PRDM1 rs2185379, unlike BRCA1, is not a prognostic marker in patients with advanced ovarian cancer
Abstract
BACKGROUND:
Ovarian cancer (OC) is mostly diagnosed in advanced stages with high incidence-to-mortality rate. Nevertheless, some patients achieve long-term disease-free survival. However, the prognostic markers have not been well established.
OBJECTIVE:
The primary objective of this study was to analyse the association of the suggested prognostic marker rs2185379 in PRDM1 with long-term survival in a large independent cohort of advanced OC patients.
METHODS:
We genotyped 545 well-characterized advanced OC patients. All patients were tested for OC predisposition. The effect of PRDM1 rs2185379 and other monitored clinicopathological and genetic variables on survival were analysed.
RESULTS:
The univariate analysis revealed no significant effect of PRDM1 rs2185379 on survival whereas significantly worse prognosis was observed in postmenopausal patients (HR
CONCLUSIONS:
Unlike age at diagnosis, OC histology or gBRCA1 status, rs2185379 in PRDM1 is unlikely a marker of long-term survival in patients with advance OC.
1.Introduction
Ovarian cancer (OC) is mostly diagnosed in advanced stages (70%), leading to a high incidence-to-mortality rate. Although patients with advanced OC achieve remission with maximum debulking surgery and chemotherapy, recurrence, usually incurable, often occurs within 3 years and the 5-year survival of late-stage OC is below 30% [1]. Nevertheless, some OC patients achieve long-term disease-free survival despite the diagnosis at advanced stage. Thus, attempts are being made to identify further prognostic markers of long-term survival. Recently, the polymorphism rs2185379 in PRDM1 has been associated with long-term recurrence-free survival in Japanese advanced OC patients and, based on the mouse model, heterozygous rs2185379 was suggested to induce initial differentiation of T lymphocytes in antitumor immune response [2]. However, the precise impact of rs2185379 on protein function or possible linkage to another variant with prognostic significance is unknown. The minor allele frequency of rs2185379 in GnomAD varies between populations from 2% in Latino America to 3.1% in European non-Finnish, 5.9% in East Asian and 7% in African American.
The PRDM1 (positive regulatory domain zinc finger protein 1; OMIM*603423) codes for the BLIMP1 (B lymphocyte-induced maturation protein 1) transcription factor that is involved in the regulation of antitumor immunity. It was recently shown that BLIMP1 enhances transcription of USP22 deubiquitinating enzyme leading to decreased degradation of SPI1 transcription factor and subsequent enhanced expression of programmed death ligand 1 (PD-L1), which leads to infiltrated CD8+ T cell exhaustion and memory responses [3]. BLIMP1 was shown to play a role in the development of malignant lymphoma, leukemia, and some non-haematopoietic cancers, including breast and colorectal cancer, hepatocellular carcinoma, or glioma as reviewed in [4]. In particular, decreased expression of PRDM1 correlates with a poor prognosis in lung cancer [5]. However, little is known about the role of PRDM1 in ovarian cancer. Zhang et al. suggested that tumour-infiltrating T lymphocytes can improve the long-term outcome of patients with advanced OC [6].
In this work, we explored the association of heterozygous rs2185379 PRDM1 variant with long-term survival of advanced OC and compared the effect of rs2185379 with selected clinicopathological and genetic factors influencing the prognosis of OC patients.
