Post-exertional malaise among people with long COVID compared to myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)
Abstract
BACKGROUND:
Long COVID describes a condition with symptoms that linger for months to years following acute COVID-19. Many of these Long COVID symptoms are like those experienced by patients with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS).
OBJECTIVE:
We wanted to determine if people with Long COVID experienced post-exertional malaise (PEM), the hallmark symptom of ME/CFS, and if so, how it compared to PEM experienced by patients with ME/CFS.
METHODS:
A questionnaire that asked about the domains of PEM including triggers, experience, recovery, and prevention was administered to 80 people seeking care for Long COVID at Bateman Horne Center. Their responses were compared to responses about PEM given by 151 patients with ME/CFS using chi-square tests of independence.
RESULTS:
All but one Long COVID respondent reported having PEM. There were many significant differences in the types of PEM triggers, symptoms experienced during PEM, and ways to recover and prevent PEM between Long COVID and ME/CFS. Similarities between Long COVID and ME/CFS included low and medium physical and cognitive exertion to trigger PEM, symptoms of fatigue, pain, immune reaction, neurologic, orthostatic intolerance, and gastrointestinal symptoms during PEM, rest to recover from PEM, and pacing to prevent PEM.
CONCLUSION:
People with Long COVID experience PEM. There were significant differences in PEM experienced by people with Long COVID compared to patients with ME/CFS. This may be due to the newness of Long COVID, not knowing what exertional intolerance is or how to manage it.
1Introduction
Little attention and resources have been dedicated to the chronic consequences of acute infection even though examples of post-infectious sequelae from epidemics around the world are common. The ongoing Chikungunya virus epidemic has caused more than 10 million cases of chronic, debilitating rheumatic disease [1]. Ebola virus disease survivors suffer from fatigue, insomnia, and depression [2]. Post-West Nile virus sequelae include muscle weakness, memory loss, and difficulties with activities of daily living [3]. The long term effects from the 2003 severe acute respiratory syndrome (SARS) outbreak included chronic fatigue, pain, weakness, depression, and sleep disturbance symptoms [4]. People that recover from infectious mononucleosis, epidemic polyarthritis and Q fever experience disabling chronic fatigue, pain, and brain fog with 11% of people meeting criteria for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) 12 months after acute illness [5].
Many patients can pinpoint the time when they got a flu-like illness, did not recover and progressed to ME/CFS [6]. It has been difficult to directly link ME/CFS to a specific pathogen as the precipitating infection often occurred years before a diagnosis of ME/CFS is made [7]. Furthermore, there are no objective diagnostic tests and ME/CFS diagnosis relies on medical history, physical examination, and the presence of the core symptoms of impaired ability to function in association with fatigue, frequent and moderate to severe post-exertional malaise (PEM), unrefreshing sleep, and either cognitive impairment or orthostatic intolerance [8]. Of all these, PEM is pathognomonic of ME/CFS and necessary to make a diagnosis of ME/CFS. PEM is a worsening of symptoms (e.g., fatigue, weakness, orthostatic intolerance) and signs (e.g., heart rate variation, temperature dysregulation) after minimal physical or cognitive exertion, which can occur up to 72 hours after exertion and persist 24 hours or more. It can be triggered by daily activities such as sitting at the dining table, standing to make a salad, taking a shower, driving a car, grocery shopping, listening to a lecture, socializing, having a conversation, reading, or cleaning the house [9].
It is estimated that 10-30% of people remain ill for many months after acute COVID-19 with an illness similar to ME/CFS [10, 11]. Given that PEM is sine qua non for ME/CFS diagnosis, we wanted to know if PEM occurred in Long COVID. To do so, we compared PEM questionnaire responses from individuals that self-reported Long COVID to ME/CFS patients participating in an ongoing clinical research study.
2Methods
A PEM questionnaire (Fig. 1) was administered online to people who were seeking clinical care for Long COVID at Bateman Horne Center. The PEM questionnaire was originally developed for clinical use by Bateman Horne Center providers and was used in a 2020 research study with ME/CFS patients and originally included open-ended text fields to elicit responses about PEM triggers, symptoms, recovery and prevention [9]. The same PEM questionnaire was used in this study except multiple choice responses about PEM triggers, symptoms, recovery and prevention were employed for statistical analysis. REDCap was used to administer the PEM questionnaire [12]. Only multiple choice questions were analyzed in this study.
