Approaches for Increasing Cerebral Efflux of Amyloid-β in Experimental Systems
Abstract
Amyloid protein-β (Aβ) concentrations are increased in the brain in both early onset and late onset Alzheimer’s disease (AD). In early onset AD, cerebral Aβ production is increased and its clearance is decreased, while increased Aβ burden in late onset AD is due to impaired clearance. Aβ has been the focus of AD therapeutics since development of the amyloid hypothesis, but efforts to slow AD progression by lowering brain Aβ failed until phase 3 trials with the monoclonal antibodies lecanemab and donanemab. In addition to promoting phagocytic clearance of Aβ, antibodies lower cerebral Aβ by efflux of Aβ-antibody complexes across the capillary endothelia, dissolving Aβ aggregates, and a “peripheral sink” mechanism. Although the blood-brain barrier is the main route by which soluble Aβ leaves the brain (facilitated by low-density lipoprotein receptor-related protein-1 and ATP-binding cassette sub-family B member 1), Aβ can also be removed via the blood-cerebrospinal fluid barrier, glymphatic drainage, and intramural periarterial drainage. This review discusses experimental approaches to increase cerebral Aβ efflux via these mechanisms, clinical applications of these approaches, and findings in clinical trials with these approaches in patients with AD or mild cognitive impairment. Based on negative findings in clinical trials with previous approaches targeting monomeric Aβ, increasing the cerebral efflux of soluble Aβ is unlikely to slow AD progression if used as monotherapy. But if used as an adjunct to treatment with lecanemab or donanemab, this approach might allow greater slowing of AD progression than treatment with either antibody alone.
The hallmark neuropathological findings in Alzheimer’s disease (AD) are amyloid-β protein (Aβ), containing senile plaques (SPs), and tau protein, containing neurofibrillary tangles. The amyloid hypothesis [1, 2] suggested that deposition of insoluble Aβ as SPs initiated AD-type neurodegeneration, with tau pathology and neuronal loss developing downstream. Following reports of weak associations between SP counts and cognitive impairment in AD patients [3–5] and the finding that Aβ oligomers rather than fibrillar Aβ may be the most neurotoxic Aβ conformation [6, 7], the hypothesis was revised to suggest that Aβ oligomers may initiate AD pathology [8]. The amyloid hypothesis led to multiple approaches which attempted to slow AD’s clinical progression by lowering brain Aβ levels [9–13], most recently via administration of monoclonal anti-Aβ antibodies [14–16]. These approaches failed until clinical trials with the monoclonal anti-Aβ antibodies lecanemab [17, 18] and donanemab [19, 20]. A third monoclonal antibody, aducanumab, reduced brain levels of insoluble (PET-detectable) Aβ in a phase 1b trial [21] but its two phase 3 trials produced conflicting results regarding its ability to slow AD progression [22].
In phase 3 trials, lecanemab slowed AD’s clinical progression by 27% [18], while donanemab slowed disease progression by 35% for subjects with low/medium cerebral tau levels and by 22% when subjects with high tau levels were included in the analysis [20]. Although these effects were statistically significant (p < 0.001), questions were raised as to whether they were clinically meaningful [23–26]. The findings in the lecanemab and donanemab trials are encouraging, but additional approaches may be needed to further slow disease progression. More effective targeting of Aβ oligomers may be required; the effects of the antibodies on brain Aβ oligomer levels in AD subjects in the clinical trials are unknown because the PET radioligands currently used to detect cerebral Aβ bind only to insoluble Aβ [27]. Another option would be targeting of downstream neuropathological processes in addition to Aβ [28–30]. A third approach would be to combine the administration of anti-Aβ monoclonal antibodies with interventions to increase the efflux of soluble Aβ from the brain. In late-onset AD (LOAD), which accounts for 90–95% of AD cases [31], the increase in cerebral Aβ has been attributed to impaired removal of Aβ [32]. Aβ is cleared from the brain by enzymatic degradation, including the endosomal-lysosomal system, the ubiquitin-proteasome system, and autophagy [33], and soluble Aβ can leave the brain via the blood-brain barrier (BBB) [34], the blood-cerebrospinal fluid (CSF) barrier (BCSFB) [35], glymphatic (paravascular) drainage [36], and intramural periarterial (IPAD) drainage, also referred to as perivascular drainage [37–41]) (Table 1). This review discusses experimental approaches for increasing cerebral Aβ efflux, clinical applications of these approaches, and the effects of these approaches in clinical trials in which they have been evaluated with subjects with AD or mild cognitive impairment (MCI). Although most studies of cerebral Aβ efflux have used either monomeric Aβ or Aβ species whose levels of aggregation were unclear [42], a study in C57BL/6 mice comparing clearance of monomeric and low molecular weight oligomeric Aβ40 from brain interstitial fluid (ISF) to CSF suggested that efflux of these Aβ conformations from the brain may be via similar routes [42].
Table 1
Mechanisms of clearance of soluble Aβ from the brain
| Clearance mechanism | Structure/location | Alteration in AD | Comments |
| Blood-brain barrier (BBB) | Capillary endothelial cells separated by tight junctions, adherens junctions, and gap junctions | Aβ clearance decreased by 30% | LRP1 and ABCB1 are key transcytosis-related transporters of Aβ across BBB; paracellular transport of Aβ also occurs |
| Blood-cerebro-spinal fluid barrier (BCSFB) | Choroid epithelial cells, separated by tight junctions, adherens junctions, and desmo-somes; also arachnoid membrane and circumventricular organs | Impaired Aβ clearance may precede Aβ deposition in LOAD | Choroid plexus epithelial cells enclose stroma containing peripheral blood capillaries; LRP1 and ABCB1 are BCSFB efflux transporters |
| Glymphatic (paravascular) drainage | Paravascular space surrounding cerebral arteries and veins | Reduced in transgenic mouse models of AD; decreased AQP4 adjacent to AD cerebral vessels suggests decreased glymphatic drainage | Unclear if impaired glymphatic drainage contributes to increased Aβ burden in AD |
| Intramural periarterial drainage (IPAD) | Perivascular space within basement membrane of cerebral artery smooth muscle cells | Frequent presence of CAA in AD suggests reduced IPAD clearance of Aβ | Pattern of Aβ deposition in CAA is similar to pattern of Aβ drainage in IPAD |
ABCB1, ATP-binding cassette sub-family B member 1; AD, Alzheimer’s disease; AQP4, aquaporin-4; BBB, blood-brain barrier; BCSFB, blood-cerebrospinal fluid barrier; CAA, cerebral amyloid angiopathy; IPAD, intramural periarterial drainage; LOAD, late onset Alzheimer’s disease; LRP1, low density lipoprotein receptor-related protein 1.
BBB CLEARANCE OF Aβ
The BBB (Fig. 1) consists of capillary endothelial cells separated by tight junctions, adherens junctions, and gap junctions [43]. Because of its tight junctions the BBB is less permeable than peripheral blood vessels to solutes [44–46]. Adherens junctions are organized similarly to tight junctions and influence paracellular permeability to solutes [43, 47]. The BBB is supported by astrocytes, pericytes, and extracellular matrix components [48–50]. Cellular elements of the BBB are in contact with the basement membrane (produced by endothelial cells and pericytes), which encloses the pericytes and attaches astrocytic endfeet processes [51]. The basement membrane undergoes age-related alterations [52] including microvascular fibrosis [53], changes in proportions of collagen IV, agrin, and laminin [54], and lipid accumulation [51]. The percentage of Aβ cleared by the BBB was estimated by Qosa et al. to be 62% [55] and by Goulay et al. to be 85% [56], although Roberts et al. suggested that direct transport of Aβ across the human BBB may account for only 25% of its clearance from the brain [57]. Distinguishing between Aβ’s clearance via the BBB and its clearance by other pathways may be difficult because of technical limitations [58]. In AD, BBB clearance of Aβ is decreased by approximately 30% [59]. Impaired BBB clearance of Aβ is thought to play a role in the pathogenesis of cerebral amyloid angiopathy (CAA) as well as AD [60].
Fig. 1
The blood-brain barrier (BBB). The BBB consists of capillary endothelial cells surrounded by extracellular matrices formed by cellular basement membrane (shared with pericytes) and astrocytic endfeet. Tight junctions, adherens junctions, and gap junctions are present between the endothelial cells. The tight junctions limit passage of solutes between the cerebral microvasculature and brain parenchyma. Junctional proteins in tight junctions and adherens junctions are shown in the black box. Aβ can cross the BBB via transcytosis (membrane-bound carrier-mediated transport, facilitated by LRP1 and ABCB1) as well as via paracellular transport. (From: Engelhardt S, Patkar S, Ogunshola OO. Cell-specific blood-brain barrier regulation in health and disease: a focus on hypoxia. Br J Pharmacol. 2014 Mar;171(5):1210-30 [British Pharmacological Society; publisher: Wiley-Blackwell, Hoboken, NJ, USA]). Permission to use obtained via RightsLink.
![The blood-brain barrier (BBB). The BBB consists of capillary endothelial cells surrounded by extracellular matrices formed by cellular basement membrane (shared with pericytes) and astrocytic endfeet. Tight junctions, adherens junctions, and gap junctions are present between the endothelial cells. The tight junctions limit passage of solutes between the cerebral microvasculature and brain parenchyma. Junctional proteins in tight junctions and adherens junctions are shown in the black box. Aβ can cross the BBB via transcytosis (membrane-bound carrier-mediated transport, facilitated by LRP1 and ABCB1) as well as via paracellular transport. (From: Engelhardt S, Patkar S, Ogunshola OO. Cell-specific blood-brain barrier regulation in health and disease: a focus on hypoxia. Br J Pharmacol. 2014 Mar;171(5):1210-30 [British Pharmacological Society; publisher: Wiley-Blackwell, Hoboken, NJ, USA]). Permission to use obtained via RightsLink.](https://content.iospress.com:443/media/jad/2024/100-2/jad-100-2-jad240212/jad-100-jad240212-g001.jpg)
In addition to transcytosis (membrane-bound carrier-mediated transport) of Aβ across the BBB, paracellular transport of monomeric Aβ across the BBB through the BBB’s tight junctions has been described. Interestingly, Aβ was found to transiently downregulate the tight junction-associated proteins claudin-5 and occludin in the Tg2576 transgenic mouse model of AD, thus increasing its clearance across the BBB via this mechanism [61].
The length and conformation of Aβ influence its BBB clearance. Aβ42 is cleared via the BBB more slowly than Aβ40 [62] and BBB clearance of aggregated Aβ has been suggested to be less effective than clearance of monomeric Aβ [63]. (In the study by McIntee et al. discussed above [42] in which clearance of monomeric and low molecular weight oligomeric Aβ40 from ISF to CSF was examined, efflux was faster for monomers than for oligomers). The effects of oligomeric and fibrillar Aβ on cerebral microvascular endothelial cells may differ, because Aβ oligomers induce apoptosis of these cells while exposure of these cells to fibrillar Aβ increases BBB permeability but does not induce apoptosis [64]. Normal BBB functioning in the hippocampus may be required to maintain normal cognition, based on a study which found that hippocampal BBB damage was associated with early cognitive decline in human subjects irrespective of the presence or absence of AD-type pathology [65].
Low-density lipoprotein receptor-related protein 1 (LRP1) and ATP-binding cassette sub-family B member 1 (ABCB1, also known as P-glycoprotein 1) have been suggested to be key transporters in BBB clearance of cerebral Aβ [66, 67]. (However, Ito et al. [68] concluded that LRP1 was not important for clearance of Aβ40 across the mouse BBB). LRP1 is expressed on the abluminal surface of the BBB while ABCB1 is expressed on its luminal surface. The expression of these transporters on the BBB decreases during normal aging [69–71] and AD [72–75]. LRP1 and ABCB1 have been suggested to be functionally related via phosphatidylinositol-binding clathrin assembly protein (PICALM) [66], therefore in AD, PICALM downregulation by Aβ may contribute to the decreased BBB clearance of Aβ [76, 77]. Oxidation of LRP1 may also play a role in this decrease [78]. Because LRP1 binds other ligands including apolipoprotein E (apoE) and amyloid-β protein precursor (AβPP) [79] in addition to Aβ, therapeutic interventions to increase LRP1’s expression in AD could have unintended (and possibly deleterious) consequences [80] unless this upregulation is specific for LRP1’s binding to Aβ, as in the site-mutagenesis approach suggested by Sagare et al. [81].
ApoE also plays a role, possibly a negative one, in clearing Aβ across the BBB. This may be true to a greater extent for apoE4 than for apoE2 and apoE3 [82–85]. ApoE competes with Aβ for binding to LRP1 and for LRP1-mediated transport across the BBB [58], and apoE-Aβ complexes are cleared from the brain more slowly than unbound Aβ [86]. Of relevance is that increasing apoE’s lipidation increases its efficiency of binding to Aβ, and this decreases the ability of Aβ to aggregate [87]. The ABC transporter ABCA1 regulates lipidation of cerebral apoE [87, 88]. Another apolipoprotein, apolipoprotein J (apoJ, also known as clusterin), also influences Aβ’s BBB clearance by binding to it [89] and mediating its clearance via low-density lipoprotein-related protein 2 (LRP2) [62, 90]. LRP2 does not bind to Aβ but it does bind to Aβ-apoJ complexes [91]. Aβ’s binding to apoJ increases its BBB clearance [62, 92], and viral vector-mediated increased expression of apoJ in astrocytes led to decreased cortical and hippocampal Aβ levels in the APP/PS1 transgenic mouse model of AD [93]. ApoJ’s role in the pathogenesis of AD is unclear; although it increases BBB clearance of Aβ and limits Aβ aggregation and neurotoxicity [91, 94, 95], genome-wide association studies have found some single nucleotide polymorphisms of the CLU (clusterin) gene (which encodes for apoJ) to be associated with increased risk for AD [96, 97]. ApoJ concentrations are increased in AD hippocampus [98] possibly as a compensatory response to the increased levels of Aβ.
Approaches which have been used to improve BBB clearance of Aβ in experimental systems are shown in Table 2. Therapeutic increasing of Wnt/β-catenin signaling, which plays a role in maintaining BBB function [99, 100], has also been suggested as an approach [101, 102] but no specific activators of this mechanism are known to cross the BBB [102]. Wnt/β-catenin signaling is reduced in AD brain [103].
Table 2
Experimental approaches for increasing BBB clearance of Aβ
| ADAM10 inhibitiona [104] |
| Amylin expression reductionb [105] |
| Annexin A1c [106, 107] |
| Astaxanthin (antioxidant)d [108] |
| Catalpole [109] |
| Copper diacetyl bis(4-methyl-3-thiosemicarbazone)f [110] |
| Dihydropyridine L-type calcium channel blockersg [111] |
| 1α, 25-dihydroxyvitamin D3 h [112] |
| Endothelial progenitor cellsi [113] |
| Exercisej [114] |
| Kallikrein-8 inhibitionk [115] |
| LDLRl [116] |
| Mesenchymal stem cellsm [117] |
| MMP-9 inhibitionn [118] |
| Muscarinic acetylcholine receptor inhibition° [119] |
| Nedd4 knockdownp [120] |
| Olive leaf extractq [121] |
| PICALMr [77, 122] |
| PCSK9 inhibitions [123] |
| Somatostatint [124] |
| α-tocopherolu [125] |
ADAM10, A Disintegrin And Metalloproteinase Domain containing protein 10; LDLR, Low-density lipoprotein receptor; MMP-9, matrix metalloproteinase-9; Nedd4, Neuronal precursor cell-expressed developmentally downregulated 4; PICALM, phosphatidylinositol binding clathrin assembly protein); PCSK9, proprotein convertase subtilisin/kexin type 9). aADAM10 inhibition improved BBB-mediated clearance of Aβ in an in vitro BBB model by reducing proteolytic shedding of LRP1 at the endothelial cell surface [104]. bLowering of systemic amylin concentration by treatment with amylin-specific antisense microRNAs reversed amylin-induced lowering of LRP1 expression in an in vitro model of the BBB [105]. cAdministration of Annexin A1 restored normal BBB functioning in mice lacking Annexin A1 [106], and also in 5xFAD and Tau-P301 L mice [107]. dThe antioxidant astaxanthin was suggested as a possible therapy for increasing BBB clearance of Aβ because it increased in vitro expression of ABCA1, ABCG1, and LRP1 [108]. eIn an in vitro BBB model exposed to fibrillar Aβ42, catalpol increased the expression of LRP1, ABCB1, and tight junction proteins, decreased the expression of MMP-2, MMP-9, and the receptor for advanced glycation end products (RAGE), and enhanced the efflux of soluble Aβ [109]. fTreatment of mice with the PET imaging agent copper diacetyl bis(4-methyl-3-thiosemicarbazone) increased the expression of P-gp (ABCB1) in brain microvasculature [110]. gThe dihydropyridine L-type calcium channel blockers nilvadipine and nitrendipine lowered brain Aβ in Tg PS1/APPsw mice and increased Aβ clearance in an in vitro BBB model [111]. hIntraperitoneal administration of 1α,25-dihydroxyvitamin D3 increased 24-h clearance of 125I-labeled human Aβ40 from mouse brain [112]. iTransplantation of endothelial progenitor cells into the hippocampus of APP/PS1 mice upregulated tight junction proteins and promoted Aβ clearance [113]. jIn TgCRND8 mice, late running (five months of wheel running, started four months after disease onset) increased BBB clearance of Aβ [114]. kInhibiting the serine protease kallikrein-8 in a mouse transgenic model of AD increased BBB clearance of Aβ [115]. lIncreasing the expression of the apoE receptor LDLR (by developing LDLR transgenic mice) increased BBB-mediated clearance of 125I-Aβ. This was suggested to be mediated in part by LRP1 [116]. mInfusion of mesenchymal stem cells to spontaneously hypertensive rats resulted in remodeling of microvasculature, in part by activation of transforming growth factor-β and angiopoietin 1 signaling pathways [117]. nThe matrix metalloproteinase 9 (MMP9) inhibitor SB-3CT prevented lipoprotein receptor shedding in Aβ42-treated human brain microvascular endothelial cells, and increased Aβ clearance via the BBB in C57BL/6 mice expressing human apoE4 [118]. oPirenzepine, a selective muscarinic acetylcholine receptor inhibitor, increased BBB clearance of Aβ in AβPPPS1, hAβPPSL, and AβPP/PS1 mice [119]. pNEDD4-1 is a ubiquitin E3 ligase that ubiquitinates P-gp (ABCB1); siRNA-mediated knockdown of Nedd4 expression in CHO-APP cells increased their P-gp expression and their secretion of Aβ [120]. qAdministration of olive leaf extract to 5xFAD mice for three months improved BBB integrity and increased cerebral Aβ clearance [121]. rPICALM was found to regulate BBB transcytosis and clearance of Aβ by regulating, in endothelial cells, PICALM/clathrin-dependent internalization of Aβ bound to LRP1 and guiding of Aβ to the endosomal regulators Rab5 and Rab11. Increasing of PICALM expression in AD brain endothelial cells (by adenoviral-mediated transfer of PICALM) increased transcytosis of Aβ [77]. Treatment of PICALM-deficient 5XFAD mice with the anti-malaria drug artesunate increased cerebral capillary PICALM expression and prevented brain Aβ accumulation [122]. sPCSK9 binds to low-density lipoprotein receptor proteins, promoting their lysosomal degradation. Treatment of 5xFAD mice with anti-PCSK9 antibodies reduced cerebral Aβ [123]. tSomatostatin prevented Aβ-induced BBB permeability in human CMEC/D3 (human temporal lobe microvessel) cells through its effects (upregulation) on LRP1 and tight junction proteins [124]. uα-tocopherol upregulated LRP1 and ABCB1 in an in vitr o model of the BBB and in 5XFAD mice, reducing cortical and hippocampal Aβ by 75% and 59% respectively [125].
BCSFB CLEARANCE OF Aβ
The term BCSFB refers to the barrier posed by the tight junctions between choroid epithelial cells to passage of solutes between CSF and blood [126]. Although the choroid plexus (Fig. 2) is a major component of the BCSFB, the BCSFB also includes the arachnoid membrane and circumventricular organs [127]. The choroid plexus lines the lateral ventricles, third ventricle, and fourth ventricle, and is the main source of CSF [128, 129], secreting about 80% of CSF [130]. Its functions include transport of glucose and other nutrients from blood to CSF, reabsorption and elimination of brain metabolic waste products from CSF, synthesis of proteins including transthyretin (TTR), cytokines, growth factors, and neurotrophic factors, regulation of trafficking of immune cells into the brain, and maintenance of CNS homeostasis by modulation of solute exchange between CSF and brain parenchyma [129, 131–134]. Choroid plexus blood flow has been estimated to be 5-fold [135] or 10-fold [136] higher than in the cerebral vasculature. The choroid plexus includes an epithelial cell monolayer whose microvilli extend into the ventricles and whose basolateral side rests on basal lamina. These cells surround and enclose stroma containing peripheral blood capillaries [137, 138]. Dendritic cells, fibroblasts, macrophages, and lymphocytes are also present in the stroma [139, 140]. The surface area of the choroid plexus epithelial cell microvilli is approximately half of the surface area of the BBB [141]. Stromal capillaries in the BCSFB are fenestrated, unlike BBB capillaries, allowing free movement of molecules up to 800 kDa between stroma and peripheral blood [135]. However, tight junctions, adherens junctions, and desmosomes are present between the apical borders of the epithelial cells [127, 133]. The Aβ in CSF is thought to originate primarily from ISF draining into the ventricles [142]. Active transfer of solutes between CSF and peripheral blood occurs in both directions across the choroid plexus [143–145] although for Aβ, movement from CSF to peripheral blood predominates [145]. Permeability of Aβ at the choroid plexus is approximately 10-fold greater than its permeability in either direction of the BBB [146].
Fig. 2
The choroid plexus in the lateral ventricle. Epithelial cells of the choroid plexus rest on a basement membrane and contain microvilli projecting into the lumen of the lateral ventricle. Tight junctions are present between cells near their apical surfaces. (From: Kaur C, Rathnasamy G, Ling EA. The Choroid Plexus in Healthy and Diseased Brain. J Neuropathol Exp Neurol 2016;75(3):198–213; copyright Oxford University Press. Reproduced with permission of Oxford University Press).
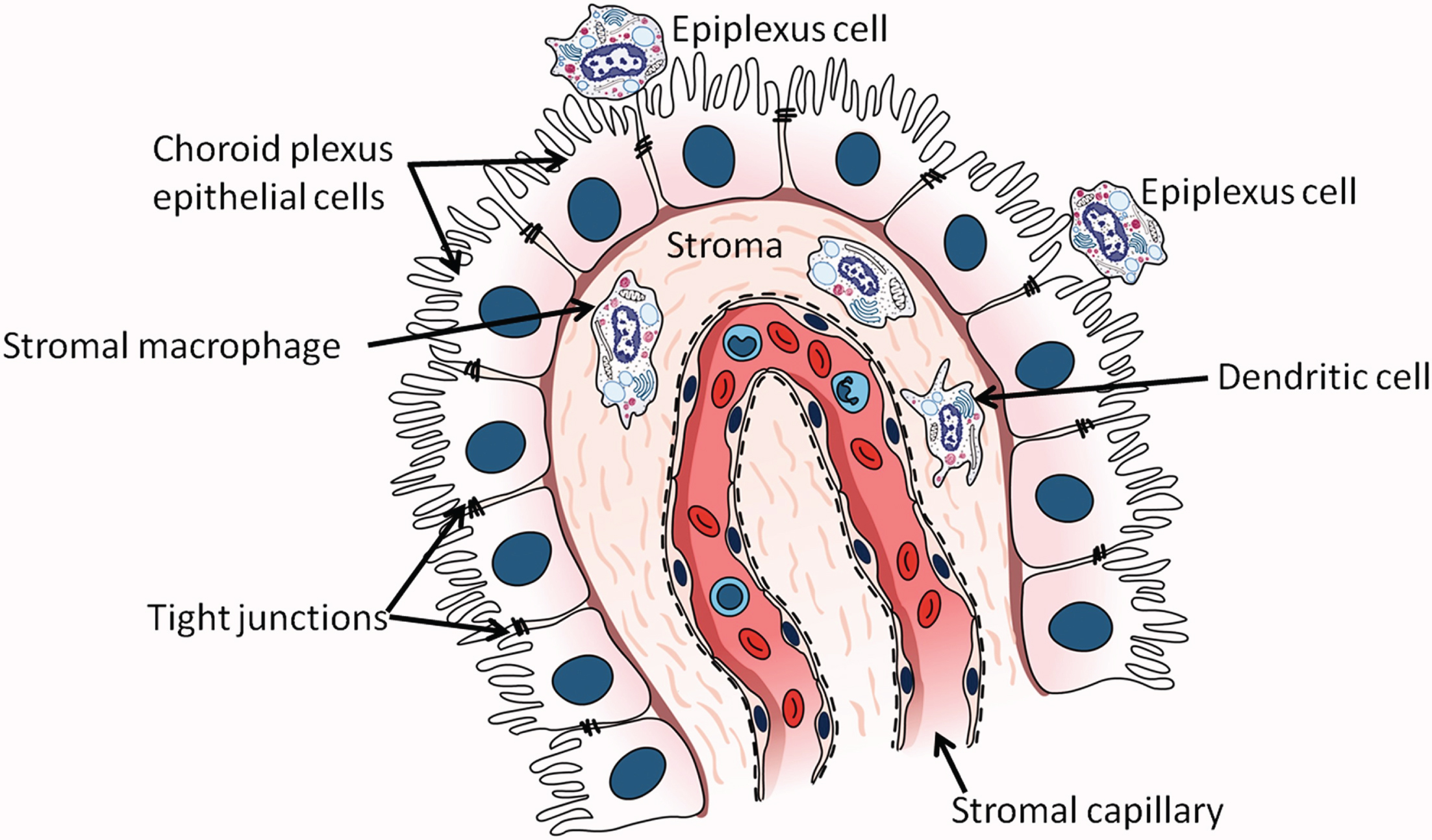
The BCSFB undergoes age-related structural and functional changes. Structural changes including loosening of tight junctions, thickening and flattening of epithelial cell basal membrane, deposition of fibrillar inclusions (Biondi bodies) and lipofuscin, and stromal thickening and calcification [139, 147, 148]. Functional changes include decreases in protein synthesis, ion transport, and clearance of CSF proteins [129, 130, 149–152]. BCSFB capillary permeability also decreases during normal aging [153, 154]. Conflicting results have been published as to whether CSF production is reduced with normal aging [150, 155].
