Efficacy and safety of kinesiology tape for hemiplegic shoulder pain: A systematic review and meta-analysis of randomized controlled trials
Abstract
OBJECTIVE:
The aim of this study was to examine the efficacy and safety of kinesiology tape in treating hemiplegic shoulder pain.
METHODS:
Web of Science, MEDLINE, Embase, Cochrane Library, six other English databases and three Chinese databases (CNKI, VIP, Wan Fang) were searched for randomized controlled trials published prior to December 13, 2020 in English or Chinese on the use of kinesiology tape for hemiplegic shoulder pain.
RESULTS:
Fourteen randomized controlled trials (679 patients) of good PEDro quality (6.43
CONCLUSION:
KT relieved pain and improved the ROM, DAH and FMA-UE score in patients with HSP to a greater extent than did the sham KT or blank control conditions. The effects on independence in activities of daily living and quality of life and whether this method is superior to active treatment in patients with HSP were not verified. More rigorous, reasonably designed RCTs with large sample sizes are still needed in the future.
1.Introduction
Hemiplegic shoulder pain (HSP) is a common complication that occurs in a significant proportion of stroke patients. Nadler et al. reported that 17% of surviving stroke patients developed shoulder pain within the first week and 22–40% developed it within the first 4–6 months [1]. In the general population, the prevalence of HSP ranges from 6.9% to 26% for point prevalence and up to 66.7% for lifetime prevalence. Differences in inclusion criteria and measurement approaches across previous studies may be causes of these wide ranges of incidence rates of HSP [2]. HSP can hinder an individual’s ability to perform muscle contractions and use the limb [3]. In addition, HSP makes it difficult for individuals to exercise, slows motor function recovery and delays the rehabilitation process [4]. Decreasing athletic ability due to HSP can influence individuals’ ability to perform activities of daily living and limit social participation [5]. The precise etiology of HSP is not very clear. Some studies have reported that it might involve both peripheral and central neuropathic mechanisms [2, 6], while others have reported that is associated with altered somatosensory function and reduced cognitive-evaluative cortical somatosensory processing [6, 7, 8]. Currently, a common view is that rotator cuff injuries, subluxation of the humeral head, motor weakness, and spasticity are the primary contributors to poststroke HSP [9]. Despite the limited understanding of the etiology of HSP, various treatments, such as neuromuscular electrical stimulation (NMES), acupuncture, strapping, sling, handling, shoulder positioning, massage and pharmacological therapy, have been studied for the treatment of HSP over the past few decades [10]. Nevertheless, none of these treatments have been shown to have significant efficacy or strong supporting evidence [11].
Kinesiology tape (KT) is a common tool used in sports and rehabilitation [12]. Because hypotonicity in the early stages of stroke is an initiating factor for HSP, KT is usually used as a therapeutic tool for HSP to support the shoulder muscles [13, 14]. A systematic review with a large sample size confirmed there is an association between shoulder subluxation and HSP [15]. Although KT can relieve pain and improve shoulder motor function [16], there is still no conclusive evidence to support the routine application of KT for HSP [2]. Thus, we conducted a systematic review and meta-analysis to examine the efficacy and safety of KT for HSP.
2.Methods
2.1Search strategy
Electronic databases (Web of Science, MEDLINE, Embase, Cochrane Library, Wiley, Springer, Sciences Direct, Karger, PEDro, Scopus and CNKI, VIP, Wan Fang) were searched for articles published prior to December 13, 2020. The search terms were based on the PICOS principle (in Web of Science): (“Stroke” OR “shoulder pain” OR “hemiplegic shoulder pain” OR “shoulder subluxation” OR “shoulder-hand syndrome” OR “hand-shoulder syndrome” OR “hemiplegic hand”) AND (“Kinesio Taping” OR “kinesio tex tape” OR “Kinesiology tape” OR “Kinesio Tape” OR “Kinesiology Taping” OR “kinesio tex taping” OR “kinesiological taping” OR “Functional Fascial Taping” OR “Shoulder taping” OR “strapping”) AND (“placebo control” OR “bank control” OR “rehabilitation” OR “conventional treatment”) AND (“randomized controlled trials” OR “RCT” OR “randomized trials” OR “controlled trials”) in the title or abstract. The keywords were modified for the other databases (Supplementary Table 1). In addition, the reference lists of the retrieved articles were manually searched and reviewed. If discrepancies occurred, a consensus was reached through consultation. The searches were limited to studies published in English or Chinese.
2.2Selection criteria
Study eligibility was determined independently by two authors (LLX and ZY) according to the following criteria. (1) For the participants, all of the participants had been diagnosed with ischemic or hemorrhagic stroke by computerized tomography or magnetic resonance imaging and were clinically confirmed to have HSP. (2) For the interventions and comparisons, the KT technique was used in the treatment groups, and a sham KT or blank control group was included. (3) For the outcomes, the primary outcome in the study was pain, as assessed by the visual analog scale (VAS) and the numerical pain rating scale (NPRS). The secondary outcomes included the range of motion (ROM), acromion humeral distance (AHD, distance from the lower margin of the acromion to the head of the humerus), Fugl-Meyer assessment for upper extremities (FMA-UE) score, individual activity (IA, as assessed by the Barthel index (BI), modified Barthel index (MBI), Shoulder Pain and Disability Index (SPADI), and Action Research Arm Test (ARAT)), quality of life (QOL) and adverse events (AE). (4) For the study design, all the only randomized controlled trials (RCTs) that were in English or Chinese and aimed to investigate the efficacy and safety of KT were included. The exclusion criteria were as follows: (1) for the patients, the trial included patients with other conditions leading to hemiplegia (e.g., head injury, shoulder subluxation); (2) for the interventions, the trial administered complex treatment without specifying the sole effects of KT; (3) for the outcomes, the required data for this study were unavailable; and (4) for the design, the study was not an RCT. Disagreements were resolved by two authors (LLX and ZY) through discussion and consultation when necessary. A flowchart of the search process for this review was created in accordance with the 2009 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [17].
