Enhanced Brain Clearance of Tau and Amyloid-β in Alzheimer’s Disease Patients by Transcranial Radiofrequency Wave Treatment: A Central Role of Vascular Endothelial Growth Factor (VEGF)
Abstract
Background:
While drainage/removal of fluid and toxins from the brain by cerebrospinal fluid (CSF) directly into venous blood is well-known, a second drainage route has recently been (re)discovered—meningeal lymphatic vessels (mLVs)—which are responsible for up to half of total brain fluid/toxin drainage. The cytokine vascular endothelial growth factor (VEGF) increases mLV diameter and numbers to increase mLV drainage, resulting in increased mLV drainage. Alzheimer’s disease (AD) is characterized by low plasma and CSF levels of VEGF.
Objective:
To determine if non-invasive transcranial radiofrequency wave treatment (TRFT), through modulation of VEGF levels in blood and CSF, can affect removal of toxins tau and amyloid-β (Aβ) from the brain.
Methods:
Eight mild/moderate AD subjects were given twice-daily 1-hour TRFT sessions at home by their caregivers. Blood and CSF samples were taken at baseline and following completion of 2 months of TRFT.
Results:
In plasma and/or CSF, strong baseline correlations between VEGF levels and AD markers (t-tau, p-tau, Aβ1-40, Aβ1-42) were eliminated by TRFT. This effect was primarily due to TRFT-induced increases in VEGF levels in AD subjects with low or unmeasurable “baseline” VEGF levels. These increased VEGF levels were associated with increased clearance/drainage of tau and Aβ from the brain, likely through VEGF’s actions on mLVs.
Conclusions:
A new mechanism of TRFT is identified (facilitation of brain tau and Aβ clearance via VEGF) that is likely contributory to TRFT’s reversal of cognitive impairment in AD subjects. TRFT may be particularly effective for cognitive benefit in AD subjects who have low VEGF levels.
INTRODUCTION
Although cerebrospinal fluid (CSF) has long been known to drain fluid and soluble toxins from the brain such as tau and amyloid-β (Aβ) via the brain’s venous sinuses, only recently has an additional drainage route for CSF and toxins been found—meningeal lymphatic vessels (mLVs) located in the brain’s meninges.1,2 The human brain’s mLVs are comprised of “dorsal” and “basal” lymphatic vessels.3 Both mLV vessel locations represent a crucial “alternative” drainage route for CSF to leave the brain4, with mLV-containing CSF then being discharged into cervical lymph nodes (Fig. 1).5 Indeed, mLV drainage of brain CSF and toxins accounts for around half of total brain CSF flow from the brain.5,6
Fig. 1
Meningeal lymphatic vessels (mLVs), located within the brain’s meninges/dura, are comprised of both “Dorsal” and “Basal” lymphatic components (green vessels). Collectively, these lymphatic vessels account for up to half of total brain CSF drainage out of the brain, and thus a substantial amount of toxin drainage/clearance from the brain. CSF within mLVs is transported to cervical lymph nodes and then into the venous circulation. Note the close parallel of mLVs to venous sinuses (blue) within the brain. Yellow circles depict the approximate head surface locations for the four radiofrequency emitters of a MemorEM device on the left side of the head.
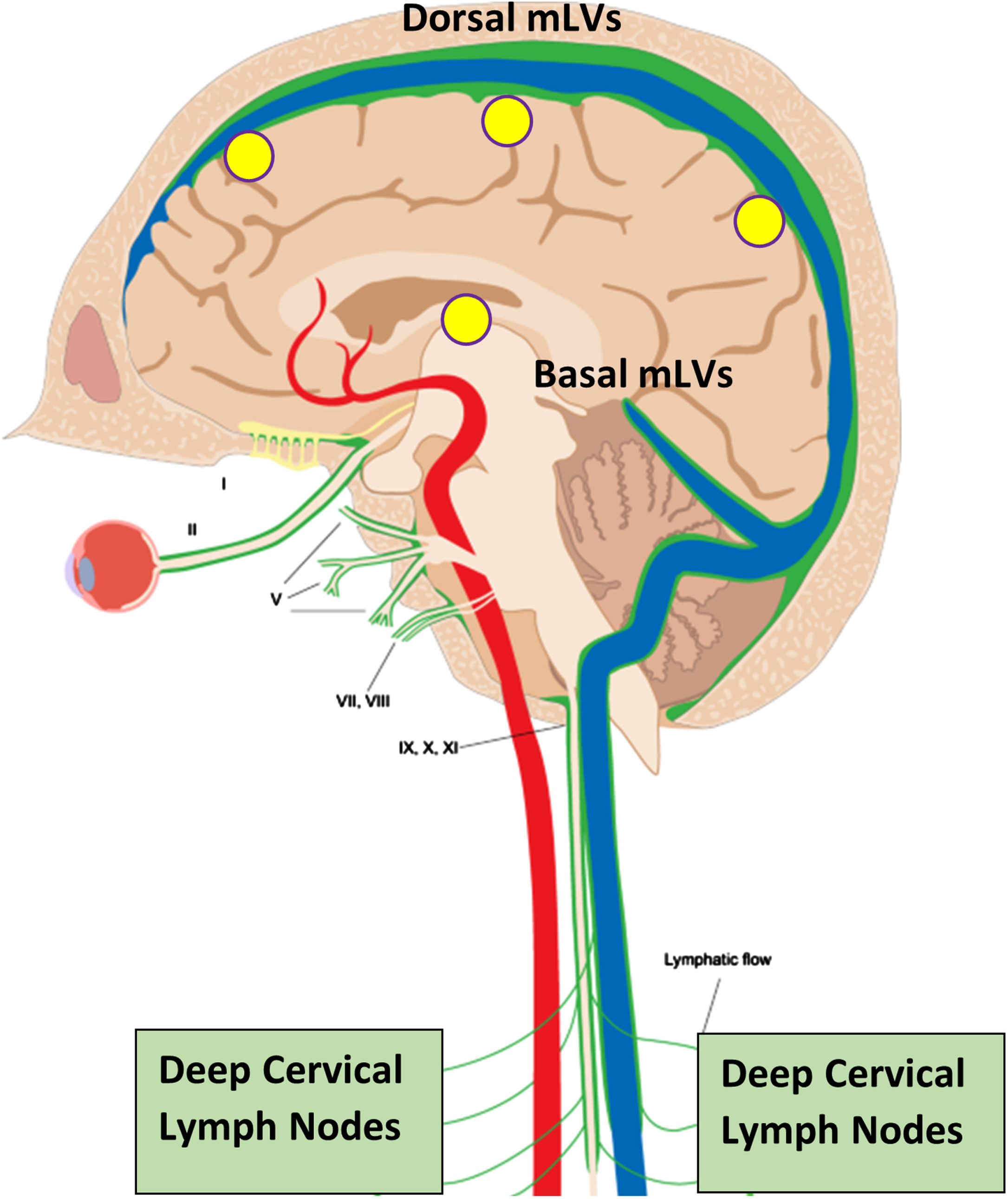
The diameter and number of mLVs can vary. As such, any dilation or lymphangeogenesis (generation of new lymphatic vessels), respectively, would increase lymph flow through the mLVs to enhance CNS flow and thus toxin clearance from the brain (Fig. 2, far right). Conversely, in aging and Alzheimer’s disease (AD), decreased CSF flow through mLVs would hinder toxin clearance from the brain, allowing buildup of toxins in brain parenchyma. Thus, mLVs have been recently suggested as a new target for AD therapeutics,7 specifically to increase CSF flow through mLVs to enhance brain clearance of soluble tau and Aβ that otherwise accumulate in the brain’s parenchyma and neurons.
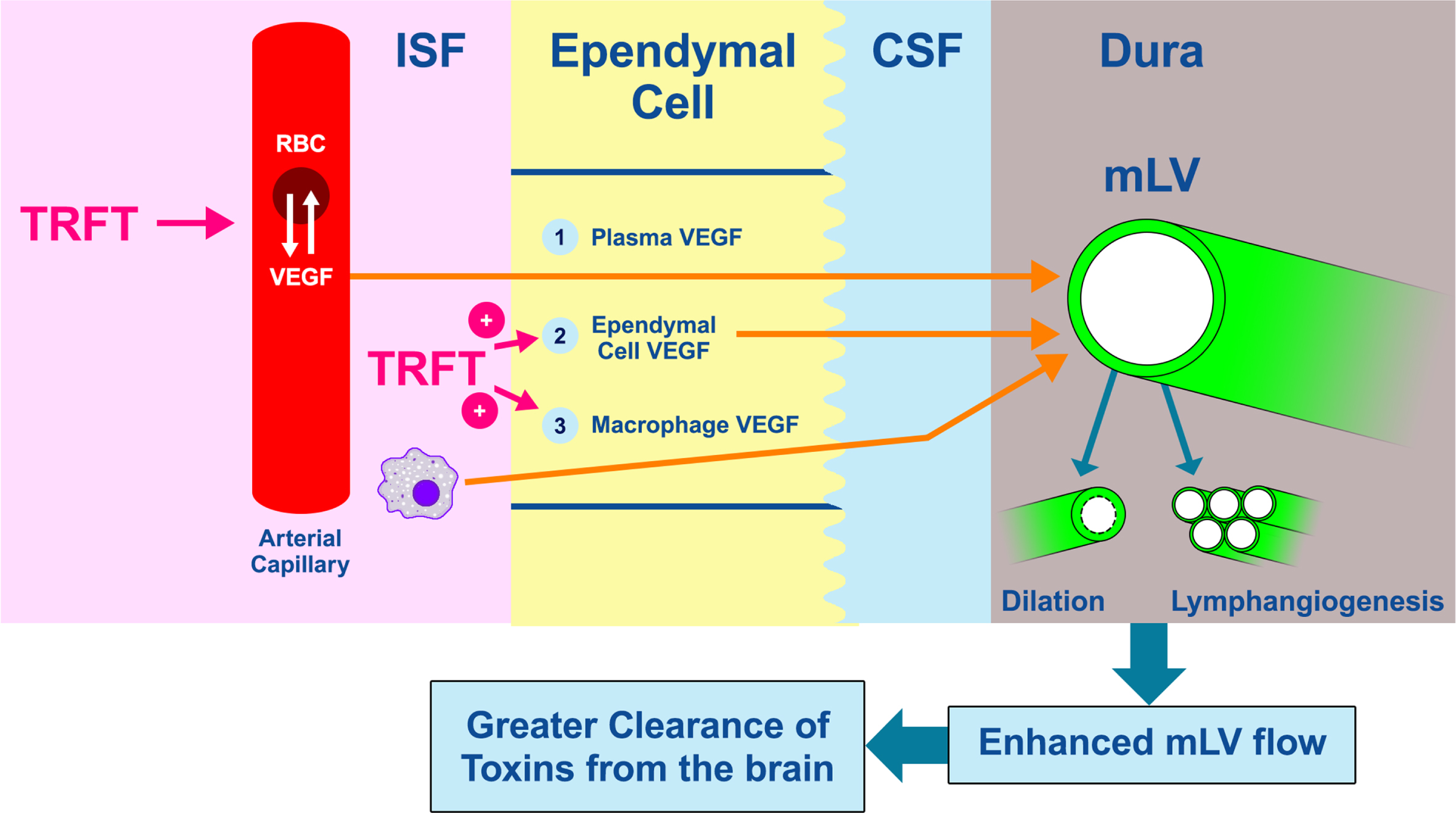
Fig. 2
Dilation of mLVs or an increase in their numbers (lymphangiogenesis) will increase MLV flow of CSF (and thus toxins) out of the brain (far right). Vascular endothelial growth factor (VEGF) is a critical cytokine that targets both of these processes to increase CSF flow and toxin removal from the brain. The three likely sources of VEGF that modulate mLV diameter and vessel numbers are shown as: 1) VEGF (both A and C sub-types) in blood plasma of choroid plexus capillaries that diffuses into choroid plexus ependymal cells, then into CSF, and finally to mLVs located in the brain’s dura, 2) epithelial ependymal cells lining the choroid plexus make and release VEGF into the CSF they produce, which then goes to mLVs, and 3) resident macrophages within the choroid plexus interstitial fluid secrete VEGF-C, which then follows the same route as plasma VEGF across ependymal cells, then to mLVs. TRFT likely affects all three of these VEGF sources through re-balancing of VEGF between RBCs and plasma, and stimulating VEGF release from ependymal cells and macrophages. To facilitate understanding of this figure, “VEGF” is meant to indicate VEGF-A and/or VEGF-C.
