Distinguishing Semantic Variant Primary Progressive Aphasia from Alzheimer’s Disease
Abstract
The differentiation of semantic variant primary progressive aphasia from dementia and Alzheimer’s disease can be difficult, particularly when the semantic anomia is pronounced. This report describes a patient who presented with complaints of memory loss and proved to have prominent semantic loss of all types of nouns, common and proper, concrete and abstract, yet continued to live independently and maintain his activities of daily living. The evaluation was consistent for semantic variant primary progressive aphasia with degradation of semantic knowledge and focal anterior temporal atrophy and hypometabolism. This report summarizes the literature and discusses the differential diagnosis of this disorder from Alzheimer’s disease and related dementias.
INTRODUCTION
Clinicians who see patients with neurocognitive disorders may fail to recognize those with prominent semantic loss due to semantic variant primary progressive aphasia (svPPA). This relatively rare frontotemporal lobar degeneration can evolve to a “semantic dementia” (SD) when loss of the ability to understand words progresses to a more general loss of object meaning [1–3]. Patients with svPPA present with “memory complaints” related to loss of semantic words or knowledge, and clinicians misdiagnose them as having Alzheimer’s disease (AD) or a related dementia [2, 4]. It is important to differentiate svPPA as it differs in management, clinical course, and the explanations and education provided patients and their families. We present a patient with extensive loss of the meaning of words and discuss the distinctive clinical features that help in differentiating his disorder from the much more common AD.
CASE REPORT
A 64-year-old, high-school educated, right-handed man complained of progressive “memory difficulties” of several years’ duration. He felt quite distressed over losing his past and “everything” he had previously in memory. When asked to give examples of his memory loss, the patient replied, “I don’t know what an ‘example’ is?” During the interview, he went on to deny knowing what the following words/items meant: education, seizures, energy, and concentration. When asked about his serving in the army, he responded, “I don’t know what ‘army’ is“. He could not remember his birthdate (transposing the numbers in the year), the content of his prior jobs, his daughter’s name (“my young lady”), or the names of other known people. When asked to describe his process of paying a bill, he stated “I do it when I see it all and go there.”
His former girlfriend and daughters reported that the patient had lost interest in activities and complained of guilt over his memory loss but did not appear depressed. They stressed that the patient lived independently and could do all his activities of daily living including driving a car and getting his necessities. The patient did not have a history of psychiatric disease, drug abuse, or head trauma but did have unmedicated hypertension.
On examination, his spontaneous connected speech was fluent with normal flow and prosody and without word finding pauses or corrections. Despite his normal fluency, his speech was empty, often lacking critical nouns. His comprehension at the sentence level was intact except when he did not understand specific words. Single word comprehension proved impaired for words, such as “ring”, “telephone”, and “check”. When asked the time, he looked to the clock in his room and correctly told the time but was unable to understand the word “clock”. On a 10-item reading screen, he demonstrated “reading letter-by-letter” of irregular words such as “pint”, “colonel”, bouquet” and “mortgage”.
His language impairment compromised the rest of his mental status examination. He would frequently say “I’m sorry I can’t help you”, repeatedly apologizing for inability to understand the tasks. The Montreal Cognitive Assessment (MoCA) was difficult to score because of his persistent “I don’t know” responses; however, he missed all five verbal memory items and two of the three naming items. He could not do “F” word generation and produced only three animal names in a minute, stating that he could “see more in his head but couldn’t remember what they were called”. During testing, he demonstrated memory for recent events, such as meeting the examiners, clinic activities, prior testing, and getting to the exam room; however, the patient could not elaborate on the current president or historical items, such as the Watergate affair, the events of “9/11”, or Hurricane Katrina. Visuospatial constructions were intact, but face recognition testing showed impairment in the recognition of famous faces. His physical and neurological examinations were otherwise normal apart from mild hypertension.