2.3Data extraction
Information on the following items was extracted from the included studies: first author, publication year, study period, age, sex, stroke duration, KT and control treatment protocols and measurement data. Based on the International Classification of Functioning, Disability and Health model (ICF) methods, data for the following four domains were extracted for this review: structure and function (pain, ROM, AHD, FMA-UE score), individual activities (MBI, SPADI, ARAT), social participation (QOL) and AEs. If the data sets overlapped or were duplicated, the articles with more information were retained. Disagreements were settled by discussion and consensus between the two authors (LLX and ZY).
2.4Quality assessment
The methodological quality of all eligible RCTs was assessed independently by the two reviewers (LLX and ZY) using the Physiotherapy Evidence Database Scale (PEDro) [18]. The PEDro scale has been shown to have good interpretability and internal and external validity and contains 11 dichotomous (yes or no) items on the eligibility criteria, random allocation, concealment of allocation, group similarity at baseline, blinding of the therapists, blinding of the assessors, availability of key outcome measures of more than 85% of the subjects, intention-to-treat analysis, between-group statistical comparisons, and point measures and measures of variability. One point is awarded when a criterion is satisfied, and the maximum score is 10 points. Studies with scores of 9–10 points were considered to have ‘excellent’ quality, those with scores of 6–8 were of ‘good’ quality, those with scores of 4–5 were of ‘fair’ quality, and those with scores of less than 4 were of ‘poor’ quality. We considered RCTs with a score of
2.5Statistical analysis
The effect size of the continuous data was expressed as the standard mean difference (SMD) and 95% confidence interval (CI). The mean difference (MD) and pooled standard deviation (SD) were calculated based on the methods [20]. MD
Table 1
Methodological quality of the included studies. (PEDro)
| ID | Author | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Scores |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [23] | Gabriela Lopes Dos Santos (2019) | Yes | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 8 |
| [24] | Lin Yang (2018) | Yes | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 7 |
| [25] | Yen-Chang Huang (2017) | Yes | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 7 |
| [26] | Anja Hochsprung (2017) | Yes | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 4 |
| [27] | Yu-Chi Huang (2016) | Yes | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 7 |
| [28] | Subhasish Chatterjee (2016) | Yes | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 7 |
| [29] | Paolo Pillastrini (2016) | Yes | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 8 |
| [30] | Min-Yeong Heo (2015) | Yes | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 7 |
| [31] | Jeyaraj D Pandian (2013) | Yes | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 5 |
| [32] | Deng-Yao Li (2013) | Yes | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 6 |
| [33] | Wen-Yu Zhu (2019) | Yes | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 6 |
| [34] | Fang Wang (2018) | Yes | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 6 |
| [35] | Bo-Han Shi (2018) | Yes | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 6 |
| [36] | Li-Sheng Zhao (2017) | Yes | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 6 |
Notes: 1, Eligibility criteria; (the item does not contribute to total score, *This score has been confirmed*) 2, Random allocation; 3, Concealed allocation; 4, Baseline comparability; 5, Blind subjects; 6, Blind therapists; 7, Blind assessors; 8, Adequate follow-up; 9, Intention-to-treat analysis; 10, Between-group comparisons; 11, Point estimates and variability.
Figure 1.
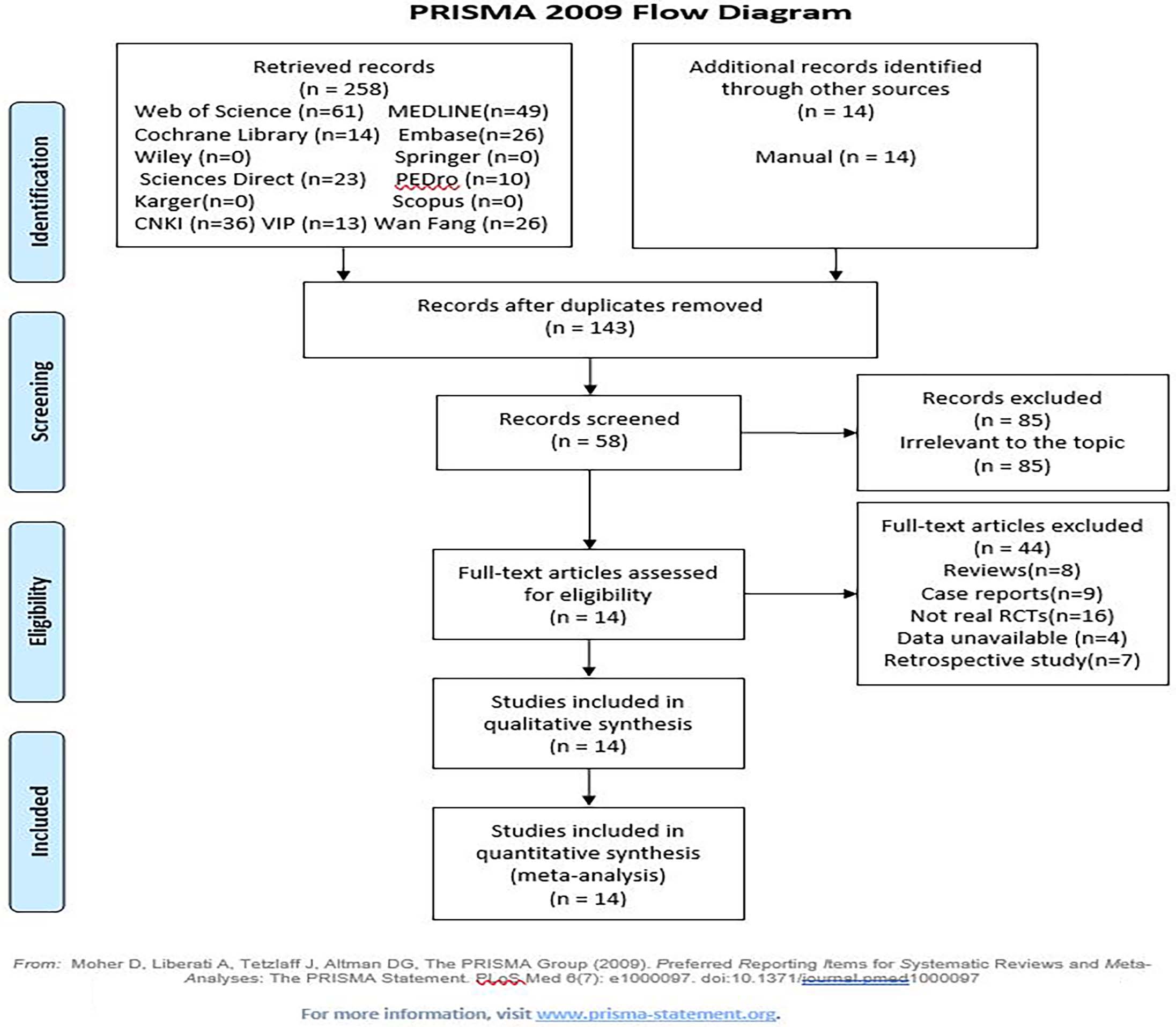
3.Results
3.1Study selection and characteristics
A total of 272 relevant references were initially retrieved. After the titles, abstracts and full texts were screened carefully, 14 studies [23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36] with 679 patients were ultimately included in the meta-analysis. A flowchart of the search process is shown in Fig. 1. The included trials were published from 2013 to 2020. Therapeutic KT was used in the experimental groups, and either sham KT or conventional rehabilitation methods such as exercise therapy and electrotherapy (blank control group) were performed in the control groups.
