP has advantages in the treatment of chronic injury.
The largest advantage of this study is its novelty. Currently, no meta-analysis exists that studies the efficacy of PRP injection in the treatment of FS. However, this study has various limitations. First, the sample sizes were small, the heterogeneity of some studies was high, which may lead to overestimation of the therapeutic effect. However, since there were fewer included studies, it is impossible to carry out a subgroup analysis. In order to obtain more accurate results, more randomized controlled trials should be conducted with larger sample sizes. Second, the lack of the different protocols used in PRP preparation and administration. Finally, included studies contained methodological defects, which may affect the persuasive power.
5.Conclusions
The duration of this study was limited to 3 months, hence, the long-term effect is not clear. Overall, available evidence suggests that for patients with FS, PRP can effectively relieve pain and improve ROM in patients with FS in the short term (3 months), compared to other non-operative treatments. However, this form of therapy is limited by quantity, quality and follow-up time of the included studies. Therefore, the above conclusions must be confirmed by conducting additional high-quality studies.
Ethical approval
Not applicable.
Funding
None to report.
Informed consent
Not applicable.
Author contributions
RH and S-ZY designed and performed the experiments, collected and analyzed data, and wrote the manuscript. H-MF and HD provided valuable advice on the design of this study. DH and LY provided valuable advice on research and guided the completion of this experiment. All authors read and approved the final manuscript.
Acknowledgments
The authors thank all participants in the study.
Conflict of interest
The authors have no conflicts of interest to declare.
References
[1] | Ewald A. Adhesive capsulitis: A review. Am Fam Physician. (2011) ; 83: : 417–422. |
[2] | Pal B, Anderson J, Dick WC, Griffiths ID. Limitation of joint mobility and shoulder capsulitis in insulin and non-insulin dependent diabetes mellitus. Br J Rheumatol. (1986) ; 25: (2): 147–51. |
[3] | Neviaser AS, Neviaser RJ. Adhesive capsulitis of the shoulder. J Am Acad Orthop Surg. 274 (2011) ; 19: (9): 536–42. |
[4] | Hand GCR, Athanasou NA, Matthews T, Carr AJ. The pathology of frozen shoulder. J Bone Joint Surg Br. (2007) ; 89: (7): 928–32. |
[5] | Brue S, Valentin A, Forssblad M, Werner S, Mikkelsen C, Cerulli G. Idiopathic adhesive Capsulitis of the shoulder: A review. Knee Surg Sports Traumatol Arthrosc. (2007) ; 15: : 1048–54. |
[6] | Holloway GB, Schenk T, Williams GR, Ramsey ML, Iannotti JP. Arthroscopic capsular release for the treatment of refractory postoperative or post-fracture shoulder stiffness. J Bone Joint Surg Am. (2001) ; 83-A: : 1682–7. |
[7] | Kivimki J, Pohjolainen T. Manipulation under anesthesia for frozen shoulder with and without steroid injection. Arch Phys Med Rehabil. (2001) ; 82: : 1188–90. |
[8] | Nicholson GP. Arthroscopic capsular release for stiff shoulders: Effect of etiology on outcomes. Arthroscopy. (2003) ; 19: : 40–9. |
[9] | Pajareya K, Chadchavalpanichaya N, Painmanakit S, Kaidwan C, Puttaruksa P, Wongsaranuchit Y. Effectiveness of physical therapy for patients with adhesive capsulitis: A randomized controlled trial. J Med Assoc Thai. (2004) ; 87: : 473–80. |
[10] | Waterbrook AL, Balcik BJ, Goshinska AJ. Blood Glucose Levels After Local Musculoskeletal Steroid Injections in Patients With Diabetes Mellitus: A Clinical Review. Sports Health. (2017) : 372–374. |
[11] | Marx RE. Platelet-rich plasma: Evidence to support its use. J Oral Maxillofac Surg. (2004) ; 62: (4): 489–496. |
[12] | Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. (2004) ; 91: (1): 4–15. |
[13] | Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA. Platelet-rich plasma: From basic science to clinical applications. Am J Sports Med. (2009) ; 37: (11): 2259–2272. |
[14] | Senet P, Bon FX, Benbunan M, et al. Randomized trial and local biological effect of autologous platelets used as adjuvant therapy for chronic venous leg ulcers. J Vasc Surg. (2003) ; 38: (6): 1342–1348. |
[15] | Kon E, Filardo G, Di Martino A, Marcacci M. Platelet-rich plasma (PRP) to treat sports injuries: Evidence to support its use. Knee Surg Sports Traumatol Arthrosc. (2011) ; 19: (4): 516–527. |
[16] | Hall MP, Band PA, Meislin RJ, et al. Platelet-rich plasma: Current concepts and application in sports medicine. Journal Am Acad Orthop Surg. (2009) ; 17: (10): 602–608. |
[17] | Lin J. Platelet-rich plasma injection in the treatment of frozen shoulder: A randomized controlled trial with 6-month follow-up. Int J Clin Pharmacol Ther. (2018) ; 56: (8): 366–371. |
[18] | Kothari SY, Srikumar V, Singh N. Comparative efficacy of platelet rich plasma injection, corticosteroid injection and ultrasonic therapy in the treatment of periarthritis shoulder. J Clin Diagn Res. (2017) ; 11: (5): RC15–RC18. |