The choroid plexus has an extensive capacity for taking up Aβ from CSF [145, 156]. Aβ transporters LRP1, ABCB1, LRP2, and RAGE are expressed on BCSFB epithelium [35, 138, 157]. A study of age-related changes in the expression of Aβ transporters on the rat choroid plexus found that LRP1 and ABCB1 increased, while LRP2 decreased and RAGE was unchanged [35]. LRP1 and ABCB1 are thought to function at the BCSFB as Aβ efflux transporters, while LRP2 mediates movement of Aβ in a bi-directional manner from both CSF and blood into the choroid plexus [35]. The role of RAGE with regard to Aβ transport at the BCSFB is unclear [35]; the authors of an in vitro study of Aβ uptake from artificial CSF by the rat choroid plexus concluded that RAGE did not appear to be involved in Aβ uptake into the choroid plexus [145]. The choroid plexus can also produce Aβ and can degrade it due to the presence of insulin degrading enzyme, neprilysin, and endothelin-converting enzyme-1 in choroidal epithelial cells [146, 156].
The age-related structural changes in the BCSFB described above are exacerbated in AD [158, 159]. Binding of immunoglobulins and C1q, the first component of the classical complement activation pathway, to choroid epithelial basement membrane has also been reported in AD brain [160]. Functional changes in the BCSFB which occur in AD include increases in pro-inflammatory gene expression [161] and in the expression of genes encoding for interferons, mammalian target of rapamycin (mTOR) signaling, peroxisome proliferator-activated receptors (PPARs), and acute phase proteins [162], decreased expression of genes encoding for ion transporters, tight junction proteins, and proteins involved in mitochondrial ATP synthesis, and reduced mitochondrial enzyme activity [163, 164]. Decreased BCSFB expression of vascular endothelial growth factor (VEGF) was reported in the APP/PS1 transgenic mouse model of AD [165]. Impaired functioning of the choroid plexus was suggested to be an early pathogenic event in LOAD, possibly preceding deposition of cerebral Aβ [132]. The relationship between BCSFB dysfunction and AD progression is unclear, with some studies concluding that changes in permeability of the BCSFB barrier did not correlate with AD severity [166, 167]. Aβ is deposited in the AD choroid plexus, perhaps due in part to its decreased degradation by choroid epithelial cells [141]. The presence of Aβ in the AD choroid plexus is associated with increased local levels of reactive oxygen species [164] which may induce apoptotic cell death [164, 168]. Aβ42 oligomers increase BCSFB permeability by activating MMPs [169].
CSF production decreases in AD [170, 171]. CSF turnover is also reduced by about 50% [172], which may contribute to the development of AD due to slower clearance of cerebral waste products [127]. The expression of Aβ transporters on the choroid plexus may be altered in AD although not necessarily decreased. A transcriptomic study found increased expression of LRP1, perhaps in compensation for reduced clearance of Aβ from cerebral capillaries [162], and a similar finding was reported in 3xTg-AD mice [142]. However, no differences were found for CSF LRP1 concentrations between AD patients and age-matched control subjects [173] although LRP2 concentrations were reported to be reduced in AD choroid plexus [174] and CSF [175]. No reports were found of changes in ABCB1 or RAGE levels on the BCSFB or in CSF in AD, although in 3xTg-AD mice immunoreactivity for RAGE on the choroid plexus was increased while TTR expression was decreased [142].
TTR requires further mention with regard to its expression and function on the AD choroid plexus. It is produced in the CNS primarily by the choroid plexus [176] while in the periphery it is produced mainly by the liver. In peripheral blood it binds to thyroxine and vitamin A [177, 178]. TTR expression is regulated by 17-beta-estradiol [179]. The level of TTR production by the choroid plexus reflects the health status of the BCSFB [143]. TTR was reported to be the major Aβ-binding protein in CSF [180]. Its possible neuroprotective role in AD was reviewed by Saponaro et al. [176] and Gião et al. [181]; TTR’s binding to Aβ prevents Aβ aggregation and inhibits Aβ-mediated neurotoxicity [182–186] and it may facilitate Aβ efflux from the brain via the BBB by interacting with LRP1 [180]. TTR may also participate in neuronal regeneration [187] and angiogenesis [188]. One study reported decreased TTR levels in AD CSF [189] but a later study found no evidence for altered choroid plexus production of TTR in AD [190]. TTR tetramers have been suggested to be unstable in AD, which could reduce their ability to bind Aβ [181].
The choroid plexus detects both peripheral and CNS inflammatory signals [133] and serves as a gateway for trafficking of immune cells into the CSF [191]. Tumor necrosis factor (TNF) has been suggested to be the main upstream inflammatory mediator in the AD choroid plexus, via its signaling through TNF receptor 1 (TNFR1) [126]. Systemic inflammation, a possible risk factor for AD [192–194], may increase BCSFB permeability. In the AppNL - G - F (APP knock-in) mouse model of AD, low-grade inflammation induced by intraperitoneal injections of lipopolysaccharide (LPS) reduced the immunoreactivity of tight junction proteins in choroid plexus epithelial cells [195]. An earlier study found that sepsis, induced by injection of a lethal dose of LPS, extensively damaged BCSFB permeability [196]. In vitro measurements in the study cited above involving low-grade LPS-induced peripheral inflammation [195] found that interleukin-1β, whose hippocampal concentration was increased in the APP knock-in mice after LPS administration, reduced the transport of Aβ across choroid plexus epithelial cells.
Multiple approaches have been suggested or explored in experimental systems for increasing BCSFB functioning in AD. These approaches are shown in Table 3.
Table 3
Experimental approaches for increasing BCSFB functioning
| Choroid plexus epithelial cellsa [197, 198] |
| CSF production enhancementb [159, 199, 200] |
| Environmental enrichmentc [201] |
| Foxp3(+) regulatory T cell depletiond [202] |
| Growth factor supplementatione [159] |
| Gut microbiotaf [203] |
| Ligustilide (upregulator of Klotho)g [204] |
| LRP2 h [174, 175] |
| Lycopenei [205, 206] |
| MMP inhibitionj [169] |
| Stem cell-derived thymic epithelial cellsk [207] |
| TNF signaling inhibitionl [126] |
| TTR tetramer stabilizationm [208] |
CSF, cerebrospinal fluid; LRP2, low density lipoprotein-related protein 2; MMP, Matrix metalloproteinase; TNF, tumor necrosis factor; TTR, transthyretin. aCerebral implantation of choroid plexus epithelial cells in APP/PS1 mice resulted in decreases in Aβ deposition, tau hyperphosphorylation, and astrocyte immunoreactivity [197]; implantation of microencapsuled choroid plexus epithelial cells into Aβ-treated rat brain decreased apoptosis and gliosis while increasing neurogenesis [198]. bJohanson et al. [159] and Wostyn et al. [200] suggested that therapeutic strategies stimulating increased CSF secretion by the choroid plexus should be tested; for example, chronic administration of caffeine to rats increased CSF production [199]. cThe choroid plexus secretes an anti-inflammatory microRNA, miR-146a, into CSF. 5XFAD mice provided with environmental enrichment increased hippocampal expression of miR-146a, and this was associated with downregulation of nuclear factor-kappa B [201]. dRegulatory T-cells (Treg) have been suggested to cause systemic immunosuppression in AD, inhibiting the entry of immune cells (monocyte-macrophages and T cells) into the CNS. Transient depletion of Foxp3 + Tregs in 5XFAD mice increased the expression of leukocyte trafficking molecules in choroid plexus and reduced SP counts in hippocampus (dentate gyrus) and cerebral cortex [202]. eThe choroid plexus synthesizes and secretes growth factors including FGF2 and TGFβ. Pharmacological manipulation of the choroid plexus (to modify the expression of choroid epithelial cell proteins) and/or supplementation with growth factors was suggested as a therapeutic approach for AD [159]. fMice lacking gut microbiota were found to have increased BCSFB permeability, which could be reversed with gut microbiota recolonization or short-chain fatty acid (SCFA) supplementation. Treatment of AppNL - G - F mice with SCFAs lowered their brain Aβ levels [203]. gKlotho is a multifunctional protein with membrane-bound, secreted, and intracellular forms. Its functions include regulation of oxidative stress, growth factor signaling, and ion homeostasis [209]. Treatment of 10-month-old senescence-accelerated mouse prone-8 (SAMP8) mice with the herbal compound ligustilide upregulated choroid plexus expression of Klotho and decreased memory deficits, Aβ42 accumulation, and tau phosphorylation [204]. hIncreasing of LRP2 expression on choroid plexus has been suggested as an approach for increasing BCSFB clearance of cerebral Aβ [174, 175]. iIn Aβ42-treated rats, the anti-oxidant lycopene blocked activation of NF-κB p65 and toll-like receptor 4 (TLR4) on the choroid plexus, inhibiting production of pro-inflammatory cytokines [205]. Similar results were found when administration of lycopene was combined with transplantation of human amniotic epithelial cells [206]. jOligomeric Aβ42, injected into C57BL/6 mouse cerebral ventricles, increased inflammatory gene expression and reduced BCSFB integrity. The BCSFB damage was prevented by co-administration into the cerebral ventricles of a broad-spectrum MMP inhibitor, GM6001 [169]. kMouse embryonic stem cells were induced in vitro to differentiate into thymic epithelial cell precursors, then transplanted into 3XTg-AD mice. This increased the expression of leukocyte homing and trafficking molecules in the choroid plexus [207]. lTNF was found by microarray analysis to be the main inflammatory mediator upstream of the choroid plexus in AD choroid plexus specimens. TNF signaling via TNFR1 was blocked in APP/PS1 mice by crossing the mice with TNFR1-deficient mice. This lack of TNF signaling decreased the expression of the inflammatory mediators IL6 and CXCL9 in the choroid plexus. [126]. mTTR is synthesized by the choroid plexus [210]. Increasing the expression of TTR in APP23 mice by crossing them with mice overexpressing human TTR resulted in normalization of the “APP23 behavioral phenotype” (as measured with Barnes maze testing) and lower hemi-brain levels of SDS-soluble and formic acid-soluble Aβ [208].
GLYMPHATIC (PARAVASCULAR) CLEARANCE OF Aβ
In 2012, Iliff et al. described a pathway for cerebral clearance of Aβ and other solutes via the paravascular space. They referred to this pathway as “glymphatic” in reference to glial-associated lymphatic drainage [211]. In the present review the glymphatic system is considered to be separate from intramural periarterial drainage, also known as perivascular drainage (discussed in the following section), although some investigators have suggested that the two systems may be the same pathway studied under different conditions [212, 213]. (See discussion of both systems by Bacyinski et al. [214]). The paravascular space, also known as the Virchow-Robin space, surrounds the cerebral vasculature (both arteries and veins), while the perivascular space is present within the middle layers of the basement membrane of arterial smooth muscle cells. A schematic drawing of the two systems is shown in Fig. 3. The use of the terms “paravascular” and “perivascular” is confusing, and glymphatic drainage is referred to as “perivascular” in some reviews [215–219]. The glymphatic system has been the subject of other recent reviews in addition to the one by Bacyinski et al.; see, for example, Jessen et al. [220], Bakker et al. [213], Benveniste et al. [221], Plog and Nedergaard [217], and Abbott et al. [222].
Fig. 3
The glymphatic and intramural periarterial (IPAD) systems. In the glymphatic (paravascular) system, indicated by the blue arrow, Aβ and other solutes in interstitial fluid are cleared from the brain via the paravascular (Virchow-Robin) space, which surrounds both cerebral arteries and veins. This is facilitated by the water channel protein aquaporin-4 which is present in the astrocytic endfoot processes lining the cerebral microvasculature. CSF in the glymphatic system enters the brain through para-arterial spaces, mixes with interstitial fluid, then leaves the brain via paravenous spaces. In the IPAD system, indicated by the green arrow, solutes (including Aβ) and interstitial fluid exit the brain via basement membranes of capillaries and arteries. Drainage of solutes via IPAD is in the opposite direction to that of cerebral blood flow. The pattern of drainage of solutes via IPAD is similar to the pattern of deposition of Aβ found in CAA, i.e., along cerebral arteries and capillaries. (From: Saito S, Yamamoto Y, Ihara M. Development of a Multicomponent Intervention to Prevent Alzheimer’s Disease. Front Neurol. 2019;10 : 490 [publisher: Frontiers Media S.A].) This figure is copyrighted by Drs. Saito, Yamamoto, and Ihara, who permitted its reproduction.
![The glymphatic and intramural periarterial (IPAD) systems. In the glymphatic (paravascular) system, indicated by the blue arrow, Aβ and other solutes in interstitial fluid are cleared from the brain via the paravascular (Virchow-Robin) space, which surrounds both cerebral arteries and veins. This is facilitated by the water channel protein aquaporin-4 which is present in the astrocytic endfoot processes lining the cerebral microvasculature. CSF in the glymphatic system enters the brain through para-arterial spaces, mixes with interstitial fluid, then leaves the brain via paravenous spaces. In the IPAD system, indicated by the green arrow, solutes (including Aβ) and interstitial fluid exit the brain via basement membranes of capillaries and arteries. Drainage of solutes via IPAD is in the opposite direction to that of cerebral blood flow. The pattern of drainage of solutes via IPAD is similar to the pattern of deposition of Aβ found in CAA, i.e., along cerebral arteries and capillaries. (From: Saito S, Yamamoto Y, Ihara M. Development of a Multicomponent Intervention to Prevent Alzheimer’s Disease. Front Neurol. 2019;10 : 490 [publisher: Frontiers Media S.A].) This figure is copyrighted by Drs. Saito, Yamamoto, and Ihara, who permitted its reproduction.](https://content.iospress.com:443/media/jad/2024/100-2/jad-100-2-jad240212/jad-100-jad240212-g003.jpg)
The finding in the initial study of the glymphatic pathway by Iliff et al. [211] that cerebral clearance of radiolabeled Aβ40, after its intrastrial injection, was decreased by 55% in mice lacking the water channel protein aquaporin-4 (AQP4) compared to its clearance in wildtype mice suggested that drainage via the paravascular space may play an important role in Aβ clearance from the brain. A more recent study in C57BL/6 mice found that inhibiting AQP4 function resulted in increased accumulation of Aβ40 around cerebral vessels [223]. AQP4 is present in the astrocytic endfoot processes lining the cerebral microvasculature [224] and is thought to facilitate movement of fluid through the glymphatic system [211, 225, 226]. Decreased AQP4 localization adjacent to cerebral vessels has been reported in AD [227]. In the glymphatic system, CSF enters the brain through para-arterial spaces [228] and after mixing with ISF [220] it leaves the brain through paravenous spaces [211, 217]. Drainage of CSF and its solutes occurs by multiple routes including cervical lymphatics, arachnoid granulations, the choroid plexus, peripheral blood, and possibly dural lymphatics [36, 171, 214, 217, 229–231]. Movement of CSF from the subarachnoid space into the brain, as well as efflux of ISF from the brain, has been suggested to be driven by bulk flow rather than diffusion [217, 228, 232–234] although this has been challenged by some investigators [218, 235–237]. The rate of fluid flow through the glymphatic system is thought to be regulated in part by pulsing of cerebral arteries, with reductions in arterial pulsatility due to cerebrovascular pathology possibly contributing to the decreased Aβ cerebral clearance in AD [36]. Vasomotion (low-frequency contractions and dilations of smooth muscle cells) has also been suggested to influence glymphatic flow [238]. Interestingly, sleep increases fluid flow through the glymphatic system by expanding the interstitial space (due to reduced noradrenergic tone), thereby reducing resistance to fluid movement [239].
In a study of elderly cognitively normal subjects, some genetic variants of AQP4 were found to modify the effects of sleep-related parameters on brain levels of Aβ [240], further supporting a role for the glymphatic system in clearance of brain Aβ. Glymphatic clearance of Aβ was decreased by 40% in old mice, with age-related lowering of perivascular AQP4 polarization and cerebral arterial pulsatility suggested to be contributing factors [241]. Reductions in glymphatic inflow (2-fold) and glymphatic clearance (1.2-fold) of intracisternally injected Aβ were found in the APP/PS1 mouse model of AD [242]. These decreases were attributed in part to disruption of glymphatic transport by Aβ oligomers, which co-localized with AQP4-expressing astrocytes and may have contributed to depolarization of AQP4 from astrocytic endfeet. Glymphatic transport of Aβ42 was less efficient than for Aβ40, possibly because of the greater propensity of Aβ42 to aggregate. Notably, reduction in glymphatic drainage of Aβ in APP/PS1 mice was detected in 3-4-month-old mice, prior to the development of extensive Aβ pathology. In another study in APP/PS1 mice, AQP4 knockout resulted in increased numbers of cortical and hippocampal plaques, as well as deposition of Aβ in cortical and leptomeningeal vessels [243]. In the tg-ArcSwe mouse model of AD, which develops perivascular as well as neuropil SPs, loss of astrocyte depolarization was found at sites of perivascular amyloid deposition [244]. Similar findings were reported in APP/PS1 mice [243]. Further, experimentally induced impairment of glymphatic drainage was found to increase Aβ deposition in APP/PS1 mice [245] and in 5xFAD and J20 mice [246]. These findings suggest that reduced glymphatic clearance of Aβ may contribute to the increased cerebral Aβ burden in AD. However, the feasibility of slowing the development of Aβ pathology by therapeutic increasing of glymphatic functioning has been questioned. Bakker et al. [213] noted poor correlation between the preferential deposition of Aβ along arteries and its clearance along veins, and Saito et al. [247] argued that Aβ rarely accumulates in the venous system, and that arterial Aβ accumulation is most prominent within the tunica media, not the paravascular space. van Veluw et al. [248], citing literature from studies in patients with CAA and animal models of CAA, noted that Aβ clearance is more likely to occur along arteries than veins.
Glymphatic drainage is decreased in experimental models of other CNS disorders in addition to AD including stroke [249], traumatic brain injury [250], and microinfarcts [251]. However, a review published in 2018 [217] noted that there had been no therapeutic efforts to improve glymphatic functioning in these disorders. The possibility has been suggested, with regard to idiopathic normal pressure hydrocephalus (in which glymphatic drainage is also compromised [252]), that improving sleep quality might reduce the development of this condition by improving glymphatic function [219].
Approaches to increase glymphatic drainage of Aβ have been investigated in experimental models. These approaches are shown in Table 4.
Table 4
Experimental approaches for increasing glymphatic drainage of cerebral Aβ. Although the number of experimental approaches for increasing glymphatic drainage of cerebral Aβ is less than for BBB and BCSFB clearance of cerebral Aβ, two of the approaches, melatonin and omega-3 polyunsaturated fatty acids, have been evaluated in AD clinical trials and are used to treat many other conditions
| AT1 receptor deficiencya [253] |
| 5-Caffeoylquinic acidb [254] |
| Electroacupuncturec [255] |
| Exercised [256, 257] |
| Melatonine [258] |
| NBPf [259, 260] |
| Omega-3 polyunsaturated fatty acidsg [261] |
| Photobiomodulationh [262] |
| Theta-burst stimulationi [263] |
| VEGF-Cj [246] |
AT1, angiotensin II type 1; NBP, L-3-n-butylphthalide; VEGF-C, vascular endothelial growth factor C. aTraumatic brain injury to mice was reported to decrease the polarized localization of AQP4 at endfoot processes of reactive astrocytes [264]. Deficiency in angiotensin II type 1 (AT1) receptors (induced by knockout of AT1 in C57BL/6 mice) inhibited AQP4 depolarization after traumatic brain injury, improving glymphatic drainage of Aβ [253]. bTreatment of APP/PS2 mice with 5-caffeoylquinic acid, also known as chlorogenic acid, resulted in upregulation of LRP1 and normalization of perivascular AQP4 polarization in the hippocampus, together with decreased hippocampal Aβ deposition [254]. cA study in SAMP8 mice subjected to electroacupuncture for 8 weeks suggested that this treatment might improve AQP4 polarity and glymphatic drainage [255]. dWheel running improved glymphatic clearance, including astrocytic AQP4 expression and polarization, in aged mice [256]. Similar findings were reported in 3-month-old but not 7-month-old APP/PS1 mice [257]. eAdministration of melatonin to Tg2576 mice increased drainage of cerebral Aβ into cervical and axillary lymph nodes. This was associated with decreased brain levels of oligomeric Aβ40 [258]. The melatonin-induced increase in lymphatic drainage of Aβ was suggested to be due to enhanced glymphatic clearance [265]. fNBP (L-3-n-Butylphthalide), an extract from Chinese celery, improved glymphatic clearance of cerebral Aβ in wild-type mice by increasing cerebral pulsation. Administration of NBP to APP/PS1 mice reduced Aβ deposition and parenchymal Aβ levels [260]. An earlier study with 3xTg-AD mice found that treatment with NBP decreased diffuse but not fibrillar plaques [259]. gCerebral clearance of Aβ in fat-1 transgenic mice was increased by treatment with omega-3 polyunsaturated fatty acids. This effect was abolished in AQP4-knockout mice. Omega-3 polyunsaturated fatty acids were found to inhibit astrocyte activation and to maintain AQP4 polarization after Aβ injection [261]. hA review of photobiomodulation therapy indicated that it promoted increased glymphatic clearance of cerebral Aβ via meningeal lymphatic vessels [262]. iContinuous theta-burst stimulation increased glymphatic fluid transport in APP/PS1 mice. This effect was attributed to improved AQP4 polarization [263]. jTreatment of old C57BL/6J mice with VEGF-C, delivered by adenoviral vector or transcranial injection, resulted in increased lymphatic vessel diameter and improved meningeal lymphatic drainage of a tracer into deep cervical lymph nodes. This effect could not be replicated in J20 or 5XFAD mice [246].
Melatonin is used to improve the onset, duration, and quality of sleep [266]. The possibility that it may increase glymphatic drainage deserves further mention. Sleep disturbances are common in AD patients [267] so glymphatic clearance of Aβ might be increased in these individuals if their quality of sleep could be improved [215]. Melatonin concentrations in CSF are decreased in AD patients and decrease further during progression of the disease [268]. Melatonin was suggested as a therapeutic approach for AD (discussed below) based on its effects in the APP/PS1 transgenic mouse model of AD [269].
INTRAMURAL PERIARTERIAL DRAINAGE (IPAD) CLEARANCE OF Aβ
In 1974 Cserr and Ostrach examined clearance of Blue Dextran from mouse brain after injecting the dye into the caudate nucleus [233]. They found that the dye was “transported away from the injection site by bulk flow of cerebral ISF, possibly along the course of cerebral blood vessels.” Weller, Carare, and colleagues explored this pathway further [270–272] and found that drainage of solutes from brain parenchyma occurred along basement membranes of capillaries and arteries, but no intracerebrally injected dye was detected in the walls of veins. In contrast to glymphatic drainage, the drainage pattern observed in these studies was in the opposite direction to that of cerebral blood flow. Studies in experimental animals suggested that solutes draining from the brain along blood vessel walls emptied into cervical lymph nodes [234] via dural lymphatic vessels [273]. An age-related impairment in this mechanism in mice was suggested to be due to decreased amplitude of arterial pulsations and/or changes in the composition of basement membranes [274, 275]. Clearance of Aβ40 from the brain via IPAD is approximately 6-fold slower than its LRP1-mediated clearance across the BBB [62]. IPAD was initially suggested to be driven by arterial pulsation, specifically the refractory or “reflection” wave that follows each main pulse wave [276], but this was challenged by later studies suggesting that the driving force behind IPAD may be vasomotion produced by spontaneous contraction and relaxation of smooth muscle cells [38, 41, 277]. Analysis of a computational model of IPAD led to the conclusion that drainage of solutes by both diffusion and bulk flow occurred in IPAD [278].
Studies in mice with intracerebrally injected Aβ have shown that its drainage follows the same pattern as tracer dyes [279]. A similar pattern of deposition of Aβ along cerebral arteries and capillaries is found in CAA, reflecting failure of Aβ to be cleared from these vessels [279–282]. Clinical sequelae of CAA include lobar intracerebral hemorrhages, cerebral microbleeds, hemorrhagic and ischemic stroke, cognitive impairment, and gait impairment [283–286]. CAA is present in up to 90% of AD patients [287–290] and is associated with more rapid progression of AD [291]. ApoE, particularly apoE4, may impair IPAD-mediated clearance of Aβ [292]. ApoE4-expressing astrocytes secrete more fibrinectin and less laminin than apoE3-expressing astrocytes, promoting aggregation of Aβ on cerebrovascular basement membranes [293]. In CAA, apoE co-localizes with Aβ [294–296].
Several approaches have been suggested or investigated in experimental systems for increasing IPAD clearance of Aβ. These are shown in Table 5.
Table 5
Experimental approaches for increasing IPAD clearance of Aβ
| Anticoagulantsa [297] |
| APOA-I knockoutb [298] |
| Chitinc [299] |
| Cilostazold [300] |
| Correction of ApoE4/Aβ/laminin interactionse [301] |
| Ergothioneinef [302] |
| Fasudil hydrochlorideg [303] |
| Ghrelinh [50, 304–306] |
| Lysyl oxidase inhibitioni [307] |
| Ponezumabj [308] |
| Taxifolink [309, 310] |
APOA-I, apolipoprotein A-I; ApoE4, apolipoprotein E4. aThe possibility was suggested that direct oral anticoagulants (DOAC) might mitigate hypoperfusion-enhanced neurodegenerative processes including reduced perivascular clearance of Aβ [311]. The DOAC dabigatran etexilate lowered cerebral Aβ in TgCRND8 mice [297]. bKnockout of the APOA-I gene, which encodes for apolipoprotein A-I (apoA-I), increased IPAD-mediated clearance of cerebral Aβ in Tg2576 mice, and decreased cerebrovascular and parenchymal levels of insoluble Aβ [298]. ApoA-I is the main structural protein of high-density lipoprotein and is present in CSF [312]. cIntracerebroventricular injection of chitin in TgCRND8 mice stimulated perivascular macrophages and reduced Aβ42-labeled cortical blood vessels [299]. dThe selective type 3 phosphodiesterase inhibitor cilostazol increased perivascular drainage (i.e., IPAD-mediated clearance) of Aβ1 - 40 in Tg-SwDI mice [300]. eApoE was found to co-localize with Aβ in basement membrane drainage pathways in arterial walls. The attachment of apoE/Aβ complexes to laminin in the basement membrane was weaker for apoE4/Aβ complexes than for apoE3/Aβ complexes, suggesting that perivascular clearance of apoE4/Aβ complexes may be less efficient than for other isoforms of apoE. Therapeutic correction for apoE4/Aβ/laminin interactions was suggested as a possible approach to increase the efficiency of Aβ clearance [301]. fTreatment with the antioxidant ergothioneine reduced the concentration of Aβ in neuroretinas of 5XFAD mice. Ergothioneine was suggested to increase Aβ clearance by blood-derived phagocytic macrophages and via perivascular drainage [302]. gFasudil hydrochloride is a selective Rho- associated, coiled-coil containing protein kinase (ROCK) inhibitor which stimulates the PI3K/Akt/eNOS pathway [313]. Similar to the effect of acetylcholine, stimulation of this pathway induces vasodilation by activating endothelial nitric oxide synthase, promoting synthesis of nitric oxide. Treatment with fasudil hydrochloride increased IPAD in the hippocampus of both control mice (C57BL/6) and mice previously treated with mu-saporin, an immunotoxin which targets cholinergic neurons [303]. hTreatment of 3xTg-AD mice with the gastrointestinal hormone ghrelin upregulated capillary microRNAs miR126 and 145 in hippocampus and cerebral cortex, and lowered cerebral Aβ oligomer concentrations. This reduction was suggested to be due to increased perivascular clearance of Aβ, possibly due to activated endothelium and increased pericyte coverage of capillaries. Decreased expression of RAGE may also have been a contributing factor [50]. Treatment of 5XFAD mice with the ghrelin agonist MK-0677 reduced Aβ deposition in the frontal cortex [305] but in another study no decrease in cerebral Aβ was found when ghrelin was administered to this transgenic mouse model of AD [306]. (See also: Moon et al., 2014 [304]). iLysyl oxidase participates in remodeling of extracellular matrix (ECM) by catalyzing crosslinking of ECM components including collagen IV, laminin, and fibronectin. Inhibition of lysyl oxidase was suggested to improve IPAD clearance of Aβ [307]. jChronic administration of the anti-Aβ monoclonal antibody Ponezumab, which binds a C-terminal epitope of Aβ40 (Aβ33 - 40) and is thought to promote “peripheral sink” Aβ clearance [314], to PSAPP mice reduced CAA-type pathology [308]. kTaxifolin is a plant flavonoid with anti-oxidant and anti-inflammatory effects [310]. Taxifolin treatment of a mouse model of CAA, Tg-SwDI mice, inhibited formation of oligomeric Aβ and reduced cerebrovascular Aβ immunoreactivity [309]. Taxifolin was suggested in that study to have increased Aβ clearance via IPAD and the BBB.
