EGFR, HLA-G, CD70, c-MET, and NY-ESO1 as potential biomarkers in high grade epithelial ovarian carcinoma
Abstract
High grade epithelial ovarian carcinoma is an aggressive tumor. Treatment includes platinum therapy, however it recurs in most patients due to therapy resistance. In this project, we study the immunohistochemical (IHC) expression of five potential biomarkers/prognostic markers in high grade epithelial ovarian carcinoma: EGFR, HLA-G, CD70, c-MET, and NY-ESO1. A cohort of 274 patients is used. We compare the IHC expression with age, stage, ascites status, family history of cancer, disease free survival (DFS) and overall survival (OS). EGFR expression is significantly correlated with family history and worse OS. HLA-G is associated with worse OS. To confirm the results of EGFR and HLA-G, a second separated cohort of 248 patients is used. Positive EGFR expression again shows worse OS, while HLA-G expression has worse prognostic trend. CD70 has a worse OS trend. C-MET and NY-ESO1 do not have any clinical correlations. EGFR can potentially serve as target in future clinical immune therapy trials.
1.Background
Among all gynecological cancer types, ovarian carcinoma has the worst prognosis and the highest mortality rate [1]. Histologically, it consists of several distinct subtypes, including high grade serous carcinoma, endometroid carcinoma, clear cell carcinoma, and mucinous carcinoma. Rare high grade subtypes include malignant mixed Mullerian tumor (MMMT), undifferentiated carcinoma, and mixed type carcinoma [2]. Due to the aggressiveness of the disease and the absence of early symptoms, the five-year survival rate is less than 50% [3]. Current first-line treatment includes cytoreductive surgery and platinum-based chemotherapy. However, patients frequently experience recurrence due to platinum resistance [4, 5].
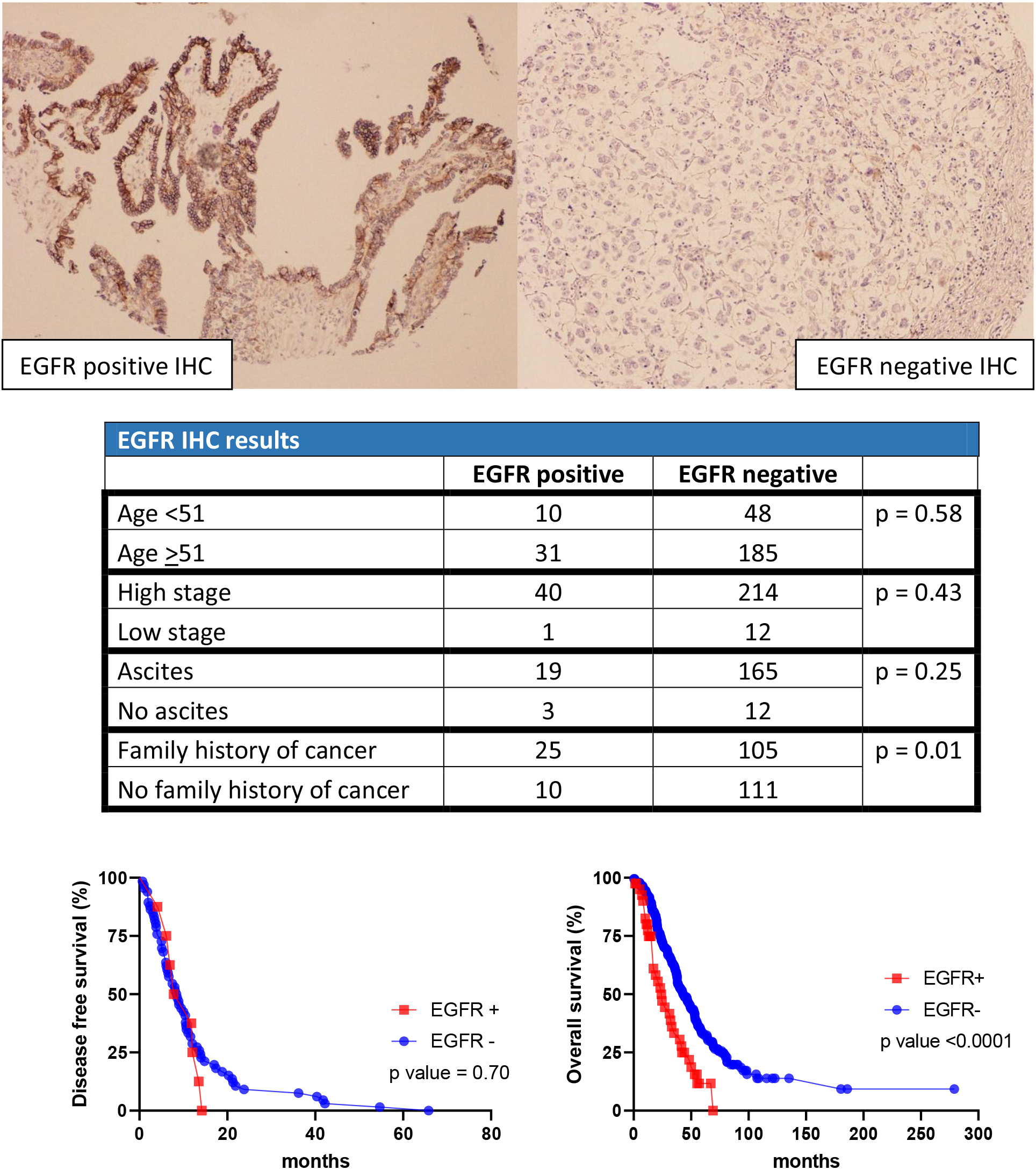
Figure 1.
EGFR results in cohort 1.