Both dorsal and basal mLVs are critical for the clearance of tau and Aβ from the brain, although dorsal mLVs appear more important for such clearance.8,9 Regarding Aβ, PET studies have shown that reduced CSF clearance in AD patients is associated with increased brain Aβ deposition.10 As well, blocking mLV drainage in AD transgenic mice increases their AD-related characteristics, including brain Aβ accumulation and cognitive impairment.11 Regarding tau, mLV drainage dysfunction has been reported to play a role in tau aggregation in the brain.9,12,13 For example, human tau injections into the brain of both wild-type and transgenic mice lacking a functional central nervous system (CNS) lymphatic system showed a significantly higher amount of tau retention in the brains of these transgenic mice.9
It is clear that therapeutic interventions to augment mLV function through an increase in mLV dilation and/or lymphangiogenesis are highly desirable to clear soluble tau and Aβ from the brain. In this regard, the cytokine vascular endothelial growth factor-C (VEGF-C) and its receptor VEGFR-3 primarily act to dilate mLVs and to increase mLV lymphangiogenesis (Fig. 2).14 Whereas early studies demonstrated that VEGF-A and its receptors VEGFR-1 and VEGFR-2 selectively stimulate angiogenesis (generation of new blood vessels), recent studies also indicate a direct lymphangiogenic role of VEGF-A15 as well as promotion of macrophages to release VEGF-C.16,17 The stimulatory effect of VEGF-A on VEGF-C release is an indirect route for VEGF-A to enhance both lymphangiogenesis and mLV dilation. Figure 2 depicts three likely sources of VEGF that can affect mLV flow and toxin clearance from the brain. It should be noted that VEGF-C and VEGF-A largely parallel one another in plasma concentrations, with an approximate 30:1 ratio.18
A variety of mouse studies have been done to investigate the effects of direct (invasive) VEGF administration to the brain on mLVs and clearance of brain toxins. For example, direct brain treatment of aged mice with VEGF-C enhances mLV diameter and meningeal lymphatic drainage of CSF macromolecules.19 In another study, intracerebroventricular infusions of VEGF-C induced lymphoangiogensis of mLVs in APP+PS1 transgenic mice, resulting in greater clearance of Aβ from their brains as evidenced by lower soluble Aβ levels in CSF and brain.20 These studies of “brain” VEGF administration in mice clearly show that there is resulting lymphogenesis and increased mLV vessel diameter; both would increase lymph flow through the mLV’s to increase brain clearance of CSF and toxins.
Regarding human clinical studies, one study found that serum VEGF levels in AD subjects is 30% lower than in aged controls; a 5-fold lower level of VEGF was present in AD subjects with the greatest dementia scores compared to those AD subjects with the least dementia scores.21 Another clinical study involved measurement of VEGF in CSF of normal aged, mild cognitive impairment (MCI), and AD subjects.22 Irrespective of group, higher VEGF levels were strongly correlated with greater baseline hippocampal volume, lesser hippocampal atrophy over time, and lesser memory decline over time. Most striking was that a sub-set of “normal aged” subjects in this study who had high VEGF levels as well as the same levels of CSF t-tau and Aβ1-42 found in the AD subset, showed no clinical cognitive impairment.22 Thus, high VEGF levels in CSF (and possibly plasma) could stave off MCI and AD-related cognitive impairment irrespective of CSF t-tau or Aβ1-42 levels.
Up until now, there had been no human studies that concurrently modulated both plasma and CSF/brain VEGF levels and effects on brain clearance of toxins. Even beyond that, the present clinical study details effects of a new bioengineered, non-invasive technology on brain clearance of tau and Aβ through proposed modulation of both blood and brain VEGF levels. The technology, called transcranial radiofrequency wave treatment (TRFT), has been shown to both stop and reverse AD cognitive decline in small clinical trials, including a TRFT-induced reversal of cognitive impairment that was previously reported for AD subjects in the present study.23,24 Three “disease-modifying” mechanisms of TRFT action have been identified for explaining these cognitive benefits: 1) brain disaggregation of soluble oligomers of Aβ and tau, 2) brain mitochondrial enhancement, and 3) re-balancing of the brain and blood’s immune system.23,25–28
The current study presents clinical evidence for a fourth mechanism of TRFT action – modulating soluble tau and Aβ clearance/drainage from the brain through TRFT-induced changes in VEGF levels within both plasma and brain (Fig. 2). Our results indicate that AD patients with low VEGF levels in plasma and/or brain should benefit from TRFT because it increases clearance of both tau and Aβ from the brain, presumably through TRFT-induced increases in VEGF levels in and around mLVs. Confirmatory involvement of mLVs in TRFT’s effects to clear tau and Aβ from the brain must nonetheless await studies that directly measure mLV flow through MRI. It is proposed that this fourth mechanism of TRFT action (facilitation of brain tau and Aβ clearance) contributes to the cognitive benefits of TRFT in AD subjects.
METHODS
Subjects
Eight subjects with mild-moderate AD were enrolled in a 2-month clinical protocol at the University of South Florida Health/Byrd Alzheimer’s Institute (Tampa, FL). Subjects were required to be diagnosed with mild or moderate AD according to the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria. At screening, subjects were at least 63 years of age (mean of 70.8), had a Mini-Mental State Exam (MMSE) score range of 16–26 (mean of 19.5), and a baseline Alzheimer’s Disease Assessment Scale-cog (ADAS-cog) 13 score range of 26.7–62 (mean of 36.7). In addition, subjects were required to have a Hachinski test score lower than 4 and a Global Deterioration Score above 2. Additional demographics, characteristics, AD-verification analyses, and exclusion criteria for the subjects participating in this study can be found in [23]. For each AD subject, a caregiver (spouse, family member, etc.) with non-impaired mental abilities/motor skills needed to be identified to be responsible for administering the daily treatments to the subject. Most AD patients of this study were receiving AD medication prior to TRFT. As such, if they were being medicated with a cholinesterase inhibitor and/or memantine, subjects were required to have been on such medication for at least 3 months prior to screening, then on a stable dose for at least the 60 days prior to screening and maintained on that dose throughout the period of this study. In strict accordance with the informed consent regulations of the USF Health/Byrd Alzheimer’s Institute, all subjects gave their consent to be in this study. This study was conducted according to the guidelines of the Declaration of Helsinki, and the clinical study’s protocol was approved by the Western Institutional Review Board (WIRB) or the WIRB-Copernicus Group (WCG-IRB) as Protocol #20152640. The clinical protocol for this study (NCT02958930) is listed on http://www.clinicaltrials.gov.
The investigational device
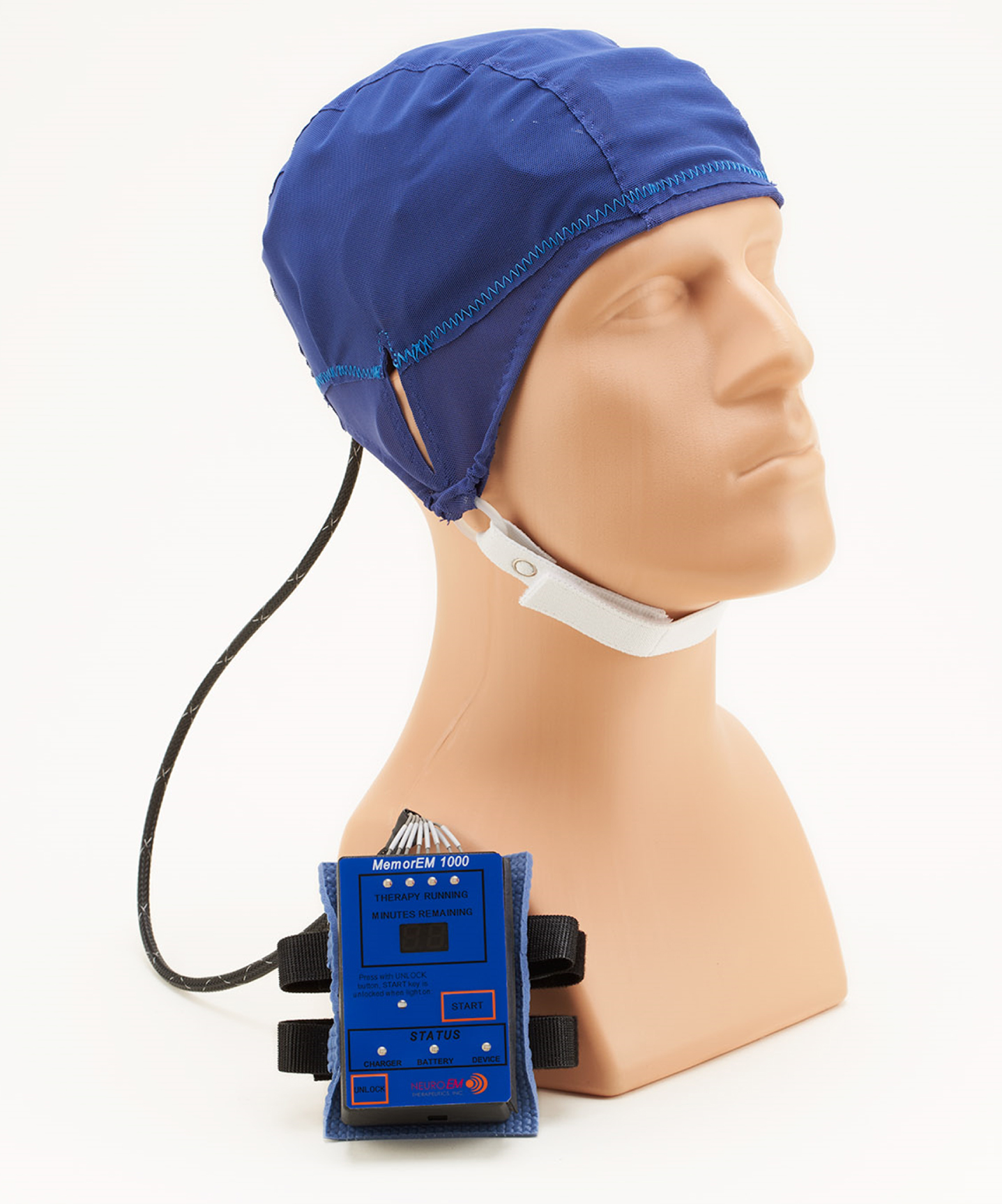
The MemorEM head device is self-contained and has been designed for in-home treatment, allowing for near-complete mobility and comfort in any given 1-h treatment, and requires at least a 7-h interval between two daily treatments (Fig. 3). When running a treatment, the device transmits radiofrequency waves sequentially through the 8 head emitters (embedded between the two head cap layers) at 915 MHz frequency every 4.6 ms (e.g., a pulse repetition rate to each antenna of 217 Hz). Figure 1 shows where the four emitters would be located on the head surface for the left side of the head. Power levels (specific absorption rate, SAR) for each emitter were set at an average of 1.6 W/kg. At this frequency and power level, human head computer simulations show that the eight emitters collectively provide both global and penetrating TRFT to the entire human forebrain.23,24,28 The MemorEM head device and this clinical trial protocol were both approved as “non-significant risk” by the Western Institutional Review Board and in March 2020, the MemorEM device was designated by FDA as its first “Breakthrough Device” for the treatment of AD cognitive decline.
Fig. 3
A MemorEM device being worn by a mannequin human head. The control panel/battery box is worn on the subject’s upper arm and is wired via a cable to eight radiofrequency wave emitters (four on each side of the head) embedded between the device’s two-layered head cap. Emitters collectively provide full forebrain TRFT, while allowing near complete mobility during any given 1-hour treatment.
