Development of an International SMA Bulbar Assessment for Inter-professional Administration
Abstract
Background:
Progressive weakness can affect bulbar muscles in individuals with moderate to severe forms of spinal muscular atrophy (SMA). The paucity of standardized, valid bulbar assessments capturing clinically significant deficits in SMA impedes the ability to monitor function, facilitate intervention, or detect treatment response.
Objective:
To fill this void, an international multidisciplinary team gathered to develop an agreed upon consensus-derived assessment of bulbar function in SMA for inter-professional administration to enhance our ability to monitor disease progression, support clinical management, and evaluate treatment effects.
Methods:
Fifty-six international clinicians experienced in SMA were invited and engaged using the Delphi method over multiple rounds of web-based surveys to establish consensus.
Results:
Serial virtual meetings occurred with 42 clinicians (21 speech and language therapists, 11 physical therapists, 5 neurologists, 4 occupational therapists, and 1 dentist). Seventy-two validated assessments of bulbar function were identified for potential relevance to individuals with SMA (32 accessible objective, 11 inaccessible objective, 29 patient-reported outcomes). Delphi survey rounds (n = 11, 15, 15) achieved consensus on individual items with relevance and wording discussed. Key aspects of bulbar function identified included: oral intake status, oral facial structure and motor strength, swallowing physiology, voice & speech, and fatigability.
Conclusions:
Multidisciplinary clinicians with expertise in bulbar function and SMA used Delphi methodology to reach consensus on assessments/items considered relevant for SMA across all age groups. Future steps include piloting the new scale moving towards validation/reliability. This work supports the advancement of assessing bulbar function in children and adults with SMA by a variety of professionals.
INTRODUCTION
Spinal muscular atrophy (SMA) is an autosomal recessive motor neuron disease caused by degeneration of lower motor neurons in the spinal cord and brainstem and is characterized by progressive proximal muscle weakness. The most common form of SMA is caused by mutations in the 5q13 survival motor neuron (SMN1) gene. The disease affects an estimated 10–16 per 100,000 infants [1]. Individuals with SMA present with a broad phenotypic range of disease severity with clinical classification based on age of onset and maximal function achieved [2]. The more severe forms of SMA, who never achieve the ability to sit (type 1) and those who can sit independently (type 2), exhibit progressive weakness that can affect bulbar muscles that impair communication, including deficits in voice and articulation, as well as swallowing. Individuals who can walk independently and may lose this ability over time (type 3) can develop bulbar deficits in adulthood [3]. These deficits have serious adverse effects on health, well-being and social participation [4–7].
In this new era of disease modifying therapies for SMA, several treatment options have been clinically proven to be effective, approved for commercial use, and are markedly altering the clinical course, functional outcomes, and evolving phenotypic expression. There is limited objective data however regarding the impacts of these therapies on bulbar function [8]. Despite the appreciated significance of bulbar impairment, there has historically been little systematic evaluation of its integrity within the clinical arena. This is in contrast to internationally utilized disease-specific assessments that are used to evaluate gross and fine motor physiology and function [9–12]. One of the reasons for the void in systematic bulbar assessment is the scarcity of standardized and validated SMA assessment tools that examine integrity across all bulbar domains. Available assessments frequently are isolated to just evaluating one component, such as swallowing, or communication. Those that evaluate across multiple bulbar domains are often limited to just a few questions on each that do not provide sufficient information regarding bulbar integrity. Other reasons for this gap in routine bulbar assessment among patients with SMA is the shortage of clinicians with expertise in bulbar physiology and function (ex. speech language pathologists) who see patients within the interdisciplinary neuromuscular clinic and/or the lack of resources to apply specialized instrumental assessments. This leaves regular bulbar assessment of SMA patients to other healthcare providers that often do not have the expertise to perform a refined assessment. These gaps in assessing bulbar integrity have clinically significant repercussions including the inability to systematically monitor function, facilitate timely rehabilitation intervention, and capture pharmaceutical treatment effects [6, 13].
To fill this void, a multidisciplinary team of international SMA experts was gathered with the purpose of developing an internationally applicable assessment of bulbar physiology and function for all individuals with SMA that can be performed by clinicians, without required specialized equipment or training, to enhance our ability to monitor disease progression, support clinical management, and evaluate treatment effects. Having a disease-specific assessment could provide an accurate picture of the individuals state that can guide a clinician to take a more invasive diagnostic pathway. This first report describes the process of establishing expert consensus on relevant bulbar assessments to include in the evaluation of individuals with SMA. This effort is part of a project to create a global scale for multi-center use in SMA.
MATERIALS AND METHODS
Procedures
Tool development was completed by conducting a modified Delphi method among a workgroup of clinicians and researchers with expertise in SMA and/or bulbar physiology and function. Specifically, 56 international clinicians with the aforementioned expertise, identified by their publication of scientific contributions, participation in SMA scientific conferences, frequent management of SMA patients, or expertise in a bulbar domain, were invited to participate in the workgroup. Workgroup members participated in a series of virtual meetings with specifications of tool development as outlined below:
Workgroup Mission: Upon initiation of workgroup meetings, and throughout the deliberation process, members were repeatedly oriented to the workgroup mission of developing an international assessment of bulbar physiology and function in SMA to identify patterns of disease progression, inform clinical management, and evaluate treatment response that could be applied across the lifespan for both pediatric and adult individuals. To maximize international utilization where clinician and financial resources are highly variable, a key component of tool development was centered around designing the metric in a way that did not require administration by a clinician with specialty bulbar knowledge or instrumentation (i.e., speech language pathologist, videofluoroscopy, or endoscopy). This was done not to discount the importance of specialty clinicians or instrumental assessments, which have clear importance in this population. Instead, it was done to encourage standard assessment where it currently is not being conducted, and in cases where staffing prohibits the bulbar expert to be part of the SMA multidisciplinary team, to enable streamlined referrals to those with bulbar expertise for a more refined assessment. Participants were invited to share their clinical experience of bulbar impairment in SMA. A collective virtual platform was established for collaboration and distribution of relevant literature and input on clinical bulbar assessment tools for consideration for SMA.
Domains of SMA Bulbar Assessment: The initial step in development was to establish the domains that compromise ‘bulbar’ physiology and function in SMA that would require assessment. These domains were used to focus the subsequent literature review of bulbar assessments as outlined below.
Review Current Bulbar Assessments and Identify Gaps: A literature review was completed by lead workgroup members to provide an initial list of published bulbar assessments. Due to the scarcity of bulbar assessment publications specific to SMA, it was determined referenced assessments did not need to be specific to their use in SMA for initial inclusion. This list was presented to workgroup members to identify additional assessments not initially included, or in cases where assessments did not exist, development of items to inquire into unmet areas. All workgroup members were provided a series of focused presentations that outlined specifications of these assessments to ensure they possessed adequate understanding of the assessment to make an educated valuation on its relevance to SMA and appropriateness to be included in the tool as outlined below.