Therefore, immunotherapy and targeted therapies can potentially serve as new treatments for ovarian carcinoma. Immunotherapy enhances the attack of immune system on neoplastic cells through different approaches: immunostimulatory cytokines, tumor antigen vaccines, antibodies targeting immunosuppressive ligands expressed by tumor cells and immune checkpoint inhibition. The immune system utilizes the immune checkpoints (e.g., CTLA-4, PD-1, HHLA2, B7-H4, and TIM-3) to recognize self-cells versus foreign-cells. The expression of immune checkpoints within the tumor cells helps evade the attack by T cells. The cytotoxicity of T cells toward tumor cells is restored by blocking the immune checkpoints [6, 7].
Membrane proteins make up approximately 30% of total human proteins and they play roles in various physiological and pathological functions [8]. Tumor derived membrane proteins may serve as potential biomarkers in early diagnosis. They can also be used to assess disease progression and prediction for treatment response [8]. EGFR, HLA-G, CD70, c-MET and NY-ESO1 have been found to play certain roles in tumor genesis and immune evasion in different tumor types. Reports have shown membranous expression of these markers in ovarian carcinoma. However, the clinical significance is not fully understood.
Figure 2.
HLA results in cohort 1.
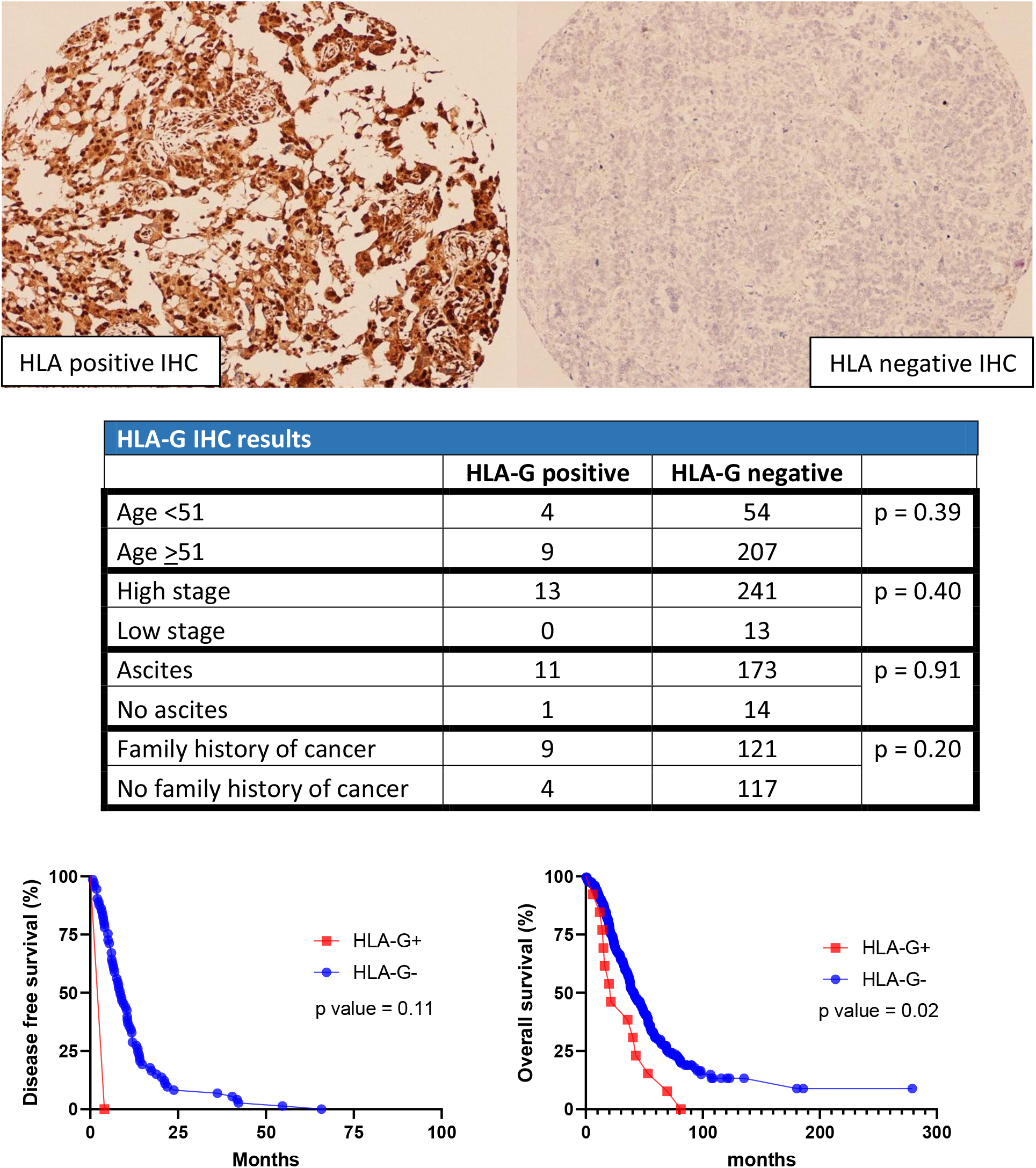
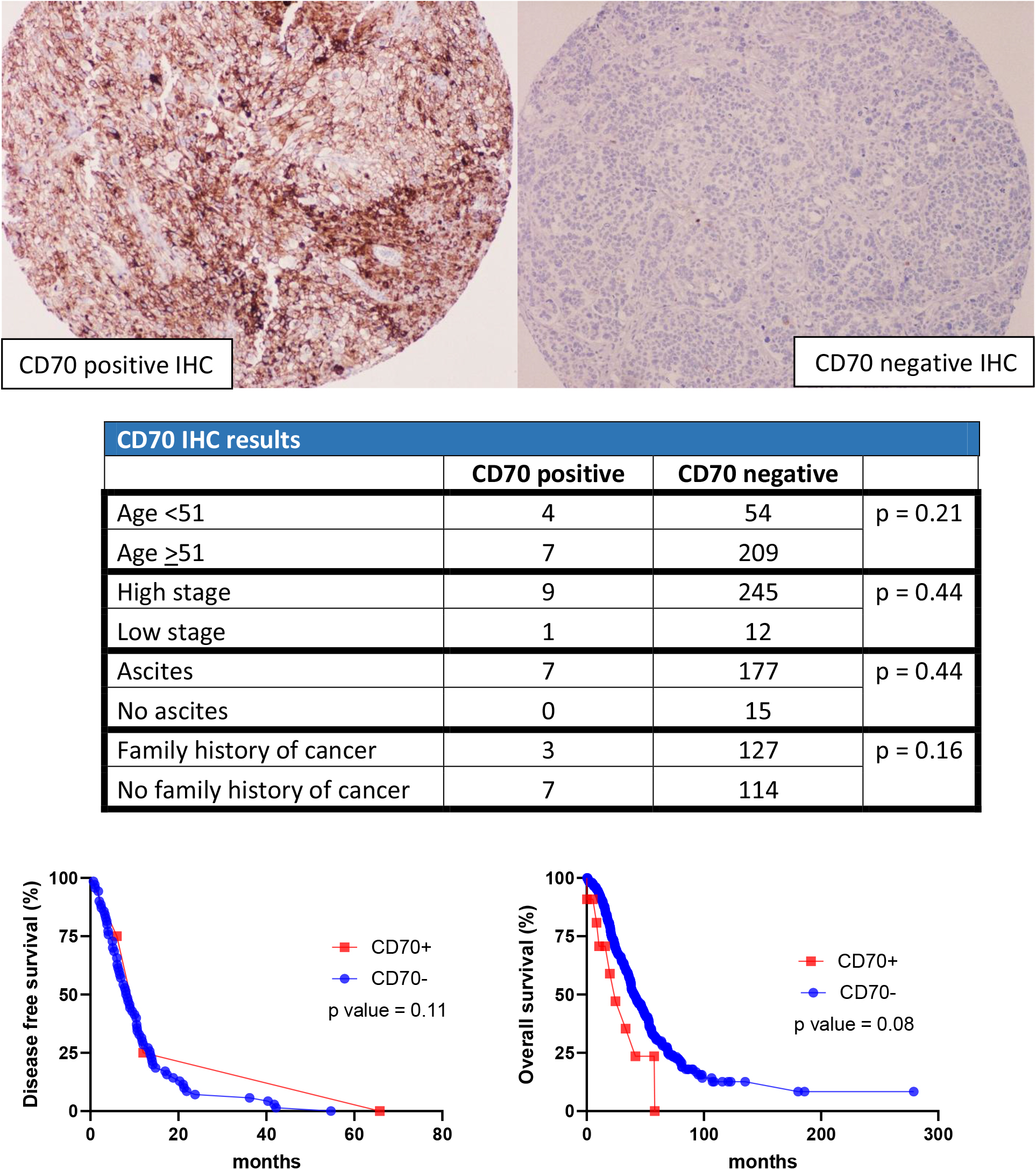
Figure 3.
CD70 results in cohort 1.
2.Objective
In our study, we conduct IHC studies of five previously mentioned markers with associated immunotherapy on two large cohorts of ovarian carcinoma. Subsequently, we correlate their expression with the survival rates.
3.Materials and methods
3.1Study population
In this study, two cohorts of patients were utilized. The first cohort comprised a total of 418 female patients with various ovarian tumor types, with 274 of them diagnosed with high grade ovarian carcinoma. The second cohort included 453 female patients with different ovarian tumors, of which 248 were diagnosed with high grade ovarian carcinoma. Patient data was collected from electronic medical records and laboratory information systems spanning from 1987 to 2006. We extracted information such as ovarian cancer diagnoses, patient ages, AJCC stages, ascites presence, family history, DFS intervals, OS times, and survival statuses.
