Effectiveness of non-pharmacological conservative therapies in adults with fibromyalgia: A systematic review of high-quality clinical trials
Abstract
BACKGROUND:
Fibromyalgia is a chronic condition characterized by generalized pain. Several studies have been conducted to assess the effects of non-pharmacological conservative therapies in fibromyalgia.
OBJECTIVE:
To systematically review the effects of non-pharmacological conservative therapies in fibromyalgia patients.
METHODS:
We searched MEDLINE, Cochrane library, Scopus and PEDro databases for randomized clinical trials related to non-pharmacological conservative therapies in adults with fibromyalgia. The PEDro scale was used for the methodological quality assessment. High-quality trials with a minimum score of 7 out of 10 were included. Outcome measures were pain intensity, pressure pain threshold, physical function, disability, sleep, fatigue and psychological distress.
RESULTS:
Forty-six studies met the inclusion criteria. There was strong evidence about the next aspects. Combined exercise, aquatic exercise and other active therapies improved pain intensity, disability and physical function in the short term. Multimodal therapies reduced pain intensity in the short term, as well as disability in the short, medium and long term. Manual therapy, needling therapies and patient education provided benefits in the short term.
CONCLUSIONS:
Strong evidence showed positive effects of non-pharmacological conservative therapies in the short term in fibromyalgia patients. Multimodal conservative therapies also could provide benefits in the medium and long term.
1.Introduction
Fibromyalgia (FM) is a chronic pain condition characterized by generalized musculoskeletal pain, hyperalgesia and allodynia, commonly associated with other symptoms, such as fatigue, poor sleep quality, anxiety and depression [1, 2]. These clinical manifestations have an impact on the quality of life and social environment of the patients [3]. The worldwide prevalence of FM has been estimated at 2.1%, affecting specially women [2, 4].
The etiopathogenesis of FM is not completely known but the central sensitization is the most accepted hypothesis [4, 5]. For this reason, the diagnosis is based on the clinical criteria described by the American College of Rheumatology [6, 7, 8].
Current clinical guidelines for the management of patients with FM recommended multimodal conservative treatments to improve the pain-related symptoms, the physical function and the quality of life [9, 10]. Among the conservative treatments, clinical guidelines include non-pharmacological therapies such as Exercise Therapy (ET), mind-body therapies, Patient Education (PE), Manual Therapy (MT), Needling Therapies (NT), balneotherapy and multimodal therapies [9, 10]. Recently, several randomized clinical trials (RCTs) and systematic reviews have analyzed the effects of these types of non-pharmacological conservative treatments [9, 11, 12, 13, 14, 15, 16, 17]. However, these systematic reviews did not consider methodological quality or included RCTs with low methodological quality, which leads to weak or biased conclusions [9, 17, 18, 19].
To the best of our knowledge, there are no studies that provide a broad perspective of non-pharmacological conservative treatments for the management of patients with FM. Therefore, the aim of this systematic review of high-quality RCTs was to analyze the effects of non-pharmacological conservative treatment on pain intensity, Pressure Pain Threshold (PPT), physical function, disability, sleep, fatigue, depression and anxiety in patients with FM.
2.Methods
2.1Design
A systematic review of high-quality RCT was carried out following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [20]. The study protocol has been recorded on the International Prospective Register of Systematic Reviews (PROSPERO) with ID CRD42020154111.
2.2The review question
The literature search was performed from October 2019 to January 2020, using MEDLINE, Cochrane Library, Scopus and PEDro. The following search terms: “fibromyalgia”, “therapeutics”, “physical therapy modalities”, “combined physical therapy” and “exercise” linked with the Boolean operators AND and OR were combined to perform the search strategy with no limits on publication dates. Studies were considered from inception until January 2020. The search strategy is shown in detail in the Appendix.
Inclusion criteria were defined following the PICOS method:
• Population: patients diagnosed with FM by a rheumatologist according to the American College of Rheumatology criteria.
• Intervention: non-pharmacological conservative treatments (ET, MT, NT, PE, mind-body therapies, whole-body vibration, balneotherapy, electrotherapy and multimodal therapies).
• Comparison: sham techniques, usual care, no intervention or a non-pharmacological conservative therapy different than the intervention group.
• Outcomes: pain intensity, PPT, physical function, disability, sleep, fatigue, depression and anxiety.
• Study design: RTCs with a minimum score of 7 in the PEDro scale, corresponding to high methodological quality [21].
The studies were excluded if they: included patients with concomitant conditions, included healthy subjects, used surgical or pharmacological interventions as their primary intervention, did not explain or did not control basic pharmacological treatment prescribed by a medical doctor.
2.3Data collection process
Potentially relevant studies were screened by two independent reviewers that selected studies based on title and abstract. Once agreement was reached, full text of relevant studies were screened and evaluated according to PEDro scale by the same reviewers. A third reviewer solved doubts or disagreements.
The two reviewers extracted data from the studies independently. The PRISMA checklist was used to collect relevant aspects from the studies and included information on study design, sample size, subject characteristics, intervention type, single session duration, frequency of sessions, total number of sessions, total time of intervention, follow-up time frame and outcome measures assessing pain intensity, PPT, physical function, disability, sleep, fatigue, depression and anxiety in the short (
2.4Data synthesis and analysis
Methodological quality of studies was evaluated using PEDro scale checklist. PEDro scale is based on the Delphi checklist, developed by Verhagen and colleagues at Epidemiology Department of Maastricht University [23]. This scale has 11 items, the first item is related to external validity and is not taken into account for the final score, the rest of the items allow a total score out of 10. The final score is established based on the number of items satisfied. In this review, only “high” quality studies were included. A score of 7 or above was considered to be “high” quality, a score between 5–6 was considered “fair” quality and a score of 4 or below was considered “poor” quality [21]. The PEDro scale has shown to be a valid measure of methodological quality of clinical trials [24].
Data extraction and methodological quality analysis of the selected studies were carried out by two independent reviewers following the same methodology and a third reviewer outside of the first process decided in case of disagreement.
Qualitative analysis of this review is based on the scientific evidence levels for the results classification [25]. The evidence was categorized into four levels, according to the results and the methodological quality of the studies:
• Strong evidence: represents concordant results from multiple RCTs (at least two) with good methodological quality.
• Moderate evidence: represents concordant results from multiple RCTs with low methodological quality, controlled clinical trials, or a high-quality RCT.
• Contradictory evidence: represents conflicting results from RCTs or controlled clinical trials.
• No evidence: there are no RCTs or controlled clinical trials.
3.Results
Initial searches identified 3985 studies (2417 MEDLINE, 1035 Cochrane library, 284 Scopus and 249 PEDro). After removing duplicates, the title and the abstract were screened, and 292 studies were considered relevant to full-text screening. Finally, a total of 46 studies that met the inclusion criteria were included [11, 12, 13, 14, 15, 16, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65]. The flowchart diagram is shown in Fig. 1.
Figure 1.
Flow diagram.
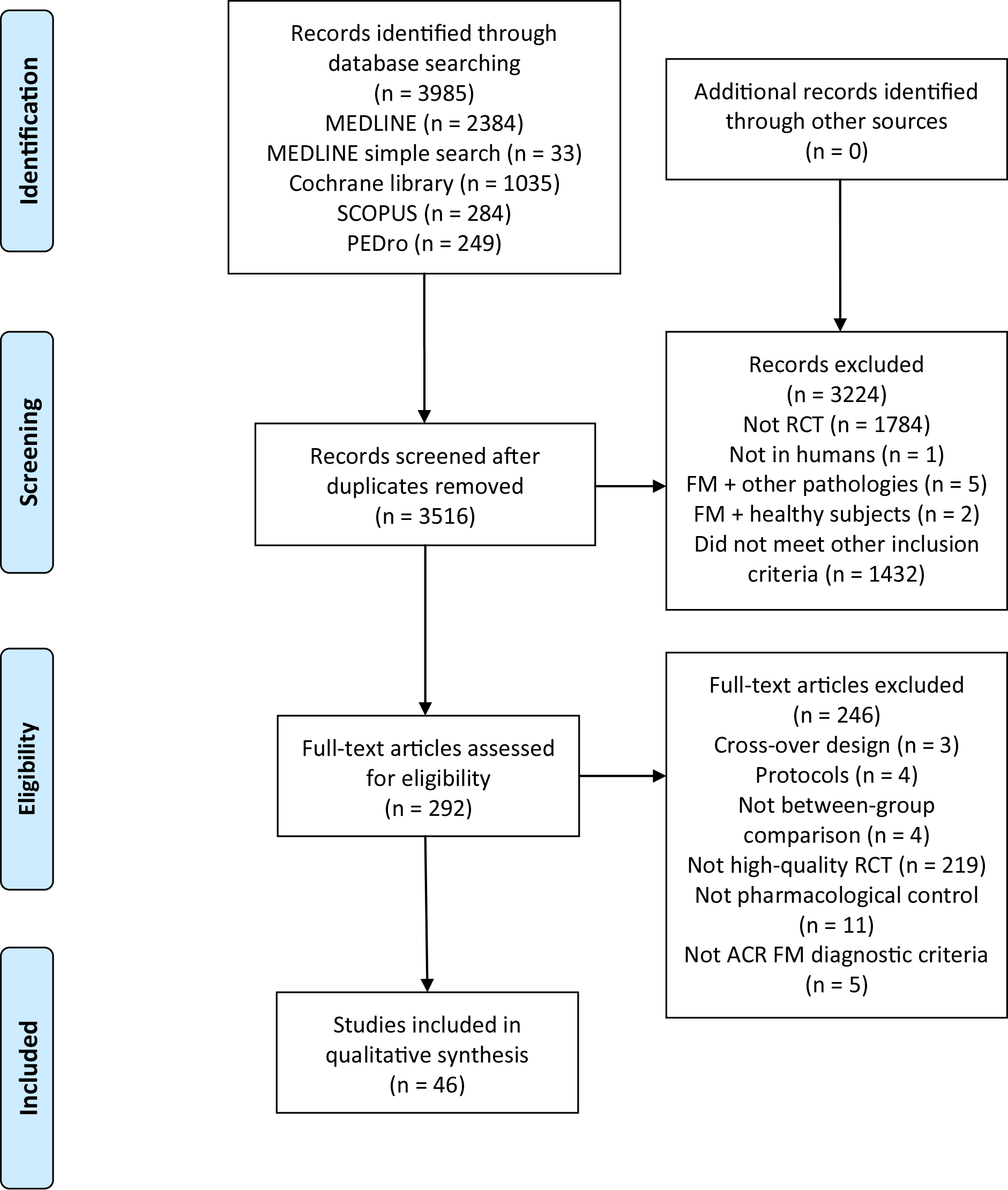
In total, 3384 participants were examined in the trials. Most studies recruited from 15 to 50 participants [11, 13, 14, 15, 16, 26, 27, 28, 29, 30, 32, 33, 35, 36, 37, 38, 39, 40, 41, 42, 43, 45, 46, 47, 48, 50, 51, 52, 53, 54, 55, 56, 58, 59, 60, 61, 62, 63]. The studies were done in Europe [12, 14, 15, 26, 27, 28, 29, 30, 31, 36, 40, 41, 42, 43, 49, 50, 51, 53, 59, 60, 62, 65], America [11, 13, 16, 32, 34, 37, 39, 44, 45, 46, 47, 48, 52, 54, 55, 56, 57, 58, 61, 63, 64] and Asia [33, 35, 38]. There were different recruitment sources: private clinics, FM associations, hospitals, rehabilitation clinics, primary care centers, research centers databases or local population through advertisements in newspapers or radio. In most studies, the interventions were performed by physical therapists, medical doctors and/or psychologists.