Enhancing the efflux mechanisms discussed in this review offers potential therapeutic options for lowering the concentrations of soluble Aβ conformations in the brain. Whether this approach would slow AD progression is unknown. Previous attempts to slow AD progression by lowering brain levels of soluble Aβ failed (with the exception of lecanemab, which preferentially binds to soluble aggregates (protofibrils) as discussed below); these include the monoclonal antibodies solanezumab [15] and ponezumab [314], both of which targeted monomeric Aβ, as well as BACE1 inhibitors [315] and γ-secretase inhibitors [316]. The failure of these approaches suggests that increasing cerebral efflux of Aβ is unlikely to slow disease progression if used as monotherapy. But if used as an adjunct to lecanemab or donanemab, this might allow greater slowing of AD progression than can be achieved by treatment with either antibody alone. Antibody binding to Aβ can promote its clearance from the brain by several mechanisms: (a) activation of microglia via binding of antibody Fc fragments to the microglial Fc receptor (FcR), thereby increasing phagocytic uptake (and subsequent proteolytic degradation) of Aβ [317] (although this is true for fibrillar Aβ, microglial uptake of soluble Aβ may be via fluid-phase macropinocytosis [318], although whether it occurs by phagocytosis or fluid-phase macropinocytosis is unclear [319]), (b) activation of the classical complement pathway by crosslinking C1q between adjacent Fc fragments of IgG molecules (bound, in this case, to Aβ) [320] (complement activation results in cleavage of C3 to generate C3b; when bound by microglial complement receptor CR1, C3b is cleaved to iC3b, which functions as an opsonin, promoting phagocytosis when bound to the microglial complement receptor CR3 [321], (c) binding of antibody-Aβ immune complexes to the BBB’s neonatal FcR, which facilitates their crossing the BBB [34], (d) antibody-mediated dissolving of fibrillar Aβ [322], and (e) a “peripheral sink” mechanism in which antibody binding to Aβ in peripheral blood alters the equilibrium between systemic and brain Aβ levels, inducing increased BBB-mediated efflux of Aβ from the brain [323]. (Some investigators have challenged the validity of the peripheral sink hypothesis [324, 325). Donanemab binds to a fibril-specific N-terminal epitope on pyroglutamate-modified Aβ 326], promoting microglial phagocytosis of Aβ [327]. Lecanemab binds large soluble Aβ aggregates (protofibrils) and has lower selectivity for fibrils [328]; the mechanism(s) by which it increases clearance of cerebral Aβ is less clear, although BBB clearance (via binding of lecanemab-Aβ complexes to the BBB’s neonatal FcR) might be involved.
A related question is whether therapeutic increasing of cerebral Aβ efflux would lower the risk of developing amyloid-related imaging abnormalities (ARIA) in subjects treated with lecanemab or donanemab. ARIA is indicated by detection, on MRI, of either edema and effusion (ARIA-E; vasogenic edema in the parenchyma or sulcal effusions in leptomeninges [329]) or bleeding, as indicated by microhemorrhages and/ hemosiderin deposition (ARIA-H). ARIA-E reflects extravasation of proteinaceous fluid while ARIA-H reflects extravasation of erythrocytes [330]. The incidence of ARIA increases when subjects are treated with monoclonal antibodies targeting Aβ’s N-terminal amino acids, and apoE4-positive subjects have an increased risk for developing ARIA when treated with these antibodies [331]. In lecanemab’s phase 3 trial, the incidence of ARIA-E was 12.6% for lecanemab-treated subjects and 1.7% for placebo-treated subjects [17] while in donanemab’s phase 3 trial ARIA-E was detected in 24.0% of donanemab-treated and 2.1% of placebo-treated subjects, and ARIA-H was found in 31.4% of donanemab-treated and 13.6% of placebo-treated subjects [20]. The mechanisms underlying ARIA are incompletely understood; it has been suggested to be related to a temporary increase in cerebrovascular Aβ due to antibody mobilization of Aβ from SPs, causing increased vascular fragility and permeability [332, 333]. Increased production of inflammatory cytokines as a consequence of antibody activation of microglia was also suggested to contribute to development of ARIA [334, 335]. Treatment-induced ARIA-E is generally asymptomatic and resolves without treatment [336], although it has been associated with confusion, visual disturbances, headache, and gait abnormalities [333]. Whether the cerebral microhemorrhages indicated by ARIA-H can influence cognitive performance is not clear [337]. Barakos et al. [338], discussing the development of ARIA in patients treated with anti-Aβ monoclonal antibodies, noted that “there may be a recovery of vessel wall integrity and an increase in effective perivascular drainage with continued treatment, and, therefore, some clinical trials have observed that the risk of ARIA is highest early in treatment and subsequently decreases in patients who continue dosing.” Therefore, approaches that increase IPAD (perivascular) - mediated efflux of Aβ (Table 5) might reduce the incidence of ARIA in patients treated with lecanemab or donanemab. As stated above, CAA is often present in AD [287–290]. MRI findings of ARIA-H are similar to those of CAA [330], so increasing IPAD-mediated clearance of cerebral Aβ might also reduce CAA-associated pathology in AD patients.
Some of the experimental approaches discussed above would be problematic in AD patients. For example, the use of viral vectors in human gene therapy trials poses substantial risks [339, 340], intracerebroventricular administration of therapeutic agents would be less desirable than systemically-administered agents, and the use of some anticoagulants has been associated with increased risk for intracerebral hemorrhage [341]. Of note with regard to the possibility of treating AD patients with anti-coagulants, cerebral microbleeding was reported in 29% of AD patients [337] so it would be a concern with this approach [342]. Nevertheless, most of the experimental approaches listed in Tables 2–5 have been used in clinical settings. Clinical applications found for the experimental approaches discussed above are shown in Tables 6–9.
Table 6
Clinical applications of experimental approaches for improving BBB clearance of cerebral Aβ. Clinical trials are identified by their ClinicalTrials.gov Identifier
| Approach | Clinical trials and/or clinical use |
| ADAM10 inhibition | Pediatric glioma [343] |
| Annexin A1 | None found |
| Catalpol | Colon cancer [344] |
| Dihydropyridine L-type | Mood stabilization [345], hypertension [calcium channel blockers [346, 347], bipolar disorder [348], transfusion-dependent thalassemia [349], schizophrenia [350] |
| 1α,25-dihydroxyvitamin D3 | Ischemic stroke [351], chronic renal disease/secondary hyperparathyroidism [352], acute myelogenous leukemia [353], prostate, breast, and colorectal cancer, melanoma, myelodysplasia [354], osteoporosis [355, 356], bone disease in patients with hepatic cirrhosis [357] |
| Endothelial progenitor cells | Ischemic heart disease, pulmonary arterial hypertension, decompensated liver cirrhosis [358], bone repair [359], diabetic patients with nonhealing ulcers [360], limb ischemia [361] |
| Exercise | Effects on cancer patients during and after treatment [362], effects on biomarkers in older adults after hospital discharge [363] |
| Extra-virgin olive oila | MCI [364] |
| Kallikrein-8 inhibition | Hereditary angioedema [365, 366] |
| LDLR | Familial hypercholesterolemia (NCT02651675) |
| Mesenchymal stem cells | Hematological disease, graft-versus-host disease, organ transplantation, diabetes, inflammatory diseases; hepatic, renal, pulmonary, cardiovascular, bone/cartilage, neurological, and autoimmune diseases [367] |
| MMP-9 inhibition | Ulcerative colitis [368], rheumatoid arthritis [369], gastric and gastroesophageal junction adenocarcinoma [370], macular degeneration (NCT04504123) |
| Muscarinic acetylcholine receptor inhibition | Depression [371], diabetic neuropathy (NCT03050827), myopia [372] |
| PICALM upregulationb | Malaria [373] |
| Somatostatinc | Polycystic kidney and liver disease [374], neuroendocrine tumors (NCT05701241), somatotropinomas, thyrotropinomas, gastroenteropancreatic neuroendocrine tumors; corticotropinomas, gonadotropinomas, prolactinomas; other endocrine diseases (congenital hyperinsulinism, Graves’ orbitopathy, diabetic retinopathy, diabetic macular edema), non-endocrine tumors (breast, colon, prostate, lung, and hepatocellular); digestive diseases (chronic refractory diarrhea, hepatorenal polycystosis, gastrointestinal hemorrhage, intestinal fistula) [375] |
| α-tocopherol | ADd [376, 377], nonalcoholic fatty liver |
| (NCT04801849); prevention studies: cancer, atherosclerosis, cataracts, age-related macular degeneration, oral squamous cell carcinoma, lung cancer [378] |
AD, Alzheimer’s disease; ADAM10, A disintegrin-like and metalloproteases 10; LDLR, Low-density lipoprotein receptor; MCI, mild cognitive impairment; MMP-9, matrix metalloproteinase-9; PICALM, phosphatidylinositol binding clathrin assembly protein). aSix-month treatment of MCI patients with extra-virgin olive oil (NCT03824197) reduced BBB permeability and improved clinical dementia rating (CDR) and behavioral scores [364]. bThe antimalarial drug artesunate increases PICALM expression at the BBB [122]. cSomatostatin is not used clinically because of its short half-life. Somatostatin analogs, which have longer half-lives, are used [375]. dIn clinical trials, Sano et al. [376] concluded that treatment with α-tocopherol slowed AD progression, and a similar conclusion was reached by Dysken et al. [377].
Table 7
Clinical applications of experimental approaches for improving BCSFB clearance of cerebral Aβ. Clinical trials are identified by their ClinicalTrials.gov Identifier
| Approach | Clinical trials and/or clinical use |
| Choroid plexus epithelial cells | None founda |
| CSF production enhancement | None foundb |
| Environmental enrichment | Stroke [379–381] |
| Foxp3(+) regulatory T cell depletion | Carcinoembryonic antigen-expressing malignancies [382], melanoma [383], T cell leukemia-lymphoma [384], esophageal cancer [385], T-cell lymphoma [386, 387] |
| Growth factor supplementation | Temporomandibular joint osteoarthritis (NCT00646763, NCT04731233), lower limb ischemia [388], tibial osteotomy [389], femoral head osteonecrosis [390], tibial shaft fractures [391], lower limb ischemia [388], periodontal regeneration [392], glucocorticoid-resistant acute hearing loss [393] |
| Ligustilidec | Menopause [394, 395] |
| LRP2 | None found. |
| Lycopene | Prostate cancer [396], cardiovascular disease [397], gum disease (NCT02263352), subarachnoid hemorrhage (NCT00905931), carotid atheroma (NCT01102504) |
| MMP inhibition | Refractory metastatic cancer (NCT00001683), wet age-related macular degeneration (NCT04504123), breast cancer [398], gastric cancer, diabetic foot ulcers, multiple sclerosis [399] |
| Stem cell-derived thymic epithelial cells | None found. |
| TNF signaling inhibition | Lung adenocarcinoma [400], melanoma (NCT03293784), ankylosing spondylitis [401], rheumatoid arthritis [402 (NCT00837434)], many other immune-mediated inflammatory diseases [403, 404] |
| TTR tetramer stabilization | Cardiac amyloidosis [405, 406], Parkinson’s disease [407], familial amyloid polyneuropathy [408] |
CSF, cerebrospinal fluid; Foxp3, forkhead box P3; LRP2, low-density lipoprotein receptor-related protein 2; MMP, matrix metalloproteinase; TNF, tumor necrosis factor; TTR, transthyretin. aNo clinical trials or human applications involving transplant of choroid plexus epithelial cells were found, although this approach continues to be investigated in experimental systems (see reviews by Jang and Lehtinen, 2022 [409], and Liu et al., 2022 [410]). bNo clinical trials or human applications were found in which the intent was to increase CSF production; however, the effects of caffeine, whose chronical consumption increased CSF production in rats [199], have been well studied (reviewed by Cappelletti et al., 2015 [411]). cLigustilide is the main active ingredient in the root of the herb Dong quai. Pure ligustilide has not been tested in humans due to its poor stability and bioavailability [412].
Table 8
Clinical applications of experimental approaches for improving glymphatic/paravascular clearance of cerebral Aβ. Note the large number of clinical applications of melatonin and omega-3 polyunsaturated fatty acids. Clinical trials are identified by their ClinicalTrials.gov Identifier
| 5-Caffeoylquinic acid | Menopause-induced dyslipidemia (NCT03019263), impaired glucose tolerance (NCT02621060), vascular function [413], non-alcoholic fatty liver in type 2 diabetes (NCT02929901), recurrent high-grade glioma [414] |
| L-3-n-Butylphthalide | ADa [415], subcortical vascular cognitive impairmentb [416], acute ischemic stroke [417, 418], amyotrophic lateral sclerosis [419], Parkinson’s disease-related dementia [420] |
| Melatonin | ADc [421], sleep disturbances [422], metabolic syndrome (NCT01038921), neuropathic pain [423], lung cancer [424], multiple sclerosis (NCT03498131), solid tumors [425, 426], migraine prevention [427], septic shock [428], ocular, hematological, gastrointestinal, and cardiovascular diseases, diabetes, rheumatoid arthritis, fibromyalgia, chronic fatigue syndrome, infectious diseases, neurological diseases, aging, depression, anesthesia, hemodialysis, in vitro fertilization, neonatal care [429] |
| Omega-3 polyunsaturated fatty acids (PUFA) | ADd [430–433], cardiovascular disease [434], prevention of coronary heart disease [435], COVID-19 [436], periodontitis (NCT04477395), Parkinson’s disease [437], attention deficit hyperactivity disorder [438], cancere [439], schizophrenia [440], chronic fatigue in relapsing-remitting multiple sclerosis [441], non-alcoholic fatty liver [442] |
| VEGF-C | Neovascular age-related macular degeneration [443, 444] |
AD, Alzheimer’s disease; ADAS-cog, Alzheimer’s Disease Assessment Scale; ADCS-ADL, Alzheimer’s Disease Cooperative Study – Activities of Daily Living; CDR-SB, Clinical Dementia Rating sum of boxes; CIBIC-Plus, Clinician’s Interview-Based Impression of Change Plus caregiver input; COVID-19, Coronavirus Disease of 2019; PUFA, polyunsaturated fatty acids; VEGF-C, vascular endothelial growth factor C. aWang et al. [415] treated patients with mild-to-moderate AD with dl-3-n-butylphthalide plus donepezil (n = 49) or donepezil alone (n = 43) for 48 weeks (ClinicalTrials.gov Identifier: NCT02711683). Changes in scores for Alzheimer’s Disease Assessment Scale (ADAS-cog) and Alzheimer’s Disease Cooperative Study - Activities of Daily Living (ADCS-ADL) were significantly different between the two treatment groups. Wang et al. concluded that the patients treated with dl-3-n-butylphthalide plus donepezil had slower cognitive decline and better performance of activities of daily living than the patients treated with donepezil alone. bJia et al. [416] conducted a randomized, double-blind, placebo-controlled trial in which patients with subcortical vascular cognitive impairment without dementia (n = 281) were treated for six months with dl-3-n-butylphthalide or placebo. Statistically significant differences (improved functioning) vs. placebo were found for ADAS-cog and Clinician’s Interview-Based Impression of Change Plus caregiver input (CIBIC-Plus) scores. cA meta-analysis of seven randomized controlled clinical trials in which AD patients were treated with melatonin found no improvement in measures of cognitive functioning [421]. dFreund-Levi et al. [430] conducted a randomized, double-blind, placebo-controlled clinical trial involving omega-3 supplementation of patients with mild-to-moderate AD. Statistically significant treatment effects were found for depressive symptoms in non-apoE4 carriers, and for symptoms of agitation in apoE4 carriers. Chiu et al. [431] performed a similar study in which subjects with mild-to-moderate AD or MCI were treated with omega-3 PUFA or placebo. Statistically significant improvement for treatment vs. placebo groups was found in ADAS-cog scores for the MCI patients. In contrast, a clinical trial by Quinn et al. [432] involving supplementation of mild-to-moderate AD patients with the omega-3 fatty acid docosahexaenoic acid found no statistically significant effects on ADAS-cog or Clinical Dementia Rating sum of boxes (CDR-SB) scores. Burckhardt et al. [433], in a systematic review, concluded that there was “no convincing evidence for the efficacy of omega-3 PUFA supplements in the treatment of mild to moderate AD.” eNabavi et al. [439] reviewed the effects of omega-3 therapy on breast cancer, colorectal cancer, leukemia, gastric cancer, pancreatic cancer, esophageal cancer, prostate cancer, lung cancer, head and neck cancer, and cancer-related cachexia.
Table 9
Clinical applications of experimental approaches for improving IPAD clearance of cerebral Aβ. Note the large number of clinical applications of cilostazol. Clinical trials are identified by their ClinicalTrials.gov Identifier
| Anticoagulants | Prevention of stroke in nonvalvular atrial fibrillation, prevention and treatment of venous thromboembolism [445], prevention of deep vein thrombosis after knee or hip replacement surgery [446] |
| Chitin | Tissue engineering, wound dressing, drug delivery, cancer diagnosis [447], cancer treatment (used as vehicle for drug delivery [448] |
| Cilostazol | MCIa [449], ADb [450], prevention of restenosis and repeat revascularization after percutaneous coronary intervention [451], prevention of vascular mortality and cardiovascular events in patients with stable intermittent claudication [452], ischemic leg ulcers, chronic arterial occlusion, prevention of recurrence of cerebral infarction, chronic atrial fibrillation with episodes of bradycardia [453], prevention of stroke recurrence (NCT00202020), fatty liver disease (NCT04761848), prevention of peripheral neuropathy (NCT05298696), lower extremity revascularization (NCT02374957), Reynaud’s syndrome (NCT00048776), recurrent stroke with intracranial artery stenosis (NCT00333164) |
| Correction of apoE4/Aβ/laminin interactions | Nothing found |
| MicroRNA targeting of cerebral vessels (with ghrelin agonists) | ADc [454], cachexia [455], obesity, gastrectomy, esophagectomy [456], sarcopenia [457], concussion (NCT04558346), malnutrition [458], alcoholism (NCT01779024) |
| Ponezumab | ADd [314], CAA [459] |
Aβ, amyloid β-protein; AD, Alzheimer’s disease; apoE4, apolipoprotein E4; CAA, cerebral amyloid angiopathy; MCI, mild cognitive impairment. aTaguchi et al. [449] performed a retrospective analysis to examine the effects of cilostazol on cognitive functioning. The analysis included all patients treated at their hospital with cilostazol. Changes in Mini-Mental State Examination (MMSE) scores over > 6 months were compared between subjects who continued to receive cilostazol and subjects who stopped cilostazol treatment. Patients taking acetylcholinesterase inhibitors were excluded. Slowing in the decrease in MMSE scores was found for MCI patients but not for non-cognitively impaired individuals or AD patients. bIhara et al. [450] also performed a retrospective study, comparing changes in MMSE scores over 28–30 months in patients with dementia (MMSE < 27; type of dementia not specified) between subjects treated with donepezil and cilostazol vs. those receiving donepezil alone. Subjects were subdivided into mild dementia (MMSE 22–26) and moderate-to-severe dementia (MMSE < 21). Slowing of the decrease in MMSE scores was found for the patients with mild dementia. Tai et al. [460] performed a small prospective study, comparing 12-month changes in MMSE scores in patients with “stable AD” between subjects taking cilostazol and acetylcholinesterase inhibitors and subjects not receiving cilostazol. The “cilostazol add-on” group was found to have less decrease in MMSE scores than the subjects not taking cilostazol, although no effect of cilostazol was found on CDR-SB scores. Cilostazol-associated slowing of cognitive decline was also reported in small pilot studies for patients with moderate AD [461] and patients with both AD and cerebrovascular disease [462]. In contrast, a 24-week prospective study by Lee et al. [463] found no significant differences in measures of cognition or global functioning between AD patients treated with cilostazol plus donepezil vs. those treated with donepezil alone, although cilostazol was suggested to slow the decrease in regional cerebral metabolism in the AD patients. cA double-blind clinical trial investigating the effects of the ghrelin agonist MK677 in patients with mild-to-moderate AD found no slowing of disease progression [454]. dA one-year clinical trial with Ponezumab in patients with mild-to-moderate AD found no effects on disease progression [314].
A few approaches for increasing cerebral Aβ efflux have been investigated for their effects on slowing of cognitive decline in AD and/or MCI patients. Clinical trials have suggested that α-tocopherol (Table 6) may slow AD progression [376, 377]. Treatment of AD patients with L-3-n-butylphthalide (Table 8) as an adjunct to donepezil was found to slow cognitive decline and loss of the ability to perform activities of daily living to a greater extent than treatment with donepezil alone [415]. A meta-analysis of findings in clinical trials of melatonin (Table 8) in AD patients found no evidence for slowing of cognitive decline [421] and a similar conclusion was reached for omega-3 PUFA (Table 8) in a systematic review [433]. Cilostazol (Table 9) was reported to slow AD-related cognitive decline in prospective studies [460–462] but another study found no effects of cilostazol on cognition or global functioning in AD patients [463]. A phase 2 trial with cilostazol in patients with MCI (NCT02491268) started in 2015 with an expected completion date of December 1, 2020 but results have not been posted on ClinicalTrials.gov. In a retrospective study, treatment with cilostazol was associated with slowing of cognitive decline in MCI patients but not AD patients [449] while in another retrospective study cilostazol slowed the decrease in MMSE scores in patients with mild dementia but not moderate-to-severe dementia (type of dementia not specified) [450]. No slowing of AD clinical progression was found in clinical trials involving the ghrelin agonist MK-677 [454] or the anti-Aβ monoclonal antibody Ponezumab (Table 9) [314].
CONCLUSIONS
Many approaches have been used in experimental systems to increase the efflux of Aβ from the brain. Most of these approaches are used to treat other conditions but few of them have been investigated for possible benefits in AD patients. Although lecanemab and donanemab slow the clinical progression of early-stage AD, there may be a ceiling to their ability to do so. Therapeutic interventions to increase Aβ efflux from the brain, if used as an adjunct to treatment with these antibodies, might allow further slowing of AD progression.
AUTHOR CONTRIBUTIONS
David Loeffler (Conceptualization; Writing – original draft; Writing – review & editing).
ACKNOWLEDGMENTS
The author has no acknowledgements to report.
FUNDING
Funding was provided by donations to the Beaumont Foundation to support Alzheimer’s research in the Beaumont Research Institute.
CONFLICT OF INTEREST
The author has no conflict of interest to report.