Item Selection, Categorization, and Refinement via Modified Delphi Method: Established bulbar assessments underwent two stages of vetting by workgroup members, both using modified Delphi method to establish consensus (defined as agreement among >80% of respondents). This method prompts expert opinion using anonymous consecutive survey voting process methodology to condense judgements into a consensus [14] and is commonly used for a lack of availability of evidence, conflicting evidence, or if expert consensus if required for decision making in healthcare [14]. To allow all participants to discuss and interpret the results of the voting rounds, virtual conferences were held following the survey-based voting (Fig. 1). The voting surveys were distributed through the International SMA Consortium (iSMAC) Network and were developed and delivered through both QuestionPro [QuestionPro, Inc. Free Online Survey Software and Tools | QuestionPro®. https://www.questionpro.com/; Austin, Texas, USA] (first voting round) and Jotform [Jotform Inc\copyright. Jotform: Free Online Form Builder & Form Creator. https://www.jotform.com/; San Francisco, California, USA] (all remaining voting rounds). The first stage of assessment refinement was focused on selecting which of the established bulbar assessments had one or more items within it that was relevant to SMA and also could be performed by clinicians, without required specialized equipment or training. Once consensus was established on the subset of assessments to more thoroughly review, workgroup members reviewed each individual item of the identified assessments to select the specific ones that were relevant to SMA and should be included. Lastly, areas for which items did not exist from the literature review but were felt to be areas that warranted evaluations were developed by the workgroup content experts in that area and once again voted upon to develop the assessment.
Fig. 1
Flowchart of modified Delphi methodology and survey round details.
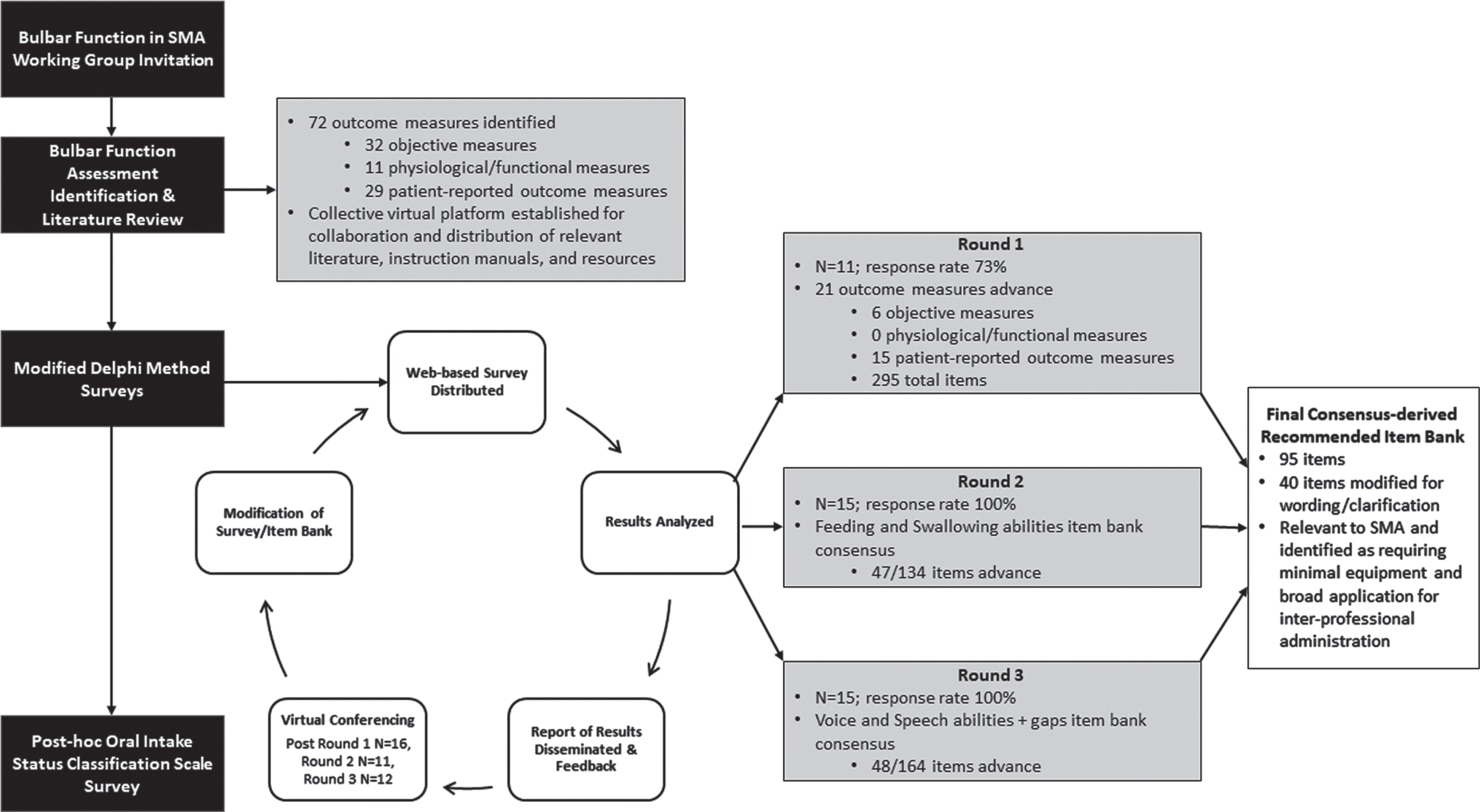
Item Categorization by Domain and Age Appropriateness: Selected items were reviewed for which domain they were most relevant to for sub-grouping. Once placed in their respective domains, they were reviewed for their applicability by patient age to enable different versions of the assessment to be administered. Based on the nature of the items, workgroup members voted on age cut-offs for the test versions. Selected items were then evaluated and voted upon for their relevance in administration into each of these age groups. For example, the question ‘does your jaw get tired when chewing’ is not applicable for an infant who has not yet started solid foods. Items that would have relevance to another age bracket if wording was modified were refined to enable continuous assessment across the age span whenever possible.
Data analysis
Descriptive statistics, including frequencies and percentages were used to analyze demographic data. Data collected from all rounds of the modified Delphi web-based survey responses were analyzed using the percentages of voting responses for each category.
RESULTS
Fifty-six participants were invited to the workgroup. Forty-two (75.0%) participants attended at least one virtual workgroup meeting, which included 21 speech and language pathologists, 11 physical therapists, 5 neurologists, 4 occupational therapists, and 1 dentist (Table 1). Fifteen (26.8%) of the 42 clinicians decided to join and partake in three modified Delphi method survey rounds to facilitate tool development. Attendance of >50% of virtual workgroup meetings was required of the 15 clinicians. The survey response rate was 73% for round 1 and 100% for rounds 2 and 3 (Fig. 1). Participants took part in 12 virtual workgroup meetings beginningJune 2020.
