Two symptoms can accurately identify post-exertional malaise in myalgic encephalomyelitis/chronic fatigue syndrome
Abstract
BACKGROUND:
Post-exertional malaise (PEM) is the hallmark symptom of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) yet its diverse manifestations make it difficult to recognize. Brief instruments for detecting PEM are critical for clinical and scientific progress.
OBJECTIVE:
To develop a clinical prediction rule for PEM.
METHOD:
49 ME/CFS and 10 healthy, sedentary subjects recruited from the community completed two maximal cardiopulmonary exercise tests (CPETs) separated by 24 hours. At five different times, subjects reported symptoms which were then classified into 19 categories. The frequency of symptom reports between groups at each time point was compared using Fisher’s exact test. Receiver operating characteristics (ROC) analysis with area under the curve calculation was used to determine the number of different types of symptom reports that were sufficient to differentiate between ME/CFS and sedentary groups. The optimal number of symptoms was determined where sensitivity and specificity of the types of symptom reports were balanced.
RESULTS:
At all timepoints, a maximum of two symptoms was optimal to determine differences between groups. Only one symptom was necessary to optimally differentiate between groups at one week following the second CPET. Fatigue, cognitive dysfunction, lack of positive feelings/mood and decrease in function were consistent predictors of ME/CFS group membership across timepoints.
CONCLUSION:
Inquiring about post-exertional cognitive dysfunction, decline in function, and lack of positive feelings/mood may help identify PEM quickly and accurately. These findings should be validated with a larger sample of patients.
1Introduction
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a chronic, debilitating medical condition which affects millions of people worldwide [1], often striking them in their prime educational and working periods [2, 3] and leaving them disabled for years [4]. Despite its high prevalence and impact, no objective test to diagnose the condition currently exists. Instead, ME/CFS is diagnosed based on fulfillment of symptom-based diagnostic criteria and elimination of other potential causes of the patients’ symptoms [5]. For the last 3 decades, the most-used case definition internationally has been the US Centers for Disease Control and Prevention’s (CDC) Fukuda 1994 CFS criteria [6]. Although the Fukuda criteria mandated that patients experience a specific set of symptoms accompanying severe, function-limiting fatigue (e.g., unrefreshing sleep, sore throat, problems thinking, etc.), in practice, most clinicians have tended to focus only or primarily on fatigue. Many medical conditions and lifestyle factors (e.g., major depressive disorder, hypothyroidism, overwork, poor diet, family obligations) may cause chronic fatigue, leading some clinicians to hesitate diagnosing ME/CFS. Consequently, up to 95% of people affected are estimated to remain in limbo for extended periods, with no diagnosis and appropriate management, or to be misdiagnosed with another condition, like major depressive disorder [7, 8].
In contrast, clinicians and researchers specializing in ME/CFS have long-believed that post-exertional malaise (PEM), rather than fatigue, is the unique, distinguishing feature of ME/CFS. Starting in 2003, these experts began publishing alternative diagnostic criteria such as the Canadian Consensus Criteria [9] which mandated PEM in addition to or instead of fatigue. To resolve these issues and to incorporate scientific advances since 1994, the United States government asked that the National Academy of Medicine (NAM) convene a committee to develop updated, evidence-based diagnostic criteria for ME/CFS. This new set of criteria, named Systemic Exertion Intolerance Disease, was released in 2015: patients were required to experience substantial, function-limiting fatigue; post-exertional malaise; and unrefreshing sleep plus either orthostatic intolerance or cognitive dysfunction [10]. Additionally, symptoms must have been present for at least 6 months and be of at least moderate intensity during at least 50% of that time. Shortly thereafter, the US CDC incorporated the new criteria into its educational materials [11] and the US National Institutes of Health began encouraging researchers applying for its ME/CFS-targeted grants to use it in their research [12].
Post-exertional malaise is exacerbation of some or all a patient’s ME/CFS symptoms following physical, cognitive, positional, and/or emotional exertion. The type, intensity, duration, and frequency of exertion needed to precipitate PEM can be minimal. Activities of daily living (ADLs), like eating, showering, cooking, or driving, are sufficient PEM triggers for some patients [13, 14] while others struggle to attend school or work even part-time consistently. Common PEM symptoms include physical and cognitive fatigue, widespread muscle pain, multi-joint pain, problems thinking, disturbed sleep, sore throat, tender cervical/axillary lymph nodes, headache, and flu-like feelings [14–16]. Some patients may also experience worsened mood, gastrointestinal upset, problems urinating, heightened sensitivity (e.g., to sound, light, and certain substances), and other neurologic issues. PEM may follow exertional challenges immediately or be delayed by hours to days and can last hours, days, weeks, or even longer [14–16]. Therefore, PEM patterns vary not only between patients but also within individual patients from one episode to another.
Even with recent criteria, unfamiliarity with and diversity in the presentation of PEM may make it difficult for clinicians to diagnose ME/CFS quickly and accurately. In its recommendations, the NAM report stated “the development of brief in-office tests for detecting PEM [is] critical”; currently, no such instrument exists [10]. The 54-item symptom survey of the DePaul Symptoms Questionnaire (DSQ) [17] contains a 5-item subscale pertaining to PEM. The US NIH’s Common Data Elements (CDE) Working Group for ME/CFS [18] recommended this subscale as an interim, mandatory measure of PEM for all ME/CFS studies. However, the CDE PEM subgroup recognized that the subscale was not designed to be a stand-alone measure and has not been validated for PEM. Further, a 2018 survey [19] of almost 800 patients spearheaded by patient-advocates found that although 92% felt the NAM definition of PEM fit their experiences, only about a third felt the DSQ PEM subscale was adequate. Since then, Dr. Leonard Jason and his team have added 5 additional items to create a 10-item questionnaire focused on PEM [20], which was able to accurately identify 80% of study participants affected by either ME/CFS, multiple sclerosis, or post-polio syndrome. The questionnaire still needs to be tested in larger samples of ME/CFS patients, on more control groups unaffected by ME/CFS, and, ideally, against objective measures of PEM.
In addition to clinical diagnosis, a short, well-validated PEM questionnaire would also benefit research by helping to recruit suitable study participants and to assess the effects of clinical interventions. Like the NAM and NIH, the US Food and Drug Administration, in their draft guidance for the pharmaceutical industry, drew attention to the importance of PEM and noted that “patient-report instruments optimal for measurement of fatigue or other symptoms” do not exist at the moment [21].
Over the last decade we have conducted multiple studies using open-ended questionnaires to pinpoint salient aspects of PEM [16, 22–25]. After a standardized bicycle exercise challenge, ME/CFS patients experienced some symptoms which healthy, sedentary subjects did not experience at all (e.g., sore throat, tender cervical and axillary lymph nodes) or at much lower rates (e.g., pain, sleep disturbances) [16, 24]. Taking more than 24 hours to recover from the challenge was an excellent marker of potential ME/CFS with a positive likelihood ratio of 11 and a negative likelihood ratio of 0.22 [25]. A cluster of four symptom categories (based on fatigue-, pain-, immune- and sleep-related symptoms in the Canadian Consensus Criteria [9]) could classify 92% of ME/CFS patients and 88% of healthy controls accurately [25]. A similar constellation of symptoms and low rate of recovery within 24 hours were confirmed by one of us (LC) in a survey of a separate group of ME/CFS subjects based at Stanford University Medical Center [26]. Only 9% of subjects reported returning to their baseline level of health in that time period. That survey also found that only 42% of subjects qualified for the 4-symptom cluster identified earlier. Differences observed could be due to some subjects in the Stanford study being predominately older (mean age of 51.6±12.5 years), with more male representation (in 20% of the cases), and greater disease severity (unable to withstand a maximal exercise test).
