Effects of hyaluronic acid injections on pain and functioning in patients affected by tendinopathies: A narrative review
Abstract
BACKGROUND:
Tendinopathies are overuse tendon injuries showing load-dependant pain, stiffness, weakness of movement in the affected area, and impairment in the movements. The scientific interest on the role of Hyaluronic Acid (HA) for the management of tendinopathies has been increased due to its anti-inflammatory and lubricative properties.
OBJECTIVE:
To collect evidence regarding the effectiveness and safety of HA injections in reducing pain in patients affected by tendinopathies.
METHODS:
A scientific literature search was conducted using the PubMed, Medline and PEDro electronic databases. The databases were searched since their inception until July 2021. The search was limited to English language articles. Different combinations of the terms and MeSH terms “tendinopathy”, “tendinosis”, “tendinitis”, “hyaluronic acid”, “hyaluronate”, “infiltration”, “hyaluronic injections”, “viscosupplementation” connected with various boolean operators were used for other electronic databases.
RESULTS:
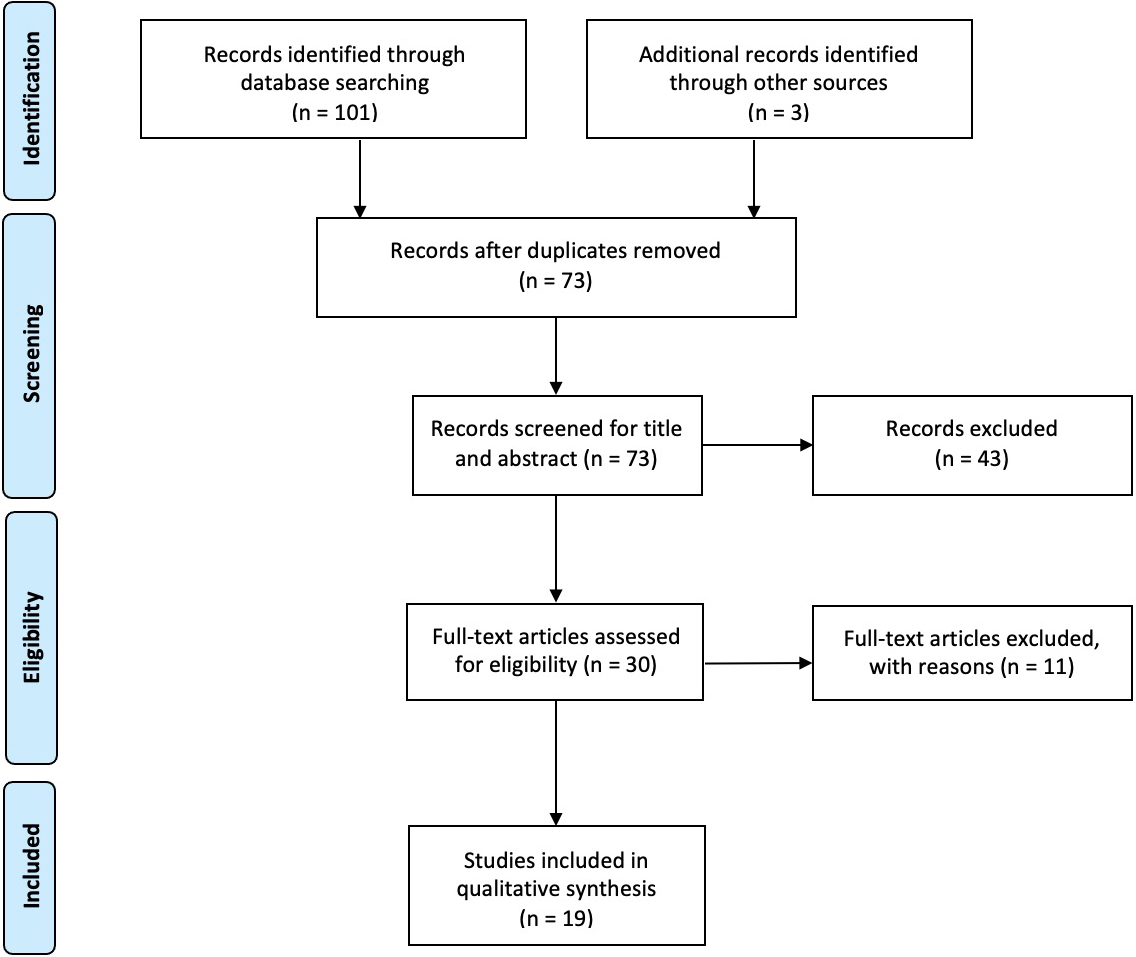
One hundred and one records were identified from the selected databases plus three additional papers identified by the authors through other sources. After removing duplicated papers and title/abstract screening, 19 studies were included in our review (eight papers on shoulder, three on elbow, four on hand, one on knee, and three on ankle).
CONCLUSION:
The results showed that none of the studies report severe adverse effects and most of them support the use of HA injections in tendinopathies, with a special attention to pain reduction and functional assessment. Further studies are warranted to better investigate effects and methods of administration of HA in tendinopathies.
1.Introduction
Tendinopathies are tendon injuries due to overuse showing load-dependant pain, stiffness, weakness of movement in the affected area, and impairment in the movements [1]. There is a dissociation between pain and pathology in tendinopathy: tendons might appear normal at imaging but painful and, conversely, tendon degeneration can often be pain-free [1]. As suggested by Maffulli et al. [2], the term tendinopathy can be used as a general descriptor of the clinical conditions arising from tendon overuse, independently from histopathological damage. The term “tendinopathy” should include different pathologies such as tendinitis, tendinosis, and para-tendinitis (which includes peri-tendinitis, tenosynovitis and tenovaginitis) [2, 3]. Focusing on different changes that may occur in tendon structure in tendinopathies, it could be distinct: tendinitis, inflammation and pathological changes of tendons; tendinosis, non-inflammatory tendon degeneration; peri-tendinitis, inflammation due to friction against a surface during tendon movement; tenosynovitis and tenovaginitis, single or double-layer inflammation of tendon sheath [2, 3]. Therefore, considering all these different definitions, Maffulli et al. [2] suggested to use the term “tendinopathy” as a general descriptor of all these clinical conditions.
Treatment for tendinopathies should be individualized on physical activity and main characteristics (training level, type of pain, timing of symptoms’ arousal) of the subjects involved. Early rehabilitation, physical agent modalities (laser therapy, ultrasound therapy, extracorporeal shock waves), non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids, oxygen-ozone therapy, and hyaluronic acid (HA) injections are considered the main conservative treatments for tendinopathies [4, 5, 6, 7].
In this context, the scientific interest on the role of HA for the management of tendinopathies has been increased due to its anti-inflammatory and lubricative properties [8, 9]. HA is a polysaccharide present in extra cellular matrix of many tissues and several chronic pathologies can modify HA concentration in synovial fluid [10, 11, 12]. Balasz and Derlinger [13] firstly introduced HA injections as therapy to reduce symptoms, to improve biomechanical function and to promote healing in intra-articular injuries without relevant secondary effects [13, 14, 15].