3.2Tissue microarray (TMA) construction and Immunohistochemistry
Formalin-fixed, paraffin-embedded (FFPE) tissue blocks from the two cohorts were retrieved from the MD Anderson Cancer Center storage room. The tissue blocks had been stored under ambient conditions at approximately 24∘C. H&E stained sections from each patient were reviewed by a pathologist to identify representative tumor areas. TMAs were constructed by taking one to two core samples from the identified areas on the FFPE blocks and assembled on the TMA-paraffin blocks. The extraction of the tissue from FFPE blocks and the mapping of the TMA blocks were performed using a precision instrument (Beecher Instruments, Silver Spring, MD, USA). For each case, one to two 1 mm wide and 5 mm deep samples were collected and mapped in the TMA blocks. The final TMAs consisted of seven blocks, each containing from 100 to 148 punches. Four-micrometer thick sections from each TMA block were stained with EGFR, CD70, c-MET, HLA-G, and NY-ESO1 IHC.
EGFR antibody clone 31G7 from Abnova with a 1:20 dilution, CD70 antibody clone E3Q1A from Cell Signaling with a 1:50 dilution, c-MET antibody clone SP44 from Ventana with a 1:1 dilution, HLA-G antibody clone 3H2678 from US Biological Life Sciences with a 1:100 dilution, and NY-ESO1 antibody clone E978 from Santa Cruz Biotechnology with a 1:100 dilution were used to perform immunohistochemical stains on the TMAs. HLA-G was incubated in Citrate buffer, while EGFR, NY-ESO1, c-Met, and CD70 were incubated in Tris-EDTA buffer. The staining process was performed at MD Anderson Cancer Center immunohistochemical laboratory.
The results were stratified as either negative (no staining or non-specific staining) or positive (
Descriptive statistics, Kaplan Meier curves, and survival analysis were performed using GraphPad Prism v.8.4.3 software (La Jolla, CA).