Table 1
Clinicopathological characteristics and identified genotypes
| All OC pts | Survival | Survival | rs2185379 | rs2185379 | ||
|---|---|---|---|---|---|---|
| Mean age at dg | 57.5 | 58.3 | 54.2 | 55 | 55.5 | |
| Menoactivity | Pre | 165 | 51 | 92 | 149 | 12 |
| Post | 389 | 131 | 145 | 358 | 25 | |
| NA | 1 | 0 | 0 | 1 | 0 | |
| Histology | Clear cell | 3 | 0 | 0 | 3 | 0 |
| Endometrioid | 15 | 3 | 8 | 13 | 1 | |
| Mucinous | 5 | 1 | 3 | 5 | 0 | |
| Other | 25 | 8 | 14 | 22 | 2 | |
| Serous | 497 | 165 | 208 | 456 | 34 | |
| 447 | 154 | 178 | 408 | 33 | ||
| 45 | 9 | 25 | 43 | 1 | ||
| 5 | 2 | 5 | 5 | 0 | ||
| Undifferentiated | 6 | 4 | 2 | 5 | 0 | |
| NA/unclassified | 4 | 1 | 2 | 4 | 0 | |
| Grade | Poorly differentiated (Grade3, High) | 489 | 170 | 197 | 446 | 34 |
| Well differentiated (Grade1, Low) | 52 | 10 | 31 | 48 | 3 | |
| NA | 14 | 2 | 9 | 14 | 0 | |
| Stage | IIIA | 52 | 10 | 26 | 50 | 1 |
| IIIB | 72 | 17 | 36 | 69 | 2 | |
| IIIC | 349 | 123 | 152 | 313 | 30 | |
| IV | 82 | 32 | 23 | 76 | 4 | |
| Surgery | Primary surgery | 343 | 87 | 176 | 316 | 22 |
| Interval Debulking | 202 | 89 | 60 | 183 | 15 | |
| No | 9 | 6 | 1 | 8 | 0 | |
| NA | 1 | 0 | 0 | 1 | 0 | |
| Residual disease | Not reported | 371 | 86 | 176 | 341 | 26 |
| Tumour | 80 | 45 | 22 | 70 | 7 | |
| Tumour | 70 | 34 | 20 | 65 | 3 | |
| NA | 34 | 17 | 19 | 32 | 1 | |
| Vital status | Alive in complete remission | 249 | x | 145 | 223 | 22 |
| Alive with disease | 53 | x | 26 | 52 | 1 | |
| Dead | 248 | 182 | 66 | 229 | 14 | |
| Missing | 5 | 0 | 0 | 4 | 0 | |
| Chemo | Adjuvant only | 331 | 85 | 167 | 305 | 21 |
| Chemo yes, no operation performed | 7 | 6 | 0 | 6 | 0 | |
| NA | 7 | 0 | 5 | 7 | 0 | |
| Neoadjuvant | 203 | 89 | 61 | 184 | 15 | |
| No | 7 | 2 | 4 | 6 | 1 | |
| Mutation | neg | 369 | 125 | 138 | 342 | 24 |
| BRCA1 | 112 | 33 | 63 | 100 | 7 | |
| BRCA2 | 48 | 17 | 22 | 42 | 5 | |
| RAD51C/D/BRIP1 | 14 | 2 | 9 | 13 | 1 | |
| MMR | 1 | 1 | 0 | 1 | 0 | |
| Other (PALB2, ATM, CHEK2, TP53) | 11 | 4 | 5 | 10 | 0 | |
| MINAS | 4 | 3 | 1 | 4 | 0 | |
| PRDM1 | rs2185379 | 37 | 9 | 15 | ||
| rs2185379 | 508 | 168 | 219 | |||
| rs2185379_NA | 10 | 5 | 3 |
*Alive patients diagnosed in 2018 or later (e.g.
2.Methods
2.1Patients
Five-hundred-and-fifty-five patients diagnosed with advanced staged OC (FIGO stages III/IV) with available DNA were enrolled regardless of familial cancer history or OC histology (Table 1). All the patients were previously tested for OC cancer predisposition [7]. Genotyping of PRDM1 rs2185379 was successfully performed in 545 of them. Clinicopathological data were obtained during genetic counselling or retrieved from patients’ records. Vital status for the estimation of survival function using the Kaplan-Meier curve was available for 541 patients. All patients were Caucasians of Czech origin. Written informed consent was obtained from all patients. The study was approved by the Ethics Committee of the General University Hospital in Prague (approval number 92/14) and performed in accordance with the Declaration of Helsinki.
2.2Genotyping
We performed genotyping of rs2185379 (NM_001198: c.220G>A; p.Gly74Ser) from DNA derived from peripheral blood using the high resolution melting analyses (primers: 5'-GTGGACAGAGGCTGAGTTTGA-3'; 5’-TCACTGTTGGTGGCATACTTGA-3') on LightCycler 480 System (Roche). Each run included negative and positive control with genotype previously confirmed by whole exome sequencing. All positive samples were confirmed by Sanger sequencing.