Although rapidly cleared from injected site, the role of HA depends on multiple interactions with microenvironment: not only mechanical effects, but also interaction with cell transduction and homeostasis regulation mechanisms. Several studies [15, 16, 17] have shown the interactions between HA and CD44, a membrane receptor capable of stimulating endogenous HA production (function known as viscoinduction) and probably involved in tissue repair and healing mechanisms, by counteracting also potential harmful mechanisms, such as inflammatory cells chemotaxis, prostaglandin E2 (PGE2) expression and free radicals’ production. Further mechanisms contributing to anti-nociceptive effect are the inhibition of arachidonic acid release from fibroblasts and the activation of opioid receptors [18].
However, to date, there is still no agreement in the scientific literature on the effects of HA in treating tendinopathies due to a lack of evidence. Injective therapy is not new in this pathology and has often been performed with corticosteroids: these are not free from complications [19, 20] and its efficacy seems to be limited on the long-term period. HA has been considered as a new alternative approach due to the properties previously described, but no specific guidelines on the argument have been developed. Thus, the present narrative review [21] explores the studies concerning the use of HA injections in tendinopathies, with a particular attention to the safety and the reported effectiveness, collecting evidence of pain reduction and focusing on what needs to be improved.
2.Methods
2.1Search strategy
A scientific literature search was conducted using the PubMed, Medline and PEDro electronic databases. The databases were searched since their inception until July 2021. The search was limited to English language articles. Different combinations of the terms and MeSH terms “tendinopathy”, “tendinosis”, “tendinitis”, “hyaluronic acid”, “hyaluronate”, “infiltration”, “hyaluronic injections”, “viscosupplementation” connected with various boolean operators were used for other electronic databases. The analysis was performed independently and synchronously by two researchers in Physical and Rehabilitation Medicine (PRM). PRISMA guidelines were followed to write this review [22].
Figure 1.
Study flowchart.
2.2Selection criteria
Two authors screened titles and abstracts of all papers identified after duplicates removal for inclusion. Then, if a consensus was not achieved, the disagreement was resolved by consulting a further reviewer.
Papers consistent with the following parameters were considered eligible according to the following PICO model:
1. P) Participants: subjects affected by tendinopa- thies;
2. I) Intervention: treatment with HA in single or multiple treatment as main intervention;
3. C) Comparator: none
4. O) Outcomes: pain and disability.
This narrative review aimed to include papers with the following study designs: randomised controlled trials (RCTs), case-control studies, and cohort studies. On the other hand, letter to authors, editorials, technical notes, case reports, case series, case reports, in vitro studies, animal studies and narrative reviews, systematic reviews and meta-analysis were excluded. Papers involving animals, in a language other than English, and without a PRM topic were excluded.
2.3Data extraction and synthesis
Two researchers in PRM independently assessed and extracted data from full-text documents into Excel. Any disagreement between the two reviewers was solved by collegial discussion among them. The following data were extracted: 1) title; 2) authors; 3) publication year; 4) design; 5) characteristics of study participants (age, sex, type of tendinopathy, previous interventions, time from symptoms’ onset); 6) intervention; 7) outcomes; 8) timing of outcomes.
3.Results
Altogether, 101 records were identified from the selected databases plus three additional papers identified by the authors through other sources. After removing duplicated papers, 73 studies were considered eligible and screened for title and abstract. Forty-three studies were excluded by title/abstract screening and 11 by full-text screening. Finally, 19 studies were included in this review for the qualitative synthesis; out of 19 articles, there were different tendinopathies studies involved: eight papers on shoulder, three on elbow, four on hand, one on knee, and three on ankle.
3.1Shoulder tendinopathies
Kim et al. [23] compared the effectiveness of HA and corticosteroid injection in patients affected by subacromial impingement. Outcomes on study were Visual Analogue Scale (VAS), Range of Motion (ROM), American Shoulder and Elbow Surgeons standardized assessment form (ASES) questionnaire and number of patients who required rescue medication. They evaluated these outcomes at baseline, three, six and 12 weeks. They found that subacromial HA injection improves functional outcomes and can reduce pain in a similar or better way than corticosteroids, at least in the short term.
Penning et al. [24] compared the effectiveness of HA
Table 1
Main characteristics of the studies included
| Study | Disease characteristics | Intervention | Outcomes measures | Timing of outcomes | Adverse events |
|---|---|---|---|---|---|
| Kim et al. [23] | Patients with subacromial impingement without rotator cuff tear (previously evaluated also at US or MRI), with pain for at least 3 months without improvement despite other conservative therapies | US guided subacromial injection of hyaluronate (hyruan plus: 300,000,000 dalton average molecular weight, sodium hyaluronate 20 mg, 2 ml) for 3 weeks compared with a 1-time corticosteroid injection (dexamethasone disodium phosphate; 5 mg, 1 ml) | VAS, ROM, American Shoulder and Elbow Surgeons standardized assessment form (ASES) and number of patients who required rescue medication | 3, 6 and 12 weeks. For 21 patients was added a 24-week ASES evaluation | Induration, musculoskeletal pain, nasopharyngitis, and cough |
| Penning et al. [24] | Patient with clinical diagnose of subacromial impingement, pain for more than 6 weeks | Three treatment groups with subacromial injections. All received 8 ml lidocaine 1%. Lidocaine was followed by 2 ml of HA in the first group; 2 ml triamcinolone acetonide 10 mg/ml in the second; 2 ml NaCl 0.9% (placebo) in the third | VAS, painful arc, ROM, Constant shoulder score, patient-specific disability, shoulder disability questionnaire, shoulder pain score, functional mobility test | Baseline, 3, 6, 12, 26 weeks | Mild adverse reactions: increase of pain after the injection, headaches and a haematoma at the site of injection |
| Özgen et al. [25] | Patients clinically and MRI diagnosed for supraspinatus tendinitis | Intra-articular 2 ml (16 mg) of Sodium Hyaluronate G–F 20 (6 | VAS scale, ROM, functional assessment questionnaire | Baseline, 3 weeks, 3 months, 4 years | None |
| Merolla et al. [26] | Rotator Cuff (RC) tendinopathy, with clinical and MRI diagnosis, pain for at least 4 months | SportVis preparation, containing STABHA (Soft Tissue Adapted Biocompatible Hyaluronic Acid) of 1 million of daltons in a pre-filled syringe (12 mg/1.2 ml) Versus Physiotherapy 30 days, 3 times a week | Constant-Murley Score (CS), Visual analog pain Scale (VAS), Oxford Shoulder Score (OSS), Patient Global assessment (PGA) | Baseline, 2, 4, 12, 24 weeks | None |
| Frizziero et al. [27] | Painful non-calcific rotator cuff tendinopathy: Clinical and Instrumental (US or MRI) diagnosis and pain for at least 3 months not responding adequately to conventional therapy with NSAIDs and/or physiotherapy | Two groups: one treated with sub-acromial injections of LMW-HA (500–730 kDa, HYALGAN | Disability of Arm, Shoulder and Hand (DASH) questionnaire and Constant-Murley Score | Baseline, end of first treatment, 3 months follow up | None |