General protocol
This was a single arm, single performance site study wherein 2 months of daily TRFT administration occurred in patients with mild-to-moderate AD. All screening events occurred within two weeks of treatment initiation, and all baseline measures were attained within one week of treatment initiation. Following the baseline clinical visit and for the purposes of this study, a succeeding visit occurred on Day 60, after 2 months of daily treatments. Throughout the 2-month treatment period, subjects were given twice-daily TRFT treatment for 1 h each (early morning and late afternoon), as administered and supervised by the caregiver. Baseline (BL) and Day 60 clinical visits actually involved 2 clinical visits each. The first clinical visit involved ADAS-cog13 administration and withdrawal of 20 ml of blood for later VEGF and AD marker analyses. At the second clinical visit, 15 ml of CSF was attained via spinal tap. On the clinical visits of blood collection, blood samples (collected at baseline and treatment Day 60) were divided into two 10 ml BD k2-EDTA tubes and centrifuged at 300 g for 10 min. The plasma (upper layer) for each tube was transferred into a new 15 ml tube, then centrifuged at 2000 g for 10 min. One ml volumes of the top plasma layer were aliquoted into 1.5 ml tubes and stored at –80°C for future analysis. The two 15-ml samples of CSF collected at baseline and Day 60 were each aliquoted after collection into 1.5 ml tubes, then frozen and stored at –80°C until analysis. At the end of the study, plasma and CSF samples were thawed completely on ice, with samples being mixed well on vortex and centrifuged at 2000 g for 10 min to precipitate any debris. Plasma and CSF measurements were performed in duplicate and averaged for each sample.
Blood and CSF analyses
VEGF. Multiplex kits from Millipore (Cat HCYTOMAg-60K) were used to detect/measure VEGF in plasma and CSF samples. These kits measure the VEGF-A sub-type, which has been shown to not only directly induce lymphangiogenesis [29], but also to stimulate the release of the VEGF-C sub-type from resident macrophages.16 VEGF-C also induces lymphangiogenesis as well as evokes dilation of lymphatic vessels.20,30 VEGF-C and VEGF-A largely parallel one another in plasma concentrations, with an approximate 30:1 ratio.18 All measurements were read on Bio-Rad Bio-Plex MAGPIX Reader. Cytokine levels in plasma and CSF (including VEGF levels) were inadvertently not determined for one of the eight AD subjects in this study.
Human total tau (t-tau). Instructions were followed according to those provided for the Thermo Fisher Human Tau (total) kit (Cat: KHB0041). Standard, Streptavidin-HRP, and wash buffer solutions were prepared according to the menu. For each well, 100μl of standard and plasma or CSF sample (undiluted) were added, incubated overnight at 4°C with shaking, then washed 4 times with wash buffer. Detection antibody (100μl/well) was then added, followed by incubation for 1 h at room temperature. Plates were washed 4 times with wash buffer, then 100μl of diluted streptavidin-PE was added to each well, followed by incubation for 1 h at room temperature with shaking. Next, plates were washed 4 times, followed by addition of 100μl of stabilized chromogen to each well. The reaction was allowed to occur for 10 min, then 100μl of stop solution was added to each well, followed by plate reading on the BioTek Synergy H4 reader.
Human phospho-tau (p-tau). Instructions were followed according to those provided for the Thermo Fisher Human p-tau (pT231) phosphoELISA kit (Cat: KHB8051). Standard, anti-rabbit IgG HRP, and wash buffer solutions were prepared according to the menu. For each well, 100μl of standard and plasma or CSF sample (undiluted) was added, incubated overnight at 4°C with shaking, then washed 4 times with wash buffer. Detection antibody (100μl /well) was then added, followed by incubation for 1 h at room temperature. Plates were washed 4 times, then 100μl of diluted anti-rabbit IgG HRP was added to each well, followed by incubation for 1 h at room temperature with shaking. Next, plates were washed 4 times, followed by addition of 100μl of stabilized chromogen to each well to allow reaction to occur for 10 min. Then 100μl of stop solution was added to each well, followed by plate reading on the BioTek Synergy H4 reader.
Human Aβ1-40, Aβ1-42. Antibodies (goat anti-human Aβ1-42, and goat anti-human Aβ31-40 specific antibody) were purchased from MegaNano BioTech., Inc. FL. Instructions were followed according to those provided. Standard, detection antibody, anti-rabbit IgG HRP, and wash buffer were all prepared according to the menu. For each well, 50μl standard and plasma or CSF (1:100 diluted for Aβ determinations) sample were added to appropriate wells, then 50μl of detection antibody for Aβ1-40 or Aβ1-42 was added to each well. Incubation occurred overnight at 4°C with shaking. After 4 washes, 100μl of diluted anti-rabbit IgG HRP was added to each well, followed by incubation for 1 h at room temperature with shaking. This was followed by 4 washes with wash buffer, then addition of 100μl of stabilized chromogen to each well to allow reaction for 10 min. Stop solution (100μl /well) was then added and plates read on the BioTeck Synergy H4 reader.
Statistical analysis
To determine effects of 2 months of daily TRFT, all baseline values were statistically compared to 2-month (Day 60) values with Paired t-tests at p < 0.05 or lower level of significance. For correlation analyses, correlation coefficients (r) were calculated and level of significance determined from Pearson’s correlation table. Plasma or CSF data from a subject on a given measure was sometimes omitted due to undetectable baseline readings, inconsistent duplicate values, or as a clear outlier (Grubb’s single outlier test).
RESULTS
ADAS-cog scores strongly correlate with CSF levels of t-tau, p-tau, and VEGF
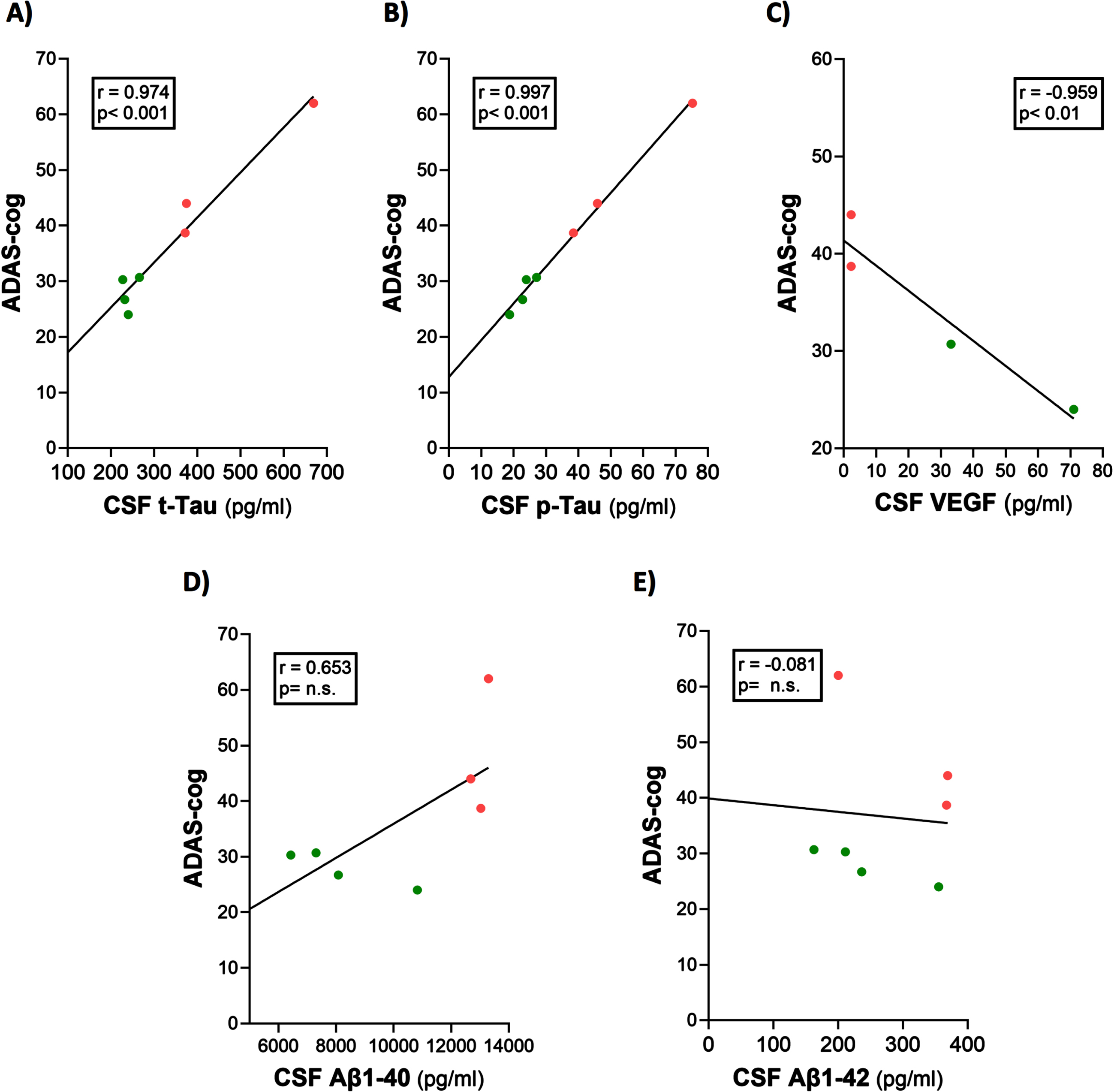
Baseline ADAS-cog scores were clearly correlated with baseline CSF levels of t-tau, p-tau, and VEGF in the mild/moderate AD subjects of this study (Fig. 4A–C). Indeed, ADAS-cog scores for the seven AD subjects plotted could be divided into two non-overlapping groups based on their ADAS-cog scores: higher scores (48.2±7.1; n = 3) and lower scores (27.9±1.6; n = 4), with a significant difference (p = 0.022) between groups. Higher levels of CSF t-tau and p-tau were seen in those AD subjects with higher ADAS-cog scores (poorer cognitive performance). Conversely, subjects with higher CSF levels of VEGF showed lower ADAS-cog scores (better cognitive performance). Thus, ADAS-cog score was a good index of CSF/brain levels of these three markers and vice versa. There were no correlations between baseline CSF levels of Aβ1-40 and Aβ1-42 versus ADAS-cog performance (Fig. 4D, E), although the correlation involving Aβ1-40 was significant at p < 0.05 (one-tailed).
Fig. 4
ADAS-cog scores strongly correlate with CSF levels of t-tau, p-tau, and VEGF. (A–C) Higher levels of t-tau and p-tau correlated with poorer ADAS-cog performance, while higher levels of VEGF correlated with better ADAS-cog performance. The correlation between Aβ1-40 and cognition (D) was significant (p < 0.05) with one-tailed analysis. Red dots represent AD subjects with higher (poorer) ADAS-cog scores, while green dots represent AD subjects with lower (better) ADAS-cog scores.
TRFT re-balances VEGF levels in both plasma and CSF
In both plasma and CSF, the change in VEGF levels induced by 2 months of TRFT was inversely dependent on baseline VEGF levels (Fig. 5A, B). If baseline levels of VEGF in plasma or CSF were low, TRFT induced an increase in those levels (green circles). If baseline levels of VEGF in plasma or CSF were high, TRFT induced a decrease in those levels (red circles). Thus, VEGF levels in both plasma and CSF were “re-balanced” by TRFT, with AD subjects bearing low baseline VEGF benefiting from TRFT with enhanced VEGF levels. This re-balancing of VEGF by TRFT is underscored in the bar graph of Fig. 5C wherein baseline plasma VEGF is shown for AD subjects divided into two groups—those with low and those with higher VEGF levels at baseline. Although there was a large difference in plasma VEGF levels between these two groups at baseline, TRFT re-balanced VEGF levels by increasing levels in the low baseline group and decreasing levels in the higher baseline group. The result was no low versus high group difference after 2 months (D60) of TRFT, as shown in the bar graph of Fig. 5C.
Fig. 5
TRFT re-balances VEGF levels in both plasma and CSF. If baseline VEGF levels in plasma (A) or CSF (B) were low, TRFT increased those levels (green circles). By contrast, if VEGF levels were high, TRFT induced a decrease in those levels (red circles). Re-balancing of VEGF by TRFT is also evident in bar graph format (C), wherein AD subjects were divided into two groups – low or high baseline (BL) plasma VEGF levels. The large difference in plasma VEGF between these two groups at BL was completely eliminated by 2 months of TRFT.