Table 1
Multidisciplinary representation of the Bulbar Function in SMA Working Group
| Working Group Clinical Disciplines | N = 42 | USA | Europe |
| Speech and language pathologists, n (%) | 21 (50) | 14 (33) | 7 (17) |
| Physical therapists, n (%) | 11 (26) | 6 (14) | 5 (12) |
| Neurologists, n (%) | 5 (12) | 1 (2) | 4 (10) |
| Occupational therapists, n (%) | 4 (10) | 3 (7) | 1 (2) |
| Dentists, n (%) | 1 (2) | 0 | 1 (2) |
USA, United States of America.
Workgroup members identified five bulbar domains of assessment relevant to SMA that were used to guide the literature review of assessments evaluating these areas: 1) Oral Intake Status, 2) Oral Facial Structure and Motor Strength, 3) Swallowing Physiology, 4) Voice & Speech, and 5) Fatigability (Fig. 2). Specifics of the nature of assessments included within the five key domains for evaluation of bulbar function in SMA can be found in Table 2. Literature review within these domains revealed 72 assessments that were considered for inclusion in the metric (Table 3). These included what were categorized as ‘accessible’ or ‘inaccessible’ objective physiologic measures based on their ability to be utilized globally without specialized training or equipment and patient-reported outcomes. The initial refinement of these assessments to identify those that had at least one relevant item yielded inclusion of 21 outcomes for more refined vetting. From these 21 assessments, 298 items were vetted in the second round of voting for their appropriateness of inclusion based on content and redundancy with other items. Consensus was achieved on including 95 of these items to be part of the final item bank, of which six items were derived from accessible objective outcome measures, 83 items from patient-reported outcome measures, and six items were identified as gaps and created from expert suggestion. Supplemental Table 1 provides the psychometric characteristics of the agreed upon assessments of bulbar function included in the final item bank.
Fig. 2
Five key domains for evaluation of bulbar function in SMA. Circles include the number of items from final consensus-derived item bank per domain.
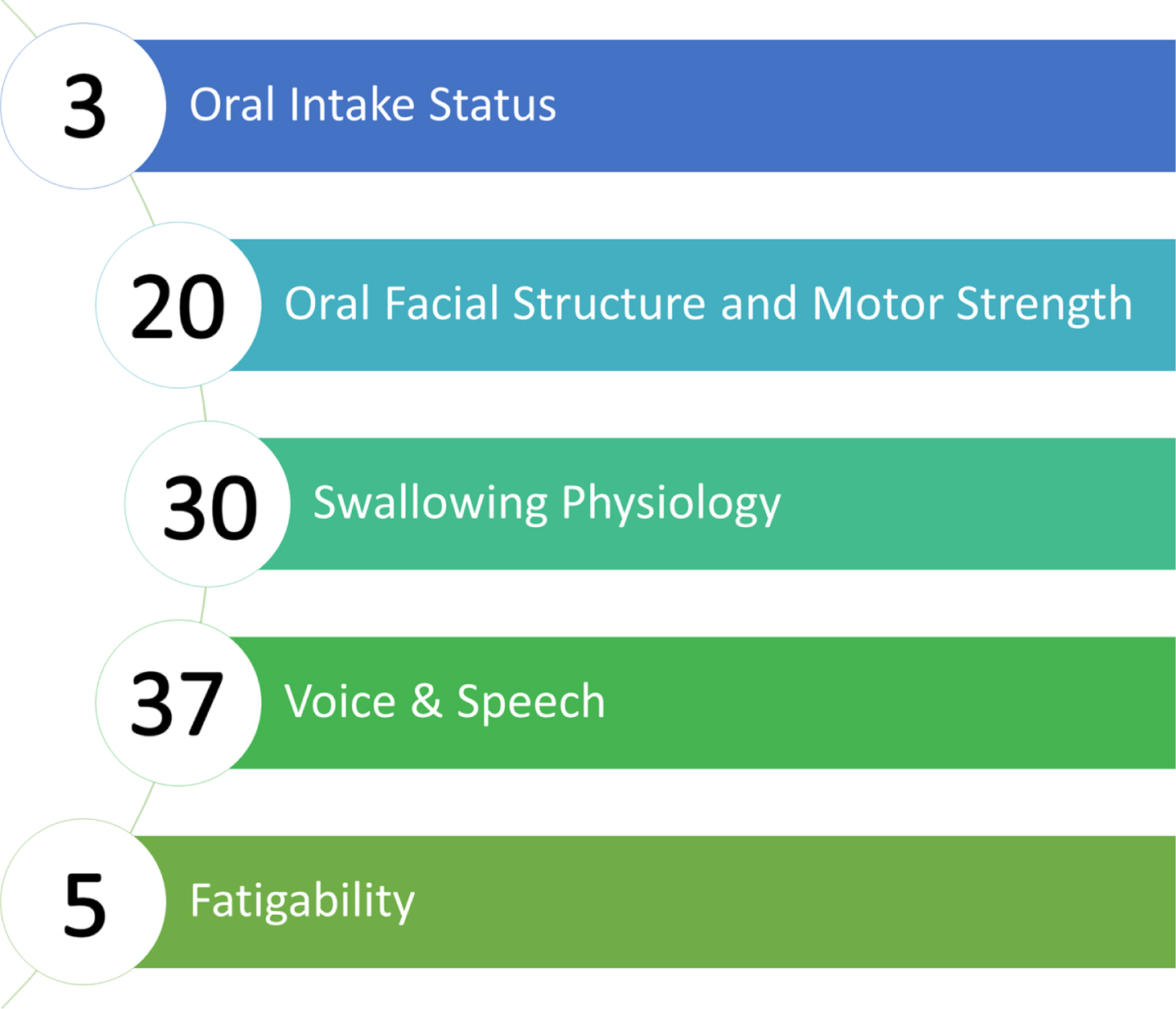
Table 2
Specifics of the nature of assessments included within the five key domains for evaluation of bulbar function in SMA
| Key Bulbar Domain | Outcome Specifics |
| Oral Intake Status | Outcomes categorizing oral intake status by amount of nutrition orally consumed, characteristics of the oral nutrition (ex. thickened liquids) and the use of alternative nutritive regimens (ex. gastrostomy) |
| Oral Facial Structure and Motor Strength | Quantification of oral-motor movements using accessible methods (ex. measuring mouth opening) and patient or parent-reported deficits relating to this ability (ex. opening mouth to bite). |
| Swallowing Physiology | Visual inspection of swallowing characteristics as well as patient or parent-reported outcomes associated with deficits including perceived difficulty, respiratory implications, and signs of impairment. |
| Voice &Speech | Patient and parent-reported outcomes associated with the patient’s loudness, intelligibility, social implications of communication deficits, and achievement of developmental norms. |
| Fatigability | Questions spanning the aforementioned areas specific to reports of decline in ease or integrity of performance with time (ex. tired when eating). |
Of the six objective outcome measures, three oral intake classification scales were identified as relevant for SMA though redundant with each other: Functional Oral Intake Scale, Oral and Swallowing Abilities Test, and Neuromuscular Disease Swallowing Status Scale. Consensus could not be achieved on which scale should be included with clinicians reporting they required firsthand experience in utilizing all three scales to allow them to make an educated decision. Final selection of these tools therefore occurred after expert clinicians explored the utility of all three scales clinically, of which the Function Oral Intake Scale-Great Ormand Street Version achieved the majority of the higher proportion of votes due to its utility across the age continuum as well as concerns with the other scales having unclear delineation of how to categorize patients and based on wording.
