Clinical application of enhanced recovery after surgery in lumbar disk herniation patients undergoing dynamic stabilization and discectomy
Abstract
BACKGROUND:
Enhanced recovery after surgery (ERAS) has been demonstrated to improve early postoperative outcomes and is becoming a crucial component of any perioperative management paradigm.
OBJECTIVE:
To investigate the effect of an ERAS protocol on lumbar disk herniation (LDH) patients undergoing dynamic stabilization and discectomy.
METHODS:
A total of 119 lumbar disk herniation (LDH) patients undergoing Dynesys dynamic stabilization and discectomy were divided into the ERAS (n1
RESULTS:
Both the ERAS and control groups had significantly decreased visual analog scale (VAS) score and Oswestry Disability Index (ODI) and increased Japanese Orthopaedic Association (JOA) score at postoperative 1 week, 1 month and 3 months compared with preoperative scores. Moreover, the ERAS group had lower postoperative VAS score and ODI and higher postoperative JOA score and rate of improved JOA score compared with the control group. Intraoperative blood loss, operation time, ambulation time and length of stay were all lower in the ERAS group than in the control group.
CONCLUSIONS:
The ERAS protocol designed was feasible for LDH patients undergoing dynamic stabilization and discectomy with significantly improved perioperative outcomes.
1.Introduction
Enhanced recovery after surgery (ERAS) is an evidence-based multidisciplinary perioperative care protocol designed to accelerate the recovery process and minimize the loss of functional capacity through improving the quality of perioperative care [1]. It was first introduced into clinical practice by Kehlet and Wilmore in 2002 [2]. Since then a variety of ERAS protocols have been developed in many surgical disciplines and have demonstrated to improve early postoperative outcomes. Therefore, ERAS is becoming a crucial component of any perioperative management paradigm. However, few studies have reported its application in the surgical treatment of lumbar disk herniation (LDH).
The Oswestry Disability Index (ODI) is one of the most frequently utilized patient-reported outcome measures for spinal disorders [3, 4]. It consists of ten questions categorized into two aspects, including the pain level and its disabling effect on the activities of daily living. The Japanese Orthopaedic Association (JOA) score is a widely used disease-specific outcome tool for evaluation of postoperative neurological changes [5], consisting of six domains: sensory function in the trunk, sensory function in upper extremities, sensory function in lower extremities, motor function in lower extremities, motor function in upper extremities and bladder function. Additionally, visual analog scale (VAS) can be applied in the assessment of lower back pain and leg pain in patients with central lumbar spinal stenosis [6]. Therefore, in this study we investigated the feasibility and effect of an ERAS protocol for LDH patients undergoing dynamic stabilization and discectomy with the above outcome measures.
2.Patients and methods
2.1Patients
All 61 LDH patients undergoing Dynesys dynamic stabilization and discectomy between March 2014 and March 2015 at the People’s Hospital of Xinjiang Uygur Autonomous Region were retrospectively enrolled as the ERAS group, and all 70 LDH patients undergoing the same surgical treatment between February 2013 and February 2014 were retrospectively enrolled as the control group. The ERAS group received an ERAS protocol which was implemented from March 2014, and the control group received a traditional care protocol. All procedures were performed in compliance with relevant laws and institutional guidelines and approved by the Ethics Committee of the People’s Hospital of Xinjiang Uygur Autonomous Region.
Inclusion criteria: (1) spinal stenosis secondary to disk herniation at two levels or less and disk herniation at two levels or less combined with intervertebral instability; (2) complete medical records, including demographic data, American Society of Anesthesiologists (ASA) physical status, preoperative and followed-up VAS, ODI and JOA scores; (3) informed consent.
Exclusion criteria: (1) disk herniation at more than two adjacent levels; (2) disk degeneration at multiple levels or discontinuous levels; and (3) combined with internal medicine diseases such as uncontrolled hypertension or diabetes mellitus, and chronic bronchitis combined with pulmonary heart disease.