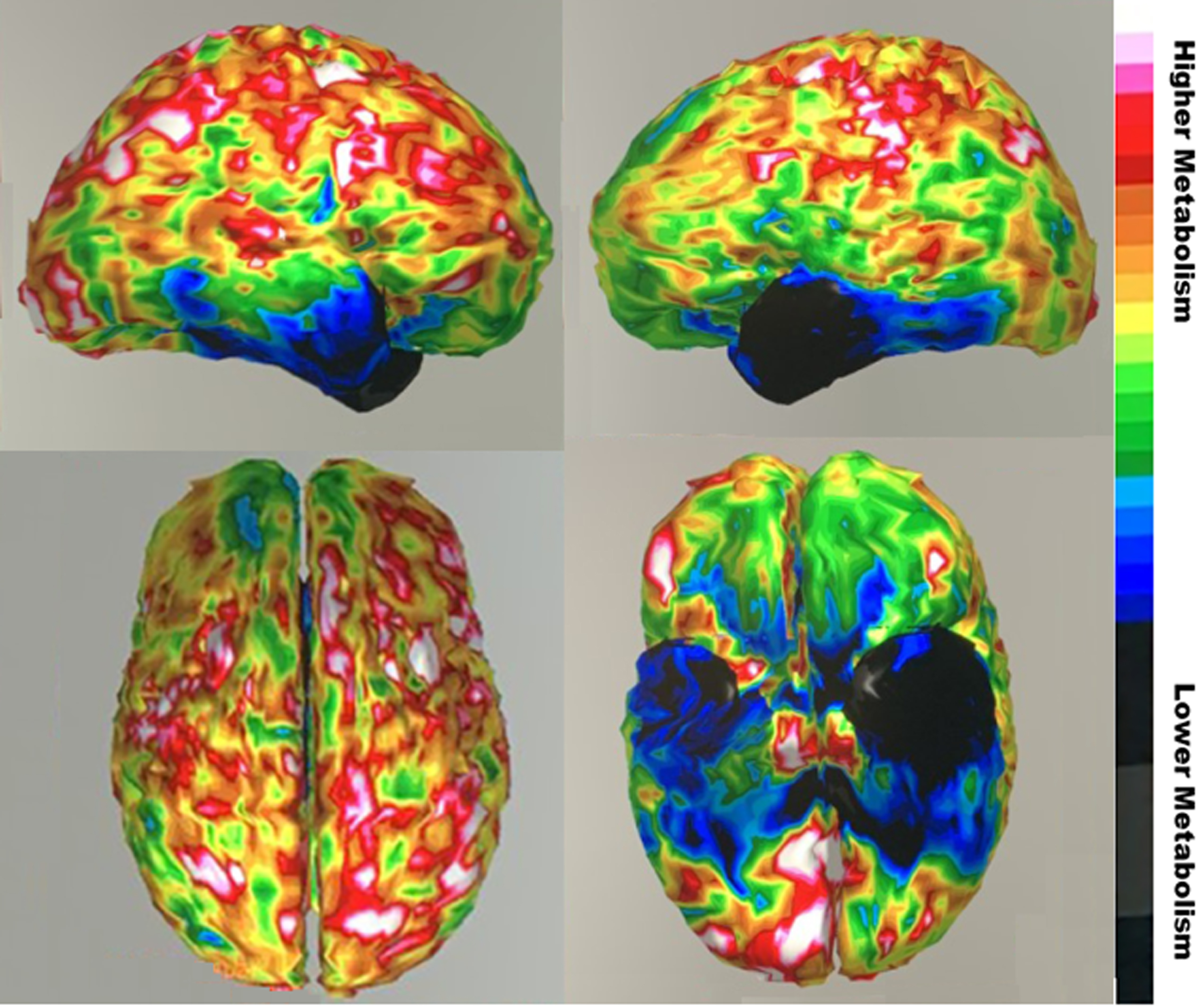
His laboratory results were all normal except for neuroimaging. Magnetic resonance imaging (MRI) of the brain showed subtle chronic encephalomalacia of the left anterior temporal lobe, with less prominent involvement of the right anterior temporal lobe (Fig. 1). He had a fluorodeoxy-glucose positron emission tomography (FDG-PET) scan of the brain, which demonstrated asymmetrically decreased metabolism in both temporal lobes (left side worse than the right side) (Fig. 2). There was more minor decreased metabolic activity in the adjacent inferior frontal regions.
Fig. 1
Magnetic resonance imaging (T1-weighted axial images on left and corresponding FLAIR images on right) showing anterior temporal atrophy involving temporal poles, more prominently on the left. Informed consent for images obtained.
