Can scoliosis lead to spinal cord ischaemia? Early diagnosis and rehabilitation: A paradigmatic case report and literature review
Abstract
INTRODUCTION:
Scoliosis is frequently associated with pain and radiculopathy, but it is not considered a possible cause of acute spinal cord injury (SCI). Here we present a case report in which scoliosis was apparently linked to spinal cord ischaemia.
CASE PRESENTATION:
A 20-year-old woman with conservatively treated severe scoliosis presented with acute spinal cord infarction, which occurred during a spinal flexion while she was tidying up the bed. Other causes of SCI were excluded. Early rehabilitation was started and the patient progressively regained motor and sensory functions, with an AIS reduction from A to C. Bowel and bladder disorders persisted and were autonomously managed with a trans-anal irrigation device and intermittent catheterisation after voluntary micturition.
DISCUSSION:
Early detection and management of spinal curvature disorders are essential in preventing long-term complications of scoliosis. Although the aetiology of spinal cord ischaemia in severe scoliosis should be better clarified, this rare case report suggests that scoliosis might be involved in its pathogenesis. Thus, we recommend early diagnosis of spinal curvature disorders and adequate rehabilitative treatment in order to prevent potential subsequent neurological complications.
1.Introduction
Scoliosis can be defined as the curvature of the spine in the coronal plane associated with vertebrae rotation. Depending on the severity of the disease, treatment might be rehabilitative or surgical. The surgical option is necessary for patients with a very high degree of spinal curvature which poses a possible impact on cardiorespiratory functions [1].
A retrospective review of the Scoliosis Research Society database reported an overall complication rate of 13.4% in patients undergoing surgery for adult degenerative or idiopathic scoliosis [2]. When considering said complications, neurological complications may often lead to functional impairment and disabling conditions, such as post-surgical irreversible spinal cord injury (SCI) which is frequently related to ischemic pathogenesis. Nevertheless, it has already been hypo- thesised that an association between spinal curvature disorders and SCI is present [2]. To date, scoliosis managed without surgical treatment has never been related to non-traumatic SCI.
We therefore present a case report of a young woman affected by scoliosis, which seemingly led to spinal cord ischemia. We aim to highlight the role of an early diagnosis and adequate rehabilitation in the management of such paradigmatic cases such as the present one.
2.Case report
A 20-year-old Caucasian woman (body mass index
In the emergency room, neurological examination revealed sensory and motor deficits in both lower limbs with bilateral absent tendon reflexes, associated with lack of light touch and pain sensation at the anal mucocutaneus junction (S4-S5 dermatome) on both sides, lack of voluntary anal contraction, and deep anal sensation (American Spinal Injury Association Impairment Scale – AIS A). Moreover, the patient had complete urinary retention.
A chest, abdomen, and pelvis computed tomography was performed, showing only severe scoliosis. Subsequently, a spinal cord magnetic resonance imaging (MRI) revealed a signal alteration characterised by intramedullary hyperintensity on T2-weighted sequences, without contrast enhancement and associated with the swelling of the spinal cord, encompassing the vertebral levels from T11 to L1 (Fig. 1).
Figure 1.
Spinal cord magnetic resonance imaging with contrast shows acute spinal cord infarction between the 11
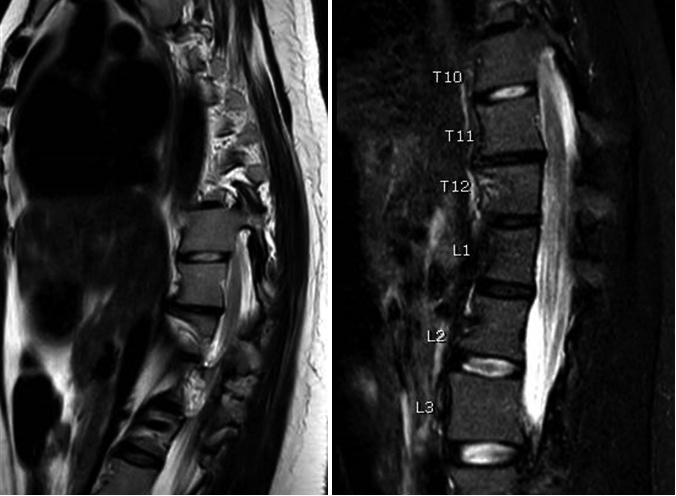
Figure 2.
Three-dimensional reconstructed computed tomography angiogram underlines a severe grade of scoliosis without any vascular alterations.