Fig. 1
PEM questionnaire.
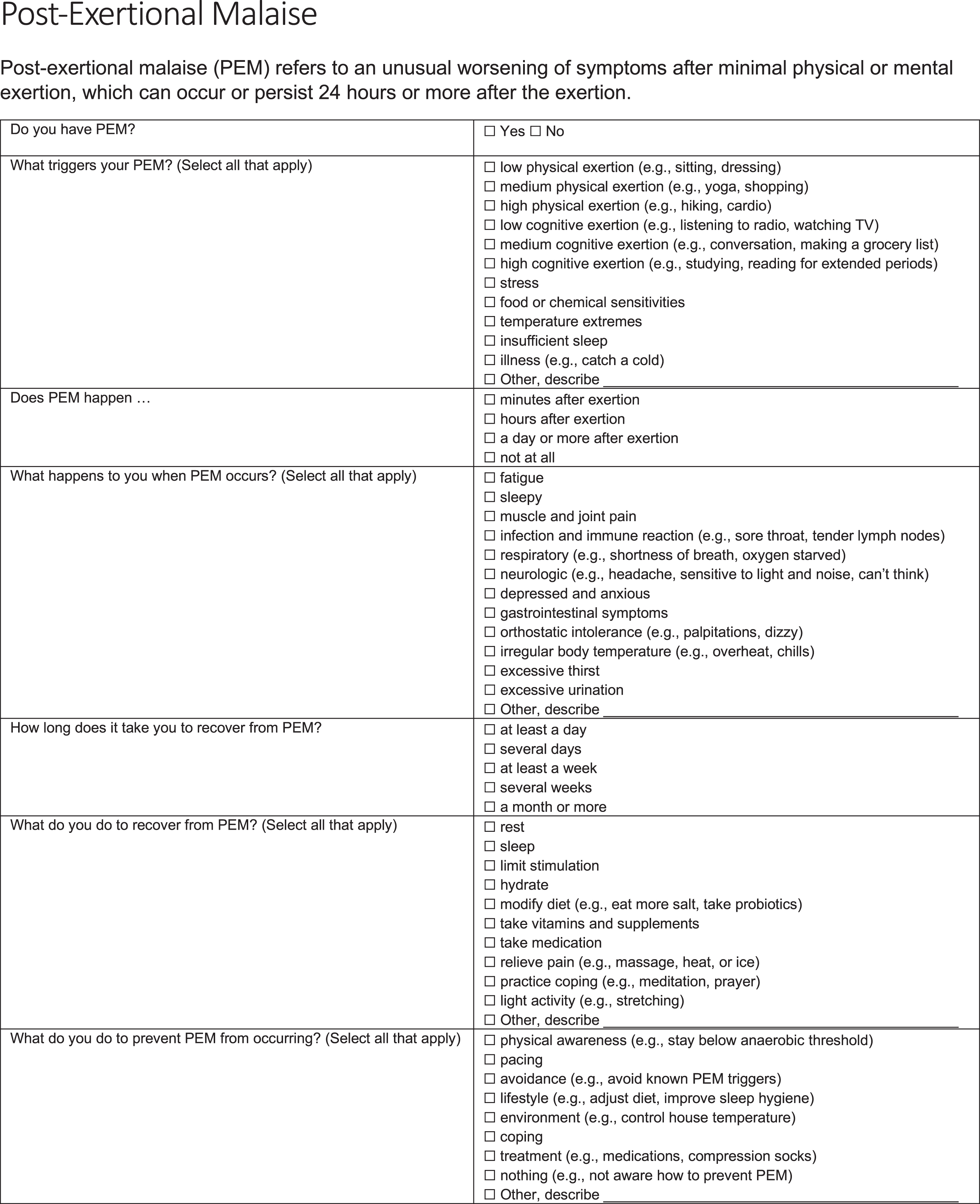
There were 80 respondents of 227 people seeking care that consented to have their survey responses analyzed and compared to other diseases treated at the Bateman Horne Center which is allowed under HIPAA privacy laws and regulations. The PEM questionnaire responses from these 80 Long COVID respondents were compared to 151 ME/CFS patients from an ongoing research study [9, 13]. ME/CFS patients were clinically evaluated and met Fukuda research criteria for ME/CFS, the Canadian consensus criteria, and the IOM clinical diagnostic criteria for ME/CFS [8, 14, 15]. The ME/CFS patients are part of an ongoing NIH-funded study titled, “Topological Mapping of Immune, Microbiota, Metabolomic and Clinical Phenotypes to Reveal ME/CFS Disease Mechanisms” (U54NS105539) and approved by The Jackson Laboratory Institutional Review Board study number 17-JGM-13. This study was conducted according to United States federal regulations for the protection of human subjects as codified in 45 CFR 46.