3.1Methodological quality of assessment
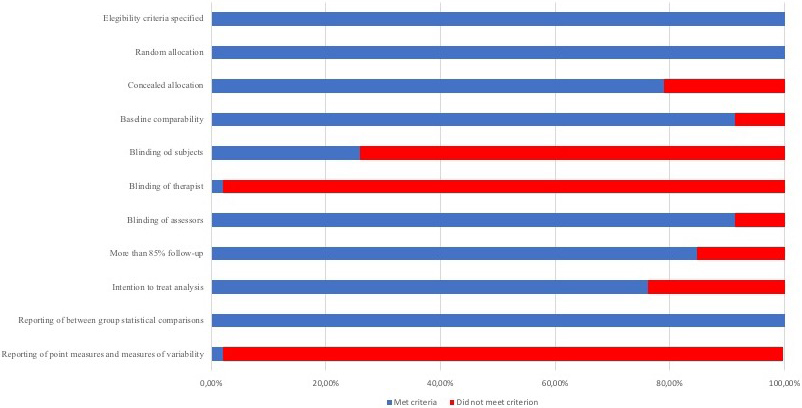
According to the PEDro scale, all of the studies included presented high-quality. Twenty-four studies showed a score of 7 [15, 28, 29, 30, 32, 33, 34, 35, 36, 38, 41, 42, 43, 44, 48, 49, 53, 54, 56, 57, 62, 63, 65], 22 showed a score of 8 [11, 12, 13, 14, 16, 26, 31, 37, 40, 45, 46, 47, 50, 51, 52, 55, 58, 59, 60, 61, 64] and no one presented a score of 9 or 10. Most of the studies met the criteria for random allocation, similar baseline characteristics between groups, blinded assessors and between-group statistical comparisons. Nevertheless, just one study met the criteria for blinded therapists [14]. Methodological quality of the included studies is shown in Table 1. Figure 2 provides the risk of bias across the included studies.
Table 1
Scoring of included studies according to the PEDro scale
| Reference | Items | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||
| Andrade, 2019 [11] | Y | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 8/10 |
| Ang, 2013 [57] | Y | Y | N | Y | N | N | Y | Y | Y | Y | Y | 7/10 |
| Assefi, 2010 [61] | Y | Y | Y | Y | Y | N | Y | N | Y | Y | Y | 8/10 |
| Assis, 2006 [47] | Y | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 8/10 |
| Bagdatli, 2015 [38] | Y | Y | N | Y | N | N | Y | Y | Y | Y | Y | 7/10 |
| Baptista, 2012 [45] | Y | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 8/10 |
| Baumueller, 2017 [60] | Y | Y | Y | Y | Y | N | Y | Y | N | Y | Y | 8/10 |
| Castro-Sanchez, 2019 [12] | Y | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 8/10 |
| Castro-Sanchez, 2017 [36] | Y | Y | Y | Y | N | N | Y | Y | N | Y | Y | 7/10 |
| Castro-Sanchez, 2014 [30] | Y | Y | Y | Y | N | N | N | Y | Y | Y | Y | 7/10 |
| Castro-Sanchez, 2011 [31] | Y | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 8/10 |
| Ceca, 2017 [29] | Y | Y | Y | Y | N | N | Y | N | Y | Y | Y | 7/10 |
| Collado-Mateo, 2017 [42] | Y | Y | N | Y | N | N | Y | Y | Y | Y | Y | 7/10 |
| Collado-Mateo, 2017 [43] | Y | Y | Y | Y | N | N | Y | Y | N | Y | Y | 7/10 |
| Corrales, 2011 [50] | Y | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 8/10 |
| Da Costa, 2005 [52] | Y | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 8/10 |
| Fernandes, 2016 [46] | Y | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 8/10 |
| Fioravanti, 2018 [14] | Y | Y | Y | N | Y | Y | N | Y | Y | Y | Y | 8/10 |
| Gowans, 2001 [48] | Y | Y | N | Y | N | N | Y | Y | Y | Y | Y | 7/10 |
| Gur, 2002 [33] | Y | Y | N | Y | Y | N | Y | Y | N | Y | Y | 7/10 |
| Harris, 2005 [37] | Y | Y | Y | Y | Y | N | Y | N | Y | Y | Y | 8/10 |
| Hooten, 2012 [56] | Y | Y | Y | Y | N | N | N | Y | Y | Y | Y | 7/10 |
| Hsu, 2010 [63] | Y | Y | Y | Y | N | N | N | Y | Y | Y | Y | 7/10 |
| Ide, 2008 [54] | Y | Y | Y | Y | N | N | Y | Y | N | Y | Y | 7/10 |
| Karatay, 2018 [35] | Y | Y | Y | Y | Y | N | Y | Y | N | Y | N | 7/10 |
| Larsson, 2015 [49] | Y | Y | Y | Y | N | N | Y | N | Y | Y | Y | 7/10 |
| Lauche, 2016 [59] | Y | Y | Y | N | Y | N | Y | Y | Y | Y | Y | 8/10 |
| Lemstra, 2005 [58] | Y | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 8/10 |
| Lumley, 2017 [64] | Y | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 8/10 |
| Mannerkorpi, 2010 [51] | Y | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 8/10 |
| Martin-Martinez, 2019 [41] | Y | Y | N | Y | N | N | Y | Y | Y | Y | Y | 7/10 |
| Mist, 2018 [34] | Y | Y | N | Y | N | N | Y | Y | Y | Y | Y | 7/10 |
| Moretti, 2012 [39] | Y | Y | Y | N | Y | N | Y | Y | N | Y | Y | 7/10 |
| Olivares, 2011 [40] | Y | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 8/10 |
| Panton, 2013 [32] | Y | Y | N | Y | Y | N | Y | Y | N | Y | Y | 7/10 |
| Paolucci, 2016 [53] | Y | Y | Y | Y | N | N | Y | Y | N | Y | Y | 7/10 |
| Torres, 2015 [28] | Y | Y | Y | Y | N | N | Y | Y | N | Y | Y | 7/10 |
| Van-Ittersum, 2017 [65] | Y | Y | Y | Y | N | N | Y | N | Y | Y | Y | 7/10 |
| Van-Oosterwijck, 2013 [26] | Y | Y | N | Y | Y | N | Y | Y | Y | Y | Y | 8/10 |
| Simister, 2018 [13] | Y | Y | Y | Y | Y | N | Y | Y | N | Y | Y | 8/10 |
| Silva, 2019 [16] | Y | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 8/10 |
| Thieme, 2006 [27] | Y | Y | Y | N | Y | N | Y | N | Y | Y | Y | 7/10 |
| Villafaina, 2019 [15] | Y | Y | N | Y | N | N | Y | Y | Y | Y | Y | 7/10 |
| Vitorino, 2006 [55] | Y | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 8/10 |
| Wang, 2018 [44] | Y | Y | Y | Y | N | N | Y | N | Y | Y | Y | 7/10 |
| Wicksell, 2013 [62] | Y | Y | Y | Y | N | N | N | Y | Y | Y | Y | 7/10 |
Out of ten; Y
Figure 2.