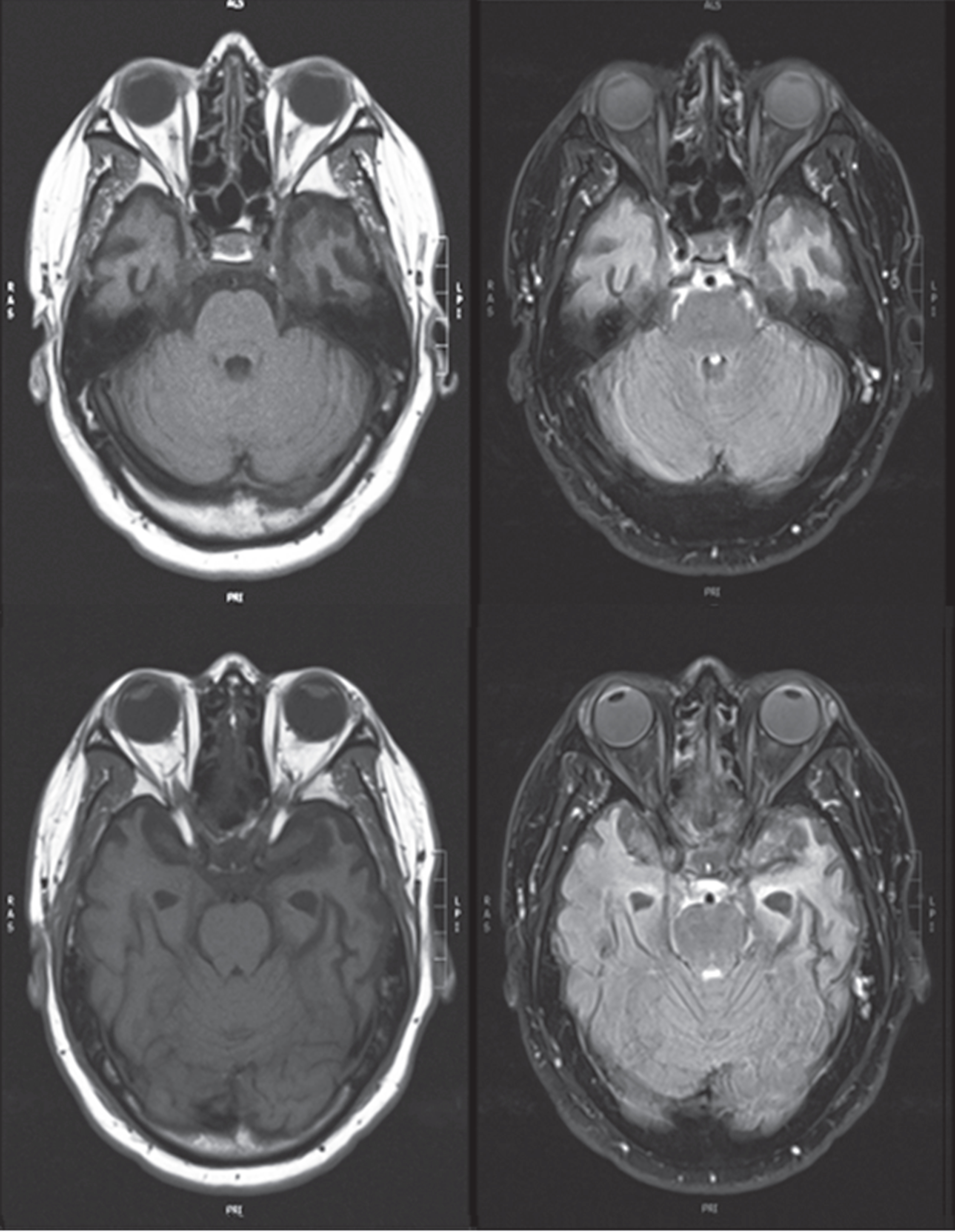
Fig. 2
Fluorodeoxy-glucose positron emission tomography (FDG-PET) images showing severe hypometabolism (blue region) in anterior temporal regions, worse on left, with somer extension into the inferior frontal region. (Top sagittal view right hemisphere on left and left hemisphere on right; color bar indicates degree of metabolism). Informed consent for images obtained.
The patient underwent limited neuropsychological assessment because of his language difficulty. On the Boston Diagnostic Aphasia Battery, he could count up to ten and state the days of the week and the alphabet up to “T”, but he was unable to state the months of year. Overall fluency and basic auditory comprehension were intact when he understood the individual words. Word and sentence repetition were also intact except for some semantic paraphasic errors. On naming, he knew letters and numbers but missed colors (identified gray as “black” and could not name pink and purple). Basic word discrimination was severely impaired, but the patient could demonstrate use of most objects that he could not name. On semantic probes he correctly answered 7/18 yes-no questions (below chance level) and could not understand complex ideational material. He could do only one of four tasks of identifying the name of pictures among four visually presented word options (chance level). His errors were often semantically related to the image such as “telescope” for “binoculars” or “pounds” for “weight.” He was able to orally read the individual letters in words but misread the irregular words, and he had similar problems with writing. In addition to language impairments, he demonstrated loss of autobiographical data related to his prior life and activities, but visuoperceptual abilities and motor control were intact.