Table 2
Characteristics of the included studies
| Study ID first author (year) | Patients (E/C) | Intervention | Comparison | Outcomes | Study design |
|---|---|---|---|---|---|
| [23] Gabriela Lopes Dos Santos (2019) | Cases: 13 (6/7) Age: 40–75 Stroke Duration: | E: Muscles: deltoid Width: 5 cm Shape: NR Tension: 10%–15% Time/Frequency: 3 D, taping 3 days C: Blank control | E: True KT | ROM | RCT |
| [24] Lin Yang (2018) | Cases: 19 (10/9) Age: 60 | E: Muscles: Supraspinatus, deltoid, teres minor, biceps Width: 5 cm Shape: I shape Tension: First 4 cm: no tension, the rest: 15%–50% Time/Frequency: 10–12 h/Day C: Sham KT Tension: neutral The other parameters were same with E group | E: True KT | NPRS, ROM, ADH | RCT |
| [25] Yen-Chang Huang (2017) | Cases: 21 (11/10) Age: 59 | E: Muscles: Supraspinatus, deltoid, biceps Width: 5 cm Shape: I shape Tension: 15%–75% Time/Frequency: Taping 3 days without 1 day C: Sham KT Tension: neutral The other parameters were same with E group | E: True KT | NPRS, ROM, SPADI | RCT |
| [26] Anja Hochsprung (2017) | Cases: 14 (7/7) Age: 63.71 | E: Muscles: deltoid Width: 5 cm Shape: I shape Tension: 10%–100% Time/Frequency: 4 W, taping 6 days without 1 day C: Sham KT Time: 10 minutes The other parameters were same with E group | E: True KT | VAS, BI, BBS, ARAT | RCT |
| [27] Yu-Chi Huang (2016) | Cases: 44 (21/23) Age: 60.4 | E: Muscles: Supraspinatus, deltoid Width: NR Shape: I shape Tension: 20%–30% Time/Frequency: Taping 3 days without 1 day C: Sham KT Tension: neutral The other parameters were same with E group | E: True KT | VAS, ROM, ADH, FMA-UE, MBI, SS-QOL | RCT |
| [28] Subhasish Chatterjee (2016) | Cases: 30 (15/15) Age: 63.20/62.80 Stroke Duration: acute stroke | E: Muscles: deltoid Width: NR Shape: NR Tension: NR Time/Frequency: 6 W, 3 days a week C: Blank control | E: True KT | VAS, ROM, ADH, FMA-UE | RCT |
| [29] Paolo Pillastrini (2016) | Cases: 31 (16/15) Age: 66 | E: Muscles: supraspinatus, deltoid, pectoralis major Width: 5 cm Shape: W-shape; Y-shape Tension: NR Time/Frequency: 15 minutes, 4 W, once a week C: Blank control | E: True KT | VAS, ROM, MAS | RCT |
| [30] Min-Yeong Heo (2015) | Cases: 36 (18/18) Age: 57.1 | E: Muscles: supraspinatus, pectoralis Width: NR Shape: NR Tension: NR Time/Frequency: 8 W C: Blank control | E: True KT | VAS, ADH | RCT |
|
Table 2, continued | |||||
|---|---|---|---|---|---|
| Study ID first author (year) | Patients (E/C) | Intervention | Comparison | Outcomes | Study design |
| [31] Jeyaraj D Pandian (2013) | Cases: 162 (80/82) Age: 59 | E: Muscles: NR Width: NR Shape: NR Tension: NR Time/Frequency: NR C: Sham KT Tension: neutral The other parameters were same with E group | E: True KT | VAS, ROM, SPADI | RCT |
| [32] Deng-Yao Li (2020) | Cases: 60 (30/30) Age: 65.5 | E: Muscles: supraspinatus, deltoid, trapezius Width: 5 cm Shape: Y shape; I shape Tension: 10%–50% Time/Frequency: 5 days a week for 4 weeks C: Blank control | E: True KT | VAS, FMA-UE, MBI | RCT |
| [33] Wen-Yu Zhu (2019) | Cases: 93 (47/46) Age: 62.43 | E: Muscles: supraspinatus, deltoid, trapezius Width: 5 cm Shape: X shape; I shape Tension: 10%–50% Time/Frequency: 24 hours, 5 per week for 3 weeks C: Blank control | E: True KT | VAS, FMA-UE | RCT |
| [34] Fang Wang (2018) | Cases: 60 (30/30) Age: 50.93 | E: Muscles: supraspinatus, deltoid, biceps, trapezius Width: 5 cm Shape: I shape; Y shape; X shape Tension: 10%–30% Time/Frequency: 3 days, 8 sessions in 4 weeks C: Sham KT Tension: neutral The other parameters were same with E group | E: True KT | VAS, FMA-UE, MBI | RCT |
| [35] Bohan Shi (2018) | Cases: 56 (28/28) Age: 65.62 | E: Muscles: supraspinatus, deltoid Width: NR Shape: X shape; I shape Tension: NR Time/Frequency: 2 days/session, 7 sessions with a day rest, for 6 weeks C: Blank control | E: True KT | VAS, ADH, FMA-UE | RCT |
| [36] Li-Sheng Zhao (2017) | Cases: 40 (20/20) Age: 66.45 | E: Muscles: supraspinatus, deltoid, trapezius, Width: 5 cm Shape: I shape Tension: 25%–30% Time/Frequency: NR C: Sham KT Tension: neutral The other parameters were same with E group | E: True KT | ADH, FMA-UE | RCT |
AHD: Acromion humeral distance; ARAT: Action Research Arm Test (0–57); BBS: Berg Balance Scale (0–56); BI: Barthel Index (0–100); C: Control group; CT: Conventional treatment; D: Day; E: Experimental group; FMA-UE: Fugl-Meyer assessment for upper extremity (0–66); KT: Kinesiology tape; MAS: Modified Ashworth Scale (0–4); MBI: Modified Bathel Index (0–100); NMES: Neuromuscular electrical stimulation; NPRS: Numerical pain rating scale (0–10); NR: No report; RCT: Randomized controlled trials; ROM: Range of motion; SPADI: Shoulder Pain and Disability Index (0–100); SS-QoL: Stroke-Specific Quality of Life (49–245); VAS: Visual analogue scale (0–10); W: Week.