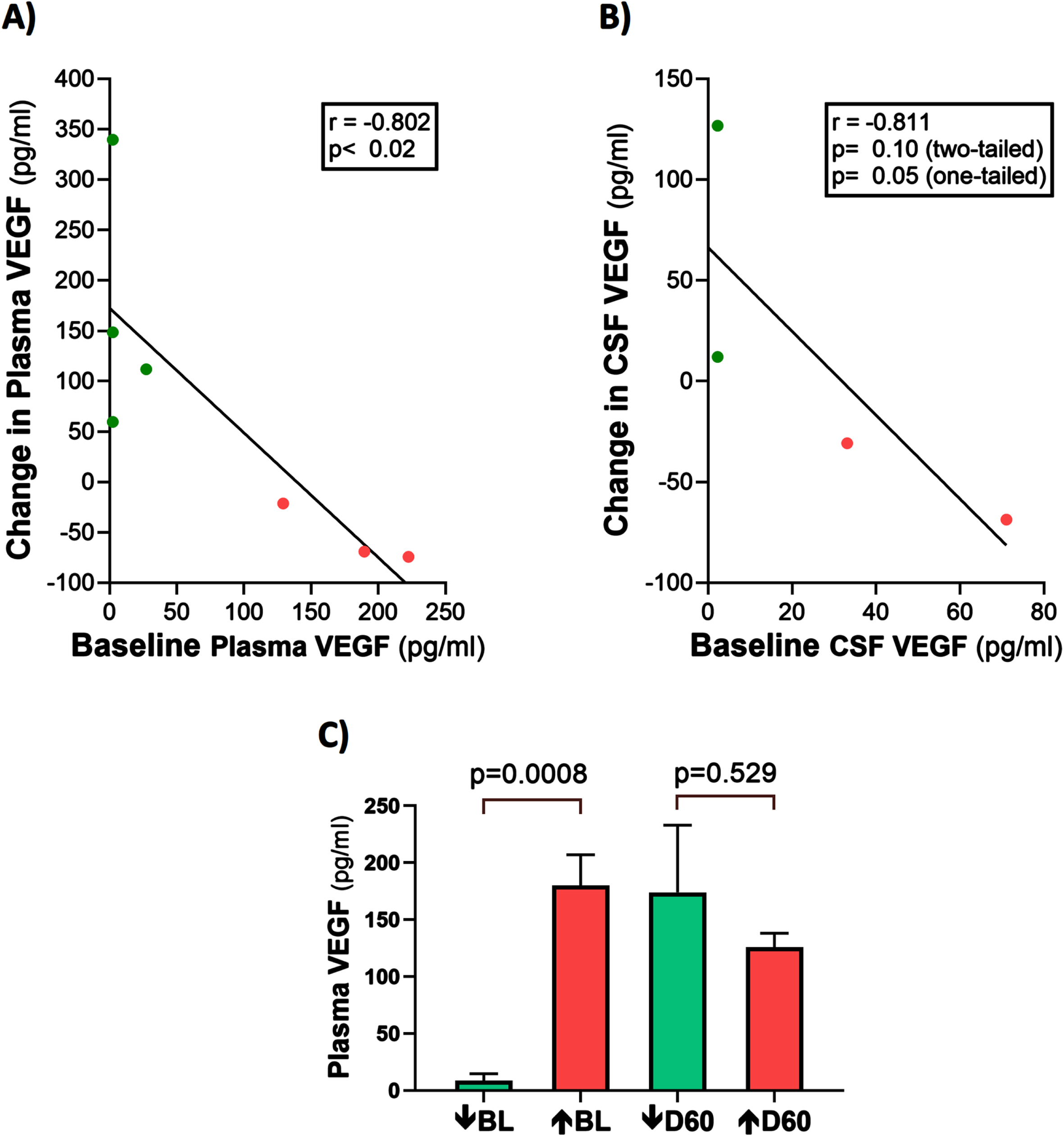
It should be mentioned that half of the AD subjects in this study had VEGF levels in their CSF that were below detection levels pre- and post-treatment. The reasons for this are three-fold: 1) plasma baseline levels were already relatively low, with two subjects even having undetectable VEGF at baseline, 2) the concentration gradient of plasma toward CSF for VEGF, as exemplified by CSF concentrations of baseline VEGF-A being well below those of baseline plasma, and 3) the VEGF-A sub-type was measured, which is only around 1/30th the plasma concentration of the VEGF-C sub-type [18]. This last reason was probably the most important reason for lack of VEGF detection in plasma.
Plasma t-tau, Aβ1-40, and Aβ1-42 levels are correlated with plasma VEGF levels: TRFT re-balances these plasma AD markers to eliminate their correlations with plasma VEGF
At baseline, three AD markers in plasma (t-tau, Aβ1-40, and Aβ1-42) were directly correlated with plasma VEGF levels (Fig. 6A–C). This suggests that higher levels of plasma VEGF are resulting in increased clearance of all three AD markers from brain/CSF into plasma. Daily TRFT for 2 months eliminated these three correlations (Fig. 6D–F). For t-tau and Aβ1-42, the correlations with VEGF were eliminated by: 1) robust TRFT-induced increases in t-tau and Aβ1-42 levels for those AD subjects with low baseline levels (green circles) and 2) small or no TRFT-induced decreases in t-tau and Aβ1-42 levels for those AD subjects with high baseline levels. This “re-balancing” of plasma t-tau and Aβ1-42 levels by TRFT is also evident in bar graphs showing means of the low baseline versus high baseline groups at baseline compared to after 2 months (D60) of TRFT (Fig. 7A, B). A “re-balancing” effect of TRFT is likewise very evident when showing the relationship between baseline t-tau levels in plasma and the effect of TRFT on them (Fig. 8A). With one exception, a re-balancing of TRFT on t-tau occurred wherein lower t-tau levels were increased and higher t-tau levels were decreased. TRFT’s re-balancing effect on Aβ1-40 (Fig. 6F) was not significant in bar graph format (Fig. 7C) because the low Aβ1-40 baseline level group: 1) did not differ from the high baseline group, and 2) did not show a TRFT-induced increase on Day 60. Parenthetically, plasma p-tau levels were below detection in this clinical study.
Fig. 6
Plasma t-tau, Aβ1-40, and Aβ1-42 levels are directly correlated with plasma VEGF levels (A–C): TRFT re-balances these AD markers to eliminate their correlations with VEGF (D–F). Subjects with low baseline (BL) levels of VEGF are indicated by green circles, while those with high BL VEGF levels are indicated by red circles. It is evident that this re-balancing by TRFT primarily involved an increase in AD marker levels in subjects with low BL levels of VEGF.
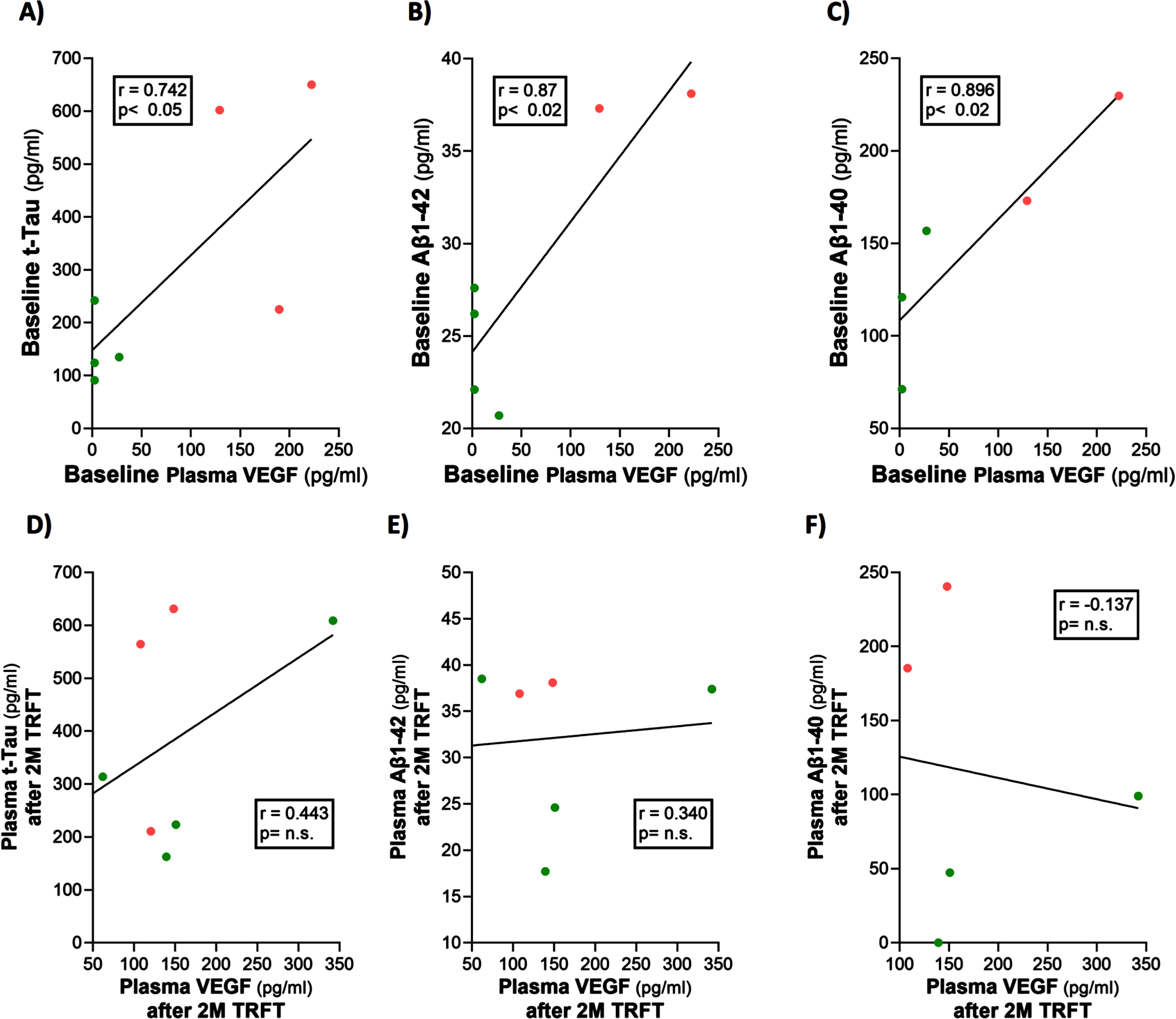
Fig. 7
Re-balancing of plasma t-tau and Aβ1-42 levels by TRFT. In bar graph format, re-balancing of both plasma t-tau and Aβ1-42 is evident following 2 months of TRFT (D60)—more specifically, the significant differences between low versus high BL VEGF groups were eliminated (A,B). TRFT’s re-balancing effect on Aβ1-40 (see Fig. 6C, D) was not evident in bar graph format (C).
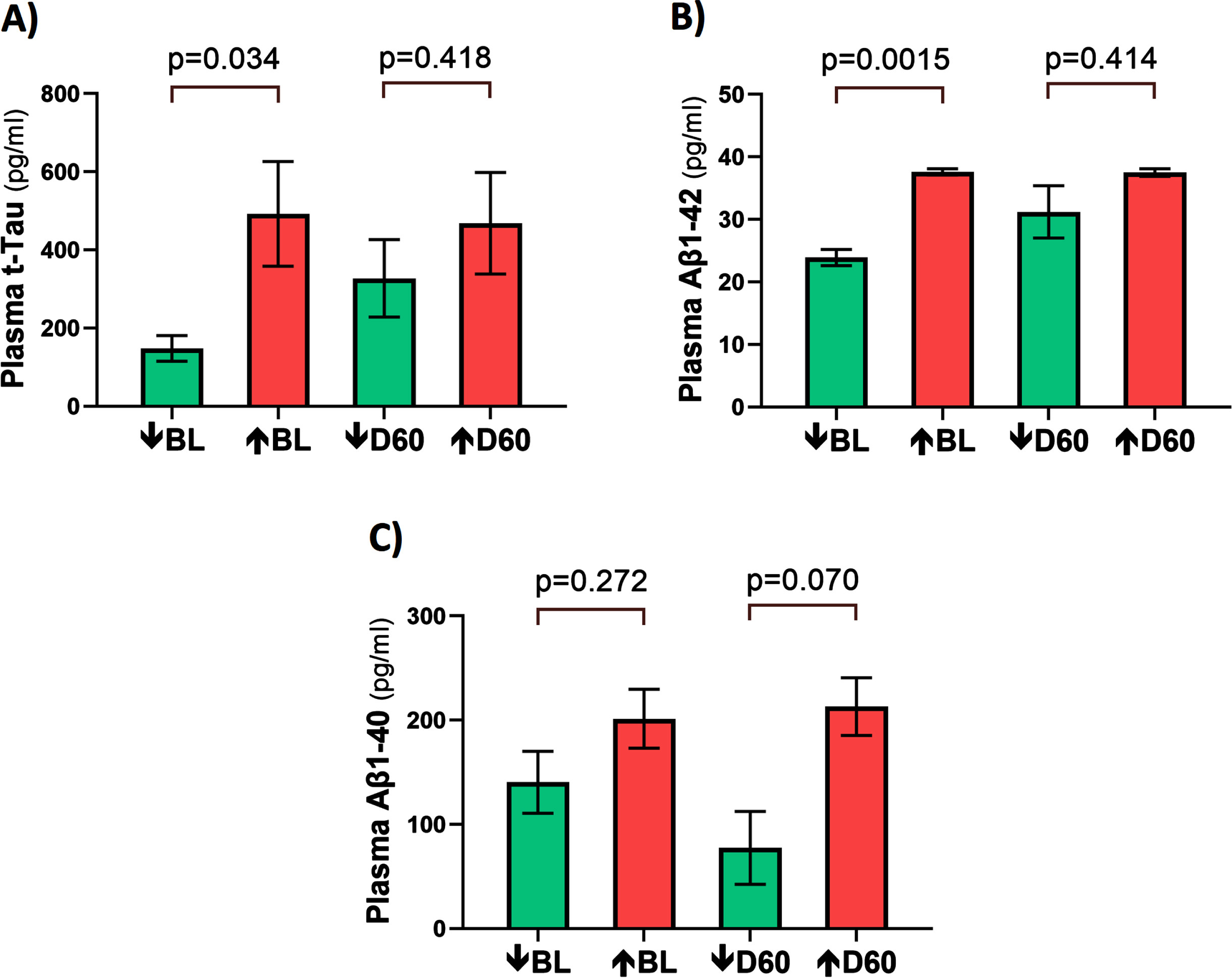
Fig. 8
TRFT re-balances plasma (A) and CSF (B) t-tau levels. With the exception of one AD subject (lower right green) in both plasma and CSF, lower t-tau levels were increased/stable with TRFT and higher t-tau levels were decreased.