Table 1
General data of the ERAS group and control group
| ERAS group (n1 | Control group (n2 |
|
| |
|---|---|---|---|---|
| Age (years) | 52.94 | 54.12 | 0.658 | 0.504 |
| Sex (male/female) | 1.55 (34/22) | 1.63 (39/24) | 0.018 | 0.894 |
| BMI | 23.18 | 22.91 | 0.329 | 0.713 |
| ASA physical status (I/II) | 50/6 | 55/8 | 0.112 | 0.737 |
| Preoperative VAS score | 7.06 | 7.01 | 0.227 | 0.805 |
| Preoperative ODI | 41.33 | 43.72 | 1.295 | 0.201 |
| Preoperative JOA score | 3.37 | 3.29 | 0.185 | 0.847 |
ERAS: enhanced recovery after surgery, BMI: body mass index, ASA: American Society of Anesthesiologists, VAS: visual analog scale, ODI: Oswestry Disability Index, JOA: Japanese Orthopaedic Association.
2.2ERAS protocol
(1) Preoperative counselling was performed. The contents of preoperative education mainly included the aim and procedure of the ERAS protocol, discharge criteria, pain coping strategies, information about perioperative treatment and a follow-up plan. (2) The patients received analgesic therapy with oral etoricoxib 120 mg the day before surgery. Postoperative analgesia was performed with the combination of parecoxib sodium and morphine. Oral etoricoxib analgesia was resumed after the patients could take oral diet by themselves on the second day after surgery. (3) Preoperative fasting and water deprivation for 2 h, and regular diet after anesthesia awareness. (4) No urethral catheterization was performed for the patients having an estimated operating time less than 3 h. (5) Continuous epidural anesthesia was performed. Gastrointestinal discomfort should be avoided. The patients’ lower limbs had sensation and movement at the end of the operation through strictly controlling the time of anesthesia and drug dose. (6) The lower limb muscles were activated and rolling over was exercised from 4 h after surgery, and standing with lumbar support was exercised from 8 h after surgery, and moderate activities were administered from 24 h after surgery. Activation of the lower limb muscles included active movement of the ankle and knee joint, and exercise of the quadriceps femoris and anterior tibialis muscles. Within the first three days after surgery, patients were asked to stand three times each day with half an hour each time. Within 4–7 days after surgery, patients were asked to stand 3–5 times each day with a total time of 2 h. The criterion of moderate activities was that patients felt no discomfort. (7) Physical exercise was performed, and the limb pneumatic pump was employed to improve microcirculation in order to prevent from deep vein thrombosis. Pulse electric frequency was used to promote wound healing in order to prevent from bleeding and inflammation three times a day. Intermittent wave was given, the ferquency was 50 Hz, and the duration was 20–30 mins each time. (8) Intraoperative nerve electrophysiological monitoring was performed in order to prevent from nerve damage. (9) Preoperative and intraoperative tranexamic acid, postoperative physical cold and adjustable negative pressure suction were used to reduce perioperative bleeding. (10) A review was performed with an X-ray machine on the second day after surgery, and moderate activities were administered with lumbar support. Review was performed monthly within three months after surgery, nonlaborious work was restored from one month after surgery, and normal life was restored and previous work was resumed from three months after surgery.
2.3Traditional care protocol
The traditional care protocol mainly included traditional informed consent, preoperative enema, preoperative fasting for 12 h and water deprivation for 4 h, postoperative fasting for 1 d and fluid for 1 d, and postoperative autonomy activities of lower limbs.
2.4Evaluation of clinical outcomes
VAS score and ODI were evaluated at preoperation, postoperative 1 week, 1 month and 3 months. The JOA score was evaluated preoperatively, postoperative 1 month and 3 months. The rate of the improved JOA score (RIS)
2.5Statistical analysis
Statistical analysis was performed using the SPSS version 20.0 for Windows (IBM Corp., Armonk, NY, USA). Significance was set at
3.Results
3.1General data
Five patients were excluded including 3 cases for lost follow-up and 2 cases for incomplete medical records in the ERAS group, and 56 patients were finally included in the ERAS group. Seven patients were excluded including 4 cases for lost follow-up and 3 cases for incomplete medical records in the control group, and 63 patients were finally included in the control group. The ERAS group included 34 males and 22 females with an average age of (52.94
Table 2
Preoperative and postoperative JOA score and RIS of the ERAS group and control group
| JOA score | RIS (%) | ||||
| Preoperative | Postoperative 1 month | Postoperative 3 months | Postoperative 1 month | Postoperative 3 months | |
| ERAS group | 3.37 | 19.35 | 27.93 | 65.38 | 95.82 |
| Control group | 3.29 | 16.12 | 23.28 | 52.69 | 81.74 |
|
| 0.185 | 7.251 | 6.569 | 9.560 | 8.611 |
|
| 0.847 | ||||
JOA: Japanese Orthopaedic Association, RIS: rate of the improved JOA score, ERAS: enhanced recovery after surgery.