Among the included RCTs, the KT protocols differed substantially from each other. The tape remained in place from 15 minutes to 3 days, and the treatment duration ranged from 3 days to 8 weeks. The tape (5 cm width) was cut into a Y shape, X shape, or W shape or was not cut (I shape) and stretched with a tension of 10%–100% over the following four muscles: the supraspinatus, deltoid, brachii and trapezius. The sham tapes were applied at the same place with no tension. The conventional rehabilitation methods included physical therapy (passive and active assistance and active exercises), occupational therapy and electrotherapy. The outcomes included (1) pain (11 studies); (2) ROM (7 studies); (3) AHD (7 studies); (4) the FMA-UE score (7 studies); (5) IA (5 studies); (6) QOL (1 study); and (7) AE (0 studies). The characteristics of the included studies are detailed in Table 1. Based on the PEDro scale of bias tool, 12 studies had “good” methodological quality (6–8 scores). The quality assessment results are summarized in Table 2.
4.Synthesis of results
4.1Effect size of pain
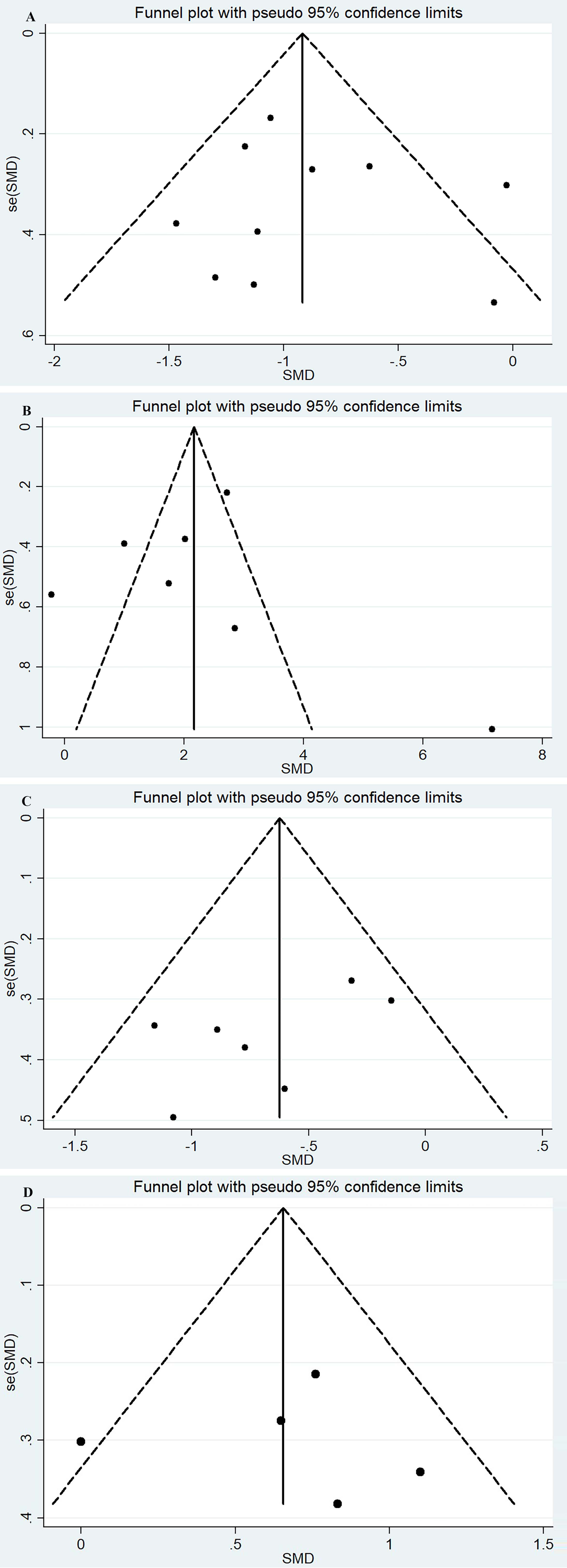
Pain data assessed with VAS and NPRS were merged due to the high correlation [37]. The pooled effect size of pain (11 RCTs [24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34], 570 patients) showed significant heterogeneity (I
Figure 2.