The purpose of this study was to combine self-reported PEM symptoms and timing in response to 2-day cardiopulmonary exercise test challenge into a short set of questions clinicians and researchers can use to quickly and accurately determine if a subject experiences PEM and thus qualifies for newer criteria. Furthermore, instead of relying on a preconceived classification of symptoms based on the CCC, we constructed a coding schema based on a review of the literature, clinician-researchers’ experiences (LC and TD), and our prior studies.
2Methods
2.1Participants
This study involved a retrospective review of data from a testing facility specializing in the evaluation of functional capacity. Subjects with ME/CFS were either formally screened using the Fukuda 1994 criteria and self-reported PEM or had been diagnosed by their physician with ME/CFS. Control subjects were recruited from the community. They could not participate in an exercise program or perform more than 30 minutes of moderate physical activity on a weekly basis. All subjects completed two days of exercise testing for inclusion in the study. This study was reviewed and approved by University of the Pacific’s Institutional Review Board (Stockton, CA, USA).
2.2Procedure
2.2.1Two-day cardiopulmonary exercise testing
Subjects were asked to refrain from all forms of physical activity for at least 24 hours before the first cardiopulmonary exercise test. An electrically braked cycle ergometer was adjusted and Hans Rudolph mask fitted to each subject. Subjects were given a minute to practice pedaling and then were instructed to pedal at a constant cadence until exhaustion. Workload was increased by 15-20 watts per minute. The test was concluded once subjects stopped pedaling or when maximal effort criteria was met. Exercise duration ranged from 5 to 15 minutes. After the test, subjects were instructed to remain seated for 2 to 5 minutes to recover. An investigator was present to monitor each subject before, during, and after the test. Subjects returned to the facility 24 hours later to perform the second exercise test which was conducted in a similar manner. Complete details of this procedure have been described elsewhere [27].
2.2.2Recovery questionnaire
At the beginning of the 2-day CPET, subjects were given an open-ended questionnaire to complete over the days of testing and into the week following both tests. This questionnaire has been described elsewhere [25]. Briefly, subjects described how they felt immediately and 24 hours after each exercise test as well as 7 days after both tests. They were also asked to specify how long it took them to recover from the two-day CPET. Subjects chose from a range of answers (e.g., less than 1 day, 2 days, 7 days, still not recovered, etc.). Completed questionnaires were mailed, faxed or emailed to the testing facility seven days after the second exercise test.
2.2.3Categorization of symptom responses
The process for coding recovery questionnaires has been detailed elsewhere [24]. Briefly, questionnaires were digitized and labeled by subjects’ study identification numbers. A coding schema, based on a review of literature and the authors’ clinical/research experiences, was developed prior to the evaluation of the questionnaires. Next, two reviewers (LJM and LC), who were blinded to demographic information and subjects’ diagnoses, tested the schema on a small, random subset of the surveys. The schema was then refined reiteratively to assure that it reflected the experiences of subjects thoroughly and accurately. A total of nineteen codes, or symptom categories, were established. The symptom categories were Cardiopulmonary, Cognitive Dysfunction, Cold Limbs, Decrease in Function, Fatigue, Flu-like Symptoms, Gastrointestinal, Headache, Increase in Sensitivity, Light-headedness, Mood, Muscle/Joint Pain, Neurologic, Pain (for generalized pain or pain not fitting into another category), Sleep Disturbances, Temperature Control, Tingling, Weakness, and Positive Feelings. LJM and LC independently reviewed each subjects’ questionnaire using Excel spreadsheets which listed the nineteen symptom categories at each of the 5 timepoints. Upon completion of coding, the reviewers’ results were compared to identify any differences. After 4 rounds of coding, the average discrepancy rate was 1.67% which equates to an average of two differences within each survey.
2.2.4Statistical analysis
Descriptive statistics were calculated, including means, standard deviations, and 95% confidence intervals (95% CIs) for continuous data, as well as counts, proportions, medians and interquartile ranges for binomial data. Chi-square statistics (Fisher’s exact test) were calculated to assess the statistical significance of differences in the proportion of subjects in each group reporting each symptom at each time point during and after the 2-day CPET that was abstracted from the open-ended recovery questionnaire (i.e., immediately after CPET 1, 24 hours after CPET 1, immediately after CPET 2, 24 hours after CPET 2, and 1 week following CPET 2).
Symptom responses were considered as a binomial variables for clinimetric analysis (i.e., present vs. not present). For each symptom report that was significantly different in frequency between groups, clinimetric statistics with 95% CIs were calculated to predict membership in the ME/CFS vs. sedentary control groups at each time point. These clinimetric statistics included sensitivity, specificity, positive and negative likelihood ratios, and positive and negative predictive values. The optimal number of types of symptoms necessary to differentiate between groups at each time point was then determined using receiver operating characteristics curve (ROC) analysis. Areas under the curves (AUCs) with 95% confidence intervals were used to assess the significance of association between symptom clustering and group membership. The minimum types of symptoms necessary to differentiate between groups was established where sensitivity (true positive rate) and specificity (true negative rate) each were maximized. Because positive feelings/mood are common after exercise in non-disabled people, symptom reports were reverse coded as ‘absence of positive feelings/mood’ for clinimetric analysis to ensure similar directionality in all symptom reports. Statistical significance for all analyses was determined at α≤0.05.
3Results
3.1Demographics
Forty-nine ME/CFS subjects were recruited: 15 fulfilled Fukuda 1994 criteria and self-reported PEM while 34 arrived with a physician diagnosis of ME/CFS. Ten sedentary, but otherwise healthy, control subjects from the community were also recruited for the study. Eighty percent of participants affected by ME/CFS were female and the mean (standard deviation) age was 41.3 (7); for the control group, these numbers were respectively 70% and 35.3 (12.2).
3.2General symptomatic response to 2-day CPET
Twenty-four hours after the second CPET, subjects with ME/CFS reported 15.4 symptoms (standard deviation [SD]: 7.7; 95% CI: 13.4-17.6) compared to 5.6 symptoms (SD: 1.7; 95% CI: 4.3-6.6) for matched sedentary control subjects (p < .001). At one-week follow-up, patients with ME/CFS reported 5.0 symptoms (SD: 1.7; 95% CI: 4.3-6.6) compared to 0.2 symptoms (SD: 0.1; 95% CI: -0.1-0.5) for matched sedentary control subjects (p < 0.001). Overall, patients with ME/CFS indicated 19.2 symptoms (SD: 10.0; 95% CI: 16.4-22.1) compared to 7.1 symptoms for matched sedentary control subjects (SD: 2.4; 95% CI: 5.5-8.7; p < 0.001). All control subjects recovered within a day or less. In contrast, 49% of patients with ME/CFS took a mean of 4.5 days (p < 0.01) while the remaining 51% had not recovered yet by 7 days after the second CPET.
Symptom reports at each time point during and after the 2-day CPET task appear in Table 1. Fatigue was reported significantly more frequently by patients with ME/CFS compared to control subjects at all timepoints. In addition, there were between-groups difference in the frequencies of symptoms at each time point.