2.3Statistical analysis
The effect of monitored variables on survival was analysed using Kaplan-Meier analysis and Cox regression in R studio (libraries survival, ranger, ggplot2, ggfortify).
3.Results
We performed genotyping of 545 well-characterized advanced-stage OC patients that were previously tested for OC cancer predisposition [7]. We identified 37 (6.8%) OC patients heterozygous for rs2185379 and no homozygote of the alternative allele (consistent with Hardy-Weinberg equilibrium).
Figure 1.
The effect of monitored variables on survival of patients diagnosed with advanced OC. Figure 1 shows univariate survival analysis of the PRDM1 rs2185379 status (Fig. 1A), menoactivity status (Fig. 1B), OC histology (Fig. 1C), germline BRCA1 mutation status (Fig. 1D), and multivariate analysis interrogating the effect of PRDM1 rs2185379 and the menoactivity status (Fig. 1E).
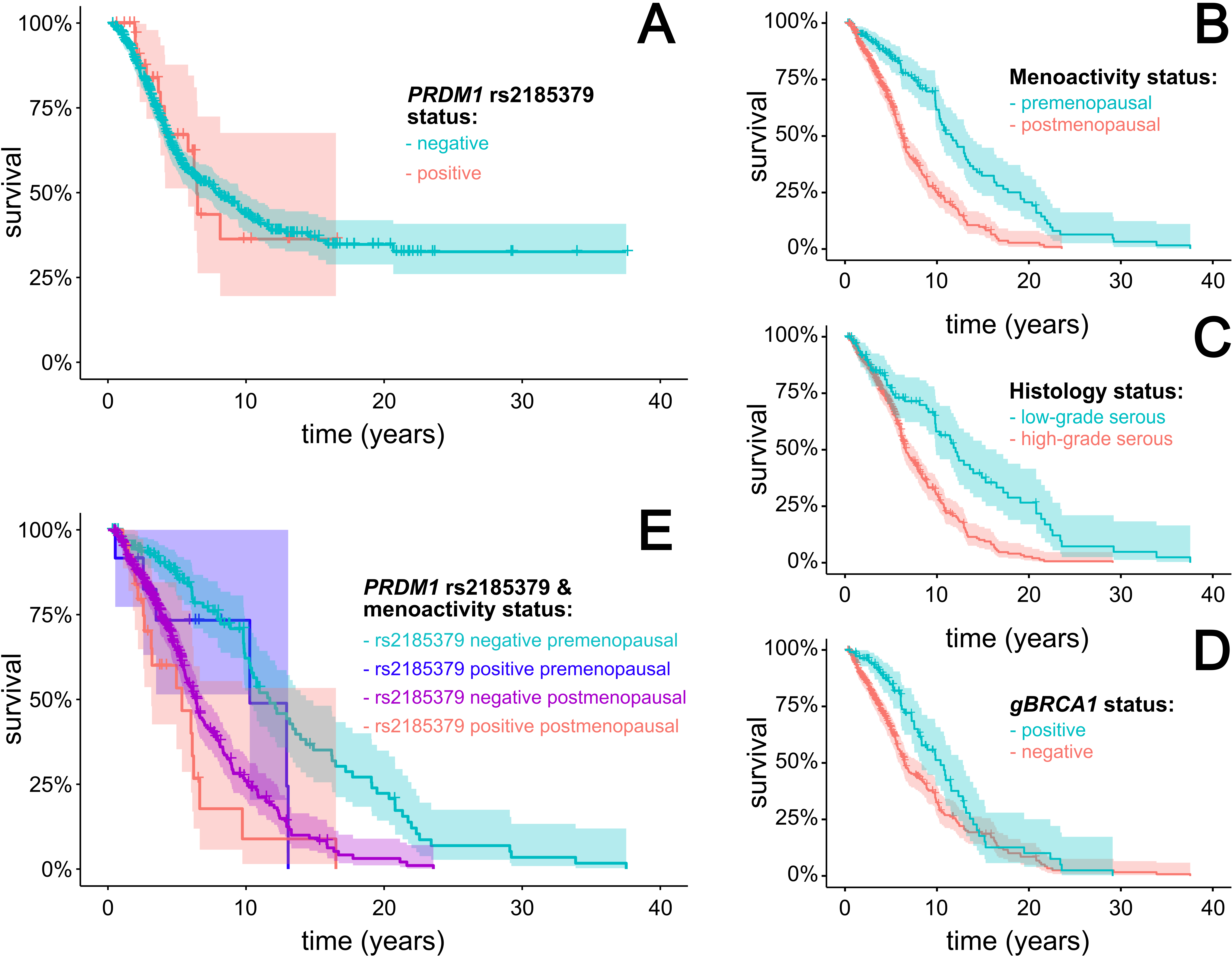
Subsequently, we performed univariate survival analysis of PRDM1 rs2185379 status as well as of the individual clinicopathological characteristics and presence of pathogenic/likely pathogenic variants in OC predisposition genes. Despite the numerically higher frequency of PRDM1 rs2185379 heterozygotes among long-term survivors (6.4%) compared to short-term survivors (5.1%;
The multivariate analysis interrogating the effect of PRDM1 rs2185379 with other analysed significant prognostic factors revealed that PRDM1 rs2185379 was marginally associated with worse survival in postmenopausal women with hazard ratio 1.54 (95%CI 1.01–2.38;
4.Discussion
Identification of the genetic and non-genetic factors modulating the prognosis of patients with advanced OC is an important prerequisite for improving the unsatisfactory outcomes of these patients. Here, we analyzed the rs2185379 in the PRDM1 gene in 545 well-characterized advanced OC patients. Recently, Mitamura and colleagues described association of rs2185379 with an excellent OC prognosis and suggested that the PRDM1 polymorphism is involved in the anticancer T-lymphocyte immunity [2]. Contrary to this report that analyzed only a small group of 24 advanced OC patients of the Japanese origin, we found lack of the association between rs2185379 and a long-term survival in our 545 Caucasian OC patients of the Czech origin.