Informed consent was obtained from the patient and his next of kin (daughter).
DISCUSSION
This patient had severe svPPA with loss of semantics or meaning for all types of nouns (common, proper, single, plural, concrete, abstract, collective), yet he retained sufficient functional ability to live alone and maintain his activities of daily living. svPPA is known as the “what is” disease because of its prominent deficits in single word comprehension. Clinicians often confuse svPPA with the much more common AD, particularly since both disorders can present with word finding difficulty and semantic deficits [5, 6]. Familiarity and assessment of the distinctive features of svPPA can help clinicians distinguish this disorder.
svPPA patients present with a period of predominant semantic deficits in words or language without other cognitive deficits [7, 8]. Although patients with svPPA most commonly have an age of onset of <65 years, up to 46% can present after age 65 [1]. This disorder has a duration of about 13.2±2.6 years [8]. It is almost entirely sporadic with focal atrophy involving the inferolateral anterior temporal lobes (ATLs) with “type C” transactive response DNA binding protein 43 (TDP-43) neuronal inclusions in about 89% [9]. The clinical diagnostic criteria for svPPA include the presence of semantic anomia (word-finding difficulty without improvement with phonemic or category cuing) and impaired single-word comprehension [7]. In addition, there are at least three of the following: Impaired object knowledge including faces, surface dysgraphia or dyslexia (decreased ability to write or read irregular words with “sounding-out” regularization errors), intact repetition, and intact speech production [7, 10]. The semantic impairment eventually extends beyond language to involve a multimodal impairment in the knowledge of objects, faces, and persons [1, 11, 12].
svPPA is a unique frontotemporal degeneration that affects the semantic system in the ATLs with progressive deterioration of semantic knowledge [13]. The inferolateral ATLs are the downstream hub of the “what” pathway for concepts, functioning as a convergence “hub-and-spoke” for integrating disparate sensory-motor features into multimodal representations of knowledge [14–17]. In svPPA, the prominence of semantic anomia, particularly for concrete words [18], originates from the more common involvement of the left ATL which focuses on language [19–21]. In contrast, predominant involvement of the right-sided ATL results in socioemotional, visceral, nonverbal semantic, and person-specific semantic loss [22, 23], changes which may have been masked in this patient because of his prominent semantic aphasia.
The key to recognizing svPPA is a careful evaluation of their language and aphasia. The most sensitive clinical screens for svPPA are the loss of nouns in conversational speech, decreased single-word comprehension, and surface dysgraphia or dyslexia in writing or reading. Spontaneous speech may sound empty of substantive or specific nouns with overgeneralization to generic terms that convey less specific information [24, 25]. The noun omissions are not associated with word searching or attempts at correction and are not helped by phonemic or contextual cues [26, 27]. In fact, the patients appear perplexed over their imprecision. The anomia starts with loss of low frequency and atypical words, and the word loss errors are constant over time. There are loss of subordinates (falls back on superordinate categories, e.g., “animal” for “mouse”), increased coordinates (modulated by familiarity and typicality), occasional differential semantic category impairment (e.g., living more than non-living objects), and semantic (but not phonemic or sound-based) paraphasic errors. There is also graded specialization in the ATLs for concrete versus abstract words [28–30], and the usual “concreteness effect,” or superior processing of imageable concrete words may be reversed in svPPA, namely, a better performance with abstract words with their greater semantic diversity [25, 28, 29, 31]. Finally, fully testing for svPPA involves asking patients to describe a named item, select an item from an array, identify or sort associated items that go together, and draw a specific item. Writing and reading irregular words further screens for surface dysgraphia and dyslexia, which may result because the ATL degeneration impairs semantic integration of exceptional or irregular word forms [32, 33].
Clinicians need to consider svPPA in the differential diagnosis of AD and other dementias (Table 1) [1]. In comparison to AD, svPPA patients have more prominent difficulties on confrontational naming, irregular word reading, and face recognition, and better episodic memory and visuospatial skills [34]. The presence of some hippocampal atrophy in svPPA may challenge the specificity of hippocampal atrophy for AD; however, studies suggest that svPPA primarily involves the anterior hippocampus and a functional semantic memory network and spares the more posterior hippocampus affected by AD [35–37]. As svPPA involves the right ATL, patients have more face recognition difficulties when compared to patients with AD [38]. As the disease progresses to SD, the semantic loss affects all modalities and patients develop personality and behavioral changes. These include rigidity in routines, a tendency to bizarre food choices or fads, restlessness and disinhibition, overreactions to pain, impoverished concepts of self, and compulsive clock watching and or obsessional interests in numbers and puzzles [39–42].