| Meloni et al. [28] | Patients with clinical, US and MRI diagnosis of supraspinatus tendinosis | Intralesional US guided injection of sodium hyaluronate (2 ml, P.M. 500–700.000, 20 mg/2 ml) together with 2 ml of 1% lidocaine and 2 ml of 0.9% sodium chloride solution, versus 4 ml of 0.9% sodium chloride solution US guided injection, together with 2 ml of 1% lidocaine. Both groups underwent one injection per week for 4 weeks | ROM, VAS and degree of discomfort with a 10-level scale, echografic aspect | Baseline, 3, 6, 12 months for US control, Variable for clinical evaluation | None |
| Moghtaderi et al. [29] | Patients with clinical and US diagnosis of rotator cuff disease with pain from at least 6 months | Two groups of treatment, both with 3 weekly intralesional injection: the first with 20 mg/ml of sodium hyaluronate, the second with 2 mL of 0.9% saline solution | VAS, Constant score | 12 week follow up for Constant score. VAS was valued 1 week after each treatment | None |
| Mohebbi et al. [30] | Patients with rotator cuff tears diagnosed at physical exam and imaging with pain for at least 6 weeks and not more than 36 months | Intralesional single injection of 20 mg (2 mL) High Molecular Wighted-HA (HMW-HA) 1% ( | VAS, ROM, DASH questionnaire, World Health Organization Quality of Life-Bref (WHOQOL-Bref) questionnaire | Baseline, 1, 4 weeks, 3 months. Another measure at 6 months for pain | None |
|
Table 1, continued | |||||
|---|---|---|---|---|---|
| Study | Disease characteristics | Intervention | Outcomes measures | Timing of outcomes | Adverse events |
| Bernetti et al. [31] | Humeral epicondylitis | Corticosteroid injections versus single corticosteroid injection followed by three LMW-HA injections | |||
| Tosun et al. [32] | Lateral epicondylitis with pain of at least 3 months, local tenderness and positivity to clinical provocative tests | Single injection of a 1.6 mL total dose mixture comprising of 1 mL of triamcinolone acetonide (40 mg/mL) and 0.6 mL of prilocaine HCl Versus Single injection of a 1.6 mL total dose mixture, comprised of 1 mL of an HA | Patient-Rated Tennis Elbow Evaluation (PRTEE) questionnaire and Minimum Clinically Important Difference (MCID) | Baseline, 3, 6 months | Early-onset pain (immediately or in a few days after the injection) solved with ice and acetaminophen |
| Apaydin et al. [33] | Patients with lateral epicondyalgia for at least 6 months | Single dose of 30 mg/2 mL 1500 kDa high-molecular-weight of HA versus Three doses of a dextrose solution at weeks 0, 3 and 6 | VAS, Q-DASH, grip strenght | Baseline, 6, 12 weeks | Early-onset pain (immediately or in a few days after the injection) solved with ice and acetaminophen |
| Orlandi et al. [34] | De Quervain’s Disease diagnosed clinically | Three groups of treatment with different US-guided injections into the first dorsal compartment of the wrist: the first with steroid injection (1 ml methylprednisolone acetate, 40 mg/mL); the second with steroid injection (1 ml methylprednisolone acetate) and a 15-day delayed 2-ml saline 0.9 % injection; the third with steroid injection (1 ml methylprednisolone acetate) and a 15-day delayed low-weight HA injection (0.8 %, 16 mg/2 ml) | VAS scale, quick-DASH questionnaire and retinaculum thickness US evaluation | Baseline, 1 month for VAS and quick-DASH 3 months for VAS, quick-DASH, US retinaculum evaluation | None |
| Liu et al. [35] | Trigger finger diagnosed clinically of at least grade 1 on the Quinnell grading scale | US-guided injections at the mouth of the pulley, into the sheets of the flexor tendon. Two different drugs: 1 cc of 10-mg/mL triamcinolone acetonide and 1 cc of HA | VAS scale, and function valued at Michigan Hand Outcome Questionnaire (MHQ), total activity motion (TAM) scale, and strength measured by a dynamometer | Baseline, 3 weeks, and 3 months | None |
| Callegari et al. [36] | Patients with clinical signs and US-confirmed diagnose of stenosing tenosynovitis of the flexor tendons | Distally to A1 pulley, into the sheath of the flexor tendons, a 40 mg/1mL methylprednisolone acetate with 0.8 mL lidoacine chlorhydrate 2% injection, plus a 10-day delayed 1 mL 0.8% HA injection with same technique versus Open surgery conventional technique | Quinnell-Green classification and 3 different scales to assess disability, pain, and satisfaction | 6 weeks, 3, 6 and 12 months | Early-onset pain (immediately or in a few days after the injection) solved with ice and acetaminophen |
| Kanchanathepsak et al. [37] | Patients with trigger finger and Quinnell grade I, II or III15) and onset of the symptoms less than 6 month | Two treatment groups, both injected with 0.5% lidocaine over the A1 pulley of the affected digit and then: in the first group 1 mL of LMWHA; in the second group 1 mL of 10 mg/ml of triamcinolone acetate, both beneath A1 pulley | Quinell grading, VAS score of pain DASH score and complications | 1, 3, 6 months, plus one phone call after 1 week only for early adverse reaction | Early-onset pain (immediately or in a few days after the injection) solved with ice and acetaminophen |
|
Table 1, continued | |||||
|---|---|---|---|---|---|
| Study | Disease characteristics | Intervention | Outcomes measures | Timing of outcomes | Adverse events |
| Muneta et al. [38] | Professional and semiprofessional athlete patients with patellar tendinopathy, who suffered pain for minimum 2 months and were graded as stage 2 or 3 by Blazina’s classification | 25 mg of hyaluronan in 2.5 mL (superpurified hyaluronate) were used for injections. Four patients were injected bilaterally, and 9 patients (10 knees) underwent two or more therapy injections (separated by at least 3 months) | Modified Roles and Maudsley score with a 4-grade subjective satisfaction | Variable due to patients’ entrance in study | Dullness |
| Lynen et al. [39] | Patients with painful Achilles midportion tendinopathy for at least 6 weeks and a VAS pain score on Huskisson scale of at least 40/100 mm | Two peritendinous HA injections (40 mg/2 ml | VAS scale, a 5-point scale (VISA-A) and a scale for patients’ and examiners’ impression of beneficial effects | Baseline, 4 weeks, 3 and 6 months | Transient moderate tendon pain in one patient |
| Gorelick et al. [40] | Patients clinically diagnosed for Achilles’ tendinopathy with a reliable sonography examination | Three different interventions: one group with rest, splint, NSAIDs, and physiotherapy; the second with corticosteroid injection (betamethasone dipropionate five mg. and betamethasone sodium phosphate two mg); the third with single sodium hyaluronate 2% (40 mg/2.0 ml) injection | Foot and Ankle Disability scale (FADI) and VAS pain score | Baseline, 6 and 12 months. | None |
| Kumai et al. [41] | Patients affected by enthesopathies (16 lateral epicondylitis, 15 patellar tendinopathy, 15 insertional Achilles’ tendinopathy and 16 plantar fasciitis | Single injection of 25 mg of High Molecular-weighted HA (2700 kDa) in the affected area | VAS | Baseline, 1 week | None |
Abbreviations HA
outcomes of interest were pain reduction (valued at VAS scale), and other functional outcomes, such as painful arc, ROM, Constant shoulder score, patient-specific disability, shoulder disability questionnaire, shoulder pain score, functional mobility test. They evaluated this outcome at baseline, three, six, 12, 26 weeks, and found that it was not possible to show convincing results of HA injections in subacromial impingement. Corticosteroid seemed to be effective in short-term, and all the treatments had similar long-term effects (placebo had the best results).