REFERENCES
[1] | Hardy J , Allsop D ((1991) ) Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol Sci 12: , 383–388. |
[2] | Hardy JA , Higgins GA ((1992) ) Alzheimer’s disease: The amyloid cascade hypothesis. Science 256: , 184–185. |
[3] | Lue LF , Kuo YM , Roher AE , Brachova L , Shen Y , Sue L , Beach T , Kurth JH , Rydel RE , Rogers J ((1999) ) Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer’s disease. Am J Pathol 155: , 853–862. |
[4] | McLean CA , Cherny RA , Fraser FW , Fuller SJ , Smith MJ , Beyreuther K , Bush AI , Masters CL ((1999) ) Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann Neurol 46: , 860–856. |
[5] | Lesné SE , Sherman MA , Grant M , Kuskowski M , Schneider JA , Bennett DA , Ashe KH ((2013) ) Brain amyloid-β oligomers in ageing and Alzheimer’s disease. Brain 136 (Pt 5): , 1383–1398. |
[6] | Kayed R , Head E , Thompson JL , McIntire TM , Milton SC , Cotman CW , Glabe CG ((2003) ) Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300: , 486–489. |
[7] | Lesné S , Koh MT , Kotilinek L , Kayed R , Glabe CG , Yang A , Gallagher M , Ashe KH ((2006) ) A specific amyloid-beta protein assembly in the brain impairs memory. Nature 440: , 352–357. |
[8] | Walsh DM , Selkoe DJ ((2007) ) A beta oligomers - a decade of discovery. J Neurochem 101: , 1172–1184. |
[9] | Gilman S , Koller M , Black RS , Jenkins L , Griffith SG , Fox NC , Eisner L , Kirby L , Rovira MB , Forette F , Orgogozo JM ; AN1792(QS-21)-201 Study Team ((2005) ) Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology 64: , 1553–1562. |
[10] | Aisen PS , Gauthier S , Ferris SH , Saumier D , Haine D , Garceau D , Duong A , Suhy J , Oh J , Lau WC , Sampalis J ((2011) ) Tramiprosate in mild-to-moderate Alzheimer’s disease - a randomized, double-blind, placebo-controlled, multi-centre study (the Alphase Study). Arch Med Sci 7: , 102–111. |
[11] | Moussa-Pacha NM , Abdin SM , Omar HA , Alniss H , Al-Tel TH ((2020) ) BACE1 inhibitors: Current status and future directions in treating Alzheimer’s disease. Med Res Rev 40: , 339–384. |
[12] | Green RC , Schneider LS , Amato DA , Beelen AP , Wilcock G , Swabb EA , Zavitz KH ; Tarenflurbil Phase 3 Study Group ((2009) ) Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: A randomized controlled trial. JAMA 302: , 2557–2564. |
[13] | Imbimbo BP , Panza F , Frisardi V , Solfrizzi V , D’Onofrio G , Logroscino G , Seripa D , Pilotto A ((2011) ) Therapeutic intervention for Alzheimer’s disease with γ-secretase inhibitors: Still a viable option? Expert Opin Investig Drugs 20: , 325–341. |
[14] | Panza F , Solfrizzi V , Imbimbo BP , Giannini M , Santamato A , Seripa D , Logroscino G ((2014) ) Efficacy and safety studies of gantenerumab in patients with Alzheimer’s disease. Expert Rev Neurother 14: , 973–986. |
[15] | Doody RS , Thomas RG , Farlow M , Iwatsubo T , Vellas B , Joffe S , Kieburtz K , Raman R , Sun X , Aisen PS , Siemers E , Liu-Seifert H , Mohs R ; Alzheimer’s Disease Cooperative Study Steering Committee; Solanezumab Study Group ((2014) ) Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N Engl J Med 370: , 311–321. |
[16] | Salloway S , Sperling R , Fox NC , Blennow K , Klunk W , Raskind M , Sabbagh M , Honig LS , Porsteinsson AP , Ferris S , Reichert M , Ketter N , Nejadnik B , Guenzler V , Miloslavsky M , Wang D , Lu Y , Lull J , Tudor IC , Liu E , Grundman M , Yuen E , Black R , Brashear HR ; Bapineuzumab 301 and 302 Clinical Trial Investigators ((2014) ) Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med 370: , 322–333. |
[17] | Swanson CJ , Zhang Y , Dhadda S , Wang J , Kaplow J , Lai RYK , Lannfelt L , Bradley H , Rabe M , Koyama A , Reyderman L , Berry DA , Berry S , Gordon R , Kramer LD , Cummings JL ((2021) ) A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer’s disease with lecanemab, an anti-Aβ protofibril antibody. Alzheimers Res Ther 13: , 80. |
[18] | van Dyck CH , Swanson CJ , Aisen P , Bateman RJ , Chen C , Gee M , Kanekiyo M , Li D , Reyderman L , Cohen S , Froelich L , Katayama S , Sabbagh M , Vellas B , Watson D , Dhadda S , Irizarry M , Kramer LD , Iwatsubo T ((2023) ) Lecanemab in early Alzheimer’s disease. N Engl J Med 388: , 9–21. |
[19] | Mintun MA , Lo AC , Duggan Evans C , Wessels AM , Ardayfio PA , Andersen SW , Shcherbinin S , Sparks J , Sims JR , Brys M , Apostolova LG , Salloway SP , Skovronsky DM ((2021) ) Donanemab in early Alzheimer’s disease. N Engl J Med 384: , 1691–1704. |
[20] | Sims JR , Zimmer JA , Evans CD , Lu M , Ardayfio P , Sparks J , Wessels AM , Shcherbinin S , Wang H , Monkul Nery ES , Collins EC , Solomon P , Salloway S , Apostolova LG , Hansson O , Ritchie C , Brooks DA , Mintun M , Skovronsky DM ; TRAILBLAZER-ALZ 2 Investigators ((2023) ) Donanemab in early symptomatic Alzheimer disease: The TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA 330: , 512–527. |
[21] | Sevigny J , Chiao P , Bussière T , Weinreb PH , Williams L , Maier M , Dunstan R , Salloway S , Chen T , Ling Y , O’Gorman J , Qian F , Arastu M , Li M , Chollate S , Brennan MS , Quintero-Monzon O , Scannevin RH , Arnold HM , Engber T , Rhodes K , Ferrero J , Hang Y , Mikulskis A , Grimm J , Hock C , Nitsch RM , Sandrock A ((2016) ) The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 537: , 50–56. |
[22] | Budd Haeberlein S , Aisen PS , Barkhof F , Chalkias S , Chen T , Cohen S , Dent G , Hansson O , Harrison K , von Hehn C , Iwatsubo T , Mallinckrodt C , Mummery CJ , Muralidharan KK , Nestorov I , Nisenbaum L , Rajagovindan R , Skordos L , Tian Y , van Dyck CH , Vellas B , Wu S , Zhu Y , Sandrock A ((2022) ) Two randomized phase 3 studies of aducanumab in early Alzheimer’s disease. J Prev Alzheimers Dis 9: , 197–210. |
[23] | Walsh S , Merrick R , Milne R , Brayne C ((2021) ) Aducanumab for Alzheimer’s disease? BMJ 374: , n1682. |
[24] | Alzforum, Donanemab Confirms: Clearing Plaques Slows Decline—By a Bit. March 19, 2021, accessed August 22, 2023. https://www.alzforum.org/news/conference-coverage/donanemab-confirms-clearing-plaques-slows-decline-bit |
[25] | Ebell MH , Barry HC ((2022) ) Why physicians should not prescribe aducanumab for Alzheimer disease. Am Fam Physician 105: , 353–354. |
[26] | Prillaman M ((2022) ) Alzheimer’s drug slows mental decline in trial - but is it a breakthrough? Nature 610: , 15–16. |
[27] | Fang XT , Hultqvist G , Meier SR , Antoni G , Sehlin D , Syvänen S ((2019) ) High detection sensitivity with antibody-based PET radioligand for amyloid beta in brain. Neuroimage 184: , 881–888. |
[28] | Musiek ES , Holtzman DM ((2015) ) Three dimensions of the amyloid hypothesis: Time, space and ‘wingmen’. Nat Neurosci 18: , 800–806. |
[29] | Lansdall CJ ((2014) ) An effective treatment for Alzheimer’s disease must consider both amyloid and tau. Biosci Horizons 7: , hzu002. |
[30] | Salloway SP , Sevingy J , Budur K , Pederson JT , DeMattos RB , Von Rosenstiel P , Paez A , Evans R , Weber CJ , Hendrix JA , Worley S , Bain LJ , Carrillo MC ((2020) ) Advancing combination therapy for Alzheimer’s disease. Alzheimers Dement (N Y) 6: , e12073. |
[31] | Zhang Q , Sidorenko J , Couvy-Duchesne B , Marioni RE , Wright MJ , Goate AM , Marcora E , Huang KL , Porter T , Laws SM ; Australian Imaging Biomarkers and Lifestyle (AIBL) Study; Sachdev PS, Mather KA , Armstrong NJ , Thalamuthu A , Brodaty H , Yengo L , Yang J , Wray NR , McRae AF , Visscher PM ((2020) ) Risk prediction of late-onset Alzheimer’s disease implies an oligogenic architecture. Nat Commun 11: , 4799. |
[32] | Mawuenyega KG , Sigurdson W , Ovod V , Munsell L , Kasten T , Morris JC , Yarasheski KE , Bateman RJ ((2010) ) Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science 330: , 1774. |
[33] | Ji XR , Cheng KC , Chen YR , Lin TY , Cheung CHA , Wu CL , Chiang HC ((2018) ) Dysfunction of different cellular degradation pathways contributes to specific β-amyloid42-induced pathologies. FASEB J 32: , 1375–1387. |
[34] | Deane R , Bell RD , Sagare A , Zlokovic BV ((2009) ) Clearance of amyloid-beta peptide across the blood-brain barrier: Implication for therapies in Alzheimer’s disease. CNS Neurol Disord Drug Targets 8: , 16–30. |
[35] | Pascale CL , Miller MC , Chiu C , Boylan M , Caralopoulos IN , Gonzalez L , Johanson CE , Silverberg GD ((2011) ) Amyloid-beta transporter expression at the blood-CSF barrier is age-dependent. Fluids Barriers CNS 8,: , 21. |
[36] | Iliff JJ , Wang M , Zeppenfeld DM , Venkataraman A , Plog BA , Liao Y , Deane R , Nedergaard M ((2013) ) Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J Neurosci 33: , 18190–18199. |
[37] | Weller RO , Subash M , Preston SD , Mazanti I , Carare RO ((2008) ) Perivascular drainage of amyloid-beta peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer’s disease. Brain Pathol 18: , 253–266. |
[38] | Diem AK , MacGregor Sharp M , Gatherer M , Bressloff NW , Carare RO , Richardson G ((2017) ) Arterial pulsations cannot drive intramural periarterial drainage: Significance for Aβ drainage. Front Neurosci 11: , 475. |
[39] | Diem AK , Carare RO , Weller RO , Bressloff NW ((2018) ) A control mechanism for intra-mural peri-arterial drainage via astrocytes: How neuronal activity could improve waste clearance from the brain. PLoS One 13: , e0205276. |
[40] | Albargothy NJ , Johnston DA , MacGregor-Sharp M , Weller RO , Verma A , Hawkes CA , Carare RO ((2018) ) Convective influx/glymphatic system: Tracers injected into the CSF enter and leave the brain along separate periarterial basement membrane pathways. Acta Neuropathol 136: , 139–152. |
[41] | Aldea R , Weller RO , Wilcock DM , Carare RO , Richardson G ((2019) ) Cerebrovascular smooth muscle cells as the drivers of intramural periarterial drainage of the brain. Front Aging Neurosci 11: , 1. |
[42] | McIntee FL , Giannoni P , Blais S , Sommer G , Neubert TA , Rostagno A , Ghiso J ((2016) ) In vivo differential brain clearance and catabolism of monomeric and oligomeric Alzheimer’s Aβ protein. Front Aging Neurosci 8: , 223. |
[43] | Stamatovic SM , Johnson AM , Keep RF , Andjelkovic AV ((2016) ) Junctional proteins of the blood-brain barrier: New insights into function and dysfunction. Tissue Barriers 4: , e1154641. |
[44] | Kniesel U , Wolburg H ((2000) ) Tight junctions of the blood-brain barrier. Cell Mol Neurobiol 20: , 57–76. |
[45] | Daneman R , Prat A ((2015) ) The blood-brain barrier. Cold Spring Harb Perspect Biol 7: , a020412. |
[46] | Lochhead JJ , Yang J , Ronaldson PT , Davis TP ((2020) ) Structure, function, and regulation of the blood-brain barrier tight junction in central nervous system disorders. Front Physiol 11: , 914. |
[47] | Bazzoni G , Dejana E ((2004) ) Endothelial cell-to-cell junctions: Molecular organization and role in vascular homeostasis. Physiol Rev 84: , 869–901. |
[48] | Obermeier B , Daneman R , Ransohoff RM ((2013) ) Development, maintenance and disruption of the blood-brain barrier. Nat Med 19: , 1584–1596. |
[49] | Yao Y , Chen ZL , Norris EH , Strickland S ((2014) ) Astrocytic laminin regulates pericyte differentiation and maintains blood brain barrier integrity. Nat Commun 5: , 3413. |
[50] | Fu L , Jiang G , Weng H , Dick GM , Chang Y , Kassab GS ((2020) ) Cerebrovascular miRNAs correlate with the clearance of Aβ through perivascular route in younger 3xTg-AD mice. Brain Pathol 30: , 92–105. |
[51] | Ceafalan LC , Fertig TE , Gheorghe TC , Hinescu ME , Popescu BO , Pahnke J , Gherghiceanu M ((2019) ) Age-related ultrastructural changes of the basement membrane in the mouse blood-brain barrier. J Cell Mol Med 23: , 819–827. |
[52] | Erdő F , Krajcsi P ((2019) ) Age-related functional and expressional changes in efflux pathways at the blood-brain barrier. Front Aging Neurosci 11: , 196. |
[53] | Farkas E , Luiten PG ((2001) ) Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog Neurobiol 64: , 575–611. |
[54] | Candiello J , Cole GJ , Halfter W ((2010) ) Age-dependent changes in the structure, composition and biophysical properties of a human basement membrane. Matrix Biol 29: , 402–410. |
[55] | Qosa H , Abuasal BS , Romero IA , Weksler B , Couraud PO , Keller JN , Kaddoumi A ((2014) ) Differences in amyloid-β clearance across mouse and human blood-brain barrier models: Kinetic analysis and mechanistic modeling. Neuropharmacology 79: , 668–678. |
[56] | Goulay R , Mena Romo L , Hol EM , Dijkhuizen RM ((2020) ) From stroke to dementia: A comprehensive review exposing tight interactions between stroke and amyloid-β formation. Transl Stroke Res 11: , 601–614. |
[57] | Roberts KF , Elbert DL , Kasten TP , Patterson BW , Sigurdson WC , Connors RE , Ovod V , Munsell LY , Mawuenyega KG , Miller-Thomas MM , Moran CJ , Cross DT 3rd , Derdeyn CP , Bateman RJ ((2014) ) Amyloid-β efflux from the central nervous system into the plasma. Ann Neurol 76: , 837–844. |
[58] | Kanekiyo T , Xu H , Bu G ((2014) ) ApoE and Aβ in Alzheimer’s disease: Accidental encounters or partners? Neuron 81: , 740–754. |
[59] | Krohn M , Lange C , Hofrichter J , Scheffler K , Stenzel J , Steffen J , Schumacher T , Brüning T , Plath AS , Alfen F , Schmidt A , Winter F , Rateitschak K , Wree A , Gsponer J , Walker LC , Pahnke J ((2011) ) Cerebral amyloid-β proteostasis is regulated by the membrane transport protein ABCC1 in mice. J Clin Invest 121: , 3924–3931. |
[60] | Gireud-Goss M , Mack AF , McCullough LD , Urayama A ((2020) ) Cerebral amyloid angiopathy and blood-brain barrier dysfunction. Neuroscientist 27: , 668–684. |
[61] | Keaney J , Walsh DM , O’Malley T , Hudson N , Crosbie DE , Loftus T , Sheehan F , McDaid J , Humphries MM , Callanan JJ , Brett FM , Farrell MA , Humphries P , Campbell M ((2015) ) Autoregulated paracellular clearance of amyloid-β across the blood-brain barrier. Sci Adv 1: , e1500472. |
[62] | Bell RD , Sagare AP , Friedman AE , Bedi GS , Holtzman DM , Deane R , Zlokovic BV ((2007) ) Transport pathways for clearance of human Alzheimer’s amyloid beta-peptide and apolipoproteins E and J in the mouse central nervous system. J Cereb Blood Flow Metab 27: , 909–918. |
[63] | Ito S , Ohtsuki S , Kamiie J , Nezu Y , Terasaki T ((2007) ) Cerebral clearance of human amyloid-beta peptide (1-40) across the blood-brain barrier is reduced by self-aggregation and formation of low-density lipoprotein receptor-related protein-1 ligand complexes. J Neurochem 103: , 2482–2490. |
[64] | Parodi-Rullán R , Ghiso J , Cabrera E , Rostagno A , Fossati S ((2020) ) Alzheimer’s amyloid β heterogeneous species differentially affect brain endothelial cell viability, blood-brain barrier integrity, and angiogenesis. Aging Cell 19: , e13258. |
[65] | Nation DA , Sweeney MD , Montagne A , Sagare AP , D’Orazio LM , Pachicano M , Sepehrband F , Nelson AR , Buennagel DP , Harrington MG , Benzinger TLS , Fagan AM , Ringman JM , Schneider LS , Morris JC , Chui HC , Law M , Toga AW , Zlokovic BV ((2019) ) Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med 25: , 270–276. |
[66] | Storck SE , Hartz AMS , Bernard J , Wolf A , Kachlmeier A , Mahringer A , Weggen S , Pahnke J , Pietrzik CU ((2018) ) The concerted amyloid-beta clearance of LRP1 and ABCB1/P-gp across the blood-brain barrier is linked by PICALM. Brain Behav Immun 73: , 21–33. |
[67] | Wang D , Chen F , Han Z , Yin Z , Ge X , Lei P ((2021) ) Relationship between amyloid-β deposition and blood-brain barrier dysfunction in Alzheimer’s disease. Front Cell Neurosci 15: , 695479. |
[68] | Ito S , Ueno T , Ohtsuki S , Terasaki T ((2010) ) Lack of brain-to-blood efflux transport activity of low-density lipoprotein receptor-related protein-1 (LRP-1) for amyloid-beta peptide(1-40) in mouse: Involvement of an LRP-1-independent pathway. J Neurochem 113: , 1356–1363. |
[69] | Shibata M , Yamada S , Kumar SR , Calero M , Bading J , Frangione B , Holtzman DM , Miller CA , Strickland DK , Ghiso J , Zlokovic BV ((2000) ) Clearance of Alzheimer’s amyloid-β(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest 106: , 1489–1499. |
[70] | Bartels AL , Kortekaas R , Bart J , Willemsen AT , de Klerk OL , de Vries JJ , van Oostrom JC , Leenders KL ((2009) ) Blood-brain barrier P-glycoprotein function decreases in specific brain regions with aging: A possible role in progressive neurodegeneration. Neurobiol Aging 30: , 1818–1824. |
[71] | Osgood D , Miller MC , Messier AA , Gonzalez L , Silverberg GD ((2017) ) Aging alters mRNA expression of amyloid transporter genes at the blood-brain barrier. Neurobiol Aging 57: , 178–185. |
[72] | Kang DE , Pietrzik CU , Baum L , Chevallier N , Merriam DE , Kounnas MZ , Wagner SL , Troncoso JC , Kawas CH , Katzman R , Koo EH ((2000) ) Modulation of amyloid beta-protein clearance and Alzheimer’s disease susceptibility by the LDL receptor-related protein pathway. J Clin Invest 106: , 1159–1166. |
[73] | Wijesuriya HC , Bullock JY , Faull RL , Hladky SB , Barrand MA ((2010) ) ABC efflux transporters in brain vasculature of Alzheimer’s subjects. Brain Res 1358: , 228–238. |
[74] | Shinohara M , Fujioka S , Murray ME , Wojtas A , Baker M , Rovelet-Lecrux A , Rademakers R , Das P , Parisi JE , Graff-Radford NR , Petersen RC , Dickson DW , Bu G ((2014) ) Regional distribution of synaptic markers and APP correlate with distinct clinicopathological features in sporadic and familial Alzheimer’s disease. Brain 137: , 1533–1549. |
[75] | Chiu C , Miller MC , Monahan R , Osgood DP , Stopa EG , Silverberg GD ((2015) ) P-glycoprotein expression and amyloid accumulation in human aging and Alzheimer’s disease: Preliminary observations. Neurobiol Aging 36: , 2475–2478. |
[76] | Baig S , Joseph SA , Tayler H , Abraham R , Owen MJ , Williams J , Kehoe PG , Love S ((2010) ) Distribution and expression of picalm in Alzheimer disease. J Neuropathol Exp Neurol 69: , 1071–1077. |
[77] | Zhao Z , Sagare AP , Ma Q , Halliday MR , Kong P , Kisler K , Winkler EA , Ramanathan A , Kanekiyo T , Bu G , Owens NC , Rege SV , Si G , Ahuja A , Zhu D , Miller CA , Schneider JA , Maeda M , Maeda T , Sugawara T , Ichida JK , Zlokovic BV ((2015) ) Central role for PICALM in amyloid-β blood-brain barrier transcytosis and clearance. Nat Neurosci 18: , 978–987. |
[78] | Owen JB , Sultana R , Aluise CD , Erickson MA , Price TO , Bu G , Banks WA , Butterfield DA ((2010) ) Oxidative modification to LDL receptor-related protein 1 in hippocampus from subjects with Alzheimer disease: Implications for Aβ accumulation in AD brain. Free Radic Biol Med 49: , 1798–1803. |
[79] | Zlokovic BV , Deane R , Sagare AP , Bell RD , Winkler EA ((2010) ) Low-density lipoprotein receptor-related protein-1: A serial clearance homeostatic mechanism controlling Alzheimer’s amyloid beta-peptide elimination from the brain. J Neurochem 115: , 1077–1089. |
[80] | Shinohara M , Tachibana M , Kanekiyo T , Bu G ((2017) ) Role of LRP1 in the pathogenesis of Alzheimer’s disease: Evidence from clinical and preclinical studies. J Lipid Res 58: , 1267–1281. |
[81] | Sagare AP , Deane R , Zlokovic BV ((2012) ) Low-density lipoprotein receptor-related protein 1: A physiological Aβ homeostatic mechanism with multiple therapeutic opportunities. Pharmacol Ther 136: , 94–105. |
[82] | Deane R , Sagare A , Hamm K , Parisi M , Lane S , Finn MB , Holtzman DM , Zlokovic BV ((2008) ) apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest 118: , 4002–4013. |
[83] | Castellano JM , Kim J , Stewart FR , Jiang H , DeMattos RB , Patterson BW , Fagan AM , Morris JC , Mawuenyega KG , Cruchaga C , Goate AM , Bales KR , Paul SM , Bateman RJ , Holtzman DM ((2011) ) Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci Transl Med 3: , 89ra57. |
[84] | Liu S , Breitbart A , Sun Y , Mehta PD , Boutajangout A , Scholtzova H , Wisniewski T ((2014) ) Blocking the apolipoprotein E/amyloid β interaction in triple transgenic mice ameliorates Alzheimer’s disease related amyloid β and tau pathology. J Neurochem 128: , 577–591. |
[85] | Ma Q , Zhao Z , Sagare AP , Wu Y , Wang M , Owens NC , Verghese PB , Herz J , Holtzman DM , Zlokovic BV ((2018) ) Blood-brain barrier-associated pericytes internalize and clear aggregated amyloid-β42 by LRP1-dependent apolipoprotein E isoform-specific mechanism. Mol Neurodegener 13: , 57. |
[86] | Bell RD , Zlokovic BV ((2009) ) Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer’s disease. Acta Neuropathol 118: , 103–113. |
[87] | Elali A , Rivest S ((2013) ) The role of ABCB1 and ABCA1 in beta-amyloid clearance at the neurovascular unit in Alzheimer’s disease. Front Physiol 4: , 45. |
[88] | Hirsch-Reinshagen V , Zhou S , Burgess BL , Bernier L , McIsaac SA , Chan JY , Tansley GH , Cohn JS , Hayden MR , Wellington CL ((2004) ) Deficiency of ABCA1 impairs apolipoprotein E metabolism in brain. J Biol Chem 279: , 41197–41207. |
[89] | Foster EM , Dangla-Valls A , Lovestone S , Ribe EM , Buckley NJ ((2019) ) Clusterin in Alzheimer’s disease: Mechanisms, genetics, and lessons from other pathologies. Front Neurosci 13: , 164. |
[90] | Nelson AR , Sagare AP , Zlokovic BV ((2017) ) Role of clusterin in the brain vascular clearance of amyloid-β. Proc Natl Acad Sci U S A 114: , 8681–8682. |
[91] | Hammad SM , Ranganathan S , Loukinova E , Twal WO , Argraves WS ((1997) ) Interaction of apolipoprotein J-amyloid β-peptide complex with low density lipoprotein receptor-related protein-2/megalin. A mechanism to prevent pathological accumulation of amyloid β-peptide. J Biol Chem 272: , 18644–18649. |
[92] | Wojtas AM , Kang SS , Olley BM , Gatherer M , Shinohara M , Lozano PA , Liu CC , Kurti A , Baker KE , Dickson DW , Yue M , Petrucelli L , Bu G , Carare RO , Fryer JD ((2017) ) Loss of clusterin shifts amyloid deposition to the cerebrovasculature via disruption of perivascular drainage pathways. Proc Natl Acad Sci U S A 114: , E6962–E6971. |
[93] | Wojtas AM , Sens JP , Kang SS , Baker KE , Berry TJ , Kurti A , Daughrity L , Jansen-West KR , Dickson DW , Petrucelli L , Bu G , Liu CC , Fryer JD ((2020) ) Astrocyte-derived clusterin suppresses amyloid formation in vivo. Mol Neurodegener 15: , 71. |
[94] | Yerbury JJ , Poon S , Meehan S , Thompson B , Kumita JR , Dobson CM , Wilson MR ((2007) ) The extracellular chaperone clusterin influences amyloid formation and toxicity by interacting with prefibrillar structures. FASEB J 21: , 2312–2322. |
[95] | Narayan P , Meehan S , Carver JA , Wilson MR , Dobson CM , Klenerman D ((2012) ) Amyloid-β oligomers are sequestered by both intracellular and extracellular chaperones. Biochemistry 51: , 9270–9276. |