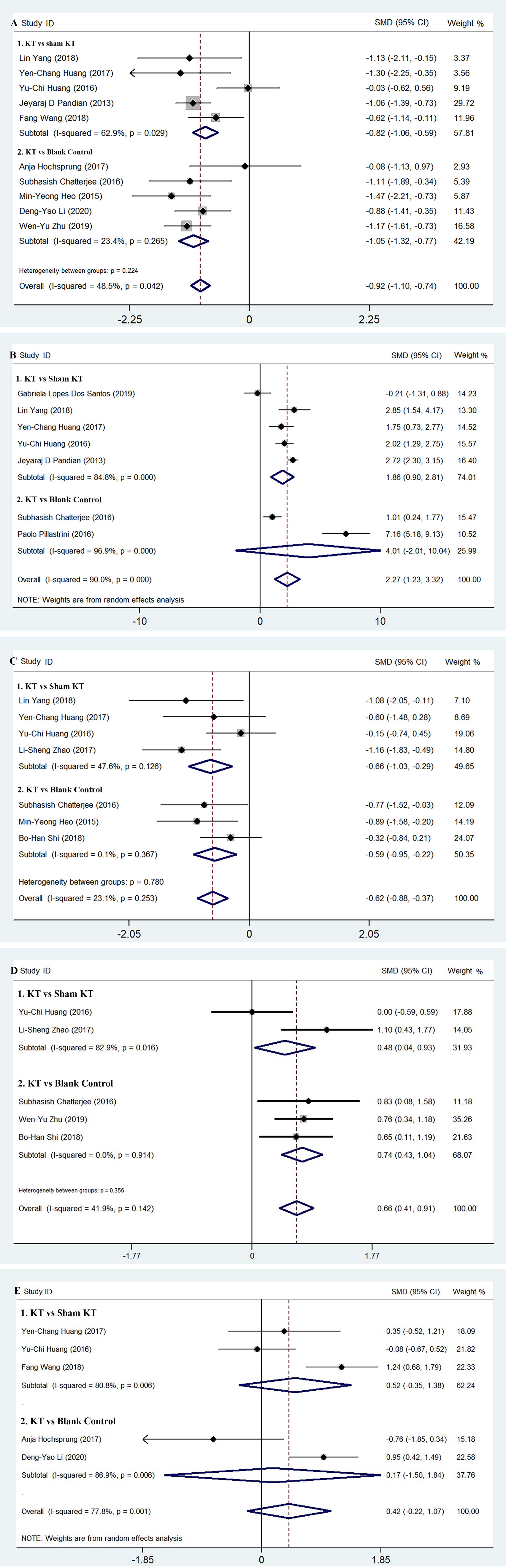
Figure 3.
4.2Effect size of ROM
The effect size of ROM (7 studies, 320 patients) [23, 24, 25, 27, 28, 29, 31] was pooled with a random model and yielded positive results with significant heterogeneity (I
4.3Effect size of AHD
The pooled effect size of AHD (7 RCTs, 246 patients, with fixed model) [24, 25, 27, 28, 30, 35, 36] showed that the KT reduced the distance from the acromion to the humeral head and prevented subluxation of the shoulder joint in patients with HSP without significant heterogeneity (I
4.4Effect size of FMA-UE
The FMA-UE data extracted from 7 studies [27, 28, 32, 33, 34, 35, 36], (383 patients) were pooled with a random effect model and showed positive results with considerable heterogeneity (I
4.5Effect size of IA and QOL
The IA data were synthesized from the BI, MBI, and SPADI data (5 RCTs [25, 26, 27, 32, 34], 232 patients). A higher score indicates more severe pain and disability on the SPADI scale, while it indicates more independence on the BI and MBI. Hence, the MD of the SPADI score was subtracted by the maximum (100) and reversed in the same direction as BI and MBI. The pooled effect size (including subgroup analysis) with a random effect model showed no improvement in individual daily living activity (SMD 0.42, 95% CI
4.6Adverse events
In all included studies, it was reported that no adverse events related to KT therapy occurred. Therefore, it is safe to use KT for the treatment of HSP.
5.Discussion
With the present meta-analysis, KT could relieve pain, improve the range of motion, reduce AHD and improve FMA-UE for HSP compared to sham KT or blank control. However, KT did not improve daily living activity or quality of life in patients with HSP, and a KT value superior to active treatment control was still not concluded.
Kinesiology tape is commonly used to treat musculoskeletal disorders [9], such as acromion impingement pain, neck pain, low back pain, and knee pain [38, 39, 40, 41, 42]. In particular, several studies have shown that bandaging with tape has a positive effect on shoulder pain [43, 44]. This might be related to the fact that KT treatment reduced soft tissue inflammation, improved muscle strength and alleviated joint pain. However, the mechanisms of KT in HSP remain uncertain. ROM limitation of the shoulder joint might be the main dysfunction in patients with HSP, and ROM limitation mainly contributes to shoulder pain [45, 46]. Previous studies reported two potential mechanisms of KT treatment in HSP. The gate control theory considered that KT can increase excitatory afferent stimulation and can cut off or block part of the pain signal conduction to the central nervous system so that subjective pain perception is reduced [47]. Another potential mechanism is that KT can stimulate cutaneous mechanoreceptors to enhance proprioception [49, 50], in turn activating large-diameter fibers (including A-beta fibers) to activate inhibitory interneurons to block pain signals conducted by small-diameter fibers, such as C- and A-delta fibers.
In the present study, 3–6 weeks of KT treatments relieved pain, improved ROM of the shoulder joint and improved FMA-UE for HSP compared with the sham or blank control. The results were similar with the previous studies. Jae-Man Yang et al. confirmed that KT relieved shoulder pain by creating wrinkles to reduce the pressure of mechanoreceptors and to increase the space underlying the tissues through its lifting effects, which in turn increased blood circulation [50]. The other two studies reported that shoulder movement was restricted in all directions [45] in HSP patients and increased significantly after KT treatment [51].