Once all items were established, each was categorized into one of the five key domains of bulbar function (Fig. 2) and item refinement commenced. Workgroup members identified three age groups for version development: 0 to <2 years, 2 to 12 years, and >12 years. Based on age of administration, 79 items classified for caregiver administration and 71 items classified for self-report. Modification to wording was determined to be required and completed on 40 of the 95 items to aid in use across the age continuum and inclarity.
DISCUSSION
This workgroup of multidisciplinary clinicians with expertise in SMA bulbar physiology and function used a modified Delphi methodology to reach consensus on assessments and items considered relevant for SMA across all phenotypes and age groups and organized into five domains. Given the availability of disease modifying therapies for SMA there is a critical need for quality real-world data regarding their effect on bulbar physiology and function. Further validation studies are needed to support routine use of an internationally agreed upon consensus-derived assessment of bulbar function for inter-professional administration to enhance our ability to illuminate patterns of disease progression, inform clinical management, and evaluate response to therapies. To our knowledge, no previous works have been made to derive expert consensus on the assessment of bulbar function in SMA. The development of an international SMA bulbar function assessment for inter-professional administration can promote greater availability and access of an assessment that evaluates an important domain of clinical care for individuals with SMA.
Our review of the SMA literature found numerous published manuscripts that utilized a total of 21 bulbar assessments collectively applied in individuals with all SMA types and ages (Table 3) [3–6, 15–33]. These included 16 ‘accessible’ or ‘inaccessible’ objective and five patient-reported outcome measures. However, very few assessments were reported to be individually utilized across the disease spectrum with varying SMA phenotypes and across all age groups [5, 23, 34].
Table 3
Assessments of bulbar function considered for relevance for SMA
| Assessments of Bulbar Function | Published Data in SMA | Total Items | Number of Items Achieved Consensus | Items Required Modification for Wording/ Clarification |
| Accessible Objective Measures | ||||
| 6 Minute Mastication Test (6MMT) | van der Heul AMB, 2022 [3] | |||
| 90 ml continuous drinking (number of swallows) / 3oz water test / timed test of swallowing | 1 | 1 | ||
| Active Maximal Mouth Opening (aMMO) | van der Heul AMB, 2022 [3] | 1 | 1 | |
| Morris E, 2020 [15] | ||||
| van der Heul AMB, 2019 [34] | ||||
| van Bruggen HW, 2016 [18] | ||||
| Wadman RI, 2014 [23] | ||||
| van Bruggen HW, 2011 [17] | ||||
| Granger MW, 1999 [33] | ||||
| Center for Neurologic Study Bulbar Function Scale (CNS-BFS) | ||||
| Diadochokinesis (DDK) | ||||
| Duration of “a" | ||||
| Dysphagia Limit Test/volume swallowed at once | ||||
| Functional Oral Intake Scale (FOIS) | van der Heul AMB, 2022 [3] | 1 | 1 | |
| Karaduman Chewing Performance Scale (KCPS) | ||||
| Lip strength (IOPI) | ||||
| Maximum voluntary bite force (MVBF) | van Bruggen HW, 2016 [18] | |||
| Granger MW, 1999 [33] | ||||
| Kruse T, 2020 [26] | ||||
| Kruse T, 2020 [27] | ||||
| Mixing Ability Test | van Bruggen HW, 2016 [18] | |||
| Neuromuscular Disease Swallowing Status Scale (NdSSS) | Choi Y, 2020 [29] | 1 | 1 | 1 |
| Nijmegen Dysarthria Scale | van den Engel-Hoek L, 2009 [20] | |||
| Observation list (fatigue, unsafe swallow, regurgitation of food, respiratory system) | van der Heul AMB, 2020 [22] | 7 | 1 | 1 |
| Oral and Swallowing Abilities Test (OrSAT) Level of Impairment | Berti B, 2021 [6] | 1 | 1 | |
| Oral Feeding Skills (OFS) | ||||
| Oral Mastication and Feeding Scale | ||||
| Oral-Motor Feeding Rating Scale (OMFRS) | ||||
| Passive Maximal Mouth Opening (pMMO) | Morris E, 2020 [15] | |||
| van Bruggen HW, 2011 [17] | ||||
| Quick Screen for Voice | ||||
| Reading passage | ||||
| Sucking pressures | ||||
| Test of Masticating and Swallowing Solids (TOMASS) | van der Heul AMB, 2022 [3] | |||
| Test of Masticating and Swallowing Solids pediatric version (TOMASS-C) | ||||
| Time of feeding in 24 hours | ||||
| Time to complete bottle feeding | ||||
| Tongue strength (IOPI) | ||||
| Viking Speech Scale | ||||
| Voice volume | ||||
| Volume consumed in 5 mins | ||||
| Volume consumed in overall feeding | ||||
| Inaccessible Objective Measures | ||||
| Bolus residue scale | ||||
| Cervical auscultation | ||||
| Flexible Endoscopic Evaluation of Swallowing (FEES) | ||||
| MRI of the temporomandibular joint and associatedmuscles | Wadman RI, 2014 [23] | |||
| Penetration-Aspiration Scale (PAS) | van der Heul AMB, 2020 [22] | |||
| Choi Y, 2020 [31] | ||||
| Pharyngeal metrics | ||||
| Pharyngeal residue IDDSI scale | ||||
| Surface EMG of masseter, orbicularis, temporal, submental muscles | van den Engel-Hoek L, 2009 [20] | |||
| Granger MW, 1999 [33] | ||||
| Ultrasound of submental, masticatory, and tonguemuscles | van der Heul AMB, 2022 [3] | |||
| Video Fluoroscopic Swallowing Study (VFSS) | Weststrate H, 2022 [25] | |||
| van der Heul AMB, 2022 [3] | ||||
| Wadman RI, 2021 [24] | ||||
| van der Heul AMB, 2020 [22] | ||||
| Choi Y, 2020 [31] | ||||
| Chen K, 2020 [29] | ||||
| Cha T, 2010 [28] | ||||
| van den Engel-Hoek L, 2009 [20] | ||||
| van den Engel-Hoek L, 2008 [19] | ||||
| Suzukia Y, 2007 [16] | ||||
| Yale Pharyngeal Residue Severity Rating Scale | ||||
| Patient-Reported Outcome Measures | ||||
| Center for Neurological Study - Bulbar function Scale (CNS-BFS) | ||||
| Child Oral and Motor Proficiency Scale (ChOMPS) | ||||
| Developmental Speech Milestones | 15 | 11 | ||
| Diagnostic Questionnaire for Dysphagia and Dysarthria in (pediatric) Patients with Neuromuscular Disease (DDD(p)NMD) | van der Heul AMB, 2022 [3] | 41 | 11 | 2 |