Figure 1.
Preoperative and postoperative VAS score of ERAS group and control group. VAS: visual analog scale;
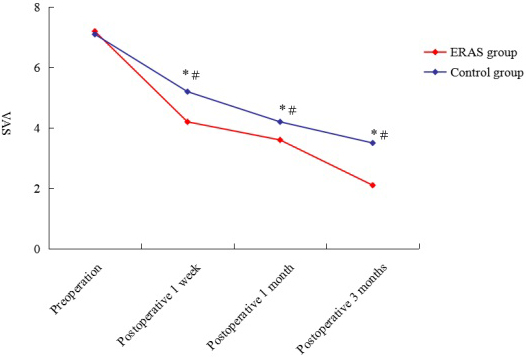
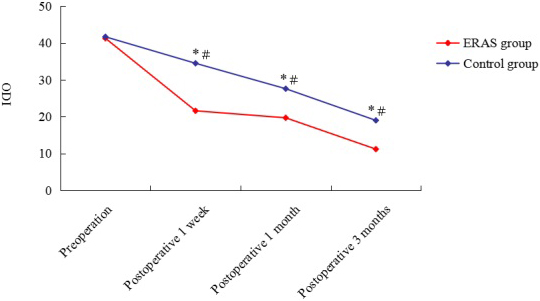
Figure 2.
Preoperative and postoperative ODI of ERAS group and control group. ODI: Oswestry Disability Index;
3.2VAS score
As demonstrated in Fig. 1, the postoperative VAS score was significantly lower than preoperative score in both the ERAS and control groups. Moreover, the VAS score was higher in the control group than in the ERAS group at postoperative 1 week, 1 month and 3 months.
3.3ODI
As demonstrated in Fig. 2, the postoperative ODI was significantly lower than preoperative DOI in both the ERAS and control groups. The ODI was higher in the control group than in the ERAS group at postoperative 1 week, 1 month and 3 months.
3.4JOA score
As demonstrated in Table 2, the postoperative JOA score was significantly higher than preoperative score. Moreover, the JOA score was higher in the ERAS group than in the control group at postoperative 1 month and 3 months. The RIS was higher in the ERAS group than in the control group at postoperative 1 month and 3 months.
Table 3
Intraoperative blood loss, operation time, ambulation time and length of stay of the ERAS and control group
| ERAS group (n1 | Control group (n2 |
|
| |||
|---|---|---|---|---|---|---|
| Intraoperative blood loss (mL) | 90.52 | 150.01 | ||||
| Operation time (h) | 2.55 | 3.25 | 0.003 | |||
| Ambulation time (h) | 30.62 | 48.22 | ||||
| Length of stay (day) | 7.12 | 9.66 | 0.025 | |||
ERAS: enhanced recovery after surgery.
3.5Intraoperative blood loss, operation time, ambulation time and length of stay
As demonstrated in Table 3, intraoperative blood loss, operation time, ambulation time and length of stay were all lower in the ERAS group than in the control group.
3.6Complications
The mean follow-up time was (49.32
4.Discussion
LDH is one of the most common spinal pathologies characterized by neurological dysfunction and debilitating pain [8]. Early conservative management is applied to patients without serious symptoms. However, surgery is performed if symptoms last for more than six weeks or are correlated with unbearable pain or neurological deficit. Moreover, 31% of patients receiving conservative management eventually undergo surgery during one year of follow-up [9]. Surgery can provide faster relief from back pain symptoms in LDH patients compared with conservative management, but does not demonstrate a benefit over conservative management in midterm and long-term follow-up [10, 11]. The primary surgical intervention for LDH is discectomy, which can be performed through many techniques aiming at neural decompression and prevention of recurrent herniation.