The present study also verified the value of KT in improving subluxation due to HSP. The pooled effect size of AHD showed that after 3–6 weeks of KT treatment, the distance between the acromion and humeral head was significantly shortened. Muscle weakness or paralysis after strokes can lead to poor shoulder protection and are associated with frequent soft tissue injuries of the affected shoulders during daily living activities or rehabilitation [52]. The most important muscles in preventing subluxation of the glenohumeral joint are the posterior fibers of the deltoid, supraspinatus, and infraspinatus [53]. The weak or paralyzed muscles could not maintain the head of the humerus in the glenoid fossa, and subluxation occurred. Furthermore, it will lead to overstretching, injury, or edema in the soft tissue. The current results confirmed that KT provided mechanical support to the subluxed shoulder and maintained persistent proprioceptive feedback and sensory stimulation, which increased sensory input from mechanoreceptors to primary somatosensories in the contralateral cortex through the thalamus [54, 55]. It reminded patients themselves to handle the upper extremity properly and prevented or mitigated subluxation. Likewise, a sling can also provide mechanical support. Moreover, it brings benefits to patients with HSP in shoulder subluxation and walking efficiency [56]. One study showed that 15–20 minutes of exercise could cause rigid tape to lose its ability to restrain the joints [57]. Therefore, kinesiology tape would be a better choice for treatment that not only can provide effective support for the shoulder but also has no restriction. Meanwhile, its elastic properties are consistent with kinesiology and are little affected by movement.
Unfortunately, the present study showed that KT could not improve individual daily living activity or the quality of life in patients with HSP. The difference between KT and active treatment, such as NMES, acupuncture, shouldering, sling, etc., was still not verified.
Compared with two reviews published recently [58, 59], our study verified similar results that KT works in relieving pain and in improving shoulder ROM, shoulder subluxation and FMA-UE for HSP, but there were still some differences. First, the present review performed a more comprehensive literature search (14 RCTs in 13 databases) than the previous 2 reviews (9 studies in 7 databases; 8 studies in 4 databases). Second, the present review conducted a more informative investigation on the effects of KT on HSP compared to sham or blank controls, including pain, ROM, AHD, FMA-UE, individual daily living activity and quality of life based on the ICF foundation.
5.1Limitations
There were several limitations in the present study. First, the number of RCTs and the sample size were still small. Second, all the studies included in this meta-analysis reported only short-term effects (3–4 weeks). Third, this study focused on patients with chronic convalescent stroke within 1 to 6 months after stroke onset. Therefore, no conclusions can be drawn for patients with acute or subacute stroke. Fourth, there was diversity of and variation in the KT protocols. The selected taping muscles, taping time and frequency, treatment duration and taping shape and tension showed significant heterogeneity in the included studies. Therefore, more rigorously designed RCTs with larger sample sizes and with longer follow-up evaluations are needed in the future. Standardized protocols of KT therapy should also be recommended.
5.2Implication
To the best of our knowledge, this is the first meta-analysis to evaluate the effectiveness and safety of KT therapy versus sham KT for treating HSP. In the present study, after rigorous meta-analysis, it was concluded that KT did have significant effects on reducing pain, increasing ROM, and shortening the subluxation distance of the shoulder joint but not on improving daily living activity and quality of life for hemiplegic shoulder pain without side effects. Interestingly, we also found that four muscles (supraspinatus, deltoid, brachii and trapezius) were most commonly used to treat HSP. The findings were similar to those of other published reviews.
6.Conclusions
This systematic meta-analysis provided currently available evidence to confirm the KT value for relieving pain and to improve the ROM, DAH and FMA-UE in patients with HSP compared with sham KT or blank controls. The effects of KT on daily living activity and quality of life and the value superior to active treatment in patients with HSP were not verified. More rigorous, reasonably designed RCTs with large sample sizes are still needed in the future.
Funding
This research was financially supported by the Science and Technology Department of Sichuan Province (Grant No. 2016SZ0039), the Medical Association of Sichuan Province (Grant No. S15063) and the Chengdu Science and Technology Bureau, Chengdu, China (Grant No. 2019-YF05-00061-SN). The funding sources had no involvement in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Supplementary data
The supplementary files are available to download from http://dx.doi.org/10.3233/BMR-200323.
Conflict of interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
[1] | Nadler M, Pauls MM. Shoulder orthoses for the prevention and reduction of hemiplegic shoulder pain and subluxation: systematic review. Clin Rehabil. (2016) ; 110. |
[2] | Vuagnat H, Chantraine A. Shoulder pain in hemiplegia revisited: Contribution of functional electrical stimulation and other therapies. J Rehabil Med. (2003) ; 35: : 49-56. |
[3] | Hodges PW, Ervilha UF, Graven-Nielsen T. Changes in motor unit firing rate in synergist muscles cannot explain the maintenance of force during constant force painful contractions. The Journal of Pain. (2008) ; 9: (12): 1169-1174. |