[19] | Ghaffar NAA, Ghanem MA. Combined platelet rich plasma intra-articular shoulder injection and stellate ganglion block. A new technique for management of chronic Postmastectomy shoulder pain syndrome. Srilankan Journal of Anaesthesiology. (2019) ; 27: (2): 133–138. |
[20] | Barman A, Mukherjee S, Sahoo J, et al. Single intra-articular platelet-rich plasma versus corticosteroid injections in the treatment of adhesive capsulitis of the shoulder: A cohort study. Am J Phys Med Rehabil. (2019) ; 98: (7): 549–557. |
[21] | Neviaser AS, Hannafin JA. Adhesive capsulitis: A review of current treatment. The American Journal of Sports Medicine. (2010) ; 38: (11): 2346–2356. |
[22] | Laver L, Marom N, Dnyanesh L, Mei-Dan O, Espregueira-Mendes J, Gobbi A. PRP for degenerative cartilage disease: A systematic re-view of clinical studies. Cartilage. (2017) ; 8: (4): 341. |
[23] | Lee HR, Park KM, Joung YK, Park KD, Do SH. Platelet-rich plas-ma loaded hydrogel scaffold enhances chondrogenic differentiation and maturation with upregulation of CB1 and CB2. J Control Release. (2012) ; 159: (3): 332–7. |
[24] | Eviaser JS. Adhesive capsulitis of theshoulder. J Bone Joint Surg Am. (1945) ; 27: : 211–222. |
[25] | Rodeo SA, Hannafin JA, Tom J, Warren RF, Wickiewicz TL. Immunolocalization of cytokines and their receptors in adhesive capsulitis of the shoulder. J Orthop Res. (1997) ; 15: : 427–36. |
[26] | Havva TÇ, Çaǧlar K, Emel G. Effects of platelet-rich injection on adhesive capsulitis: An interventional case series. Erciyes Med J. (2019) ; 41: (1): 102–104. |
[27] | Hammond JW, Hinton RY, Curl LA, et al. Use of autologous platelet – rich plasma to treat muscle strain injuries. Am J Sports Med. (2009) ; 37: (6): 1135–1142. |
[28] | Bubnov R, Yevseenko V, Semeniv I. Ultrasound guided injections of Platelets rich Plasma for muscle injury in professional athletes Comparative study. Med Ultrason. (2013) ; 15: (2): 101–105. |
[29] | Alok A, Buddhadeb N, Harshal S. Management of adhesive capsulitis of shoulder joint by single platelet rich plasma injection. Journal of Orthopedics, Traumatology and Rehabilitation. (2019) ; 11: (1): 62–65. |
[30] | Monto RR. Platelet rich plasma treatment for chronic Achilles tendinosis. Foot Ankle Int. (2012) ; 33: : 379–385. |
[31] | Gosens T, Den Oudsten BL, Fievez E, van ’t Spi-jker P, Fievez A. Pain and activity levels before and after platelet-rich plasma injection treatment of patellar tendinopathy: A prospective cohort study and the influence of previous treatments. Int Orthop. (2012) ; 36: : 1941–1946. |
[32] | Umatheepan B, Ravi D, Lucas A. Efficacy of platelet-rich plasma injections in pain associated with chronic tendinopathy: A systematic review. Phys Sportsmed. (2015) ; 43: (3): 253–261. |
[33] | Bölük ŞH, İkbali AS, Özen S. Platelet-rich plasma injection in a patient with adhesive capsulitis due to chronic kidney disease. Agri. (2020) ; 32: (2): 113–114. |
[34] | Thanasas C, Papadimitriou G, Charalambidis C, et al. Platelet-rich plasma versus autologous whole blood for the treatment of chronic lateral elbow epicondylitis: A randomized controlled clinical trial. American Journal of Sports Medicine. (2011) ; 39: (10): 2130–4. |
[35] | Gosens T, Peerbooms JC, van Laar W, et al. Ongoing positive effect of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: A double-blind randomized controlled trial with 2-year follow-up. Am J Sports Med. (2011) ; 39: (6): 1200–8. |
[36] | Jongh T, Rommers GM, Dekker R, et al. Examination of the shoulder. Nederlands Tijdschrift Voor Geneeskunde. (2011) ; 155: (12): A2659. |
[37] | Feusi O, Karol A, Fleischmann T, et al. Platelet-rich plasma as a potential prophylactic measure against frozen shoulder in an in vivo shoulder contracture model. Archives of Orthopaedic and Trauma Surgery. (2020) ; (12). |
[38] | Chen X, Jones IA, Togashi R, et al. Use of platelet-rich plasma for the improvement of pain and function in rotator cuff tears: A systematic review and meta-analysis with bias assessment. The American Journal of Sports Medicine. (2019) ; (8): 036354651988142. |
[39] | Bennell KL, Paterson KL, Metcalf BR, Duong V, Eyles J, Kasza J, et al. Effect of intra-articular platelet-rich plasma vs placebo injection on pain and medial tibial cartilage volume in patients with knee osteoarthritis: The RESTORE randomized clinical trial. JAMA. (2021) Nov 23; 326: (20): 2021–2030. |
[40] | Keene DJ, Alsousou J, Harrison P, Hulley P, Wagland S, Parsons SR, et al. Platelet rich plasma injection for acute Achilles tendon rupture: PATH-2 randomised, placebo controlled, superiority trial. BMJ. (2019) Nov 20; 367: : l6132. |
[41] | Min JL, Kang SY, Oh S, et al. Allogenic pure platelet-rich plasma therapy for adhesive capsulitis: A bed-to-bench study with propensity score matching using a corticosteroid control group. The American Journal of Sports Medicine. (2021) ; 49: (1): 036354652110186. |