| Kooi-van Es M, 2020 [4] | ||||
| van der Heul AMB, 2019 [34] | ||||
| Drooling Impact Scale (DIS) | Shoval HA, 2018 [43] | 10 | 4 | |
| Eating Assessment Tool-10 (EAT-10) | 10 | 4 | 4 | |
| Egen Klassifikation Scale Version 2 (EK2) | 17 | 1 | ||
| Gothenburg Trismus Questionnaire (GTQ) | 29 | 2 | 2 | |
| Infant &Child Feeding &Swallowing Tool | ||||
| Intelligibility in Context Scale (ICS) | 7 | 7 | 7 | |
| Jaw Functional Limitation Scale-20 | 20 | 9 | 9 | |
| Mandibular Function Impairment Questionnaire (MFIQ) | van Bruggen HW, 2016 [18] | |||
| van Bruggen HW, 2011 [17] | ||||
| Mouth Impairment and Disability Assessment (MIDA) | ||||
| Nordic Orofacial Test - Screening (NOT-S) | ||||
| Oral and Swallowing Abilities Test (OrSAT) | D’Silva AM, 2022 [32] | 13 | 6 | 2 |
| Berti B, 2021 [6] | ||||
| Parent assessment of swallowing ability (PASA) | ||||
| Pediatric Eating Assessment Tool (PediEAT) | ||||
| Pediatric Eating Assessment Tool-10 (PediEAT-10) | 10 | 5 | 5 | |
| Pediatric Evaluation of Disability Inventory-Computer Adaptive Test (PEDI-CAT) | ||||
| Pediatric Voice Handicap Index (p-VHI) | 23 | 6 | ||
| Questionnaire for Feeding and Swallowing Difficulties in Spinal Muscular Atrophy | Chen Y, 2012 [30] | 17 | 3 | 3 |
| Screeninglist Physician of the DDD-pNMD | ||||
| Kooi-van Es M, 2020 [4] | 9 | 5 | 4 | |
| Kooi-van Es M, 2020 [5] | ||||
| Speech Handicap Index (SHI) | 30 | 7 | ||
| The M.D. Anderson Dysphagia Inventory (MDADI) | ||||
| The Swallowing Quality of Life instrument (SWAL-QOL) | ||||
| Visual Analogue Scale (VAS 0-10) to assess masticatory muscle pain and fatigue | ||||
| Vocal development landmarks interview (VLDI) | ||||
| Voice Handicap Index (VHI) | 30 | 2 | ||
| Voice Handicap Index-10 (VHI-10) | ||||
| Expert Suggested (evaluation of swallowing domains including vomiting, food refusal, satiation, incomplete bolus clearance, and saliva management interventions) | 6 | |||
| Final Item Bank Total | 95 | 40 |
Achieved Consensus. Required Modification for Wording/ Clarification.
Objective measures of bulbar physiology and function offer a sensitive, valid, and reliable representation of bulbar integrity without influence by patient perception. In the current investigation objective measures were categorized as being accessible or inaccessible based on their ability to be scaled for international utilization. Specifically, assessments were determined to be inaccessible for international use if they required specialized training or equipment for execution. For example, a videofluoroscopic swallow study was categorized as inaccessible as it requires specialized speech language pathology training and a videofluoroscopic suite, despite reported use in SMA [3, 16, 19, 20, 22, 24, 25, 28, 29, 31] with description of events [22, 31]. Other such inaccessible assessments used in previous SMA investigations include MRI [23], surface EMG [20, 33] and ultrasound [3]. Despite the strongly appreciated benefits of these tools, they were excluded from the assessment battery due to their inability to be feasibly executed internationally where clinical set-ups, resources, and inter-professional supports vary widely. Emerging technologies including wearable devices or remote applications to assess bulbar function are becoming increasingly more prevalent to allow for remote monitoring and assessment. Many of these are in the early concept phase however, the ability to assess bulbar impairment in different settings remains promising.
In contrast, we included those objective assessments that were determined to be accessible for widespread international utilization. These included questions pertaining to oral intake status, assessment of liquid intake, and measures of mandibular range of motion that provided the benefits of sensitivity of objective physiologic assessment while also enabling feasibility for use across practice settings. Previous studies in SMA have investigated performance [18], endurance and efficiency of mastication [3], active [3, 15, 17, 18, 23, 33, 34] and passive [15, 17] mandibular range of motion, bite force [18, 26, 27, 33], oral motor performance and dysarthria [20], and classification scales of functional oral intake status [3, 6, 31]. An important distinction to make is that these accessible assessments are not meant to be a replacement for the inaccessible assessments when available. Instrumental assessments such as the videofluoroscopic swallow study are crucial in the identification of internal physiologic deficits as research in untreated patients with SMA indicates profound physiologic swallowing impairments would often go unnoticed by caregivers and common clinicians due to high rates of silent aspiration [13]. Of equal importance is the value that these instrumental assessments provide in identifying the underlying muscle groups that should be targeted for therapeutic swallowing interventions through the execution of varying oropharyngeal and respiratory maneuvers that target improvement in neuromuscular strength and control. Such interventions have been found to be effective at improving physiologic and functional swallowing deficits in patients following stroke [35–39], with Parkinson’s disease [40], amyotrophic lateral sclerosis [41], or presbyphagia [42]. Another intervention in SMA includes botulinum toxin injection into the salivary glands, a procedure still sometimes performed to control excessive oral secretions [16, 43]. Whenever available, clinical teams are encouraged to use both routine clinical and instrumental assessments with the collaboration of experts in bulbar physiology and function to provide a thorough understanding of integrity. Future work developing standardized add-on modules to guide this assessment when deficits are identified in the primary questionnaire is warranted to promote the standardization of thorough and sensitive assessments capable of detecting more subtle changes.
