Comparing an e-Health program vs home rehabilitation program in patients with non-specific low back pain: A study protocol randomized feasibility trial
Abstract
BACKGROUND:
There is little evidence on the reliability of the web application-based rehabilitation systems to treat chronic low back pain (CLBP).
METHODS:
This protocol describes a double-blind, randomized controlled feasibility trial of an e-Health intervention developed to support the self-management of people with CLBP in primary care physiotherapy. Three Hospitals with primary care for outpatients will be the units of randomisation, in each Hospital the participants will be randomized to one of two groups, a pragmatic control group receiving either the usual home program based on electrostimulation and McKenzie Therapy and e-Health intervention. Patients are followed up at 2 and 6 months. The primary outcomes are (1) acceptability and demand of the intervention by GPs, physiotherapists and patients and (2) feasibility and optimal study design/methods for a definitive trial. Secondary outcomes will include analysis in the clinical outcomes of pain, disability, fear of movement, quality of life, isometric resistance of the trunk flexors, lumbar anteflexion and lumbar segmental range of motion.
DISCUSSION:
The specific e-Health programs to home could increase adherence to treatment, prevent stages of greater pain and disability, and improve the painful symptomatology.
CONCLUSIONS:
The e-Health programs could be an effective healthcare tool that can reach a large number of people living in rural or remote areas.
1.Background
Non-specific low back pain (LBP) is one of the most common health problems worldwide and is the leading cause of years suffering from a disability in western countries [1]. Non-specific LBP is characterized by a mechanical pain of musculoskeletal origin, with no defined cause, which lasts more than 12 weeks [2, 3].
The most recent review in the adult general population estimated the global point prevalence at 11.9%, the monthly prevalence at 23.2%, and the annual prevalence at 38%, being greater in females and individuals aged between 40 to 80 years [4, 5]. In Spain, LBP ranks first among the causes of temporary disability in the population over 50 years of age [6]. Furthermore, as the population ages over the coming decades, it is more than likely that the number of people with non-specific LBP will increase significantly.
In European countries, the total cost of LBP has been estimated at 1.7% to 2.1% of gross domestic product [6, 7], since it is among the health problems responsible for the majority of sick leave and the top five most expensive disorders of the musculoskeletal system [8, 9]. In Spain, LBP accounts for over 2 million visits to a Primary Care Center (PCC) per year [9].
Although the European international guidelines recommend non-pharmacological treatments for chronic musculoskeletal conditions [10, 11, 12], the health services require strong evidence of their clinical and profitability before the implementation of widespread rehabilitation programs.
Several possible interventions exist for the treatment and management of LBP, including exercise, patient education, manual therapy and electrotherapy; these are often used alone or combined [10, 13, 14, 15, 16, 17, 18].
Transcutaneous electrical nerve stimulation (TENS) is an inexpensive non pharmacological intervention used in chronic pain conditions. The research evidence, TENS reduces hyperalgesia through both peripheral and central mechanisms, reducing the need for medication in these patients [19, 20]. However, the different systematic reviews have examined the efficacy of TENS for low back pain with conflicting results [21, 22, 23, 24, 25, 26, 27, 28].
Also exercises are recommended in all guidelines for chronic LBP; these determine that patients with chronic LBP should exercise and maintain a physically active lifestyle [2, 13]. The specific back exercise programs have been found to be moderately effective in reducing pain and improving function in chronic LBP, especially if programs are individually designed/tailored and supervised by a physiotherapist [16, 17].
The McKenzie Therapy (MT) is a treatment in which exercise is prescribed individually based on the classification made of patients with low back pain [29]. This method of diagnosis and mechanical therapy associated with an educational component has been considered a more effective intervention in reducing pain and disability than other standard therapies in the short term [30, 31, 32]. However, the availability of secondary rehabilitation centers in the public health system could be insufficient to meet the demand of these patients in a supervised way [33].
The interventions performed electronically have been shown to be effective in patients with chronic musculoskeletal disease [34], since they can provide educational information beyond traditional paper-based media, such as audio and video material that patients can consult at any time. This facilitates goal setting, adherence, self-monitoring and behavioral and symptom-related feedback [35, 36, 37, 38]. Also, the studies suggest that interventions supported by virtual materials are more accessible to patients than many traditional face-to-face services [38, 39].
These data lead us to believe that patients who have the support of an online platform to perform the intervention at home, have the potential to obtain greater adherence and long-term effects than those who perform the same physiotherapy intervention without supervision in the home and without computerized support. There is also the need to explore the effectiveness, adherence, usefulness and support of interventions in primary care supported by an online platform designed exclusively for patients with chronic LBP.
The aim of this randomized controlled trial is to evaluate the feasibility of providing an e-Health rehabilitation program through a web platform performing electroanalgesia and an exercise program following the MT for patients with chronic LBP in primary care, compared with the same home rehabilitation program but without the support of an electronic program.
2.Objectives
Our primary objectives are: (1) to evaluate the acceptability and demand of the e-Health intervention for patients and physiotherapists for the optimization of their design, development and delivery; (2) to analyze the feasibility of the trial procedures. See Table 1 below for details on feasibility aspects.
Table 1
Feasibility aspects related to e-Health intervention and trial procedures
| Feasibility | e-Health intervention | Trial procedures |
|---|---|---|
| Acceptability | ||
| Participants | The extent to which participants who have received the intervention through a web application consider that the content and support materials (web application and initial learning sessions) are appropriate and satisfactory to obtain the expected results. | The extent to which participants believe that their eligibility, outcome measures, follow-up and intervention by the physiotherapist have been satisfactory. |
| Physiotherapists | The extent to which the physiotherapists who have administered the intervention consider that the training, content and support materials are appropriate to meet their needs and those of their patients within the primary care service. | The extent to which physiotherapists who have participated in the trial consider recruitment, outcome measures, evaluation follow-ups and appropriate and satisfactory intervention procedures. |
| General practitioners | The extent to which the GPs who have carried out the first screening of patients, consider that the eligibility criterio and recruitment are suitable for detecting the potential sample. | |
| Demand | ||
| Participants | The extent to which participants adhere to the e-Health intervention, complying with the weekly sessions. | The extent to which participants perceive the burden of participating in follow-up and completing specific outcome measures within the trial. |
| Physiotherapists | The extent to which physiotherapists perceive the demand to complete their tasks required to participate in the trial, including intervention procedures. | The extent to which physiotherapists perceive the demand of completing their required tasks for participating in the trial. |
| Practicality | ||
| Physiotherapists | The factors that influence the implementation of the e-Health intervention in a variety of health environments due to variations in personnel, facilities, equipment and the environment. | |
| Adaptation | ||
| Participants | The extent to which the content of the e-Health intervention, support materials and learning classes should be modified to improve their acceptability and implementation for a future definitive trial | The extent to which recruitment, follow-up procedures and the number and outcome measures should be modified during/at the end of the trial to improve its acceptability and implementation for a definitive future trial. |
| Physiotherapists | The extent to which the e-Health intervention training, the content of the program, the classes previously conducted for its learning and the support materials (web application) should be modified during/at the end of the test to improve its acceptability and implementation for a definitive future proof. | The extent to which the recruitment and fidelity procedures of the trial, including the tasks of physiotherapists, should be modified during/at the end of the trial to improve the acceptability and implementation of a definitive future trial. |
The secondary objectives are: (3) to assess medium-term changes in pain intensity, disability, fear of movement, quality of life, resistance of the trunk flexors, lumbar segmental range of motion in both arms.