Survey responses were exported into Microsoft Excel for analysis. Chi-square tests of independence were used to compare PEM responses between the Long COVID and ME/CFS groups, where α= 0.05. Independence was analyzed in the responses for PEM triggers, PEM experience, PEM recovery, and PEM prevention. The characteristics of Long COVID respondents and ME/CFS patients are presented as descriptive statistics only since information was collected in different studies and questions were asked in slightly different ways.
3Results
The demographics of Long COVID survey respondents and ME/CFS patients were similar (Table 1). At least 75% of Long COVID respondents received a diagnosis of COVID-19 by a health care worker during the acute illness and 65% (52 of 79) reported having at least one positive test for SARS-CoV-2 nucleic acid or for SARS-CoV-2 antibodies. One of the 80 respondents reported not taking any COVID-19 tests during acute COVID-19. Forty-eight percent of Long COVID respondents had at least one dose of a COVID-19 vaccine. Most Long COVID respondents (67%) indicated they had been sick for more than 6 months.
Table 1
Characteristics of long COVID respondents (N = 80) and ME/CFS patients (N = 151)
| Long COVID | ME/CFS | |
| N (%) | N (%) | |
| Sex at birth | ||
| Male | 15 (19%) | 40 (27%) |
| Female | 65 (81%) | 111 (74%) |
| Ethnicity/background | ||
| Asian | 0 | 2 (1%) |
| Black | 0 | 0 |
| Latino | 3 (4%) | 4 (3%) |
| White | 72 (90%) | 147 (97%) |
| Mixed | 1 (1%) | 2 (1%) |
| Arab | 0 | 0 |
| Other | 3 (4%) | 0 |
| Unknown | 1 (1%) | 0 |
| Education | ||
| Less than high school | 2 (3%) | 2 (1%) |
| High school | 22 (28%) | 17 (11%) |
| Vocational/specialized | 4 (5%) | 47 (31% |
| University | 52 (65%) | 85 (56%) |
PEM was present in all but one Long COVID respondent and PEM onset and recovery time was similar for Long COVID and ME/CFS patients (Table 2). PEM started minutes after exertion for 28% of Long COVID and 35% of ME/CFS patients, hours after exertion for 40% of Long COVID and 41% of ME/CFS patients, and a day or more after exertion for 25% of Long COVID and 30% of ME/CFS patients. Both groups indicated that it would take at least a day (31% Long COVID, 39% ME/CFS) to several days (44% Long COVID, 48% ME/CFS) to recover from PEM. A smaller percentage of Long COVID respondents (16%) and ME/CFS patients (21%) indicated that it could take at least a week to a month or more to recover from PEM.
Table 2
These three questions of the PEM questionnaire were presented the exact same way to both the long COVID respondents (N = 80) and ME/CFS patients (N = 151)
| Long COVID N (%) | ME/CFS N (%) | P-value | |
| Do you have PEM? | |||
| Yes | 79 (99%) | 151 (100%) | 0.93 |
| No | 1 (1%) | 0 | 0.12 |
| PEM onset | |||
| Minutes after exertion | 22 (28%) | 53 (35%) | 0.33 |
| Hours after exertion | 33 (41%) | 60 (40%) | 0.87 |
| A day or more after exertion | 24 (30%) | 38 (25%) | 0.49 |
| No answer | 1 (1%) | 0 | 0.17 |
| How long does it take to recover from PEM? | |||
| At least a day | 31 (39%) | 47 (31%) | 0.34 |
| Several days | 35 (44%) | 72 (48%) | 0.68 |
| At least a week | 7 (9%) | 20 (13%) | 0.34 |
| Several weeks | 3 (4%) | 8 (5%) | 0.61 |
| A month or more | 3 (3%) | 4 (3%) | 0.65 |
| No answer | 1 (1%) | 0 | 0.17 |
PEM triggers, symptoms, recovery, and prevention were compared between the groups (Table 3). Low and medium physical and cognitive exertion were similar PEM triggers for both groups. Significantly more Long COVID respondents reported that PEM was triggered by high physical and cognitive exertion, stress, food or chemical sensitivities, temperature extremes, insufficient sleep or illness compared to ME/CFS patients.