3.3Institutional review board statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of MD Anderson Cancer Center (protocol code: LAB11-0418, date of approval: 6/7/2011).
Table 1
Clinical information of cohort 1
| Number of patients | 274 | |||
|---|---|---|---|---|
| Types of carcinoma | 210 high grade serous | 11 MMMT | 44 mixed type carcinoma | 9 undifferentiated carcinoma |
| Age range | 27.5–92.4 years old | |||
| Stages | 13 low stage (I & II) | 254 high stage (III & IV) | ||
| Ascites | 184 with ascites | 15 without ascites | ||
| Family history of any cancer types | 130 with family history | 121 without family history | ||
| Disease free survival range | 2.4–65.8 months | |||
| Overall survival time | 0.3–279 months | |||
| Survival status | 186 died from ovarian carcinoma | 88 alive/died from other causes | ||
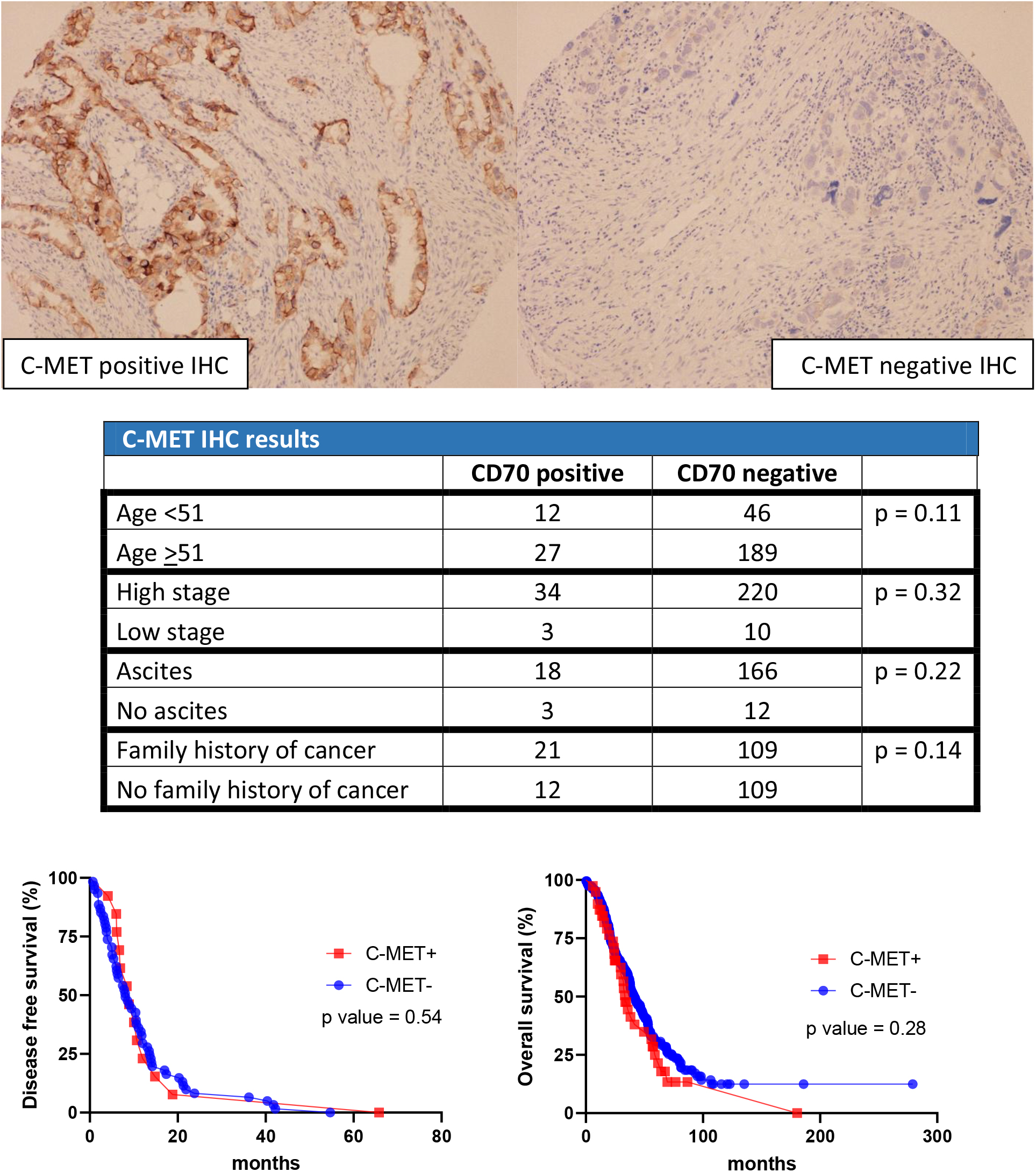
Figure 4.
C-MET results in cohort 1.
4.Results
In the first cohort, 274 out of 418 patients were diagnosed with high grade ovarian carcinoma. The age ranged from 21.7 years to 92.4 years. The diagnoses were further categorized into 210 high grade serous carcinoma, 11 MMMT, 44 mixed malignant carcinoma, and 9 undifferentiated carcinoma. Of the 274 patients, 254 had advanced AJCC stage at the time of diagnosis (stages III & IV) and 13 had low stage (stages I & II). Documentation of ascites was recorded for 199 patients: 184 with clinical symptoms of ascites versus 15 without. Detailed family cancer history was recorded for 251 patients: 130 with family history of some forms of cancer versus 121 without. The disease-free survival time (from treatment to recurrence of or death due to ovarian carcinoma) ranged from 2.4 months to 65.8 months. The overall survival time (from treatment to death due to ovarian carcinoma) ranged from 0.3 months to 279 months. 186 patients died due to high grade ovarian carcinoma, and 88 patients either survived or died due to other causes (Table 1).