Table 1
Symptom expression after serial cardiopulmonary exercise testing (CPET) in individuals with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS; n = 49) and control subjects (n = 10) at five time points: immediately after the first CPET, 24 hours after the first CPET, immediately after the second CPET, 24 hours after the second CPET, and 1 week after the second CPET. The top value in each cell is the number of subjects endorsing a symptom; parenthetical values are within-group symptom prevalence. Bolded values signify statistically significant differences in symptom prevalence between ME/CFS and control subjects
| Symptom | Group | Test 1 | Test 2 | Any time point | |||
| Immediately | 24 hours | Immediately | 24 hours | 1 week | |||
| Cardiopulmonary symptoms | ME/CFS | 7 (14.3%) | 3 (6.1%) | 12 (24.5%) | 7 (14.3%) | 10 (20.4%) | 19 (38.8%) |
| Control | 1 (9.1%) | 0 (0.0%) | 1 (9.1%) | 0 (0.0%) | 0 (0.0%) | 1 (9.1%) | |
| Cognitive dysfunction | ME/CFS | 14* (28.6%) | 20† (40.8%) | 19 (38.8%) | 16* (32.7%) | 24† (50.0%) | 30† (61.2%) |
| Control | 0 (0.0%) | 0 (0.0%) | 1 (9.1%) | 0 (0.0%) | 0 (0.0%) | 1 (9.1%) | |
| Cold limbs | ME/CFS | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Control | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Decrease in function | ME/CFS | 12 (24.5%) | 9 (18.4%) | 16* (32.7%) | 17* (34.7%) | 13* (26.5%) | 30§ (61.2%) |
| Control | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Fatigue | ME/CFS | 40† (81.6%) | 38† (77.6%) | 39† (79.6%) | 31† (63.3%) | 29† (59.2%) | 47§ (95.9%) |
| Control | 4 (36.4%) | 3 (27.3%) | 3 (27.3%) | 1 (9.1%) | 0 (0.0%) | 6 (54.5%) | |
| Flu-like symptoms | ME/CFS | 2 (4.1%) | 6 (12.2%) | 6 (12.2%) | 5 (10.2%) | 8 (16.3%) | 13* (26.5%) |
| Control | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Gastrointestinal disturbance | ME/CFS | 6 (12.2%) | 3 (6.1%) | 5 (10.2%) | 6 (12.2%) | 7 (14.3%) | 14* (28.6%) |
| Control | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Headache | ME/CFS | 15 (30.6%) | 13* (26.5%) | 13* (26.5%) | 15* (30.6%) | 10 (20.4%) | 28† (57.1%) |
| Control | 1 (9.1%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (9.1%) | |
| Increase in sensitivity | ME/CFS | 0 (0.0%) | 2 (4.1%) | 2 (4.1%) | 1 (2.0%) | 1 (2.0%) | 3 (6.1%) |
| Control | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Light-headedness | ME/CFS | 14 (28.6%) | 4 (8.2%) | 9 (18.4%) | 6 (12.2%) | 6 (12.2%) | 23 (46.9%) |
| Control | 1 (9.1%) | 0 (0.0%) | 1 (9.1%) | 0 (0.0%) | 0 (0.0%) | 2 (18.2%) | |
| Mood disturbance | ME/CFS | 2 (4.1%) | 3 (6.1%) | 7 (14.3%) | 4 (8.2%) | 6 (12.2%) | 14* (28.6%) |
| Control | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Muscle/joint pain | ME/CFS | 17* (34.7%) | 28 (57.1%) | 20 (40.8%) | 19 (38.8%) | 19* (38.8%) | 40† (81.6%) |
| Control | 0 (0.0%) | 3 (27.3%) | 2 (18.2%) | 2 (18.2%) | 0 (0.0%) | 4 (36.3%) | |
| Neurologic symptoms | ME/CFS | 1 (2.0%) | 1 (2.0%) | 1 (2.0%) | 3 (6.1%) | 4 (8.2%) | 7 (14.3%) |
| Control | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Pain | ME/CFS | 8 (16.3%) | 15* (30.6%) | 7 (14.3%) | 16* (32.7%) | 16* (32.7%) | 26† (53.1%) |
| Control | 1 (9.1%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (9.1%) | |
| Positive feelings/mood | ME/CFS | 4 (8.2%) | 5 (10.2%) | 3 (6.1%) | 3 (6.1%) | 1 (2.0%) | 9 (18.4%) |
| Control | 5† (45.5%) | 7§ (63.6%) | 6§ (54.5%) | 8§ (72.7%) | 0 (0.0%) | 8§ (72.7%) | |
| Sleep disturbances | ME/CFS | 10 (20.4%) | 15 (30.6%) | 8 (16.3%) | 18* (36.7%) | 13* (26.5%) | 28† (57.1%) |
| Control | 1 (9.1%) | 1 (9.1%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (9.1%) | |
| Temperature control | ME/CFS | 7 (14.3%) | 1 (2.0%) | 3 (6.1%) | 4 (8.2%) | 4 (8.2%) | 11 (22.4%) |
| Control | 1 (9.1%) | 0 (0.0%) | 1 (9.1%) | 0 (0.0%) | 0 (0.0%) | 1 (9.1%) | |
| Tingling | ME/CFS | 2 (4.1%) | 2 (4.1%) | 4 (8.2%) | 2 (4.1%) | 4 (8.2%) | 5 (10.2%) |
| Control | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Weakness | ME/CFS | 18 (36.7%) | 6 (12.2%) | 10 (20.4%) | 10 (20.4%) | 11 (22.4%) | 27* (55.1%) |
| Control | 2 (18.2%) | 1 (9.1%) | 2 (18.2%) | 0 (0.0%) | 0 (0.0%) | 2 (18.2%) | |
* - statistically significant difference in proportions between groups, p≤.05. † - statistically significant difference in proportions between groups, p≤.01. § - statistically significant difference in proportions between groups, p≤.001.
3.2.1Immediately after CPET 1
Positive feelings and mood were reported by significantly fewer patients with ME/CFS compared to sedentary control subjects, while cognitive dysfunction and muscle and joint pain were reported by significantly more patients with ME/CFS than sedentary control subjects.
3.2.2Twenty-four hours after CPET 1
Cognitive dysfunction, headache, and pain were reported significantly more frequently by patients with ME/CFS compared to sedentary control subjects. Patients with ME/CFS reported positive feelings and mood significantly less often than sedentary control subjects.
3.2.3Immediately after CPET 2
Patients with ME/CFS reported a decrease in function and headache more frequently than sedentary control subjects. Patients with ME/CFS reported positive feelings and mood significantly less frequently than sedentary control subjects.
3.2.4Twenty-four hours after CPET 2
Patients with ME/CFS reported cognitive dysfunction, decrease in function, headache, pain, sleep disturbances, and worsened symptoms significantly more frequently than sedentary control subjects. Patients with ME/CFS reported positive feelings and mood significantly less frequently than sedentary control subjects.
3.2.5One week following CPET 2
Cognitive dysfunction, decrease in function, muscle and joint pain, pain, and sleep disturbances were reported significantly more frequently by patients with ME/CFS than sedentary control subjects. Patients with ME/CFS reported positive feelings and mood significantly less frequently than sedentary control subjects.
3.2.6At any time during and after the CPET task
Patients with ME/CFS reported cognitive dysfunction, decrease in function, flu-like symptoms, gastrointestinal disturbance, headache, mood disturbance, muscle and joint pain, pain, sleep disturbances, and weakness more frequently during the 2-day CPET task compared to sedentary control subjects. Patients with ME/CFS also reported positive feelings and mood significantly less frequently than sedentary control subjects.
3.3Clinimetric properties of individual PEM symptoms
At all time points during and after CPET, all symptoms except fatigue generally demonstrated high specificity and low sensitivity (Table 2). Fatigue had higher sensitivity that generally increased over each of the five time points in this study.