Özgen et al. [25] compared the effect of Sodium Hyaluronate with physical therapy (transcutaneous electrical nerve stimulation TENS, ultrasound therapy and hot pack) in patients diagnosed with supraspinatus tendinitis clinically and using Magnetic Resonance Imaging (MRI). Since previous trials have shown its positive effects, both groups added physical activity as treatment: this consisted in exercise programs of stretching and strengthening recommended to both groups. They measured the effectiveness by assessing ROM of the affected joint, pain severity with VAS scale and functional assessment with the “Society of American Shoulder and Elbow Surgeons Rating Scale” questionnaire at baseline, 3rd week, 3rd month and 4th year post-treatment. Despite the positive role played by exercise programs, since results in recovery in ROM and pain relief is similar to other studies with no exercise associated, they conclude that both therapies had similar short- and long-term effects.
Merolla et al. [26] compared the efficacy of HA subacromial injections with physiotherapy in patient with rotator cuff (RC) tendinopathy. Forty-eight (48) patients were enrolled, randomized and splitted in two groups. The primary valued outcome was reduction in VAS of pain; the secondary outcomes were comparison of Constant-Murley Score (CS), Oxford Shoulder Score (OSS) and Patient Global Assessment (PGA) between two groups. These outcomes were valued at two, four, 12 and 24 weeks. Pain assessment scores were statistically different at week four and 12, while at baseline, week two, and at week 24 there was no statistical difference. Similar differences were found at functional scores, with a CS and OSS score improvement at week four and 12 in the HA group compared to physiotherapy group.
Frizziero et al. [27] compared a three-week LWM-HA injection therapy (one injection per week) with Extracorporeal Shockwaves (ESWT) therapy in patients with painful non-calcific rotator cuff tendinopathy. Outcomes of interest were pain level and function, assessed with the Disability of Arm, Shoulder and Hand (DASH) and Constant-Murley questionnaires at baseline, at the end of first treatment and at third month follow-up. They conclude that both therapies are effective until three months follow-up.
Meloni et al. [28] compared the effectiveness of Sodium Hyaluronate (SH) with Sodium Chloride in patients with clinical, US and MRI diagnosis of supraspinatus tendinosis. They evaluated changes in ROM, VAS and degree of discomfort with a ten-level scale, plus a follow-up ultrasound evaluation. They found that SH had higher improvements of clinical symptoms and functional parameters than SC.
Moghtaderi et al. [29] compared the effectiveness of intralesional Sodium Hyaluronate (SH) with placebo (0.9% saline solution) in patients with clinical and US diagnosis of Rotator Cuff disease, who suffered of pain (not responsive to conservative treatment) from at least six months and were excluded for a complete tendon tear. At the end of the treatment, there was a significant decrease of VAS score meaning that subacromial injections of SH are effective in treating rotator cuff disease without complete tears.
Mohebbi et al. [30] compared the effectiveness of a single injection of high molecular weighted HA (HMW-HA) versus low molecular HA (LMW-HA) in patients diagnosed for rotator cuff tendinopathy at physical exam and imaging. The primary outcome was pain, assessed by VAS; secondary outcomes were the changes in terms of shoulder’s ROM, DASH functional questionnaire and WHOQOL-Bref questionnaire of quality of life (QoL). They found that there were not differences in efficacy between the two drugs, with benefits on pain, ROM, QoL and reduction of disability: these beneficial effects were higher in first three months, even though partially present at six months.
3.2Elbow tendinopathies
Bernetti et al. [31] compared the long-term effectiveness of local corticosteroids injection versus a protocol of one infiltration of local corticosteroid followed by three infiltrations of low molecular weight HA in 11 patients practicing tennis as a hobby, who were diagnosed humeral epicondylitis. They found that the better way of treatment was the combination of methylprednisolone acetate 40 mh/ml with 0.8 ml lidocaine plus injection of 1 ml of low HA ten days later and once a week for two more times.
Tosun et al. [32] compared a single injection treatment effectiveness of triamcinolone with the mixture HA
Apaydin et al. [33] evaluated pain reduction and function and grip strength in patients with lateral epicondylalgia treated with single HA injection versus triple dextrose prolotherapy (DPT). The pain was assessed with VAS scale, function with quick Disabilities of the Arm, Shoulder, Hand questionnaire (quick-DASH), and hand grip strength test, through a dynamometer. All outcomes were assessed at baseline, six and 12 weeks. They found that DPT and HA were both capable to improve pain, grip strength and function but DPT was more effective over 12 weeks.
3.3Hand tendinopathies
Orlandi et al. [34] analyzed pain reduction, functional improvement and retinaculum thickness at baseline, one month, three months and six months in patients with De Quervain’s disease (DQD) treated with three different injective therapies: single steroid injection, steroid injection followed by a 15 days-delayed saline injection and steroid injection followed by a 15 days-delayed HA injection. Pain was evaluated with VAS, functional improvement with quick-DASH questionnaire and retinaculum thickness with ultrasound evaluation. They found that pain and functional improvement were statistically different compared to baseline and these results were better in the steroid
Liu et al. [35] compared the effectiveness at baseline, three week and three months of a single steroid injection with single HA injection in patients diagnosed for trigger finger. The main outcome was the Quinnell trigger finger disease severity score; other outcomes were pain, valued with VAS scale, and function valued with Michigan Hand Outcome Questionnaire (MHQ), total activity motion (TAM) scale, and strength measured by a dynamometer. They found that HA improved Quinell score in a similar way as steroids. Regarding functional improvement, they found that HA had more persistent improvement at MHQ at three months than steroids, whose functional effectiveness was similar to HA at three-week follow-up.
Callegari et al. [36] compared the effectiveness of a corticosteroid injections plus a ten days-delayed HA injection with surgery in patients diagnosed for trigger finger. They defined as “satisfactory” outcomes at baseline, six weeks, three, six and 12 months. These outcomes were: patients’ symptoms change assessed with Quinnell-Green classification and then disability, pain and satisfaction with other three different scales. They found comparable satisfactory outcomes in corticosteroid
Kanchanathepsak et al. [37] compared the effectiveness of Low Molecular Weighted HA with Corticosteroid (CS) in trigger digit. Quinell grading, VAS score of pain DASH score and complications were collected at one, three and six months follow up. They concluded that HA and CS have similar therapeutic effects in trigger digit, despite higher CS’ results in early phase.