The second category of questions we included are those pertaining to patient-reported outcomes. These are essential in understanding specific needs and goals of the individual with SMA and/or caregiver and can deliver unique person-focused information. Published literature in SMA have included questionnaires to identify feeding and swallowing problems [3, 5, 30, 34], including screening for signs of dysphagia and dysarthria [4, 5], to document impairments of the function of the masticatory system [17, 18], and to record oral abilities, swallowing and feeding in young type 1 SMA patients [6, 32]. Our methodology for the final consensus-derived item bank recognized key areas of bulbar function where the patient experience would be critical to evaluate bulbar impairment. Experiences of dysarthria, speech intelligibility, and gaining developmental speech milestones were identified as important aspects to assess globally in SMA. Experts’ consensus also found oral facial structure and motor strength, including jaw functioning, chewing, fatigability, dysphagia and swallowing abilities, and the impact of drooling from the patient experience to be integral as well. Further research into how these patient-reported outcomes reflect physiologic integrity are desperately warranted. In contrast to gross and fine motor function, where an individual can visually compare their ability to perform a task compared to peers, the internal nature of many bulbar functions makes comparing integrity relative to peers, and therefore perception of deficit, more difficult. Further, the gradual degradation of function that often starts at a very early age likely facilitates an ‘acclimation’ both reducing a patient’s perception of impairments as well as inadvertent modifications to activities of daily living that limit the individual’s need to perform at maximum capacity. For example, if an individual has profound dysarthria that greatly limits their intelligibility by unknown listeners, but spends the majority of their time with their caregiver who has learned their unique communication methods, they may not perceive a significant burden. Patients’ perception of their deficits is also likely to evolve over time as social expectations change with age and across environments. In other populations of children with congenital motor speech disorders (e.g., cerebral palsy), reduced speech intelligibility has been shown to have negative impacts on participation and quality of life throughout childhood, with increasing social impacts reported during adolescence [44]. Research understanding patients’ priorities for maximizing their participation in life activities is critical as the field moves forward and determines how to approach rehabilitation.
Though the majority of accessible objective and patient-reported assessments were selected from previously established bulbar assessments, six of the included items were developed within our workgroup after determination that there were gaps of relevance to SMA. These areas included evaluation of swallowing domains including vomiting, food refusal, satiation, incomplete bolus clearance, and saliva management interventions.
Given the rapidly growing landscape of literature, it is feasible that validated assessments of bulbar function may have been mistakenly omitted. It is the hope that our multidisciplinary clinical representation from the USA and Europe provided a comprehensive perspective for the assessment of bulbar function in SMA which included speech and language therapists, physical therapists, neurologists, occupational therapists, and a dentist. It is conceivable that participants may have had possible bias in the selection of assessments and items from their clinical experience and practice. The virtual conferences following the voting rounds allowed clinicians to discuss results and share feedback to account for the varying degrees of practice and clinical experience and support an open forum to discourse merits and irrelevance.
Additional work is needed to incorporate patient and caregiver perspectives as well as input from patient organizations and industry partners to support face validity. Cognitive interviews will ensue to confirm understanding and appropriateness of individual items, strength of responses, and meaningfulness of the patient as well as help recognize gaps in the assessment of bulbar function. Future steps include piloting the assessment internationally moving towards validation, reliability, and responsiveness of the new scale. Establishing the psychometric properties will be critical to help screen and recognize individuals with SMA in need of interventions for their bulbar function impairments. Real-world data collection of bulbar impairment in SMA from international data pooling through partners will aid in optimizing data quality and establishing clinical meaningfulness.
This workgroup will support the advancement of assessing bulbar function in children and adults with SMA by a variety of health professionals and lay a foundation towards understanding bulbar function impairment and providing evidence-based care. This effort could improve the screening and identification of early onset bulbar impairment and advance the availability of clinical services and interventions. This assessment will continue to be refined with the hopes to capture what is meaningful for individuals with SMA.
ACKNOWLEDGMENTS
We gratefully thank the international SMA Consortium (PNCR-USA, SMA REACH-UK, SMA Telethon-Italy). We thank all clinicians who participated in the Bulbar Function in SMA Working Group: Miranda Clements, Lone Bech Christensen, Sunita Palecanda, Sarah Stranberg, Lenie van den Engel-Hoek, Valeria Sansone, Eiri Inenaga, Katie Walsh, Callan Amelot, Peter Watson, Donnielle Rome-Martin, Tim Estilow, Anna Mayhew, Elizabeth Maczek, Ulrike Schara-Schmidt, Stefano Usai, Gilliam Welsher, Elisa Liu, Renee Hill, Ali West, Kimberly Grenawitzke, Jenny Doxtad, Kayla Hernandez, Teresea Pitts, and Nicky Sedwick. The authors wish to thank the Cure SMA Industry Collaboration for the funding that supported this project. The members of the Cure SMA Industry Collaboration during the time of program development were, Astellas, Biogen, Cytokinetics Inc., Genentech/ Roche Pharmaceuticals, Novartis Gene Therapies, Novartis, and Scholar Rock, Inc. This work was supported by CureSMA and had no involvement in the preparation of the article, study design, collection, analysis and interpretation of data, or in the writing of the report.
CONFLICTS OF INTEREST
S. D. Y. has been a member of advisory boards for Biogen, Roche/Genentech, and Scholar Rock; received personal compensation for activities with Biogen, Cure SMA, and Scholar Rock as a consultant; and received research support from Cure SMA.
K. M. has received grant funding and consulting income from Biogen and AveXis but has no financial interest in these companies. She is founder and scientific director of nuBorn Medical and Science Stand. These relationships have been reviewed and managed by the University of Minnesota in accordance with its conflict of interest policies.
E. J. has no conflict of interest to report.
M. V. has no conflict of interest to report.
T. D. has been an advisory board member of Cure SMA, Biogen, Cytokinetics, Roche, Scholar Rock, Sarepta, Bristol Myers Squibb, Audentes, and Novartis and served as a consultant for Roche, Audentes, and Novartis.
M. B. has received research support from the Danish Parkinson’s Foundation and the Danish Dental Association and serves as a consultant at the Danish Headcenter, rigshospitalet.dk
U. W. has no conflict of interest to report.
A. P. has received research support from the SMA Foundation; serves as a consultant for Biogen; and has served on advisory boards for AveXis, Biogen, Roche, and Scholar Rock.
C. C. has no conflict of interest to report.
K. H. has been an advisory board member for Roche previously.
L. F. has no conflict of interest to report.
A. B. has no conflict of interest to report.
K. A. has no financial or non-financial conflicts of interest related to this work. She receives grant funding from the NIH for research on pediatric dysarthria.