Table 2
Diagnostic accuracy of symptom expression to predict group membership (myalgic encephalomyelitis/chronic fatigue syndrome; ME/CFS vs. control) after cardiopulmonary exercise testing (CPET) at five time points: immediately after the first CPET, 24 hours after the first CPET, immediately after the second CPET, 24 hours after the second CPET, 1 week after the second CPET, and any time point during the CPET series. Analysis included for symptoms demonstrating significant between-groups differences in frequency as shown in Table 1. Hatched boxes indicate non-significant difference in frequency of the symptom between groups at that timepoint. Parenthetical values are 95% confidence intervals
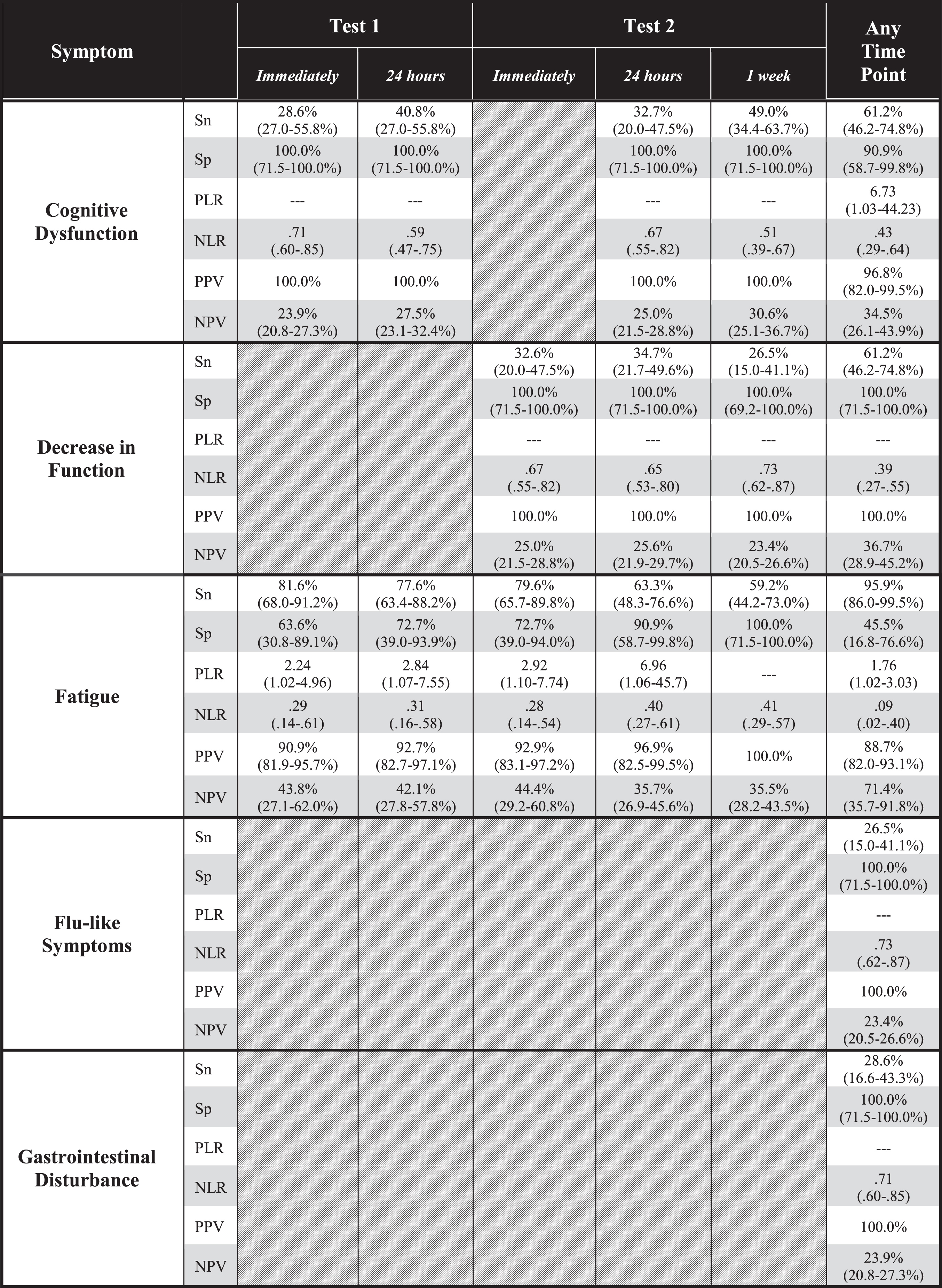 |
Sn: sensitivity; Sp: specificity; PLR: positive likelihood ratio; NLR: negative likelihood ratio; PPV: positive predictive value; NPV: negative predictive value.
3.4Clinimetric properties of PEM symptom clusters
ROC analysis indicated significant associations between symptom reports and group membership (Table 3). AUC calculations ranged between .855 (95% CI: .746-.965, p < .001) immediately after CPET 1 and .949 (95% CI: .895-1.000, p < .001). 7 days after CPET 2, suggesting the strength of association generally increased across all time points during the 2-day CPET task. Cluster sizes for possible symptoms also generally increased across all time points during the 2-day CPET task.
Table 3
Diagnostic accuracy of symptom clusters at various timepoints during serial cardiopulmonary exercise testing (CPET). To assure consistency in directionality, “positive feelings/mood” was re-phrased to “absence of positive feelings/mood” for this analysis
| Timepoint | Criteria | AUC (95% CI) | p-value | Sensitivity | Specificity |
| Immediately after CPET 1 | Any two of the following symptoms: •Cognitive dysfunction •Fatigue •Muscle/joint pain •Absence of positive feelings/mood | .855 (.746-.965) | <.001 | .857 | .636 |
| 24 hours after CPET 1 | Any two of the following symptoms: •Cognitive dysfunction •Fatigue •Headache •Pain •Absence of positive feelings/mood | .898 (.813-.983) | <.001 | .878 | .818 |
| Immediately after CPET 2 | Any two of the following symptoms: •Decrease in function •Fatigue •Headache •Absence of positive feelings/mood | .865 (.754-.977) | <.001 | .816 | .727 |
| 24 hours after CPET 2 | Any two of the following symptoms: •Cognitive dysfunction •Decrease in function •Fatigue •Headache •Pain •Absence of positive feelings/mood •Sleep disturbances | .927 (.861-.992) | <.001 | .878 | .909 |
| 1 week after CPET 2 | Any one of the following symptoms: •Cognitive dysfunction •Decrease in function •Fatigue •Muscle/joint pain •Pain •Sleep disturbances | .949 (.895-1.000) | <.001 | .898 | 1.000 |
AUC: area under the receiver operating characteristics curve; CI: confidence interval; CPET: cardiopulmonary exercise test.
Diagnostic accuracy was optimal with two symptoms for the first four time points during the 2-day CPET task: immediately after CPET 1, 24 hours after CPET 1, immediately after CPET 2, and 24 hours after CPET 2 (Table 3). Sensitivity (Sn) and specificity (Sp) were .857 and .636, .878 and .818, .816 and .727, and .878 and .909 for each of the first four time points during the 2-day CPET task, respectively. The composition of symptom clusters varied somewhat but had in common fatigue, cognitive dysfunction, pain, headache, and absence of positive feelings or mood. At 7 days following CPET 2, diagnostic accuracy was optimal with only one symptom of a list consisting of cognitive dysfunction, decrease in function, fatigue, muscle/joint pain, pain, and sleep disturbances (Sn:.898, Sp: 1.000).