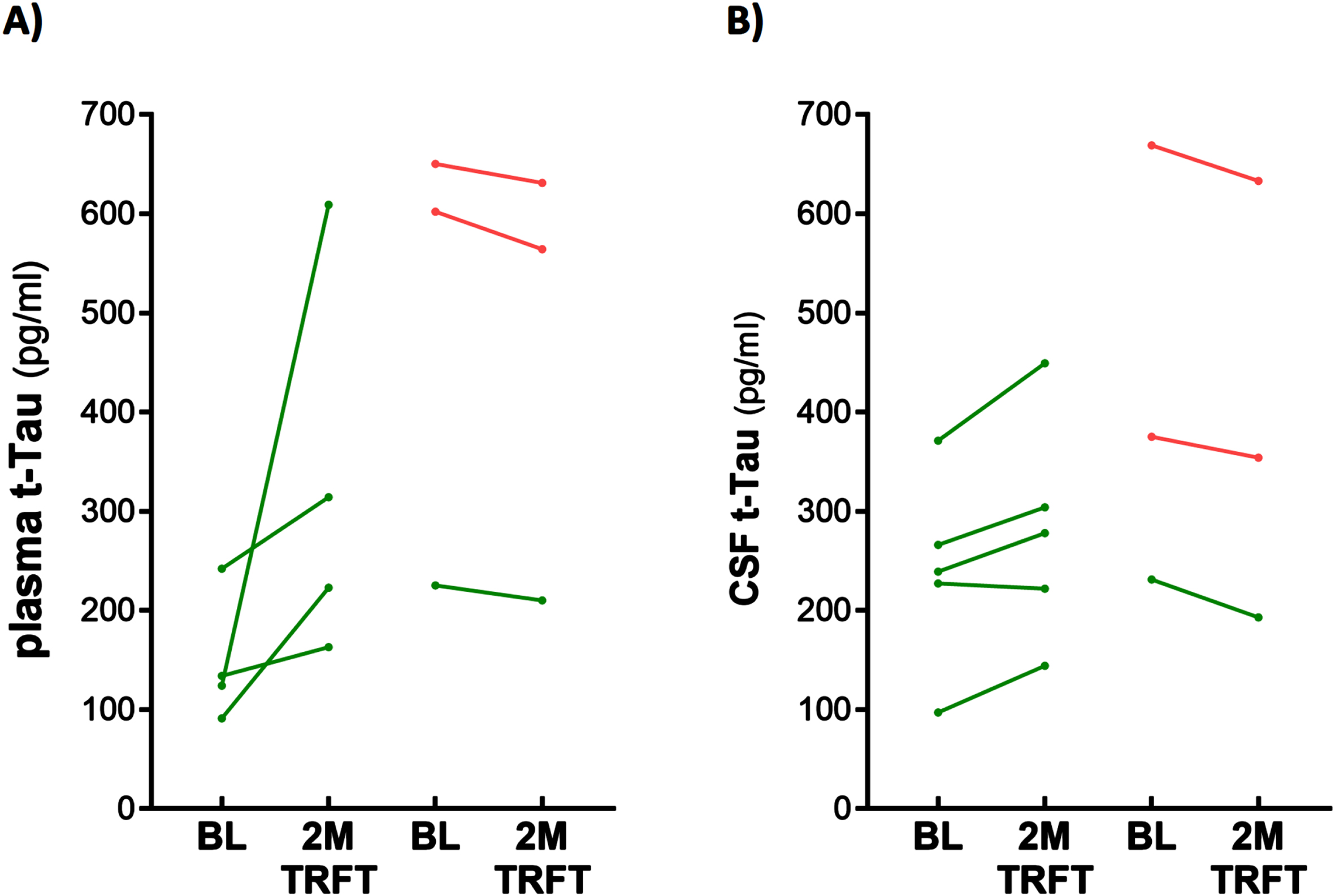
CSF t-tau and p-tau levels are correlated with VEGF levels in CSF: TRFT re-balances these CSF AD markers to eliminate their correlations with CSF VEGF
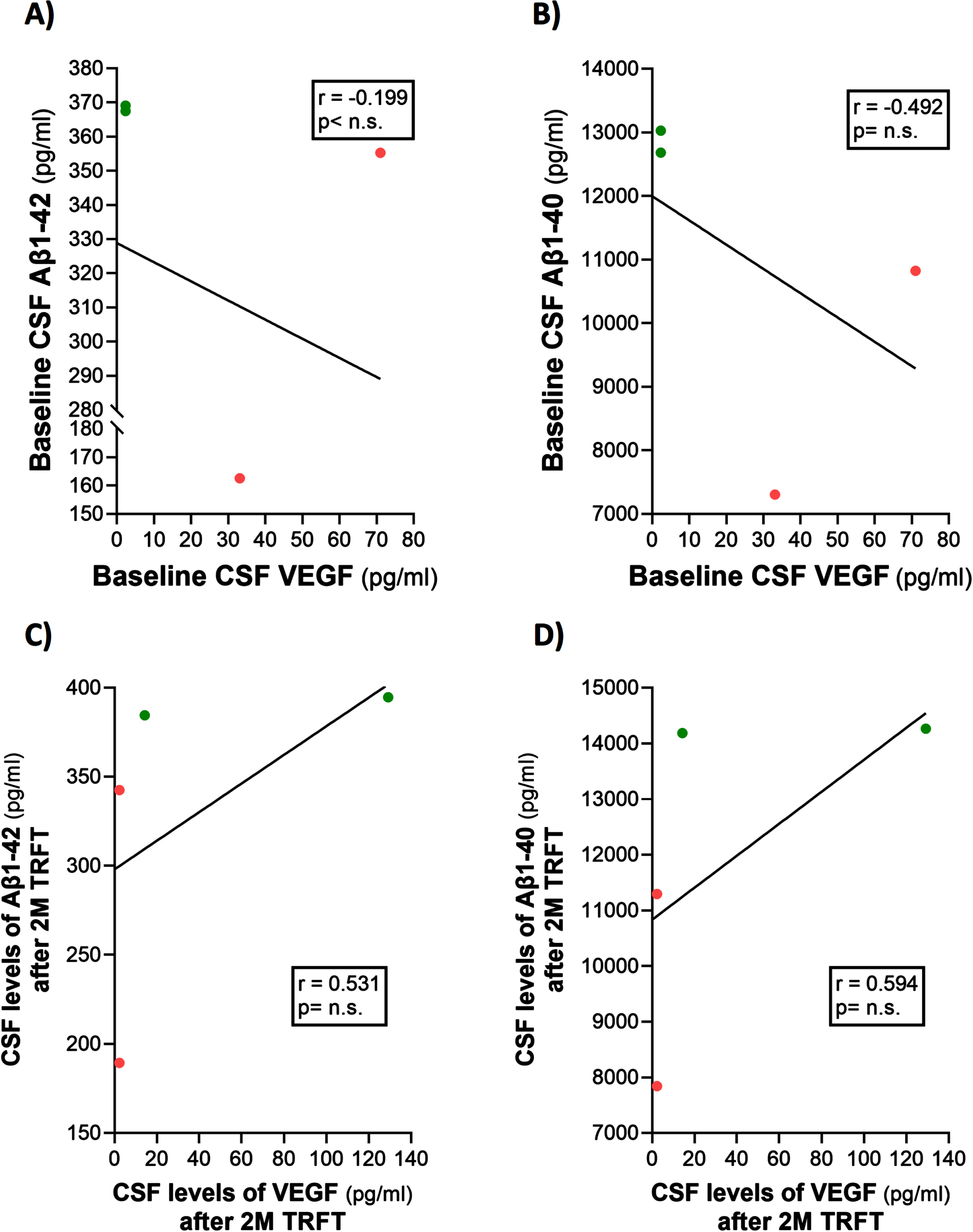
Only four of the 8 AD subjects of this study had measurable CSF levels of VEGF. The reasons for this were indicated in the second sub-section of this Results section. Thus, caution should be taken for any interpretation of the CSF VEGF results. However, even with only four subjects having measurable levels of VEGF in CSF, evidence for VEGF within CSF/mLV increasing “baseline” drainage of t-tau and Aβ1-42 was apparent in comparing: 1) averaged plasma t-tau levels in low versus high CSF VEGF subjects (98 versus 387 pg/ml) and 2) averaged plasma Aβ1-42 levels in low versus high CSF VEGF subjects (21.7 versus 32.2 pg/ml). Also, there were strong negative correlations between baseline CSF levels of VEGF and baseline CSF levels of t-tau and p-tau (Fig. 9A, B). TRFT eliminated these two correlations at Day 60 by dramatically increasing CSF VEGF levels in the two subjects with low baseline VEGF levels and by decreasing CSF levels in the two subjects with higher baseline VEGF levels (Fig. 9C, D). Although only half of AD subjects in this study had measurable VEGF in CSF, all eight subjects had measurable CSF levels of t-tau at both baseline and after TRFT (Day 60). For these eight subjects, Fig. 8B shows the relationship between their baseline t-tau levels in CSF and the effect of TRFT. With one exception, a re-balancing of TRFT on CSF t-tau occurred wherein lower t-tau levels were increased/stable and higher t-tau levels were decreased. This is essentially the same TRFT effect as seen for t-tau levels in plasma (Fig. 8A). Thus, TRFT re-balances t-tau in both plasma and CSF. Interestingly, TRFT had no effect on the “baseline” gradient of t-tau between plasma and CSF, wherein half of AD subjects had greater t-tau levels in CSF than plasma and the other half had just the opposite. TRFT had no effect on CSF levels of Aβ1-40 and Aβ1-42, largely because there were no correlations between VEGF levels and these AD markers at baseline for the small number of subjects involved (Fig. 10).
Fig. 9
CSF t-tau and p-tau levels are directly correlated with CSF levels of VEGF (A, B): TRFT re-balances these AD markers to eliminate their correlations with VEGF (C, D).