3.4Knee tendinopathies
Muneta et al. [38] evaluated the effectiveness of a HA injection in professional and semi-professional athlete patients with patellar tendinopathy. All patients were graded stage two or three by Blazina’s classification (had pain during and after activity). They were stratified in four clinical levels and injected differently due to this stratification. They conclude that HA injection therapy is optional but effective in athlete patients with patellar tendinopathy and speculate that injection therapy effectiveness may be different due to different disease classification.
3.5Ankle and foot tendinopathies
Lynen et al. [39] compared the safety and the efficacy of two HA peri-tendinous injection with ESWT with standardized parameters in patients with midportion painful Achilles’ tendinopathy. Their primary outcome was percentage changes in three months VAS compared to baseline; secondary outcomes were intensity of clinical parameters evaluated at five-point scale, Victorian Institute of Sports Assessment Achilles questionnaire (VISA-A) and a scale for patients’ and examiners’ impression of beneficial effects. They found at four week, three months and six months controls that HA had a higher impact than ESWT on pain and VISA-A. HA was superior to ESWT also at clinical impact at three months, although at three week and six months follow-up results were comparable [34].
Gorelick et al. [40] reviewed 56 patients with insertional Achilles Tendinopathy (AT), splitted in three groups, treated with three different approaches: one with corticosteroid injection, the second with single HA injection and the third with rest, splint, NSAIDs, and physiotherapy. Foot and Ankle Disability Index (FADI) questionnaire and VAS pain score were used to assess patients’ condition. After one year, patients treated by a single HA injection compared with other treatments showed better and more lasting results, according to FADI and VAS scores.
Kumai et al. [41] treated patients affected by enthesopathies (16 with lateral epicondylitis, 15 with patellar tendinopathy, 15 with insertional Achilles’ tendinopathy and 16 with plantar fasciitis) with a single injection of 25 mg of high molecular weight HA (2700 kDa) in the vicinity of tendon/ligament attachment site in the affected area, without local anaesthetic. Their outcomes were VAS pain scale. They found that HA may be effective on pain reduction in enthesopathies.
Several studies [23, 26, 30, 35, 36, 38, 39] assessed the safety of HA. No severe complications such as aggravation of symptoms or infections, hemarthrosis or synovitis were reported in the selected studies; when present, adverse events were often reported as induration [30], dullness [38] or as a early-onset pain (immediately or in a few days after the injection) solved with ice and acetaminophen [32, 33, 36, 37]. In one study, Kim [23] reports that patients who underwent HA injections experienced musculoskeletal pain, nasopharyngitis, and cough.
Adverse events were recorded in a special section in two studies [24, 39]: Lynen [39] reports transient moderate tendon pain in one patient treated with HA injection; Penning [24] reports “mild adverse reactions” in which are included: increase of pain after the injection, headaches and a haematoma at the site of injection, not specifying if related to HA or to the control groups.
No long-term complications clearly related to HA injections are reported in the studies included. Although the follow up period varies and, in many studies, it is not over a few months, none of the studies suggest that there might be major long-term complications related to HA injections. For these reasons it might speculate that HA injections are safe.
4.Discussion
Tendinopathies are multifactorial, common pathologies that can affect both active and sedentary population [42, 43, 44, 45]. The role of the conservative treatment of tendinopathies has been increasingly supported by scientific evidence over the last years, and HA has been showed to be an intriguing therapeutic option [42, 43, 44, 45, 46, 47, 48].
By the present narrative review, it is reported that HA has positive impact not only in terms of pain reduction but also in improving functioning in patients with tendinopathies.
Nevertheless, in scientific community there is no unanimous consensus on this treatment, maybe due to lack of standardization in the therapeutic process. Even this narrative review pointed out that HA preparations are different in the studies included, such as LMW HA [31, 37], sodium hyaluronate [25, 28, 29, 40], average molecular weight HA [23], or other types, with different molecular weight and different dosages. There is also not a unique way of administration, which could be intra-articular [25], intralesional [28, 29], or other type. Moreover, there is also a high difference in number of administrations (from single to three, or more) and timing of injections (most time weekly). In this context, Osti et al. [49] suggested that differences in HA preparations were important on influencing cell activity increasement, tendon cell apoptosis reduction, in a dose-dependent and not molecular weight-dependent way, as also supported in one of the included studies [30].
In almost all studies assessed [23, 25, 26, 27, 28, 29, 30, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41], comparing to baseline, HA has been demonstrated to be effective on pain relief, although in some studies [24, 33, 35, 37] the comparison had better results than HA. All studies but three [27, 32, 38] analyzed pain reduction with Visual Analogue Scale (VAS) and found improvement in its values. Improvements were also found in the scales used in the other three studies: the Patient-Rated Tennis Elbow Tendinopathy (PRTEE) [32], a questionnaire which measures pain and disability of the forearm; the Roles and Maudsley score [27, 28], a score of subjective pain feeling and satisfaction. Such studies evaluate pain relief, so they don’t determine the role of the intervention in the healing process.
In the study by Kanchanathepsak et al. [37], authors conclude that HA injections in trigger digits have similar therapeutic effects than corticosteroids, which have better results on the measured outcomes only in the early phase. Corticosteroids, according to the European guidelines for trigger digits are the standard treatment and seem to have a poorer prognosis in patients with specific pathologies [50, 51], so authors still promote HA as alternative to corticosteroids. Similar conclusions in Liu et al. [35]. In Penning et al. [24] diagnosis and injections have been performed with no ultrasound or other imaging guide: authors speculate that this might be one of the reasons why placebo seems to have better results than corticosteroids and HA injection for the treatment of shoulder impingement, with a no statistically significative difference between treatments. Ultrasound has been proven as a safe method to improve the accuracy of site injection [52, 53].
In the study by Apaydin et al. [33], prolotherapy has been judged to be more effective than HA injections in lateral epicondylalgia: authors believe that the absence of ultrasound guidance might be a limitation of this study. Moreover, the group treated with prolotherapy underwent a triple injection with a solution containing a small part of lidocaine (compared to a single HA injection without lidocaine): as shown by other studies [54, 55, 56, 57] local anesthetic injection might have a role in pain reduction.
Effects of HA on pain have been already specifically analyzed in intra-articular injections in OA [58, 59, 60, 61] and confirmed to be effective on functional improvement even in subjects suffering from tendinopathies, as shown by this narrative review.
However, albeit effects of HA are still unknown in patients with tendinopathies, the potential molecular effects might result in functional and kinematic improvement, with walking pattern changes measured at instrumental gait analysis, as already reported by Paoloni et al. [61] in OA patients.
Individual characteristics have been also hypothesized to be important in the effectiveness of HA. Indeed, Frizziero et al. [60] in a study on HA injection in detraining-associated tendons’ damage, splitted tendon rats by training levels and Gallorini et al. [17] suggested that may be plausible to personalize the HA treatment according to the patients’ characteristics.
This review is not free from limitations: first, it is not possible to compare the effectiveness of HA among different anatomical districts; second, there is a high variability in terms of study design, control groups, follow-up evaluations.