Risk of bias across studies presented by percent that met the PEDro scale criteria.
3.2Characteristics of the studies: interventions and outcomes
The most used modality of the intervention was ET. Different types of ET were applied: Five studies used aerobic training [11, 44, 46, 47, 51], 4 studies used exergames [15, 41, 42, 43], 4 aquatic training [11, 46, 47, 54], 2 used strengthening training [16, 49] and the rest of studies used a combination of different types of ET [15, 41, 42, 43, 45, 48, 50, 52, 53, 60].
The most investigated therapies after ET were PE [13, 26, 27, 62, 63, 64, 65], NT [12, 34, 35, 36, 37], MT [12, 28, 29, 30, 31] and multimodal therapies [38, 39, 55, 56, 57, 58]. Finally, other interventions were used such as laser therapy [32, 33], whole-body vibration [40], balneotherapy [14], cupping [59], relaxation [16, 49] and reiki [61]. The included therapies were classified into active and passive interventions.
The frequency and the total number of sessions varied widely across all studies for ET, PE and MT. ET sessions ranged from 10 to 69 over 4 to 24 weeks [11, 15, 16, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54]. When ET was combined with other therapies, the number of sessions ranged from 9 to 18 over 3 to 6 weeks [55, 56, 57, 58]. Studies in which PE was applied in isolation or combined with other therapies, the number of sessions ranged from 2 to 15 over 2 to 15 weeks [13, 26, 27, 34, 38, 53, 56, 57, 58, 62, 63, 64, 65]. NT sessions ranged from 4 to 20 over 4 to 10 weeks [12, 34, 35, 36, 37]. MT sessions ranged from 5 to 40 over 5 to 20 weeks [12, 28, 29, 30, 31].
In relation to the assessment of the outcomes of the studies, 35 studies assessed disability using the Fibromyalgia Impact Questionnaire (FIQ) [11, 12, 13, 14, 16, 26, 27, 29, 30, 32, 34, 35, 38, 39, 40, 42, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 57, 59, 60, 62, 65], the Pain Disability Index (PDI) [49, 58, 62], the Fibromyalgia Assessment Status (FAS) and the Health Assessment Questionnaire (HAQ) [53]. Thirty studies assessed pain intensity with the visual analogue scale (VAS) [11, 12, 14, 15, 16, 30, 34, 35, 36, 39, 45, 46, 47, 49, 51, 52, 54, 58, 59, 61], the numerical rating scale (NRS) [27, 33, 37, 62], the Brief Pain Inventory (BPI) [28, 57, 63, 64] and the pain severity subscale of the Multidimensional Pain Inventory (PS-MPI) [56]. Sixteen studies assessed depression using the Beck Depression Inventory (BDI) [11, 12, 35, 38, 44, 45, 47, 48, 50, 58, 60, 62], the Centre for Epidemiologic Studies Depression Scale (CES-D) [13, 14, 30, 64] and the depression subscale of the Hospital Anxiety and Depression Scale (HADS-D) [12, 44]. Twelve studies assessed sleep quality using the Pittsburgh Sleep Quality Index (PSQI) [11, 13, 30, 44, 54, 59, 64], the VAS [61], the Post Sleep Inventory (PSI) [39], the Medical Outcomes Study Sleep Scale (MOS) [63], the NRS [33], the total sleep time (TST) and the total nap time (TNT) [55]. Eleven studies assessed fatigue with the Multidimensional Fatigue Inventory (MFI) [37, 51, 59, 63], the VAS [11, 61], the Fatigue Impact Scale (FIS) [12], the NRS [33], the Fatigue Severity Scale (FSS) [28], the Global Fatigue Index (GFI) [34] and the short form of the PROMIS fatigue scale [64]. Nine studies assessed anxiety using the State-Trait Anxiety Inventory (STAI) [12, 14, 45, 48, 62], the anxiety subscale of the Hospital Anxiety and Depression Scale (HADS-A) [12, 44], the Hamilton Anxiety Rating Scale (HAM-A) [54], the Beck Anxiety Inventory (BAI) [11] and the Generalized Anxiety Disorder-7 scale (GAD-7) [64]. Nine studies assessed pressure pain thresholds (PPT) with algometry [11, 12, 26, 30, 31, 36, 56, 60, 63]. Twelve studies assessed function using the 6 Minutes Walking Test (6MWT) [13, 16, 44, 45, 48, 49, 51, 57], the Timed Up and Go test (TUG) [16, 41, 43, 46], the Sit to Stand test [13, 44], the Arm Curl Test (ACT) [41] and the Continuous Scale Physical Functioning Performance (CS-PFP) [32].
3.3Effects of interventions
The results of studies that included active therapies as a primary intervention, such as ET, mind-body therapies, whole body vibration and multimodal active therapies are shown in Table 2. The results of studies that used passive therapies such as PE, MT, NT, laser therapy, balneotherapy and multimodal passive therapies are shown in Table 3.
3.3.1Pain intensity
Regarding active therapies, there was strong evidence that showed that strengthening, swimming and other aquatic exercise therapies were effective for reducing pain intensity in the short term [11, 16, 49, 54]. Moreover, strong evidence showed that ET combined with PE reduced significantly pain intensity in the short term [56, 57]. Moderate evidence suggested that exergames, dance and combined ET reduced significantly pain in the short term [15, 45, 52]. Aquatic ET, combined types of ET and the combination of ET with PE and MT were effective in the medium and long term [11, 52, 58]. There was contradictory evidence about walking interventions in the short term [46, 47, 51].
For passive therapies, strong evidence suggested that MT [28, 30] and NT were effective for improve pain intensity in the short term [12, 34, 35, 36]. Moderate evidence showed that balneotherapy, laser and the combination of ultrasounds with electrotherapy reduced significantly pain intensity in the short term [14, 33, 39]. Moderate evidence suggested that affective self-awareness intervention and motivational interviewing for encourage exercise reduced pain intensity in the short and long term [57, 63].
3.3.2PPT
Moderate evidence suggested that aerobic aquatic ET increased significantly PPT in the short and long term [11]. In addition, strength or aerobic ET in addition to cognitive-behavioral therapy increased significantly PPT in the short term [56]. Trapezius exercises guided by electromyogram biofeedback were effective for the increase of local PPT in the short term [60].
Strong evidence showed that dry needling increased significantly PPT in more than half of the body points evaluated in the short term [12, 36]. Regarding PE, moderate evidence showed that self-awareness intervention was effective for increasing PPT in the long term [63].
3.3.3Physical function
Strong evidence showed that exergames were effective for improving physical function in the short term [41, 43]. For distance walked in 6 minutes, moderate evidence suggested that dance and Nordic walking were effective in the short and long term [45, 51]. Furthermore, motivational interviewing to encourage ET,
Table 2
Results of studies that included active therapies as a primary intervention
| Author | Sample (N) | Interventions | Total number of session | Session per week | Single session duration | Variables | Results | Follow-up |
|---|---|---|---|---|---|---|---|---|
| EXERCISE THERAPY | ||||||||
| Martín-Martínez et al. (2019) | G1: 54.04 | G1: exergame G2: usual care | 48 | 2 | 60 min | ACT TUG Sit and reach | G1 obtained better scores compared to G2 ( | |
