Reduced Dose Intravesical Bacillus Calmette-Guérin: Why It Might Not Matter
Abstract
When it comes to the treatment of patients with non-muscle-invasive bladder cancer (NMIBC) with intravesical bacillus Calmette-Guérin (BCG), two questions must be considered: 1) what dose to give, and 2) for how long? The issue of optimal dose and duration has been the subject of several randomized trials and is especially pertinent in the context of a global BCG shortage. Despite this, there appears to be uncertainty as to whether BCG dose or duration may be compromised in the event of shortage. As such, we wish to summarize the available evidence as an aid to the practicing urologist.
BACKGROUND
One of the earliest randomized trials to address the question of BCG dose was conducted by the CUETO group who compared full dose BCG (81mg) with one-third dose (27mg) in intermediate- and high-risk patients with NMIBC [1]. Both dose cohorts received a total of 12 instillations –a 6-week induction course followed by a further six instillations given fortnightly –and found similar recurrence rates (29.4% in the full dose arm vs. 30.7% in the reduced dose arm) with no significant difference in time to first recurrence (p = 0.586). Similarly, both cohorts had similar rates of progression to muscle-invasive disease (11.5% vs. 13.3%). When looking for clues as to subgroup analysis, they observed a trend towards increased efficacy with full dose BCG against progression in patients with multifocal disease (p = 0.048). In a later study, the CUETO group addressed the same question in patients with high-risk NMIBC (G3/T1/Tis), and once again demonstrated that one-third dose BCG was as effective as full dose [2]. Subsequently, the EORTC 30962 study examined the use of full- and one-third dose OncoTICE® BCG given as either a 1- or 3-year maintenance course in 1355 patients with intermediate- and high-risk NMIBC [3]. At a median follow-up of 7.1 years, the authors reported that full dose BCG was not superior to one-third dose BCG (5-year disease-free rate: 61.7% vs. 58.5%, p = 0.092). When looking at subgroups, in high-risk patients, full dose BCG given for 3-years reduced recurrences compared with full dose for 1-year; however, there were no differences in progression or survival. Additionally, a recent retrospective analysis of 563 patients with intermediate- and high-risk NMIBC treated with either reduced or full dose adequate BCG during the BCG shortage provided real-world experience that the use of one-third dose BCG was not associated with adverse oncological outcomes [4]: time to recurrence (p = 0.449), time to progression (0.716) and cancer-specific survival (p = 0.320) was similar in both groups.
The results of these clinical trials are not unexpected. Each trial evaluated full and reduced dose BCG with regards to the clinically used supply, which is by weight of the lyophilized powder. What is not widely known is that the number of viable BCG organisms contained in clinical supply BCG is expressed as a range with an almost ten fold variation in amount of organisms in each vial. For example, using the data from TICE® BCG (Merck, USA), the main strain in North America and many other parts of the world, we know that one vial of TICE® BCG contains between 1 to 8×108 colony forming units (CFU) [4]. Put in other terms, each milligram of lyophilized BCG contains 2 million to 16 million CFUs of actual BCG organisms. Given this wide variation in CFUs, using one-third dose will not necessarily result in suboptimal dosing.
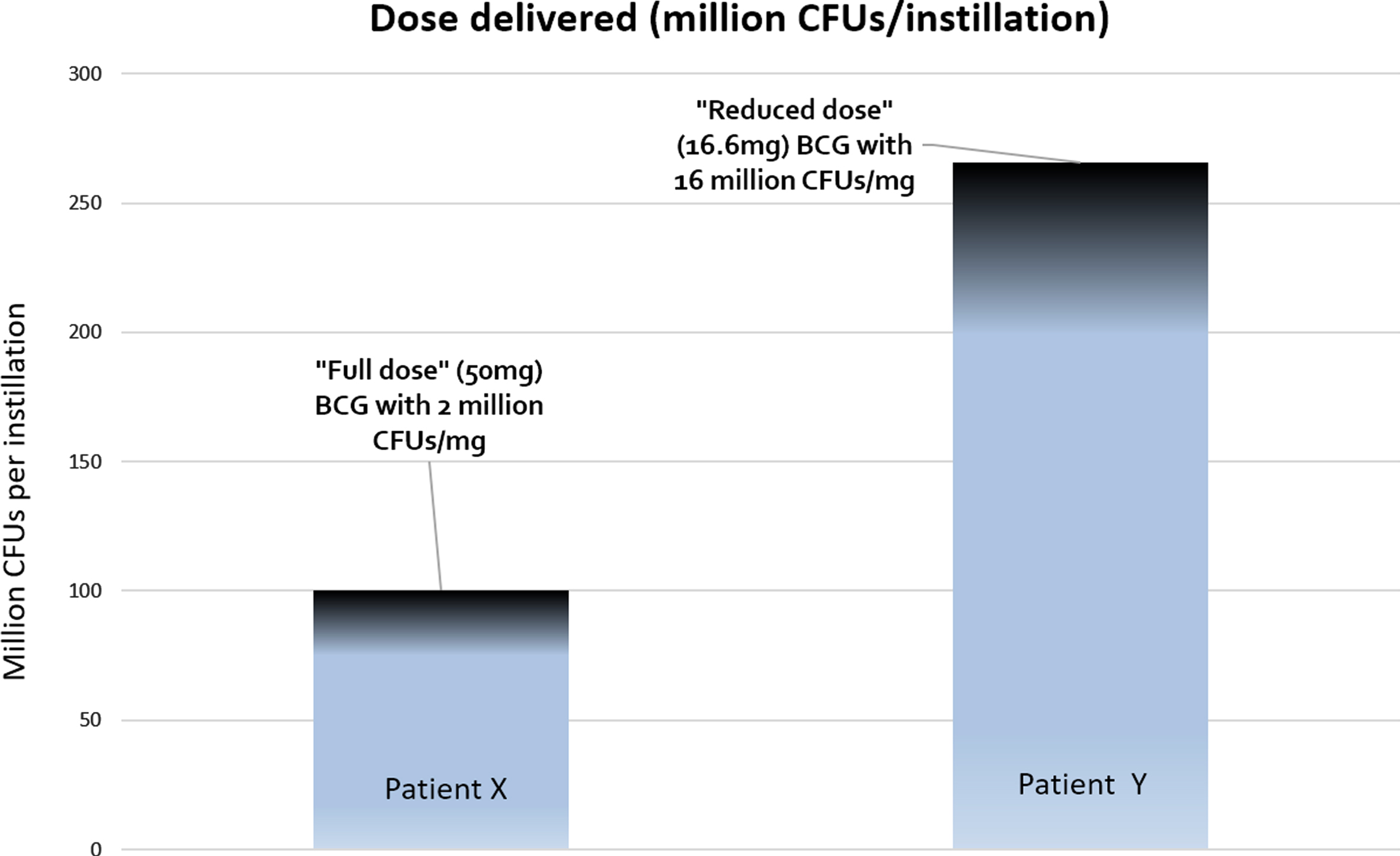