3.Materials and methods
This randomized controlled feasibility trial with an allocation ratio 1:1, double-blind, clinical trial divided into two groups (Fig. 1) was designed to assess the methodology proposed for use in a definitive RCT. This study protocol followed the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) Guidelines (Additional file 1) [40]. The trial was registered under registration number: NCT04283370.
The GPs of three Hospital Centers (HC) of the Andalusian Health Service that include physical therapy in primary care for outpatients have agreed to participate. These HCs are publicly financed and include the metropolitan areas of Southern Spain.
3.1Participants
A Consolidated Standards of Reporting Trials (or CONSORT) [40] flow diagram is provided in Fig. 1. Eligible participants are intentionally selected by GPs according to the eligibility criteria provided by the research team.
Figure 1.
Design and flow of participants through the trial.
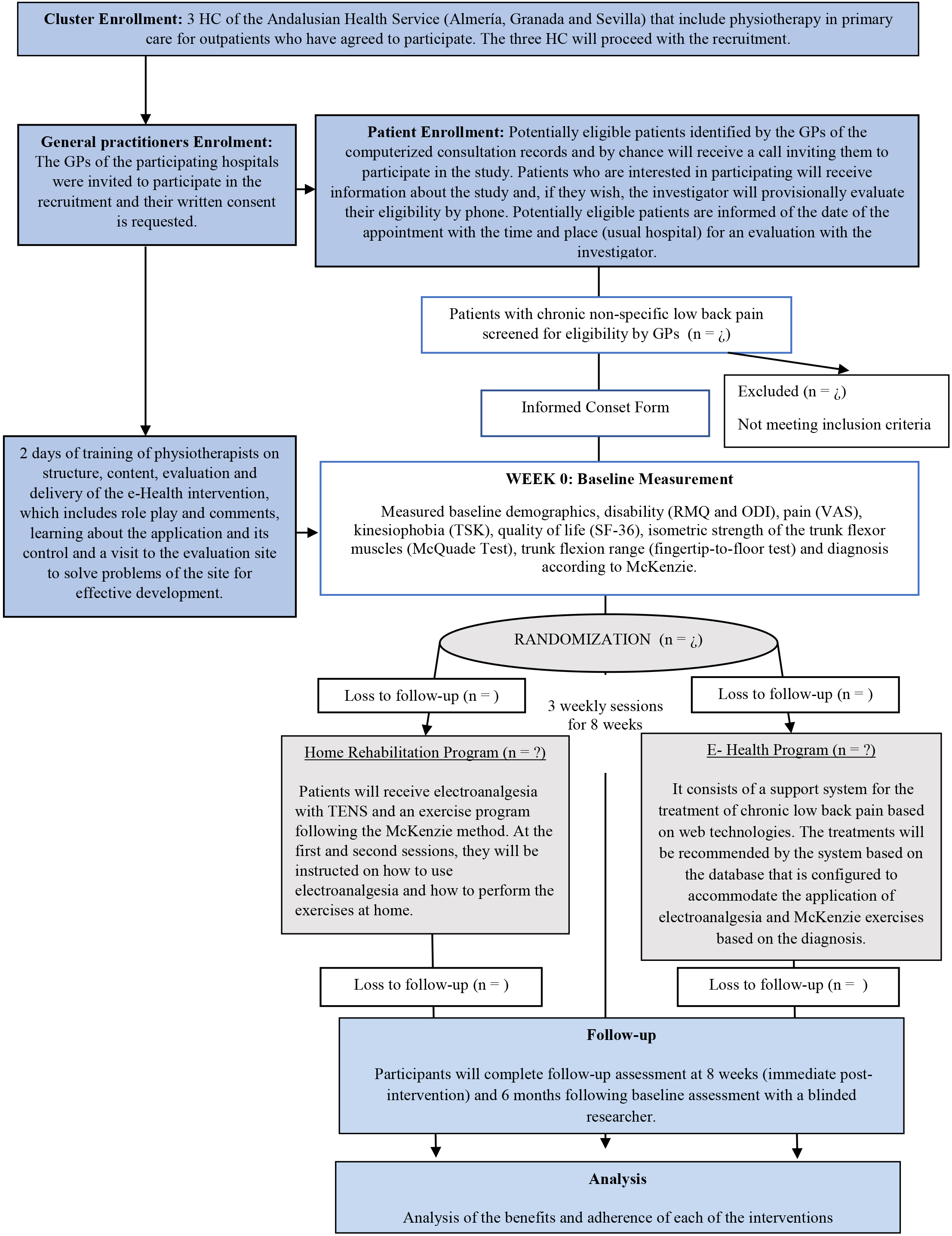
The inclusion criteria will be: individuals of both genders aged between 30 and 67 years old; presenting with low back pain for at least 3 months; low back pain disability
3.2Randomization
The computer software (Epidat 4.2) will automate the randomization process for this trial. The randomization sequence is automatically generated, using a table of random numbers generated by the software, which randomizes participants to each intervention group. As the software randomizes participants, a researcher will inform the participants about their assignment. The feasibility protocol will remain blind to the assignment until the full analysis is completed.
3.3Interventions
Consenting participants will be randomly assigned in two groups to receive electro-analgesia therapy with TENS and MT exercise through an e-Health program (telemedicine) or through a home rehabilitation program. Within both groups, patients will be distributed in three subgroups (postural, dysfunction and disorder) according to the therapeutic classification of MT. Both groups will perform three sessions per week, to complete a total of 24 sessions over eight weeks.
3.3.1Home rehabilitation program
It consists of a home rehabilitation program. Participants will receive TENS and an exercise program following the MT. At the first and second sessions, 1-hour per session, patients will be instructed by a RPT on how to use electroanalgesia and how to perform the exercises.
Each participant will be instructed in the use of a portable TENS (TENStem eco basic, schwa-medico Medizinische Apparate Vertriebsgesellschaft mbH, Wetzlarer/35630/Ehringshausen, Deutschland) of low frequency, high phase duration (80 Hz/200
Figure 2.
Description of the McKenzie exercises prescribed for home.