[96] | Harold D , Abraham R , Hollingworth P , Sims R , Gerrish A , Hamshere ML , Pahwa JS , Moskvina V , Dowzell K , Williams A , Jones N , Thomas C , Stretton A , Morgan AR , Lovestone S , Powell J , Proitsi P , Lupton MK , Brayne C , Rubinsztein DC , Gill M , Lawlor B , Lynch A , Morgan K , Brown KS , Passmore PA , Craig D , McGuinness B , Todd S , Holmes C , Mann D , Smith AD , Love S , Kehoe PG , Hardy J , Mead S , Fox N , Rossor M , Collinge J , Maier W , Jessen F , Schürmann B , Heun R , van den Bussche H , Heuser I , Kornhuber J , Wiltfang J , Dichgans M , Frölich L , Hampel H , Hüll M , Rujescu D , Goate AM , Kauwe JS , Cruchaga C , Nowotny P , Morris JC , Mayo K , Sleegers K , Bettens K , Engelborghs S , De Deyn PP , Van Broeckhoven C , Livingston G , Bass NJ , Gurling H , McQuillin A , Gwilliam R , Deloukas P , Al-Chalabi A , Shaw CE , Tsolaki M , Singleton AB , Guerreiro R , Mühleisen TW , Nöthen MM , Moebus S , Jöckel KH , Klopp N , Wichmann HE , Carrasquillo MM , Pankratz VS , Younkin SG , Holmans PA , O’Donovan M , Owen MJ , Williams J ((2009) ) Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet 41: , 1088–1093. |
[97] | Lambert JC , Heath S , Even G , Campion D , Sleegers K , Hiltunen M , Combarros O , Zelenika D , Bullido MJ , Tavernier B , Letenneur L , Bettens K , Berr C , Pasquier F , Fiévet N , Barberger-Gateau P , Engelborghs S , De Deyn P , Mateo I , Franck A , Helisalmi S , Porcellini E , Hanon O ; European Alzheimer’s Disease Initiative Investigators; de Pancorbo MM, Lendon C , Dufouil C , Jaillard C , Leveillard T , Alvarez V , Bosco P , Mancuso M , Panza F , Nacmias B , Bossù P , Piccardi P , Annoni G , Seripa D , Galimberti D , Hannequin D , Licastro F , Soininen H , Ritchie K , Blanché H , Dartigues JF , Tzourio C , Gut I , Van Broeckhoven C , Alpérovitch A , Lathrop M , Amouyel P ((2009) ) Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet 41: , 1094–1099. |
[98] | May PC , Lampert-Etchells M , Johnson SA , Poirier J , Masters JN , Finch CE ((1990) ) Dynamics of gene expression for a hippocampal glycoprotein elevated in Alzheimer’s disease and in response to experimental lesions in rat. Neuron 5: , 831–839. |
[99] | Engelhardt B , Liebner S ((2014) ) Novel insights into the development and maintenance of the blood-brain barrier. Cell Tissue Res 355: , 687–699. |
[100] | Liebner S , Dijkhuizen RM , Reiss Y , Plate KH , Agalliu D , Constantin G ((2018) ) Functional morphology of the blood-brain barrier in health and disease. Acta Neuropathol 135: , 311–336. |
[101] | Liu L , Wan W , Xia S , Kalionis B , Li Y ((2014) ) Dysfunctional Wnt/β-catenin signaling contributes to blood-brain barrier breakdown in Alzheimer’s disease. Neurochem Int 75: , 19–25. |
[102] | Jia L , Piña-Crespo J , Li Y ((2019) ) Restoring Wnt/β-catenin signaling is a promising therapeutic strategy for Alzheimer’s disease. Mol Brain 12: , 104. |
[103] | Liu CC , Tsai CW , Deak F , Rogers J , Penuliar M , Sung YM , Maher JN , Fu Y , Li X , Xu H , Estus S , Hoe HS , Fryer JD , Kanekiyo T , Bu G ((2014) ) Deficiency in LRP6-mediated Wnt signaling contributes to synaptic abnormalities and amyloid pathology in Alzheimer’s disease. Neuron 84: , 63–77. |
[104] | Shackleton B , Crawford F , Bachmeier C ((2016) ) Inhibition of ADAM10 promotes the clearance of Aβ across the BBB by reducing LRP1 ectodomain shedding. Fluids Barriers CNS 13: , 14. |
[105] | Verma N , Velmurugan GV , Winford E , Coburn H , Kotiya D , Leibold N , Radulescu L , Despa S , Chen KC , Van Eldik LJ , Nelson PT , Wilcock DM , Jicha GA , Stowe AM , Goldstein LB , Powel DK , Walton JH , Navedo MF , Nystoriak MA , Murray AJ , Biessels GJ , Troakes C , Zetterberg H , Hardy J , Lashley T , Despa F ((2023) ) Aβ efflux impairment and inflammation linked to cerebrovascular accumulation of amyloid-forming amylin secreted from pancreas. Commun Biol 6: , 2. |
[106] | Cristante E , McArthur S , Mauro C , Maggioli E , Romero IA , Wylezinska-Arridge M , Couraud PO , Lopez-Tremoleda J , Christian HC , Weksler BB , Malaspina A , Solito E ((2013) ) Identification of an essential endogenous regulator of blood-brain barrier integrity, and its pathological and therapeutic implications. Proc Natl Acad Sci U S A 110: , 832–841. |
[107] | Ries M , Watts H , Mota BC , Lopez MY , Donat CK , Baxan N , Pickering JA , Chau TW , Semmler A , Gurung B , Aleksynas R , Abelleira-Hervas L , Iqbal SJ , Romero-Molina C , Hernandez-Mir G , d’Amati A , Reutelingsperger C , Goldfinger MH , Gentleman SM , Van Leuven F , Solito E , Sastre M ((2021) ) Annexin A1 restores cerebrovascular integrity concomitant with reduced amyloid-β and tau pathology. Brain 144: , 1526–1541. |
[108] | Babalola JA , Lang M , George M , Stracke A , Tam-Amersdorfer C , Itxaso I , Lucija D , Tadic J , Schilcher I , Loeffler T , Flunkert S , Prokesch M , Leitinger G , Lass A , Hutter-Paier B , Panzenboeck U , Hoefler G ((2023) ) Astaxanthin enhances autophagy, amyloid beta clearance and exerts anti-inflammatory effects in in vitro models of Alzheimer’s disease-related blood brain barrier dysfunction and inflammation. . Brain Res 1819: , 148518. |
[109] | Liu C , Chen K , Lu Y , Fang Z , Yu G ((2018) ) Catalpol provides a protective effect on fibrillary Aβ1-42 -induced barrier disruption in an in vitro model of the blood-brain barrier. Phytother Res 32: , 1047–1055. |
[110] | Pyun J , Koay H , Runwal P , Mawal C , Bush AI , Pan Y , Donnelly PS , Short JL , Nicolazzo JA ((2023) ) Cu(ATSM) increases P-glycoprotein expression and function at the blood-brain barrier in C57BL6/J mice. Pharmaceutics 15: , 2084. |
[111] | Paris D , Bachmeier C , Patel N , Quadros A , Volmar CH , Laporte V , Ganey J , Beaulieu-Abdelahad D , Ait-Ghezala G , Crawford F , Mullan MJ ((2011) ) Selective antihypertensive dihydropyridines lower Aβ accumulation by targeting both the production and the clearance of Aβ across the blood-brain barrier. Mol Med 17: , 149–162. |
[112] | Ito S , Ohtsuki S , Nezu Y , Koitabashi Y , Murata S , Terasaki T ((2011) ) 1α,25-Dihydroxyvitamin D3 enhances cerebral clearance of human amyloid-β peptide(1-40) from mouse brain across the blood-brain barrier. Fluids Barriers CNS 8: , 20. |
[113] | Zhang S , Zhi Y , Li F , Huang S , Gao H , Han Z , Ge X , Li D , Chen F , Kong X , Lei P ((2018) ) Transplantation of in vitro cultured endothelial progenitor cells repairs the blood-brain barrier and improves cognitive function of APP/PS1 transgenic AD mice. J Neurol Sci 387: , 6–15. |
[114] | Herring A , Münster Y , Metzdorf J , Bolczek B , Krüssel S , Krieter D , Yavuz I , Karim F , Roggendorf C , Stang A , Wang Y , Hermann DM , Teuber-Hanselmann S , Keyvani K ((2016) ) Late running is not too late against Alzheimer’s pathology. Neurobiol Dis 94: , 44–54. |
[115] | Herring A , Münster Y , Akkaya T , Moghaddam S , Deinsberger K , Meyer J , Zahel J , Sanchez-Mendoza E , Wang Y , Hermann DM , Arzberger T , Teuber-Hanselmann S , Keyvani K ((2016) ) Kallikrein-8 inhibition attenuates Alzheimer’s disease pathology in mice. Alzheimers Dement 12: , 1273–1287. |
[116] | Castellano JM , Deane R , Gottesdiener AJ , Verghese PB , Stewart FR , West T , Paoletti AC , Kasper TR , DeMattos RB , Zlokovic BV , Holtzman DM ((2012) ) Low-density lipoprotein receptor overexpression enhances the rate of brain-to-blood Aβ clearance in a mouse model of β-amyloidosis. Proc Natl Acad Sci U S A 109: , 15502–15507. |
[117] | Nakazaki M , Sasaki M , Kataoka-Sasaki Y , Oka S , Suzuki J , Sasaki Y , Nagahama H , Hashi K , Kocsis JD , Honmou O ((2019) ) Intravenous infusion of mesenchymal stem cells improves impaired cognitive function in a cerebral small vessel disease model. Neuroscience 408: , 361–377. |
[118] | Shackleton B , Ringland C , Abdullah L , Mullan M , Crawford F , Bachmeier C ((2019) ) Influence of matrix metallopeptidase 9 on beta-amyloid elimination across the blood-brain barrier. Mol Neurobiol 56: , 8296–8305. |
[119] | Paganetti P , Antoniello K , Devraj K , Toni N , Kieran D , Madani R , Pihlgren M , Adolfsson O , Froestl W , Schrattenholz A , Liebner S , Havas D , Windisch M , Cirrito JR , Pfeifer A , Muhs A ((2014) ) Increased efflux of amyloid-β peptides through the blood-brain barrier by muscarinic acetylcholine receptor inhibition reduces pathological phenotypes in mouse models of brain amyloidosis. J Alzheimers Dis 38: , 767–786. |
[120] | Chai AB , Callaghan R , Gelissen IC ((2022) ) The ubiquitin E3 ligase Nedd4 regulates the expression and amyloid-β peptide export activity of P-glycoprotein. Int J Mol Sci 23: , 1019. |
[121] | Abdallah IM , Al-Shami KM , Yang E , Wang J , Guillaume C , Kaddoumi A ((2022) ) Oleuropein-rich olive leaf extract attenuates neuroinflammation in the Alzheimer’s disease mouse model. ACS Chem Neurosci 13: , 1002–1013. |
[122] | Kisler K , Sagare AP , Lazic D , Bazzi S , Lawson E , Hsu CJ , Wang Y , Ramanathan A , Nelson AR , Zhao Z , Zlokovic BV ((2023) ) Anti-malaria drug artesunate prevents development of amyloid-β pathology in mice by upregulating PICALM at the blood-brain barrier. Mol Neurodegener 18: , 7. |
[123] | Mazura AD , Ohler A , Storck SE , Kurtyka M , Scharfenberg F , Weggen S , Becker-Pauly C , Pietrzik CU ((2022) ) PCSK9 acts as a key regulator of Aβ clearance across the blood-brain barrier. Cell Mol Life Sci 79: , 212. |
[124] | Paik S , Somvanshi RK , Kumar U ((2019) ) Somatostatin maintains permeability and integrity of blood-brain barrier in β-amyloid induced toxicity. Mol Neurobiol 56: , 292–306. |
[125] | Elfakhri KH , Duong QV , Langley C , Depaula A , Mousa YM , Lebeouf T , Cain C , Kaddoumi A ((2018) ) Characterization of hit compounds identified from high-throughput screening for their effect on blood-brain barrier integrity and amyloid-β clearance: In vitro and in vivo studies. Neuroscience 379: , 269–280. |
[126] | Steeland S , Gorlé N , Vandendriessche C , Balusu S , Brkic M , Van Cauwenberghe C , Van Imschoot G , Van Wonterghem E , De Rycke R , Kremer A , Lippens S , Stopa E , Johanson CE , Libert C , Vandenbroucke RE ((2018) ) Counteracting the effects of TNF receptor-1 has therapeutic potential in Alzheimer’s disease. EMBO Mol Med 10: , e8300. |
[127] | Damkier HH , Brown PD , Praetorius J ((2013) ) Cerebrospinal fluid secretion by the choroid plexus. Physiol Rev 93: , 1847–1892. |
[128] | Speake T , Whitwell C , Kajita H , Majid A , Brown PD ((2001) ) Mechanisms of CSF secretion by the choroid plexus. Microsc Res Tech 52: , 49–59. |
[129] | Serot JM , Béné MC , Faure GC ((2003) ) Choroid plexus, aging of the brain, and Alzheimer’s disease. Front Biosci 8: , s515–s521. |
[130] | Redzic ZB , Preston JE , Duncan JA , Chodobski A , Szmydynger-Chodobska J ((2005) ) The choroid plexus-cerebrospinal fluid system: From development to aging. Curr Top Dev Biol 71: , 1–52. |
[131] | Spuch C , Carro E ((2011) ) The p75 neurotrophin receptor localization in blood-CSF barrier: Expression in choroid plexus epithelium. BMC Neurosci 12: , 39. |
[132] | Krzyzanowska A , Carro E ((2012) ) Pathological alteration in the choroid plexus of Alzheimer’s disease: Implication for new therapy approaches. Front Pharmacol 3: , 75. |
[133] | Raha-Chowdhury R , Henderson JW , Raha AA , Vuono R , Bickerton A , Jones E , Fincham R , Allinson K , Holland A , Zaman SH ((2019) ) Choroid plexus acts as gatekeeper for TREM2, abnormal accumulation of ApoE, and fibrillary tau in Alzheimer’s disease and in Down syndrome dementia. J Alzheimers Dis 69: , 91–109. |
[134] | Verheggen ICM , Freeze WM , de Jong JJA , Jansen JFA , Postma AA , van Boxtel MPJ , Verhey FRJ , Backes WH ((2021) ) Application of contrast-enhanced magnetic resonance imaging in the assessment of blood-cerebrospinal fluid barrier integrity. Neurosci Biobehav Rev 127: , 171–183. |
[135] | Hubert V , Chauveau F , Dumot C , Ong E , Berner LP , Canet-Soulas E , Ghersi-Egea JF , Wiart M ((2019) ) Clinical imaging of choroid plexus in health and in brain disorders: A mini-review. Front Mol Neurosci 12: , 34. |
[136] | Keep RF , Jones HC ((1990) ) A morphometric study on the development of the lateral ventricle choroid plexus, choroid plexus capillaries and ventricular ependyma in the rat. Brain Res 56: , 47–53. |
[137] | Strazielle N , Ghersi-Egea JF ((2000) ) Choroid plexus in the central nervous system: Biology and physiopathology. J Neuropathol Exp Neurol 59: , 561–574. |
[138] | Kaur C , Rathnasamy G , Ling EA ((2016) ) The choroid plexus in healthy and diseased brain. J Neuropathol Exp Neurol 75: , 198–213. |
[139] | Marques F , Sousa JC , Sousa N , Palha JA ((2013) ) Blood-brain-barriers in aging and in Alzheimer’s disease. Mol Neurodegener 8: , 38. |
[140] | Ghersi-Egea JF , Strazielle N , Catala M , Silva-Vargas V , Doetsch F , Engelhardt B ((2018) ) Molecular anatomy and functions of the choroidal blood-cerebrospinal fluid barrier in health and disease. Acta Neuropathol 135: , 337–361. |
[141] | Balusu S , Brkic M , Libert C , Vandenbroucke RE ((2016) ) The choroid plexus-cerebrospinal fluid interface in Alzheimer’s disease: More than just a barrier. Neural Regen Res 11: , 534–537. |
[142] | González-Marrero I , Giménez-Llort L , Johanson CE , Carmona-Calero EM , Castañeyra-Ruiz L , Brito-Armas JM , Castañeyra-Perdomo A , Castro-Fuentes R ((2015) ) Choroid plexus dysfunction impairs beta-amyloid clearance in a triple transgenic mouse model of Alzheimer’s disease. Front Cell Neurosci 9: , 17. |
[143] | Segal MB ((2000) ) The choroid plexuses and the barriers between the blood and the cerebrospinal fluid. Cell Mol Neurobiol 20: , 183–196. |
[144] | Monro OR , Mackic JB , Yamada S , Segal MB , Ghiso J , Maurer C , Calero M , Frangione B , Zlokovic BV ((2002) ) Substitution at codon 22 reduces clearance of Alzheimer’s amyloid-beta peptide from the cerebrospinal fluid and prevents its transport from the central nervous system into blood. Neurobiol Aging 23: , 405–412. |
[145] | Crossgrove JS , Li GJ , Zheng W ((2005) ) The choroid plexus removes beta-amyloid from brain cerebrospinal fluid. Exp Biol Med (Maywood) 230: , 771–776. |
[146] | Crossgrove JS , Smith EL , Zheng W ((2007) ) Macromolecules involved in production and metabolism of beta-amyloid at the brain barriers. Brain Res 1138: , 187–195. |
[147] | Wen GY , Wisniewski HM , Kascsak RJ ((1999) ) Biondi ring tangles in the choroid plexus of Alzheimer’s disease and normal aging brains: A quantitative study. Brain Res 832: , 40–46. |
[148] | Serot JM , Foliguet B , Béné MC , Faure GC ((2001) ) Choroid plexus and ageing in rats: A morphometric and ultrastructural study. Eur J Neurosci 14: , 794–798. |
[149] | Ferrante F , Amenta F ((1987) ) Enzyme histochemistry of the choroid plexus in old rats. Mech Ageing Dev 41: , 65–72. |
[150] | May C , Kaye JA , Atack JR , Schapiro MB , Friedland RP , Rapoport SI ((1990) ) Cerebrospinal fluid production is reduced in healthy aging. Neurology 8: , 500–503. |
[151] | Kvitnitskaia-Ryzhova TI , Shkapenko AL ((1992) ) A comparative ultracytochemical and biochemical study of the ATPases of the choroid plexus in aging. Tsitologiia 34: , 81–87. |
[152] | Preston JE ((2001) ) Ageing choroid plexus-cerebrospinal fluid system. Microsc Res Tech 52: , 31–37. |
[153] | Chen RL , Kassem NA , Redzic ZB , Chen CP , Segal MB , Preston JE ((2009) ) Age-related changes in choroid plexus and blood-cerebrospinal fluid barrier function in the sheep. Exp Gerontol 44: , 289–96. |
[154] | Bouzerar R , Chaarani B , Gondry-Jouet C , Zmudka J , Balédent O ((2013) ) Measurement of choroid plexus perfusion using dynamic susceptibility MR imaging: Capillary permeability and age-related changes. Neuroradiology 55: , 1447–1454. |
[155] | Gideon P , Thomsen C , Ståhlberg F , Henriksen O ((1994) ) Cerebrospinal fluid production and dynamics in normal aging: A MRI phase-mapping study. Acta Neurol Scand 89: , 362–366. |
[156] | Behl M , Zhang Y , Zheng W ((2009) ) Involvement of insulin-degrading enzyme in the clearance of beta-amyloid at the blood-CSF barrier: Consequences of lead exposure. Cerebrospinal Fluid Res 6: , 11. |
[157] | Maślińska D , Laure-Kamionowska M , Taraszewska A , Deręgowski K , Maśliński S ((2011) ) Immunodistribution of amyloid beta protein (Aβ) and advanced glycation end-product receptors (RAGE) in choroid plexus and ependyma of resuscitated patients. Folia Neuropathol 49: , 295–300. |
[158] | Serot JM , Béné MC , Foliguet B , Faure GC ((2000) ) Morphological alterations of the choroid plexus in late-onset Alzheimer’s disease. Acta Neuropathol 99: , 105–108. |
[159] | Johanson C , McMillan P , Tavares R , Spangenberger A , Duncan J , Silverberg G , Stopa E ((2004) ) Homeostatic capabilities of the choroid plexus epithelium in Alzheimer’s disease. Cerebrospinal Fluid Res 1: , 3. |
[160] | Serot JM , Bene MC , Faure GC ((1994) ) Comparative immunohistochemical characteristics of human choroid plexus in vascular and Alzheimer’s dementia. Hum Pathol 25: , 1185–1190. |
[161] | Kant S , Stopa EG , Johanson CE , Baird A , Silverberg GD ((2018) ) Choroid plexus genes for CSF production and brain homeostasis are altered in Alzheimer’s disease. Fluids Barriers CNS 15: , 34. |
[162] | Stopa EG , Tanis KQ , Miller MC , Nikonova EV , Podtelezhnikov AA , Finney EM , Stone DJ , Camargo LM , Parker L , Verma A , Baird A , Donahue JE , Torabi T , Eliceiri BP , Silverberg GD , Johanson CE ((2018) ) Comparative transcriptomics of choroid plexus in Alzheimer’s disease, frontotemporal dementia and Huntington’s disease: Implications for CSF homeostasis. Fluids Barriers CNS 15: , 18. |
[163] | Cottrell DA , Blakely EL , Johnson MA , Ince PG , Turnbull DM ((2001) ) Mitochondrial enzyme-deficient hippocampal neurons and choroidal cells in AD. Neurology 57: , 260–264. |
[164] | Vargas T , Ugalde C , Spuch C , Antequera D , Morán MJ , Martín MA , Ferrer I , Bermejo-Pareja F , Carro E ((2010) ) Abeta accumulation in choroid plexus is associated with mitochondrial-induced apoptosis. Neurobiol Aging 31: , 1569–1581. |
[165] | Spuch C , Antequera D , Portero A , Orive G , Hernández RM , Molina JA , Bermejo-Pareja F , Pedraz JL , Carro E ((2010) ) The effect of encapsulated VEGF-secreting cells on brain amyloid load and behavioral impairment in a mouse model of Alzheimer’s disease. Biomaterials 31: , 5608–5618. |
[166] | Frölich L , Kornhuber J , Ihl R , Fritze J , Maurer K , Riederer P ((1991) ) Integrity of the blood-CSF barrier in dementia of Alzheimer type: CSF/serum ratios of albumin and IgG. Eur Arch Psychiatry Clin Neurosci 240: , 363–366. |
[167] | Hampel H , Müller-Spahn F , Berger C , Haberl A , Ackenheil M , Hock C ((1995) ) Evidence of blood-cerebrospinal fluid-barrier impairment in a subgroup of patients with dementia of the Alzheimer type and major depression: A possible indicator for immunoactivation. Dementia 6: , 348–354. |
[168] | Perez-Gracia E , Blanco R , Carmona M , Carro E , Ferrer I ((2009) ) Oxidative stress damage and oxidative stress responses in the choroid plexus in Alzheimer’s disease. Acta Neuropathol 118: , 497–504. |
[169] | Brkic M , Balusu S , Van Wonterghem E , Gorlé N , Benilova I , Kremer A , Van Hove I , Moons L , De Strooper B , Kanazir S , Libert C , Vandenbroucke RE ((2015) ) Amyloid β oligomers disrupt blood-CSF barrier integrity by activating matrix metalloproteinases. J Neurosci 35: , 12766–12778. |
[170] | Silverberg GD , Heit G , Huhn S , Jaffe RA , Chang SD , Bronte-Stewart H , Rubenstein E , Possin K , Saul TA ((2001) ) The cerebrospinal fluid production rate is reduced in dementia of the Alzheimer’s type. Neurology 57: , 1763–1766. |
[171] | Johanson CE , Duncan JA 3rd , Klinge PM , Brinker T , Stopa EG , Silverberg GD ((2008) ) Multiplicity of cerebrospinal fluid functions: New challenges in health and disease. Cerebrospinal Fluid Res 5: , 10. |
[172] | Silverberg GD , Mayo M , Saul T , Rubenstein E , McGuire D ((2003) ) Alzheimer’s disease, normal-pressure hydrocephalus, and senescent changes in CSF circulatory physiology: A hypothesis. Lancet Neurol 2: , 506–511. |
[173] | Liu Q , Zhang J , Tran H , Verbeek MM , Reiss K , Estus S , Bu G ((2009) ) LRP1 shedding in human brain: Roles of ADAM10 and ADAM17. Mol Neurodegener 4: , 17. |
[174] | Alvira-Botero X , Carro EM ((2010) ) Clearance of amyloid-β peptide across the choroid plexus in Alzheimer’s disease. Curr Aging Sci 3: , 219–229. |
[175] | Spuch C , Antequera D , Pascual C , Abilleira S , Blanco M , Moreno-Carretero MJ , Romero-López J , Ishida T , Molina JA , Villarejo A , Bermejo-Pareja F , Carro E ((2015) ) Soluble megalin is reduced in cerebrospinal fluid samples of Alzheimer’s disease patients. Front Cell Neurosci 9: , 134. |
[176] | Saponaro F , Kim JH , Chiellini G ((2020) ) Transthyretin stabilization: An emerging strategy for the treatment of Alzheimer’s disease? Int J Mol Sci 21: , 8672. |
[177] | Ingbar SH ((1958) ) Pre-albumin: A thyroxinebinding protein of human plasma. Endocrinology 63: , 256–259. |
[178] | Raz A , Goodman DS ((1969) ) The interaction of thyroxine with human plasma prealbumin and with the prealbumin-retinol-binding protein complex. J Biol Chem 244: , 3230–3237. |
[179] | Quintela T , Gonçalves I , Baltazar G , Alves CH , Saraiva MJ , Santos CR ((2009) ) 17beta-estradiol induces transthyretin expression in murine choroid plexus via an oestrogen receptor dependent pathway. Cell Mol Neurobiol 29: , 475–483. |
[180] | Alemi M , Gaiteiro C , Ribeiro CA , Santos LM , Gomes JR , Oliveira SM , Couraud PO , Weksler B , Romero I , Saraiva MJ , Cardoso I ((2016) ) Transthyretin participates in beta-amyloid transport from the brain to the liver–involvement of the low-density lipoprotein receptor-related protein 1? Sci Rep 6: , 20164. |
[181] | Gião T , Saavedra J , Cotrina E , Quintana J , Llop J , Arsequell G , Cardoso I ((2020) ) Undiscovered roles for transthyretin: From a transporter protein to a new therapeutic target for Alzheimer’s Disease. Int J Mol Sci 21: , 2075. |
[182] | Schwarzman AL , Gregori L , Vitek MP , Lyubski S , Strittmatter WJ , Enghilde JJ , Bhasin R , Silverman J , Weisgraber KH , Coyle PK , Zagorski MG , Talafous J , Eisenberg M , Saunders AM , Roses AD , Goldgaber D ((1994) ) Transthyretin sequesters amyloid beta protein and prevents amyloid formation. Proc Natl Acad Sci U S A 91: , 8368–8372. |
[183] | Schwarzman AL , Goldgaber D ((1996) ) Interaction of transthyretin with amyloid beta-protein: Binding and inhibition of amyloid formation. Ciba Found Symp 199: , 146–160. |
[184] | Tang YP , Haslam SZ , Conrad SE , Sisk CL ((2004) ) Estrogen increases brain expression of the mRNA encoding transthyretin, an amyloid beta scavenger protein. J Alzheimers Dis 6: , 413–420. |
[185] | Du J , Murphy RM ((2010) ) Characterization of the interaction of β-amyloid with transthyretin monomers and tetramers. Biochemistry 49: , 8276–8289. |
[186] | Li X , Zhang X , Ladiwala AR , Du D , Yadav JK , Tessier PM , Wright PE , Kelly JW , Buxbaum JN ((2013) ) Mechanisms of transthyretin inhibition of β-amyloid aggregation in vitro. J Neurosci 33: , 19423–19433. |