Dynamic stabilization for the lumbar spine, aiming at either securing the physiological segmental motion of the spine or preventing the loading of the intervertebral disc and facet joint, has been conducted for almost 30 years. Currently, multiple pedicle-based systems have been employed to stabilize the spine while allowing limited mobility without fusion. Dynesys dynamic stabilization system was designed by Dubois and Graf and approved in the USA in 2009, which can provide spinal stabilization and alignment for patients with degenerative spondylolisthesis and radiculopathy, spinal stenosis or the other stenosing lesion [12]. Biomechanical and in vitro studies have demonstrated that this system is associated with restrained amount of flexibility through polycarbonate urethane spacers and polyethylene terephthalate cords [13, 14]. In vivo, this system allows movement at the instrumented level, although reduced, with no markedly increased mobility at the adjacent segments [15]. In this study, all patients received Dynesys dynamic stabilization and discectomy for corresponding lesion segments through the posterior approach.
The application of surgery is limited by complications associated with surgery, long postoperative recovery period, long length of hospital stay and decreased patients’ satisfaction, despite advances in surgical concept and technique. ERAS is designed to promote patient’s rehabilitation through improving the quality of perioperative care [1]. Plenty of ERAS protocols aiming at different surgical disciplines have been developed and proven to be able to improve early postoperative outcomes. Quiny et al. demonstrated that ERAS was associated with shorter LOS and reduced postoperative complications for emergency abdominal surgery [16]. Zhu et al. indicated that ERAS could significantly reduce the incidence of postoperative complications and LOS for patients undergoing total hip arthroplasty (THA) or total knee arthroplasty (TKA), but it had no significant effect on 30-day readmission rate [17]. Deiss et al. reported long-term outcomes of ERAS for colorectal surgery. Their results confirmed long-term (6 months after surgery) benefits of ERAS [18]. Li et al. developed an ERAS protocol for spinal surgery [19]. Their results showed that this ERAS protocol was safe and feasible for patients receiving laminoplasty, and could reduce LOS without increasing the incidence of complications. Braga et al. evaluated the effect of ERAS on short-term outcomes of patients receiving pancreaticoduodenectomy [20]. Their results demonstrated that ERAS could promote early recovery after pancreaticoduodenectomy.
In our study, an ERAS protocol was developed for LDH patients receiving Dynesys dynamic stabilization and discectomy. Our results demonstrated that this ERAS protocol could reduce postoperative VAS score and ODI, increase postoperative JOA score and RIS, and decrease intraoperative blood loss, operation time, ambulation time and length of stay. Therefore, the ERAS protocol was feasible for LDH patients receiving dynamic stabilization and discectomy, and significantly improved perioperative outcomes.
Studies show that preoperative education can decrease preoperative anxiety and enhance patients’ satisfaction. Patients may have obvious perioperative anxiety and feel vulnerable due to fear of the serious complications of spinal surgery, which has an adverse impact on functional recovery and postoperative pain [21]. A preoperative education for anxiety and depression may significantly reduce postoperative pain, enhance patients’ satisfaction and promote patient’s rehabilitation [22]. A study on 175 patients undergoing spinal surgery demonstrated that 87% of the patients reported preoperative anxiety, and faith in the surgeon’s explanation of the procedure and the medical staff could help to overcome preoperative anxiety [21]. Preoperative education about pain coping strategies, details of the surgery, estimated LOS and details of recovery process can significantly alleviate patients’ anxiety, and thus avoid unnecessary stress [23]. In our study, preoperative education introduced the goal and procedure of the ERAS protocol, details of the surgery and pain coping strategies.
Early postoperative activities are helpful in promoting patient’s rehabilitation. Getting out of bed “early” can reduce the LOS and the incidence of perioperative complications such as pulmonary embolism, deep venous thrombosis, atelectasis, pneumonia, sepsis, urinary tract infections, and so on [24]. For patients undergoing lumbar surgery, an intense rehabilitation program started on the day of surgery significantly enhances patients’ satisfaction and shortens the LOS without increasing the risks of pain and complications [25]. For patients with an age greater than 65 years undergoing elective spinal surgery, early ambulation within 24 h after surgery can significantly shorten the LOS and decrease the incidence of perioperative complications [26]. In this study, early postoperative activities were administered. The lower limb muscles were activated and rolling over was exercised from 4 h after surgery, and standing with lumbar support was exercised from 8 h after surgery, and moderate activities were administered from 24 h after surgery.
Decreased tissue trauma and intraoperative blood loss have a positive influence on recovery after surgery [27]. In our study, preoperative and intraoperative tranexamic acid, postoperative physical cold and adjustable negative pressure suction were used to reduce perioperative bleeding. The results demonstrated that intraoperative blood loss was lower in the ERAS group than in the control group, which was associated with improved perioperative outcomes of the ERAS group.