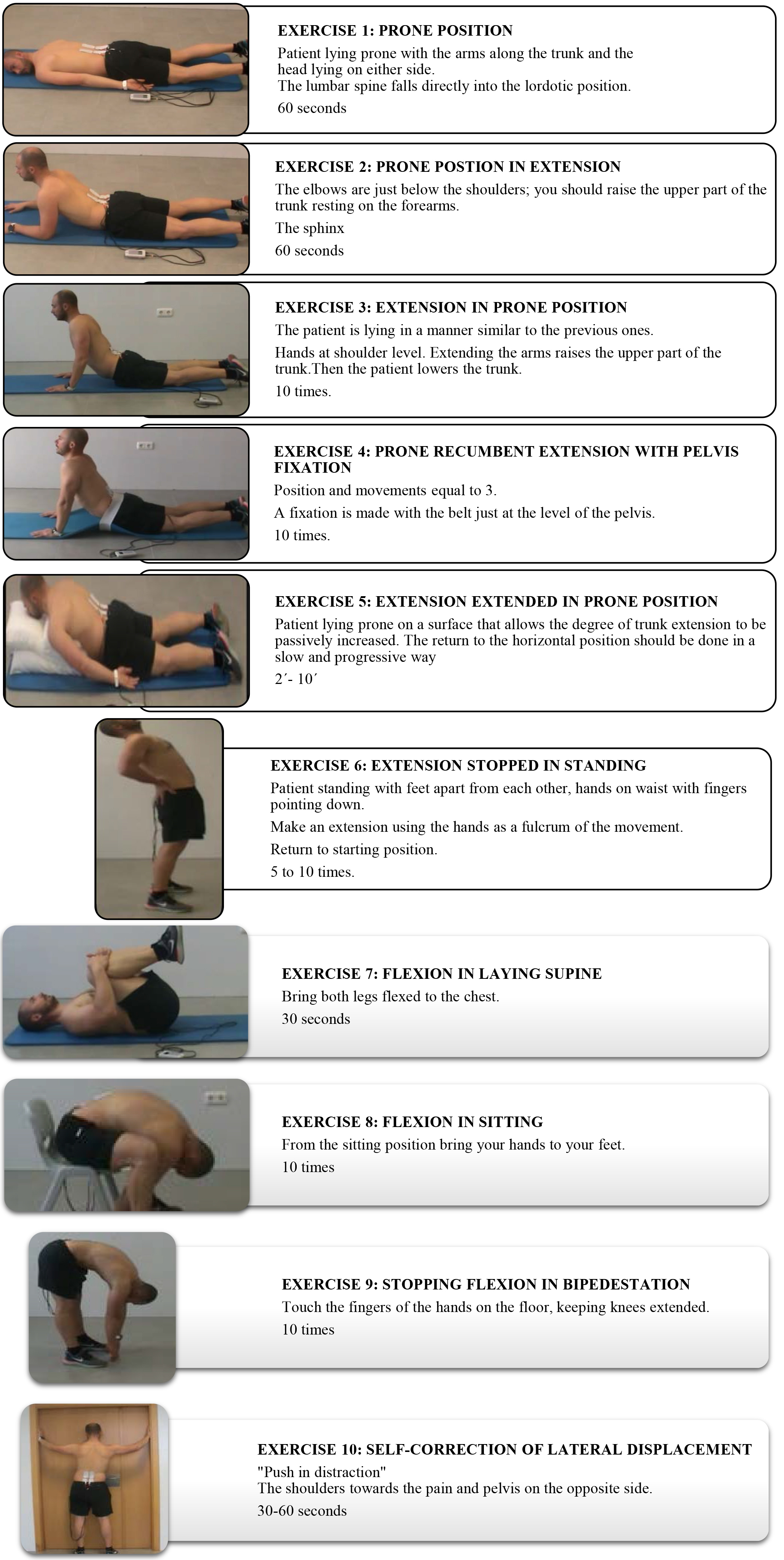
The exercise program will be individualized according to the results obtained in the initial evaluation, and which consists of the following exercises (Fig. 2):
Patients with Postural Syndrome: exercises 1, 2 and 3. Patients with Dysfunction Syndrome: exercises 3, 6 and 7. Patients with Disorder Syndrome (DS): according to the dysfunction syndrome, the following exercises are established for each subgroup:
• DS 1: exercises 1-4 and 6, with extension in recumbency.
• DS 2: the exercises will begin in the prone position and patients will continue with the DS1 protocol and exercise 5.
• DS 3: DS1 protocol, exercise 7 and rotation maintained for 2 minutes.
• DS 4: exercise 7, exercise 2 and 3, and DS 1 protocol.
• DS 5: exercise 7 and 8, and exercise 1–3.
• DS 6: DS4 Protocol, and then the protocol of DS1 and DS3.
• DS 7: exercises 7–10.
3.3.2e-Health program
It is a support system for the treatment of chronic LBP based on web technology, accredited as a health web. This system has a structure based on four sections: database treatment, database of user profiles, recommendations, and feedback procedures. This system allows users to register and enter a subject and modify an electroanalgesia and exercise treatment plan according to the symptomatic evolution of pain. It is based on an initial patient assessment system (Fig. 3).
Figure 3.
Treatment plan according to the symptomatic evolution of pain.
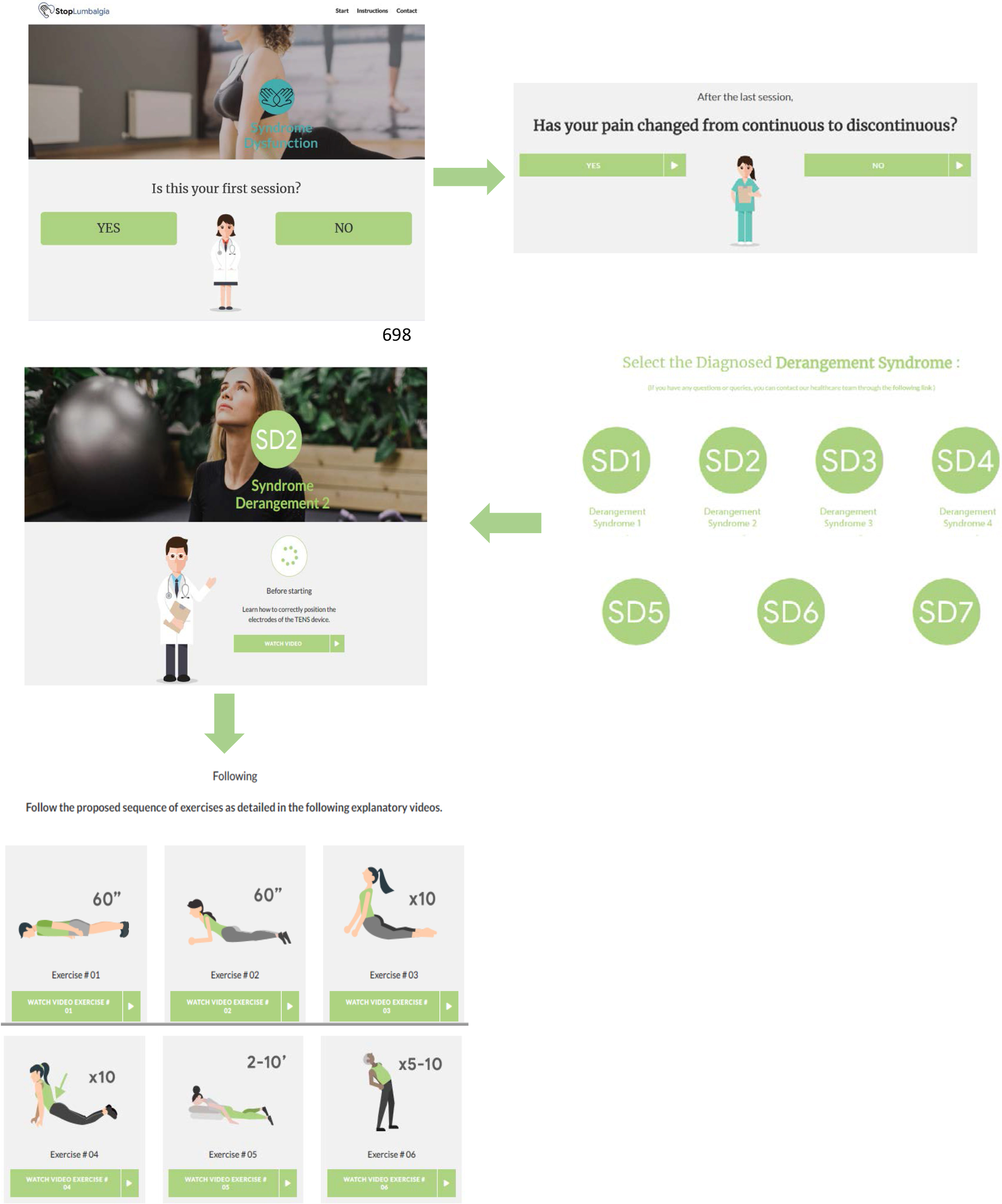
A multimedia database will be developed with examples of specific treatments (according to symptomatic evolution) for postural syndrome, dysfunction syndrome and derangement syndrome. The videos of the application will be shown to patients with combined electroanalgesia and exercise therapy; patients can access the platform using their computer or mobile devices with internet access. The database is configured to accommodate the application of TENS and MT exercises based on the diagnosis according to the Mckenzie method, so that the treatments will be recommended by the system individually. The system calculates and recalculates the recommendations and updates the values of the relevant recommendations for the recommended treatments.