In a hypothetical situation, patient X receives full dose BCG from a vial containing 2 million CFUs of BCG per milligram. Thus s/he received 50×2 = 100 million CFUs per instillation (Fig. 1). Patient Y on the other hand, receives ‘reduced dose’ BCG from a vial containing 16 million CFUs of BCG. Thus s/he received 16.6×16 = 256 million CFUs per instillation, which is greater than the ‘full dose’ received by patient X. Of course, each manufacturer of BCG might have their own range of CFU variation (e.g. Onco-BCG® from the Serum Institute of India has an even greater variation of 1–19.2×108 CFUs per vial) so we urge readers to read up on the specifics of this variation for the strain of BCG used in their centers (eg from manufacturer data sheets).
Fig. 1
Patient X receives “full dose” (BCG 50 mg) from a vial containing 2 million CFUs/mg, thus receiving 100 CFUs per instillation. Patient Y receives “reduced-dose” BCG (16.6 mg) from a vial containing 16 million CFUs/mg, thus receiving 256 million CFUs per instillation. In this situation, the patient receiving 1/3rd dose actually is getting 2.5 times the dose of the ‘full dose’ patient.
IMPLICATIONS
It is important to clarify that this letter serves to provide an explanation as to why patients treated with reduced dose BCG may not actually receive fewer CFUs than patients receiving full dose BCG. With this in mind, what are the implications for our patients? Firstly, patients may be reassured that if they do receive reduced dose BCG, based on data from RCTs, this likely has no major impact on their disease-related outcomes. Indeed, recent American Urological Association/Society of Urologic Oncology guidelines for the treatment of NMIBC recommend the use of reduced dose BCG for maintenance treatment [5]. Secondly, during these times of BCG shortage, splitting a vial of BCG amongst three patients allows more patients to receive BCG. Since the preponderance of data suggests that receipt of maintenance BCG is an important component of the durability of response –especially with regards to decreasing progression rates –it is preferable that patients receive a reduced dose (either one-half or one-third dose) with the standard 6 + 3 schedule rather than full dose BCG given over a shorter duration. We acknowledge that some institutions may be hesitant to employ split-vial dosing given that manufacturers stipulate BCG should be administered within 2 hours of reconstitution. However, it has been shown that BCG remains viable for at least 8 hours and, in some cases, up to 72 hours after reconstitution [6]. Furthermore, whilst the 3 weekly maintenance schedule is important, this timing is an approximation rather than a rigid prescription. As such, providers have some flexibility around the scheduling of maintenance treatments and may split the dose based on when maximal patients are available. With that said, one-third dose appears to be the minimum required for clinical effectiveness as demonstrated by the CUETO group who showed that one-sixth dose BCG was inferior to one-third dose [7]. Finally, this carries practical implications for the conduct of clinical trials for BCG unresponsive disease. While the United States Food and Drug Administration does not specify that patients must receive full dose BCG in order to qualify for trials of agents in the BCG-unresponsive setting, many sponsors are hesitant to include patients who have received one-third dose BCG for induction and/or maintenance during these times of shortage. We believe, as previously stated [8], that these patients should be allowed to enroll in trials for the aforementioned reasons.