| Collado Mateo et al. (2017) | G1: 52.52 | G1: exergame G2: usual care | 16 | 2 | 60 min | FIQ | G1 obtained better scores compared to G2 ( | |
| Villafaina et al. (2019) | G1: 54.04 | G1: exergame G2: usual care | 48 | 2 | 60 min | VAS pain | G1 obtained better scores compared to G2 ( | |
| Collado Mateo et al. (2017) | G1: 52.43 | G1: exergame G2: usual care | 16 | 2 | 60 min | TUG | G1 obtained better scores compared to G2 ( | |
| Baptista et al. (2012) | G1: 49.5 ( | G1: dance G2: usual care | 32 | 2 | 1 hour | VAS pain 6MWT FIQ BDI STAI | G1 obtained better scores compared to G2 for VAS pain, 6MWT and FIQ ( | G1 improved compared to G2 for VAS pain, 6MWT and FIQ at 32 weeks ( |
| Andrade et al. (2019) | G1: 48 | G1: aerobic aquatic training G2: usual care | 32 | 2 | 45 min | VAS pain VAS fatigue PPT FIQ BDI BAI PSQI | G1 improved compared to G2 for PPT, VAS pain and FIQ ( | G1 improved compared to G2 for PPT, VAS pain and FIQ ( |
| Fernandes et al. (2016) | G1: 48.3 | G1: swimming G2: walking | 36 | 3 | 50 min | VAS pain FIQ TUG | G1 and G2 improved in VAS pain, FIQ and TUG ( | |
| Assis et al. (2006) | G1: 42.17 | G1: swimming G2: walking or jogging | 45 | 3 | 60 min | VAS pain BDI FIQ | G1 and G2 improved in all variables ( | |
| Gowans et al. (2001) | G1: 46.7 | G1: aerobic and stretching exercises G2: usual care | 69 | 3 | 30 min | BDI 6MWT STAI FIQ | G1 obtained better scores compared to G2 in all variables ( | |
| Larsson et al. (2015) | G1: 50.81 | G1: strengthening training G2: relaxation therapy | 30 | 2 | FIQ VAS pain 6MWT PDI | G1 improved compared to G2 for FIQ, VAS pain, 6MWT and PDI ( | ||
| Corrales et al. (2011) | G1: 55.48 | G1: aerobic and muscle strength training G2: usual care | 48 | 2 | 45 min | FIQ BDI | G1 improved compared to G2 for FIQ ( | |
|
Table 2, continued | ||||||||
|---|---|---|---|---|---|---|---|---|
| Author | Sample (N) | Interventions | Total number of session | Session per week | Single session duration | Variables | Results | Follow-up |
| Mannerkorpi et al. (2010) | G1: 48 | G1: nordic walking G2: low intensive walking | 30 | 2 | 20 min | 6MWT FIQ VAS pain MFI | G1 improved in 6MWT and FIQ. G1 improved compared to G2 for 6MWT and MFI-RM ( | G1 improved in 6MWT, MFI-GF and MFI-PF ( |
| Da Costa et al. (2005) | G1: 49.2 | G1: aerobic, stretch- ing and strength train- ing G2: usual care | 4 guided sessions | 4 sessions for 12 weeks | 60-120 min/week self-managed exercise | FIQ VAS upper body pain VAS low body pain | G1 improved in FIQ and VAS upper body pain ( | G1 improved compared to G2 for VAS upper body pain at 6 and 12 months and for FIQ at 12 months ( |
| Paolucci et al. (2016) | G1: 49.3 | G1: perceptive reha- bilitation program G2: aerobic, propri- oceptive and posture exercises G3: usual care | 10 | 2 | 60 min | FIQ FAS HAQ | G1 improved compared to G3 for FAS and HAQ ( | G1 and G2 improved compared to G3 for FIQ, FAS and HAQ ( |
| Ide et al. (2008) | G1: 46.61 | G1: aquatic respira- tory exercise | 16 | 4 | 60 min | VAS pain FIQ HAM-A PSQI | G1 improved in all variables ( | |
| Silva et al. (2018) | G1: 49.40 | G1: sophrology relax- ation therapy G2: strengthening training | 24 | 2 | 40 min | VAS pain 6MWT TUG FIQ | G1 and G2 improved in VAS pain and TUG ( | |
| Baumueller et al. (2017) | G1: 55.4 | G1: trapezius mus- cle strain and relaxation guided by electromyogram | 14 | 3 sessions for 3 weeks. 1 session for 5 weeks | No data | FIQ PPT BDI | G1 improved compared to G2 for PPT ( | No between-groups differences were found in any variable at 3 months. |
| MIND-BODY THERAPIES | ||||||||
| Wang et al. (2018) | G1: 53 | G1: 1 Tai chi session (12 w) G2: 2 Tai chi sessions (12 w) G3: 1 Tai chi session (24 w) G4: 2 Tai chi sessions (24 w) G5: 2 aerobic exer- cise sessions (24 w) | 12 or 24 | 1 or 2 | 60 min | FIQR HADS-A HADS-D PSQI BDI-II Sit-to-stand 6MWT | G1, G2, G3 and G4 improved compared to G5 for FIQR and HADS-A ( | G3 and G4 improved compared to G5 for FIQR and HADS-A at 52 weeks ( |
|
Table 2, continued | ||||||||
|---|---|---|---|---|---|---|---|---|
| Author | Sample (N) | Interventions | Total number of session | Session per week | Single session duration | Variables | Results | Follow-up |
| WHOLE BODY VIBRATION | ||||||||
| Olivares et al. (2011) | G1: 52.4 | G1: whole body vibration G2: usual care | 36 | 3 | 30 min | FIQ | G2 worsened and G1 had no changes in FIQ ( | |
| MULTIMODAL ACTIVE THERAPIES | ||||||||
| Vitorino et al. (2006) | G1: 48.9 | G1: aquatic exercise and relaxation G2: conventional physiotherapy | 9 | 3 | 60 min | TST TNT | G1 and G2 improved in all variables ( | |
| Hooten et al. (2012) | G1: 47.3 | G1: strength training and CBT G2: aerobic training and CBT | 15 | 5 | G1: 25–30 min | MPI-PSS PPT | G1 and G2 improved in all variables ( | |
| Ang et al. (2013) | G1: 46.0 | G1: motivational in- terviewing to encour- age exercise | 12 weeks of intervention | 2 to 4 | 12 to 30 min | FIQ BPI 6MWT (not measured at 3 months follow up) | G1 and G2 improved in all variables ( | G1 and G2 improved in all variables at 3 and 6 months ( |
| Lemstra et al. (2005) | G1: 49.70 | G1: exercise, pain and stress manage- ment lectures, educa- tion lecture, dietary lecture and massage G2: usual care | 18 exercise 2 pain and stress management 1 education lecture 1 dietary lecture 2 massage 6 weeks | No data | 20 min–3 hours | VAS pain PDI BDI | G1 obtained better scores in all variables compared to G2 ( | G1 obtained better scores compared to G2 for PDI at 21 weeks ( |
Abbreviations: G: group; FM: Fibromyalgia; w: weeks; n: number of subjects per group; CBT: Cognitive Behavioural Therapy; FIQ: Fibromyalgia Impact Questionnaire; FIQ-R: revised version of Fibromyalgia Impact Questionnaire; PDI: Pain Disability Index; FAS: Fibromyalgia Assessment Status; HAQ: Health Assessment Questionnaire; VAS: Visual Analogue Scale; NRS: Numerical Rating Scale; BPI: Brief Pain Inventory; PS-MPI: Multidimensional Pain Inventory-Pain Severity Subscale; BDI: Beck Depression Inventory; CES-D: Center for Epidemiologic Studies Depression Scale; HADS-D: Hospital Anxiety and Depression Scale – Depression Subscale; PSQI: Pittsburgh Sleep Quality Index; PSI: Post Sleep Inventory; TST: Total Sleep Time; TNT: Total Nap Time; MFI: Multidimensional Fatigue Inventory; MFI-RM: Multidimensional Fatigue Inventory-Reduced Motivation; MFI-GF: Multidimensional Fatigue Inventory-General Fatigue; MFI-PF: Multidimensional Fatigue Inventory-Physical Fatigue; FIS: Fatigue Impact Scale; FIS; FSS: Fatigue Severity Scale; GFI: Global Fatigue Index; HADS-A: Hospital Anxiety and Depression Scale – Anxiety Subscale; STAI: State-Trait Anxiety and Depression Scale; HAM-A: Hamilton Anxiety Rating Scale; BAI: Beck Anxiety Inventory; GAD-7: Generalized Anxiety Disorder-7 Scale; PPT: Pressure Pain Threshold; 6MWT: 6 Minutes Walking Test; TUG: Timed Up and Go; ACT: Arm Curl Test.