G. B. has been Principal investigator in SMA trials sponsored by AveXis, Roche, Novartis Gene Therapy and Scholar Rock; has received compensation for participation at symposia and scientific advisory boards from Biogen, Roche and AveXis/Novartis Gene Therapy; his Institution has received grant donations for the purchase of equipment from Roche and grant support from Novartis Gene Therapy; he is one of the PIs of the SMA REACH who is supported by Biogen and Roche.
R. F. has served as a consultant to Affinia, AveXis, Biogen, Capricor, Catabasis, Cytokinetics, Dyne, Genentech, Neurogene, n-Lorem Foundation, Novartis, Roche, Sarepta, Scholar Rock on SMA related topics and with no financial interests in these companies; has received SMA clinical trial funding from Biogen/Ionis, AveXis/Novartis, Genentech/Roche, Cytokinetics, and Scholar Rock; research funding from Biogen, Cure SMA, Genentech, SMA Foundation, Muscular Dystrophy Association, National Institutes of Health; has served on data safety monitoring boards for the AveXis AVXS-101 START study, Roche MOONFISH study, and Ionis Angelman HALOS study; and has received royalty payments from Children’s Hospital of Philadelphia for licensing fees obtained for use of the CHOP INTEND motor function scale. Dr. Finkel is an Editorial Board Member of this journal, but was not involved in the peer-review process nor had access to any information regarding its peer-review.
G. C. reports personal fees from BIOGEN S.R.L. ITALIA, ROCHE, GENESIS PHARMA, AVEXIS, Biologix, outside the submitted work.
R. M. L. has research funding from Biogen and Roche; has been member of advisory board for AveXis (Novartis) and has served as a consultant for Biogen, Roche and Novartis.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-221672.
REFERENCES
[1] | Mercuri E , Sumner CJ , Muntoni F , et al. Spinal muscular atrophy. Nat Rev Dis Prim. (2022) ;8: :52. 10.1038/s41572-022-00380-8. |
[2] | Dubowitz V . Chaos in the classification of SMA: A possible resolution. Neuromuscul Disord. (1995) ;5: :3–5. 10.1016/0960-8966(94)00075-K. |
[3] | van der Heul AMB , van Eijk RPA , Wadman RI , et al. Mastication in patients with spinal muscular atrophy types 2 and 3 is characterized by abnormal efficiency, reduced endurance, and fatigue. Dysphagia. (2022) ;37: :715–23. 10.1007/s00455-021-10351-y. |
[4] | Kooi-van Es M , Erasmus CE , de Swart BJM , et al. Dysphagia and dysarthria in children with neuromuscular diseases, a prevalence study. J Neuromuscul Dis. (2020) ;7: :287–95. 10.3233/JND-190436. |
[5] | Kooi-van Es M , Erasmus CE , Houwen S , et al. Early detection of dysphagia and dysarthria in children with neuromuscular disorders: Diagnostic accuracy of a Screeninglist for Physicians. J Pediatr Rehabil Med. (2020) ;13: :17–23. 10.3233/PRM-180569. |
[6] | Berti B , Fanelli L , de Sanctis R , et al. Oral and swallowing abilities tool (OrSAT) for type 1 SMA patients: Development of a new module. J Neuromuscul Dis. (2021) ;8: :589–601. 10.3233/JND-200614. |
[7] | Mercuri E , Finkel RS , Muntoni F , et al. Diagnosis and management of spinal muscular atrophy: Part Recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul Disord. (2018) ;28: :103–15. 10.1016/j.nmd.2017.11.005. |
[8] | Sumner CJ , Crawford TO . Two breakthrough gene-targeted treatments for spinal muscular atrophy: Challenges remain. J Clin Invest. (2018) ;128: :3219–27. 10.1172/JCI121658. |
[9] | Glanzman AM , McDermott MP , Montes J , et al. Validation of the children’s hospital of philadelphia infant test of neuromuscular disorders (CHOP INTEND). Pediatr Phys Ther. (2011) ;23: :322–6. 10.1097/PEP.0b013e3182351f04. |
[10] | O’Hagen JM , Glanzman AM , McDermott MP , et al. An expanded version of the Hammersmith Functional Motor Scale for SMA II and III patients. Neuromuscul Disord. (2007) ;17: :693–7. 10.1016/j.nmd.2007.05.009. |
[11] | Mazzone ES , Mayhew A , Montes J , et al. Revised upper limb module for spinal muscular atrophy: Development of a new module. Muscle and Nerve. (2017) ;55: :869–74.10.1002/mus.25430. |
[12] | Dunaway Young S , Montes J , Kramer SS , et al. Six-minute walk test is reliable and valid in spinal muscular atrophy. Muscle Nerve. (2016) ;54: :836–42. 10.1002/mus.25120. |
[13] | McGrattan KE , Graham RJ , DiDonato CJ , et al. Dysphagia phenotypes in spinal muscular atrophy: The past, present, and promise for the future. Am J speech-language Pathol. (2021) ;30: :1008–22. 10.1044/2021_AJSLP-20-00217. |
[14] | Steurer J . The delphi method: An efficient procedure to generate knowledge. Skeletal Radiol. (2011) ;40: :959–61. 10.1007/s00256-011-1145-z. |
[15] | Lloyd Morris EH , Estilow T , Glanzman AM , et al. Improving temporomandibular range of motion in people with duchenne muscular dystrophy and spinal muscular atrophy. Am J Occup Ther Off Publ Am Occup Ther Assoc. (2050) ;74: :80p1–80p10. 10.5014/ajot.2020.030825. |
[16] | Suzukia Y , Sano N , Shinonaga C , et al. Successful botulinum toxin treatment of dysphagia in a spinal muscular atrophy type 2 patient. Brain Dev. (2007) ;29: :662–5. 10.1016/j.braindev.2007.04.003. |
[17] | van Bruggen HW , van den Engel-Hoek L , van der Pol WL , et al. Impaired mandibular function in spinal muscular atrophy type II: Need for early recognition. J Child Neurol. (2011) ;26: :1392–6. 10.1177/0883073811407696. |
[18] | van Bruggen HW , Wadman RI , Bronkhorst EM , et al. Mandibular dysfunction as a reflection of bulbar involvement in SMA type 2 and 3. Neurology. (2016) ;86: :552–9. 10.1212/WNL.0000000000002348. |
[19] | van den Engel-Hoek L , de Swart BJM , Erasmus CE , et al. Is head balance a major determinant for swallowing problems in patients with spinal muscular atrophy type 2? J Child Neurol. (2008) ;23: :919–21. 10.1177/0883073808315418. |