ACKNOWLEDGMENTS
This letter was supported by the Wayne B. Duddlesten Professorship in Cancer Research and the Raymond and Maria Floyd Bladder Cancer Research Foundation to Ashish M. Kamat and The Urology Foundation-Fulbright Scholar Award to Niyati Lobo.
FUNDING
The authors report no funding
AUTHOR CONTRIBUTIONS
Conception: A.M.K.
Performance of work: A.M.K., N.L.
Writing the article: A.M.K., N.L., S.P.L., R.L., J.T.M., J.P., J.A.W., M.R., A.B.S., S.S.C., N.D.S., G.D.S, C.P.D., R.S.S., D.L.L.
CONFLICTS OF INTEREST
Ashish M. Kamat, MD
Consultant/Advisory Board
Arquer Diagnostics, Asieris, Biological Dynamics, Bristol Myers Squibb, CG Oncology, H3 Biomedicine/Eisai, Engene, FerGene, Imagin Medical, Janssen, Medac, Merck, Photocure, ProTara, Seattle Genetics, Sessen Bio, Theralase, US Biotest, Urogen Inc, Roche, TMC Innovation
Grants/Research Support
Adolor, BMS, FKD Industries, FerGene, Heat Biologics, Merck, Photocure, SWOG, NIH/GU SPORE, AIBCCR, Janssen (+Taris), Seattle Genetics
Patent
CyPRIT (Cytokine Predictors of Response to Intravesical Therapy)
Board Member
International Bladder Cancer Group (IBCG)
Dr. Kamat is an Editorial Board Member of this journal, but was not involved in the peer-review process nor had access to any information regarding its peer-review.
Colin P. Dinney, MD
Consultant
STIMIT Corp., UroGen Pharma
Dr. Dinney is an Editorial Board Member of this journal, but was not involved in the peer-review process nor had access to any information regarding its peer-review.
Seth P. Lerner, MD
Consultant/Advisory Board
Aura Bioscience, BMS, C2iGenomics, FerGene, Genentech, Merck, Pfizer/EMD Serono, Stimit, UroGen, Vaxiion, Verity
Honoraria
Annenberg, Clinical Care Options, Grand Rounds Urology, Ology, UroToday
Clinical trials
Endo, FKD, JBL (SWOG), Genentech (SWOG), QED Therapeutics, UroGen, Vaxiion, Viventia
Patent
TCGA classifier
Dr. Lerner is an Editorial Board Member of this journal, but was not involved in the peer-review process nor had access to any information regarding its peer-review.
Gary D. Steinberg, MD
Clinical Trial
Merck, BMS, Janssen, CG Oncology, Pfizer, PhotoCure, Fidia, Seagen, Protara
Scientific advisor/consultant within the past 5 years for the following companies: Heat Biologics; CG Oncology; PhotoCure; Merck; Roche/Genentech; Ciclomed; Taris Biomedical (Now Janssen); MDxHealth; Fidia Farmaceuticals; Urogen, Ferring; Aduro; Boston Scientific; Bristol Myers Squibb; Astra Zeneca; Pfizer, Janssen; Epivax Therapeutics; Natera; FKD; EnGene Bio; SesenBio; BioCanCell (Now Archiano); Nucleix; Ipsen; Combat Medical; Astellas; Fergene; Dendreon; Abbvie; Seattle Genetics; Verity Pharmaceuticals, Regeneron, STIMIT, Vyriad
Equity stock/options: Epivax Therapeutics, Urogen
Dr. Steinberg is an Editorial Board Member of this journal, but was not involved in the peer-review process nor had access to any information regarding its peer-review.
Angela B. Smith, MD
Funding: AHRQ, Genentech, PCORI, BCAN
Consultant/Advisor: Merck, Urogen, Ambu, Fergene
Dr. Smith is an Editorial Board Member of this journal, but was not involved in the peer-review process nor had access to any information regarding its peer-review.
Donald Lamm, MD
Ongoing Research Trials: Nanology, CG Oncology
Dr. Lamm is an Editorial Board Member of this journal, but was not involved in the peer-review process nor had access to any information regarding its peer-review.
Roger Li, MD
Research support: Predicine; Decipher Biosciences.
Clinical trial protocol committee: CG Oncology
Scientific advisor/consultant: BMS, Ferring, Fergene, Arquer Diagnostics, Urogen Pharma.