[187] | Fleming CE , Saraiva MJ , Sousa MM ((2007) ) Transthyretin enhances nerve regeneration. J Neurochem 103: , 831–839. |
[188] | Shao J , Yao Y ((2016) ) Transthyretin represses neovascularization in diabetic retinopathy. Mol Vis 22: , 1188–1197. |
[189] | Hansson SF , Andréasson U , Wall M , Skoog I , Andreasen N , Wallin A , Zetterberg H , Blennow K ((2009) ) Reduced levels of amyloid-beta-binding proteins in cerebrospinal fluid from Alzheimer’s disease patients. J Alzheimers Dis 16: , 389–397. |
[190] | Bergen AA , Kaing S , ten Brink JB ; Netherlands Brain Bank, Gorgels TG , Janssen SF ((2015) ) Gene expression and functional annotation of human choroid plexus epithelium failure in Alzheimer’s disease. BMC Genomics 16: , 956. |
[191] | Schwerk C , Tenenbaum T , Kim KS , Schroten H ((2015) ) The choroid plexus-a multi-role player during infectious diseases of the CNS. Front Cell Neurosci 9: , 80. |
[192] | Engelhart MJ , Geerlings MI , Meijer J , Kiliaan A , Ruitenberg A , van Swieten JC , Stijnen T , Hofman A , Witteman JC , Breteler MM ((2004) ) Inflammatory proteins in plasma and the risk of dementia: The rotterdam study. Arch Neurol 61: , 668–672. |
[193] | Tan ZS , Beiser AS , Vasan RS , Roubenoff R , Dinarello CA , Harris TB , Benjamin EJ , Au R , Kiel DP , Wolf PA , Seshadri S ((2007) ) Inflammatory markers and the risk of Alzheimer disease: The Framingham Study. Neurology 68: , 1902–1908. |
[194] | Bermejo P , Martín-Aragón S , Benedí J , Susín C , Felici E , Gil P , Ribera JM , Villar AM ((2008) ) Differences of peripheral inflammatory markers between mild cognitive impairment and Alzheimer’s disease. Immunol Lett 117: , 198–202. |
[195] | Xie J , Gorlé N , Vandendriessche C , Van Imschoot G , Van Wonterghem E , Van Cauwenberghe C , Parthoens E , Van Hamme E , Lippens S , Van Hoecke L , Vandenbroucke RE ((2021) ) Low-grade peripheral inflammation affects brain pathology in the AppNL-G-Fmouse model of Alzheimer’s disease. Acta Neuropathol Commun 9: , 163. |
[196] | Vandenbroucke RE , Dejonckheere E , Van Lint P , Demeestere D , Van Wonterghem E , Vanlaere I , Puimège L , Van Hauwermeiren F , De Rycke R , Mc Guire C , Campestre C , López-Otin C , Matthys P , Leclercq G , Libert C ((2012) ) Matrix metalloprotease 8-dependent extracellular matrix cleavage at the blood-CSF barrier contributes to lethality during systemic inflammatory diseases. J Neurosci 32: , 9805–9816. |
[197] | Bolos M , Antequera D , Aldudo J , Kristen H , Bullido MJ , Carro E ((2014) ) Choroid plexus implants rescue Alzheimer’s disease-like pathologies by modulating amyloid-β degradation. Cell Mol Life Sci 71: , 2947–2955. |
[198] | Aliaghaei A , Digaleh H , Khodagholi F , Ahmadiani A ((2015) ) Encapsulated choroid plexus epithelial cells actively protect against intrahippocampal Aβ-induced long-term memory dysfunction: Upregulation of effective neurogenesis with the abrogated apoptosis and neuroinflammation. J Mol Neurosci 56: , 708–721. |
[199] | Han ME , Kim HJ , Lee YS , Kim DH , Choi JT , Pan CS , Yoon S , Baek SY , Kim BS , Kim JB , Oh SO ((2009) ) Regulation of cerebrospinal fluid production by caffeine consumption. BMC Neurosci 10: , 110. |
[200] | Wostyn P , Audenaert K , De Deyn PP ((2011) ) Choroidal proteins involved in cerebrospinal fluid production may be potential drug targets for Alzheimer’s disease therapy. Perspect Medicin Chem 5: , 11–17. |
[201] | Nakano M , Kubota K , Hashizume S , Kobayashi E , Chikenji TS , Saito Y , Fujimiya M ((2020) ) An enriched environment prevents cognitive impairment in an Alzheimer’s disease model by enhancing the secretion of exosomal microRNA-146a from the choroid plexus. Brain Behav Immun Health 9: , 100149. |
[202] | Baruch K , Rosenzweig N , Kertser A , Deczkowska A , Sharif AM , Spinrad A , Tsitsou-Kampeli A , Sarel A , Cahalon L , Schwartz M ((2015) ) Breaking immune tolerance by targeting Foxp3(+) regulatory T cells mitigates Alzheimer’s disease pathology. Nat Commun 6: , 7967. |
[203] | Xie J , Bruggeman A , De Nolf C , Vandendriessche C , Van Imschoot G , Van Wonterghem E , Vereecke L , Vandenbroucke RE ((2023) ) Gut microbiota regulates blood-cerebrospinal fluid barrier function and Aβ pathology. EMBO J 42: , e111515. |
[204] | Kuang X , Chen YS , Wang LF , Li YJ , Liu K , Zhang MX , Li LJ , Chen C , He Q , Wang Y , Du JR ((2014) ) Klotho upregulation contributes to the neuroprotection of ligustilide in an Alzheimer’s disease mouse model. Neurobiol Aging 35: , 169–178. |
[205] | Liu CB , Wang R , Yi YF , Gao Z , Chen YZ ((2018) ) Lycopene mitigates β-amyloid induced inflammatory response and inhibits NF-κB signaling at the choroid plexus in early stages of Alzheimer’s disease rats. J Nutr Biochem 53: , 66–71. |
[206] | Xu Z , Liu C , Wang R , Gao X , Hao C , Liu C ((2021) ) A combination of lycopene and human amniotic epithelial cells can ameliorate cognitive deficits and suppress neuroinflammatory signaling by choroid plexus in Alzheimer’s disease rat. J Nutr Biochem 88: , 108558. |
[207] | Zhao J , Su M , Lin Y , Liu H , He Z , Lai L ((2020) ) Administration of amyloid precursor protein gene deleted mouse ESC-derived thymic epithelial progenitors attenuates Alzheimer’s pathology. Front Immunol 11: , 1781. |
[208] | Buxbaum JN , Ye Z , Reixach N , Friske L , Levy C , Das P , Golde T , Masliah E , Roberts AR , Bartfai T ((2008) ) Transthyretin protects Alzheimer’s mice from the behavioral and biochemical effects of Abeta toxicity. Proc Natl Acad Sci U S A 105: , 2681–2686. |
[209] | Kim JH , Hwang KH , Park KS , Kong ID , Cha SK ((2015) ) Biological role of anti-aging protein Klotho. J Lifestyle Med 5: , 1–6. |
[210] | Sousa JC , Cardoso I , Marques F , Saraiva MJ , Palha JA ((2007) ) Transthyretin and Alzheimer’s disease: Where in the brain? Neurobiol Aging 28,: , 713–718. |
[211] | Iliff JJ , Wang M , Liao Y , Plogg BA , Peng W , Gundersen GA , Benveniste H , Vates GE , Deane R , Goldman SA , Nagelhus EA , Nedergaard M ((2012) ) A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med 4: , 147ra111. |
[212] | Tarasoff-Conway JM , Carare RO , Osorio RS , Glodzik L , Butler T , Fieremans E , Axel L , Rusinek H , Nicholson C , Zlokovic BV , Frangione B , Blennow K , Ménard J , Zetterberg H , Wisniewski T , de Leon MJ ((2015) ) Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol 11: , 457–470. |
[213] | Bakker EN , Bacskai BJ , Arbel-Ornath M , Aldea R , Bedussi B , Morris AW , Weller RO , Carare RO ((2016) ) Lymphatic clearance of the brain: Perivascular, paravascular and significance for neurodegenerative diseases. Cell Mol Neurobiol 36: , 181–194. |
[214] | Bacyinski A , Xu M , Wang W , Hu J ((2017) ) The paravascular pathway for brain waste clearance: Current understanding, significance and controversy. Front Neuroanat 11: , 101. |
[215] | Kyrtsos CR , Baras JS ((2015) ) Modeling the role of the glymphatic pathway and cerebral blood vessel properties in Alzheimer’s disease pathogenesis. PLoS One 10: , e0139574. |
[216] | Gupta A , Iadecola C ((2015) ) Impaired Aβ clearance: A potential link between atherosclerosis and Alzheimer’s disease. Front Aging Neurosci 7: , 115. |
[217] | Plog BA , Nedergaard M ((2018) ) The glymphatic system in central nervous system health and disease: Past, present, and future. Annu Rev Pathol 13: , 379–394. |
[218] | Cheng Y , Haorah J ((2019) ) How does the brain remove its waste metabolites from within? Int J Physiol Pathophysiol Pharmacol 11: , 238–249. |
[219] | Reeves BC , Karimy JK , Kundishora AJ , Mestre H , Cerci HM , Matouk C , Alper SL , Lundgaard I , Nedergaard M , Kahle KT ((2020) ) Glymphatic system impairment in Alzheimer’s disease and idiopathic normal pressure hydrocephalus. Trends Mol Med 26: , 285–295. |
[220] | Jessen NA , Munk AS , Lundgaard I , Nedergaard M ((2015) ) The glymphatic system: A beginner’s guide. Neurochem Res 40: , 2583–2599. |
[221] | Benveniste H , Liu X , Koundal S , Sanggaard S , Lee H , Wardlaw J ((2019) ) The glymphatic system and waste clearance with brain aging: A review. Gerontology 65: , 106–119. |
[222] | Abbott NJ , Pizzo ME , Preston JE , Janigro D , Thorne RG ((2018) ) The role of brain barriers in fluid movement in the CNS: Is there a ‘glymphatic’ system? Acta Neuropathol 135: , 387–407. |
[223] | Rosu GC , Catalin B , Balseanu TA , Laurentiu M , Claudiu M , Kumar-Singh S , Daniel P ((2020) ) Inhibition of aquaporin 4 decreases amyloid Aβ40 drainage around cerebral vessels. Mol Neurobiol 57: , 4720–4734. |
[224] | Mathiisen TM , Lehre KP , Danbolt NC , Ottersen OP ((2010) ) The perivascular astroglial sheath provides a complete covering of the brain microvessels: An electron microscopic 3D reconstruction. Glia 58: , 1094–1103. |
[225] | Mestre H , Hablitz LM , Xavier AL , Feng W , Zou W , Pu T , Monai H , Murlidharan G , Castellanos Rivera RM , Simon MJ , Pike MM , Plá V , Du T , Kress BT , Wang X , Plog BA , Thrane AS , Lundgaard I , Abe Y , Yasui M , Thomas JH , Xiao M , Hirase H , Asokan A , Iliff JJ , Nedergaard M ((2018) ) Aquaporin-4-dependent glymphatic solute transport in the rodent brain. Elife 7: , e40070. |
[226] | Mader S , Brimberg L ((2019) ) Aquaporin-4 water channel in the brain and its implication for health and disease. Cells 8: , 90. |
[227] | Zeppenfeld DM , Simon M , Haswell JD , D’Abreo D , Murchison C , Quinn JF , Grafe MR , Woltjer RL , Kaye J , Iliff JJ ((2017) ) Association of perivascular localization of aquaporin-4 with cognition and Alzheimer disease in aging brains. JAMA Neurol 74: , 91–99. |
[228] | Rennels ML , Gregory TF , Blaumanis OR , Fujimoto K , Grady PA ((1985) ) Evidence for a ‘paravascular’ fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res 326: , 47–63. |
[229] | Ghersi-Egea JF , Gorevic PD , Ghiso J , Frangione B , Patlak CS , Fenstermacher JD ((1996) ) Fate of cerebrospinal fluid-borne amyloid beta-peptide: Rapid clearance into blood and appreciable accumulation by cerebral arteries. J Neurochem 67: , 880–883. |
[230] | Fujiyoshi M , Tachikawa M , Ohtsuki S , Ito S , Uchida Y , Akanuma S , Kamiie J , Hashimoto T , Hosoya K , Iwatsubo T , Terasaki T ((2011) ) Amyloid-β peptide(1-40) elimination from cerebrospinal fluid involves low-density lipoprotein receptor-related protein 1 at the blood-cerebrospinal fluid barrier. J Neurochem 118: , 407–415. |
[231] | Louveau A , Smirnov I , Keyes TJ , Eccles JD , Rouhani SJ , Peske JD , Derecki NC , Castle D , Mandell JW , Lee KS , Harris TH , Kipnis J ((2015) ) Structural and functional features of central nervous system lymphatic vessels. Nature 523: , 337–341. |
[232] | Cserr HF , Ostrach LH ((1974) ) Bulk flow of interstitial fluid after intracranial injection of blue dextran 2000. Exp Neurol 45: , 50–60. |
[233] | Cserr HF , Cooper DN , Suri PK , Patlak CS ((1981) ) Efflux of radiolabeled polyethylene glycols and albumin from rat brain. Am J Physiol 240: , F319–F328. |
[234] | Szentistvanyi I , Patlak CS , Ellis RA , Cserr HF ((1984) ) Drainage of interstitial fluid from different regions of rat brain. Am J Physiol 246: , F835–F844. |
[235] | Jin BJ , Smith AJ , Verkman AS ((2016) ) Spatial model of convective solute transport in brain extracellular space does not support a “glymphatic” mechanism. J Gen Physiol 148: , 489–501. |
[236] | Smith AJ , Yao X , Dix JA , Jin BJ , Verkman AS ((2017) ) Test of the ‘glymphatic’ hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. Elife 6: , e27679. |
[237] | Holter KE , Kehlet B , Devor A , Sejnowski TJ , Dale AM , Omholt SW , Ottersen OP , Nagelhus EA , Mardal KA , Pettersen KH ((2017) ) Interstitial solute transport in 3D reconstructed neuropil occurs by diffusion rather than bulk flow. Proc Natl Acad Sci U S A 114: , 9894–9899. |
[238] | Greenberg SM , Bacskai BJ , Hernandez-Guillamon M , Pruzin J , Sperling R , van Veluw SJ ((2020) ) Cerebral amyloid angiopathy and Alzheimer disease - one peptide, two pathways. Nat Rev Neurol 16: , 30–42. |
[239] | Xie L , Kang H , Xu Q , Chen MJ , Liao Y , Thiyagarajan M , O’Donnell J , Christensen DJ , Nicholson C , Iliff JJ , Takano T , Deane R , Nedergaard M ((2013) ) Sleep drives metabolite clearance from the adult brain. Science 342: , 373–377. |
[240] | Rainey-Smith SR , Mazzucchelli GN , Villemagne VL , Brown BM , Porter T , Weinborn M , Bucks RS , Milicic L , Sohrabi HR , Taddei K , Ames D , Maruff P , Masters CL , Rowe CC , Salvado O , Martins RN , Laws SM ; AIBL Research Group ((2018) ) Genetic variation in Aquaporin-4 moderates the relationship between sleep and brain Aβ-amyloid burden. Transl Psychiatry 8: , 47. |
[241] | Kress BT , Iliff JJ , Xia M , Wang M , Wei HS , Zeppenfeld D , Xie L , Kang H , Xu Q , Liew JA , Plog BA , Ding F , Deane R , Nedergaard M ((2014) ) Impairment of paravascular clearance pathways in the aging brain. Ann Neurol 76: , 845–861. |
[242] | Peng W , Achariyar TM , Li B , Liao Y , Mestre H , Hitomi E , Regan S , Kasper T , Peng S , Ding F , Benveniste H , Nedergaard M , Deane R ((2016) ) Suppression of glymphatic fluid transport in a mouse model of Alzheimer’s disease. Neurobiol Dis 93: , 215–225. |
[243] | Xu Z , Xiao N , Chen Y , Huang H , Marshall C , Gao J , Cai Z , Wu T , Hu G , Xiao M ((2015) ) Deletion of aquaporin-4 in APP/PS1 mice exacerbates brain Aβ accumulation and memory deficits. Mol Neurodegener 10: , 58. |
[244] | Yang J , Lunde LK , Nuntagij P , Oguchi T , Camassa LM , Nilsson LN , Lannfelt L , Xu Y , Amiry-Moghaddam M , Ottersen OP , Torp R ((2011) ) Loss of astrocyte polarization in the tg-ArcSwe mouse model of Alzheimer’s disease. J Alzheimers Dis 27: , 711–722. |
[245] | Wang L , Zhang Y , Zhao Y , Marshall C , Wu T , Xiao M ((2019) ) Deep cervical lymph node ligation aggravates AD-like pathology of APP/PS1 mice. Brain Pathol 29: , 176–192. |
[246] | Da Mesquita S , Louveau A , Vaccari A , Smirnov I , Cornelison RC , Kingsmore KM , Contarino C , Onengut-Gumuscu S , Farber E , Raper D , Viar KE , Powell RD , Baker W , Dabhi N , Bai R , Cao R , Hu S , Rich SS , Munson JM , Lopes MB , Overall CC , Acton ST , Kipnis J ((2018) ) Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature 560: , 185–191. |
[247] | Saito S , Yamamoto Y , Ihara M ((2019) ) Development of a multicomponent intervention to prevent Alzheimer’s disease. Front Neurol 10: , 490. |
[248] | van Veluw SJ , Hou SS , Calvo-Rodriguez M , Arbel-Ornath M , Snyder AC , Frosch MP , Greenberg SM , Bacskai BJ ((2020) ) Vasomotion as a driving force for paravascular clearance in the awake mouse brain. Neuron 105: , 549–561.e5. |
[249] | Gaberel T , Gakuba C , Goulay R , Martinez De Lizarrondo S , Hanouz JL , Emery E , Touze E , Vivien D , Gauberti M ((2014) ) Impaired glymphatic perfusion after strokes revealed by contrast-enhanced MRI: A new target for fibrinolysis? Stroke 45: , 3092–3096. |
[250] | Iliff JJ , Chen MJ , Plog BA , Zeppenfeld DM , Soltero M , Yang L , Singh I , Deane R , Nedergaard M ((2014) ) Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci 34: , 16180–16193. |
[251] | Wang M , Ding F , Deng S , Guo X , Wang W , Iliff JJ , Nedergaard M ((2017) ) Focal solute trapping and global glymphatic pathway impairment in a murine model of multiple microinfarcts. J Neurosci 37: , 2870–2877. |
[252] | Ringstad G , Vatnehol SAS , Eide PK ((2017) ) Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain 140: , 2691–2705. |
[253] | Yang L , Chen Z , Wan X , Liu M , Wu J , Chen Y , Zhang G , Fan Z ((2023) ) Angiotensin II type 1 receptor deficiency protects against the impairment of blood-brain barrier in a mouse model of traumatic brain injury. Int J Neurosci 133: , 604–611. |
[254] | Ishida K , Misawa K , Nishimura H , Hirata T , Yamamoto M , Ota N ((2020) ) 5-caffeoylquinic acid ameliorates cognitive decline and reduces Aβ deposition by modulating Aβ clearance pathways in APP/PS2 transgenic mice. Nutrients 12: , 494. |
[255] | Liang PZ , Li L , Zhang YN , Shen Y , Zhang LL , Zhou J , Wang ZJ , Wang S , Yang S ((2021) ) Electroacupuncture improves clearance of amyloid-β through the glymphatic system in the SAMP8 mouse model of Alzheimer’s disease. Neural Plast 2021: , 9960304. |
[256] | He XF , Liu DX , Zhang Q , Liang FY , Dai GY , Zeng JS , Pei Z , Xu GQ , Lan Y ((2017) ) Voluntary exercise promotes glymphatic clearance of amyloid beta and reduces the activation of astrocytes and microglia in aged mice. Front Mol Neurosci 10: , 144. |
[257] | Liu Y , Hu PP , Zhai S , Feng WX , Zhang R , Li Q , Marshall C , Xiao M , Wu T ((2022) ) Aquaporin 4 deficiency eliminates the beneficial effects of voluntary exercise in a mouse model of Alzheimer’s disease. Neural Regen Res 17: , 2079–2088. |
[258] | Pappolla MA , Matsubara E , Vidal R , Pacheco-Quinto J , Poeggeler B , Zagorski M , Sambamurti K ((2018) ) Melatonin treatment enhances Aβ lymphatic clearance in a transgenic mouse model of amyloidosis. Curr Alzheimer Res 15: , 637–642. |
[259] | Peng Y , Sun J , Hon S , Nylander AN , Xia W , Feng Y , Wang X , Lemere CA ((2010) ) L-3-n-butylphthalide improves cognitive impairment and reduces amyloid-beta in a transgenic model of Alzheimer’s disease. J Neurosci 30: , 8180–8189. |
[260] | Zhang B , Li W , Zhuo Y , Xiang H , Li W , Liu H , Xie L , Gao Q , Tan S ((2021) ) L-3-n-butylphthalide effectively improves the glymphatic clearance and reduce amyloid-β deposition in Alzheimer’s transgenic mice. J Mol Neurosci 71: , 1266–1274. |
[261] | Ren H , Luo C , Feng Y , Yao X , Shi Z , Liang F , Kang JX , Wan JB , Pei Z , Su H ((2017) ) Omega-3 polyunsaturated fatty acids promote amyloid-β clearance from the brain through mediating the function of the glymphatic system. FASEB J 31: , 282–293. |
[262] | Salehpour F , Khademi M , Bragin DE , DiDuro JO ((2022) ) Photobiomodulation therapy and the glymphatic system: Promising applications for augmenting the brain lymphatic drainage system. Int J Mol Sci 23: , 2975. |
[263] | Wu C , Lin T , Ding Q , Zhang N , Ou ZT , Cai GY , Chen HY , Xu JY , Li G , Pei Z , Xu GQ , Lan Y ((2022) ) Continuous theta-burst stimulation promotes paravascular CSF-interstitial fluid exchange through regulation of aquaporin-4 polarization in APP/PS1 mice. Mediators Inflamm 2022: , 2140524. |
[264] | Ren Z , Iliff JJ , Yang L , Yang J , Chen X , Chen MJ , Giese RN , Wang B , Shi X , Nedergaard M ((2013) ) ‘Hit & Run’ model of closed-skull traumatic brain injury (TBI) reveals complex patterns of post-traumatic AQP4 dysregulation. J Cereb Blood Flow Metab 33: , 834–845. |
[265] | Spinedi E , Cardinali DP ((2019) ) Neuroendocrine-metabolic dysfunction and sleep disturbances in neurodegenerative disorders: Focus on Alzheimer’s disease and melatonin. Neuroendocrinology 108: , 354–364. |
[266] | Xie Z , Chen F , Li WA , Geng X , Li C , Meng X , Feng Y , Liu W , Yu F ((2017) ) A review of sleep disorders and melatonin. Neurol Res 39: , 559–565. |
[267] | Peter-Derex L , Yammine P , Bastuji H , Croisile B ((2015) ) Sleep and Alzheimer’s disease. Sleep Med Rev 19: , 29–38. |
[268] | Zhou JN , Liu RY , Kamphorst W , Hofman MA , Swaab DF ((2003) ) Early neuropathological Alzheimer’s changes in aged individuals are accompanied by decreased cerebrospinal fluid melatonin levels. J Pineal Res 35: , 125–130. |
[269] | O’Neal-Moffitt G , Delic V , Bradshaw PC , Olcese J ((2015) ) Prophylactic melatonin significantly reduces Alzheimer’s neuropathology and associated cognitive deficits independent of antioxidant pathways in AβPP(swe)/PS1 mice. Mol Neurodegener 10: , 27. |
[270] | Carare RO , Bernardes-Silva M , Newman TA , Page AM , Nicoll JA , Perry VH , Weller RO ((2008) ) Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: Significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol Appl Neurobiol 34: , 131–144. |
[271] | Weller RO , Djuanda E , Yow HY , Carare RO ((2009) ) Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol 117: , 1–14. |
[272] | Hawkes CA , Jayakody N , Johnston DA , Bechmann I , Carare RO ((2014) ) Failure of perivascular drainage of β-amyloid in cerebral amyloid angiopathy. Brain Pathol 24: , 396–403. |
[273] | Aspelund A , Antila S , Proulx ST , Karlsen TV , Karaman S , Detmar M , Wiig H , Alitalo K ((2015) ) A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med 212: , 991–999. |
[274] | Hawkes CA , Härtig W , Kacza J , Schliebs R , Weller RO , Nicoll JA , Carare RO ((2011) ) Perivascular drainage of solutes is impaired in the ageing mouse brain and in the presence of cerebral amyloid angiopathy. Acta Neuropathol 121: , 431–443. |
[275] | Hawkes CA , Gatherer M , Sharp MM , Dorr A , Yuen HM , Kalaria R , Weller RO , Carare RO ((2013) ) Regional differences in the morphological and functional effects of aging on cerebral basement membranes and perivascular drainage of amyloid-β from the mouse brain. Aging Cell 12: , 224–236. |
[276] | Schley D , Carare-Nnadi R , Please CP , Perry VH , Weller RO ((2006) ) Mechanisms to explain the reverse perivascular transport of solutes out of the brain. J Theor Biol 238: , 962–974. |
[277] | Di Marco LY , Farkas E , Martin C , Venneri A , Frangi AF ((2015) ) Is vasomotion in cerebral arteries impaired in Alzheimer’s disease? J Alzheimers Dis 46: , 35–53. |
[278] | Diem AK , Tan M , Bressloff NW , Hawkes C , Morris AW , Weller RO , Carare RO ((2016) ) A simulation model of periarterial clearance of amyloid-β from the brain. Front Aging Neurosci 8: , 18. |
[279] | Morris AW , Carare RO , Schreiber S , Hawkes CA ((2014) ) The cerebrovascular basement membrane: Role in the clearance of β-amyloid and cerebral amyloid angiopathy. Front Aging Neurosci 6: , 251. |
[280] | Yamaguchi H , Yamazaki T , Lemere CA , Frosch MP , Selkoe DJ ((1992) ) Beta amyloid is focally deposited within the outer basement membrane in the amyloid angiopathy of Alzheimer’s disease. An immunoelectron microscopic study. Am J Pathol 141: , 249–259. |
[281] | Weller RO , Massey A , Newman TA , Hutchings M , Kuo YM , Roher AE ((1998) ) Cerebral amyloid angiopathy: Amyloid beta accumulates in putative interstitial fluid drainage pathways in Alzheimer’s disease. Am J Pathol 153: , 725–733. |
[282] | Preston SD , Steart PV , Wilkinson A , Nicoll JA , Weller RO ((2003) ) Capillary and arterial cerebral amyloid angiopathy in Alzheimer’s disease: Defining the perivascular route for the elimination of amyloid beta from the human brain. Neuropathol Appl Neurobiol 29: , 106–117. |
[283] | Yamada M , Tsukagoshi H , Otomo E , Hayakawa M ((1987) ) Cerebral amyloid angiopathy in the aged. J Neurol 234: , 371–376. |
[284] | Yamada M , Naiki H ((2012) ) Cerebral amyloid angiopathy. Prog Mol Biol Transl Sci 107: , 41–78. |
[285] | Greenberg SM , Gurol ME , Rosand J , Smith EE ((2004) ) Amyloid angiopathy-related vascular cognitive impairment. Stroke 35: , 2616–2619. |