5.Conclusions
This study showed that the ERAS protocol was safe and feasible for LDH patients receiving dynamic stabilization and discectomy and significantly improved perioperative outcomes.
Conflict of interest
None to report.
References
[1] | Moningi S, Patki A, Padhy N, Ramachandran G. Enhanced recovery after surgery: an anesthesiologist’s perspective. J Anaesthesiol Clin Pharmacol. (2019) ; 35: (Suppl 1): S5-S13. doi: 10.4103/joacp.JOACP_238_16. |
[2] | Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg. (2002) ; 183: (6): 630-641. doi: 10.1016/s0002-9610(02)00866-8. |
[3] | Liu H, Tao H, Luo Z. Validation of the simplified Chinese version of the oswestry disability index. Spine (Phila Pa 1976). (2009) ; 34: (11): 1211-1216; discussion 1217. doi: 10.1097/BRS.0b013e31819e2b34. |
[4] | Vavken P, Ganal-Antonio AK, Quidde J, Shen FH, Chapman JR, Samartzis D. Fundamentals of clinical outcomes assessment for spinal disorders: clinical outcome instruments and applications. Global Spine J. (2015) ; 5: (4): 329-338. doi: 10.1055/s-0034-1396046. |
[5] | Yonenobu K, Abumi K, Nagata K, Taketomi E, Ueyama K. Interobserver and intraobserver reliability of the japanese orthopaedic association scoring system for evaluation of cervical compression myelopathy. Spine (Phila Pa 1976). (2001) ; 26: (17): 1890-1894; discussion 1895. doi: 10.1097/00007632-200109010-00014. |
[6] | Xue J, Chen H, Zhu B, Li X, Ouyang Z, Li S, et al. Percutaneous spinal endoscopy with unilateral interlaminar approach to perform bilateral decompression for central lumbar spinal stenosis: radiographic and clinical assessment. BMC Musculoskelet Disord. (2021) ; 22: : 236. doi: 10.1186/s12891-021-04100-3. |
[7] | Mayhew D, Mendonca V, Murthy BVS. A review of ASA physical status – historical perspectives and modern developments. Anaesthesia. (2019) ; 74: (3): 373-379. doi: 10.1111/anae.14569. |
[8] | Ammerman J, Watters WC, Inzana JA, Carragee G, Groff MW. Closing the treatment gap for lumbar disc herniation patients with large annular defects: a systematic review of techniques and outcomes in this high-risk population. Cureus. (2019) ; 11: (5): e4613. doi: 10.7759/cureus.4613. |
[9] | Gadjradj PS, Arts MP, van Tulder MW, Rietdijk WJR, Peul WC, Harhangi BS. Management of symptomatic lumbar disk herniation: an international perspective. Spine (Phila Pa 1976). (2017) ; 42: (23): 1826-1834. doi: 10.1097/BRS.0000000000002294. |
[10] | Gugliotta M, da Costa BR, Dabis E, Theiler R, Jüni P, Reichenbach S, et al. Surgical versus conservative treatment for lumbar disc herniation: a prospective cohort study. BMJ Open. (2016) ; 6: (12): e012938. doi: 10.1136/bmjopen-2016-012938. |
[11] | Bailey CS, Rasoulinejad P, Taylor D, Sequeira K, Miller T, Watson J, et al. Surgery versus conservative care for persistent sciatica lasting 4 to 12 months. N Engl J Med. (2020) ; 382: (12): 1093-1102. doi: 10.1056/NEJMoa1912658. |
[12] | Würgler-Hauri CC, Kalbarczyk A, Wiesli M, Landolt H, Fandino J. Dynamic neutralization of the lumbar spine after microsurgical decompression in acquired lumbar spinal stenosis and segmental instability. Spine (Phila Pa 1976). (2008) ; 33: (3): E66-72. doi: 10.1097/BRS.0b013e31816245c0. |
[13] | Lin HM, Pan YN, Liu CL, Huang LY, Huang CH, Chen CS. Biomechanical comparison of the K-ROD and Dynesys dynamic spinal fixator systems-A finite element analysis. Biomed Mater Eng. (2013) ; 23: (6): 495-505. doi: 10.3233/BME-130766. |