Morgan Roupret, MD
Consultant/Advisory Board
Arquer diagnostic, Ferring, Roche, BMS, Astellas
Neal D. Shore, MD
Consulting
Abbvie, Amgen, Astellas, Astra Zeneca, Bayer, BMS, Boston Scientific, Clovis Oncology, Cold Genesys, Dendreon, Exact Imaging, Exact Sciences, FerGene, Foundation Medicine, Genesis Care, Invitae, Janssen, MDxhealth, Merck, Myovant, Myriad, Nymox, Pacific Edge, Pfizer, Phosphorous, Propella, Sanofi Genzyme, Sesen Bio, Tolmar, Urogen
Speaker’s Bureau
Astellas, AstraZeneca, Bayer, Clovis Oncology,Foundation Medicine, Janssen, Merck, Pfizer, Guardant Health
Sam S. Chang, MD
Consultant
Pacific Edge Lantheus, Prokarium, KDx Diagnostics, CGOncology, Merck, Pfizer, UroGen, UroToday, Urovant, Virtuoso Surgical, Janssen
Dr. Chang is an Editorial Board Member of this journal, but was not involved in the peer-review process nor had access to any information regarding its peer-review.
J. Alfred Witjes, MD
Advisor/Lecturer
Sanofi, Ipsen, Astra Zeneca, Ferring,Beijene, Janssen, Nucleix, Merck, Astellas, BMS.
Dr. Witjes is an Editorial Board Member of this journal, but was not involved in the peer-review process nor had access to any information regarding its peer-review.
Joan Palou, MD
Clinical Trial
Cepheid, Combat BRS, Arquer Diagnostics LTD, IDL Biotech AB.
Consultant
Pfizer, Astra Zeneca, Merck, Janssen, BMS
Honoraria
Combat BRS, Olympus, Sanofi Pasteur, Cepheid
Dr. Palou is an Editorial Board Member of this journal, but was not involved in the peer-review process nor had access to any information regarding its peer-review.
Robert S. Svatek, MD, MSCI
Consultant: Fergene, UroGen
Research support: JBL, Merck, Pacific Edge, Decipher
Dr. Svatek is an Editorial Board Member of this journal, but was not involved in the peer-review process nor had access to any information regarding its peer-review.
Niyati Lobo and Justin T. Matulay declare no conflicts of interest.
REFERENCES
[1] | Martınez-Pineiro JA , Flores N , Isorna S et al. Long-term follow-up of a randomized prospective trial comparing a standard 81mg dose of intravesical bacille Calmette-Guerin with a reduced dose of 27mg in superficial bladder cancer. BJU Int (2002) ;89: :671–80. |
[2] | Martınez-Pineiro JA , Martınez-Pineiro L , Solsona E , et al. Has a 3-fold decreased dose of bacillus Calmette-Guerin the same efficacy against recurrences and progression of T1G3 and Tis bladder tumors than the standard dose? Results of a prospective randomized trial. J Urol (2005) ;174: :1242–7. |
[3] | Oddens J , Brausi M , Sylvester R et al. Final Results of an EORTC-GU Cancers Group Randomized Studyof Maintenance Bacillus Calmette-Guerin in Intermediate- and High-risk Ta, T1 PapillaryCarcinoma of the Urinary Bladder: One-third Dose Versus Full Dose and 1 Year Versus 3 Years of Maintenance. Eur Urol (2013) ;63: :462–72. |
[4] | Lobo N , Bree KK , Hensley PJ et al. Reduced Dose BCG in an Era of BCG Shortage: Real World Experience from a Tertiary Cancer Centre. BJU International 2021. Doi: 10.1111/bju.15661. |
[5] | Chang SS , Boorjian SA , Chou R et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO Joint Guideline (2020). https://www.auanet.org/guidelines/guidelines/bladder-cancer-non-muscle-invasive-guideline Accessed 11th November 2021. |
[6] | Brooks N , Nagaraju S , Matulay J et al. Bacillus Calmette-Guerin retains clinically relevant viability for up to 72 hours after reconstitution: potential implications for clinical practice in times of shortage. Eur Urol Oncology (2020) ;S2588-9311: :30046–8. |
[7] | Ojea A , Nogueira JL , Solsona E et al. A multicentre, randomized prospective trial comparing three intravesical adjuvant therapies of intermediate-risk superficial bladder cancer: low-dose bacillus Calmette-Guerin (27mg) versus very low dose bacillus Calmette-Guerin (13.5mg) versus mitomycin C. Eur Urol (2007) ;52: :1398–406. |
[8] | Li R , Lerner SP , Kamat AM . The who, what, when, where and why of bacillus Calmette-Guerin-unresponsive bladder cancer. Eur Urol (2021) ;79: :437–9. |