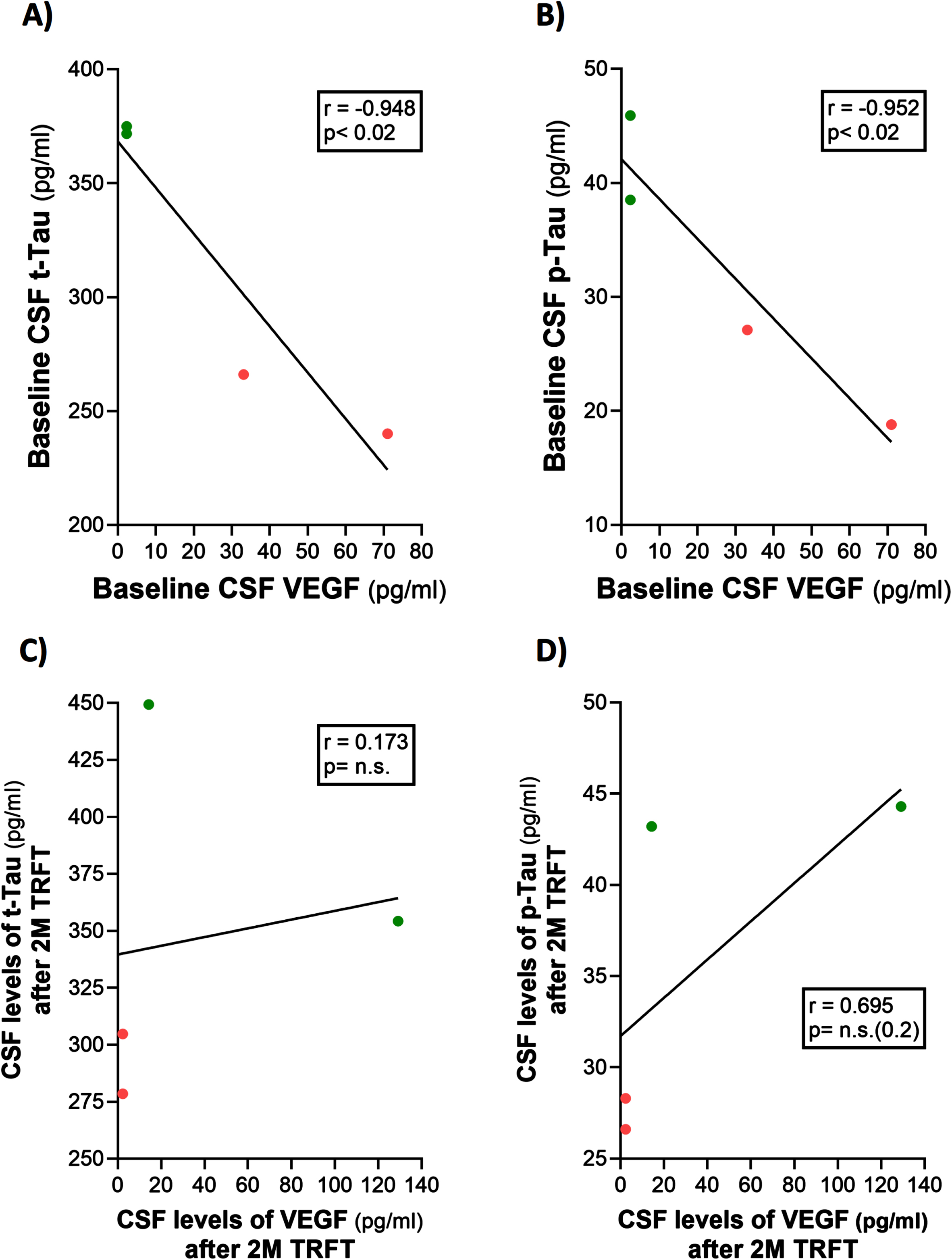
Fig. 10
TRFT has no effect on CSF levels of Aβ1-40 and Aβ1-42.
DISCUSSION
The present study is the first to clinically evaluate the cytokine VEGF on brain clearance of tau and Aβ in human subjects, specifically those with mild/moderate AD. Moreover, this study provides the first evidence for TRFT as a new and non-invasive therapeutic that “clinically” modulates VEGF levels in and around mLVs, thus impacting their flow and ensuing removal of toxins such as Aβ and tau from the brain. These TRFT effects would seem to be particularly beneficial to the majority of AD subjects who have low or unmeasurable VEGF levels in plasma because such subjects in the present study also had low cognitive performance at baseline.21 TRFT induced elevations in both plasma and CSF levels of VEGF in these low-VEGF individuals, which was associated with (and likely contributed to) the much better cognitive performance these AD subjects showed after TRFT.23 Though accurate measurement of mLV flow in humans presently requires MRI imaging, it seems apparent that TRFT-enhanced levels of VEGF in and around mLVs likely increased their flow through vessel dilation and enhanced lymphangiogenesis to facilitate removal of Aβ and tau from the brain. Such facilitated toxin removal by TRFT is evidenced by elevated levels of Aβ and tau in plasma of “low baseline” VEGF subjects following the 2 months of TRFT.
In this Discussion, the importance of mLVs and their modulation by VEGF is considered first—this, in order to put the ensuing detailed discussion of this clinical study’s TRFT-induced effects on VEGF and brain toxin clearance into proper perspective.
Importance of mLVs for CSF/ISF drainage from the brain
The recently (re-)discovered mLV system in the human brain’s dura is comprised of both dorsal and basal lymphatic vessels in close apposition to the brain’s venous sinuses (Fig. 1).3 Dorsal mLVs appear to be more important for toxin removal from the brain, with MRI showing they are located around almost all dural venous-parasagittal structures.3 Although both the CSF/venous sinus pathway and mLVs drain CSF from the brain, the proportion of this CSF drained by mLVs has been reported to be a surprising 30–50% of the total outflow.6 Indeed, there is even evidence that the main route of CSF outflow is through mLVs rather than through the CSF/venous sinus pathway.6
Underscoring how important mLVs are for clearance/drainage of toxins and other molecules from the brain is a study in AD transgenic mice showing that impaired lymphatic drainage of CSF decreases Aβ clearance and thus increases the Aβ burden in the brain.31 In another study, inhibition of VEGFR3 (the receptor specifically on mLVs that is activated by VEGF-C) leads to degeneration of mLVs.32 Consistent with these results, some studies have demonstrated that boosting the function of mLVs could lessen brain Aβ deposition in various transgenic AD mouse models. For example, intracerebroventricular (icv) injection of recombinant VEGF-C, which promoted mLV lymphangiogenesis, also reduced the accumulation/levels of deposited and soluble brain Aβ, and alleviated the cognitive deficits of APP+PS1 mice.20 Additionally, repetitive transcranial magnetic stimulation (rTMS) treatment in 5xFAD transgenic mice reduced brain Aβ deposition by improving the drainage of Aβ via mLVs.33 Regarding these last two studies that measured “insoluble” Aβ deposition, brain insoluble Aβ deposition into neuritic plaques is no longer thought to be a primary pathogenic cause of AD’s memory impairment.34 Rather, “soluble” levels of Aβ and tau (measured in the present study) are now viewed by many researchers as a primary cause of AD cognitive decline, specifically the oligomeric species of Aβ and tau.34–36 In any event, the importance of mLVs for drainage of CSF and brain toxins appears to be fairly well established from animal studies.9,11
Modulation of mLVs by VEGF
VEGF is a cytokine well-known for its ability to stimulate tissue angiogenesis.29 However, with the recent (re-)discovery of lymphatic vessels in the brain’s dura (i.e., mLVs), a second function of VEGF is now becoming prominently researched – namely, VEGF’s targeted ability to enhance mLV flow (via mLV dilation and lymphanagiogenesis) to remove/drain toxins from the brain.14 Studies have shown that direct brain treatment of aged mice with VEGF results in enhanced mLV diameter, increased lymphoangiogenesis of mLVs, and greater clearance of Aβ from their brains.8,20 Both mLV dilation and mLV lymphoangiogenesis increase lymph flow through mLVs, resulting in increased brain drainage of CSF and toxins within it.
There appear to be at least three sources of VEGF that can influence mLV diameter and vessel numbers (Fig. 2). First, VEGF (both A and C sub-types) in blood plasma of choroid plexus capillaries diffuses down its concentration gradient into choroid plexus (CP) ependymal cells, then into CSF, and finally around and into mLVs located in the brain’s dura. Second, ependymal cells lining the choroid plexus and bordering CSF make and release VEGF into the CSF they produce, which then goes around and in mLVs.37,38 Third, resident macrophages within the CP interstitial fluid secrete VEGF-C, which then follows the same route as plasma VEGF across ependymal cells, then into and around mLVs.17
Actions of VEGF-A and VEGF-C on mLVs
The present study measured VEGF-A in plasma and CSF, but not VEGF-C. This was inadvertent because the utilized cytokine kit indicated measurement of “VEGF” without indicating that the VEGF-A subtype was being measured. Nonetheless, recent studies have demonstrated that VEGF-A has the same lymphangiogenic actions as VEGF-C on mLVs, although having much lower levels in plasma and presumably low levels in CSF/ISF as well.18 Nonetheless, VEGF-A appears to induce formation of greatly enlarged lymphatic vessels, which would facilitate mLV drainage.29,39,40
All but one of the AD subjects in this study had higher “baseline” VEGF levels in plasma than in CSF, indicating a flux of VEGF was occurring into CP ependymal cells and then into mLVs. Even at that, plasma VEGF-A levels were relatively low, resulting in even lower or unmeasurable levels in CSF. Certainly, the 30:1 ratio of VEGF-C to VEGF-A in plasma also was contributory to the low VEGF-A levels in plasma and CSF.18 Given our inadvertent choice of measuring VEGF-A in this study, it would have been better to have also measured VEGF-C in hind-sight. Nonetheless, TRFT’s effects on VEGF-A levels should be paralleled by the same effects on VEGF-C levels in both blood and CSF.
TRFT regulates VEGF to influence mLV function
Although one clinical study reported plasma VEGF levels to be slightly elevated or no different from aged controls,41 another study showed that VEGF in serum of AD subjects is 30% lower than in controls, with greatest severity of AD linked to 5-fold lower levels of VEGF.21 Yet another clinical study measured VEGF in CSF of normal aged, MCI, and AD subjects, finding that higher VEGF levels were strongly correlated with greater baseline hippocampal volume, lesser hippocampal atrophy over time, and lesser memory decline over time.22 Incredibly, a subset of “normal aged” subjects in this study who had the same high levels of CSF t-tau and Aβ1-42 as in the AD subset showed no clinical manifestations of AD. It could be argued that the high CSF levels of VEGF in these normal aged subjects staved off AD-related neurodegeneration and associated cognitive impairment. If true, high VEGF levels in CSF would be protective against AD irrespective of CSF t-tau or Aβ1-42 levels.
In the present study, an initial set of correlation analyses showed that baseline CSF/brain levels of t-tau, p-tau, and VEGF were all excellent indices of cognitive performance (ADAS-cog score) in AD subjects. Higher t-tau and p-tau levels in CSF were correlated with poorer cognitive performance, while higher VEGF levels in CSF were correlated with better cognitive performance. Thus, a therapeutic that can reduce t-tau or p-tau levels in CSF, and/or increase VEGF levels therein, could beneficially impact cognitive performance in AD subjects. The present study provides evidence that such a therapeutic is TRFT in view of its ability to: 1) modulate all three of these markers in CSF and/or plasma, and 2) reverse cognitive impairment in the same AD subjects, as we have previously reported.23
If TRFT influences mLV function and flow, we reasoned that it would need to modulate the VEGF levels in and around mLV vessels. In this capacity, a 2-month period of daily TRFT re-balanced VEGF levels in both plasma and CSF of AD subjects. Specifically, if AD subjects had low baseline VEGF levels, TRFT increased those levels and vice versa such that there were no differences between these two groups in VEGF levels in plasma or CSF after TRFT. This was especially important for the AD subjects with low baseline VEGF because the TRFT-induced increase in their VEGF levels were associated with (and likely contributory to) their better cognitive performance after TRFT.