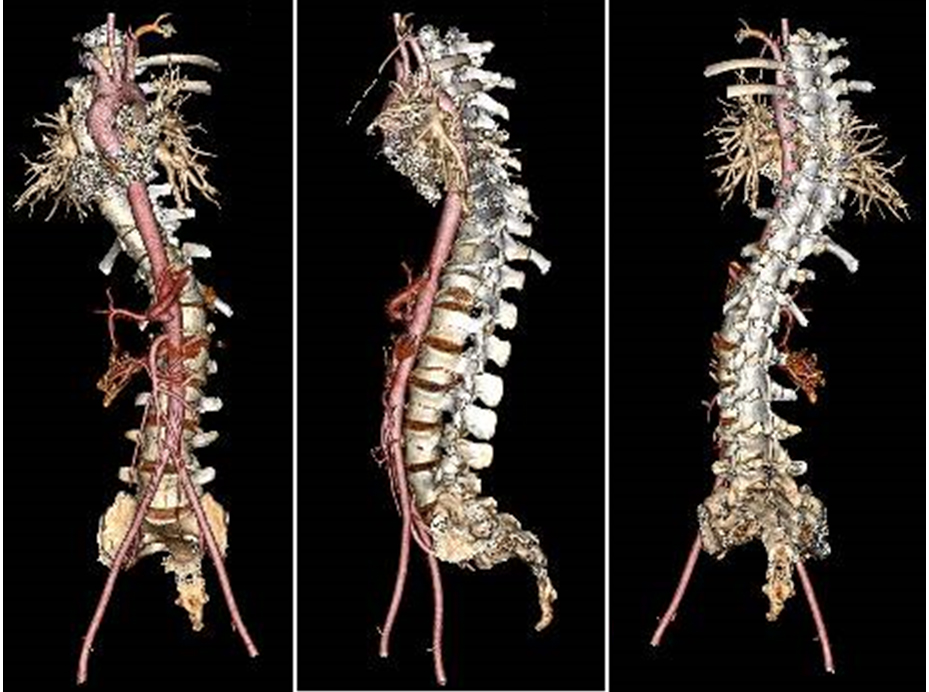
Assuming this was an acute spinal cord infarction, a computed tomography angiography (CTA) was performed and no aorta, intercostal or radicular arteries dissections, or any other vascular alterations were detected (Fig. 2). Empirical pharmacological treatment was started; 40 mg (4000 I.U.) of enoxaparin and 100 mg of acetylsalicylic acid 100 mg were administered daily, along with methylprednisolone 30 mg/kg bolus and intravenous hydration.
After admission, other causes of SCI, such as vasculitis, multiple sclerosis, and neuromyelitis optica spectrum disorders were excluded. Furthermore, a blood coagulation screening was performed which showed the presence of a Factor II heterozygous mutation.
The patient concurrently underwent bed-based rehabilitation which focused on the prevention of complications due to prolonged bed rest and immobilisation, such as pain, contractures, pressure ulcers, correct posture education and passive range of motion (ROM) exercises. Furthermore, active work for the upper limbs and trunk exercises were started in order to minimise the twisting and rotation of the spine.
After seven days from the acute onset, the patient presented initial restoration of sensitivity in the lower limbs and perianal region, allowing the diagnosis of an incomplete D10 AIS B lesion.
After admission to the Rehabilitation Unit, the patient progressively recovered motor functions, being able to walk autonomously with a walking frame along with an assist orthosis to support her right foot dorsiflexion and knee extension. In addition, the sensory function was partially restored, however in the L3-S1 dermatomes in her right lower limb some sensory deficits persisted. Unfortunately, neurogenic bowel and bladder dysfunction remained. She gained partial autonomy thanks to a trans-anal irrigation device and voluntary micturition followed by intermittent catheterisation.
Clinical improvement was also observed in assessed outcome measures. In fact, after six months, an AIS reduction from B to C with bilateral T10 motor and sensory levels was detected. This was accompanied by an improvement from 15 to 76 of the Spinal Cord Independence Measure (SCIM) and a raise in the Functional Independence Measurement (FIM) from 57 to 113, whereas the ADL Barthel Index (BI) increased from 2 to 17.
3.Discussion
Taking this report into account, the anatomic da- mage causing anterior spinal cord syndrome might be reasonably supported by both clinical and instrumental findings. Despite this, on the basis of the available information, it was not possible to identify the direct pathogenetic link leading to this spinal cord damage. In fact, it should be noted that Weidauer et al. [3] have recently reviewed the possible causes of spinal cord infarction, highlighting an unclear aetiology in 23.6% of the cases.