To demonstrate the consistency of our patient population, we analyzed previously described associations of monitored clinicopathological and genetic factors with survival in advanced OC. We observed significant survival advantage in patients diagnosed with advanced OC premenopausally, as described by Chan et al. previously [8]. Accordingly, the increasing age at diagnosis directly correlated with worse survival. Similarly, HG serous OC was associated with significantly worse prognosis compared to LG serous or to non-HG serous OC, as described in previous studies [9]. Furthermore, we observed significantly improved survival in BRCA1-positive OC patients, with the most pronounced effect in the first five years after diagnosis that disappeared after 11 years since diagnosis. Similar results were observed by McLaughlin et al. and Heemskerk-Gerritsen et al, who observed survival benefit for BRCA1- and BRCA2-positive OC patients that disappeared after 10 and 6 years after diagnosis, respectively [10, 11].
Interrogation of significant clinicopathological and genetic factors revealed a worsened survival in a subset of rs2185379 carriers diagnosed with advanced OC postmenopausally, suggesting rather an opposite, if any, effect of this genetic marker on advanced OC prognosis. The PRDM1 gene product BLIMP1 might improve survival and therapeutic response enhancing the transcription of PD-L1 [3]. Blocking PD-1/PD-1L signaling improves anticancer T-cell responses making PRDM1 a promising survival biomarker. However, rs2185379 in PRDM1 does not seem to influence the BLIMP1 function significantly, at least with its impact on survival in patients with advanced OC.
Acknowledgments
This study was supported by Ministry of Health of the Czech Republic NU20-03-00016; Charles University: COOPERATIO; Charles University: SVV260516; Programme EXCELES, ID Project No. LX22NPO5102 –Funded by the European Union – Next Generation EU.
Author contributions
Conception: SJ, KZ; Interpretation or analysis of data: HK, LS, ZP, VM; Preparation of the manuscript: SJ; Revision for important intellectual content: VM; Supervision: KZ.