4Discussion
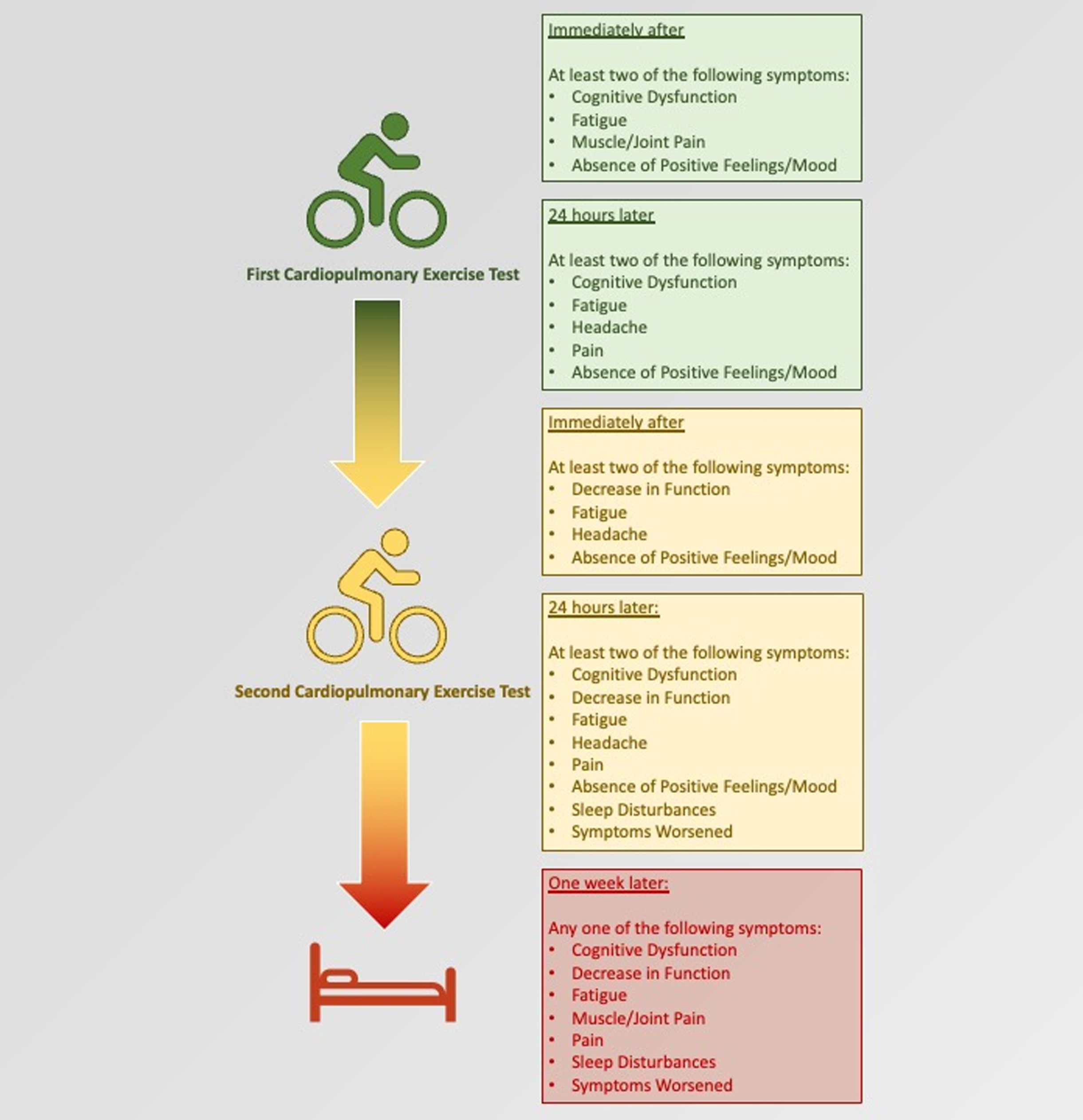
Post-exertional malaise may manifest in a multitude of ways with different symptoms, timing, and intensity. Yet, the findings of this study indicate clinicians only need to focus on the presence and duration of just a few symptom categories and prolonged duration to identify its existence. For each of the five time points assessed in this study, only 1 or 2 symptom categories were necessary to distinguish study participants affected by ME/CFS from those of healthy controls (Fig. 1). Furthermore, although fatigue showed up at all five time points and muscle/joint pain appeared twice, cognitive dysfunction, decline in function, and headaches – symptoms which are not typically induced by a short bout of physical exercise – each appeared three or more times. Subjects affected by ME/CFS also did not feel better overall after exercise. Thus, atypical symptoms may also provide a clue to PEM. This paper also reinforces symptom persistence as a key feature of PEM. Previously we found that inability to recover from a CPET within 24 hours increased the pre-test probability that a patient suffered from ME/CFS by 45% (positive likelihood ratio = 11) [25]. In this study, we found that even the existence of one symptom 7 days after CPET could predict ME/CFS group membership.
Surprisingly, even though many patients have reported problems with functioning due to PEM, formal emphasis of post-exertional changes in function have not been widespread. Most ME/CFS case definitions describe substantial decreases in functioning after onset of ME/CFS compared to the patient’s state pre-illness but do not include temporary declines in function when PEM is triggered. For example, the 1994 Fukuda criteria [6] only notes that PEM should last 24 hours or more whereas the 2003 Canadian Consensus Criteria (CCC) [9] and 2011 Myalgic Encephalomyelitis-International Consensus Criteria (ME-ICC) [28] both mention a prolonged recovery period but not recovery of function explicitly. However, beginning in 2017, the US National Institutes of Health [29] included “loss of...functional capacity” as a feature of PEM. Subsequently, a 2019 survey of 1,534 people [30] affected by ME/CFS found that “reduced stamina and functional ability” was the most common consequence of PEM (selected by 99.4%), edging out even “physical fatigue” (chosen by 98.9%).
A reduction in function after exertion might have been assumed to be such common knowledge that highlighting of this outcome seems unnecessary. It is not clear though whether clinicians ask patients about function during appointments focused on ME/CFS. Past research in general medical settings discovered that between 60% -98% of medical records contain no documentation of function [31], absence of data did not equate to absence of disability, and physicians failed to recognize or underestimated the level of patient disability by 66% [32]. Further, younger patients were less likely to be assessed and, unexpectedly, severity or chronicity of illness did not correlate with increased attention to function [30]. Generally, past research has indicated some clinicians may not be well-educated about functional assessment or pay less attention to function, particularly when the patient is younger and appears normal/healthy during a clinical encounter. Hardly any research papers focusing on PEM have measured functional changes after exertion, especially in the context of patients’ daily lives. Study participants are primarily queried about symptom intensity which may or may not affect function. Patients and caregivers also struggle to explain the impact of PEM on their lives: many are not familiar with the concepts of basic or instrumental activities of daily living that healthcare professionals are exposed to during their training. They may not know how to convey their difficulties in a meaningful and actionable way to clinicians. Yet, generally, most patients view assessment of function as a vital component of holistic care [33]. If clinicians and researchers do not query about function and patients/caregivers do not voluntarily raise the topic, it is unsurprising this important feature of PEM may be under-emphasized in research and clinical practice.
Fig. 1
Summary of symptom clusters at each time point during the study, which differentiated between people with ME/CFS and sedentary control subjects.
Questionnaires that are sensitive to post-exertional changes in symptoms and function are not yet well developed. In this study, participants could communicate in an open-ended manner about changes in functioning. For example, one person recounted they could not sit up for dinner and an accountant observed they could not perform simple math in the hours to days post-CPET. Most instruments developed to evaluate physical and cognitive capacity are designed to measure static states or changes occurring over weeks to months rather than the hours to days over which PEM frequently occurs. For example, the Medical Outcomes Survey Short Form-36 (SF-36) is the most tested and used functional outcome measure in ME/CFS populations [34] yet it may not be a suitable measure of PEM-reduced function. Haywood noted that most patient-reported outcome measures used in ME/CFS studies have not been developed with, nor tested for content validity among patients. Items comprising the SF-36 Physical Function and Role Limitations due to Physical Health subscales, respectively, ask respondents to compare their current state to a vague, possibly pre-illness state (“The following items are about activities you might do during a typical day. Does your health now limit you in these activities?”) or to determine the impact of their illness within the last 4 weeks, respectively [35, 36]. Neither of these time frames are appropriate to measure fluctuations in functioning after exertion. Whether PEM is induced by 2 CPETs separated by 1 day [24] or by ordinary, daily activity [14], 50% of subjects reported recovering within 1 week. Thus, items about changes over a 4-week time frame are inappropriate.
Other than ambulatory, bathing, and grooming activities, SF-36 items do not ask about basic or instrumental activities of daily living. Moreover, cognitive dysfunction was one of the key symptoms distinguishing between subjects with ME/CFS and healthy subjects, yet the SF-36 does not contain any times pertaining to cognitive function specifically. Finally, for the most severely affected patients, the SF-36 may not be sensitive enough to demonstrate further declines in function following activity. For 5 out of 8 SF-36 subscales, more than 10% of participants in Davenport et al. [22] reported the lowest score of “0” at baseline before exercise testing. Thus, there is no lower score participants can select if they experience post-exercise deterioration.