5.Conclusions
By the present review, we showed that none of the studies report severe adverse effects and most of them support the use of HA injections in tendinopathies (alone or associated with other therapies), with a reported effectiveness on pain reduction and functional assessment. Nevertheless, there is a lack of scientific evidence regarding the use of HA in the treatment of the most common tendinopathies: especially, there is no conformity on both the type of HA used, as well as on the dose and the way of administration of it. Further studies with a high sample size and a careful methodology are warranted to better investigate effects and methods of administration of HA in tendinopathies, which still seems to be a therapy with promising positive results.
Acknowledgments
None to report.
Conflict of interest
The authors certify that there is no conflict of interest in any way with any financial organization regarding the material discussed in the manuscript.
Ethical declaration
Approval was not sought due to the nature of the study.
References
[1] | Cook JL, Purdam CR. Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. Br J Sports Med. (2009) Jun; 43: (6): 409-16. doi: 10.1136/bjsm.2008.051193. |
[2] | Maffulli N, Khan KM, Puddu G. Overuse tendon conditions: Time to change a confusing terminology. Arthroscopy. (1998) Nov-Dec; 14: (8): 840-3. doi: 10.1016/s0749-8063(98)70021-0. |
[3] | Henniger M, Rehart S. Tendinopathien bei rheumatischen Erkrankungen [Tendinopathy in rheumatic diseases]. Unfallchirurg. (2017) Mar; 120: (3): 214-219. German. doi: 10.1007/s00113-016-0291-0. |
[4] | de Sire A, Agostini F, Lippi L, Mangone M, Marchese S, Cisari C, Bernetti A, Invernizzi M. Oxygen-ozone therapy in the rehabilitation field: State of the art on mechanisms of action, safety and effectiveness in patients with musculoskeletal disorders. Biomolecules. (2021) Feb 26; 11: (3): 356. doi: 10.3390/biom11030356. |
[5] | de Sire A, Stagno D, Minetto MA, Cisari C, Baricich A, Invernizzi M. Long-term effects of intra-articular oxygen-ozone therapy versus hyaluronic acid in older people affected by knee osteoarthritis: A randomized single-blind extension study. J Back Musculoskelet Rehabil. (2020) ; 33: (3): 347-354. doi: 10.3233/BMR-181294. |
[6] | Frizziero A, Vittadini F, Fusco A, Giombini A, Masiero S. Efficacy of eccentric exercise in lower limb tendinopathies in athletes. J Sports Med Phys Fitness. (2016) Nov; 56: (11): 1352-1358. |
[7] | Dragoni S, Bernetti A. Rectus Femoris Tendinopathy. In: Bisciotti G, Volpi P. (eds) The Lower Limb Tendinopathies. Sports and Traumatology. Springer. (2016) . pp. 67-84. |
[8] | Paoloni M, Bernetti A, Belelli A, Brignoli O, Buoso S, Caputi AP, Catani F, Coclite D, Fini M, Mantovani L, Migliore A, Napoletano A, Viora U, Santilli V. Appropriateness of clinical and organizational criteria for intra-articular injection therapies in osteoarthritis. A Delphi method consensus initiative among experts in Italy. Ann Ist Super Sanita. (2015) ; 51: (2): 131-8. doi: 10.4415/ANN_15_02_11. |
[9] | Necas J, Bartosikova L, Brauner P, Kolar J. Hyaluronic acid (hyaluronan): A review. Veterinarni Medicina. (2008) ; 53: (8): 397-411. |
[10] | Bernetti A, Agostini F, Alviti F, Giordan N, Martella F, Santilli V, Paoloni M, Mangone M. New Viscoelastic Hydrogel Hymovis MO.RE. Single Intra-articular Injection for the Treatment of Knee Osteoarthritis in Sportsmen: Safety and Efficacy Study Results. Front Pharmacol. (2021) May 28; 12: : 673988. doi: 10.3389/fphar.2021.673988. |
[11] | Santilli V, Mangone M, Paoloni M, Agostini F, Alviti F, Bernetti A. Comment on “Early Efficacy of Intra-Articular HYADD® 4 (Hymovis®) Injections for Symptomatic Knee Osteoarthritis”. Joints. (2018) Jun 14; 6: (2): 131-132. doi: 10.1055/s-0038-1660791. |
[12] | Bernetti A, Mangone M, Paolucci T, Santilli V, Verna S, Agostini F, et al. Evaluation of the efficacy of intra-articular injective treatment with reticular hyaluronic acid (Mo.Re. Technology) in amateur athletes with over-use gonarthrosis. Med Sport. (2020) ; 73: : 127-39. doi: 10.23736/S0025-7826.20.03648-0. |
[13] | Balazs EA, Denlinger JL. Viscosupplementation: A new concept in the treatment of osteoarthritis. J Rheumatol Suppl. (1993) Aug; 39: : 3-9. |
[14] | Pereira H, Sousa DA, Cunha A, Andrade R, Espregueira-Mendes J, Oliveira JM, Reis RL. Hyaluronic Acid. © Springer International Publishing AG, part of Springer Nature 2018 137 Oliveira JM et al. (eds.), Osteochondral Tissue Engineering, Advances in Experimental Medicine and Biology 1059. doi: 10.1007/978-3-319-76735-2_6. |
[15] | Abate M, Pulcini D, Di Iorio A, Schiavone C. Viscosupplementation with intra-articular hyaluronic acid for treatment of osteoarthritis in the elderly. Curr Pharm Des. (2010) ; 16: (6): 631-40. doi: 10.2174/138161210790883859. |
[16] | Bhattacharya D, Svechkarev D, Souchek JJ, Hill TK, Taylor MA, Natarajan A, Mohs AM. Impact of structurally modifying hyaluronic acid on CD44 interaction. J Mater Chem B. (2017) ; 5: (41): 8183-8192. doi: 10.1039/C7TB01895A. |
[17] | Gallorini M, Berardi AC, Gissi C, Cataldi A, Osti L. Nrf2-mediated cytoprotective effect of four different hyaluronic acids by molecular weight in human tenocytes. J Drug Target. (2020) Feb; 28: (2): 212-224. doi: 10.1080/1061186X.2019.1648476. |
[18] | Zavan B, Ferroni L, Giorgi C, Calò G, Brun P, Cortivo R, Abatangelo G, Pinton P. Hyaluronic acid induces activation of the κ-opioid receptor. PLoS One. (2013) ; 8: (1): e55510. doi: 10.1371/journal.pone.0055510. |
[19] | Tillander B, Franzén LE, Karlsson MH, Norlin R. Effect of steroid injections on the rotator cuff: An experimental study in rats. J Shoulder Elbow Surg. (1999) May-Jun; 8: (3): 271-4. doi: 10.1016/s1058-2746(99)90141-6. PMID: 10389085. |