Since many of the patients do not have a tablet to access the study platform entitled “Stop Lumbalgia”, participants in the e-Health group will be given a tablet to each. To ensure patient adherence, control of its inputs is made to the “Stop Lumbalgia” application and the time they spend on it each login. In addition, participants of both groups are called each two weeks to remind and encourage them to perform the exercises. Table 1 summarizes the content of the program e- Health.
The RPTs will complete a 2 days of training on structure, content, evaluation and delivery of the e-Health intervention, which includes role play and comments, learning about the application and its control and a visit to the evaluation site to solve problems of the site for effective development.
3.4Outcome measures
At baseline, demographic data including age, gender, education, occupational and marital status and clinical presentation according a MT evaluation will be documented.
Table 2
Variables, measures and their characteristics
| Variable | Measure | Items | Details | Reliability where available |
|---|---|---|---|---|
| Back-specific physical disability | RMDQ [43] | 24 | This is a self-reported questionnaire consisting of 24 items reflecting limitations in different activities of daily living attributed to low back pain, including walking, bending over, sitting, lying down, dressing, sleeping, self-care and daily activities. | Internal consistency range: 0.77–0.93 [43] |
| Quality of life | EuroQol EQ-5D [48] | 6 | The state of health is defined in 3 Parts. Part 1 define the health status in five dimensions (mobility, personal care, daily activities, pain/discomfort and anxiety/depression mobility). Part 2 features a VAS that records patient’s ratings of overall health. Part 3 is a demographic characterization. | Internal consistency range: 0.67–0.85 [49] |
| SF-36 Health questionnaire [46, 47] | 36 | The SF-36 is a multipurpose, short-form health survey with only 36 questions. It yields an eight-scale profile of scores as well as physical and mental health summary measures. | Internal consistency range: 0.74–0.92 [47] | |
| Disability | ODI [44] | 10 | The Oswestry disability index evaluates daily life activity limitations in 10 dimensions, each scored on a 6-point scale (0–5 points); the total points scored are expressed as a percentage, used to classify individuals as minimally disabled (0–10%), moderately disabled (20–40%), severely disabled (40–60%), crippled (60–80%), or bedbound (80–100%). | Internal consistency range: 0.69–0.87 [45] |
| Pain intensity | VAS [50] | 1 | Patients rate their average pain over the past 2 weeks on a horizontal line with 11 marks on it –from 0 to 10 –where, measuring pain severity, 0 indicates “no pain” and 10 indicates “the worst possible pain”. | Test-retest reliability: 0.67–0.96 [51] |
| Days in pain | Developed specifically for this study | The patients are asked about the number of troublesome days they have spent in pain and which have resulted in absence at work over the previous two months. | No reliability data available | |
| Fear of movement and (re)injury | TSK [52] | 17 | Patient rate beliefs about their pain on a 4-point scale ranging from strongly disagree to strongly agree. | Internal consistency range: 0.70–0.83 [53] |
| Isometric resistance of abdominal muscles | McQuade Test [54] | 1 | The purpose of this test is to compile the times of isometric resistance of the subjects by performing a trunk flexion exercise with flexed knees, and the patient supine. | Internal consistency: 0.97 [54] |
| Forward bending | Fingers-floor distance [55] | 1 | The patient flexes the trunk forward from the standing position, and the distance from the fingers to the ground is measured. | Internal consistency: 0.82 [55] |
| Lumbar mobility | Range of motion and segmental mobility [56] | This variable is quantified using the SpinalMouse | Internal consistency range: 0.92–0.95 [56] | |
| Adherence to specific activities for LBP | Questionnaire developed for this trial | 6 | Patients are asked about the number of weeks that they have fully completed the program. They are also asked for an estimate of how many days a week they did Mckenzie exercises and the electroanalgesia protocol with TENS. Patients are also asked if they stopped doing the activities because they are no longer experiencing pain. | No reliability data available |
*Abbreviations: EQ-5D-3L: EuroQol 5 Dimensions 3 Levels; RMDQ
The primary outcomes are related to the feasibility of the e- Health intervention and trial design and procedure. For participants, this will be assessed by questionnaires (see Table 2) of treatment acceptability and demand of treatment and trial participation. For physiotherapists, a variety of aspects of feasibility will be evaluated according to the expectations of the treatment questionnaires.
In addition, the feasibility of the design and trial procedures will be assessed through experimentation of methodological procedures, recruitment, intervention procedures, the number and reasons for withdrawal during the treatment process, the feasibility outcome measurement, follow-up and fidelity procedures, refining factors that influence the implementation of the intervention.
The selected secondary outcome measures are the disability will be evaluated using Roland Morris Disability Questionnaire (RMDQ) [42, 43] and the Oswestry Disability Index (ODI) [44, 45]; quality of life will be assessed using the SF-36 Quality of Life Questionnaire [46, 47] and the EuroQol (EQ) 5D-5L [48, 49], the pain intensity and days that pain incapacitates for work by the by Visual Analogic Scale (VAS) [50, 51] and an ítem developed specifically for this feasibility protocol; kinesiophobia (fear of movement and re-injury) will be assessed using the Tampa Scale Kinesiophobia (TSK) [52, 53], isometric resistance of the abdominal muscles will evaluated by the McQuade test [54]; and range of motion of the trunk in flexion and in the sagittal plane by the fingertip-to-floor test and the SpinalMouse
Measurement variables will be evaluated before the first treatment session (baseline data), after the 8-week intervention period (immediately after the last session, i.e. 2 months), and after six months after the last session (follow-up).
The patients’ adherence to the internet intervention will be explored by a brief questionnaire developed for this trial that will examine the data of use of the objective intervention automatically collected by the internet intervention e-Health. While the group that performs the program at home without electronic support will use a diary to record the same data as the e-Health group.