Table 3
Results of studies that included passive therapies as a primary intervention
| Author | Sample | Interventions | Total number of session | Session per week | Single session duration | Variables | Results | Follow-up |
|---|---|---|---|---|---|---|---|---|
| PATIENT EDUCATION | ||||||||
| Simister et al. (2018) | G1: ( | G1: acceptance and commit- ment therapy G2: usual care | 6 | 5 days per module (online) | No data | FIQ-R CES-D PSQI 6MWT Sit-to-stand | G1 improved compared to G2 for FIQ-R ( | G1 improved in FIQ-R and CES-D at 3 months ( |
| Wicksell et al. (2013) | 45.1 | G1: acceptance and commit- ment therapy G2: usual care | 12 | 1 | 90 min | PDI FIQ BDI STAI NRS pain | G1 improved compared to G2 for PDI, FIQ, BDI and STAI ( | G1 improved compared to G2 for PDI, FIQ, BDI and STAI at 3–4 months ( |
| Hsu et al. (2010) | 50.1 | G1: self-awareness interven- tion G2: usual care | 1 individual session 3 group sessions | 1 | Individual session: 90 min Group sessions: 2 hours | BPI-pain MFI PPT MOS sleep | G1 improved compared to G2 for BPI pain ( | G1 improved compared to G2 for BPI pain ( |
| Lumley et al. (2017) | G1: 48.98 | G1: emotion awareness and expression therapy G2: CBT G3: education in FM | 8 | 1 | 90 min | BPI-pain PSQI CES-D GAD-7 SF-PROMIS | G1 improved compared to G2 for WPI ( | G1 improved compared to G2 for WPI ( |
| Van Ittersum et al. (2009) | G1: 47.60 | G1: pain neuroscience education G2: relaxation | 6-weeks of self-management based in a booklet and phone calls | FIQ | No differences between groups were found. | |||
| Van Oosterwijck et al. (2013) | G1: 45.80 | G1: pain neurophysiology education G2: activity self-management education | 2 | 1 | 30 min | FIQ PPT | No differences between groups were found. | No differences between groups were found at 3 months. |
| Thieme et al. (2006) | G1: 43.23 | G1: operant behavioural therapy G2: CBT G3: attention placebo | 15 | 1 | 2 hours | NRS pain FIQ | G2 improved in NRS pain ( | G1 and G2 improved in NRS pain at 6 and 12 months. G1 improved in FIQ at 12 months ( |
| MANUAL THERAPY | ||||||||
| Torres et al. (2015) | G1: 53.0 | G1: neurodynamic mobilization program G2: information about health lifestyle | 16 | 2 | 60 min | BPI-Reactive BPI-Sensory FSS | G1 improved in all variables ( | |
|
Table 3, continued | ||||||||
|---|---|---|---|---|---|---|---|---|
| Author | Sample | Interventions | Total number of session | Session per week | Single session duration | Variables | Results | Follow-up |
| Ceca et al. (2017) | G1: ( | G1: self-myofascial release G2: usual care | 40 | 2 | 50 min | FIQ | G1 improved compared to G2 for FIQ ( | |
| Castro-Sanchez et al. (2014) | 54 | G1: manual therapy G2: usual care | 5 | 1 | 45 min | PPT FIQ VAS pain PSQI CES-D | G1 improved compared to G2 for FIQ, VAS pain, PSQI, CES-D and 10 out of 11 points of PPT ( | |
| Castro-Sanchez et al. (2011) | G1: 53.85 | G1: craniosacral therapy G2: disconnected magnetotherapy | 40 | 2 | 60 min | PPT | G1 improved compared to baseline and G2 in 13 out of 18 points ( | G1 improved compared to G2 in 9 out of 18 points at 28 weeks. G1 improved compared to G2 in 4 out of 18 points at 17 months. |
| NEEDLING THERAPIES | ||||||||
| Mist et al. (2018) | G1: 52.3 | G1: acupuncture G2: education | 20 | 2 | 40 min | FIQ-R VAS pain GFI | G1 improved in all variables ( | G1 improved in all variables at 14 weeks ( |
| Karatay et al. (2018) | G1: 34.71 | G1: acupuncture G2: sham acupuncture G3: simulated acupuncture | 8 | 2 | 30 min | VAS pain FIQ BDI | G1 ( | G1 improved compared to G2 in all variables ( |
| Castro-Sanchez et al. (2017) | G1: 46.65 | G1: dry needling G2: cross tape | 4 | 1 | No data | VAS pain PPT | G1 and G2 improved in VAS pain and 22 out of 26 points PPT ( | |
| Harris et al. (2005) | G1: 46.0 | G1: acupuncture over tradi- tional site with manual stimu- lation of the needle G2: acupuncture over tradi- tional site G3: acupuncture over nontra- ditional site with stimulation G4: acupuncture over nontraditional site | 18 | 3 sessions for 3 weeks, 6 sessions for 3 weeks, 9 sessions for 3 weeks | 20 min | NRS pain MFI | G1 | No differences between groups at 13 and 15 weeks. |
| Castro Sanchez et al. (2019) | G1: 47.37 | G1: dry needling group G2: myofascial release group | 4 | 1 | No data | PPT FIQ PSQI VAS pain STAI BDI FIS HADS-A HADS-D | G1 improved in 28 out of 46 points PPT, PSQI, STAI, BDI, VAS pain and FIS ( | |
|
Table 3, continued | ||||||||
|---|---|---|---|---|---|---|---|---|
| Author | Sample | Interventions | Total number of session | Session per week | Single session duration | Variables | Results | Follow-up |
| LASER THERAPY | ||||||||
| Panton et al. (2013) | G1: 52 | G1: laser and heat therapy G2: sham laser and heat therapy | 8 | 2 | 15 min | FIQ CS-PFP | G1 improved in all variables ( | |
| Gür et al. (2002) | G1: ( | G1: laser G2: placebo laser | 10 | 5 | 3 min at each tender point | NRS pain NRS sleep NRS fatigue | G1 and G2 improved in NRS pain ( | |
| BALNEOTHERAPY | ||||||||
| Fioravanti et al. (2018) | G1: 56.16 | G1: balneotherapy G2: control group | 12 | 6 | 15min | VAS pain FIQ STAI CES-D | G1 improved in VAS pain, FIQ, CES-D and WPI ( | G1 improved compared to G2 for VAS pain, FIQ, WPI and CES-D at 3 ( |
| MULTIMODAL PASSIVE THERAPIES | ||||||||
| Bagdatli et al. (2015) | G1: 45.17 | G1: balneotherapy | 10 | 5 | 40 min | FIQ BDI | G1 and G2 improved in FIQ and BDI ( | G1 and G2 improved in FIQ and BDI ( |
| Moretti et al. (2011) | G1: 53.2 | G1: ultrasound | G1: 12 G2: 24 | G1:1 G2:2 | Variable | VAS pain FIQ PSI | G1 and G2 showed improvements in all variables ( | |
Abbreviations: G: group; FM: Fibromyalgia; w: weeks; n: number of subjects per group; CBT: Cognitive Behavioural Therapy; FIQ: Fibromyalgia Impact Questionnaire; FIQ-R: revised version of Fibromyalgia Impact Questionnaire; PDI: Pain Disability Index; FAS: Fibromyalgia Assessment Status; HAQ: Health Assessment Questionnaire; VAS: Visual Analogue Scale; NRS: Numerical Rating Scale; BPI: Brief Pain Inventory; BDI: Beck Depression Inventory; CES-D: Center for Epidemiologic Studies Depression Scale; HADS-D: Hospital Anxiety and Depression Scale – Depression Subscale; PSQI: Pittsburgh Sleep Quality Index; PSI: Post Sleep Inventory; MOS: Medical Outcomes Study Sleep Scale; MFI: Multidimensional Fatigue Inventory; FIS: Fatigue Impact Scale; FIS; FSS: Fatigue Severity Scale; GFI: Global Fatigue Index; HADS-A: Hospital Anxiety and Depression Scale – Anxiety Subscale; STAI: State-Trait Anxiety and Depression Scale; HAM-A: Hamilton Anxiety Rating Scale; BAI: Beck Anxiety Inventory; GAD-7: Generalized Anxiety Disorder-7 Scale; PPT: Pressure Pain Threshold; 6MWT: 6 Minutes Walking Test; TUG: Timed Up and Go; ACT: Arm Curl Test; CS-PFP: Continuous Scale Physical Functioning Performance.
aerobic ET combined with FM education and combination of aerobic ET with stretching ET were effective in the short term [48, 57]. There was contradictory evidence about the effectiveness of strengthening training and relaxation for physical function in the short term [16, 49].
3.3.4Disability
For active therapies, strong evidence suggested that aerobic ET [11, 44, 46, 47, 51], combined ET [48, 50, 52, 53] and aquatic ET [11, 46, 47, 54] were effective for reducing disability in the short term. Moderate evidence suggested that dance, exergames, tai chi and combination of ET with PE and MT reduced significantly disability in the short term [43, 44, 45, 58]. In addition, moderate evidence suggested that dance, tai chi and combination of ET with PE and MT reduced disability in the medium or long term [44, 45, 58]. Moderate evidence suggested that combination of aerobic ET with FM education reduced significantly disability in the short, medium and long term [57]. There was contradictory evidence about the effectiveness of strengthening ET in the short term [16, 49].
For passive therapies, strong evidence showed that acceptance and commitment therapy reduced significantly disability in the short and medium term [13, 62], but pain neuroscience education applied in isolation did not reduce disability in the short term [26, 65]. Moderate evidence showed that FM education and motivational interviewing to encourage exercise reduced significantly disability in the short term [38, 57]. PE combined with balneotherapy was effective in the short and medium term [38]. There was contradictory evidence about the effectiveness of myofascial release and NT in the short term [12, 29, 34, 35]. However, strong evidence showed that NT were effective in the medium term [34, 35].
3.3.5Sleep
Moderate evidence suggested that the aquatic respiratory training combined with recreational activities or relaxation improved significantly sleep quality in the short term [54, 55]. Aquatic exercise plus relaxation and infrared thermotherapy combined with stretching, aerobic ET and relaxation increased significantly the total sleep time in the short term [55].
Strong evidence showed that MT improved significantly sleep quality in the short term [12, 30]. Moderate evidence showed that NT, laser therapy and the combination of ultrasounds with the interferential current improved significantly sleep quality in the short term [12, 33, 39].
3.3.6Fatigue
For ET, moderate evidence showed that Nordic walking, low intensive walking and aerobic aquatic ET did not reduce fatigue in the short term [11, 51]. Nordic walking reduced general fatigue and physical fatigue in the long term [51].
Strong evidence showed that NT reduced significantly fatigue in the short term [12, 34]. Moderate evidence showed that a neurodynamic mobilization program was effective in the short term [28], but myofascial release did not show effectiveness in the short term [12].
3.3.7Depression
Moderate evidence suggested that swimming, walking or jogging and the combination of aerobic ET with stretching were effective for reducing depression in the short term [47, 48].
Strong evidence showed that NT reduced depression significantly in the short term [12, 35]. About PE intervention, strong evidence showed that acceptance and commitment therapy was effective for reducing depression in the short term [13, 62]. Moderate evidence showed that PE combined with other therapies like MT, ET or balneotherapy reduced significantly depression in the short term [38, 58]. Moderate evidence showed that MT reduced significantly depression in the short term [30], and balneotherapy applied in isolation or combined with PE were effective in the short and medium term [14, 38].
3.3.8Anxiety
Concerning to active therapies, moderate evidence showed that aquatic respiratory ET and aerobic ET combined with stretching techniques were effective for reducing anxiety in the short term [48, 54].
For passive therapies, moderate evidence showed that PE through acceptance and commitment therapy were effective for reducing anxiety level in the short term [62]. Also moderate evidence suggested that dry needling could be effective in the short term [12].