Table 3
Long COVID respondents (N = 80) and ME/CFS patients (N = 151) responses to triggers, experience, recovery, and prevention of PEM
| Long COVID N (%) | ME/CFS N (%) | P-value | |
| Trigger | |||
| Low physical exertion | 14 (18%) | 33 (22%) | 0.49 |
| Medium physical exertion | 67 (84%) | 108 (72%) | 0.31 |
| High physical exertion | 58 (73%) | 48 (32%) | <.001 |
| Low cognitive exertion | 8 (10%) | 14 (9%) | 0.86 |
| Medium cognitive exertion | 36 (45%) | 62 (41%) | 0.66 |
| High cognitive exertion | 55 (69%) | 26 (17%) | <.001 |
| Stress | 62 (78%) | 17 (11%) | <.001 |
| Food or chemical sensitivities | 23 (29%) | 2 (1%) | <.001 |
| Temperature extremes | 37 (46%) | 7 (5%) | <.001 |
| Insufficient sleep | 62 (78%) | 7 (5%) | <.001 |
| Illness | 32 (40%) | 1 (1%) | <.001 |
| Experience | |||
| Fatigue | 77 (96%) | 130 (86%) | 0.44 |
| Sleepy | 64 (80%) | 24 (16%) | <.001 |
| Muscle and joint pain | 56 (70%) | 91 (60%) | 0.38 |
| Infection and immune reaction | 31 (39%) | 56 (37%) | 0.84 |
| Respiratory | 47 (59%) | 29 (19%) | <.001 |
| Neurologic | 57 (72%) | 103 (68%) | 0.79 |
| Depressed and anxious | 39 (49%) | 20 (13%) | <.001 |
| Gastrointestinal symptoms | 27 (34%) | 50 (33%) | 0.94 |
| Orthostatic intolerance | 51 (64%) | 88 (58%) | 0.61 |
| Irregular body temperature | 42 (53%) | 30 (20%) | <.001 |
| Excessive thirst | 24 (30%) | 5 (3%) | <.001 |
| Excessive urination | 11 (14%) | 3 (2%) | <.001 |
| Recovery | |||
| Rest | 72 (90%) | 139 (92%) | 0.88 |
| Sleep | 59 (74%) | 50 (33%) | <.001 |
| Limit stimulation | 46 (58%) | 18 (12%) | <.001 |
| Hydrate | 60 (75%) | 55 (36%) | <.001 |
| Modify diet | 36 (45%) | 43 (29%) | 0.04 |
| Take vitamins and supplements | 45 (56%) | 13 (9%) | <.001 |
| Take medication | 23 (29%) | 15 (10%) | <.001 |
| Relieve pain | 31 (39%) | 27 (18%) | 0.003 |
| Practice coping | 43 (54%) | 18 (12%) | <.001 |
| Light activity | 21 (26%) | 6 (4%) | <.001 |
| Prevention | |||
| Physical awareness | 48 (60%) | 119 (79%) | 0.11 |
| Pacing | 43 (54%) | 57 (38%) | 0.08 |
| Avoidance | 56 (70%) | 25 (17%) | <.001 |
| Lifestyle | 40 (50%) | 18 (12%) | <.001 |
| Environment | 23 (29%) | 11 (7%) | <.001 |
| Coping | 36 (29%) | 6 (9%) | <.001 |
| Treatment | 38 (31%) | 9 (14%) | <.001 |
| Nothing | 28 (22%) | 3 (4%) | <.001 |
Fatigue, muscle and joint pain, infection and immune reactions, neurologic symptoms and orthostatic intolerance were symptoms experienced at similar rates in both Long COVID respondents and ME/CFS patients. Compared to ME/CFS patients, Long COVID respondents reported being significantly more sleepy, had more respiratory symptoms, felt more depressed and anxious, and had irregular body temperature, excessive thirst, and excessive urination.