The literature includes old case reports on neurological deficits in patients affected by scoliosis, reporting that any reduction of the dural sac diameter or an increase in the angle of kyphoscoliosis caused by rapid growth during adolescence may contribute to the onset of neurological deficits [4, 5]. Moreover, these modifications may have a negative impact on spinal cord vascularisation, where the blood flows less through few arteries and is balanced between the anastomosis of the cervical and lumbar circulation [6].
Masini et al. [7] showed how a combination of angulation, compression, and traction in scoliosis could affect both spinal cord vascularisation and nerves, suggesting that the anomalous angles of the spinal curvature and the alterations of its vessels might promote turbulent flows with possible thrombus formation.
In this case report, we took three etiopathogenetic hypotheses of the spinal cord ischemia due to scoliosis into consideration. The first one was the arterial thromboembolism, an extremely rare event occurring mainly in patients with vascular damage or hypercoagulabi- lity. Even if the stretching of the segmental arteries in scoliosis is a progressive process that occurs during physiological growth in adolescents, a new position or physical exertion of the spine might improve acute anatomical distortion of vessels and might potentially result in small tears in the intima. On the other hand, it has been reported in literature that sudden movement during spinal manipulation might lead to arterial dissection of carotid or vertebral arteries, suggesting that any vascular alteration related to spinal deformation could promote injuries [8]. Although prothrombin gene mutation is considered a congenital risk factor for venous thrombosis, its role in arterial thromboembolism is still debated. Furthermore, to date, the heterozygous mutation of factor II of blood coagulation has never been related to spinal cord ischaemia [9].
The second hypothesis considered was venous thromboembolism. In fact, the peripheral venous system is valveless and different studies suggest that inferior vena cava compression by the Valsalva manoeuvre or physical activity may allow a retrograde flow of emboli through anastomoses within the spinal arterial sy- stem [10].
The third and last etiopathogenetic hypothesis for spinal cord infarction was fibrocartilaginous embolism, as described for the first time in 1961 by Naiman et al. [11] after an autopsy. This rare cause might be related to the embolisation of nucleus pulposus fragments, presumably due to a retrograde movement through the spinal arteries. There is a close relationship between scoliosis and intervertebral discs alterations, on one hand due to an increased production of matrix metalloproteinases subsequent to the altered biomechanics of the spine, on the other hand for the convective loss of large molecules such as proteoglycan degradation products [12]. In further detail, differences in weight distribution and disc composition combined with anomalous spinal angle and exertion might cause anomalous nucleus pulposus migration, resulting in fibrocartilaginous embolism.
However, spinal cord MRIs did not show disc herniation or macroscopic disc degeneration, and despite artery occlusion by a cartilage embolus, it has also been described in young patients after high axial overload to the spine and intervertebral discs, we cannot provide proof of this event in vivo.
Taking into account the potential hypotheses, we based our therapeutic approach on clinical and radiological findings, thereby starting both anti-aggregating and anticoagulant therapy in order to treat an acute va- scular disease and to prevent any other possible cause of thromboembolism. We decided concurrently to perform a 24-hour infusion of high-dose of methylprednisolone in the 8 hours following acute SCI.
In addition, we planned early rehabilitation to prevent the onset of possible complications and to restore, as soon as possible, patient autonomy in the activities of daily living. In fact, early rehabilitation should be always considered as a valuable strategy in improving muscle mass and function after SCI in order to counte- ract the common musculoskeletal impairment [13, 14].
Even though scoliosis might be frequently associated with a higher risk of neurological complications, no previous studies have reported it as a major cause of SCI. To the best of our knowledge, this case report could be considered unique, highlighting for the first time the potential association between non-surgical scoliosis and acute spinal cord ischemia. Furthermore, it opens the question of whether the risk of spinal cord ischemia might be mentioned among the possible complications of conservative treatment of scoliosis. Thus, starting from an adequate screening [15], a prompt diagnosis of scoliosis is mandatory to avoid complications.
However, the cause of spinal cord infarction remains unconfirmed, due to the impossibility of direct ana- lysis in vivo. This might well be considered the main limitation of this work.
4.Conclusion
In conclusion, even though the etiology still needs to be clarified, this paradigmatic case report and literature review suggests that scoliosis might be strictly involved in the pathogenesis of spinal cord infarction. Therefore, we recommend the early diagnosis and rehabilitative treatment of spinal curvature disorders to prevent curve progression and potential subsequent spinal cord ischemia.
Acknowledgments
The authors wish to thank Oliaro Valeria, Schizzi Rodolfo, Santi Roberto and Bolgeo Tatiana for their support in this work. Moreover, the authors thank Miss. Silcock Jessica Catherine for the language revision of the entire manuscript.