As mentioned earlier in this Discussion, the VEGF seen by mLVs comes from at least three sources: 1) plasma within arterial capillaries in the CP, 2) ependymal cells lining the CP, and 3) resident CP macrophages. TRFT is probably affecting all three of these VEGF sources, as shown in Fig. 2. First and most likely is TRFT’s ability, through the aforementioned “re-balancing” of plasma VEGF levels, to increase the fluidity/transport capacity of red blood cells (RBCs). This would result in a “re-balancing” flux of VEGF in or out of RBCs depending on baseline VEGF levels (low baseline VEGF levels would result in a flux of VEGF out of RBCs). We have previously indicated that this TRFT-induced flux in or out of RBCs occurs for many cytokines in view of the ability of RBCs to act as cytokine reserves by concentrating cytokines by an average of 12x higher than their plasma concentrations;42 for VEGF, its concentration in RBCs is 30x higher than in plasma. Secondly, TRFT could be stimulating the known release of VEGF from the CP’s ependymal cells for ensuing uptake into CSF.38 Unfortunately, the effects (if any) of radiofrequency waves on such ependymal cells is unknown. Thirdly, TRFT may be stimulating the known release of VEGF from resident macrophages within CP.38,43 Along this line, rat macrophages have been shown to be activated by exposure to radiofrequency waves of the same frequency (915 MHz) used in the present clinical study.44
Re-balancing of plasma AD markers by TRFT
Baseline plasma t-tau, Aβ1-40, and Aβ1-42 levels were all positively correlated with plasma VEGF levels. This is consistent with VEGF increasing the clearance/drainage of all three of these AD markers from the brain/CSF into plasma, presumably via VEGF increasing mLV flow. TRFT re-balanced these plasma AD markers to eliminate their correlations with plasma VEGF. This was almost totally due to the TRFT-induced increase in these AD markers observed in those AD subjects having low baseline VEGF levels in plasma. For such AD subjects, re-balancing by TRFT to increase VEGF levels in plasma (and in and around mLVs), presumably resulted in increased lymphatic drainage/clearance of t-tau, Aβ1-40, and Aβ1-42 from the brain. For AD subjects with higher plasma VEGF levels at baseline, some reduction of these three AD markers in plasma occurred during re-balancing, but not much (i.e., mLV flow was not significantly compromised). Alternatively, it is possible that TRFT induced changes in RBC levels of these three AD markers to affect their plasma levels directly, without any VEGF involvement—in other words, a plasma re-balancing of AD markers similar to that occurring for cytokines. Along this line, RBCs of senescent-accelerated mice concentrate high levels of t-tau, p-tau, and Aβ1-42.45 As well, RBCs of AD subjects have increased Aβ inside compared to controls.46 In view of the above considerations, it is presently not known which of the two sources of these three AD markers (i.e., release from RBCs or from brain drainage) is being impacted more prominently by TRFT to increase their plasma levels.
One additional point should be made regarding plasma VEGF levels. We believe it is important to measure plasma VEGF levels in AD patients since the large majority of them who bear low VEGF levels should benefit substantially from TRFT elevating their VEGF levels to increase clearance of Aβ and tau from their brains/CSF.21 Such an effect of TRFT to enhance VEGF levels could increase their cognitive performance, as was seen for the AD subjects in the present study.
Re-balancing of brain/CSF AD markers by TRFT
Although limited by the number of AD subjects with measurable CSF/brain VEGF-A levels in this study, the strong negative correlations between baseline CSF levels of VEGF and baseline CSF levels of both t-tau and p-tau are strongly suggestive of increased VEGF in CSF going the short distance to mLVs to induce increased mLV dilation and lymphangiogenesis. The resultant increase in mLV flow would clear/drain Aβ and tau from the brain and into blood vessels. Evidence for this increased baseline drainage of t-tau and Aβ1-42 by increased CSF VEGF was seen when comparing: 1) averaged “baseline” plasma t-tau levels in low versus high CSF VEGF subjects, and 2) averaged “baseline” plasma Aβ1-42 levels in low versus high CSF VEGF subjects.
Two months of daily TRFT re-balanced CSF levels of both t-tau and p-tau to eliminate their “baseline” correlations with CSF VEGF. It should be underscored that TRFT only increased CSF VEGF in 2 of the 8 AD subjects; in 2 other AD subjects, TRFT actually decreased VEGF levels in CSF. So most of VEGF’s effects on mLVs must result from: 1) the very low levels of VEGF-A in CSF, 2) stimulation by VEGF-A of VEGF-C release from ependymal cells37, and/or 3) from the much more prevalent VEGF-C itself.18 In the several AD patients with unmeasurable CSF levels of VEGF at baseline, the increased (measurable) VEGF levels in CSF as a result of TRFT likely came from both the plasma (RBC re-balancing) and the brain (stimulation of ependymal cells and/or macrophages).
TRFT rebalances blood and brain/CSF t-tau levels
For t-tau in both plasma and CSF, when baseline and 2M TRFT values were plotted out for the individual AD subjects in this study, a clear “re-balancing” pattern of TRFT on t-tau was apparent. Indeed, the plasma and CSF response to TRFT was the same as that we have previously found for 11 of 12 cytokines in plasma and 7 of 7 cytokines in CSF,28 as well as for plasma t-tau at 14–17 months into TRFT.24 With one exception for both plasma and brain, lower levels of t-tau at baseline resulted in increased t-tau levels after TRFT. Since soluble t-tau is almost totally monomeric tau, these increases may reflect a treatment-induced increase in monomeric t-tau within plasma and CSF, which would be consistent with disaggregation of oligomeric tau in the brain and re-balancing of t-tau by RBCs. Regarding the latter, RBCs in a model of AD have high internal concentrations of t-tau compared to controls and RBCs of AD subjects have much higher Aβ levels than in plasma.45,47 So the increased RBC membrane fluidity induced by TRFT for blood going through the brain likely produced a flux of t-tau out of RBCs. If baseline t-tau levels were higher in plasma and CSF at baseline, there was only a small decrease in t-tau after TRFT. This maintenance of t-tau levels (if already high) may be a beneficial for facilitating t-tau clearance in the brain and blood. Thus, TRFT re-balances both plasma and brain levels of t-tau. It should be noted that p-tau levels were not measurable in plasma and that p-tau levels in CSF did not show the same re-balancing effects by TRFT as did CSF t-tau levels.
Decreased mLV function and flow during aging and disease
Both mLV and CSF drainage from the brain become impaired during aging.19,48 Drainage capacity of meningeal immune cells is also decreased with aging.19,49 Not surprisingly, meningeal lymphatic function and CSF toxin transport become significantly less efficient in aged mice and in elderly humans.6,49 For example, MRI studies revealed age-related lymphatic output and waste removal were reduced in older human subjects compared with younger subjects.3 Additionally, old mice have a decrease in meningeal lymphatic vessel diameter, as well as decreased drainage capacity of CSF toxins/macromolecules into their cervical lymph nodes.19
Importantly, mLVs are an effective way to remove “senescent” astrocytes from the brain. In comprising most of the senescent cells in the brain, glial cells (mostly astrocytes) secrete inflammatory cytokines onto adjacent neurons.50,51 Consistent with this finding, blockage of mLVs to prevent brain senescent astrocyte removal increases brain inflammation in aged mice, while administration of VEGF improves clearance of senescent astrocytes and lessens neuroinflammation.50 Moreover, VEGF is highly expressed in senescent astrocytes, inducing them to migrate across/into mLVs.50 We believe that removal of senescent glial cells from the brain via their drainage in mLVs is much safer and much more beneficial than trying to kill the brain’s senescent cells with “senolytic” drugs. A case-in-point is the senolytic drug combination of Dasatinib+Quercitin that was given to AD subjects for 3 months.52 The study reported no beneficial cognitive effects, no changes in AD markers in plasma or brain, and evidence of increased inflammation in both brain and the periphery. We anticipated these disappointing findings involving senolytic drug administration to AD subjects, stating that senolytic drugs will increase brain inflammation and not provide beneficial neurologic actions.53
It seems apparent that improving the function of (“rejuvenating”) the brain’s mLV drainage system could be very beneficial to aged humans in general. Dysfunctional mLVs, particularly “dorsal” mLVs, play a role in a number of neurologic conditions besides AD, including Parkinson’s disease, multiple sclerosis, stroke, traumatic brain injury, brain tumors, and aging itself.54. Along this line, enhancement of meningeal lymphatic function by VEGF-C has been shown to be beneficial against brain tumors and increasing VEGF signaling in mice lessened occurrence of age-associated pathologies (e.g., sarcopenia, osteoporosis, tumors, inflammation).55,56 Pretreatment of mice with VEGF-C also promotes expansion of MLVs and alleviates viral infections in the brain.57 We propose that TRFT may be therapeutically beneficial to all of these conditions by “rejuvenating” the aged mLV system through VEGF modulation. More broadly, we propose that enhancement of meningeal lymphatic function by TRFT could prevent or lessen age-associated neurologic diseases, with the result being an extension of healthy human longevity.
Clearance/drainage of toxins from the brain: a fourth mechanism of TRFT action
In the present study, initial clinical evidence is provided for enhanced brain mLV clearance of soluble toxins (e.g., Aβ and tau) through the actions of TRFT, potentially on three sources of VEGF in CSF. TRFT modulation of VEGF release from red blood cells via “re-balancing of VEGF’s levels in plasma is probably the most contributory to enhanced mLV function, although TRFT actions to directly influence VEGF release from epithelial cells and macrophages within choroid plexus is also likely. This fourth mechanism of TRFT action is complimentary to the other three mechanisms of TRFT action that we have previously identified: 1) disaggregation of small toxic Aβ and tau oligomers within neurons and in brain parenchyma, 2) brain mitochondrial enhancement, and 3) re-balancing of the immune system’s cytokines in both the brain and blood. Regarding AD, a primary key to the occurrence and development of AD is the imbalance between tau/Aβ production and their clearance. Results from our present and earlier studies23–25,27 indicate that TRFT has a two-fold beneficial action on brain Aβ and tau. Specifically, TRFT decreases Aβ/tau aggregation (induces disaggregation) and TRFT also increases clearance/removal of these soluble toxins from the brain via VEGF modulation.
Study strengths and drawbacks
This study has a number of noteworthy strengths. Firstly, this was an initial clinical study to evaluate the relationship of plasma and CSF VEGF levels with AD marker clearance from the brain. A second strength is the convincing evidence that a therapeutic intervention (TRFT) can non-invasively affect this relationship to increase AD marker clearance from the brain. Third, the study brings in and ties mLV function to explain VEGF’s potentially beneficial effects in AD. And finally, the study shows for the first time the ability of an AD therapeutic to impact (re-balance) both plasma and brain levels of VEGF and AD markers. Collectively, we believe that these strengths show TRFT is essentially “rejuvenating” the mLV drainage system to that seen in young adulthood/middle age.
The primary drawbacks to this study are its small number of AD subjects and lack of a control (placebo) group of AD subjects. Even so, the often highly significant effects presently reported with such a small number of subjects underscores the large clinical significance of the effects reported. Future clinical studies should, nonetheless, be controlled and have larger number of subjects in order to definitively confirm the cognitive, AD/cytokine marker, and brain imaging effects of TRFT that we have reported in our single-arm studies.23,24,28 A second drawback is our inadvertent measurement of the VEGF-A sub-type rather than measuring the far more prevalent VEGF-C sub-type in plasma and CSF. Although measurement of VEGF-C would have certainly added additional insight, it is doubtful that the results and conclusions of this study would have been altered much, especially since these two VEGF isoforms parallel one another in plasma concentration.18 Lastly, not performing FLAIR MRI imaging to accurately measure mLV flow in humans necessitated us to make assumptions about mLV flow based on VEGF levels. Future TRFT clinical studies directly measuring mLV flow via FLAIR MRI are now warranted.
Conclusions
Meningial lymphatic vessels (mLVs) are a critical route for drainage of CSF and toxins (e.g., Aβ and tau) from the brain into blood for their peripheral clearance/catabolism. AD and other neurologic conditions such as Parkinson’s disease, multiple sclerosis, brain tumors, and aging itself are now known to involve impaired mLV flow. As such, maintenance or enhancement of mLV flow could be an important component to any therapeutic strategy for these conditions. The present clinical study forwards TRFT as the first therapeutic intervention that has the capacity to non-invasively modify both plasma and brain/CSF levels of the cytokine VEGF, the primary known agent that regulates mLV flow. In the case of the large majority of AD subjects who have low or undetectable VEGF levels in their blood or brain/CSF, the present study demonstrates that TRFT should increase plasma and brain VEGF levels to presumably enhance CSF/ISF flow through mLVs and thus increase brain Aβ and tau removal/drainage—this, as evidenced by the increased levels of both Aβ and tau in plasma after TRFT. Such a profound effect of TRFT is likely contributory to the cognitive benefits shown by these same AD subjects. If clinical benefits of TRFT are shown in other neurologic conditions or to increase human longevity itself, VEGF and its target mLVs may very well be playing a prominent role.
AUTHORS CONTRIBUTIONS
Gary Arendash (Conceptualization; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Writing – original draft; Writing – review & editing); Xiaoyang Lin (Data curation; Investigation); Chuanhai Cao (Data curation; Formal analysis; Investigation; Methodology).
ACKNOWLEDGMENTS
We thank the clinical staff at the USF Health/Byrd Alzheimer’s Institute for their expert behavioral evaluation of this study’s subjects and for both blood and CSF collections from those subjects. We are also most appreciative of Ning Shen (USF) and Dave Wright (Phoenix Web Designers) for graphs and graphic design, respectively. In addition, Chuck Papageorgiou (NeuroEM Therapeutics, Inc.) is also to be thanked for providing medical reviews of this manuscript prior to submission.