Table 2
IHC results for cohort 1
| Positive | Negative | |
|---|---|---|
| EGFR | 41 | 233 |
| CD70 | 11 | 263 |
| C-MET | 39 | 235 |
| HLA-G | 13 | 261 |
| NY-ESO1 | 23 | 251 |
Table 3
Clinical information of cohort 2
| Number of patients | 248 | |||
|---|---|---|---|---|
| Types of carcinoma | 218 high grade serous | 6 MMMT | 24 mixed type carcinoma | 1 undifferentiated carcinoma |
| Age range | 39.7–85.4 years old | |||
| Disease free survival range | 1–152.7 months | |||
| Overall survival time | 0.3–227 months | |||
| Survival status | 72 died from ovarian carcinoma | 176 alive/died from other causes | ||
Figure 5.
NY-ESO1 results in cohort 1.
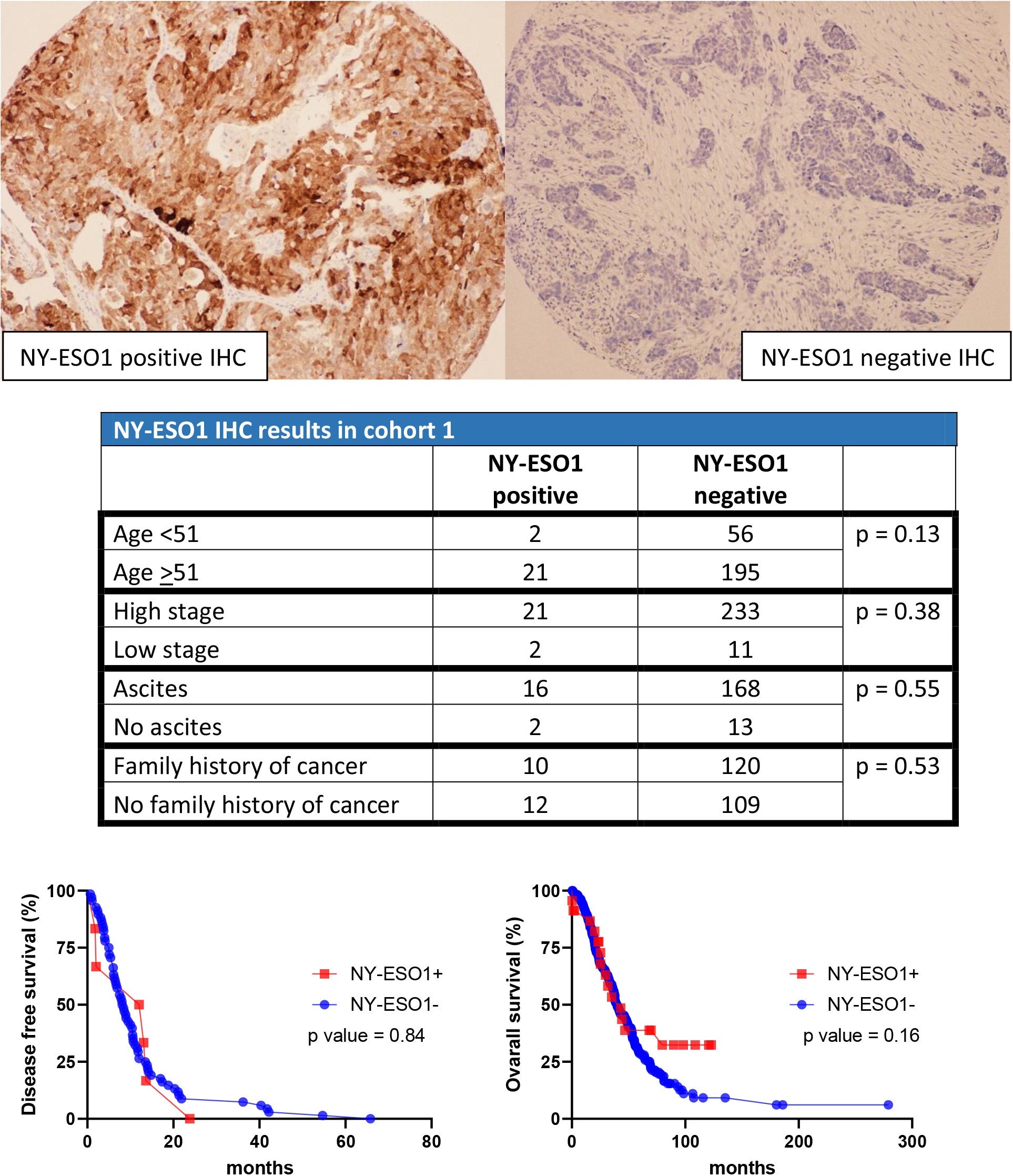
Figure 6.
EGFR expression vs DFS (left) and OS (right) in cohort 2.