3.5Timeline
The recruitment of patients started on September 05, 2020 and will be completed by April 30, 2021. All data for all follow-up occasions is expected to be collected before to October 31, 2021. The data analysis, writing of scientific manuscripts and submissions to peer-reviewed scientific journals will be carried out during 2021–22.
3.6Sample size
The sample size was calculated according to the specifications established by Willian [57]. Assuming a standard deviation of 2.5 points, a 2-tailed test, an alpha (
3.7Data analysis plan
An a priori data analysis plan will be implemented by the trial statistician on completion of data collection. The test statistician will carry out an a priori data analysis plan at the end of the data collection. For the primary analysis, as it is a feasibility study, an exhaustive descriptive analysis of the data is performed. A combination of quantitative (i.e., direct observation and audio recording by researcher, RPT and GPs self-report) and qualitative methods (interviews with intervention RPTs and participants) will be used to respond to the objectives related to the feasibility of the intervention and the trial procedures. Fidelity will be assessed and reported by separate evaluators from the outcome evaluators [63]. These data will determine the feasibility and will improve the study design for a future definitive trial.
Data will be analyzed with SPSS version 21.0 and STATA 14 software and will follow intention-to-treat principles. The analysis of the data of the secondary outcome measure will be carried out at the end of the trial and will be performed by the statistician who will remain blinded to the identification of the group until the analysis is completed. Baseline demo-graphic and clinical variables will be examined between both groups’ independent Stu-dent t-test for continuous data and
3.8Adverse effects
The risk of adverse events occurring as a consequence of the interventions in this trial is low. All activities and their intensity (specific McKenzie exercises for the lower back and TENS) will be recommended based on the individual signs and symptoms of each participant and will be described in detail in paper format or through internet support in group e-Health. Patients will be reminded that the assigned level should be comfortable for them. It will be amended quickly if the patient feels that the initial level is too high.
Participants must inform the RPTs of any adverse effects/events, and they will be responsible for communicating it immediately to the IP. The treatment will be modified or interrupted if necessary, and the type, frequency and duration of the effect will be documented if it occurs.
3.9Ethics, data security and dissemination
The protocol was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Human Research and Local Ethics Committee of the “Hospital Complex Torrecárdenas of Almeria, University Hospital Complex of Granada and Virgen Macarena de Sevilla Hospital – Andalusian Health Service” (CFS/apg).
All patients, GPs and RPTs will receive specific information on the study in writing and will have a chance to discuss procedures with a member of the study team before consenting to take part. Participants that agree to participate in the study will sign two copies of the informed consent, one that will be kept in the trial records and one for the participant. Informed consent has been obtained for the clinical and personal images and details of patients included in this study (Fig. 2).
The data collected from each patient will be stored in a closed locker in an office of the University of Almeria and only the RPTs evaluators will have access to that information. Subsequently, the data will be entered and saved by the statistician on a laptop with password protection to maintain confidentiality. Eligibility criteria, results and analysis will not be modified after registration of the first participant. The feasibility results will be published in journals indexed in the Journal Citations Report and presented at national and international conferences.
4.Discussion
In this randomized controlled trial we intend investigate the effectiveness of an e-Health programs versus home rehabilitation programs in patients with chronic LBP. The difference between both programs is that the e-Health group has constant and remote information on the exercises through a web platform.
4.1Strengths
Considering that the adherence to home exercise programmes ranges from 50% to 70% [64, 65], and that some studies have shown that patients who do not adhere to home exercise regimens benefit less from treatment than those that do [66]. That lack of adherence to treatment in patients with low back pain could be facilitated by using computer systems to make exercise programs more attractive [67]. Previous studies show that patients prefer short, simple exercise programmes, and prefer their therapist to be knowledgeable about their disease, encourage feedback, motivate them to learn, give them re-minders and monitor their results and adherence to the programme [68]. As can be seen in Table 2, also through this study we intend to know the preferences and adherence of the patient and the opinion of physiotherapists and GPs on the e-Health intervention (web applications and learning sessions), maintaining a constant feedback with the patient, and recording if the sessions are appropriate and satisfactory to obtain the expected results.
If data are obtained on patient preferences, and adequate feedback is given to achieve adherence, the new technologies could allow physical therapists to provide their patients with the treatment, follow-up and remote contact they require.
Through the specific e-Health programs at home, could increase adherence to treatment, patients could learn to control and self-manage the evolution of their LBP, preventing its evolution to stages of greater pain and disability. If the painful symptomatology improves could be cost-effective healthcare tool that can reach a large number of people living in rural or remote areas.
4.2Limitations
The main limitation of the present study is the problem of adherence to the e-Health program due to the difficulty of accessing the web application in certain population centers, such as those belonging to rural population groups. This limitation is mitigated by providing 15 tablets of 10,1” Quad Core (Supernova Qi16, Leotec) in each study center, together with a 5-session course on their use.
Authors should discuss the results and how they can be interpreted from the perspective of previous studies and of the working hypotheses. The findings and their implications should be discussed in the broadest context possible. Future research directions may also be highlighted.
5.Conclusions
The new technologies could allow physical therapists to provide their patients with the treatment, follow-up and remote contact they require. Through the specific e-Health programs at home, could increase adherence to treatment, patients could learn to control and self-manage the evolution of their LBP.
Author contributions
AMCS: Conceptualization, methodology, writing-original draft, writing-review and editing, supervision, and project administration ICLP: Conceptualization, methodology, investigation, formal analysis, and writing-review and editing EAS, GAMP, DHO, JMC and HGL: Conceptualization, methodology, and writing–review and editing. All authors read and approved the final manuscript. All authors have read and agreed to the published version of the manuscript.”
Funding
This work was supported by a research project grant (PI18/00562 Proyecto E-CEPEDOL co-funded by FEDER – European Regional Development Fund/European Social Fund “A way to make Europe”/“Investing in your future)from the Carlos III Health Institute and two grant from the Andalusian Health Service, Junta de Andalucía (PC-0185-2017 and PC-0536-2017). The funders did not have any part in the design of the study, its implementation, analysis, data interpretation an/or presentation of the results”.
Conflict of interest
The authors declare no conflict of interest.