Table 1
Semantic Variant Primary Progressive Aphasia/Semantic Dementia (svPPA/SD) versus Dementia/Alzheimer’s Disease (Dementia/AD)
| Feature | svPPA/SD [7] | Dementia/AD [4] |
| PRESENTATION | ||
| Cognitive or behavioral symptoms that interfere with the ability to function at work or at usual activities | Yes | Yes |
| Cognitive or behavioral symptoms that represent a decline for previous levels of functioning and performing | Yes | Yes |
| Insidious onset over months to years | Yes | Possible for dementia, Yes for AD |
| Clear-cut history of progressive worsening of cognition by report or observation | Yes | Possible for dementia, Yes for AD |
| Cognitive impairment is detected and diagnosed through a combination of (1) history-taking from the patient and a knowledgeable informant and (2) an objective cognitive assessment | Yes | Yes |
| The cognitive or behavioral impairment involves a minimum of two cognitive domains (memory, executive, visuospatial, language, personality and behavior) | No | Yes for dementia; can have just severe amnesia for AD |
| PROMINENT SYMPTOM(S) | ||
| Prominent initial episodic memory, visual memory, visuoperceptual impairments | No | Probable |
| Prominent, initial behavioral disturbance | No | Possible |
| Language difficulty is the most prominent clinical feature | Yes | Possible |
| Language deficits are principal cause of impaired activities of daily living | Yes | Possible |
| Aphasia (language impairment) is the most prominent deficit at symptom onset and for the initial phases of the disease | Yes | Possible |
| LANGUAGE and SEMANTIC FEATURES | ||
| Impaired confrontational naming | Yes | Possible |
| Impaired single-word comprehension | Yes | Possible |
| Impaired object knowledge | No in svPPA, Yes in SD | Possible |
| Surface dyslexia or dysgraphia (reading and writing by sound) | Probable | Possible |
| Spared repetition | Probable | Possible |
| Spared speech production (grammar and motor speech) | Probable | Possible |
| EXCLUSIONS | ||
| Not explained by delirium or medical disorders | Yes | Yes |
| Not explained by major psychiatric disorder or better accounted for by a psychiatric diagnosis | Yes | Yes |
| IMAGING | ||
| Predominant anterior temporal lobe atrophy | Yes | No |
| Predominant anterior temporal hypoperfusion or hypometabolism | Yes | No |
Neuroimaging supplements the clinical examination and helps distinguish svPPA from AD. On MRI, image-supported svPPA has inferolateral ATL involvement distinguishable from more prominent hippocampal and parietal lobe atrophy in AD [7]. svPPA has more severe and asymmetrical atrophy in temporal structures than AD [43]. FDG-PET imaging can confirm focal predominant hypometabolism localized to the ATLs as in this patient (Fig. 2). Amyloid-PET imaging is negative in svPPA and SD [44], but it can be false positive in the elderly or reflect coexistent pathology [45]. The more recent “tau” PET imaging may also fail to distinguish these disorders, possibly due to positive scans from binding to monoamine oxidase B positive astrocytes [46].
In conclusion, clinicians need to be aware of the language and other features of svPPA, and SD as it progresses, to recognize this disorder and differentiate it from AD. Along with better episodic memory and visuospatial constructions, language measures, including naming and irregular word writing and reading, may distinguish this neurocognitive disorder on presentation. Brain imaging can confirm the presence of ATL atrophy or hypometabolism. Currently, there is no specific treatment for this disorder, beyond speech therapy and symptomatic and supportive measures. Medications used specifically for AD are not indicated for svPPA or SD as they are generally ineffective, could exacerbate some symptoms, and target wrong neuropathology, e.g., the new and expensive anti-amyloid drugs for AD [47]. svPPA and SD are good targets for developing anti-TDP-43 agents with novel mechanisms of action, e.g., the myeloperoxidase inhibitor verdiperstat. More research on svPPA and SD can offer further insights into its recognition and differentiation from AD and other neurological conditions as well as potential future treatments.
ACKNOWLEDGMENTS
The authors have no acknowledgements to report.