Outside of patient-reported function, the increasing availability of inexpensive, easy-to-use, portable monitoring devices may allow accurate, quantitative measurement of function remotely. This is especially important given the delayed and prolonged nature of PEM. PEM can start or peak hours to days after the study participant has already left the research site. Returning for repeated assessments is not possible for many participants. Several previous studies have documented functional changes after a standardized challenge. For example, McCully and Black used a simple pedometer to demonstrate the number of steps walked during an exercise program deteriorated over time [37] while Natelson et al. used a custom-designed, watch-like device to show decreased activity, increased daily naps, but no change in cognitive function occurring over days after one maximal CPET [38, 39]. Others have demonstrated cognitive changes, such as decreased reaction time or working memory after a physical or orthostatic challenge [40, 41]. Although these cognitive outcomes have been assessed onsite, the advent of cognitive testing online [42] and/or via mobile devices [43] may make longitudinal assessment post-activity more feasible soon.
A lack of positive feelings/mood change following exercise is another demonstration of the maladaptive response to physical activity endured by ME patients. Popularly referred to as a “runner’s high,” athletes have described feeling “emotionally lighter”, “optimistic”, “empowered and strong,” “extreme energy”, and “incredible energy” after a workout [44]. Healthy, sedentary people [45], patients diagnosed with depression and anxiety [46], as well as those with non-clinical levels of psychological distress [47] can also reap the benefits of even short, vigorous bouts of exercise [41]. Eighty percent of our healthy, sedentary participants recounted feeling increased energy after CPET, compared to 18% of patients with ME/CFS [24]. These results are consistent with Loy et al.’s meta-analyses. They demonstrated that for ME/CFS participants, a single bout of exercise increased fatigue moderately, especially at 4 to 96 hours (4 days) after exercise [48]. For non-ME/CFS participants, even some with baseline fatigue, acute exercise boosted energy considerably even as fatigue might not decrease unless the activity was low intensity and lasted more than 20 minutes [45].
Additionally, although it did not rise to the level of statistical significance, none of the healthy participants experienced mood disturbances whereas almost a third of the sick participants did, noting increased anxiety, restlessness, “low mood” for example post-CPET. Similarly, following a 4-week exercise regimen, McCully [49] found that overall mood increased in healthy study participants but not in participants with ME/CFS, and, after a series of cognitive exercises, Arroll observed a significant increase in depressive feelings among people with ME/CFS [50]. In ME/CFS patients without mood disorders, increases in emotional lability, irritability, depression, and reduction in motivation, were also the most common, new (i.e., not present at baseline) symptoms preceding episodes of PEM [51]. Ghali [51] suggested that noticing such symptoms might assist patients in figuring out their activity parameters.
Both psychological and physiological mechanisms normally create mood elevation following physical activity, but these benefits appear to be lost for patients with ME/CFS. Negative memories of PEM likely contribute to this response [52] but abnormalities in both peripheral (co-released opioids with norepinephrine) and/or central (pro-opiomelanocortin derived) endogenous opioids to produce mood altering effects are possible [53, 54].
There is some agreement between this study’s results and our prior studies. Findings from this study reduce the number of symptoms needed to identify PEM from 3 to 2 [25]. In our 2010 study, 74% (17 out of 23) of healthy, sedentary subjects reported increased energy following CPET whereas none of the 25 ME/CFS-afflicted participants did [16]. These latter participants instead related not only worsened fatigue but temporary reductions in their ability to walk, rise out of bed, think clearly, and carry on a conversation. Our prior work and this study agree that cognitive dysfunction may be a prominent symptom [16]. In this study, cognitive dysfunction was involved in the symptom cluster that reliably separates subjects with ME/CFS and sedentary control subjects in 4 out of the 5 time periods assessed during the 2-day CPET.
Despite notable similarities, a direct comparison of this study with prior work is not possible since symptom categories were inconsistent between studies. For example, we did not include a “neuroendocrine” category this time and conversely, past studies did not explicitly examine cognitive dysfunction, reduction in function, or improved mood/energy. Univariate analysis demonstrate that pain symptoms (other than headache) and sleep disturbances were significantly associated with ME/CFS membership. However, other symptoms proved to be more significant. This is a departure from our 2011 results [25] which identified a 3-symptom cluster of pain, immune, and sleep disturbance symptoms as adequate for recognizing PEM.
Major strengths of this study include enrollment of healthy yet sedentary controls, administration of a standardized exercise stimulus repeated across 2 days, utilization of open-ended questionnaires, and monitoring of study participants for a week post-CPET. Open-ended questionnaires permitted participants to write freely about how they felt, without predetermined categories. Most studies of PEM have followed participants for 3 or fewer days even though patients have reported their PEM lasting much longer. Extending follow-up to 7 days allowed us to capture this prolonged experience.
Limitations of this study are the small sample size, absence of a single case definition used to recruit sick participants, and voluntary participation by study subjects. Because exercise exacerbates symptoms people afflicted by ME/CFS, those who are more severely ill, less passionate about partaking in research, or not threatened with loss of employment or disability benefits will be less likely to participate in any exercise study. Consequences originating from strenuous physical activity may not be equivalent to post-exertional symptoms following mild/moderate exertion, basic and instrumental activities of daily living (e.g., cooking, walking) or non-physical challenges (e.g., cognitive, orthostatic, etc.). Using one-time, open-ended, written questionnaires meant we could not clarify what participants precisely meant in some cases. For example, we could not confirm whether a lack of positive feelings after exertion referred specifically to emotions or energy. We also could not distinguish whether “lack” meant an increase in the negative feeling (worsened mood, increased fatigue) or no improvement in energy and/or mood as the participant might have experienced before they were sick. Some researchers also believe energy and fatigue may be separate concepts rather than directly opposing ones: that is, someone can experience both increased fatigue and energy at the same time [45].
Our results would benefit from further validation in larger, more diverse samples, including participants who fit specific ME/CFS criteria and control groups affected by other exertion-exacerbated, chronic medical conditions. Correlating participant-reported symptoms and function with more objective outcomes such as CPET metabolic outputs, well-validated activity of daily living scales, cognitive assessments, or daily step counts would lend further strength. Evaluating for not just presence but severity of symptoms might also increase how accurately sick versus healthy participants are classified. Operationalization of our findings into a brief self-administered questionnaire or standardized set of questions with answer choices faithfully reflecting patients’ experiences will also be needed. Inconsistent phrasing of questions and difficulty interpreting patients’ diverse responses will not improve recognition of PEM. We plan to investigate these issues in the near future and encourage other researchers to do so as well.
Recognition of PEM could also have equally important implications for patients suffering from post-acute COVID-19 syndrome (also known as Long Covid) where it may be called post-exertional symptom exacerbation (PESE) or post-exertional neuroimmune exhaustion (PENE) [55]. A recent study looking at chronic fatigue and PEM in long COVID patients found that 58.7% participants met the scoring thresholds used for people with ME/CFS on the previously mentioned DSQ-PEM [20]. Characterizing the nature of post-exertional symptom exacerbation in this population was deemed important for developing and evaluating rehabilitation strategies, including the potential benefits or harms of exercise for persons living with long-COVID [56].
5Conclusion
Although PEM is a complex phenomenon, researchers and clinicians may not have to engage in lengthy conversations or utilize complicated questionnaires to identify its existence. Medical professionals can efficiently assess for PEM by focusing on a specific set of post-exertional symptoms and the overall functional impact of those symptoms in the days following physical exertion.
Conflict of interest
The authors declare that they have no conflicts of interest.
Funding
The authors report no funding.
Acknowledgments
The authors thank the study participants for helping to advance their understanding of ME/CFS.