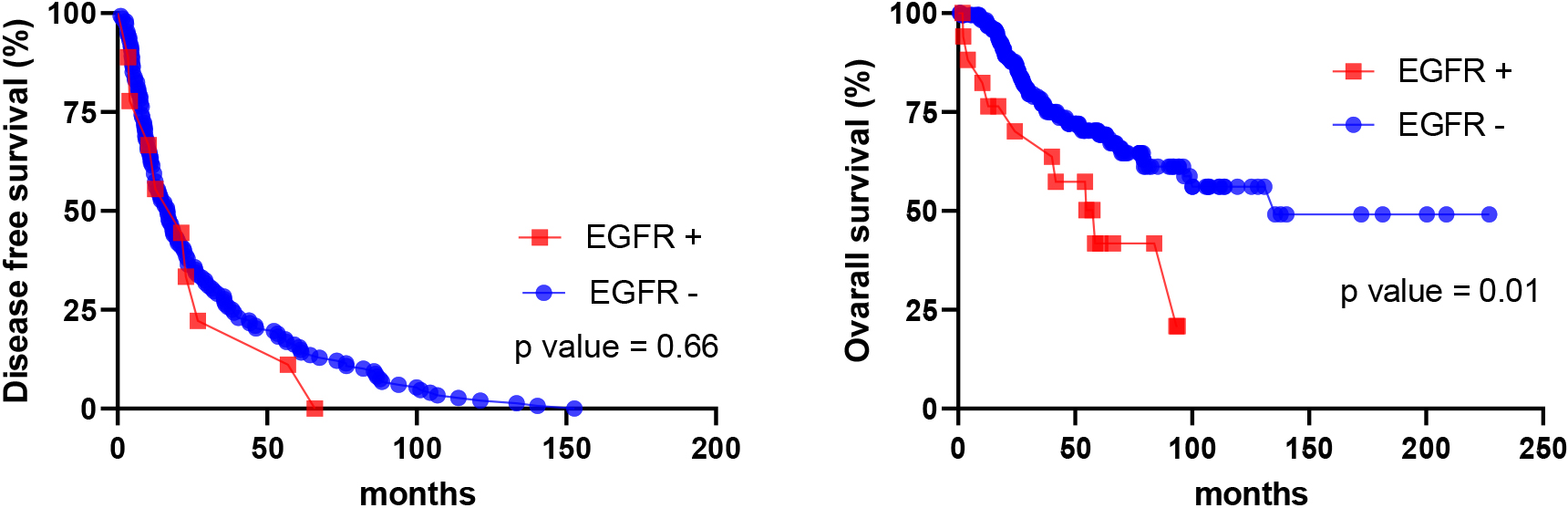
Figure 7.
HLA-G expression vs DFS (left) and OS (right) in cohort 2.
In the first cohort, 41 cases were positive for EGFR and 233 cases were negative. For HLA-G, 13 cases stained positive while 261 cases stained negative For CD70, 11 cases stained positive while 263 cases stained negative. For C-MET, 39 cases stained positive while 235 cases stained negative. For NY-ESO1, 23 cases stained positive while 251 cases stained negative (Table 2).
EGFR-positive and negative stains were stratified into different groups based on age (
The same statistical analysis was performed for HLA-G. There was no significant difference between HLA-G status versus age, AJCC stage, clinical symptoms of ascites, and family history of cancer. In addition, HLA-G staining pattern is not associated with DFS. However, with the OS, HLA-G-positive expression is associated with shorter survival compared to HLA-G negative expression (
For CD70, C-MET, and NY-ESO1 immunohistochemical stains, there was no significant difference between each individual stain versus age, AJCC stage, clinical symptoms of ascites, and family history of cancer. Furthermore, the Kaplan-Meier curve showed that there was no correlation between each stain versus DFS and OS (Figs 3–5).
A second separate cohort was used to confirm the association between DFS and OS versus EGFR and HLA-G staining patterns. In this cohort, 248 out of 453 females were diagnosed with high grade ovarian carcinoma. The age ranged from 39.7 years to 85.4 years. The diagnoses were categorized into 218 high grade serous carcinoma, 5 MMMT, 24 mixed malignant carcinoma, and 1 undifferentiated carcinoma. The DFS intervals ranged from 1 to 152.7 months, and the OS ranged from 0.6 to 227 months. 72 patients died from ovarian carcinoma, and 176 patients died from other causes or are still alive (Table 3). For EGFR, Kaplan-Meier curve showed no clinical significance with the DFS intervals (
5.Discussion/conclusion
Ovarian epithelial tumors are generally separated into two major categories: type I versus type II. Type I carcinoma follows a step-wise progression from benign histology to borderline tumors to low grade malignancy. Molecularly, these tumors are associated with KRAS, BRAF, PTEN, PIK3CA or ARID1A mutations. Clinically, they grow locally, metastasize late, and behave in a less aggressive manner [9]. Type II carcinoma, on the other hand, is frequently discovered at an advanced stage and is more aggressive. Molecularly, it is associated with TP53 mutations, hypermethylation, or BRCA1/2 mutations [10]. Histologically, it consists of high grade serous carcinoma, mixed type carcinoma, malignant mixed Mullerian tumor, and undifferentiated carcinoma. In this study, we compare the clinical data of patients with high grade carcinoma against the immunohistochemical expression of EGFR, HLA-G, CD70, NY-ESO1, and C-Met, in the hope that they can be used as biomarkers or part of the therapeutic regime in the future.
5.1EGFR
Epidermal growth factor receptor (EGFR) is a receptor tyrosine kinase protein that functions in cell development, autophagy and metabolism regulation. Mutations in EGFR have been associated with different cancer types, such as lung, colon, and pancreas [11, 12]. In our analysis, we find an association between EGFR staining and family history of different cancer types. This supports the idea that EGFR plays a vital role in neoplastic development, not limited to a specific type. Furthermore, with the association of family history, mutations in EGFR may be part of the syndromic cause for carcinoma. We also performed an analysis to associate the staining pattern of EGFR to other clinical data (age, clinical symptoms of ascites, and staging), but we found no correlation.
Additionally, we demonstrate that EGFR expression is significantly associated with a worse OS rate, with a median of 24 months versus 43.1 months in non-EGFR patients. To confirm the result, a separate cohort is used, and a similar outcome is concluded: EGFR expression is associated with a shorter median survival of 58.5 months, while the non-EGFR population is associated with a longer median survival of 135.5 months. Our result is inconsistent with Mehner’s study, which has a sample size of 488. Her study concludes that there is no association between EGFR staining patterns and survival outcomes. However, Mehner’s study incorporates different types of ovarian epithelial carcinoma (low grade serous, mucinous, endometrioid, clear cell, high grade serous, and mixed type) [13]. Our study, on the other hand, is more specific for high grade carcinoma (high grade serous, MMMT, mixed type, and undifferentiated). Thus, the difference in tumor type selection could explain the disparity between the two studies.