4.Discussion
This systematic review assessed the effects of non-pharmacological conservative treatment on pain intensity, PPT, physical function, disability, sleep, fatigue, depression and anxiety in FM patients. This review found strong evidence that active therapies reduced pain intensity and disability, and increased physical function in the short term [11, 16, 41, 43, 44, 46, 47, 48, 49, 50, 51, 52, 53, 54, 56, 57]. Our results also showed moderate evidence that active therapies improved pain intensity, disability and physical function in the long term, and sleep quality, anxiety and depression in the short term [11, 44, 45, 47, 48, 51, 52, 54, 55, 57, 58]. The main active non-pharmacological conservative treatments that improve pain, disability and/or physical function in the short and long term were water-based and land-based aerobic ET, strengthening, exergames and multimodal active therapies [11, 16, 41, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58].
These findings are in concordance with previous guidelines that recommend multimodal non-pharmaco- logical therapies as first-line intervention [9, 66]. According to this, a recent systematic review and meta-analysis concluded that ET reduces pain intensity, disability and depression. However, the effect on depression variable was small [67]. In the present review, the benefits of ET on depression showed to be moderate in the short term [47, 48].
The improvements achieved on pain-related variables with active therapies may be due to central and peripheral adaptations [68, 69, 70]. Opioid and serotonergic mechanisms could modulate pain-related symptoms, through pain processing areas in the central nervous system [42, 68, 71]. The descending pain inhibition system plays an important role in the exercise-induced analgesia observed in patients with chronic pain [68, 71]. The improvement on disability may be related to the decrease on pain intensity. Therefore, patients with less pain could adopt a more active lifestyle increasing physical function and avoiding disability [42]. Moreover, ET promotes neuroplasticity mechanisms, and that enhances the ability to improve performance in different skills [69, 70].
Strong evidence showed that passive therapies present different short-term benefits in patients with FM. MT decreased pain intensity and improved sleep quality in the short term [12, 28, 30], NT reduced pain intensity, fatigue, depression, and increased PPT in the short term [12, 34, 35, 36], and PE, through acceptance and commitment therapy, reduced disability and depression in the short term [13, 62].
The results found in this systematic review are in accordance with previous studies that concluded that MT induces immediate analgesic effects. The improvements achieved on pain intensity after MT techniques could be the result of the interaction of local, segmental and central processes that inhibit pain sensitizing mechanisms and facilitate pain inhibitory mechanisms [72, 73, 74]. MT effects could involve biomechanical mediators, such as the therapeutic procedure and the tissue adaptations, and neurophysiological mediators, including the decrease in the inflammatory environment, the excitation of the sympathetic nervous system and the modulation of afferent nerve fibers [72, 73]. The analgesic effect produced by MT could be implicated in the improvement on sleep quality.
The short-term benefits of NT on pain-related variables, fatigue and depression showed in this systematic review are in accordance with the results showed by other authors in chronic pathologies [75, 76, 77]. NT appear to reduce both peripheral and central sensitization. The insertion of the needle provokes the secretion of endogenous opioids and the increment of
Strong evidence suggested that PE, through acceptance and commitment therapy, reduces disability and depression. The results are in accordance with previous studies that showed limited evidence of the benefits of PE on pain-related variables, but the inclusion in multimodal therapies is recommended [9, 81]. The modification of behaviors and the adoption of coping strategies could reduce the negative impact of FM, improving disability and depression [13, 27]. This fact allows the patient to take more control and to be an active participant in the treatment [81, 82].
From a clinical perspective, the results achieved in this systematic review showed that different types of active therapies seem to improve pain-related variables, physical function and disability in the short and long term. In addition, passive therapies such as MT, NT and PE seem to improve different clinical features of FM in the short term. According to these results, clinicians could use different MT, NT or PE techniques to achieve immediate benefits in patients with FM, and its combination with different types of ET may contribute to further improvements in the medium and long terms.
This study presents several limitations. The main limitation is the heterogeneity of the instruments and outcome measures, and the variability in the design of the interventions that complicated the comparison between studies. Another limitation is that the consistency of the independent reviewers was not calculated during the systematic searches. Finally, only studies in English and Spanish were included, while studies in other languages were not considered, potentially excluding relevant evidence.
5.Conclusion
The result of this systematic review of high-quality clinical trials provides moderate to strong evidence that active therapies such as water-based and land-based aerobic ET, strengthening, exergames and multimodal active therapies, improved pain intensity, disability and physical function in the long term, and sleep quality, anxiety and depression in the short term in patients with FM. Strong evidence showed that passive therapies have benefits on different clinical features. MT decreased pain intensity and improved sleep quality in the short term, NT reduced pain intensity, fatigue, depression, and increased PPT in the short term, and PE through acceptance and commitment therapy reduced disability and depression in the short term.
Conflict of interest
This research is not financed, and the authors have no conflict of interest to report.
References
[1] | Bennett RM. Pain management in fibromyalgia. Pain Manag. (2016) May 1; 6: (4): 313-6. |
[2] | Cabo-Meseguer A, Cerdá-Olmedo G, Trillo-Mata JL. Fibromyalgia: prevalence, epidemiologic profiles and economic costs. Med Clin (Barc). (2017) Nov 22; 149: (10): 441-8. |
[3] | Verbunt JA, Pernot DHFM, Smeets RJEM. Disability and quality of life in patients with fibromyalgia. Health Qual Life Outcomes. (2008) Jan 22; 6: : 8. |
[4] | Marques AP, Santo A de S do E, Berssaneti AA, Matsutani LA, Yuan SLK. Prevalence of fibromyalgia: literature review update. Rev Bras Reumatol (English Ed.) (2017) Jul 1; 57: (4): 356-63. |
[5] | Sañudo JI, Corrales-Sánchez R, Sañudo B. Physical activity levels, quality of life and incidence of depression in older women with fibromyalgia. (2013) ; 6: (2): 53-60. |
[6] | Wolfe F, Clauw DJ, Fitzcharles M-A, Goldenberg DL, Häuser W, Katz RL, et al. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum. (2016) ; 46: : 319-29. |
[7] | Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, et al. The american college of rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. (2010) May; 62: (5): 600-10. |
[8] | Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The american college of rheumatology 1990 criteria for the classification of fibromyalgia. Arthritis Rheum. (1990) ; 33: (2): 160-72. |
[9] | Macfarlane GJ, Kronisch C, Dean LE, Atzeni F, Häuser W, Flub E, et al. EULAR revised recommendations for the management of fibromyalgia. Ann Rheum Dis. (2017) Feb 1; 76: (2): 318-28. |
[10] | Fitzcharles MA, Ste-Marie PA, Goldenberg DL, Pereira JX, Abbey S, Choinière M, et al. 2012 Canadian guidelines for the diagnosis and management of fibromyalgia syndrome: executive summary. Pain Res Manag. (2013) ; 18: (3): 119-26. |
[11] | Andrade CP, Zamunér AR, Forti M, Tamburús NY, Silva E. Effects of aquatic training and detraining on women with fibromyalgia: controlled randomized clinical trial. Eur J Phys Rehabil Med. (2019) Feb 1; 55: (1): 79-88. |
[12] | Castro Sánchez AM, García López H, Fernández Sánchez M, Pérez Mármol JM, Aguilar-Ferrándiz ME, Luque Suárez A, et al. Improvement in clinical outcomes after dry needling versus myofascial release on pain pressure thresholds, quality of life, fatigue, pain intensity, quality of sleep, anxiety, and depression in patients with fibromyalgia syndrome. Disabil Rehabil. (2019) Sep; 41: (19): 2235-46. |