References
[1] | CDC team takes ME/CFS around the world | Blogs | CDC. https://blogs.cdc.gov/global/2020/05/12/cdc-team-takes-me-cfs-around-the-world/. Accessed 2 Sept. 2022. |
[2] | Bakken IJ , Tveito K , Gunnes N , Ghaderi S , Stoltenberg C , Trogstad L , et al. Two age peaks in the incidence of chronic fatigue syndrome/myalgic encephalomyelitis: a population-based registry study from Norway 2008-2012. BMC Medicine. (2014) ;12: :167. doi: 10.1186/s12916-014-0167-5. |
[3] | Pheby D , Saffron L . Risk factors for severe ME/CFS. Biology and Medicine. (2009) ;1: :50–74. |
[4] | CFIDS Association of America. ME-CFS road to diagnosis survey [Internet]. USA. Solve CFS.; 2014 Jan [cited 9 May 2022]. Available from: https://solvecfs.org/wp-content/uploads/2014/01/IOM_RoadtoDiagnosisSurveyReport.pdf |
[5] | Understanding history of case definitions and criteria | healthcare providers | myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) | CDC. 26 July 2022, https://www.cdc.gov/me-cfs/healthcare-providers/case-definitions-criteria.html |
[6] | Fukuda K , Straus SE , Hickie I , Sharpe MC , Dobbins JG , Komaroff A . The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study GrouAnn Intern Med. (1994) ;121: :953–959. doi: 10.7326/0003-4819-121-12-199412150-00009. |
[7] | Jason LA , Taylor RR , Kennedy CL , Song S , Johnson D , Torres S . Chronic fatigue syndrome: occupation, medical utilization, and subtypes in a community-based sample. J Nerv Ment Dis. (2000) ;188: :568–576. doi: 10.1097/00005053-200009000-00002. |
[8] | Jason LA , Katz BZ , Sunnquist M , Torres C , Cotler J , Bhatia S . The prevalence of pediatric myalgic encephalomyelitis/chronic fatigue syndrome in a community-based sample. Child Youth Care Forum. (2020) ;49: :563–579. doi: 10.1007/s10566-019-09543-3. |
[9] | Carruthers BM , Jain AK , De Meirleir KL , Peterson DL , Klimas NG , Lerner AM , et al. Myalgic encephalomyelitis/chronic fatigue syndrome. Journal of Chronic Fatigue Syndrome. (2003) ;11: :7–115. doi: 10.1300/J092v11n01_02. |
[10] | Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome, Board on the Health of Select Populations, Institute of Medicine. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: redefining an illness. Washington (DC): National Academies Press (US); 2015. Available: http://www.ncbi.nlm.nih.gov/books/NBK274235/. |
[11] | US Centers for Disease Control and Prevention. IOM 2015 Diagnostic Criteria | Diagnosis | Healthcare Providers | Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) | CDC. 27 Apr 2021 [cited 10 May 2022]. Available: https://www.cdc.gov/me-cfs/healthcare-providers/diagnosis/iom-2015-diagnostic-criteria.html. |
[12] | US National Institutes of Health. RFA-NS-22-019: Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) Collaborative Research Centers (CRCs) (U54, Basic Experimental Studies Involving Humans Allowed) [Internet]. [cited 10 May 2022]. Available: https://grants.nih.gov/grants/guide/rfa-files/RFA-NS-22-019.html. |
[13] | Montoya JG , Dowell TG , Mooney AE , Dimmock ME , Chu L . Caring for the patient with severe or very severe myalgic encephalomyelitis/chronic fatigue syndrome. Healthcare (Basel). (2021) ;9: :1331. doi: 10.3390/healthcare9101331. |
[14] | Stussman B , Williams A , Snow J , Gavin A , Scott R , Nath A , et al. Characterization of post–exertional malaise in patients with myalgic encephalomyelitis/chronic fatigue syndrome. Front Neurol. (2020) ;11: :1025. doi: 10.3389/fneur.2020.01025. |
[15] | Chu L , Valencia IJ , Garvert DW , Montoya JG . Deconstructing post-exertional malaise in myalgic encephalomyelitis/chronic fatigue syndrome: A patient-centered, cross-sectional survey. PLoS One. (2018) ;13: :e0197811. doi: 10.1371/journal.pone.0197811. |
[16] | VanNess JM , Stevens SR , Bateman L , Stiles TL , Snell CR . Postexertional malaise in women with chronic fatigue syndrome. J Womens Health (Larchmt). (2010) ;19: :239–244. doi: 10.1089/jwh.2009.1507. |
[17] | Measures – Leonard A. Jason. [cited 5 Jun 2022]. Available: https://www.leonardjason.com/cfsme_measures-2/. |
[18] | NINDS ME/CFS Common Data Elements Working Group. Guidance for core PEM assessment: DePaul Symptoms Questionnaire subscale. US National Institutes of Health National Institute of Neurologic Disorders and Stroke. (NINDS); 2017 [cited 4 Jun 2022]. Available: https://www.commondataelements.ninds.nih.gov/sites/nindscde/files/Doc/MECFS/F2771_Guidance_for_Core_PEM_Assessment.pdf. |
[19] | Results of the poll to inform the NIH/CDC’s definition of PEM in all their future ME/CFS research. In: Science for ME [Internet]. [cited 5 Jun 2022]. Available: https://www.s4me.info/threads/results-of-the-poll-to-inform-the-nih-cdc%E2%80%99s-definition-of-pem-in-all-their-future-me-cfs-research.2221/. |
[20] | Cotler J , Holtzman C , Dudun C , Jason LA . A brief questionnaire to assess post-exertional malaise. Diagnostics (Basel). (2018) ;8: :66. doi: 10.3390/diagnostics8030066. |
[21] | US Food and Drug Administration. Guidance for industry chronic fatigue syndrome/myalgic encephalomyelitis: developing drug products for treatment. US Food and Drug Administration; Mar 2014 [cited 4 Jun 2022]. Available: https://www.fda.gov/media/87875/download. |
[22] | Davenport TE , Stevens SR , Baroni K , Van Ness JM , Snell CR . Reliability and validity of Short Form 36 Version 2 to measure health perceptions in a sub-group of individuals with fatigue. Disabil Rehabil. (2011) ;33: :2596–2604. doi: 10.3109/09638288.2011.582925. |
[23] | Snell CR , Stevens SR , Davenport TE , Van Ness JM . Discriminative validity of metabolic and workload measurements for identifying people with chronic fatigue syndrome. Phys Ther. (2013) ;93: :1484–1492. doi: 10.2522/ptj.20110368. |
[24] | Mateo LJ , Chu L , Stevens S , Stevens J , Snell CR , Davenport T , et al. Post-exertional symptoms distinguish Myalgic Encephalomyelitis/Chronic Fatigue Syndrome subjects from healthy controls. Work. (2020) ;66: :265–275. doi: 10.3233/WOR-203168. |
[25] | Davenport TE , Stevens SR , Baroni K , Van Ness M , Snell CR . Diagnostic accuracy of symptoms characterising chronic fatigue syndrome. Disabil Rehabil. (2011) ;33: :1768–1775. doi: 10.3109/09638288.2010.546936. |
[26] | Chu L , Valencia IJ , Garvert DW , Montoya JG . Deconstructing post-exertional malaise in myalgic encephalomyelitis/chronic fatigue syndrome: A patient-centered, cross-sectional survey. PLoS One. (2018) ;13: :e0197811. doi: 10.1371/journal.pone.0197811. |
[27] | Stevens S , Snell C , Stevens J , Keller B , VanNess JM . Cardiopulmonary exercise test methodology for assessing exertion intolerance in myalgic encephalomyelitis/chronic fatigue syndrome. Frontiers in Pediatrics. 2018;6.Available: https://www.frontiersin.org/article/10.3389/fped.2018.00242 |