[20] | Aström M. Partial rupture in chronic achilles tendinopathy. A retrospective analysis of 342 cases. Acta Orthop Scand. (1998) Aug; 69: (4): 404-7. doi: 10.3109/17453679808999056. PMID: 9798451. |
[21] | Gasparyan AY, Ayvazyan L, Blackmore H, Kitas GD. Writing a narrative biomedical review: Considerations for authors, peer reviewers, and editors. Rheumatol Int. (2011) Nov; 31: (11): 1409-17. doi: 10.1007/s00296-011-1999-3. Epub 2011 Jul 29. PMID: 21800117. |
[22] | Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. (2009) Jul 21; 6: (7): e1000097. doi: 10.1371/journal.pmed.1000097. Epub 2009 Jul 21. PMID: 19621072; PMCID: PMC2707599. |
[23] | Kim YS, Park JY, Lee CS, Lee SJ. Does hyaluronate injection work in shoulder disease in early stage? A multicenter, randomized, single blind and open comparative clinical study. J Shoulder Elbow Surg. (2012) Jun; 21: (6): 722-7. doi: 10.1016/j.jse.2011.11.009. |
[24] | Penning LI, de Bie RA, Walenkamp GH. The effectiveness of injections of hyaluronic acid or corticosteroid in patients with subacromial impingement: A three-arm randomised controlled trial. J Bone Joint Surg Br. (2012) Sep; 94: (9): 1246-52. doi: 10.1302/0301-620X.94B9.28750. |
[25] | Ozgen M, Fırat S, Sarsan A, Topuz O, Ardıç F, Baydemir C. Short- and long-term results of clinical effectiveness of sodium hyaluronate injection in supraspinatus tendinitis. Rheumatol Int. (2012) Jan; 32: (1): 137-44. doi: 10.1007/s00296-010-1577-0. |
[26] | Merolla G, Bianchi P, Porcellini G. Ultrasound-guided subacromial injections of sodium hyaluronate for the management of rotator cuff tendinopathy: A prospective comparative study with rehabilitation therapy. Musculoskelet Surg. (2013) Jun; 97: (Suppl 1): 49-56. doi: 10.1007/s12306-013-0259-y. |
[27] | Frizziero A, Vittadini F, Barazzuol M, Gasparre G, Finotti P, Meneghini A, Maffulli N, Masiero S. Extracorporeal shockwaves therapy versus hyaluronic acid injection for the treatment of painful non-calcific rotator cuff tendinopathies: Preliminary results. J Sports Med Phys Fitness. (2017) Sep; 57: (9): 1162-1168. doi: 10.23736/S0022-4707.16.06408-2. |
[28] | Meloni F, Milia F, Cavazzuti M, Doria C, Lisai P, Profili S, Meloni GB. Clinical evaluation of sodium hyaluronate in the treatment of patients with sopraspinatus tendinosis under echographic guide: Experimental study of periarticular injections. Eur J Radiol. (2008) Oct; 68: (1): 170-3. doi: 10.1016/j.ejrad.2007.11.001. |
[29] | Moghtaderi A, Sajadiyeh S, Khosrawi S, Dehghan F, Bateni V. Effect of subacromial sodium hyaluronate injection on rotator cuff disease: A double-blind placebo-controlled clinical trial. Adv Biomed Res. (2013) Nov 30; 2: : 89. doi: 10.4103/2277-9175. |
[30] | Mohebbi R, Rezasoltani Z, Mir M, Mohebbi M, Vatandoost S, Esmaily H. High- versus low-molecular-weight hyaluronic acid for the treatment of rotator cuff tendinopathy: A triple-blind randomized comparative trial. Ann Pharmacother. (2021) Oct; 55: (10): 1203-1214. doi: 10.1177/1060028021994297. |
[31] | Bernetti A, Mangone M, Paoloni M, Di Sante L, Murgia M, Santilli V. Corticosteroid and hyaluronic acid injection therapy in tennis elbow (lateral epicondylalgia). Medicina dello Sport. (2014) ; 67: (2): 289-295. |
[32] | Tosun HB, Gumustas S, Agir I, Uludag A, Serbest S, Pepele D, Ertem K. Comparison of the effects of sodium hyaluronate-chondroitin sulphate and corticosteroid in the treatment of lateral epicondylitis: A prospective randomized trial. J Orthop Sci. (2015) Sep; 20: (5): 837-43. doi: 10.1007/s00776-015-0747-z. |
[33] | Apaydin H, Bazancir Z, Altay Z. Injection therapy in patients with lateral epicondylalgia: Hyaluronic acid or dextrose prolotherapy? A single-blind, randomized clinical trial. J Altern Complement Med. (2020) Dec; 26: (12): 1169-1175. doi: 10.1089/acm.2020.0188. |
[34] | Orlandi D, Corazza A, Fabbro E, Ferrero G, Sabino G, Serafini G, Silvestri E, Sconfienza LM. Ultrasound-guided percutaneous injection to treat de Quervain’s disease using three different techniques: A randomized controlled trial. Eur Radiol. (2015) May; 25: (5): 1512-9. doi: 10.1007/s00330-014-3515-0. |
[35] | Liu DH, Tsai MW, Lin SH, Chou CL, Chiu JW, Chiang CC, Kao CL. Ultrasound-guided hyaluronic acid injections for trigger finger: A double-blinded, randomized controlled trial. Arch Phys Med Rehabil. (2015) Dec; 96: (12): 2120-7. doi: 10.1016/j.apmr.2015.08.421. |
[36] | Callegari L, Spanò E, Bini A, Valli F, Genovese E, Fugazzola C. Ultrasound-guided injection of a corticosteroid and hyaluronic acid: A potential new approach to the treatment of trigger finger. Drugs R D. (2011) ; 11: (2): 137-45. doi: 10.2165/11591220-000000000-00000. |
[37] | Kanchanathepsak T, Pichyangkul P, Suppaphol S, Watcharananan I, Tuntiyatorn P, Tawonsawatruk T. Efficacy comparison of hyaluronic acid and corticosteroid injection in treatment of trigger digits: A randomized controlled trial. J Hand Surg Asian Pac Vol. (2020) Mar; 25: (1): 76-81. doi: 10.1142/S2424835520500101. |
[38] | Muneta T, Koga H, Ju YJ, Mochizuki T, Sekiya I. Hyaluronan injection therapy for athletic patients with patellar tendinopathy. J Orthop Sci. (2012) Jul; 17: (4): 425-31. doi: 10.1007/s00776-012-0225-9. |
[39] | Lynen N, De Vroey T, Spiegel I, Van Ongeval F, Hendrickx NJ, Stassijns G. Comparison of peritendinous hyaluronan injections versus extracorporeal shock wave therapy in the treatment of painful achilles’ tendinopathy: A randomized clinical efficacy and safety study. Arch Phys Med Rehabil. (2017) Jan; 98: (1): 64-71. doi: 10.1016/j.apmr.2016.08.470. |
[40] | Gorelick L, Rozano-Gorelick A, Saab A, Ram E. Single hyaluronate injection in the management of insertional achilles 369 tendinopathy in comparison to corticosteroid injections and non-invasive conservative 370 treatments. Scholars Bulletin. (2015) ; 1: (1): 16-20. |