Both the Long COVID respondents and ME/CFS patients indicated that rest was the most common PEM recovery strategy, 90% and 92% respectively. Long COVID respondents were significantly more likely to use the other nine approaches to recover from PEM.
Both groups reported using physical awareness and pacing to prevent PEM. Significantly more Long COVID respondents indicated that avoidance (70% Long COVID, 17% ME/CFS), lifestyle (50% Long COVID, 12% ME/CFS), environment (29% Long COVID, 7% ME/CFS), coping (29% Long COVID, 9% ME/CFS), treatment (31% Long COVID, 14% ME/CFS) or nothing (22% Long COVID, 4% ME/CFS) were used as approaches to prevent PEM.
4Discussion
Post-exertional malaise is one of three core symptoms required to diagnosis ME/CFS and is a distinctive feature that can help differentiate or liken to ME/CFS [8]. Our goal was to determine if PEM was experienced in Long COVID respondents and if it was similar to PEM experienced by ME/CFS patients. Since PEM is not familiar to most people, the PEM questionnaire used in this study first introduced it as “an unusual worsening of symptoms after minimal physical or mental exertion, which can occur or persist 24 hours or more after the exertion” then asks, “Do you have PEM?” All but one Long COVID respondent answered “Yes” to having PEM. The onset and duration of PEM were the same for both groups with the majority indicating PEM occurred within hours of exertion and lasted for several days. Furthermore, there were no differences in the other PEM onset and duration categories. One other study found that PEM was one of the most frequent Long COVID symptoms [11].
For ME/CFS patients, physical and cognitive exertion that was easily tolerated prior to illness now triggers a worsening of disease symptoms. Low and medium physical and cognitive exertion triggered PEM for both Long COVID respondents and ME/CFS patients. Long COVID respondents indicated that high physical and cognitive exertion, stress, food or chemical sensitivities, temperature extremes, insufficient sleep and illness were significantly more likely to trigger PEM than reported by ME/CFS patients. The respondents in this study have been sick with Long COVID for less than a year and were unfamiliar with exertional intolerance whereas ME/CFS patients, who have been sick for years, have had more time to understand their illness and PEM, know what level of physical and cognitive exertion they can tolerate, and other things that can trigger PEM. We have previously shown that new onset ME/CFS patients reported that stress triggered PEM significantly more often than for patients sick with ME/CFS for >10 years [9].
The PEM questionnaire asks, “What happens to you when PEM occurs?” Long COVID respondents and ME/CFS patients both answered that fatigue, muscle and joint pain, infection and immune reaction, neurologic and gastrointestinal symptoms, and orthostatic intolerance all worsened. Fatigue, pain/musculoskeletal, orthostatic intolerance, and neurologic symptoms are a common PEM symptom constellation experienced by most ME/CFS patients [7, 16]. Long COVID respondents reported significantly more sleepiness, respiratory issues, depression and anxiety, irregular body temperature and excessive thirst than ME/CFS patients. Symptoms worsened by exertion in Long COVID may be due to the unique pathophysiology of SARS-CoV-2 infection including the ability of this virus to cause neuroinflammation [17]. Depression and anxiety, both associated with neuroinflammation, were the most frequent neuropsychiatric sequelae of COVID-19 [18]. There is increasing evidence for the role of neuroinflammation in Long COVID because of SARS-CoV-2 neurotropism and neuroimmune pathophysiology. Irregular body temperature, excessive thirst, and excessive urination may be associated with autonomic nervous system disruption that occurs after SARS-CoV-2 infection [19]. PEM symptoms experienced by Long COVID respondents but not in ME/CFS patients may be because ME/CFS patients have been sick longer and are familiar with the activities that trigger PEM. ME/CFS patients in this study are receiving treatment and this clinical care may have ameliorated their symptoms whereas Long COVID is new to both patients and providers, and they are only beginning to receive clinical care for their Long COVID symptoms.
The majority of Long COVID respondents and ME/CFS patients rested to recover from PEM; although both groups used a variety of things to recover including sleep, hydration, and diet. These make biological sense since deficits in energy utilization are known in ME/CFS [20, 21]. Most ME/CFS patients used physical awareness to prevent PEM whereas Long COVID respondents prevented PEM by avoiding triggers.