[286] | Viswanathan A , Greenberg SM ((2011) ) Cerebral amyloid angiopathy in the elderly. Ann Neurol 70: , 871–880. |
[287] | Bergeron C , Ranalli PJ , Miceli PN ((1987) ) Amyloid angiopathy in Alzheimer’s disease. Can J Neurol Sci 14: , 564–569. |
[288] | Ellis RJ , Olichney JM , Thal LJ , Mirra SS , Morris JC , Beekly D , Heyman A ((1996) ) Cerebral amyloid angiopathy in the brains of patients with Alzheimer’s disease: The CERAD experience, Part XV. Neurology 46: , 1592–1596. |
[289] | Jellinger KA ((2002) ) Alzheimer disease and cerebrovascular pathology: An update. J Neural Transm (Vienna) 109: , 813–836. |
[290] | Brenowitz WD , Nelson PT , Besser LM , Heller KB , Kukull WA ((2015) ) Cerebral amyloid angiopathy and its co-occurrence with Alzheimer’s disease and other cerebrovascular neuropathologic changes. Neurobiol Aging 36: , 2702–2708. |
[291] | Boyle PA , Yu L , Nag S , Leurgans S , Wilson RS , Bennett DA , Schneider JA ((2015) ) Cerebral amyloid angiopathy and cognitive outcomes in community-based older persons. Neurology 85: , 1930–1936. |
[292] | Hawkes CA , Sullivan PM , Hands S , Weller RO , Nicoll JA , Carare RO ((2012) ) Disruption of arterial perivascular drainage of amyloid-β from the brains of mice expressing the human APOE ɛ4 allele. PLoS One 7: , e41636. |
[293] | Keable A , O’Neill R , MacGregor Sharp M , Gatherer M , Yuen HM , Johnston DA , Weller RO , Carare RO ((2020) ) ApoE4 astrocytes secrete basement membranes rich in fibronectin and poor in laminin compared to ApoE3 astrocytes. Int J Mol Sci 21: , E4371. |
[294] | Olichney JM , Hansen LA , Galasko D , Saitoh T , Hofstetter CR , Katzman R , Thal LJ ((1996) ) The apolipoprotein E epsilon 4 allele is associated with increased neuritic plaques and cerebral amyloid angiopathy in Alzheimer’s disease and Lewy body variant. Neurology 47: , 190–196. |
[295] | Navarro A , Del Valle E , Astudillo A , González del Rey C , Tolivia J ((2003) ) Immunohistochemical study of distribution of apolipoproteins E and D in human cerebral beta amyloid deposits. Exp Neurol 184: , 697–704. |
[296] | Utter S , Tamboli IY , Walter J , Upadhaya AR , Birkenmeier G , Pietrzik CU , Ghebremedhin E , Thal DR ((2008) ) Cerebral small vessel disease-induced apolipoprotein E leakage is associated with Alzheimer disease and the accumulation of amyloid beta-protein in perivascular astrocytes. J Neuropathol Exp Neurol 67: , 842–856. |
[297] | Cortes-Canteli M , Kruyer A , Fernandez-Nueda I , Marcos-Diaz A , Ceron C , Richards AT , Jno-Charles OC , Rodriguez I , Callejas S , Norris EH , Sanchez-Gonzalez J , Ruiz-Cabello J , Ibanez B , Strickland S , Fuster V ((2019) ) Long-term dabigatran treatment delays Alzheimer’s disease pathogenesis in the TgCRND8 Mouse Model. J Am Coll Cardiol 74: , 1910–1923. |
[298] | Contu L , Carare RO , Hawkes CA ((2019) ) Knockout of apolipoprotein A-I decreases parenchymal and vascular β-amyloid pathology in the Tg2576 mouse model of Alzheimer’s disease. Neuropathol Appl Neurobiol 45: , 698–714. |
[299] | Hawkes CA , McLaurin J ((2009) ) Selective targeting of perivascular macrophages for clearance of beta-amyloid in cerebral amyloid angiopathy. Proc Natl Acad Sci U S A 106: , 1261–1266. |
[300] | Maki T , Okamoto Y , Carare RO , Hase Y , Hattori Y , Hawkes CA , Saito S , Yamamoto Y , Terasaki Y , Ishibashi-Ueda H , Taguchi A , Takahashi R , Miyakawa T , Kalaria RN , Lo EH , Arai K , Ihara M ((2014) ) Phosphodiesterase III inhibitor promotes drainage of cerebrovascular β-amyloid. Ann Clin Transl Neurol 1: , 519–533. |
[301] | Zekonyte J , Sakai K , Nicoll JA , Weller RO , Carare RO ((2016) ) Quantification of molecular interactions between ApoE, amyloid-beta (Aβ) and laminin: Relevance to accumulation of Aβ in Alzheimer’s disease. Biochim Biophys Acta 1862: , 1047–1053. |
[302] | Wijesinghe P , Whitmore CA , Campbell M , Li C , Tsuyuki M , To E , Haynes J , Pham W , Matsubara JA ((2023) ) Ergothioneine, a dietary antioxidant improves amyloid beta clearance in the neuroretina of a mouse model of Alzheimer’s disease. Front Neurosci 17: , 1107436. |
[303] | Nizari S , Wells JA , Carare RO , Romero IA , Hawkes CA ((2021) ) Loss of cholinergic innervation differentially affects eNOS-mediated blood flow, drainage of Aβ and cerebral amyloid angiopathy in the cortex and hippocampus of adult mice. Acta Neuropathol Commun 9: , 12. |
[304] | Moon M , Cha MY , Mook-Jung I ((2014) ) Impaired hippocampal neurogenesis and its enhancement with ghrelin in 5XFAD mice. J Alzheimers Dis 41: , 233–241. |
[305] | Jeong YO , Shin SJ , Park JY , Ku BK , Song JS , Kim JJ , Jeon SG , Lee SM , Moon M ((2018) ) MK-0677, a ghrelin agonist, alleviates amyloid beta-related pathology in 5XFAD mice, an animal model of Alzheimer’s disease. Int J Mol Sci 19: , 1800. |
[306] | Tian J , Wang T , Wang Q , Guo L , Du H ((2019) ) MK0677, a ghrelin mimetic, improves neurogenesis but fails to prevent hippocampal lesions in a mouse model of Alzheimer’s disease pathology. . J Alzheimers Dis 72: , 467–478. |
[307] | Kelly L , Sharp MM , Thomas I , Brown C , Schrag M , Antunes LV , Solopova E , Martinez-Gonzalez J , Rodríguez C , Carare RO ((2023) ) Targeting lysyl-oxidase (LOX) may facilitate intramural periarterial drainage for the treatment of Alzheimer’s disease. Cereb Circ Cogn Behav 5: , 100171. |
[308] | Bales KR , O’Neill SM , Pozdnyakov N , Pan F , Caouette D , Pi Y , Wood KM , Volfson D , Cirrito JR , Han BH , Johnson AW , Zipfel GJ , Samad TA ((2016) ) Passive immunotherapy targeting amyloid-β reduces cerebral amyloid angiopathy and improves vascular reactivity. Brain 139: , 563–577. |
[309] | Saito S , Yamamoto Y , Maki T , Hattori Y , Ito H , Mizuno K , Harada-Shiba M , Kalaria RN , Fukushima M , Takahashi R , Ihara M ((2017) ) Taxifolin inhibits amyloid-β oligomer formation and fully restores vascular integrity and memory in cerebral amyloid angiopathy. Acta Neuropathol Commun 5: , 26. |
[310] | Saito S , Tanaka M , Satoh-Asahara N , Carare RO , Ihara M ((2021) ) Taxifolin: A potential therapeutic agent for cerebral amyloid angiopathy. Front Pharmacol 12: , 643357. |
[311] | Grossmann K ((2020) ) Anticoagulants for treatment of Alzheimer’s disease. J Alzheimers Dis 77: , 1373–1382. |
[312] | Tsujita M , Vaisman B , Chengyu L , Vickers KC , Okuhira KI , Braesch-Andersen S , Remaley AT ((2021) ) Apolipoprotein A-I in mouse cerebrospinal fluid derives from the liver and intestine via plasma high-density lipoproteins assembled by ABCA1 and LCAT. FEBS Lett 595: , 773–788. |
[313] | Shi J , Wei L ((2013) ) Rho kinases in cardiovascular physiology and pathophysiology: The effect of fasudil. J Cardiovasc Pharmacol 62: , 341–354. |
[314] | Landen JW , Andreasen N , Cronenberger CL , Schwartz PF , Börjesson-Hanson A , Östlund H , Sattler CA , Binneman B , Bednar MM ((2017) ) Ponezumab in mild-to-moderate Alzheimer’s disease: Randomized phase II PET-PIB study. Alzheimers Dement (N Y) 3: , 393–401. |
[315] | Bazzari FH , Bazzari AH ((2022) ) BACE1 inhibitors for Alzheimer’s disease: The past, present and any future? Molecules 27: , 8823. |
[316] | De Strooper B , Chávez Gutiérrez L ((2015) ) Learning by failing: Ideas and concepts to tackle γ-secretases in Alzheimer’s disease and beyond. Annu Rev Pharmacol Toxicol 55: , 419–437. |
[317] | Wilcock DM , Munireddy SK , Rosenthal A , Ugen KE , Gordon MN , Morgan D ((2004) ) Microglial activation facilitates Abeta plaque removal following intracranial anti-Abeta antibody administration. Neurobiol Dis 15: , 11–20. |
[318] | Mandrekar S , Jiang Q , Lee CY , Koenigsknecht-Talboo J , Holtzman DM , Landreth GE ((2009) ) Microglia mediate the clearance of soluble Abeta through fluid phase macropinocytosis. J Neurosci 29: , 4252–4262. |
[319] | Zuroff L , Daley D , Black KL , Koronyo-Hamaoui M ((2017) ) Clearance of cerebral Aβ in Alzheimer’s disease: Reassessing the role of microglia and monocytes. Cell Mol Life Sci 74: , 2167–2201. |
[320] | Janeway CA Jr , Travers P ((1996) ) Immunobiology. The Immune System in Health and Disease. 2nd edition. Garland Publishing Inc, New York and London. |
[321] | Crehan H , Hardy J , Pocock J ((2012) ) Microglia, Alzheimer’s disease, and complement. Int J Alzheimers Dis 2012: , 983640. |
[322] | Solomon B , Koppel R , Frankel D , Hanan-Aharon E ((1997) ) Disaggregation of Alzheimer beta-amyloid by site-directed mAb. Proc Natl Acad Sci U S A 94: , 4109–4112. |
[323] | DeMattos RB , Bales KR , Cummins DJ , Dodart JC , Paul SM , Holtzman DM ((2001) ) Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A 98: , 8850–8855. |
[324] | Henderson SJ , Andersson C , Narwal R , Janson J , Goldschmidt TJ , Appelkvist P , Bogstedt A , Steffen AC , Haupts U , Tebbe J , Freskgård PO , Jermutus L , Burrell M , Fowler SB , Webster CI ((2014) ) Sustained peripheral depletion of amyloid-β with a novel form of neprilysin does not affect central levels of amyloid-β. Brain 137: (Pt 2), 553–564. |
[325] | Georgievska B , Gustavsson S , Lundkvist J , Neelissen J , Eketjäll S , Ramberg V , Bueters T , Agerman K , Juréus A , Svensson S , Berg S , Fälting J , Lendahl U ((2015) ) Revisiting the peripheral sink hypothesis: Inhibiting BACE1 activity in the periphery does not alter β-amyloid levels in the CNS. J Neurochem 132: , 477–486. |
[326] | Plotkin SS , Cashman NR ((2020) ) Passive immunotherapies targeting Aβ and tau in Alzheimer’s disease. Neurobiol Dis 144: , 105010. |
[327] | Demattos RB , Lu J , Tang Y , Racke MM , Delong CA , Tzaferis JA , Hole JT , Forster BM , McDonnell PC , Liu F , Kinley RD , Jordan WH , Hutton ML ((2012) ) A plaque-specific antibody clears existing β-amyloid plaques in Alzheimer’s disease mice. Neuron 76: , 908–920. |
[328] | McDade E , Cummings JL , Dhadda S , Swanson CJ , Reyderman L , Kanekiyo M , Koyama A , Irizarry M , Kramer LD , Bateman RJ ((2022) ) Lecanemab in patients with early Alzheimer’s disease: Detailed results on biomarker, cognitive, and clinical effects from the randomized and open-label extension of the phase 2 proof-of-concept study. Alzheimers Res Ther 14: , 191. |
[329] | Bracoud L , Klein G , Lyons M , Scelsi MA , Wojtowicz J , Bullain S , Purcell D , Fiebach JB , Barakos J , Suhy J ((2023) ) Validation of 3- and 5-point severity scales to assess ARIA-E. (Amst) 15: , e12503. |
[330] | Roytman M , Mashriqi F , Al-Tawil K , Schulz PE , Zaharchuk G , Benzinger TLS , Franceschi AM ((2023) ) Amyloid-related imaging abnormalities: An update. AJR Am J Roentgenol 220: , 562–574. |
[331] | Doran SJ , Sawyer RP ((2024) ) Risk factors in developing amyloid related imaging abnormalities (ARIA) and clinical implications. Front Neurosci 18: , 1326784. |
[332] | Hampel H , Elhage A , Cho M , Apostolova LG , Nicoll JAR , Atri A ((2023) ) Amyloid-related imaging abnormalities (ARIA): Radiological, biological and clinical characteristics. Brain 146: , 4414–4424. |
[333] | Sperling RA , Jack CR Jr , Black SE , Frosch MP , Greenberg SM , Hyman BT , Scheltens P , Carrillo MC , Thies W , Bednar MM , Black RS , Brashear HR , Grundman M , Siemers ER , Feldman HH , Schindler RJ ((2011) ) Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: Recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimers Dement 7: , 367–385. |
[334] | Racke MM , Boone LI , Hepburn DL , Parsadainian M , Bryan MT , Ness DK , Piroozi KS , Jordan WH , Brown DD , Hoffman WP , Holtzman DM , Bales KR , Gitter BD , May PC , Paul SM , DeMattos RB ((2005) ) Exacerbation of cerebral amyloid angiopathy-associated microhemorrhage in amyloid precursor protein transgenic mice by immunotherapy is dependent on antibody recognition of deposited forms of amyloid beta. J Neurosci 25: , 629–636. |
[335] | Adolfsson O , Pihlgren M , Toni N , Varisco Y , Buccarello AL , Antoniello K , Lohmann S , Piorkowska K , Gafner V , Atwal JK , Maloney J , Chen M , Gogineni A , Weimer RM , Mortensen DL , Friesenhahn M , Ho C , Paul R , Pfeifer A , Muhs A , Watts RJ ((2012) ) An effector-reduced anti-β-amyloid (Aβ) antibody with unique aβ binding properties promotes neuroprotection and glial engulfment of Aβ. J Neurosci 32: , 9677–9689. |
[336] | Sperling R , Salloway S , Brooks DJ , Tampieri D , Barakos J , Fox NC , Raskind M , Sabbagh M , Honig LS , Porsteinsson AP , Lieberburg I , Arrighi HM , Morris KA , Lu Y , Liu E , Gregg KM , Brashear HR , Kinney GG , Black R , Grundman M ((2012) ) Amyloid-related imaging abnormalities in patients with Alzheimer’s disease treated with bapineuzumab: A retrospective analysis. Neurol 11: , 241–249. |
[337] | Pettersen JA , Sathiyamoorthy G , Gao FQ , Szilagyi G , Nadkarni NK , St George-Hyslop P , Rogaeva E , Black SE ((2008) ) Microbleed topography, leukoaraiosis, and cognition in probable Alzheimer disease from the Sunnybrook dementia study. Arch Neurol 65: , 790–795. |
[338] | Barakos J , Purcell D , Suhy J , Chalkias S , Burkett P , Marsica Grassi C , Castrillo-Viguera C , Rubino I , Vijverberg E ((2022) ) Detection and management of amyloid-related imaging abnormalities in patients with Alzheimer’s disease treated with anti-amyloid beta therapy. J Prev Alzheimers Dis 9: , 211–220. |
[339] | Goswami R , Subramanian G , Silayeva L , Newkirk I , Doctor D , Chawla K , Chattopadhyay S , Chandra D , Chilukuri N , Betapudi V ((2019) ) Gene therapy leaves a vicious cycle. Front Oncol 9: , 297. |
[340] | Ghosh S , Brown AM , Jenkins C , Campbell K ((2020) ) Viral vector systems for gene therapy: A comprehensive literature review of progress and biosafety challenges. Applied Biosafety 25: , 7–18. |
[341] | Charidimou A , Shakeshaft C , Werring DJ ((2012) ) Cerebral microbleeds on magnetic resonance imaging and anticoagulant-associated intracerebral hemorrhage risk. Front Neurol 3: , 133. |
[342] | Martinez-Ramirez S , Greenberg SM , Viswanathan A ((2014) ) Cerebral microbleeds: Overview and implications in cognitive impairment. Alzheimers Res Ther 6: , 33. |
[343] | Rallis KS , George AM , Wozniak AM , Bigogno CM , Chow B , Hanrahan JG , Sideris M ((2022) ) Molecular genetics and targeted therapies for paediatric high-grade glioma. Cancer Genomics Proteomics 19: , 390–414. |
[344] | Fei B , Dai W , Zhao S ((2018) ) Efficacy, safety, and cost of therapy of the traditional chinese medicine, catalpol, in patients following surgical resection for locally advanced colon cancer. Med Sci Monit 24: , 3184–3192. |
[345] | Dunn RT , Frye MS , Kimbrell TA , Denicoff KD , Leverich GS , Post RM ((1998) ) The efficacy and use of anticonvulsants in mood disorders. Clin Neuropharmacol 21: , 215–235. |
[346] | Fares H , DiNicolantonio JJ , O’Keefe JH , Lavie CJ ((2016) ) Amlodipine in hypertension: A first-line agent with efficacy for improving blood pressure and patient outcomes. Open Heart 3: , e000473. |
[347] | Wang AL , Iadecola C , Wang G ((2017) ) New generations of dihydropyridines for treatment of hypertension. J Geriatr Cardiol 14: , 67–72. |
[348] | Cipriani A , Saunders K , Attenburrow MJ , Stefaniak J , Panchal P , Stockton S , Lane TA , Tunbridge EM , Geddes JR , Harrison PJ ((2016) ) A systematic review of calcium channel antagonists in bipolar disorder and some considerations for their future development. Mol Psychiatry 21: , 1324–1332. |
[349] | Gupta V , Kumar I , Raj V , Aggarwal P , Agrawal V ((2022) ) Comparison of the effects of calcium channel blockers plus iron chelation therapy versus chelation therapy only on iron overload in children and young adults with transfusion-dependent thalassemia: A randomized double-blind placebo-controlled trial. Pediatr Blood Cancer 69: , e29564. |
[350] | Vahdani B , Armani Kian A , Esmaeilzadeh A , Zenoozian S , Yousefi V , Mazloomzadeh S ((2020) ) Adjunctive raloxifene and isradipine improve cognitive functioning in patients with schizophrenia: A pilot study. J Clin Psychopharmacol 40: , 457–463. |
[351] | Lasoń W , Jantas D , Leśkiewicz M , Regulska M , Basta-Kaim A ((2022) ) Vitamin D3 and ischemic stroke: A narrative review. Antioxidants (Basel) 11: , 2120. |
[352] | Pandey R , Zella JB , Zhu JG , Plum LA , Clagett-Dame M , Blaser WJ , Bedale W , DeLuca HF , Coyne DW ((2017) ) Pharmacokinetics of a new oral vitamin D receptor activator (2-methylene-19-Nor-(20S)-1α,25-dihydroxyvitamin D3) in patients with chronic kidney disease and secondary hyperparathyroidism on hemodialysis. Drugs R D 17: , 597–605. |
[353] | Cao H , Xu Y , de Necochea-Campion R , Baylink DJ , Payne KJ , Tang X , Ratanatharathorn C , Ji Y , Mirshahidi S , Chen CS ((2017) ) Application of vitamin D and vitamin D analogs in acute myelogenous leukemia. Exp Hematol 50: , 1–12. |
[354] | Marcinkowska E , Wallace GR , Brown G ((2016) ) The use of 1α,25-dihydroxyvitamin D3 as an anticancer agent. Int J Mol Sci 17: , 729. |
[355] | Matsumoto T , Endo I ((2012) ) Eldecalcitol for the treatment of osteoporosis. Drugs Today (Barc) 48: , 189–196. |
[356] | Hagino H ((2015) ) Vitamin D3 analogs for the treatment of osteoporosis. Can J Physiol Pharmacol 93: , 327–332. |
[357] | Shiomi S , Masaki K , Habu D , Takeda T , Nishiguchi S , Kuroki T , Tanaka T , Ochi H ((1999) ) Calcitriol for bone disease in patients with cirrhosis of the liver. J Gastroenterol Hepatol 14: , 547–552. |
[358] | Keighron C , Lyons CJ , Creane M , O’Brien T , Liew A ((2018) ) Recent advances in endothelial progenitor cells toward their use in clinical translation. Front Med (Lausanne) 5: , 354. |
[359] | Kamei N , Atesok K , Ochi M ((2017) ) The use of endothelial progenitor cells for the regeneration of musculoskeletal and neural tissues. Stem Cells Int 2017: , 1960804. |
[360] | Tanaka R , Masuda H , Kato S , Imagawa K , Kanabuchi K , Nakashioya C , Yoshiba F , Fukui T , Ito R , Kobori M , Wada M , Asahara T , Miyasaka M ((2014) ) Autologous G-CSF-mobilized peripheral blood CD34+cell therapy for diabetic patients with chronic nonhealing ulcer. Cell Transplant 23: , 167–179. |
[361] | Kawamoto A , Katayama M , Handa N , Kinoshita M , Takano H , Horii M , Sadamoto K , Yokoyama A , Yamanaka T , Onodera R , Kuroda A , Baba R , Kaneko Y , Tsukie T , Kurimoto Y , Okada Y , Kihara Y , Morioka S , Fukushima M , Asahara T ((2009) ) Intramuscular transplantation of G-CSF-mobilized CD34(+) cells in patients with critical limb ischemia: A phase I/IIa, multicenter, single-blinded, dose-escalation clinical trial. Stem Cells 27: , 2857–2864. |
[362] | Knols R , Aaronson NK , Uebelhart D , Fransen J , Aufdemkampe G ((2005) ) Physical exercise in cancer patients during and after medical treatment: A systematic review of randomized and controlled clinical trials. J Clin Oncol 23: , 3830–3842. |
[363] | Carneiro MAS , Oliveira-Júnior G , Castro-E-Souza P , Oliveira AA , Nunes PRP , Izquierdo M , Cadore EL , Cyrino ES ((2022) ) Impact of exercise intervention-based changes on physical function biomarkers in older adults after hospital discharge: A systematic review with meta-analysis of randomized clinical trials. Ageing Res Rev 80: , 101673. |
[364] | Kaddoumi A , Denney TS Jr , Deshpande G , Robinson JL , Beyers RJ , Redden DT , Praticò D , Kyriakides TC , Lu B , Kirby AN , Beck DT , Merner ND ((2022) ) Extra-virgin olive oil enhances the blood-brain barrier function in mild cognitive impairment: A randomized controlled trial. Nutrients 14: , 5102. |
[365] | Busse PJ , Farkas H , Banerji A , Lumry WR , Longhurst HJ , Sexton DJ , Riedl MA ((2019) ) Lanadelumab for the prophylactic treatment of hereditary angioedema with C1 inhibitor deficiency: A review of preclinical and phase I studies. BioDrugs 33: , 33–43. |
[366] | Maetzel A , Smith MD , Duckworth EJ , Hampton SL , De Donatis GM , Murugesan N , Rushbrooke LJ , Li L , Francombe D , Feener EP , Yea CM ((2022) ) KVD900, an oral on-demand treatment for hereditary angioedema: Phase 1 study results. J Allergy Clin Immunol 149: , 2034–2042. |
[367] | Squillaro T , Peluso G , Galderisi U ((2016) ) Clinical trials with mesenchymal stem cells: An update. Cell Transplant 25: , 829–848. |
[368] | Sandborn WJ , Bhandari BR , Randall C , Younes ZH , Romanczyk T , Xin Y , Wendt E , Chai H , McKevitt M , Zhao S , Sundy JS , Keshav S , Danese S ((2018) ) Andecaliximab [anti-matrix metalloproteinase-9] induction therapy for ulcerative colitis: A randomised, double-blind, placebo-controlled, phase 2/3 study in patients with moderate to severe disease. J Crohns Colitis 12: , 1021–1029. |
[369] | Gossage DL , Cieslarová B , Ap S , Zheng H , Xin Y , Lal P , Chen G , Smith V , Sundy JS ((2018) ) Phase 1b study of the safety, pharmacokinetics, and disease-related outcomes of the matrix metalloproteinase-9 inhibitor andecaliximab in patients with rheumatoid arthritis. Clin Ther 40: , 156-165.e5. |
[370] | Shah MA , Starodub A , Sharma S , Berlin J , Patel M , Wainberg ZA , Chaves J , Gordon M , Windsor K , Brachmann CB , Huang X , Vosganian G , Maltzman JD , Smith V , Silverman JA , Lenz HJ , Bendell JC ((2018) ) Andecaliximab/GS-5745 alone and combined with mFOLFOX6 in advanced gastric and gastroesophageal junction adenocarcinoma: Results from a phase I study. Clin Cancer Res 24: , 3829–3837. |
[371] | Drevets WC , Zarate CA Jr , Furey ML ((2013) ) Antidepressant effects of the muscarinic cholinergic receptor antagonist scopolamine: A review. Biol Psychiatry 73: , 1156–1163. |
[372] | Siatkowski RM , Cotter SA , Crockett RS , Miller JM , Novack GD , Zadnik K ; U.S. Pirenzepine Study Group ((2008) ) Two-year multicenter, randomized, double-masked, placebo-controlled, parallel safety and efficacy study of 2% pirenzepine ophthalmic gel in children with myopia. J AAPOS 12: , 332–339. |
[373] | Zoller T , Junghanss T , Kapaun A , Gjorup I , Richter J , Hugo-Persson M , Mørch K , Foroutan B , Suttorp N , Yürek S , Flick H ((2011) ) Intravenous artesunate for severe malaria in travelers, Europe. Emerg Infect Dis 17: , 771–777. |
[374] | Hogan MC , Masyuk TV , Page LJ , Kubly VJ , Bergstralh EJ , Li X , Kim B , King BF , Glockner J , Holmes DR 3rd , Rossetti S , Harris PC , LaRusso NF , Torres VE ((2010) ) Randomized clinical trial of long-acting somatostatin for autosomal dominant polycystic kidney and liver disease. J Am Soc Nephrol 21: , 1052–1061. |
[375] | Gomes-Porras M , Cárdenas-Salas J , Álvarez-Escolá C ((2020) ) Somatostatin analogs in clinical practice: A review. Int J Mol Sci 21: , 1682. |
[376] | Sano M , Ernesto C , Thomas RG , Klauber MR , Schafer K , Grundman M , Woodbury P , Growdon J , Cotman CW , Pfeiffer E , Schneider LS , Thal LJ ((1997) ) A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. N Engl J Med 336: , 1216–1222. |