FUNDING
This research was funded by the Glass Charitable Foundation, Angel Investor Forum (Connecticut), NIH, and NINDS grant #12129318 for these studies and studies related to its scientific foundation. None of these funding sources were involved in the design of the studies reported in this paper, nor in the collection, analysis, or interpretation of data, nor in the writing of this manuscript.
CONFLICT OF INTEREST
The University of South Florida has a small financial interest in NeuroEM Therapeutics, a company that provided all of the financial support for this clinical trial. The interest has been reviewed and managed by the University in accordance with its Institutional Conflict of Interest policy. Gary Arendash is an Editorial Board Member of this journal but was not involved in the peer-review process of this article nor had access to any information regarding its peer-review. He also has common shares in NeuroEM Therapeutics, Inc. Chuanhai Cao and X. Lin have nothing to disclose.
DATA AVAILABILITY
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
REFERENCES
1. | Hershenhouse1 K , Shauly O , Gould D , et al. Meningeal lymphatics: a review and future directions from a clinical perspective. Neurosci Insights (2019) ; 14: : 1179069519889027. |
2. | Jiang H , Wei H , Zhou Y , et al. Overview of the meningeal lymphatic vessels in aging and central nervous system disorders. Cell Biosci (2022) ; 12: : 202. |
3. | Albayram SM , Smith G , Tufan F , et al. Non-invasive MR imaging of human brain lymphatic networks with connections to cervical lymph nodes. Nat Commun (2022) ; 13: : 203. |
4. | Da Mesquita S , Papadopoulos Z , Dykstra T , et al. Meningeal lymphatics affect microglia responses and anti-Aβ immunotherapy. Nature (2021) ; 593: : 255–260. |
5. | Ahn JH , Cho H , Kim JH , et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature (2019) ; 572: : 62–66. |
6. | Ma Q , Ineichen BV , Detmar M , et al. Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat Commun (2017) ; 8: : 1434. |
7. | Guo X , Zhang G , Peng Q , et al. Emerging roles of meningeal lymphatic vessels in Alzheimer’s disease. J Alzheimers Dis (2023) ; 94: : S355–S366. |
8. | Da Mesquita S , Louveau A , Vaccari A , et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature (2018) ; 560: : 185–191. |
9. | Patel TK , Habimana-Griffin L , Gao X , et al. Dural lymphatics regulate clearance of extracellular tau from the CNS. Mol Neurodegener (2019) ; 14: : 11. |
10. | Rasmussen MK , Mestre H and Nedergaard M. The glymphatic pathway in neurological disorders. Lancet Neurol (2018) ; 17: : 1016–1024. |
11. | Wang L , Zhang Y , Zhao Y , et al. Deep cervical lymph node ligation aggravates AD-like pathology of APP/PS1 mice. Brain Pathol (2019) ; 29: : 176–192. |
12. | Iliff JJ , Chen MJ , Plog BA , et al. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci (2014) ; 34: : 16180–16193. |
13. | Harrison IF , Ismail O , Machhada A , et al. Impaired glymphatic function and clearance of tau in an Alzheimer’s disease model. Brain (2020) ; 143: : 2576–2593. |
14. | Shibuya M . Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: a crucial target for anti- and pro-angiogenic therapies. G enes Cancer (2011) ; 12: : 1097–1105. |
15. | Halin C , Tobler NE , Vigl B , et al. VEGF-A produced by chronically inflamed tissue induces lymphangiogenesis in draining lymph nodes. Blood (2007) ; 110: : 3158–3167. |
16. | Cursiefen C , Chen L , Borges L , et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest (2004) ; 113: : 1040–1050. |
17. | Harvey NL . Deciphering the roles of macrophages in developmental and inflammation stimulated lymphangiogenesis. Vascular Cell (2012) ; 4: : 15. |
18. | Zajkowska M , Lubowicka E , Fiedorowicz W , et al. Human plasma levels of VEGF-A, VEGF-C, VEGF-D, their soluble receptor - VEGFR-2 and applicability of these parameters as tumor markers in the diagnostics of breast cancer. Pathol Oncol Res (2019) ; 25: : 1477–1486. |
19. | Da Mesquita S , Fu Z and Kipnis J. The meningeal lymphatic system: a new player in neurophysiology. Neuron (2018) ; 100: : 375–388. |
20. | Wen Y-R , Yan J-H , Wang X , et al. Induced dural lymphangiogenesis facilities soluble amyloid-beta clearance from brain in a transgenic mouse model of Alzheimer’s disease. Neural Regen Res (2018) ; 13: : 709–716. |
21. | Mateo I , Llorca J , Infante J , et al. Low serum VEGF levels are associated with Alzheimer’s disease. Acta Neurol Scand (2007) ; 116: : 56–58. |
22. | Hohman TJ , Bell SP , Jefferson AL , et al. The role of vascular endothelial growth factor in neurodegeneration and cognitive decline: exploring interactions with biomarkers of Alzheimer’s disease. JAMA Neurol (2015) ; 72: : 520–529. |
23. | Arendash G , Cao C , Abulaban H , et al. A clinical trial of transcranial electromagnetic treatment in Alzheimer’s disease: cognitive enhancement and associated changes in cerebrospinal fluid, blood, and brain imaging. J Alzheimers Dis (2019) ; 71: : 57–82. |
24. | Arendash G , Abulaban H , Steen S , et al. Transcranial electromagnetic treatment stops Alzheimer’s cognitive decline over a 2½ year period: A pilot study. Medicines (2022) ; 9: : 42. |
25. | Arendash GW , Sanchez-Ramos J , Mori T , et al. Electromagnetic field treatment protects against and reverses cognitive impairment in Alzheimer’s disease mice. J Alzheimers Dis (2010) ; 19: : 191–210. |
26. | Dragicevic N , Bradshaw P , Mamcartz M , et al. Long-term electromagnetic field treatment enhances brain mitochondrial function of both Alzheimer’s transgenic mice and normal mice: A mechanism for electromagnetic field-induced cognitive benefit? Neuroscience (2011) ; 185: : 135–149. |
27. | Arendash G , Mori T , Dorsey M , et al. Electromagnetic treatment to old Alzheimer’s mice reverses β-amyloid deposition, modifies cerebral blood flow, and provides selected cognitive benefit. PLoS One (2012) ; 7: : e35751. |
28. | Cao C , Abulaban H , Baranowski R , et al. Transcranial electromagnetic treatment “rebalances” blood and brain cytokines levels in Alzheimer’s patients: A new mechanism for reversal of their cognitive impairment. Front Aging Neurosci (2022) ; 14: : 829049. |
29. | Nagy J , Vasile E , Feng D , et al. VEGF-A induces angiogenesis, arteriogenesis, lymphangiogenesis, and vascular malformations. Cold Spring Harb Symp Quant Biol (2002) ; 67: : 227–237. |
30. | Li M-N , Jing Y-H , Wu C , et al. Continuous theta burst stimulation dilates meningeal lymphatic vessels by up-regulating VEGF-C in meninges. Neurosci Lett (2020) ; 735: : 135197. |
31. | Louveau A , Da Mesquita S and Kipnis J. Lymphatics in neurological disorders: a neuro-lympho-vascular component of multiple sclerosis and Alzheimer’s disease? Neuron (2016) ; 91: : 957–973. |
32. | Antila S , Karaman S , Nurmi H , et al. Development and plasticity of meningeal lymphatic vessels. J Exp Med (2017) ; 214: : 3645–3667. |
33. | Lin Y , Jin J , Lv R , et al. Repetitive transcranial magnetic stimulation increases the brain’s drainage efficiency in a mouse model of Alzheimer’s disease. Acta Neuropathol Commun (2021) ; 9: : 102. |
34. | Cline EN , Bicca MA , Viola KL , et al. The amyloid-β oligomer hypothesis: beginning of the third decade. J Alzheimers Dis (2018) ; 64: : S567–S610. |
35. | Guerrero-Munoz M , Gerson J and Castillo-Carranza D. Tau oligomers: The toxic player at synapses in Alzheimer’s disease. Front Cell Neurosci (2015) ; 9: : 464. |
36. | Gerson J and Kayed R. Formation and propagation of tau oligomeric seeds. Front Neurol (2013) ; 4: : 93. |
37. | Maharaj A , Walshe T , Saint-Geniez M , et al. VEGF and TGF- β are required for the maintenance of the choroid plexus and ependymal. J Exp Med (2008) ; 205: : 491–501. |
38. | Stopa E , Berzin T , Kim S , et al. Human choroid plexus growth factors: what are the implications for CSF dynamics in Alzheimer’s disease? Exp Neurol (2001) ; 167: : 40–47. |
39. | Nagy J , Vasile E , Brown L , et al. Vascular permeability factor/vascular endothelial growth factor-A (VPF/VEGF, VEGF-A) induces lymphangiogenesis as well as angiogenesis. In: Abstracts from the Annual Meeting of the American Society for Cell Biology (2001) , L69. |
40. | Nagy J , Vasile E , Feng D , et al. Vascular permeability factor/ vascular endothelial growth factor induces lymphangiogenesis as well as angiogenesis. J Exp Med (2002) ; 196: : 1497. |
41. | Elahi F , Casaletto K , La Joie R , et al. Plasma biomarkers of astrocytic and neuronal dysfunction in early- and late-onset Alzheimer’s disease. Alzheimers Dement (2020) ; 16: : 681–695. |
42. | Karsten E , Breen E and Herbert B. (2018) Red blood cells are dynamic reservoirs of cytokines Sci Rep (2020) ; 8: : 3101. |
43. | Cui J , Xu H and LehtinenM. Macrophages on the margin: Choroid plexus immune responses. Trends Neurosci (2021) ; 44: : 864–875. |
44. | Kudo M , Fujita K , Niyaz M , et al. Immunohistochemical findings that exposure to 915 MHz Global System for Mobile Communications (GSM) mobile phone microwaves activates microglia in rat brain. J Tokyo Med Univ (2007) ; 65: : 29–36. |
45. | Piccarducci R , Pietrobono D , Pellegrini C , et al. High Levels of β-amyloid, tau, and phospho-tau in red blood cells as biomarkers of neuropathology in senescence-accelerated mouse. Oxid Med Cell Longev (2019) ; 2019: : 5030475. |
46. | Lan J , Liu J , Zhao Z , et al. The peripheral blood of Aβ binding RBC as a biomarker for diagnosis of Alzheimer’s disease. Age Ageing (2015) ; 44: : 458–464. |
47. | Kiko T , Nakagawa K , Satoh A , et al. Amyloid β levels in human red blood cells. PLos One (2012) ; 7: : e49620. |
48. | Jaffe RJ , Dave RS and Byrareddy SN. Meningeal lymphatics in aging and Alzheimer’s disease. Ann Transl Med (2019) ; 7: : S2. |
49. | Kress BT , Iliff JJ , Xia M , et al. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol (2014) ; 76: : 845–861. |
50. | Li Q , Chen Y , Feng W , et al. Drainage of senescent astrocytes from brain via meningeal lymphatic routes. Brain Behav Immunity (2022) ; 103: : 85–96. |
51. | Chinta SJ , Woods G , Demaria M , et al. Cellular senescence is induced by the environmental neurotoxin paraquat and contributes to neuropathology linked to Parkinson’s disease. Cell Rep (2018) ; 22: : 930–940. |
52. | Gonzales M , Garbarino V , Kautz T , et al. Senolytic therapy in mild Alzheimer’s disease: A Phase 1 feasibility trial. Nat Med (2023) ; 29: : 2481–2488. |
53. | Arendash G and Cao C. Transcranial electromagnetic wave treatment: a fountain of healthy longevity? Int J Mol Sci (2023) ; 24: : 9652. |
54. | Jiang H , Wei H , Zhou Y , et al. Overview of the meningeal lymphatic vessels in aging and central nervous system disorders. Cell Biosci (2022) ; 12: : 202. |
55. | Song E , Mao T , Dong H , et al. VEGF-C-driven lymphatic drainage enables brain tumor immunosurveillance. Nature (2020) ; 577: : 689–694. |
56. | Grunewald M , Kumar S , Sharife H , et al. Counteracting age-related VEGFF signaling insufficiency promotes healthy aging and extends life span. Science (2021) ; 373: : eabc8479. |
57. | Li X , Qi L , Yang D , et al. Meningeal lymphatic vessels mediate neurotropic viral drainage from the central nervous system. Nat Neurosci (2022) ; 25: : 577–587. |