References
[1] | Krismer M, van Tulder M. Strategies for prevention and management of musculoskeletal conditions. Low back pain (non-specific). Best Pract Res Clin Rheumatol. (2007) ; 21: : 77-91. doi: 10.1016/j.berh.2006.08.004. PMID: 17350545. |
[2] | Delitto A, George SZ, Van-Dillen L, Whitman JM, Sowa GA. Low back pain: Clinical practice guidelines linked to the international classification of functioning, disability, and healh for the Orthopaedic section of the American Physical Therapy Association. J Orthop Phys Ther. (2012) ; 42: (4): A1-57. doi: 10.2519/jospt.2012.42.4.A1. |
[3] | Wolter T, Szabo E, Becker R, Mohadjer M, Knoeller SM. Chronic low back pain: course of disease from the patient’s perspective. Int Orthop. (2011) ; 35: : 717-724. doi: 10.1007/s00264-010-1081-x. Epub 2010 Jul 10. PMID: 20623120; PMCID: PMC3080499. |
[4] | Mamchikanti L, Singh V, Falco F, Benyamin R, Hirsch J. Epidemiology of low back pain in adults. Neuromodulation. (2014) ; 17: : 3-10. doi: 10.1007/s00264-010-1081-x. Epub 2010 Jul 10. PMID: 20623120. |
[5] | Hoy D, Bain C, Williams G, March L, Brooks P, Blyth F, Woolf A, Vos T, Buchbinder R. A systematic review of the global prevalence of low back pain. Arthritis Rheum. (2012) Jun; 64: (6): 2028-37. doi: 10.1002/art.34347. Epub 2012 Jan 9. Review. PubMed PMID: 22231424. |
[6] | Walker B. The prevalence of low back pain: a systematic review of the literature from 1966 to 1998. J Spinal Disord (2000) ; 13: : 205-17. doi: 10.1097/00002517-200006000-00003. PMID: 10872758. |
[7] | Deyo R, Cherkin D, Conrad D, et al. Cost, controversy, crisis: low back pain and the health of the public. Annu Rev Public Health. (1991) ; 12: : 141-56. doi: 10.1146/annurev.pu.12.050191.001041. PMID: 1828670. |
[8] | Van Tulder M, Koes B, Bombardier C. Low back pain. Best Pract Res Clin Rheumatol. (2002) ; 16: : 761-75. doi: 10.1053/berh.2002.0267. PMID: 12473272. |
[9] | Kovacs FM, Fernández C, Cordero A, Muriel A, González-Luján L, Gil del Real MT. Spanish Back Pain Research Network. Non-specific low back pain in primary care in the Spanish National Health Service: a prospective study on clinical outcomes and determinants of management. BMC Health Serv Res. (2006) ; 6: : 57. doi: 10.1186/1472-6963-6-57. PMID: 16707005; PMCID: PMC1479820. |
[10] | Yang H, Haldeman S, Lu ML, Baker D. Low back pain prevalence and related workplace psychosocial risk factors: a study using data from the 2010 National Health Interview Survey. J Manipulative Physiol Ther. (2016) ; 39: (7): 459-472. doi: 10.1016/j.jmpt.2016.07.004. Epub 2016 Aug 25. PMID: 27568831; PMCID: PMC5530370. |
[11] | Waddell G, Burton AK. Occupational health guidelines for the management of low back pain at work: evidence review. Occup Med. (2001) ; 51: : 124-135. doi: 10.1093/occmed/51.2.124. PMID: 11307688. |
[12] | Herman PM, Lavelle TA, Sorbero ME, Hurwitz EL, Coulter ID. Are Nonpharmacologic Interventions for Chronic Low Back Pain More Cost Effective Than Usual Care? Proof of Concept Results From a Markov Model. Spine (Phila Pa 1976). (2019) ; 44: (20): 1456-1464. doi: 10.1097/BRS.0000000000003097. PubMed PMID: 31095119; PubMed Central PMCID: PMC6779140. |
[13] | Traeger A, Buchbinder R, Harris I, Maher C. Diagnosis and management of low-back pain in primary care. CMAJ. (2017) ; 189: (45): E1386-E1395. doi: 10.1503/cmaj.170527. PMID: 29133540; PMCID: PMC5687927. |
[14] | May S. Self-management of chronic low back pain and osteoarthritis. Nat Rev Rheumatol. (2010) ; 6: : 199-209. doi: 10.1038/nrrheum.2010.26. PMID: 20357789. |
[15] | National Guideline Centre (UK). Low Back Pain and Sciatica in Over 16s: Assessment and Management. London: National Institute for Health and Care Excellence (UK); (2016) Nov. PMID: 27929617. |
[16] | Hayden JA, van Tulder MW, Malmivaara A, Koes BW. Exercise therapy for treatment of non-specific low back pain. Cochrane Database Syst Rev. (2005) ; (3): CD000335. doi: 10.1002/14651858.CD000335.pub2. PMID: 16034851. |
[17] | van Middelkoop M, Rubinstein SM, Kuijpers T, Verhagen AP, Ostelo R, Koes BW, van Tulder MW. A systematic review on the effectiveness of physical and rehabilitation interventions for chronic non-specific low back pain. Eur Spine J. (2011) ; 20: (1): 19-39. doi: 10.1007/s00586-010-1518-3. Epub 2010 Jul 18. PMID: 20640863; PMCID: PMC3036018. |
[18] | Delitto A, George SZ, Van Dillen L, Whitman JM, Sowa G, Shekelle P, Denninger TR, Godges JJ. Low back pain. J Orthop Sports Phys Ther. (2012) ; 42: (4): A1-57. doi: 10.2519/jospt.2012.42.4.A1. Epub 2012 Mar 30. PubMed PMID: 22466247; PubMed Central PMCID: PMC4893951. |
[19] | Köke AJ, Schouten JS, Lamerichs-Geelen MJ, Lipsch JS, Waltje EM, van Kleef M, Patijn J. Pain reducing effect of three types of transcutaneous electrical nerve stimulation in patients with chronic pain: a randomized crossover trial. Pain. (2004) Mar; 108: (1-2): 36-42. doi: 10.1016/j.pain.2003.11.013. PMID: 15109505. |
[20] | Jauregui JJ, Cherian JJ, Gwam CU, Chughtai M, Mistry JB, Elmallah RK, Harwin SF, Bhave A, Mont MA. A Meta-Analysis of Transcutaneous Electrical Nerve Stimulation for Chronic Low Back Pain. Surg Technol Int. (2016) ; 28: : 296-302. Review. PubMed PMID: 27042787. |
[21] | Dailey DL, Rakel BA, Vance CG, et al. Transcutaneous electrical nerve stimulation reduces pain, fatigue and hyperalgesia while restoring central inhibition in primary fibromyalgia. Pain. (2013) ; 154: : 2554-2562. doi: 10.1016/j.pain.2013.07.043. Epub 2013 Jul 27. PMID: 23900134; PMCID: PMC3972497. |
[22] | DeSantana JM, da Silva LF, De Resende MA, Sluka KA. Transcutaneous electrical nerve stimulation at both high and low frequencies activates ventrolateral periaqueductal grey to decrease mechanical hyperalgesia in arthritic rats. Neuroscience. (2009) ; 163: : 1233-1241. doi: 10.1016/j.neuroscience.2009.06.056. Epub 2009 Jul 2. PMID: 19576962; PMCID: PMC3955259. |
[23] | Johnson MI, Paley CA, Howe TE, Sluka KA. Transcutaneous electrical nerve stimulation for acute pain. Cochrane Database Syst Rev. (2015) ; (6): CD006142. doi: 10.1002/14651858.CD006142.pub3. PMID: 26075732. |