[41] | Kumai T, Muneta T, Tsuchiya A, Shiraishi M, Ishizaki Y, Sugimoto K, Samoto N, Isomoto S, Tanaka Y, Takakura Y. The short-term effect after a single injection of high-molecular-weight hyaluronic acid in patients with enthesopathies (lateral epicondylitis, patellar tendinopathy, insertional Achilles tendinopathy, and plantar fasciitis): A preliminary study. J Orthop Sci. (2014) Jul; 19: (4): 603-11. doi: 10.1007/s00776-014-0579-2. |
[42] | Kaux JF, Crielaard JM. Tendon et tendinopathie. Journal de Traumatologie du Sport. (2014) ; 31: (4): 235-240. |
[43] | Frizziero A, Vittadini F, Pignataro A, Gasparre G, Biz C, Ruggieri P, Masiero S. Conservative management of tendinopathies around hip. Muscles Ligaments Tendons J. (2016) Dec 21; 6: (3): 281-292. doi: 10.11138/mltj/2016.6.3.281. |
[44] | Loppini M, Maffulli N. Conservative management of tendinopathy: An evidence-based approach. Muscles Ligaments Tendons J. (2012) Apr 1; 1: (4): 134-7. |
[45] | Agostini F, Bernetti A, Di Giacomo G, Viva MG, Paoloni M, Mangone M, Santilli V, Masiero S. Rehabilitative good practices in the treatment of sarcopenia: A narrative review. Am J Phys Med Rehabil. (2021) Mar 1; 100: (3): 280-287. doi: 10.1097/PHM.0000000000001572. |
[46] | Masiero S, Maccarone MC, Agostini F. Health resort medicine can be a suitable setting to recover disabilities in patients tested negative for COVID-19 discharged from hospital? A challenge for the future. Int J Biometeorol. (2020) Oct; 64: (10): 1807-1809. doi: 10.1007/s00484-020-01947-4. |
[47] | Bernetti A, Mangone M, Alviti F, Paolucci T, Attanasi C, Murgia M, Di Sante L, Agostini F, Vitale M, Paoloni M. Spa therapy and rehabilitation of musculoskeletal pathologies: A proposal for best practice in Italy. Int J Biometeorol. (2020) Jun; 64: (6): 905-914. doi: 10.1007/s00484-019-01731-z. |
[48] | Costantino C, Olvirri S. Rehabilitative and infiltrative treatment with hyaluronic acid in elderly patients with rotator cuff tears. Acta Biomed. (2009) ; 80: (3): 225-9. |
[49] | Osti L, Berardocco M, di Giacomo V, Di Bernardo G, Oliva F, Berardi AC. Hyaluronic acid increases tendon derived cell viability and collagen type I expression in vitro: Comparative study of four different Hyaluronic acid preparations by molecular weight. BMC Musculoskelet Disord. (2015) Oct 6; 16: : 284. doi: 10.1186/s12891-015-0735-7. Erratum in: BMC Musculoskelet Disord. 2015; 16: 334. |
[50] | Baumgarten KM, Gerlach D, Boyer MI. Corticosteroid injection in diabetic patients with trigger finger. A prospective, randomized, controlled double-blinded study. J Bone Joint Surg Am. (2007) Dec; 89: (12): 2604-11. doi: 10.2106/JBJS.G.00230. PMID: 18056491. |
[51] | Roh YH, Oh M, Noh JH, Gong HS, Baek GH. Effect of metabolic syndrome on the functional outcome of corticosteroid injection for lateral epicondylitis: Retrospective matched case-control study. Sci Rep. (2017) Sep 7; 7: (1): 10845. doi: 10.1038/s41598-017-11179-z. PMID: 28883422; PMCID: PMC5589833. |
[52] | Godey SK, Bhatti WA, Watson JS, Bayat A. A technique for accurate and safe injection of steroid in trigger digits using ultrasound guidance. Acta Orthop Belg. (2006) Oct; 72: (5): 633-4. PMID: 17152429. |
[53] | Jeyapalan K, Choudhary S. Ultrasound-guided injection of triamcinolone and bupivacaine in the management of De Quervain’s disease. Skeletal Radiol. (2009) Nov; 38: (11): 1099-103. doi: 10.1007/s00256-009-0721-y. Epub 2009 Jun 1. PMID: 19484469. |
[54] | Plafki C, Steffen R, Willburger RE, Wittenberg RH. Local anaesthetic injection with and without corticosteroids for subacromial impingement syndrome. Int Orthop. (2000) ; 24: (1): 40-2. doi: 10.1007/s002640050010. PMID: 10774861; PMCID: PMC3619854. |
[55] | Alvarez CM, Litchfield R, Jackowski D, Griffin S, Kirkley A. A prospective, double-blind, randomized clinical trial comparing subacromial injection of betamethasone and xylocaine to xylocaine alone in chronic rotator cuff tendinosis. Am J Sports Med. (2005) Feb; 33: (2): 255-62. doi: 10.1177/0363546504267345. PMID: 15701612. |
[56] | Hsieh LF, Kuo YC, Lee CC, Liu YF, Liu YC, Huang V. Comparison between corticosteroid and lidocaine injection in the treatment of tennis elbow: A randomized, double-blinded, controlled trial. Am J Phys Med Rehabil. (2018) Feb; 97: (2): 83-89. doi: 10.1097/PHM.0000000000000814. PMID: 28816704. |
[57] | de Sire A, Marotta N, Lippi L, Scaturro D, Farì G, Liccardi A, Moggio L, Letizia Mauro G, Ammendolia A, Invernizzi M. Pharmacological treatment for acute traumatic musculoskeletal pain in athletes. Medicina (Kaunas). (2021) Nov 5; 57: (11): 1208. doi: 10.3390/medicina57111208. PMID: 34833426; PMCID: PMC8618079. |
[58] | Bannuru RR, Natov NS, Dasi UR, Schmid CH, McAlindon TE. Therapeutic trajectory following intra-articular hyaluronic acid injection in knee osteoarthritis-meta-analysis. Osteoarthritis Cartilage. (2011) Jun; 19: (6): 611-9. doi: 10.1016/j.joca.2010.. |
[59] | Yoshida M, Funasaki H, Kubota M, Marumo K. Therapeutic effects of high molecular weight hyaluronan injections for tendinopathy in a rat model. J Orthop Sci. (2015) Jan; 20: (1): 186-95. doi: 10.1007/s00776-014-0650-z. |
[60] | Frizziero A, Salamanna F, Giavaresi G, Ferrari A, MartiniL, Marini M, Veicsteinas A, Maffulli N, Masiero S, Fini M. Hyaluronic acid injections protect patellar tendon from detraining-associated damage. Histol Histopathol. (2015) Sep; 30: (9): 1079-88. doi: 10.14670/HH-11-605. |
[61] | Paoloni M, Di Sante L, Dimaggio M, Bernetti A, Mangone M, Di Renzo S, Santilli V. Kinematic and kinetic modifications in walking pattern of hip osteoarthritis patients induced by intra-articular injections of hyaluronic acid. Clin Biomech (Bristol, Avon). (2012) Aug; 27: (7): 661-5. doi: 10.1016/j.clinbiomech.2012.02.004. |