This is the first study to examine the triggers, experience, recovery, and prevention of PEM in Long COVID. A recent online survey study found that people with Long COVID who reported having PEM also reported more severe fatigue, reduced physical and social functioning, and worse health compared with a year earlier [22]. Many studies have found that Long COVID symptoms are the same as those that define ME/CFS [8]. FAIR Health studied a total of 1,959,982 healthcare claim records from COVID-19 patients and found that fatigue and malaise were among the five most common post-COVID symptoms across all ages [23]. A comprehensive review of the Long COVID literature published since January 2021 found sleep disturbances to be one of the predominant symptoms [24]. People with Long COVID also experience orthostatic intolerance [19]. Fatigue, post-exertional malaise, and cognitive dysfunction were the most frequent symptoms in people who were still sick 6 months after acute COVID-19 illness [11]. Since Long COVID is not well-defined for diagnosis and research, the ME/CFS diagnostic criteria [7] could be useful for diagnosing Long COVID patients and providing common data elements, validated instruments and patient reported outcomes for Long COVID research.
There are limitations to this study. The first version of the PEM questionnaire used free form text to elicit information about PEM triggers, experiences, recovery, and prevention which were subsequently coded into multiple choice categories that Long COVID respondents used in this current version of the questionnaire [9]. There was selection bias of the Long COVID respondents in this study. The PEM questionnaire was taken by Long COVID respondents who were not hospitalized, who were sick with lingering symptoms for at least 3 months, and who predominately non-Hispanic White females living in Utah, and who were seeking care at Bateman Horne Center, a tertiary care center for ME/CFS, fibromyalgia and post-viral illnesses. Therefore, these results may not be generalizable to other Long COVID patients. Some Long COVID respondents did not have a positive COVID test or were not clinically diagnosed with COVID-19 by a health care provider. This was mainly due to being sick early in the pandemic when testing was not available, and COVID-19 was not readily diagnosed.
5Conclusion
People with Long COVID experience PEM that is the hallmark of ME/CFS. Systemic exertional intolerance has physiological underpinnings that can be addressed with early diagnosis and management. Asking about PEM in people that have lingering symptoms following COVID-19 is essential to mitigate progression and possible development of ME/CFS.
Ethics statement
Survey respondents provided consent to analyze their answers for research and publication. The ME/CFS protocol was approved by The Jackson Laboratory Institutional Review Board (study number 17-JGM-13) and the study was carried out according to United States federal regulations for the protection of human subjects as codified in 45 CFR 46. Procedures were carried out with adequate understanding and written consent of the subjects.
Consent for publication
The manuscript does not contain any identifiable individual data in any form.
Availability of data and materials
The PEM Questionnaire is in the REDCap Shared Library and is available to other REDCap users around the world. The PEM Questionnaire will be made freely available upon request to those who do not have REDCap access. The datasets used and/or analyzed are available from the corresponding author upon reasonable request.
Conflict of interest
The authors declare that they have no conflicts of interest.
Funding
The Long COVID survey was funded by donations made to Bateman Horne Center. The ME/CFS study was funded by a grant from the National Institutes of Health (1U54NS105539). The funding sources had no involvement in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Acknowledgments
The Bateman Horne Center serves as the Clinical Core of the Collaborative Research Center (Grant project number U54NS105539) led by Derya Unutmaz, M.D. of The Jackson Laboratory. The authors want to thank everyone for their participation in this research.