[20] | van den Engel-Hoek L , Erasmus CE , van Bruggen HW , et al. Dysphagia in spinal muscular atrophy type II: More than a bulbar problem? Neurology. (2009) ;73: :1787–91. 10.1212/WNL.0b013e3181c34aa6. |
[21] | Van Der Heul AMB , Wijngaarde CA , Wadman RI , et al. Bulbar problems self-reported by children and adults with spinal muscular atrophy. J Neuromuscul Dis. (2019) ;6: :361–8. 10.3233/JND-190379. |
[22] | van der Heul AMB , Cuppen I , Wadman RI , et al. Feeding and swallowing problems in infants with spinal muscular atrophy type An observational study. J Neuromuscul Dis. (2020) ;7: :323–30. 10.3233/JND-190465. |
[23] | Wadman RI , van Bruggen HW , Witkamp TD , et al. Bulbar muscle MRI changes in patients with SMA with reduced mouth opening and dysphagia. Neurology. (2014) ;83: :1060–6. 10.1212/WNL.0000000000000796. |
[24] | Wadman RI , De Amicis R , Brusa C , et al. Feeding difficulties in children and adolescents with spinal muscular atrophy type 2. Neuromuscul Disord. (2021) ;31: :101–12. 10.1016/j.nmd.2020.12.007. |
[25] | Weststrate H , Stimpson G , Thomas L , et al. Evolution of bulbar function in spinal muscular atrophy type 1 treated with nusinersen. Dev Med Child Neurol. (2022) ;64: :907–14. 10.1111/dmcn.15171. |
[26] | Kruse T , Lehmann HC , Braumann B , et al. The maximum bite force for treatment evaluation in severely affected adult SMA patients-protocol for a longitudinal study. Front Neurol. (2020) ;11: :139. 10.3389/fneur.2020.00139. |
[27] | Kruse T , Heller R , Wirth B , et al. Maximum bite force in patients with spinal muscular atrophy during the first year of nusinersen therapy - A pilot study. Acta Myol myopathies cardiomyopathies Off J Mediterr Soc Myol. (2020) ;39: :83–9. 10.36185/2532-1900-010. |
[28] | Cha T-H , Oh D-W , Shim J-H . Noninvasive treatment strategy for swallowing problems related to prolonged nonoral feeding in spinal muscular atrophy type II. Dysphagia. (2010) ;25: :261–4. 10.1007/s00455-009-9269-1. |
[29] | Chen K-A , Widger J , Teng A , et al. Real-world respiratory and bulbar comorbidities of SMA type 1 children treated with nusinersen: 2-Year single centre Australian experience. Paediatr Respir Rev. (2021) ;39: :54–60. 10.1016/j.prrv.2020.09.002. |
[30] | Chen Y-S , Shih H-H , Chen T-H , et al. Prevalence and risk factors for feeding and swallowing difficulties in spinal muscular atrophy types II and III. J Pediatr. (2012) ;160: :447–51.e1. 10.1016/j.jpeds.2011.08.016. |
[31] | Choi Y-A , Suh DI , Chae J-H , et al. Trajectory of change in the swallowing status in spinal muscular atrophy type I. Int J Pediatr Otorhinolaryngol. (1098) ;130: :18. 10.1016/j.ijporl.2019.109818. |
[32] | D’Silva AM , Holland S , Kariyawasam D , et al. Onasemnogene abeparvovec in spinal muscular atrophy: An Australian experience of safety and efficacy. Ann Clin Transl Neurol. (2022) ;9: :339–50.10.1002/acn3.51519. |
[33] | Granger MW , Buschang PH , Throckmorton GS , et al. Masticatory muscle function in patients with spinal muscular atrophy. Am J Orthod Dentofac Orthop Off Publ Am Assoc Orthod its Const Soc Am Board Orthod. (1999) ;115: :697–702. 10.1016/s0889-5406(99)70296-9. |
[34] | van der Heul AMB , Wijngaarde CA , Wadman RI , et al. Bulbar problems self- reported by children and adults with spinal muscular atrophy. J Neuromuscul Dis. (2019) ;6: :361–8. 10.3233/JND-190379. |
[35] | Park J-S , Lee G , Jung Y-J . Effects of game-based chin tuck against resistance exercise vs head-lift exercise in patients with dysphagia after stroke: An assessor-blind, randomized controlled trial. J Rehabil Med. (2019) ;51: :749–54.10.2340/16501977-2603. |
[36] | Park J-S , An D-H , Oh D-H , et al. Effect of chin tuck against resistance exercise on patients with dysphagia following stroke: A randomized pilot study. NeuroRehabilitation. (2018) ;42: :191–7. 10.3233/NRE-172250. |
[37] | Gao J , Zhang H-J . Effects of chin tuck against resistance exercise versus Shaker exercise on dysphagia and psychological state after cerebral infarction. Eur J Phys Rehabil Med. (2017) ;53: :426–32. 10.23736/S1973-9087.16.04346-X. |
[38] | Carnaby GD , LaGorio L , Silliman S , et al. Exercise-based swallowing intervention (McNeill Dysphagia Therapy) with adjunctive NMES to treat dysphagia post-stroke: A double-blind placebo-controlled trial. J Oral Rehabil. (2020) ;47: :501–10.10.1111/joor.12928. |
[39] | Park H-S , Oh D-H , Yoon T , et al. Effect of effortful swallowing training on tongue strength and oropharyngeal swallowing function in stroke patients with dysphagia: A double-blind, randomized controlled trial. Int J Lang Commun Disord. (2019) ;54: :479–84. 10.1111/1460-6984.12453. |
[40] | Miles A , Jardine M , Johnston F , et al. Effect of Lee Silverman VoiceTreatment (LSVT LOUD®) on swallowing and cough in Parkinson’s disease: A pilot study. J Neurol Sci. (2017) ;383: :180–7. 10.1016/j.jns.2017.11.015. |
[41] | Plowman EK , Tabor-Gray L , Rosado KM , et al. Impact of expiratory strength training in amyotrophic lateral sclerosis: Results of a randomized, sham-controlled trial. Muscle Nerve. (2019) ;59: :40–6. 10.1002/mus.26292. |
[42] | Balou M , Herzberg EG , Kamelhar D , et al. An intensive swallowing exercise protocol for improving swallowing physiology in older adults with radiographically confirmed dysphagia. Clin Interv Aging. (2019) ;14: :283–8. 10.2147/CIA.S194723. |
[43] | Shoval HA , Antelis E , Hillman A , et al. Onabotulinum toxin a injections into the salivary glands for spinal muscle atrophy type I: A prospective case series of 4 patients. Am J Phys Med Rehabil. (2018) ;97: :873–8. 10.1097/PHM.0000000000000989. |
[44] | Connaghan KP , Baylor C , Romanczyk M , et al. Communication and social interaction experiences of youths with congenital motor speech disorders. Am J speech-language Pathol. (2022) ;31: :2609–27.10.1044/2022_AJSLP-22-00034. |