Preclinical study of anti-EGFR antibody shows promising results, as tumor cells exhibit increased sensitivity to NK cell-mediated antibody-dependent cellular cytotoxicity [14]. With the correlation of worse prognosis, EGFR can serve as a potential biomarker and therapeutic marker in the future.
5.2HLA-G
Human leukocyte antigen-G (HLA-G) is expressed within the placental and embryonic tissues. It plays a role in the tolerance of the maternal immune system by inhibiting the differentiation, proliferation, and cytokine release from different immune cell types. It is postulated that tumor cells use HLA-G to evade immune surveillance [15]. HLA-G mRNA and protein levels, as well as immunohistochemical expression, have been found in greater quantity in high stage and high grade epithelial ovarian cancer [16, 17]. In our analysis, we do not find any correlation between HLA-G expression and age, clinical symptoms of ascites, advanced AJCC stage, or DFS intervals. When analyzing the OS rate, a correlation between positive HLA-G expression and worse OS rate is observed (
Literature review of HLA-G associated survival rates has yielded mixed results. The conclusion from Jung’s study suggests an unfavorable prognosis, while Rutten’s study shows the contrary. The discrepancy might be due to different sample selections. Jung’s cohort includes both type I and II ovarian carcinoma, while Rutten’s cohort includes only type II ovarian carcinoma [16, 18]. In addition, both our study and Rutten’s study use tissue microarrays, but with different methods of scoring. Rutten’s study stratifies the staining results by adding the score of staining percentage (0-5) and intensity (0-3). Positive HLA-G staining has a score of
5.3CD70
CD70 is a membranous protein that belongs to the tumor necrosis factor family, its activation leads to cellular proliferation. CD70 expression is associated with cisplatin resistance and decreased survival rates in ovarian carcinoma [19]. Additionally, CD70 antibody-drug conjugate has shown to inhibit the proliferation of ovarian carcinoma cells that express CD70 both in vitro and in vivo [20]. Therefore, CD70 has the potential to be a biomarker and therapeutic marker. However, our study only has 11 cases with CD70 positivity. Analysis of CD70 expression does not correlate with age, AJCC stage, clinical symptoms of ascites, DFS, or OS rate (all
5.4C-MET
C-mesenchymal-epithelial transition factor (c-MET) is a tyrosine kinase receptor that plays a role in embryogenesis and tissue regeneration [21]. Normally, hormone regulation promotes c-MET and HGF to enhance the proliferation of the ovarian surface to repair the area damaged by ovum expulsion. In ovarian carcinoma, hormonal regulation is lost, leading to constant activation from c-MET and HGF signaling. This results in proliferation, stromal invasion, and metastasis of the carcinoma. Thus, c-MET could serve as a potential biomarker [22]. In our study, we do not find any correlation of c-MET expression and advanced age (indirectly related to hormonal regulation), clinical symptoms of ascites, or advanced stages (indirectly related to metastasis). There is also no association of c-MET with DFS or OS rate. Therefore, c-MET might not be an ideal candidate for ovarian carcinoma biomarker.
5.5NY-ESO1
New York esophageal squamous carcinoma 1 (NY-ESO1) is expressed at the fetal level within the germ cells. As the germ cells undergo spermatid differentiation, NY-ESO1 expression is physiologically lost. Its expression is affected by tumor grade, stage, or therapeutic intervention [23]. NY-ESO1 expression is also associated with poor clinical outcome in ovarian carcinoma [24]. However, our study does not find any association between NY-ESO1 expression and age, clinical symptoms of ascites, or advanced stages (indirectly related to metastasis). NY-ESO1 also does not correlate with DFS or OS rates. Therefore, NY-ESO1 might not be an ideal candidate for tumor marker.
With the total sample size of 522 from two separate cohorts, our study is the largest clinicopathological study of EGFR, HLA-G, CD70, c-MET, and NY-ESO1 expressions in high grade ovarian carcinoma. The sample size strongly supports the clinical significance of EGFR and HLA-G expression in predicting a worse prognosis. CD70, c-MET, and NY-ESO1, on the other hand, do not correlate with prognosis.
Unlike Her-2, the five immunohistochemical stains that we analyze do not have consensus interpretation. In different EGFR studies, methods that have been used include low (
We analyzed a panel of membrane markers including EGFR, HLA-G, CD70, c-MET, and NY-ESO1 using the largest cohort of ovarian cancer. We found that immunohistochemical expressions of EGFR are associated with worse prognosis. The expression of HLA-G also correlates with poor prognosis, either statistically significant or trend. Although CD70 expression has no statistically significant association with prognosis, it does follow the trend of poor prognosis. C-MET and NY-ESO1 do not have any correlation with OS rate or other clinical data.
Targeted therapy, such as EGFR inhibitors (erlotinib, cetuximab or lapatinib), has proven to be non-effective in ovarian carcinoma treatment [30]. However, the results from this study can be helpful for designing future clinical immunotherapy trials, especially for EGFR and HLA-G and their roles in immune regulation. They can be used as a prerequisite study prior to placing patients with disease recurrence on immunotherapy. In addition, if there are fruitful results in clinical trials, these biomarkers can be incorporated into routine clinical pathology practice. They are as affordable as other diagnostic immunohistochemical markers.
Acknowledgments
This research is supported in part by NIH/NIC to MD Anderson SPORT in Ovarian Cancer, Award Number 1P50CA281701-01. The authors declare no conflict of interest.
Author contributions
Conceptualization: Duc Vo and Jinsong Liu.
Interpretation or analysis of data: Duc Vo and Jinsong Liu.
Preparation of the manuscript: Duc Vo.
Revision for important intellectual content: Yan Liu, Anil K Sood, Katy Rezvani, Amir A Jazaeri.
Supervision: Jinsong Liu.