[14] | Erbulut DU, Zafarparandeh I, Ozer AF, Goel VK. Biomechanics of posterior dynamic stabilization systems. Adv Orthop. (2013) ; 2013: : 451956. doi: 10.1155/2013/451956. |
[15] | Beastall J, Karadimas E, Siddiqui M, Nicol M, Hughes J, Smith F, et al. The Dynesys lumbar spinal stabilization system: a preliminary report on positional magnetic resonance imaging findings. Spine (Phila Pa 1976). (2007) ; 32: (6): 685-690. doi: 10.1097/01.brs.0000257578.44134.fb. |
[16] | Quiney N, Aggarwal G, Scott M, Dickinson M. Survival after emergency general surgery: what can we learn from enhanced recovery programmes? World J Surg. (2016) ; 40: (6): 1283-1287. doi: 10.1007/s00268-016-3418-0. |
[17] | Zhu S, Qian W, Jiang C, Ye C, Chen X. Enhanced recovery after surgery for hip and knee arthroplasty: a systematic review and meta-analysis. Postgrad Med J. (2017) ; 93: (1106): 736-742. doi: 10.1136/postgradmedj-2017-134991. |
[18] | Deiss T, Chen LL, Sarin A, Naidu RK. Patient-reported outcomes 6 months after enhanced recovery after colorectal surgery. Perioper Med (Lond). (2018) ; 7: : 19. doi: 10.1186/s13741-018-0099-2. |
[19] | Li J, Li H, Xv ZK, Wang J, Yu QF, Chen G, et al. Enhanced recovery care versus traditional care following laminoplasty: a retrospective case-cohort study. Medicine (Baltimore). (2018) ; 97: (48): e13195. doi: 10.1097/MD.0000000000013195. |
[20] | Braga M, Pecorelli N, Ariotti R, Capretti G, Greco M, Balzano G, et al. Enhanced recovery after surgery pathway in patients undergoing pancreaticoduodenectomy. World J Surg. (2014) ; 38: (11): 2960-2966. doi: 10.1007/s00268-014-2653-5. |
[21] | Lee JS, Park YM, Ha KY, Cho SW, Bak GH, Kim KW. Preoperative anxiety about spinal surgery under general anesthesia. Eur Spine J. (2016) ; 25: (3): 698-707. doi: 10.1007/s00586-015-3788-2. |
[22] | Dunn LK, Durieux ME, Fernández LG, Tsang S, Smith-Straesser EE, Jhaveri HF, et al. Influence of catastrophizing, anxiety, and depression on in-hospital opioid consumption, pain, and quality of recovery after adult spine surgery. J Neurosurg Spine. (2018) ; 28: (1): 119-126. doi: 10.3171/2017.5.SPINE1734. |
[23] | Louw A, Diener I, Landers MR, Zimney K, Puentedura EJ. Three-year follow-up of a randomized controlled trial comparing preoperative neuroscience education for patients undergoing surgery for lumbar radiculopathy. J Spine Surg. (2016) ; 2: (4): 289-298. doi: 10.21037/jss.2016.12.04. |
[24] | Epstein NE. A review article on the benefits of early mobilization following spinal surgery and other medical/surgical procedures. Surg Neurol Int. (2014) ; 5: (Suppl 3): S66-73. doi: 10.4103/2152-7806.130674. |
[25] | Nielsen PR, Jørgensen LD, Dahl B, Pedersen T, Tønnesen H. Prehabilitation and early rehabilitation after spinal surgery: randomized clinical trial. Clin Rehabil. (2010) ; 24: (2): 137-148. doi: 10.1177/0269215509347432. |
[26] | Adogwa O, Elsamadicy AA, Fialkoff J, Cheng J, Karikari IO, Bagley C. Early ambulation decreases length of hospital stay, peri-operative complications and improves functional outcomes in elderly patients undergoing surgery for correction of adult degenerative scoliosis. Spine (Phila Pa 1976). (2017) ; 42: (18): 1420-1425. doi: 10.1097/BRS.0000000000002189. |
[27] | Zhao J, Zhang S, Li X, He B, Ou Y, Jiang D. Comparison of minimally invasive and open transforaminal lumbar interbody fusion for lumbar disc herniation: a retrospective cohort study. Med Sci Monit. (2018) ; 24: : 8693-8698. doi: 10.12659/MSM.912808. |