Conflict of interest
The authors certify that there is no conflict of interest with any financial organisation regarding the manuscript.
References
[1] | Negrini S, Dincer F, Kiekens C, Kruger L, Varela-Donoso E, Christodoulou N. Evidence based position paper on physical and rehabilitation medicine (PRM) practice for people with spinal deformities during growth. The european PRM position (UEMS PRM section). Eur J Phys Rehabil Med. (2017) ; 53: (1): 125–131. doi: 10.23736/S1973-9087.16.04406-3. |
[2] | Kuo YL, Chung CH, Huang TW, Tsao CH, Chang SY, Peng CK, Cheng WE, Chien WC, Shen CH. Association between spinal curvature disorders and injury: a nationwide population-based retrospective cohort study. BMJ Open. (2019) ; 9: (1): e023604. doi: 10.1136/bmjopen-2018-023604. |
[3] | Weidauer S, Nichtweiß M, Hattingen E, Berkefeld J. Spinal cord ischemia: aetiology, clinical syndromes and imaging features. Neuroradiology. (2015) ; 57: (3): 241–57. doi: 10.1007/s00234-014-1464-6. |
[4] | Lonstein JE, Winter RB, Moe JH, Bradford DS, Chou SN, Pinto WC. Neurologic deficits secondary to spinal deformity. A review of the literature and report of 43 cases. Spine (Phila Pa 1976). (1980) ; 5: (4): 331–355. doi: 10.1097/00007632-198007000-00007. |
[5] | Winter RB, Moe JH, Lonstein JE. The surgical treatment of congenital kyphosis. A review of 94 patients age 5 years or older, with 2 years or more follow-up in 77 patients. Spine (Phila Pa 1976). (1985) ; 10: (3): 224–231. |
[6] | Dommisse GF. The blood supply of the spinal cord. A critical vascular zone in spinal surgery. J Bone Joint Surg Br. (1974) ; 56: (2): 225–235. |
[7] | Masini M, Maranhão V. Experimental determination of the effect of progressive sharp-angle spinal deformity on the spinal cord. Eur Spine J. (1997) ; 6: (2): 89–92. |
[8] | Haldeman S, Kohlbeck FJ, McGregor M. Risk factors and precipitating neck movements causing vertebrobasilar artery dissection after cervical trauma and spinal manipulation. Spine (Phila Pa 1976). (1999) Apr 15; 24: (8): 785–94. |
[9] | De Stefano V, Chiusolo P, Paciaroni K, Casorelli I, Rossi E, Molinari M, Servidei S, Tonali PA, Leone G. Prothrombin G20210A mutant genotype is a risk factor for cerebrovascular ischemic disease in young patients. Blood. (1998) May 15; 91: (10): 3562–5. |
[10] | Sliwa JA, Maclean IC. Ischemic myelopathy: a review of spinal vasculature and related clinical syndromes. Arch Phys Med Rehabil. (1992) Apr; 73: (4): 365–72. |
[11] | Naiman JL, Donohue WL, Prichard JS. Fatal nucleus pulposus embolism of spinal cord after trauma. Neurology. (1961) ; 11: : 83–7. doi: 10.1212/WNL.11.1.83. |
[12] | Moorhouse DF, Burke M, Keohane C, Farrell MA. Spinal cord infarction caused by cartilage embolus to the anterior spinal artery. Surg Neurol. (1992) Jun; 37: (6): 448–52. |
[13] | Invernizzi M, de Sire A, Renò F, Cisari C, Runza L, Baricich A, Carda S, Fusco N. Spinal cord injury as a model of bone-muscle interactions: therapeutic implications from in vitro and in vivo studies. Front Endocrinol (Lausanne). (2020) ; 11: : 204. doi: 10.3389/fendo.2020.00204. |
[14] | Invernizzi M, de Sire A, Carda S, Venetis K, Renò F, Cisari C, Fusco N. Bone muscle crosstalk in spinal cord injuries: pathophysiology and implications for patients’ quality of life. Curr Osteoporos Rep. (2020) Aug; 18: (4): 422–431. doi: 10.1007/s11914-020-00601-7. |
[15] | Scaturro D, de Sire A, Terrana P, Costantino C, Lauricella L, Sannasardo CE, Vitale F, Mauro GL. Adolescent idiopathic scoliosis screening: could a school-based assessment protocol be useful for an early diagnosis? J Back Musculoskelet Rehabil. (2020) Nov 24. doi: 10.3233/BMR-200215. |