FUNDING
The authors have no pertinent funding for this work.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
REFERENCES
[1] | Hodges JR , Mitchell J , Dawson K , Spillantini MG , Xuereb JH , McMonagle P , Nestor PJ , Patterson K ((2010) ) Semantic dementia: Demography, familial factors and survival in a consecutive series of 100 cases. Brain 133: , 300–306. |
[2] | Kertesz A , Jesso S , Harciarek M , Blair M , McMonagle P ((2010) ) What is semantic dementia? A cohort study of diagnostic features and clinical boundaries. Arch Neurol 67: , 483–489. |
[3] | Johnson JK , Diehl J , Mendez MF , Neuhaus J , Shapira JS , Forman M , Chute DJ , Roberson ED , Pace-Savitsky C , Neumann M , Chow TW , Rosen HJ , Forstl H , Kurz A , Miller BL ((2005) ) Frontotemporal lobar degeneration: Demographic characteristics of 353 patients. Arch Neurol 62: , 925–930. |
[4] | McKhann GM , Knopman DS , Chertkow H , Hyman BT , Jack CR Jr., Kawas CH , Klunk WE , Koroshetz WJ , Manly JJ , Mayeux R , Mohs RC , Morris JC , Rossor MN , Scheltens P , Carrillo MC , Thies B , Weintraub S , Phelps CH ((2011) ) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 263–269. |
[5] | Xie SX , Libon DJ , Wang X , Massimo L , Moore P , Vesely L , Khan A , Chatterjee A , Coslett HB , Hurtig HI , Liang TW , Grossman M ((2010) ) Longitudinal patterns of semantic and episodic memory in frontotemporal lobar degeneration and Alzheimer’s disease. J Int Neuropsychol Soc 16: , 278–286. |
[6] | Reilly J , Peelle JE , Antonucci SM , Grossman M ((2011) ) Anomia as a marker of distinct semantic memory impairments in Alzheimer’s disease and semantic dementia. Neuropsychology 25: , 413–426. |
[7] | Gorno-Tempini ML , Hillis AE , Weintraub S , Kertesz A , Mendez M , Cappa SF , Ogar JM , Rohrer JD , Black S , Boeve BF , Manes F , Dronkers NF , Vandenberghe R , Rascovsky K , Patterson K , Miller BL , Knopman DS , Hodges JR , Mesulam MM , Grossman M ((2011) ) Classification of primary progressive aphasia andits variants. Neurology 76: , 1006–1014. |
[8] | Mesulam MM , Coventry CA , Bigio EH , Sridhar J , Gill N , Fought AJ , Zhang H , Thompson CK , Geula C , Gefen T , Flanagan M , Mao Q , Weintraub S , Rogalski EJ ((2022) ) Neuropathological fingerprints of survival, atrophy and language in primary progressive aphasia. Brain 145: , 2133–2148. |
[9] | Spinelli EG , Mandelli ML , Miller ZA , Santos-Santos MA , Wilson SM , Agosta F , Grinberg LT , Huang EJ , Trojanowski JQ , Meyer M , Henry ML , Comi G , Rabinovici G , Rosen HJ , Filippi M , Miller BL , Seeley WW , Gorno-Tempini ML ((2017) ) Typical and atypical pathology in primary progressive aphasia variants. Ann Neurol 81: , 430–443. |
[10] | Knibb JA , Hodges JR ((2005) ) Semantic dementia and primary progressive aphasia: A problem of categorization? Alzheimer Dis Assoc Disord 19: (Suppl 1), S7–14. |
[11] | Caixeta L , Soares VL , Soares CD ((2011) ) Hyperalgesia in semantic dementia. Arq Neuropsiquiatr 69: , 260–261. |
[12] | Snowden JS , Bathgate D , Varma A , Blackshaw A , Gibbons ZC , Neary D ((2001) )Distinct behavioural profiles in frontotemporal dementia and semantic dementia. J Neurol Neurosurg Psychiatry 70: , 323–332. |
[13] | Leyton CE , Britton AK , Hodges JR , Halliday GM , Kril JJ ((2016) ) Distinctive pathological mechanisms involved in primary progressive aphasias. Neurobiol Aging 38: , 82–92. |
[14] | Fletcher PD , Warren JD ((2011) ) Semantic dementia: A specific network-opathy. J Mol Neurosci 45: , 629–636. |
[15] | Wang Y , Collins JA , Koski J , Nugiel T , Metoki A , Olson IR ((2017) ) Dynamic neural architecture for social knowledge retrieval. Proc Natl Acad Sci U S A 114: , E3305–E3314. |
[16] | Hoffman P , Evans GA , Lambon Ralph MA ((2014) ) The anterior temporal lobes are critically involved in acquiring new conceptual knowledge: Evidence for impaired feature integration in semantic dementia. Cortex 50: , 19–31. |