[28] | Carruthers BM , van de Sande MI , De Meirleir KL , Klimas NG , Broderick G , Mitchell T , et al. Myalgic encephalomyelitis: International Consensus Criteria. Journal of Internal Medicine. (2011) ;270: :327–338. doi: 10.1111/j.1365-2796.2011.02428.x. |
[29] | United States National Institute of Neurological Disorders and Stroke Common Data Elements Project Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) Post-Exertional Malaise Subgroup. Subgroup statement of purview [Internet]. Washington DC (USA): US National Institutes of Health; 2018 [cited 4 September 2022]. Available: https://www.commondataelements.ninds.nih.gov/sites/nindscde/files/Doc/MECFS/PEM_Subgroup_Summary.pdf. |
[30] | Holtzman CS , Bhatia S , Cotler J , Jason LA . Assessment of post-exertional malaise (PEM) in patients with myalgic encephalomyelitis (ME) and chronic fatigue syndrome (CFS): a patient-driven survey. Diagnostics (Basel). (2019) ;9: :26. doi: 10.3390/diagnostics9010026. |
[31] | Bogardus ST , Towle V , Williams CS , Desai MM , Inouye SK . What does the medical record reveal about functional status? J Gen Intern Med. (2001) ;16: :728–736. doi: 10.1111/j.1525-1497.2001.00625.x. |
[32] | Calkins DR , Rubenstein LV , Cleary PD , et al. Failure of physicians to recognize functional disability in ambulatory patients. Ann Intern Med. (1991) ;114: (6):451-454. doi: 10.7326/0003-4819-114-6-451. |
[33] | Nicosia FM , Spar MJ , Neumann A , Silvestrini MC , Barrientos M , Brown RT . “The more they know, the better care they can give”: patient perspectives on measuring functional status in primary care. J Gen Intern Med. (2020) ;35: :2947–2954. doi: 10.1007/s11606-020-06075-8. |
[34] | Haywood KL , Staniszewska S , Chapman S . Quality and acceptability of patient-reported outcome measures used in chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME): a systematic review. Qual Life Res. (2012) ;21: :35–52. doi: 10.1007/s11136-011-9921-8. |
[35] | RAND Corporation. 36-Item Short Form Survey Instrument (SF-36). [cited 2 Jul 2022]. Available: https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form/survey-instrument.html |
[36] | RAND Corporation. 36-Item Short Form Survey (SF-36) Scoring Instructions. [cited 2 Jul 2022]. Available: https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form/scoring.html. |
[37] | Black CD , McCully KK . Time course of exercise induced alterations in daily activity in chronic fatigue syndrome. Dyn Med. (2005) ;4: :10. doi: 10.1186/1476-5918-4-10. |
[38] | Sisto SA , Tapp WN , LaManca JJ , Ling W , Korn LR , Nelson AJ , et al. Physical activity before and after exercise in women with chronic fatigue syndrome. QJM. (1998) ;91: :465–473. doi: 10.1093/qjmed/91.7.465. |
[39] | Yoshiuchi K , Cook DB , Ohashi K , Kumano H , Kuboki T , Yamamoto Y , et al. A real-time assessment of the effect of exercise in chronic fatigue syndrome. Physiol Behav. (2007) ;92: :963–968. doi: 10.1016/j.physbeh.2007.07.001. |
[40] | Cook DB , Light AR , Light KC , Broderick G , Shields MR , Dougherty RJ , et al. Neural consequences of post-exertion malaise in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Brain, Behavior, and Immunity. (2017) ;62: :87–99. doi: 10.1016/j.bbi.2017.02.009. |
[41] | van Campen CLMC , Rowe PC , Verheugt FWA , Visser FC . Cognitive function declines following orthostatic stress in adults with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Front Neurosci. (2020) ;14: :688. doi: 10.3389/fnins.2020.00688. |
[42] | CNS Vital Signs - Remote Test. [cited 2 Jul 2022]. Available: https://www.cnsvs.com/RemoteTest.html |
[43] | Koo BM , Vizer LM . Mobile technology for cognitive assessment of older adults: a scoping review. Innov Aging. (2019) ;3: :igy038. doi: 10.1093/geroni/igy038. |
[44] | What does a runner’s high feel like? 10 runners explain. In: ClassPass Blog [Internet]. 12 Nov 2021 [cited 5 Aug 2022]. Available: https://classpass.com/blog/what-does-a-runners-high-feel-like/. |
[45] | Loy BD , O’Connor PJ , Dishman RK . The effect of a single bout of exercise on energy and fatigue states: a systematic review and meta-analysis. Fatigue: Biomedicine, Health & Behavior. (2013) ;1: :223–242. doi: 10.1080/21641846.2013.843266. |
[46] | Md Zemberi NFN , Ismail MM , Abdullah MFIL . Exercise interventions as the primary treatment for depression: evidence from a narrative review. Malays J Med Sci. (2020) ;27: :5–23. doi: 10.21315/mjms2020.27.5.2. |
[47] | Rebar AL , Stanton R , Geard D , Short C , Duncan MJ , Vandelanotte C . A meta-meta-analysis of the effect of physical activity on depression and anxiety in non-clinical adult populations. Health Psychol Rev. (2015) ;9: :366–378. doi: 10.1080/17437199.2015.1022901. |
[48] | Loy BD , O’Connor PJ , Dishman RK . Effect of acute exercise on fatigue in people with ME/CFS/SEID: A Meta-analysis. Med Sci Sports Exerc. (2016) ;48: :2003–2012. doi: 10.1249/MSS.0000000000000990. |
[49] | McCully K , Hendrix BA , Black C , Black D , McCully K , et al. Effects of four weeks of increased physical activity in individuals with chronic fatigue syndrome. 2003 [cited 5 Aug 2022]. Available: https://www.semanticscholar.org/paper/EFFECTS-OF-FOUR-WEEKS-OF-INCREASED-PHYSICAL-IN-WITH-McCully-B.A/3e43660c602e07a959d8056e830554e5d88fece5 |
[50] | Arroll MA , Attree EA , O’Leary JM , Dancey CP . The delayed fatigue effect in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Fatigue: Biomedicine, Health & Behavior. (2014) ;2: :57–63. doi: 10.1080/21641846.2014.892755. |
[51] | Ghali , Alaa , et al. Warning Signals of Post-Exertional Malaise in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Retrospective Analysis of 197 Patients. Journal of Clinical Medicine. 2021;10(11):2517. PubMed Central, https://doi.org/10.3390/jcm10112517. |
[52] | Anderson Rachel J , Samuel Brice . The mood-enhancing benefits of exercise: memory biases augment the effect. Psychology of Sport and Exercise. (2011) ;12: (2):79-82. ScienceDirect, https://doi.org/10.1016/j.psychsport.2010.08.003. |
[53] | Chen C , Nakagawa S , An Y , Ito K , Kitaichi Y , Kusumi I . The exercise-glucocorticoid paradox: How exercise is beneficial to cognition, mood, and the brain while increasing glucocorticoid levels. Front Neuroendocrinol. (2017) ;44: :83–102. doi: 10.1016/j.yfrne.2016.12.001. |
[54] | Oosterwijck, Jessica Van, et al. The role of autonomic function in exercise-induced endogenous analgesia: a case-control study in myalgic encephalomyelitis/chronic fatigue syndrome and healthy people. Pain Physician. (2017) ;20: (3):E389–99. |
[55] | Mackay A . A paradigm for post-Covid-19 fatigue syndrome analogous to ME/CFS. Frontiers in Neurology. 2021;12:701419. DOI.org (Crossref), https://doi.org/10.3389/fneur.2021.701419. |
[56] | Twomey R , DeMars J , Franklin K , Culos-Reed S , Weatherald J , Wrightson JG . Chronic fatigue and postexertional malaise in people living with long COVID: an observational study. Phys Ther. (2022) ;102: (4):pzac005. doi: 10.1093/ptj/pzac005. |