References
[1] | Pathak H , Mohan MC , Ravindran V . Chikungunya arthritis. Clin Med. (2019) ;19: (5):381–5. |
[2] | Lötsch F , Schnyder J , Goorhuis A , Grobusch MP . Neuropsychological long-term sequelae of Ebola virus disease survivors – A systematic review. Travel Med Infect Dis. (2017) ;18: :18–23. |
[3] | Hughes JM , Wilson ME , Sejvar JJ . The Long-Term Outcomes of Human West Nile Virus Infection. Clin Infect Dis. (2007) ;44: (12):1617–24. |
[4] | Moldofsky H , Patcai J . Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post-SARS syndrome; a case-controlled study. BMC Neurol. (2011) ;11: (1):37. |
[5] | Hickie I , Davenport T , Wakefield D , Vollmer-Conna U , Cameron B , Vernon SD , et al. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ. (2006) ;333: (7568):575. |
[6] | FDA (Food and Drug Administration). (2013) . The voice of the patient: Chronic fatigue syndrome and myalgic encephalomyelitis. Bethesda, MD: Center for Drug Evaluation and Research (CDER), FDA. |
[7] | Bateman L , Bested AC , Bonilla HF , Chheda BV , Chu L , Curtin JM , et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Essentials of Diagnosis and Management. Mayo Clin Proc. (2021) ;96: (11):2861–78. |
[8] | Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness [Internet]. Washington, D.C.: National Academies Press; (2015) [cited 2022 Oct 6]. Available from: http://www.naedu/catalog/19012 |
[9] | Hartle M , Bateman L , Vernon SD . Dissecting the nature of post-exertional malaise. Fatigue Biomed Health Behav. (2021) ;9: (1):33–44. |
[10] | Nalbandian A , Sehgal K , Gupta A , Madhavan MV , McGroder C , Stevens JS , et al. Post-acute COVID-19 syndrome. Nat Med. (2021) ;27: (4):601–15. |
[11] | Davis HE , Assaf GS , McCorkell L , Wei H , Low RJ , Re’em Y , et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. eClinicalMedicine. (2021) ;38: :101019. |
[12] | Harris PA , Taylor R , Thielke R , Payne J , Gonzalez N , Conde JG . Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. (2009) ;42: (2):377–81. |
[13] | Lee J , Vernon SD , Jeys P , Ali W , Campos A , Unutmaz D , et al. Hemodynamics during the 10-minute NASA Lean Test: evidence of circulatory decompensation in a subset of ME/CFS patients. J Transl Med. (2020) ;18: (1):314. |
[14] | Fukuda K . The Chronic Fatigue Syndrome: A Comprehensive Approach to Its Definition and Study. Ann Intern Med. (1994) ;121: (12):953. |
[15] | Carruthers BM . Definitions and aetiology of myalgic encephalomyelitis: how the Canadian consensus clinical definition of myalgic encephalomyelitis works. J Clin Pathol. (2006) ;60: (2):117–9. |
[16] | Chu L , Valencia IJ , Garvert DW , Montoya JG . Deconstructing post-exertional malaise in myalgic encephalomyelitis/chronic fatigue syndrome: A patient-centered, cross-sectional survey. Nater UM, editor. PLOS ONE. (2018) ;13: (6):e0197811. |
[17] | Crook H , Raza S , Nowell J , Young M , Edison P . Long covid—mechanisms, risk factors, and management. BMJ. (2021) ;n1648. |
[18] | Schou TM , Joca S , Wegener G , Bay-Richter C . Psychiatric and neuropsychiatric sequelae of COVID-19 – A systematic review. Brain Behav Immun. (2021) ;97: :328–48. |
[19] | Dani M , Dirksen A , Taraborrelli P , Torocastro M , Panagopoulos D , Sutton R , et al. Autonomic dysfunction in ‘long COVID’: rationale, physiology and management strategies. Clin Med. (2021) ;21: (1):e63–7. |
[20] | Yamano E , Sugimoto M , Hirayama A , Kume S , Yamato M , Jin G , et al. Index markers of chronic fatigue syndrome with dysfunction of TCA and urea cycles. Sci Rep. (2016) ;6: (1):34990. |
[21] | Yamano E , Watanabe Y , Kataoka Y . Insights into Metabolite Diagnostic Biomarkers for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Int J Mol Sci. (2021) ;22: (7):3423. |
[22] | Twomey R , DeMars J , Franklin K , Culos-Reed SN , Weatherald J , Wrightson JG . Chronic Fatigue and Postexertional Malaise in People Living With Long COVID: An Observational Study. Phys Ther. (2022) ;102: (4):pzac005. |
[23] | FAIR Health. A Detailed Study of Patients with Long-Haul COVID. An Analysis of Private Healthcare Claims. |
[24] | Akbarialiabad H , Taghrir MH , Abdollahi A , Ghahramani N , Kumar M , Paydar S , et al. Long COVID, a comprehensive systematic scoping review. Infection. (2021) ;49: (6):1163–86. |