References
[1] | S. Lheureux, C. Gourley, I. Vergote and A.M. Oza, Epithelial ovarian cancer, Lancet 393: ((2019) ), 1240–1253. |
[2] | T. Mitamura, T. Zhai, K.C. Hatanaka, Y. Hatanaka, T. Amano, L. Wang, S. Tanaka and H. Watari, Germline PRDM1 Variant rs2185379 in Long-Term Recurrence-Free Survivors of Advanced Ovarian Cancer, Pharmgenomics Pers Med 15: ((2022) ), 977–984. |
[3] | Q. Li, L. Zhang, W. You, J. Xu, J. Dai, D. Hua, R. Zhang, F. Yao, S. Zhou, W. Huang, Y. Dai, Y. Zhang, T. Baheti, X. Qian, L. Pu, J. Xu, Y. Xia, C. Zhang, J. Tang and X. Wang, PRDM1/BLIMP1 induces cancer immune evasion by modulating the USP22-SPI1-PD-L1 axis in hepatocellular carcinoma cells, Nat Commun 13: ((2022) ), 7677. |
[4] | A. Casamassimi, M. Rienzo, E. Di Zazzo, A. Sorrentino, D. Fiore, M.C. Proto, B. Moncharmont, P. Gazzerro, M. Bifulco and C. Abbondanza, Multifaceted Role of PRDM Proteins in Human Cancer, Int J Mol Sci 21: ((2020) ). |
[5] | Z. Zhu, H. Wang, Y. Wei, F. Meng, Z. Liu and Z. Zhang, Downregulation of PRDM1 promotes cellular invasion and lung cancer metastasis, Tumour Biol 39: ((2017) ), 1010428317695929. |
[6] | L. Zhang, J.R. Conejo-Garcia, D. Katsaros, P.A. Gimotty, M. Massobrio, G. Regnani, A. Makrigiannakis, H. Gray, K. Schlienger, M.N. Liebman, S.C. Rubin and G. Coukos, Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer, N Engl J Med 348: ((2003) ), 203–13. |
[7] | K. Lhotova, L. Stolarova, P. Zemankova, M. Vocka, M. Janatova, M. Borecka, M. Cerna, S. Jelinkova, J. Kral, Z. Volkova, M. Urbanova, P. Kleiblova, E. Machackova, L. Foretova, J. Hazova, P. Vasickova, F. Lhota, M. Koudova, L. Cerna, S. Tavandzis, J. Indrakova, L. Hruskova, M. Kosarova, R. Vrtel, V. Stranecky, S. Kmoch, M. Zikan, L. Macurek, Z. Kleibl and J. Soukupova, Multigene Panel Germline Testing of 1333 Czech Patients with Ovarian Cancer, Cancers (Basel) 12: ((2020) ). |
[8] | J.K. Chan, R. Urban, M.K. Cheung, K. Osann, J.Y. Shin, A. Husain, N.N. Teng, D.S. Kapp, J.S. Berek and G.S. Leiserowitz, Ovarian cancer in younger vs older women: a population-based analysis, Br J Cancer 95: ((2006) ), 1314–20. |
[9] | A. Gockley, A. Melamed, A.J. Bregar, J.T. Clemmer, M. Birrer, J.O. Schorge, M.G. Del Carmen and J.A. Rauh-Hain, Outcomes of Women With High-Grade and Low-Grade Advanced-Stage Serous Epithelial Ovarian Cancer, Obstet Gynecol 129: ((2017) ), 439–447. |
[10] | J.R. McLaughlin, B. Rosen, J. Moody, T. Pal, I. Fan, P.A. Shaw, H.A. Risch, T.A. Sellers, P. Sun and S.A. Narod, Long-term ovarian cancer survival associated with mutation in BRCA1 or BRCA2, J Natl Cancer Inst 105: ((2013) ), 141–8. |
[11] | B.A.M. Heemskerk-Gerritsen, A. Hollestelle, C.J. van Asperen, I. van den Beek, W.J. van Driel, K. van Engelen, E.B. Gomez Garcia, J.A. de Hullu, M.J. Koudijs, M.J.E. Mourits, M.J. Hooning and I.A. Boere, Progression-free survival and overall survival after BRCA1/2-associated epithelial ovarian cancer: A matched cohort study, PLoS One 17: ((2022) ), e0275015. |