[13] | Simister HD, Tkachuk GA, Shay BL, Vincent N, Pear JJ, Skrabek RQ. Randomized controlled trial of online acceptance and commitment therapy for fibromyalgia. J Pain. (2018) Jul 1; 19: (7): 741-53. |
[14] | Fioravanti A, Manica P, Bortolotti R, Cevenini G, Tenti S, Paolazzi G. Is balneotherapy effective for fibromyalgia? Results from a 6-month double-blind randomized clinical trial. Clin Rheumatol. (2018) Aug 1; 37: (8): 2203-12. |
[15] | Villafaina S, Collado-Mateo D, Domínguez-Muñoz FJ, Fuentes-García JP, Gusi N. Benefits of 24-week exergame intervention on health-related quality of life and pain in women with fibromyalgia: a single-blind, randomized controlled trial. Games Health J. (2019) Dec 1; 8: (6): 380-6. |
[16] | Silva HJ de A, Assunção Júnior JC, de Oliveira FS, Oliveira JM de P, Figueiredo Dantas GA, Lins CA de A, et al. Sophrology versus resistance training for treatment of women with fibromyalgia: a randomized controlled trial. J Bodyw Mov Ther. (2019) Apr 1; 23: (2): 382-9. |
[17] | Johnson MI, Claydon LS, Herbison GP, Paley CA, Jones G. Transcutaneous Electrical Nerve Stimulation (TENS) for fibromyalgia in adults. Cochrane Database Syst Rev. (2017) Apr 28; 2017: (10): CD012172. |
[18] | Bidonde J, Busch AJ, Schachter CL, Overend TJ, Kim SY, Góes SM, et al. Aerobic exercise training for adults with fibromyalgia. Cochrane Database Syst Rev. (2017) Jun 21; 6: : CD012700. |
[19] | Kim SY, Busch AJ, Overend TJ, Schachter CL, van der Spuy I, Boden C, et al. Flexibility exercise training for adults with fibromyalgia. Cochrane Database Syst Rev. (2019) Sep 2; 2019: (9): CD013419. |
[20] | Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (prisma-p) 2015: elaboration and explanation. BMJ. (2015) Jan 2; 350: : g7647. |
[21] | Walser RF, Meserve BB, Boucher TR. The effectiveness of thoracic spine manipulation for the management of musculoskeletal conditions: a systematic review and meta-analysis of randomized clinical trials. J Man Manip Ther. (2009) Dec 18; 17: (4): 237-46. |
[22] | Ashton RE, Tew GA, Aning JJ, Gilbert SE, Lewis L, Saxton JM. Effects of short-term, medium-term and long-term resistance exercise training on cardiometabolic health outcomes in adults: Systematic review with meta-analysis. Br J Sports Med. (2020) Mar 1; 54: (6): 341-8. |
[23] | Verhagen AP, De Vet HCW, De Bie RA, Kessels AGH, Boers M, Bouter LM, et al. The delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. (1998) Dec; 51: (12): 1235-41. |
[24] | de Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother. (2009) Jan 1; 55: (2): 129-33. |
[25] | van Tulder M, Furlan A, Bombardier C, Bouter L. Updated method guidelines for systematic reviews in the cochrane collaboration back review group. Spine (Phila Pa 1976). (2003) Jun; 28: (12): 1290-9. |
[26] | Van Oosterwijck J, Meeus M, Paul L, De Schryver M, Pascal A, Lambrecht L, et al. Pain physiology education improves health status and endogenous pain inhibition in fibromyalgia: a double-blind randomized controlled trial. Clin J Pain. (2013) Oct; 29: (10): 873-82. |
[27] | Thieme K, Flor H, Turk DC. Psychological pain treatment in fibromyalgia syndrome: efficacy of operant behavioural and cognitive behavioural treatments. Arthritis Res Ther. (2006) Jul 19; 8: (4): R121. |
[28] | Torres JR, Martos IC, Sánchez IT, Rubio AO, Pelegrina AD, Valenza MC. Results of an active neurodynamic mobilization program in patients with fibromyalgia syndrome: a randomized controlled trial. Arch Phys Med Rehabil. (2015) Oct 1; 96: (10): 1771-8. |
[29] | Ceca D, Elvira L, Guzmán JF, Pablos A. Benefits of a self-myofascial release program on health-related quality of life in people with fibromyalgia: a randomized controlled trial. J Sports Med Phys Fitness. (2017) Jul 1; 57: (7–8): 993-1002. |
[30] | Castro-Sánchez AM, Aguilar-Ferrándiz ME, Matarán-Peñarrocha GA, Sánchez-Joya MDM, Arroyo-Morales M, Fernández-de-las-Peñas C. Short-term effects of a manual therapy protocol on pain, physical function, quality of sleep, depressive symptoms, and pressure sensitivity in women and men with fibromyalgia syndrome: a randomized controlled trial. Clin J Pain. (2014) Jul; 30: (7): 589-97. |
[31] | Castro-Sánchez AM, Matarán-Peñarrocha GA, Sánchez-Labraca N, Quesada-Rubio JM, Granero-Molina J, Moreno-Lorenzo C. A randomized controlled trial investigating the effects of craniosacral therapy on pain and heart rate variability in fibromyalgia patients. Clin Rehabil. (2011) Jan; 25: (1): 25-35. |
[32] | Panton L, Simonavice E, Williams K, Mojock C, Kim J-S, Kingsley JD, et al. Effects of class IV laser therapy on fibromyalgia impact and function in women with fibromyalgia. J Altern Complement Med. (2013) May 1; 19: (5): 445-52. |
[33] | Gür A, Karakoç M, Nas K, Cevik R, Saraç J, Demir E. Efficacy of low power laser therapy in fibromyalgia: a single-blind, placebo-controlled trial. Lasers Med Sci. (2002) ; 17: (1): 57-61. |
[34] | Mist SD, Jones KD. Randomized controlled trial of acupuncture for women with fibromyalgia: group acupuncture with traditional chinese medicine diagnosis-based point selection. Pain Med. (2018) ; 19: (9): 1862-71. |
[35] | Karatay S, Okur SC, Uzkeser H, Yildirim K, Akcay F. Effects of acupuncture treatment on fibromyalgia symptoms, serotonin, and substance p levels: a randomized sham and placebo-controlled clinical trial. Pain Med. (2018) ; 19: (3): 615-28. |
[36] | Castro-Sánchez AM, García-López H, Matarán-Peñarrocha GA, Fernández-Sánchez M, Fernández-Sola C, Granero-Molina J, et al. Effects of dry needling on spinal mobility and trigger points in patients with fibromyalgia syndrome. Pain Physician. (2017) ; 20: (2): 37-52. |
[37] | Harris RE, Tian X, Williams DA, Tian TX, Cupps TR, Petzke F, et al. Treatment of fibromyalgia with formula acupuncture: investigation of needle placement, needle stimulation, and treatment frequency. J Altern Complement Med. (2005) Aug; 11: (4): 663-71. |
[38] | Bağdatlı AO, Donmez A, Eröksüz R, Bahadır G, Turan M, Erdoğan N. Does addition of “mud-pack and hot pool treatment” to patient education make a difference in fibromyalgia patients? A randomized controlled single blind study. Int J Biometeorol. (2015) Dec 1; 59: (12): 1905-11. |
[39] | Moretti FA, Marcondes FB, Provenza JR, Fukuda TY, de Vasconcelos RA, Roizenblatt S. Combined therapy (ultrasound and interferential current) in patients with fibromyalgia: once or twice in a week? Physiother Res Int. (2012) Sep; 17: (3): 142-9. |
[40] | Olivares PR, Gusi N, Parraca JA, Adsuar JC, Del Pozo-Cruz B. Tilting whole body vibration improves quality of life in women with fibromyalgia: a randomized controlled trial. J Altern Complement Med. (2011) Aug 1; 17: (8): 723-8. |
[41] | Martín-Martínez JP, Villafaina S, Collado-Mateo D, Pérez-Gómez J, Gusi N. Effects of 24-week exergame intervention on physical function under single- and dual-task conditions in fibromyalgia: a randomized controlled trial. Scand J Med Sci Sports. (2019) Oct 1; 29: (10): 1610-7. |
[42] | Collado-Mateo D, Dominguez-Muñoz FJ, Adsuar JC, Garcia-Gordillo MA, Gusi N. Effects of exergames on quality of life, pain, and disease effect in women with fibromyalgia: a randomized controlled trial. Arch Phys Med Rehabil. (2017) Sep 1; 98: (9): 1725-31. |
[43] | Collado-Mateo D, Dominguez-Muñoz FJ, Adsuar JC, Merellano-Navarro E, Gusi N. Exergames for women with fibromyalgia: a randomised controlled trial to evaluate the effects on mobility skills, balance and fear of falling. PeerJ. (2017) ; 5: (4): e3211. |
[44] | Wang C, Schmid CH, Fielding RA, Harvey WF, Reid KF, Price LL, et al. Effect of tai chi versus aerobic exercise for fibromyalgia: comparative effectiveness randomized controlled trial. BMJ. (2018) ; 360: : k851. |
[45] | Baptista AS, Villela AL, Jones A, Natour J. Effectiveness of dance in patients with fibromyalgia: a randomised, single-blind, controlled study. Clin Exp Rheumatol. (2012) ; 30: (6 Suppl.74): 18-23. |
[46] | Fernandes G, Jennings F, Nery Cabral MV, Pirozzi Buosi AL, Natour J. Swimming improves pain and functional capacity of patients with fibromyalgia: a randomized controlled trial. Arch Phys Med Rehabil. (2016) ; 97: (8): 1269-75. |
[47] | Assis MR, Silva LE, Barros Alves AM, Pessanha AP, Valim V, Feldman D, et al. A randomized controlled trial of deep water running: clinical effectiveness of aquatic exercise to treat fibromyalgia. Arthritis Care Res. (2006) Feb 15; 55: (1): 57-65. |
[48] | Gowans SE, deHueck A, Voss S, Silaj A, Abbey SE, Reynolds WJ. Effect of a randomized, controlled trial of exercise on mood and physical function in individuals with fibromyalgia. Arthritis Rheum. (2001) Dec; 45: (6): 519-29. |