[4] | Yetisgin A. Clinical characteristics aecting motor recovery and ambulation in stroke patients. J PHYS THER SCI. (2017) ; 29: (2): 216-220. |
[5] | Sarquis LM, Coggon D, Ntani G, Walker-Bone K, Palmer KT, Fellid VE, et al. Classification of neck/shoulder pain in epidemiological research: a comparison of personal and occupational characteristics, disability, and prognosis among 12,195 workers from 18 countries. Pain. (2016) ; 157: : 1028-1036. |
[6] | Roosink M, Renzenbrink GJ, Geurts AC, Ijzerman MJ. Towards a mechanism-based view on poststroke shoulder pain: theoretical considerations and clinical implications. NeuroRehabilitation. (2012) ; 30: : 153-165. |
[7] | Roosink M, Buitenweg JR, Renzenbrink GJ, Geurts AC, Ijzerman MJ. Altered cortical somatosensory processing in chronic stroke: a relationship with poststroke shoulder pain. NeuroRehabilitation. (2011) ; 28: : 331-344. |
[8] | Roosink M, Van Dongen RT, Buitenweg JR, Renzenbrink GJ, Geurts AC, Ijzerman MJ. Multimodal and widespread somatosensory abnormalities in persistent shoulder pain in the first 6 months after stroke: an exploratory study. Archives of Physical Medicine and Rehabilitation. (2012) ; 93: (11): 1968-1974. |
[9] | Paci M, Nannetti L, Taiti P, Baccini M, Pasquini J, Rinaldi L. Shoulder subluxation after stroke: relationships with pain and motor recovery. Physiotherapy Research International. (2007) ; 12: (2): 95-104. |
[10] | Stolzenberg D, Siu G, Cruz E. Current and future interventions for glenohumeral subluxation in hemiplegia secondary to stroke. Topics in Rehabilitation Stroke. (2012) ; 19: (5): 444-456. |
[11] | Li Z, Alexander SA. Current evidence in the management of poststroke hemiplegic shoulder pain: a review. J Neurosci Nurs. (2015) ; 47: (1): 10-19. |
[12] | Vasudevan JM, Browne BJ. Hemiplegic shoulder pain: an approach to diagnosis and management. Phys Med Rehabil Clin N Am. (2014) ; 25: : 411-437. |
[13] | Benlidayi IC, Basaran S. Hemiplegic shoulder pain: a common clinical consequence of stroke. Pract Neurol. (2013) ; 0: : 1-4. |
[14] | Van Bladel A, Lambrecht G, Oostra KM, Vanderstraeten G, Cambier D. A randomized controlled trial on the immediate and long-term eects of arm slings on shoulder subluxation in stroke patient. EUR J PHYS REHAB MED. (2017) ; 53: (3): 400-409. |
[15] | Paci M, Nannetti L, Rinaldi LA. Glenohumeral subluxation in hemiplegia: an overview. J Rehabil Res Dev. (2005) ; 42: : 557-568. |
[16] | Ravichandran H Janakiraman B Sundaram S, Fisseha B, Gebreyesus T, Gelaw AY. Systematic review on effectiveness of shoulder taping in hemiplegia. J Stroke Cerebrovasc Dis. (2019) ; 28: (6): 1463-1473. |
[17] | Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) ; 6: (7). |
[18] | Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Physical Therapy. (2003) ; 83: (8): 713-721. |
[19] | Armijo-Olivo S, Da Costa BR, Cummings GG, Ha C, Fuentes J, Saltaji H, et al. PEDro or cochrane to assess the quality of clinical trials? A meta-epidemiological study. PLOS ONE. (2015) ; 10: (7). |
[20] | Ilyas Bakbergenuly, David C. Hoaglin, Elena Kulinskaya. Estimation in meta-analyses of mean difference and standardized mean difference. Stat Med. (2020) ; 39: (2): 171-191. |
[21] | Jonathan JD, Julian PH, Douglas GA. Analysing data and undertaking meta-analyses. The Cochrane Collaboration. (2011) ; 243296. |
[22] | Der Simonian R, Laird N. Meta-analysis in clinical trials revisited. Contemporary Clinical Trial. (2015) ; 45: : 139-145. |
[23] | Dos Santos GL, Da Silva ES, Desloovere K, Russo TL. Effects of elastic tape on kinematic parameters during a functional task in chronic hemiparetic subjects: a randomized sham-controlled crossover trial. PLoS One. (2019) ; 14: (1). |
[24] | Yang L, Yang J, He C. The Effect of Kinesiology Taping on the Hemiplegic Shoulder Pain: A Randomized Controlled Trial. J Healthc Eng. (2018) ; 2018: 8346432. |
[25] | Huang YC, Chang KH, Liou TH, Cheng CW, Lin LF, Huang SW. Effects of kinesio taping for stroke patients with hemiplegic shoulder pain: a double-blind, randomized, placebo-controlled study. J Rehabil Med. (2017) ; 49: (3): 208-215. |
[26] | Hochsprung A, Domínguez-Matito A, López-Hervás A, Herrera-Monge P, Moron-Martin S, Ariza-Martínez C, et al. Short- and medium-term effect of kinesio taping or electrical stimulation in hemiplegic shoulder pain prevention: a randomized controlled pilot trial. NeuroRehabilitation. (2017) ; 41: (4): 801-810. |
[27] | Huang YC, Leong CP, Wang L, Wang LY, Yang YC, Chuang CY, et al. Effect of kinesiology taping on hemiplegic shoulder pain and functional outcomes in subacute stroke patients: a randomized controlled study. EUR J PHYS REHAB MED. (2016) ; 52: (6): 774-781. |
[28] | Chatterjee S, Hayner KA, Arumugam N, Goyal M, Midha D, Arora A, et al. The California tri-pull taping method in the treatment of shoulder subluxation after stroke: a randomized clinical trial. North Am J Med Sci. (2016) ; 8: : 175-182. |
[29] | Pillastrini P, Rocchi G, Deserri D, Foschi P, Mardegan M, Naldi MT, et al. Effectiveness of neuromuscular taping on painful hemiplegic shoulder: a randomised clinical trial. Disabil Rehabil. (2016) ; 38: (16): 1603-1609. |
[30] | Heo MY, Kim CY, Nam CW. Influence of the application of inelastic taping on shoulder subluxation and pain changes in acute stroke patients. J Phys Ther Sci. (2015) ; 27: : 3393-3395. |
[31] | Pandian JD, Kaur P, Arora R, Vishwambaran DK, Toor G, Mathangi S, et al. Shoulder taping reduces injury and pain in stroke patients Randomized controlled trial. Neurology. (2013) ; 80: (6): 528-532. |
[32] | Li D-Y, Luo L, Xiang T, Wang X-Y, Ren C, Liu N, et al. Effect of intramuscular plaster combined with intensive training of scapula on shoulder pain after early stroke. Neural Injury and Functional Reconstruction. (2020) ; 15: (1): 55-57. |