[17] | Reilly J , Peelle JE , Garcia A , Crutch SJ ((2016) ) Linking somatic and symbolic representation in semantic memory: The dynamic multilevel reactivation framework. Psychon Bull Rev 23: , 1002–1014. |
[18] | Cousins KA , York C , Bauer L , Grossman M ((2016) ) Cognitive and anatomic double dissociation in the representation of concrete and abstract words in semantic variant and behavioral variant frontotemporal degeneration. Neuropsychologia 84: , 244–251. |
[19] | Meijboom R , Steketee RM , Ham LS , van der Lugt A , van Swieten JC , Smits M ((2017) ) Differential hemispheric predilection of microstructural white matter and functional connectivity abnormalities between respectively semantic and behavioral variant frontotemporal dementia. J Alzheimers Dis 56: , 789–804. |
[20] | Tu S , Leyton CE , Hodges JR , Piguet O , Hornberger M ((2016) ) Divergent longitudinal propagation of white matter degradation in logopenic and semantic variants of primary progressive aphasia. J Alzheimers Dis 49: , 853–861. |
[21] | Montembeault M , Chapleau M , Jarret J , Boukadi M , Laforce R Jr., Wilson MA , Rouleau I , Brambati SM ((2019) ) Differential language network functional connectivity alterations in Alzheimer’s disease and the semantic variant of primary progressive aphasia. Cortex 117: , 284–298. |
[22] | Borghesani V , DeLeon J , Gorno-Tempini ML ((2022) ) Frontotemporal dementia: A unique window on the functional role of the temporal lobes. Handb Clin Neurol 187: , 429–448. |
[23] | Younes K , Borghesani V , Montembeault M , Spina S , Mandelli ML , Welch AE , Weis E , Callahan P , Elahi FM , Hua AY , Perry DC , Karydas A , Geschwind D , Huang E , Grinberg LT , Kramer JH , Boxer AL , Rabinovici GD , Rosen HJ , Seeley WW , Miller ZA , Miller BL , Sturm VE , Rankin KP , Gorno-Tempini ML ((2022) ) Right temporal degeneration and socioemotional semantics: Semantic behavioural variant frontotemporal dementia. Brain 145: , 4080–4096. |
[24] | Mesulam MM ((2023) ) Temporopolar regions of the human brain. Brain 146: , 20–41. |
[25] | Hoffman P , Meteyard L , Patterson K ((2014) ) Broadly speaking: Vocabulary in semantic dementia shifts towards general, semantically diverse words. Cortex 55: , 30–42. |
[26] | Lukic S , Borghesani V , Weis E , Welch A , Bogley R , Neuhaus J , Deleon J , Miller ZA , Kramer JH , Miller BL , Dronkers NF , Gorno-Tempini ML ((2021) ) Dissociating nouns and verbs in temporal and perisylvian networks: Evidence from neurodegenerative diseases. Cortex 142: , 47–61. |
[27] | Bruffaerts R , Schaeverbeke J , De Weer AS , Nelissen N , Dries E , Van Bouwel K , Sieben A , Bergmans B , Swinnen C , Pijnenburg Y , Sunaert S , Vandenbulcke M , Vandenberghe R ((2020) ) Multivariate analysis reveals anatomical correlates of naming errors in primary progressive aphasia. Neurobiol Aging 88: , 71–82. |
[28] | Papagno C ((2022) ) The neural correlates of abstract and concrete words. Handb Clin Neurol 187: , 263–275. |
[29] | Hoffman P ((2016) ) The meaning of ‘life’ and other abstract words: Insights from neuropsychology. J Neuropsychol 10: , 317–343. |
[30] | Crutch SJ , Warrington EK ((2005) ) Abstract and concrete concepts have structurally different representational frameworks. Brain 128: , 615–627. |
[31] | Jefferies E , Patterson K , Jones RW , Lambon Ralph MA ((2009) ) Comprehension of concrete and abstract words in semantic dementia. Neuropsychology 23: , 492–499. |
[32] | Joyal M , Brambati SM , Laforce RJ , Montembeault M , Boukadi M , Rouleau I , Macoir J , Joubert S , Fecteau S , Wilson MA ((2017) ) The role of the left anterior temporal lobe for unpredictable and complex mappings in word reading. Front Psychol 8: , 517. |