[49] | Larsson A, Palstam A, Löfgren M, Ernberg M, Bjersing J, Bileviciute-Ljungar I, et al. Resistance exercise improves muscle strength, health status and pain intensity in fibromyalgia–a randomized controlled trial. Arthritis Res Ther. (2015) Jun 18; 17: (1): 161. |
[50] | Corrales BS, Galiano D, Carrasco L, De Hoyo M, McVeigh JG. Effects of a prolonged exercise programe on key health outcomes in women with fibromyalgia? A randomized controlled trial. J Rehabil Med. (2011) May; 43: (6): 521-6. |
[51] | Mannerkorpi K, Nordeman L, Cider A, Jonsson G. Does moderate-to-high intensity Nordic walking improve functional capacity and pain in fibromyalgia? A prospective randomized controlled trial. Arthritis Res Ther. (2010) Oct 13; 12: (5): R189. |
[52] | Da Costa D, Abrahamowicz M, Lowensteyn I, Bernatsky S, Dritsa M, Fitzcharles M-A, et al. A randomized clinical trial of an individualized home-based exercise programme for women with fibromyalgia. Rheumatology (Oxford). (2005) Nov; 44: (11): 1422-7. |
[53] | Paolucci T, Baldari C, Di Franco M, Didona D, Reis V, Vetrano M, et al. A new rehabilitation tool in fibromyalgia: the effects of perceptive rehabilitation on pain and function in a clinical randomized controlled trial. Evid Based Complement Alternat Med. (2016) ; 2016: : 7574589. |
[54] | Ide MR, Laurindo LMM, Rodrigues-Júnior AL, Tanaka C. Effect of aquatic respiratory exercise-based program in patients with fibromyalgia. Int J Rheum Dis. (2008) Aug 1; 11: (2): 131-40. |
[55] | Vitorino DF de M, de Carvalho LBC, do Prado GF. Hydrotherapy and conventional physiotherapy improve total sleep time and quality of life of fibromyalgia patients: randomized clinical trial. Sleep Med. (2006) Apr; 7: (3): 293-6. |
[56] | Hooten WM, Qu W, Townsend CO, Judd JW. Effects of strength vs aerobic exercise on pain severity in adults with fibromyalgia: a randomized equivalence trial. Pain. (2012) Apr; 153: (4): 915-23. |
[57] | Ang DC, Kaleth AS, Bigatti S, Mazzuca S, Saha C, Hilligoss J, et al. Research to encourage exercise for fibromyalgia (REEF): use of motivational interviewing design and method. Contemp Clin Trials. (2011) Jan; 32: (1): 59-68. |
[58] | Lemstra M, Olszynski WP. The effectiveness of multidisciplinary rehabilitation in the treatment of fibromyalgia: a randomized controlled trial. Clin J Pain. (2005) Mar; 21: (2): 166-74. |
[59] | Lauche R, Spitzer J, Schwahn B, Ostermann T, Bernardy K, Cramer H, et al. Efficacy of cupping therapy in patients with the fibromyalgia syndrome-a randomised placebo controlled trial. Sci Rep. (2016) Nov 17; 6: : 37316. |
[60] | Baumueller E, Winkelmann A, Irnich D, Weigl M. Electromyogram biofeedback in patients with fibromyalgia: a randomized controlled trial. Complement Med Res. (2017) Mar 1; 24: (1): 33-9. |
[61] | Assefi N, Bogart A, Goldberg J, Buchwald D. Reiki for the treatment of fibromyalgia: a randomized controlled trial. J Altern Complement Med. (2008) Nov 1; 14: (9): 1115-22. |
[62] | Wicksell RK, Kemani M, Jensen K, Kosek E, Kadetoff D, Sorjonen K, et al. Acceptance and commitment therapy for fibromyalgia: a randomized controlled trial. Eur J Pain (United Kingdom). (2013) Apr; 17: (4): 599-611. |
[63] | Hsu MC, Schubiner H, Lumley MA, Stracks JS, Clauw DJ, Williams DA. Sustained pain reduction through affective self-awareness in fibromyalgia: a randomized controlled trial. J Gen Intern Med. (2010) Oct; 25: (10): 1064-70. |
[64] | Lumley MA, Schubiner H, Lockhart NA, Kidwell KM, Harte SE, Clauw DJ, et al. Emotional awareness and expression therapy, cognitive behavioral therapy, and education for fibromyalgia: a cluster-randomized controlled trial. Pain. (2017) ; 158: (12): 2354-63. |
[65] | van Ittersum MW, van Wilgen CP, van der Schans CP, Lambrecht L, Groothoff JW, Nijs J. Written pain neuroscience education in fibromyalgia: a multicenter randomized controlled trial. Pain Pract. (2014) Nov 1; 14: (8): 689-700. |
[66] | de Miquel CA, Campayo JG, Flórez MT, Arguelles JMG, Tarrio EB, Montoya MG, et al. Interdisciplinary consensus document for the treatment of fibromyalgia. Actas Esp Psiquiatr. (2010) ; 38: (2): 108-20. |
[67] | Sosa-Reina MD, Nunez-Nagy S, Gallego-Izquierdo T, Pecos-Martín D, Monserrat J, Álvarez-Mon M. Effectiveness of therapeutic exercise in fibromyalgia syndrome: a systematic review and meta-analysis of randomized clinical trials. Biomed Res Int. (2017) ; 2017: : 1-14. |
[68] | Lima LV, Abner TSS, Sluka KA. Does exercise increase or decrease pain? Central mechanisms underlying these two phe-nomena. J Physiol. (2017) Jul 1; 595: (13): 4141-50. |
[69] | Qaisar R, Bhaskaran S, Van Remmen H. Muscle fiber type diversification during exercise and regeneration. Free Radic Biol Med. (2016) Sep 1; 98: : 56-67. |
[70] | Hötting K, Röder B. Beneficial effects of physical exercise on neuroplasticity and cognition. Neurosci Biobehav Rev. (2013) Nov; 37: (9): 2243-57. |
[71] | Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. (2002) Jun 1; 25: (6): 319-25. |
[72] | Bialosky JE, Beneciuk JM, Bishop MD, Coronado RA, Penza CW, Simon CB, et al. Unraveling the mechanisms of manual therapy: modeling an approach. J Orthop Sports Phys Ther. (2018) Jan 1; 48: (1): 8-18. |
[73] | Bishop MD, Torres-Cueco R, Gay CW, Lluch-Girbés E, Beneciuk JM, Bialosky JE. What effect can manual therapy have on a patient’s pain experience? Pain Manag. (2015) Nov; 5: (6): 455-64. |
[74] | Estébanez-de-Miguel E, Jimenez-del-Barrio S, Fortún-Agud M, Bueno-Gracia E, Caudevilla-Polo S, Malo-Urriés M, et al. Comparison of high, medium and low mobilization forces for reducing pain and improving physical function in patients with hip osteoarthritis: secondary analysis of a randomized controlled trial. Musculoskelet Sci Pract. (2019) Jun 1; 41: : 43-8. |
[75] | Zhang XC, Chen H, Xu WT, Song YY, Gu YH, Ni GX. Acupuncture therapy for fibromyalgia: a systematic review and meta-analysis of randomized controlled trials. J Pain Res. (2019) ; 12: : 527-42. |
[76] | Casanueva B, Rivas P, Rodero B, Quintial C, Llorca J, González-Gay MA. Short-term improvement following dry needle stimulation of tender points in fibromyalgia. Rheumatol Int. (2014) ; 34: (6): 861-6. |
[77] | Ceballos-Laita L, Jiménez-del-Barrio S, Marín-Zurdo J, Moreno-Calvo A, Marín-Boné J, Albarova-Corral MI, et al. Effects of dry needling on pain, pressure pain threshold and psychological distress in patients with mild to moderate hip osteoarthritis: Secondary analysis of a randomized controlled trial. Complement Ther Med. (2020) Jun 1; 51: : 102443. |
[78] | Shah JP, Danoff JV, Desai MJ, Parikh S, Nakamura LY, Phillips TM, et al. Biochemicals associated with pain and inflammation are elevated in sites near to and remote from active myofascial trigger points. Arch Phys Med Rehabil. (2008) ; Jan; 89: (1): 16-23. |
[79] | Hsieh YL, Yang SA, Yang CC, Chou LW. Dry needling at myofascial trigger spots of rabbit skeletal muscles modulates the biochemicals associated with pain, inflammation, and hypoxia. Evidence-based Complement Altern Med. (2012) ; 2012: : 342165. |
[80] | Hsieh YL, Chou LW, Joe YS, Hong CZ. Spinal cord mechanism involving the remote effects of dry needling on the irritability of myofascial trigger spots in rabbit skeletal muscle. Arch Phys Med Rehabil. (2011) Jul; 92: (7): 1098-105. |
[81] | García-Ríos MC, Navarro-Ledesma S, Tapia-Haro RM, Toledano-Moreno S, Casas-Barragán A, Correa-Rodríguez M, et al. Effectiveness of health education in patients with fibromyalgia: a systematic review. Eur J Phys Rehabil Med. (2019) May 1; 55: (2): 301-13. |
[82] | Van Koulil S, Van Lankveld W, Kraaimaat FW, Van Helmond T, Vedder A, Van Hoorn H, et al. Tailored cognitive-behavioural therapy and exercise training improves the physical fitness of patients with fibromyalgia. Ann Rheum Dis. (2011) Dec; 70: (12): 2131-3. |
Appendices
Appendix: Search strategy
MEDLINE database: “fibromyalgia” [MeSH Terms] AND (“therapeutics” [MeSH Terms] OR “physical therapy modalities” [MeSH Terms] OR (combined [All Fields] AND “physical therapy modalities” [MeSH Terms]) OR (“exercise” [MeSH Terms] OR “exercise” [All Fields]))
Cochrane database: ((fibromyalgia) AND (therapeutics OR physical therapy modalities OR combined physical therapy OR exercise)) in Title, Abstract, Keywords in Trials
Scopus database: TITLE-ABSTRACT-KEYWORDS ((fibromyalgia) AND (therapeutics OR physical AND therapy AND modalities OR combined AND physical AND therapy OR exercise)) AND (LIMITED-TO (DOCTYPE, “article”))
PEDro database:
Search 1: “fibromyalgia” AND “therapeutics”
Search 2: “fibromyalgia” AND “physical therapy modalities”
Search 3: “fibromyalgia” AND “combined physical therapy”
Search 4: “fibromyalgia” AND “exercise”