[377] | Dysken MW , Sano M , Asthana S , Vertrees JE , Pallaki M , Llorente M , Love S , Schellenberg GD , McCarten JR , Malphurs J , Prieto S , Chen P , Loreck DJ , Trapp G , Bakshi RS , Mintzer JE , Heidebrink JL , Vidal-Cardona A , Arroyo LM , Cruz AR , Zachariah S , Kowall NW , Chopra MP , Craft S , Thielke S , Turvey CL , Woodman C , Monnell KA , Gordon K , Tomaska J , Segal Y , Peduzzi PN , Guarino PD ((2014) ) Effect of vitamin E and memantine on functional decline in Alzheimer disease: The TEAM-AD VA cooperative randomized trial. JAMA 311: , 33–44. |
[378] | Tucker JM , Townsend DM ((2005) ) Alpha-tocopherol: Roles in prevention and therapy of human disease. Biomed Pharmacother 59: , 380–387. |
[379] | Janssen H , Ada L , Bernhardt J , McElduff P , Pollack M , Nilsson M , Spratt NJ ((2014) ) An enriched environment increases activity in stroke patients undergoing rehabilitation in a mixed rehabilitation unit: A pilot non-randomized controlled trial. Disabil Rehabil 36: , 255–262. |
[380] | Khan F , Amatya B , Elmalik A , Lowe M , Ng L , Reid I , Galea MP ((2016) ) An enriched environmental programme during inpatient neuro-rehabilitation: A randomized controlled trial. J Rehabil Med 48: , 417–425. |
[381] | Rosbergen IC , Grimley RS , Hayward KS , Walker KC , Rowley D , Campbell AM , McGufficke S , Robertson ST , Trinder J , Janssen H , Brauer SG ((2017) ) Embedding an enriched environment in an acute stroke unit increases activity in people with stroke: A controlled before-after pilot study. Clin Rehabil 31: , 1516–1528. |
[382] | Morse MA , Hobeika AC , Osada T , Serra D , Niedzwiecki D , Lyerly HK , Clay TM ((2008) ) Depletion of human regulatory T cells specifically enhances antigen-specific immune responses to cancer vaccines. Blood 112: , 610–618. |
[383] | Luke JJ , Zha Y , Matijevich K , Gajewski TF ((2016) ) Single dose denileukin diftitox does not enhance vaccine-induced T cell responses or effectively deplete Tregs in advanced melanoma: Immune monitoring and clinical results of a randomized phase II trial. J Immunother Cancer 4: , 35. |
[384] | Sugiyama D , Nishikawa H , Maeda Y , Nishioka M , Tanemura A , Katayama I , Ezoe S , Kanakura Y , Sato E , Fukumori Y , Karbach J , Jäger E , Sakaguchi S ((2013) ) Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+regulatory T cells, evoking antitumor immune responses in humans. Proc Natl Acad Sci U S A 110: , 17945–17950. |
[385] | Kurose K, Ohue Y, Wada H, Iida S, Ishida T, Kojima T, Doi T, Suzuki S, Isobe M, Funakoshi T, Kakimi K, Nishikawa H, Udono H, Oka M, Ueda R, Nakayama E ((2015) ) Phase Ia study of FoxP3+CD4 Treg depletion by infusion of a humanized anti-CCR4 antibody, KW-0761, in cancer patients. Clin Cancer Res 21: , 4327–4336. |
[386] | Ishida T , Joh T , Uike N , Yamamoto K , Utsunomiya A , Yoshida S , Saburi Y , Miyamoto T , Takemoto S , Suzushima H , Tsukasaki K , Nosaka K , Fujiwara H , Ishitsuka K , Inagaki H , Ogura M , Akinaga S , Tomonaga M , Tobinai K , Ueda R ((2012) ) Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: A multicenter phase II study. J Clin Oncol 30: , 837–842. |
[387] | Ogura M , Ishida T , Hatake K , Taniwaki M , Ando K , Tobinai K , Fujimoto K , Yamamoto K , Miyamoto T , Uike N , Tanimoto M , Tsukasaki K , Ishizawa K , Suzumiya J , Inagaki H , Tamura K , Akinaga S , Tomonaga M , Ueda R ((2014) ) Multicenter phase II study of mogamulizumab (KW-0761), a defucosylated anti-cc chemokine receptor 4 antibody, in patients with relapsed peripheral T-cell lymphoma and cutaneous T-cell lymphoma. J Clin Oncol 32: , 1157–1163. |
[388] | Marui A , Tabata Y , Kojima S , Yamamoto M , Tambara K , Nishina T , Saji Y , Inui K , Hashida T , Yokoyama S , Onodera R , Ikeda T , Fukushima M , Komeda M ((2007) ) A novel approach to therapeutic angiogenesis for patients with critical limb ischemia by sustained release of basic fibroblast growth factor using biodegradable gelatin hydrogel: An initial report of the phase I-IIa study. Circ J 71: , 1181–1186. |
[389] | Kawaguchi H , Jingushi S , Izumi T , Fukunaga M , Matsushita T , Nakamura T , Mizuno K , Nakamura T , Nakamura K ((2007) ) Local application of recombinant human fibroblast growth factor-2 on bone repair: A dose-escalation prospective trial on patients with osteotomy. J Orthop Res 25: , 480–487. |
[390] | Kuroda Y , Matsuda S , Akiyama H ((2016) ) Joint-preserving regenerative therapy for patients with early-stage osteonecrosis of the femoral head. Inflamm Regen 36: , 4. |
[391] | Kawaguchi H , Oka H , Jingushi S , Izumi T , Fukunaga M , Sato K , Matsushita T , Nakamura K ; TESK Group ((2010) ) A local application of recombinant human fibroblast growth factor 2 for tibial shaft fractures: A randomized, placebo-controlled trial. J Bone Miner Res 25: , 2735–2743. |
[392] | Lynch SE , Williams RC , Polson AM , Howell TH , Reddy MS , Zappa UE , Antoniades HN ((1989) ) A combination of platelet-derived and insulin-like growth factors enhances periodontal regeneration. J Clin Periodontol 16: , 545–548. |
[393] | Nakagawa T , Sakamoto T , Hiraumi H , Kikkawa YS , Yamamoto N , Hamaguchi K , Ono K , Yamamoto M , Tabata Y , Teramukai S , Tanaka S , Tada H , Onodera R , Yonezawa A , Inui K , Ito J ((2010) ) Topical insulin-like growth factor 1 treatment using gelatin hydrogels for glucocorticoid-resistant sudden sensorineural hearing loss: A prospective clinical trial. BMC Med 8: , 76. |
[394] | Hirata JD , Swiersz LM , Zell B , Small R , Ettinger B ((1997) ) Does dong quai have estrogenic effects in postmenopausal women? A double-blind, placebo-controlled trial. Fertil Steril 68: , 981–986. |
[395] | Wang CC , Cheng KF , Lo WM , Law C , Li L , Leung PC , Chung TK , Haines CJ ((2013) ) A randomized, double-blind, multiple-dose escalation study of a Chinese herbal medicine preparation (Dang Gui Buxue Tang) for moderate to severe menopausal symptoms and quality of life in postmenopausal women. Menopause 20: , 223–231. |
[396] | Sharifi-Zahabi E , Soltani S , Malekahmadi M , Rezavand L , Clark CCT , Shidfar F ((2022) ) The effect of lycopene supplement from different sources on prostate specific antigen (PSA): A systematic review and meta-analysis of randomized controlled trials. Complement Ther Med 64: , 102801. |
[397] | Gajendragadkar PR , Hubsch A , Mäki-Petäjä KM , Serg M , Wilkinson IB , Cheriyan J ((2014) ) Effects of oral lycopene supplementation on vascular function in patients with cardiovascular disease and healthy volunteers: A randomised controlled trial. PLoS One 9: , e99070. |
[398] | Radisky ES , Raeeszadeh-Sarmazdeh M , Radisky DC ((2017) ) Therapeutic potential of matrix metalloproteinase inhibition in breast cancer. J Cell Biochem 118: , 3531–3548. |
[399] | Fields GB ((2019) ) The rebirth of matrix metalloproteinase inhibitors: Moving beyond the dogma. Cells 8: , 984. |
[400] | Paik PK , Luo J , Ai N , Kim R , Ahn L , Biswas A , Coker C , Ma W , Wong P , Buonocore DJ , Lai WV , Chaft JE , Acharyya S , Massagué J , Kris MG ((2022) ) Phase I trial of the TNF-α inhibitor certolizumab plus chemotherapy in stage IV lung adenocarcinomas. Nat Commun 13: , 6095. |
[401] | Garcia-Montoya L , Emery P ((2021) ) Disease modification in ankylosing spondylitis with TNF inhibitors: Spotlight on early phase clinical trials. Expert Opin Investig Drugs 30: , 1109–1124. |
[402] | Navarro-Millán I , Curtis JR ((2013) ) Newest clinical trial results with antitumor necrosis factor and nonantitumor necrosis factor biologics for rheumatoid arthritis. Curr Opin Rheumatol 25: , 384–390. |
[403] | Sfikakis PP ((2010) ) The first decade of biologic TNF antagonists in clinical practice: Lessons learned, unresolved issues and future directions. Curr Dir Autoimmun 11: , 180–210. |
[404] | Evangelatos G , Bamias G , Kitas GD , Kollias G , Sfikakis PP ((2022) ) The second decade of anti-TNF-a therapy in clinical practice: New lessons and future directions in the COVID-19 era. Rheumatol Int 42: , 1493–1511. |
[405] | Rosenblum H , Castano A , Alvarez J , Goldsmith J , Helmke S , Maurer MS ((2018) ) TTR (transthyretin) stabilizers are associated with improved survival in patients with TTR cardiac amyloidosis. Circ Heart Fail 11: , e004769. |
[406] | Tschöpe C , Elsanhoury A ((2022) ) Treatment of transthyretin amyloid cardiomyopathy: The current options, the future, and the challenges. J Clin Med 11: , 2148. |
[407] | Artusi CA , Sarro L , Imbalzano G , Fabbri M , Lopiano L ((2021) ) Safety and efficacy of tolcapone in Parkinson’s disease: Systematic review. Eur J Clin Pharmacol 77: , 817–829. |
[408] | Berk JL , Suhr OB , Obici L , Sekijima Y , Zeldenrust SR , Yamashita T , Heneghan MA , Gorevic PD , Litchy WJ , Wiesman JF , Nordh E , Corato M , Lozza A , Cortese A , Robinson-Papp J , Colton T , Rybin DV , Bisbee AB , Ando Y , Ikeda S , Seldin DC , Merlini G , Skinner M , Kelly JW , Dyck PJ ; Diflunisal Trial Consortium ((2013) ) Repurposing diflunisal for familial amyloid polyneuropathy: A randomized clinical trial. JAMA 310: , 2658–2667. |
[409] | Jang A , Lehtinen MK ((2022) ) Experimental approaches for manipulating choroid plexus epithelial cells. Fluids Barriers CNS 19: , 36. |
[410] | Liu R , Zhang Z , Chen Y , Liao J , Wang Y , Liu J , Lin Z , Xiao G ((2022) ) Choroid plexus epithelium and its role in neurological diseases. Front Mol Neurosci 15: , 949231. |
[411] | Cappelletti S , Piacentino D , Sani G , Aromatario M ((2015) ) Caffeine: Cognitive and physical performance enhancer or psychoactive drug? Curr Neuropharmacol 13: , 71–88. |
[412] | Alzheimer’s Drug Discovery Foundation ((2018) ) Cognitive Vitality Reports: Ligustilide. https://www.alzdiscovery.org/uploads/cognitive_vitality_media/Ligustilide-Cognitive-Vitality-For-Researchers.pdf. Last updated November 30, 2018, accessed August 13, 2023. |
[413] | Mills CE , Flury A , Marmet C , Poquet L , Rimoldi SF , Sartori C , Rexhaj E , Brenner R , Allemann Y , Zimmermann D , Gibson GR , Mottram DS , Oruna-Concha MJ , Actis-Goretta L , Spencer JPE ((2017) ) Mediation of coffee-induced improvements in human vascular function by chlorogenic acids and its metabolites: Two randomized, controlled, crossover intervention trials. Clin Nutr 36: , 1520–1529. |
[414] | Kang Z , Li S , Kang X , Deng J , Yang H , Chen F , Jiang J , Zhang J , Li W ((2023) ) Phase I study of chlorogenic acid injection for recurrent high-grade glioma with long-term follow-up. Cancer Biol Med 20: , 465–476. |
[415] | Wang J , Guo X , Lu W , Liu J , Zhang H , Quan Q , Su H , Ma L , Gao F , Qu Q ((2021) ) Donepezil combined with DL-3-n-butylphthalide delays cognitive decline in patients with mild to moderate Alzheimer’s disease: A multicenter, prospective cohort study. J Alzheimers Dis 80: , 673–681. |
[416] | Jia J , Wei C , Liang J , Zhou A , Zuo X , Song H , Wu L , Chen X , Chen S , Zhang J , Wu J , Wang K , Chu L , Peng D , Lv P , Guo H , Niu X , Chen Y , Dong W , Han X , Fang B , Peng M , Li D , Jia Q , Huang L ((2016) ) The effects of DL-3-n-butylphthalide in patients with vascular cognitive impairment without dementia caused by subcortical ischemic small vessel disease: A multicentre, randomized, double-blind, placebo-controlled trial. Alzheimers Dement 12: , 89–99. |
[417] | Cui LY , Zhu YC , Gao S , Wang JM , Peng B , Ni J , Zhou LX , He J , Ma XQ ((2013) ) Ninety-day administration of dl-3-n-butylphthalide for acute ischemic stroke: A randomized, double-blind trial. Chin Med J (Engl) 126: , 3405–3410. |
[418] | Wang H , Ye K , Li D , Liu Y , Wang D ((2022) ) DL-3-n-butylphthalide for acute ischemic stroke: An updated systematic review and meta-analysis of randomized controlled trials. Front Pharmacol 13: , 963118. |
[419] | Liu M , Yao X , Huang X , Shang H , Fan D , He J , Cui L ; Chinese ALS Study Group ((2023) ) A multicenter, randomized, double blind, placebo-controlled clinical trial of DL-3-n-butylphthalide in treatment of amyotrophic lateral sclerosis. Chin Med J (Engl) 136: , 354–356. |
[420] | Zhong R , Chen Q , Zhang X , Li M , Lin W ((2019) ) L-3-n-butylphthalide soft capsules in the treatment of Parkinson disease dementia: A systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 98: , e16082. |
[421] | Wang YY , Zheng W , Ng CH , Ungvari GS , Wei W , Xiang YT ((2017) ) Meta-analysis of randomized, double-blind, placebo-controlled trials of melatonin in Alzheimer’s disease. Int J Geriatr Psychiatry 32: , 50–57. |
[422] | Costello RB , Lentino CV , Boyd CC , O’Connell ML , Crawford CC , Sprengel ML , Deuster PA ((2014) ) The effectiveness of melatonin for promoting healthy sleep: A rapid evidence assessment of the literature. Nutr J 13: , 106. |
[423] | Gilron I , Tu D , Holden RR , Moulin DE , Duggan S , Milev R ((2022) ) Melatonin for neuropathic pain: Protocol for a double-blind, randomized controlled trial. JMIR Res Protoc 11: , e40025. |
[424] | Seely D , Legacy M , Auer RC , Fazekas A , Delic E , Anstee C , Angka L , Kennedy MA , Tai LH , Zhang T , Maziak DE , Shamji FM , Sundaresan RS , Gilbert S , Villeneuve PJ , Ashrafi AS , Inculet R , Yasufuku K , Waddell TK , Finley C , Shargall Y , Plourde M , Fergusson DA , Ramsay T , Seely AJE ((2021) ) Adjuvant melatonin for the prevention of recurrence and mortality following lung cancer resection (AMPLCaRe): A randomized placebo controlled clinical trial. EClinicalMedicine 33: , 100763. |
[425] | Lissoni P , Barni S , Tancini G , Crispino S , Paolorossi F , Lucini V , Mariani M , Cattaneo G , Esposti D , Esposti G , Fraschini F ((1987) ) Clinical study of melatonin in untreatable advanced cancer patients. Tumori 73: , 475–480. |
[426] | Mills E , Wu P , Seely D , Guyatt G ((2005) ) Melatonin in the treatment of cancer: A systematic review of randomized controlled trials and meta-analysis. J Pineal Res 39: , 360–366. |
[427] | Gonçalves AL , Martini Ferreira A , Ribeiro RT , Zukerman E , Cipolla-Neto J , Peres MF ((2016) ) Randomised clinical trial comparing melatonin 3 mg, amitriptyline 25 mg and placebo for migraine prevention. J Neurol Neurosurg Psychiatry 87: , 1127–1132. |
[428] | Taher A , Shokoohmand F , Abdoli E , Mohammadi Y , Mehrpooya M ((2022) ) A pilot study on the melatonin treatment in patients with early septic shock: Results of a single-center randomized controlled trial. Ir J Med Sci 191: , 1913–1924. |
[429] | Sánchez-Barceló EJ , Mediavilla MD , Tan DX , Reiter RJ ((2010) ) Clinical uses of melatonin: Evaluation of human trials. Curr Med Chem 17: , 2070–2095. |
[430] | Freund-Levi Y , Basun H , Cederholm T , Faxén-Irving G , Garlind A , Grut M , Vedin I , Palmblad J , Wahlund LO , Eriksdotter-Jönhagen M ((2008) ) Omega-3 supplementation in mild to moderate Alzheimer’s disease: Effects on neuropsychiatric symptoms. Int J Geriatr Psychiatry 23: , 161–169. |
[431] | Chiu CC , Su KP , Cheng TC , Liu HC , Chang CJ , Dewey ME , Stewart R , Huang SY ((2008) ) The effects of omega-3 fatty acids monotherapy in Alzheimer’s disease and mild cognitive impairment: A preliminary randomized double-blind placebo-controlled study. Prog Neuropsychopharmacol Biol Psychiatr 32: , y1538–1544. |
[432] | Quinn JF , Raman R , Thomas RG , Yurko-Mauro K , Nelson EB , Van Dyck C , Galvin JE , Emond J , Jack CR Jr , Weiner M , Shinto L , Aisen PS ((2010) ) Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: A randomized trial. JAMA 304: , 1903–1911. |
[433] | Burckhardt M , Herke M , Wustmann T , Watzke S , Langer G , Fink A ((2016) ) Omega-3 fatty acids for the treatment of dementia. Cochrane Database Syst Rev 4: , CD009002. |
[434] | Kris-Etherton PM , Richter CK , Bowen KJ , Skulas-Ray AC , Jackson KH , Petersen KS , Harris WS ((2019) ) Recent clinical trials shed new light on the cardiovascular benefits of omega-3 fatty acids. Methodist Debakey Cardiovasc J 15: , 171–178. |
[435] | Shen S , Gong C , Jin K , Zhou L , Xiao Y , Ma L ((2022) ) Omega-3 fatty acid supplementation and coronary heart disease risks: A meta-analysis of randomized controlled clinical trials. Front Nutr 9: , 809311. |
[436] | Sedighiyan M , Abdollahi H , Karimi E , Badeli M , Erfanian R , Raeesi S , Hashemi R , Vahabi Z , Asanjarani B , Mansouri F , Abdolahi M ((2021) ) Omega-3 polyunsaturated fatty acids supplementation improve clinical symptoms in patients with Covid-19: A randomised clinical trial. Int J Clin Pract 75: , e14854. |
[437] | Taghizadeh M , Tamtaji OR , Dadgostar E , Daneshvar Kakhaki R , Bahmani F , Abolhassani J , Aarabi MH , Kouchaki E , Memarzadeh MR , Asemi Z ((2017) ) The effects of omega-3 fatty acids and vitamin E co-supplementation on clinical and metabolic status in patients with Parkinson’s disease: A randomized, double-blind, placebo-controlled trial. Neurochem Int 108: , 183–189. |
[438] | Chang JP , Su KP , Mondelli V , Pariante CM ((2018) ) Omega-3 polyunsaturated fatty acids in youths with attention deficit hyperactivity disorder: A systematic review and meta-analysis of clinical trials and biological studies. Neuropsychopharmacology 43: , 534–545. |
[439] | Nabavi SF , Bilotto S , Russo GL , Orhan IE , Habtemariam S , Daglia M , Devi KP , Loizzo MR , Tundis R , Nabavi SM ((2015) ) Omega-3 polyunsaturated fatty acids and cancer: Lessons learned from clinical trials. Cancer Metastasis Rev 34: , 359–380. |
[440] | Goh KK , Chen CY , Chen CH , Lu ML ((2921) ) Effects of omega-3 polyunsaturated fatty acids supplements on psychopathology and metabolic parameters in schizophrenia: A meta-analysis of randomized controlled trials. J Psychopharmacol 35: , 221–235. |
[441] | Ovcharova EM , Danovska MP , Marinova DL , Pendicheva DI , Tonchev PT , Shepherd NM ((2022) ) Role of diet and supplementation with omega-3 polyunsaturated fatty acids for managing chronic fatigue in patients with relapsing-remitting multiple sclerosis. J Biomed Clin Res 15: , 99–104. |
[442] | He XX , Wu XL , Chen RP , Chen C , Liu XG , Wu BJ , Huang ZM ((2016) ) Effectiveness of omega-3 polyunsaturated fatty acids in non-alcoholic fatty liver disease: A meta-analysis of randomized controlled trials. PLoS One 11: , e0162368. |
[443] | Solomon SD , Lindsley K , Vedula SS , Krzystolik MG , Hawkins BS ((2014) ) Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database Syst Rev 8: , CD005139. |
[444] | Jackson TL , Slakter J , Buyse M , Wang K , Dugel PU , Wykoff CC , Boyer DS , Gerometta M , Baldwin ME , Price CF ; Opthea Study Group Investigators ((2023) ) A randomized controlled trial of OPT-302, a VEGF-C/D inhibitor for neovascular age-related macular degeneration. Ophthalmology 130: , 588–597. |
[445] | Roberti R , Iannone LF , Palleria C , Curcio A , Rossi M , Sciacqua A , Armentaro G , Vero A , Manti A , Cassano V , Russo E , De Sarro G , Citraro R ((2021) ) Direct oral anticoagulants: From randomized clinical trials to real-world clinical practice. Front Pharmacol 12: , 684638. |
[446] | von Vajna E , Alam R , So TY ((2016) ) Current clinical trials on the use of direct oral anticoagulants in the pediatric population. Cardiol Ther 5: , 19–41. |
[447] | Dave U , Somanader E , Baharlouei P , Pham L , Rahman MA ((2021) ) Applications of chitin in medical, environmental, and agricultural industries. J Mar Sci Eng 9: , 1173. |
[448] | Baharlouei P , Rahman A ((2022) ) Chitin and chitosan: Prospective biomedical applications in drug delivery, cancer treatment, and wound healing. Marine Drugs 20: , 460. |
[449] | Taguchi A , Takata Y , Ihara M , Kasahara Y , Tsuji M , Nishino M , Stern D , Okada M ((2013) ) Cilostazol improves cognitive function in patients with mild cognitive impairment: A retrospective analysis. Psychogeriatrics 13: , 164–169. |
[450] | Ihara M , Nishino M , Taguchi A , Yamamoto Y , Hattori Y , Saito S , Takahashi Y , Tsuji M , Kasahara Y , Takata Y , Okada M ((2014) ) Cilostazol add-on therapy in patients with mild dementia receiving donepezil: A retrospective study. PLoS One 9: , e89516. |
[451] | Biondi-Zoccai GG , Lotrionte M , Anselmino M , Moretti C , Agostoni P , Testa L , Abbate A , Cosgrave J , Laudito A , Trevi GP , Sheiban I ((2008) ) Systematic review and meta-analysis of randomized clinical trials appraising the impact of cilostazol after percutaneous coronary intervention. Am Heart J 155: , 1081–1089. |
[452] | Robless P , Mikhailidis DP , Stansby GP ((2008) ) Cilostazol for peripheral arterial disease. Cochrane Database Syst Rev 1: , CD003748. |
[453] | Smith JA ((2002) ) Measuring treatment effects of cilostazol on clinical trial endpoints in patients with intermittent claudication. Clin Cardiol 25: , 91–94. |
[454] | Sevigny JJ , Ryan JM , van Dyck CH , Peng Y , Lines CR , Nessly ML ; MK-677 Protocol 30 Study Group ((2008) ) Growth hormone secretagogue MK-677: No clinical effect on AD progression in a randomized trial. Neurology 71: , 1702–1708. |
[455] | Mansson JV , Alves FD , Biolo A , Souza GC ((2016) ) Use of ghrelin in cachexia syndrome: A systematic review of clinical trials. Nutr Rev 74: , 659–669. |
[456] | Takiguchi S , Hiura Y , Miyazaki Y , Takata A , Murakami K , Doki Y ((2012) ) Clinical trial of ghrelin synthesis administration for upper GI surgery. Methods Enzymol 514: , 409–431. |
[457] | Giorgioni G , Del Bello F , Quaglia W , Botticelli L , Cifani C , Micioni Di Bonaventura E , Micioni Di Bonaventura MV , Piergentili A ((2022) ) Advances in the development of nonpeptide small molecules targeting ghrelin receptor. J Med Chem 65: , 3098–3118. |
[458] | Su J , Geng J , Bao J , Tang Y , Liu M , Yu H , Han Y , Huang W , Zhou S ((2016) ) Two ghrelin receptor agonists for adults with malnutrition: A systematic review and meta-analysis. Nutr J 15: , 97. |
[459] | Leurent C , Goodman JA , Zhang Y , He P , Polimeni JR , Gurol ME , Lindsay M , Frattura L , Sohur US , Viswanathan A , Bednar MM , Smith EE ; Ponezumab Trial Study Group; Greenberg SM ((2019) ) Immunotherapy with ponezumab for probable cerebral amyloid angiopathy. Ann Clin Transl Neurol 6: , 795–806. |
[460] | Tai SY , Chen CH , Chien CY , Yang YH ((2017) ) Cilostazol as an add-on therapy for patients with Alzheimer’s disease in Taiwan: A case control study. BMC Neurol 17: , 40. |
[461] | Arai H , Takahashi T ((2009) ) A combination therapy of donepezil and cilostazol for patients with moderate Alzheimer disease: Pilot follow-up study. Am J Geriatr Psychiatry 17: , 353–354. |
[462] | Sakurai H , Hanyu H , Sato T , Kume K , Hirao K , Kanetaka H , Iwamoto T ((2013) ) Effects of cilostazol on cognition and regional cerebral blood flow in patients with Alzheimer’s disease and cerebrovascular disease: A pilot study. Geriatr Gerontol Int 13: , 90–97. |
[463] | Lee JY , Lee H , Yoo HB , Choi JS , Jung HY , Yoon EJ , Kim H , Jung YH , Lee HY , Kim YK ((2019) ) Efficacy of cilostazol administration in Alzheimer’s disease patients with white matter lesions: A positron-emission tomography study. Neurotherapeutics 16: , 394–403. |