References
[1] | Z. Momenimovahed et al., Ovarian cancer in the world: Epidemiology and risk factors, Int J Womens Health 11: ((2019) ), 287–299. |
[2] | R.J. Kurman and L.M. Shih, The dualistic model of ovarian carcinogenesis: Revisited, revised, and expanded, Am J Pathol 186: (4) ((2016) ), 733–747. |
[3] | L.A. Torre et al., Ovarian cancer statistics, 2018, CA Cancer J Clin 68: (4) ((2018) ), 284–296. |
[4] | A. Gockley et al., Outcomes of women with high-grade and low-grade advanced-stage serous epithelial ovarian cancer, Obstet Gynecol 129: (3) ((2017) ), 439–447. |
[5] | L.Y. Guan and Y. Lu, New developments in molecular targeted therapy of ovarian cancer, Discov Med 26: (144) ((2018) ), 219–229. |
[6] | X. Jiang et al., Immune checkpoint: The novel target for antitumor therapy, Genes Dis 8: (1) ((2021) ), 25–37. |
[7] | S. Morand et al., Ovarian cancer immunotherapy and personalized medicine, Int J Mol Sci 22: (12) ((2021) ). |
[8] | G. Varady et al., Cell surface membrane proteins as personalized biomarkers: Where we stand and where we are headed, Biomark Med 7: (5) ((2013) ), 803–819. |
[9] | K.Y. Terada, H.J. Ahn and B. Kessel, Differences in risk for type 1 and type 2 ovarian cancer in a large cancer screening trial, J Gynecol Oncol 27: (3) ((2016) ), e25. |
[10] | M. Koshiyama, N. Matsumura and I. Konishi, Recent concepts of ovarian carcinogenesis: Type I and type II, Biomed Res Int 2014: ((2014) ), 934261. |
[11] | S. Sigismund, D. Avanzato and L. Lanzetti, Emerging functions of the EGFR in cancer, Mol Oncol 12: (1) ((2018) ), 3–20. |
[12] | W.L. Cheng et al., The Role of EREG/EGFR Pathway in Tumor Progression, Int J Mol Sci 22: (23) ((2021) ). |
[13] | C. Mehner et al., EGFR as a prognostic biomarker and therapeutic target in ovarian cancer: Evaluation of patient cohort and literature review, Genes Cancer 8: (5-6) ((2017) ), 589–599. |
[14] | N. Mallmann-Gottschalk et al., EGFR-Specific Tyrosine Kinase Inhibitor Modifies NK Cell-Mediated Antitumoral Activity against Ovarian Cancer Cells, Int J Mol Sci 20: (19) ((2019) ). |
[15] | A. Lin and W.H. Yan, Human Leukocyte Antigen-G (HLA-G) Expression in Cancers: Roles in immune evasion, metastasis and target for therapy, Mol Med 21: (1) ((2015) ), 782–791. |
[16] | Y.W. Jung et al., Correlation of human leukocyte antigen-G (HLA-G) expression and disease progression in epithelial ovarian cancer, Reprod Sci 16: (11) ((2009) ), 1103–1111. |
[17] | C. Menier et al., Human leukocyte antigen-G is expressed in advanced-stage ovarian carcinoma of high-grade histology, Hum Immunol 70: (12) ((2009) ), 1006–1009. |
[18] | M.J. Rutten et al., HLA-G expression is an independent predictor for improved survival in high grade ovarian carcinoma, J Immunol Res 2014: ((2014) ), 274584. |
[19] | N. Liu et al., Increased CD70 expression is associated with clinical resistance to cisplatin-based chemotherapy and poor survival in advanced ovarian carcinoma, Onco Targets Ther 6: ((2013) ), 615–619. |
[20] | M. Shiomi et al., CD70 antibody-drug conjugate: A potential novel therapeutic agent for ovarian cancer, Cancer Sci 112: (9) ((2021) ), 3655–3668. |
[21] | Y. Zhang et al., Function of the c-Met receptor tyrosine kinase in carcinogenesis and associated therapeutic opportunities, Mol Cancer 17: (1) ((2018) ), 45. |
[22] | Y. Kwon and A.K. Godwin, Regulation of HGF and c-MET Interaction in Normal Ovary and Ovarian Cancer, Reprod Sci 24: (4) ((2017) ), 494–501. |
[23] | A. Raza et al., Unleashing the immune response to NY-ESO-1 cancer testis antigen as a potential target for cancer immunotherapy, J Transl Med 18: (1) ((2020) ), 140. |
[24] | J.B. Szender et al., NY-ESO-1 expression predicts an aggressive phenotype of ovarian cancer, Gynecol Oncol 145: (3) ((2017) ), 420–425. |
[25] | P. de Graeff et al., The ErbB signalling pathway: protein expression and prognostic value in epithelial ovarian cancer, Br J Cancer 99: (2) ((2008) ), 341–349. |
[26] | C.K. Lin et al., The expression of six biomarkers in the four most common ovarian cancers: Correlation with clinicopathological parameters, APMIS 117: (3) ((2009) ), 162–175. |
[27] | A. Noske et al., An intracellular targeted antibody detects EGFR as an independent prognostic factor in ovarian carcinoma, BMC Cancer 11: ((2011) ), 294. |
[28] | A. De Meulenaere et al., CD70 Expression and Its Correlation with Clinicopathological Variables in Squamous Cell Carcinoma of the Head and Neck, Pathobiology 83: (6) ((2016) ), 327–333. |
[29] | N. Fuse et al., Prognostic impact of HER2, EGFR, and c-MET status on overall survival of advanced gastric cancer patients, Gastric Cancer 19: (1) ((2016) ), 183–191. |
[30] | A. Cortez et al., Advances in ovarian cancer therapy, Cancer Chemotherapy Pharmacology 81: (1) ((2018) ), 17–38. |