[33] | Zhu W-Y, Qi Q, Jiang C, Luo J, Xie H-Q. Effect of intramuscular plaster combined with rehabilitation training on shoulder hand syndrome after stroke. Chin J Phys Med Rehabil. (2019) ; 41: (8): 588-590. |
[34] | Wang F, Ye J-B, Zhao S-R, Zheng C-M, Huang C-Y. Observation on the effect of intramuscular effect sticking technique on hemiplegic shoulder pain after stroke. The Journal of Medical Theory and Practice. (2018) ; 31: (3): 452-453. |
[35] | Shi B-H, Li K-P, Hu Y-H, Xu Q, Cheng Z, Chai Z-Z, et al. The effect of intramuscular patch on shoulder pain after shoulder subluxation in stroke patients. Chinese Journal of Rehabilitation Medicine. (2018) ; 33: (3): 310-314. |
[36] | Zhao L-S, Wang J-W. Effect of kinesio taping on shoulder subluxation in hemiplegic patients after stroke. Chinese Journal of Rehabilitation Theory and Practice. (2017) ; 23: (10): 1200-1202. |
[37] | Downie WW, Leatham PA, Rhind VM, Wright V, Branco JA, Anderson JA. Studies with pain rating scales. Ann. Rheumatic Diseases. (1978) ; 37: (4): 378-381. |
[38] | Thelen MD, Dauber JA, Stoneman PD. The clinical ecacy of Kinesio Tape for shoulder pain: a randomized, double-blinded, clinical trial. Journal of Orthopaedic and Sports Physical Therapy. (2008) ; 38: (7): 389-395. |
[39] | Kaya E, Zinnuroglu M, Tugcu I. Kinesio taping compared to physical therapy modalities for the treatment of shoulder impingement syndrome. Clinical Rheumatology. (2011) ; 30: (2): 201-207. |
[40] | Gonzalez-Iglesias J, Fernandez-de-las-Penas C, Cleland J, Huijbregts P, Gutierrez-vega MR. Short-term eects of cervical kinesio taping on pain and cervical range of motion in patients with acute whiplash injury: a randomized clinical trial. Journal of Orthopaedic and Sports Physical Therapy. (2009) ; 39: (7): 515-521. |
[41] | Bae SH, Lee JH, Oh KA, Kim KY. The effects of kinesio taping on potential in chronic low back pain patients anticipatory postural control and cerebral cortex. J Phys Ther Sci. (2013) ; 25: (11): 1367-1371. |
[42] | Nemitalla MA, Costa LOP, Fukuda TY, De Freitas DG, Salomao EC, Monteiro RL, et al. Efficacy of adding the kinesio taping method to guideline-endorsed conventional physiotherapy in patients with chronic nonspecific low back pain: a randomized controlled trial. BMC Musculoskelet Disord. (2013) ; 14: : 301. |
[43] | Heo MY, Kim CY, Nam CW. Influence of the application of inelastic taping on shoulder subluxation and pain changes in acute stroke patients. J Phys Ther Sci. (2015) ; 27: (11): 3393-3395. |
[44] | Pillastrini P, Rocchi G, Deserri D, Foschi P, Mardegan M, Naldi MT, et al. Effectiveness of neuromuscular taping on painful hemiplegic shoulder: a randomized clinical trial. Disabil Rehabil. (2016) ; 38: (16): 1603-1609. |
[45] | Suriya-amarit D, Gaogasigam C, Siriphorn A, Boonyong S. Effect of interferential current stimulation in management of hemiplegic shoulder pain. Arch Phys Med Rehabil. (2014) ; 95: : 1441-1446. |
[46] | Hodges PW, Ervilha UF, Graven-Nielsen T. Changes in motor unit ring rate in synergist muscles cannot explain the maintenance of force during constant force painful contractions. Journal of Pain. (2008) ; 9: (12): 1169-1174. |
[47] | Jaraczewska E, Long C. Kinesio taping in stroke: improving functional use of the upper extremity in hemiplegia. Topics in Stroke Rehabilitation. (2006) ; 13: (3): 31-42. |
[48] | Konishi Y. Tactile stimulation with Kinesiology tape alleviates muscle weakness attributable to attenuation of Ia afferents. J Sci Med Sport. (2013) ; 16: : 45-48. |
[49] | Bravi R, Quarta E, Cohen EJ, Gottard A, Minciacchi D. A little elastic for a better performance: Kinesiotaping of the motor effector modulates neural mechanisms for rhythmic movements. Front Syst Neurosci. (2014) ; 8: : 181. |
[50] | Jae-Man Y, Jung-Hoon L. Is kinesio taping to generate skin convolutions effective for increasing local blood circulation? Med Sci Monit. (2018) ; 24: : 288-293. |
[51] | Thelen MD, Dauber JA, Stoneman PD. The clinical efficacy of kinesio tape for shoulder pain: a randomized, double-blinded, clinical trial. J Orthop Sports Phys Ther. (2008) ; 38: : 389-395. |
[52] | Huang YC, Liang PJ, Pong YP, Leong CP, Tseng CH. Physical findings and sonography of hemiplegic shoulder in patients after acute stroke during rehabilitation. J rehabil Med. (2010) ; 42: : 21-26. |
[53] | Cuéllar R, Ruiz-Ibán MA, Cuéllar A. Anatomy and biomechanics of the unstable shoulder. Open Orthop J. (2017) ; 11: : 919-933. |
[54] | Dos Santos GL, Souza MB, Desloovere K, Russo TL. Elastic tape improved shoulder joint position sense in chronic hemiparetic subjects: a randomized sham-controlled crossover study. PLoS One. (2017) ; 12: (1). |
[55] | Roijezon U, Clark NC, Treleaven J. Proprioception in musculoskeletal rehabilitation. Part 1: Basic science and principles of assessment and clinical interventions. Manual Therapy. (2015) ; 20: (3): 368-77. |
[56] | Han SH, Kim T, Jang SH, Kim MJ, Park S, Yoon SI. The eect of an arm sling on energy consumption while walking in hemiplegic patients: a randomized comparison. Clinical Rehabilitation. (2011) ; 25: (1): 36-42. |
[57] | Bragg RW, Macmahon JM, Overom EK, Yerby SA, Matheson GO, Carter DR, et al. Failure and fatigue characteristics of adhesive athletic tape. Medicine and Sciencein Sports and Exercise. (2002) ; 34: (3): 403-410. |
[58] | Deng P, Zhao Z, Zhang S, Xiao T, Li Y. Effect of kinesio taping on hemiplegic shoulder pain: A systematic review and meta-analysis of randomized controlled trials. Clinical Rehabilitation. (2020) ; 00(0): 1-15. doi: 10.1177/0269215520964950, Online ahead of print. |
[59] | Ravichandran H, Janakiraman B, Sundaram S, Fisseha B, Gebreyesus T, Gelaw AY. Systematic review on effectiveness of shoulder taping in hemiplegia. J Stroke Cerebrovasc Dis. (2019) ; 28: (6): 1463-1473. |