[24] | Khadilkar A, Odebiyi DO, Brosseau L, Wells GA. Transcutaneous electrical nerve stimulation (TENS) versus placebo for chronic low-back pain. Cochrane Database Syst Rev. (2008) ; 2008: (4): CD003008. doi: 10.1002/14651858.CD003008.pub3. PMID: 18843638; PMCID: PMC7138213. |
[25] | Machado LA, Kamper SJ, Herbert RD, Maher CG, McAuley JH. Analgesic effects of treatments for non-specific low back pain: a meta-analysis of placebo-controlled randomized trials. Rheumatology (Oxford). (2009) ; 48: (5): 520-7. doi: 10.1093/rheumatology/ken470. Epub 2008 Dec 24. PMID: 19109315. |
[26] | Claydon LS, Chesterton LS, Barlas P, Sim J. Dose-specific effects of transcutaneous electrical nerve stimulation (TENS) on experimental pain: a systematic review. Clin J Pain. (2011) ; 27: (7): 635-47. doi: 10.1097/AJP.0b013e31821962b4. PMID: 21562411. |
[27] | Vance CG, Dailey DL, Rakel BA, Sluka KA. Using TENS for pain control: the state of the evidence. Pain Manag. (2014) ; 4: (3): 197-209. doi: 10.2217/pmt.14.13. PMID: 24953072; PMCID: PMC4186747. |
[28] | Deyo RA, Walsh NE, Martin DC, Schoenfeld LS, Ramamurthy S. A controlled trial of transcutaneous electrical nerve stimulation (TENS) and exercise for chronic low back pain. N Engl J Med. (1990) ; 322: (23): 1627-34. doi: 10.1056/NEJM199006073222303. PMID: 2140432. |
[29] | Mann SJ, Lam JC, Singh P. McKenzie Back Exercises. 2020 Jul 10. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; (2020) . PMID: 30969542. |
[30] | Clare HA, Adams R, Maher CG. A systematic review of efficacy of McKenzie therapy for spinal pain. Aust J Physiother. (2004) ; 50: (4): 209-16. doi: 10.1016/s0004-9514(14)60110-0. PMID: 15574109. |
[31] | Busanich BM, Verscheure SD. Does McKenzie therapy improve outcomes for back pain? J Athl Train. (2006) ; 41: (1): 117-9. PMID: 16619104; PMCID: PMC1421491. |
[32] | Garcia AN, Costa Lda C, da Silva TM, Gondo FL, Cyrillo FN, Costa RA, Costa LO. Effectiveness of back school versus McKenzie exercises in patients with chronic nonspecific low back pain: a randomized controlled trial. Phys Ther. (2013) ; 93: (6): 729-47. doi: 10.2522/ptj.20120414. Epub 2013 Feb 21. PMID: 23431213. |
[33] | Sebbag E, Felten R, Sagez F, Sibilia J, Devilliers H, Arnaud L. The world-wide burden of musculoskeletal diseases: a systematic analysis of the World Health Organization Burden of Diseases Database. Ann Rheum Dis. (2019) ; 78: (6): 844-848. doi: 10.1136/annrheumdis-2019-215142. Epub 2019 Apr 15. PMID: 30987966. |
[34] | Mbada CE, Olaoye MI, Dada OO, Ayanniyi O, Johnson OE, Odole AC, Ishaya GP, Omole OJ, Makinde MO. Comparative Efficacy of Clinic-Based and Telerehabilitation Application of Mckenzie Therapy in Chronic Low-Back Pain. Int J Telerehabil. (2019) ; 11: (1): 41-58. doi: 10.5195/ijt.2019.6260. PMID: 31341546; PMCID: PMC6597146. |
[35] | Cottrell MA, O’Leary SP, Raymer M, Hill AJ, Comans T, Russell TG. Does telerehabilitation result in inferior clinical outcomes compared with in-person care for the management of chronic musculoskeletal spinal conditions in the tertiary hospital setting? A non-randomised pilot clinical trial. J Telemed Telecare. (2019) , doi: 10.1177/1357633X19887265. Epub ahead of print. PMID: 31771410. |
[36] | Hurley MV, Walsh N, Bhavnani V, Britten N, Stevenson F. Health beliefs before and after participation on an exercised-based rehabilitation programme for chronic knee pain: doing is believing. BMC Musculoskelet Disord. (2010) ; 11: : 31. doi: 10.1186/1471-2474-11-31. PMID: 20149236; PMCID: PMC2828988. |
[37] | Palacín-Marín F, Esteban-Moreno B, Olea N, Herrera-Viedma E, Arroyo-Morales M. Agreement between telerehabilitation and face-to-face clinical outcome assessments for low back pain in primary care. Spine (Phila Pa 1976). (2013) ; 38: (11): 947-52. doi: 10.1097/BRS.0b013e318281a36c. PMID: 23238489. |
[38] | Geraghty AWA, Stanford R, Stuart B, Little P, Roberts LC, Foster NE, Hill JC, Hay EM, Turner D, Malakan W, Leigh L, Yardley L. Using an internet intervention to support self-management of low back pain in primary care: findings from a randomised controlled feasibility trial (SupportBack). BMJ Open. (2018) ; 8: (3): e016768. doi: 10.1136/bmjopen-2017-016768. PMID: 29525768; PMCID: PMC5879455. |
[39] | Lindberg B, Nilsson C, Zotterman D, Söderberg S, Skär L. Using Information and Communication Technology in Home Care for Communication between Patients, Family Members, and Healthcare Professionals: A Systematic Review. Int J Telemed Appl. (2013) ; 2013: : 461829. doi: 10.1155/. Epub 2013 Apr 10. PMID: 23690763; PMCID: PMC3649237. |
[40] | Chan A-W, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, Hróbjartsson A, Mann H, Dickersin K, Berlin J, Doré C, Parulekar W, Summerskill W, Groves T, Schulz K, Sox H, Rockhold FW, Rennie D, Moher D. SPIRIT 2013 Statement: Defining standard protocol items for clinical trials. Ann Intern Med. (2013) ; 158: (3): 200-207. doi: 10.7326/0003-4819-158-3-201302050-00583. PMID: 23295957; PMCID: PMC5114123. |
[41] | Mokhtari T, Ren Q, Li N, Wang F, Bi Y, Hu L. Transcutaneous Electrical Nerve Stimulation in Relieving Neuropathic Pain: Basic Mechanisms and Clinical Applications. Curr Pain Headache Rep. (2020) Feb 18; 24: (4): 14. doi: 10.1007/s11916-020-0846-1. PMID: 32072323. |
[42] | Roland M, Fairbank J. The Roland-Morris Disability Questionnaire and the Oswestry Disability Questionnaire. Spine (Phila Pa 1976). 2000: ; 25: 3115-24. doi: 10.1097/00007632-200012150-00006. Erratum in: Spine 2001; 26: (7): 847. PMID: 11124727. |
[43] | Müller U, Duetz MS, Roeder C, Greenough CG. Condition-specific outcome measures for low back pain. Part I: validation. Eur Spine J. (2004) ; 13: (4): 301-13. doi: 10.1007/s00586-003-0665-1. Epub 2004 Mar 17. PMID: 15029488; PMCID: PMC3468051. |
[44] | Ferrer M, Pellisé F, Escudero O, Alvarez L, Pont A, Alonso J, Deyo R. Validation of a minimum outcome core set in the evaluation of patients with back pain. Spine (Phila Pa 1976). (2006) ; 31: (12): 1372-9. discussion 1380. doi: 10.1097/. PMID: 16721302. |