[33] | Wilson SM , Brambati SM , Henry RG , Handwerker DA , Agosta F , Miller BL , Wilkins DP , Ogar JM , Gorno-Tempini ML ((2009) ) The neural basis of surface dyslexia in semantic dementia. Brain 132: , 71–86. |
[34] | Mendez MF , Chavez D , Desarzant RE , Yerstein O ((2020) ) Clinical features of late-onset semantic dementia. Cogn Behav Neurol 33: , 122–128. |
[35] | Chapleau M , Aldebert J , Montembeault M , Brambati SM ((2016) ) Atrophy in Alzheimer’s disease and semantic dementia: An ALE meta-analysis of voxel-based morphometry studies. J Alzheimers Dis 54: , 941–955. |
[36] | Chapleau M , Montembeault M , Boukadi M , Bedetti C , Laforce R Jr., Wilson M , Brambati SM ((2019) ) The role of the hippocampus in the semantic variant of primary progressive aphasia: A resting-state fcMRI study. Hippocampus 29: , 1127–1132. |
[37] | La Joie R , Landeau B , Perrotin A , Bejanin A , Egret S , Pelerin A , Mezenge F , Belliard S , de La Sayette V , Eustache F , Desgranges B , Chetelat G ((2014) ) Intrinsic connectivityidentifies the hippocampus as a main crossroad between Alzheimer’sand semantic dementia-targeted networks. Neuron 81: , 1417–1428. |
[38] | Luzzi S , Baldinelli S , Ranaldi V , Fabi K , Cafazzo V , Fringuelli F , Silvestrini M , Provinciali L , Reverberi C , Gainotti G ((2017) ) Famous faces and voices: Differential profiles in early right and left semantic dementia and in Alzheimer’s disease. Neuropsychologia 94: , 118–128. |
[39] | Ahmed RM , Irish M , Kam J , van Keizerswaard J , Bartley L , Samaras K , Hodges JR , Piguet O ((2014) ) Quantifying the eating abnormalities in frontotemporal dementia. JAMA Neurol 71: , 1540–1546. |
[40] | Fletcher PD , Downey LE , Golden HL , Clark CN , Slattery CF , Paterson RW , Rohrer JD , Schott JM , Rossor MN , Warren JD ((2015) ) Pain and temperature processing in dementia: A clinical and neuroanatomical analysis. Brain 138: , 3360–3372. |
[41] | Hutchings R , Hodges JR , Piguet O , Kumfor F , Boutoleau-Bretonniere C ((2015) ) Why should i care? Dimensions of socio-emotional cognition in younger-onset dementia. J Alzheimers Dis 48: , 135–147. |
[42] | Van Langenhove T , Leyton CE , Piguet O , Hodges JR ((2016) ) Comparing longitudinal behavior changes in the primary progressive aphasias. J Alzheimers Dis 53: , 1033–1042. |
[43] | Kobayashi R , Hayashi H , Kawakatsu S , Shibuya Y , Morioka D , Ohba M , Yoshioka M , Sakamoto K , Kanoto M , Otani K ((2022) ) Comparing medial temporal atrophy between early-onset semantic dementia and early-onset Alzheimer’s disease using voxel-based morphometry: A multicenter MRI study. Curr Alzheimer Res 19: , 503–510. |
[44] | Brown EE , Graff-Guerrero A , Houle S , Mizrahi R , Wilson AA , Pollock BG , Mulsant BH , Felsky D , Voineskos AN , Tang-Wai DF , Verhoeff NP , Freedman M , Ismail Z , Chow TW ((2016) ) Amyloid deposition in semantic dementia: A positron emission tomography study. Int J Geriatr Psychiatry 31: , 1064–1074. |
[45] | Santos-Santos MA , Rabinovici GD , Iaccarino L , Ayakta N , Tammewar G , Lobach I , Henry ML , Hubbard I , Mandelli ML , Spinelli E , Miller ZA , Pressman PS , O’Neil JP , Ghosh P , Lazaris A , Meyer M , Watson C , Yoon SJ , Rosen HJ , Grinberg L , Seeley WW , Miller BL , Jagust WJ , Gorno-Tempini ML ((2018) ) Rates of amyloid imaging positivity in patients with primary progressive aphasia. JAMA Neurol 75: , 342–352. |
[46] | Kobayashi R , Hayashi H , Kawakatsu S , Ishiki A , Okamura N , Arai H , Otani K ((2018) ) [(18)F]THK-5351 PET imaging in early-stage semantic variant primary progressive aphasia: A report of two cases and a literature review. BMC Neurol 18: , 109. |
[47] | Kerchner GA , Tartaglia MC , Boxer A ((2011) ) Abhorring the vacuum: Use of Alzheimer’s disease medications in frontotemporal dementia. Expert Rev Neurother 11: , 709–717. |