[45] | Chiarotto A, Maxwell LJ, Terwee CB, Wells GA, Tugwell P, Ostelo RW. Roland-Morris Disability Questionnaire and Oswestry Disability Index: Which Has Better Measurement Properties for Measuring Physical Functioning in Nonspecific Low Back Pain? Systematic Review and Meta-Analysis. Phys Ther. (2016) ; 96: (10): 1620-1637. doi: 10.2522/ptj.20150420. Epub 2016 Apr 14. PMID: 27081203. |
[46] | Ware JE. SF-36 health survey update. Spine (Phila Pa 1976). 2000: ; 25: (24): 3130-3139. doi: 10.1097/00007632-200012150-00008. PMID: 11124729. |
[47] | Vilagut G, Ferrer M, Rajmil L, Rebollo P, Permanyer-Miralda G, Quintana JM, Santed R, Valderas JM, Ribera A, Domingo-Salvany A, Alonso J. El Cuestionario de Salud SF-36 español: una década de experiencia y nuevos desarrollos [The Spanish version of the Short Form 36 Health Survey: a decade of experience and new developments]. Gac Sanit. (2005) ; 19: (2): 135-50. Spanish. doi: 10.1157/13074369. PMID: 15860162. |
[48] | Rabin R, Charro F. EQ-SD: a measure of health status from the EuroQol Group. Ann Med. (2009) ; 33: (5): 337-4. doi: 10.3109/07853890109002087. PMID: 11491192. |
[49] | Ye Z, Sun L, Wang Q. A head-to-head comparison of EQ-5D-5 L and SF-6D in Chinese patients with low back pain. Health Qual Life Outcomes. (2019) ; 17: (1): 57. doi: 10.1186/s12955-019-1137-6. PMID: 30971265; PMCID: PMC6458837. |
[50] | Crichton N. Information point: Visual analogue scale (VAS). J Clin Nurs. (2001) ; 10: (5): 706-706. |
[51] | Kahl C, Cleland JA. Visual analogue scale, numeric pain rating scale, and the McGill pain questionnaire: an overview of psychometric properties. Phys Ther Rev. (2005) ; 10: : 123-8. doi: 10.1179/108331905X55776. |
[52] | Kori SH, Miller RP, Todd DD. Kinesiophobia: a new view of chronic pain behavior. Pain Manag. (1990) ; 3: : 35-43. doi: 10.1179/108331905X55776. |
[53] | Swinkels-Meewisse EJ, Swinkels RA, Verbeek AL, Vlaeyen JW, Oostendorp RA. Psychometric properties of the Tampa Scale for kinesiophobia and the fear-avoidance beliefs questionnaire in acute low back pain. Man Ther. (2003) ; 8: (1): 29-36. doi: 10.1054/math.2002.0484. PMID: 12586559. |
[54] | McGill SM, Childs A, Liebenson C. Endurance times for low back stabilization exercises: clinical targets for testing and training from a normal database. Arch Phys Med Rehabil. (1999) ; 80: (8): 941-4. doi: 10.1016/s0003-9993(99)90087-4. PMID: 10453772. |
[55] | Frost M, Sutckey S, Samelley LA. Dorman G. Reliability of measuring trunk motions in centimetres. Phys Ther. (1982) ; 62: : 1431-1438. doi: 10.1093/ptj/62.10.1431. PMID: 7122701. |
[56] | Post RB, Leferink VJM. Spinal mobility: sagittal range of motion measured with the SpinalMouse, a new non-invasive device. Archives of Orthopaedic and Trauma Surgery. (2004) ; 124: (3): 187-192. doi: 10.1007/s00402-004-0641-1. Epub 2004 Feb 14. PMID: 14968367. |
[57] | Willan AR. Analysis, sample size, and power for estimating incremental net health benefit from clinical trial data. Control Clin Trials. (2001) ; 22: (3): 228-37. doi: 10.1016/s0197-2456(01)00110-6. PMID: 11384787. |
[58] | Ostelo RW, de Vet HC. Clinically important outcomes in low back pain. Best Pract Res Clin Rheumatol. (2005) ; 19: (4): 593-607. doi: 10.1016/j.berh.2005.03.003. PMID: 15949778. |
[59] | Browne RH. On the use of a pilot sample for sample-size determination. Stat Med (1995) ; 14: : 1933-40. doi: 10.1002/sim.4780141709. PMID: 8532986. |
[60] | Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract. (2004) ; 10: : 307-12. doi: 10.1111/j..2002.384.doc.x. PMID: 15189396. |
[61] | Shih WJ, Ohman-Strickland PA, Lin Y. Analysis of pilot and early phase studies with small sample sizes. Stat Med (2004) ; 23: : 1827-42. doi: 10.1002/sim.1807. PMID: 15195318. |
[62] | Lonsdale C, Hall AM, Williams GC, McDonough SM, Ntoumanis N, Murray A, Hurley DA. Communication style and exercise compliance in physiotherapy (CONNECT): a cluster randomized controlled trial to test a theory-based intervention to increase chronic low back pain patients’ adherence to physiotherapists’ recommendations: study rationale, design, and methods. BMC Musculoskelet Disord. (2012) ; 13: : 104. doi: 10.1186/1471-2474-13-104. PMID: 22703639; PMCID: PMC3475041. |
[63] | Borelli B. The assessment, monitoring and enhancement of treatment fidelity in public health clinical trials. J Public Health Dent (2011) ; 71: : S52-63. |
[64] | Holden MA, Haywood KL, Potia TA, Gee M, McLean S. Recommendations for exercise adherence measures in muscu-loskeletal settings: a systematic review and consensus meeting (protocol). Syst Rev. (2014) ; 3: : 10. doi: 10.1186/2046-4053-3-10. PMID: 24512976; PMCID: PMC3923391. |
[65] | Medina-Mirapeix F, Escolar-Reina P, Gascon-Canovas JJ, Montilla-Herrador J, Jimeno-Serrano FJ, Collins SM. Predictive factors of adherence to frequency and duration components in home exercise programs for neck and low back pain: an observational study. BMC Musculoskelet Disord. (2009) ; 10: : 155. doi: 10.1186/1471-2474-10-155. PMID: 19995464; PMCID: PMC2796992. |
[66] | Thomas KS, Muir KR, Doherty M, Jones AC, O’Reilly SC, Bassey EJ. Home based exercise programme for knee pain and knee osteoarthritis: randomised controlled trial. BMJ. (2002) ; 325: (7367): 752. doi: 10.1136/bmj.325.7367.752. PMID: 12364304; PMCID: PMC128377. |
[67] | Palazzo C, Klinger E, Dorner V, Kadri A, Thierry O, Boumenir Y, Martin W, Poiraudeau S, Ville I. Barriers to home-based exercise program adherence with chronic low back pain: Patient expectations regarding new technologies. Ann Phys Rehabil Med. (2016) ; 59: (2): 107-13. doi: 10.1016/j.rehab.2016.01.009. Epub 2016 Apr 1. PMID: 27050664. |
[68] | Escolar-Reina P, Medina-Mirapeix F, Gascón-Cánovas JJ, Montilla-Herrador J, Jimeno-Serrano FJ, de Oliveira Sousa SL, del Baño-Aledo ME, Lomas-Vega R. How do care-provider and home exercise program characteristics affect patient adherence in chronic neck and back pain: a qualitative study. BMC Health Serv Res. (2010) ; 10: : 60. doi: 10.1186/1472-6963-10-60. PMID: 20219095; PMCID: PMC2847560. |




